Abstract
Voltage-gated sodium channels (VGSC) are a well-established drug target for anti-epileptic, anti-arrhythmic and pain medications due to their presence and important roles that they play in excitable cells. Recently, their presence has been recognized in non-excitable cells such as cancer cells and their overexpression has been shown to be associated with metastatic behavior in a variety of human cancers. The neonatal isoform of the VGSC subtype, Nav1.5 (nNav1.5) is overexpressed in the highly aggressive human breast cancer cell line, MDA-MB-231. The activity of nNav1.5 is known to promote the breast cancer cell invasion in vitro and metastasis in vivo, and its expression in primary mammary tumors has been associated with metastasis and patient death. Metastasis development is responsible for the high mortality of breast cancer and currently there is no treatment available to specifically prevent or inhibit breast cancer metastasis. In the present study, a 3D-QSAR model is used to assist the development of low micromolar small molecule VGSC blockers. Using this model we have designed, synthesized and evaluated five small molecule compounds as blockers of nNav1.5-dependent inward currents in whole-cell patch-clamp experiments in MDA-MB-231 cells. The most active compound identified from these studies blocked sodium currents by 34.9 ± 6.6 % at 1 μM. This compound also inhibited the invasion of MDA-MB-231 cells by 30.3 ± 4.5 % at 1 μM concentration without affecting the cell viability. The potent small molecule compounds presented here have the potential to be developed as drugs for breast cancer metastasis treatment.
Keywords: Breast cancer, nNav1.5, voltage-gated sodium channel, sodium current, cancer cell invasion, metastasis
Graphical Abstract

1. Introduction
Despite the increased awareness and advances in treatment, breast cancer remains as the leading cause of cancer death in women.1–2 Though the survival rate for patients diagnosed with localized breast cancer has improved significantly, it is the metastatic form of the disease that is mainly associated with mortality.3–4 Metastasis development has a complex mechanism which involves the acquisition of the ability by cancer cells to degrade and migrate through extracellular matrices (ECM) to distant tissues.5–7 The biggest hurdle in controlling breast cancer associated mortality is the lack of proper treatment for metastasis targeting and prevention.8–9 Thus, there is an urgent need to identify new druggable therapeutic targets for the treatment and prevention of breast cancer metastasis. One such promising anti-metastasis drug target is the voltage-gated sodium channels (VGSC).10–14 VGSCs have long been identified and characterized in excitable cells in which they are responsible for the initiation and propagation of action potentials.15–16 In recent years, VGSC expression has been found to be profoundly and aberrantly enhanced in some non-excitable cells, including a variety of aggressive human cancers of epithelial origin such as lung, prostate, ovarian, colon and breast cancer, and this overexpression is associated with cancer cell invasiveness.11, 17–22 Voltage-gated sodium channels are abnormally expressed in tumors, often as neonatal isoforms.23 These channels are not expressed in the non-aggressive cancer cells or normal tissue, providing selectivity in targeting metastatic cancer cells. Their expression levels and activities are related to the aggressiveness of the metastatic form of the cancer giving us the prospect of developing drugs that could specifically inhibit the neonatal VGSC leaving the adult isoform fully functional in the excitable cells.15
Nine different VGSC isoforms have been identified, and are classified as Nav1.1-Nav1.9.24–25 The major VGSC isoform expressed in aggressive tumor biopsies and metastatic breast cancer cells, such as triple-negative [estrogen receptors (ER−), progesterone receptor (PR−), human epidermal growth receptor 2 (HER-2−)] MDA-MB-231 cells,22 is nNav1.5, a neonatal splice variant showing a substitution of seven amino acids in the first domain of the channel compared to the adult isoform.26 In a normal scenario, VGSCs open and inactivate within a few milliseconds under membrane depolarization but in cancer cells nNav1.5 remains in a partially activated and non-inactivated state at the resting membrane potential of cells (around −40mV, because of a window of voltage between −60 and −20 mV), resulting in a continuous inward flow of Na+ ions (called window current).22 Literature reports strongly support the involvement of VGSC in breast cancer cell invasiveness; where overexpression of nNav1.5 (SCN5A gene) and low expression of auxiliary β4-subunit (SCN4B gene) promote the development of metastasis.10, 27–29 Metastasis development depends on numerous abilities acquired by highly aggressive cancer cells, one of which is their ability to degrade ECM mediated by various extracellular proteases such as matrix metalloproteinases and cysteine cathepsins.30–32 Investigations on the mechanistic role of nNav1.5 in metastasis revealed that nNav1.5 along with the Na+/H+ exchanger type 1 (NHE1) and caveolin-1 are all colocalized in the invasive structures of MDA-MB-231 cells, called invadopodia.28 NHE1, often overexpressed and over-activated in breast cancer cells, is the central regulator of intracellular and perimembrane pH augmenting the ECM proteolysis.33 nNav1.5 and NHE1 are functionally coupled to enhance H+ efflux, acidifying the extracellular pH.28 The extracellular matrix proteolytic activity in MDA-MB-231 cells is induced by acidic cysteine cathepsins B and S, which are released as soluble proforms in the extracellular microenvironment and are activated in an acidic pH.27, 31 Thus, nNav1.5 displays a persistent activity at the membrane potential of breast cancer cells, which is responsible for the increased ECM proteolysis and cancer cell invasiveness. Since the functional activity, and not simply the presence, of nNav1.5 channels is required to promote breast cancer cell invasiveness, nNav1.5 constitute a promising target for the breast cancer metastasis drug discovery.34 It is important to note that numerous clinically used drugs target various subtypes of VGSCs in excitable tissues. 35 These drugs have acceptable toxicity as find use as local anesthetics, antiarrhythmics, anticonvulsants, and for treating neuropathic pain.36 Similar clinical utility can also be anticipated for nNav1.5 blockers for the treatment of breast cancer, particularly since nNav1.5 is a neonatal variant not normally found in normal adult tissues.
Designing ligands for VGSCs has been, and continues to be, difficult since detailed structural information of the drug binding site for this integral membrane protein remain largely unclear. In recent years, we have developed a highly predictive, comprehensive 3D-QSAR model for the design of VGSC ligands using Comparative Molecular Field Analysis (CoMFA).37 This 3D-QSAR model samples the differences in steric and electrostatic fields surrounding each ligand in the training set, in 3D space, and correlates these changes with its biological activity.38 The training set for this model utilized several classes of drugs targeting sodium channels, including local anesthetics, anticonvulsants and antiarrhythmics.37 A total of 67 compounds were used to train this comprehensive 3D-QSAR model, which well covered 3D space and spanned over 4 orders of magnitude in biological activity. This model was used to design a large number of new small organic molecules, not yet synthesized or evaluated, with predicted VGSC activities near 100 nM. Several of these were then synthesized and evaluated for VGSC binding activity against a mixed population of VGSCs. Potency predictions by this model have been highly accurate for all compounds that were evaluated. Here we synthesized five compounds (Fig. 1) predicted to have low nanomolar VGSC binding and evaluated these for the inhibition of nNav1.5 currents and inhibition of invasion using MDA-MB-231 cells.
Figure 1.
Compounds evaluated for the inhibition of nNav1.5 currents in breast cancer MDA-MB-231 cells.
2. Results and Discussion
2.1. Chemistry
Two straightforward approaches were employed for the synthesis of the target compounds 1–5. Compounds 1–3 were synthesized by N-alkylation of the suitable amines by treatment with 4,4-di-(4-fluorophenyl)butyl chloride (6) in the presence of Et3N in DMSO at 80°C for 7–8 h as outlined in Scheme 1. Compound 1 was prepared by alkylation of 3-(N-piperidino)propylamine with compound 6 in DMSO in 60% yield. Alkylation of 2-phenethylamine with compound 6 yielded compound 2 in 58% yield and a similar alkylation of 3-phenylpropylamine with compound 6 afforded the target compound 3 in 60% yield.
Scheme 1.

Synthesis of compounds 1, 2 and 3.
Compounds 4 and 5 were synthesized via a reductive amination procedure as outlined in Schemes 2 and 3, respectively. The synthesis of compound 4 began with the reduction of 4-(4-fluorophenyl)-4-oxobutanoic acid (7) using borane/tert-butylamine complex and AlCl3 in anhydrous CH2Cl2 resulting in the formation of 4-(4-fluorophenyl)butan-1-ol (8) in 50% yield. Compound 8 was oxidized with PCC in CH2Cl2 to furnish 4-(4-fluorophenyl)butan-1-al (9) in 52% yield.39 The aldehyde (9) was then subjected to reductive amination using 3-(N-piperdino)propylamine in the presence of NaBH(OAc)3 in anhydrous CH2Cl2 resulting in the formation of compound 4 in 30% yield.
Scheme 2.
Synthesis of compound 4
Scheme 3.
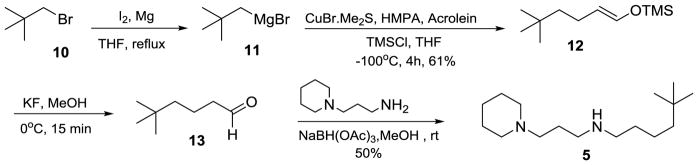
Synthesis of compound 5
The synthesis of compound 5 started with the preparation of the Grignard reagent 11 by refluxing a solution of neopentyl bromide (10) in anhydrous THF with Mg metal in the presence of iodine. The resulting neopentyl magnesium bromide was reacted with acrolein via Michael addition using CuBr-Me2S, TMSCl, and HMPA in anhydrous THF at −100°C to provide (5,5-dimethylhex-1-enyloxy)trimethylsilane (12) with 61% yield.40 The next two steps, removal of the TMS group from compound 12 and the reductive amination were carried out as a one pot due to the instability of the intermediate aldehyde, 5,5-dimethylhexanal (13). The TMS group was removed by stirring the compound 12 with KF in MeOH at 0°C, and the aldehyde 13 thus obtained was reacted with 3-(N-piperidino)propylamine in MeOH in the presence of NaBH(OAc)3 to afford the compound 5 in 50% yield.
2.2. Biological evaluation
The mechanistic studies so far on the involvement of nNav1.5 in breast cancer progression reveal that they promote cell invasion without playing any role in cell proliferation. Therefore, the cytotoxicity of compounds was first evaluated to avoid misinterpretation of the effects of compounds on invasion or channel currents. Cell viability was evaluated using a colorimetric MTS assay. All the biological evaluations were carried out using the highly aggressive human breast cancer cell line, MDA-MB-231. Compounds were tested at a range of concentrations from 1.0 to 25 μM, as compared to a control containing no drug. All solutions contained 2% PBS and 0.2% DMSO. Cell viability was assessed after 24 hours of drug treatment. Maximum non-toxic concentrations were determined for each compound and none of the compounds affected cell viability at concentrations <5 μM (Table 1). Compound 1 displayed a maximum non-toxic concentration of 5 μM while compound 4 displayed least cytotoxicity with a maximum non-toxic concentration of 25 μM. Maximum non-toxic concentrations for compounds 2, 3 and 5 were found to be about 10 μM. For compounds 1 and 4, these values are much higher than the concentrations required (0.1 μM and 1 μM) to produce significant nNav1.5 current blockade and cell invasion inhibition (Table 1, Figs. 2A and 3A).
Table 1.
Cell viability, nNav1.5 current blockade and invasion inhibition of compounds 1–5 in MDA-MB-231 cells
| Compd No | Maximum nontoxic concentrationa | % Blockade of INa peak currentsb | % Inhibition of cell invasionc |
|---|---|---|---|
| 1 | 5 μM | 34.9 ± 6.6 % at 1 μM (n = 6) | 30.3 ± 4.5 % at 1 μM |
| 2 | 10 μM | ~30 % at 10 μM (n = 2) | NEe |
| 3 | 10 μM | ~30 % at 10 μM (n = 2) | NEe |
| 4 | >25 μM d | 25.0 ± 3.3 % at 1 μM (n = 21) | 28.0 ± 5.7 % at 1 μM |
| 5 | 10 μM | ~30 % at 10 μM (n = 2) | NEe |
| TTX22 | 30 μM | > 95% | 31.5 ± 3.8 % at 30 μM |
Determined by five measurements,
Evaluated in ‘n’ cells and expressed as mean ± SEM,
Average of seven repeats, expressed as mean ± SEM,
Maximum concentration tested,
Not evaluated.
Figure 2. Effects of compound 1 on voltage-gated sodium currents from MDA-MB-231 cells.
A) representative recordings showing steady-state block of NaV1.5 currents by increasing concentrations (from 0.1 to 100 μM) of compound 1. Whole-cell patch-clamp recordings were obtained in MDA-MB-231 cells and sodium currents were evoked by 30 ms duration voltage steps to −5 mV from a holding potential of −100 mV, applied every 2 s. The dotted line represents the zero current level. B) Time course of NaV1.5 channels block by compound 1. Peak currents generated for a depolarization step from −100 to −5 mV were normalized to control amplitude (before drug exposure), and defined as the fraction of INa remaining. C) Concentration-response relationships for the effect of compound 1 on NaV1.5 channels. Percent blocked current was calculated from peak current measurements from step voltages from −100 to −5 mV in the presence of compound 1 concentrations indicated in the figure. Data from these experiments were fitted using a Hill equation (IC50 = 4.8 ± 1.2 μM; Hill’s slope = 0.56 ± 0.09). D) Compound 1 slows down the activation and inactivation kinetics of NaV1.5 currents. Currents like those illustrated in panel A were fitted with two exponentials functions, one for the activation and the other for the inactivation of the current at −5 mV, and the respective time constants (τ) were plotted. Columns, means from six cells before (control) and after a steady-state block (compound 1, at 10 μM). *, statistical significance with a Student’s t test (P < 0.05) represent mean values. E) I–V curves in absence and presence of 10 μM of compound 1. The inset shows the normalized current-voltage relationship obtained from same cells, where values were normalized to the maximal current recorded in each condition. F) Activation- of peak Na+ conductance (filled circles) and steady-state inactivation-voltage relationships (filled squares) of NaV1.5 channels from MDA-MB-231 cells in absence and presence of 10 μM compound 1. Smooth lines are fits to Boltzmann functions and V1/2 values were calculated for each condition. While the V1/2-activation voltage were not significantly different, −33.0 ± 1.2 mV in control condition and to − 34.9 ± 2.8 mV in presence of compound 1, the V1/2-inactivation voltage was significantly shifted to hyperpolarized values from − 71.6 ± 0.4 mV in control condition, to − 88.6 ± 3.4 mV in presence of compound 1 (p<0.01, Mann-Whitney Rank Sum test). All data were obtained from 6 cells.
Figure 3. Effects of compound 4 on voltage-gated sodium currents from MDA-MB-231 cells.
A) Representative recordings showing steady-state block of NaV1.5 currents by increasing concentrations (from 0.1 to 100 μM) of compound 4. Whole-cell patch-clamp recordings were obtained in MDA-MB-231 cells and sodium currents were evoked by 30 ms duration voltage steps to −5 mV from a holding potential of −100 mV, applied every 2 s. The dotted line represents the zero current level. B) Time course of NaV1.5 channels block by compound 4. Peak currents generated for a depolarization step from −100 to −5 mV were normalized to control amplitude (before drug exposure), and defined as the fraction of INa remaining, only a slight fraction of the initial current amplitude was recovered after drug washing (17.4 ± 3.1 %). C) concentration-response relationships for the effect of compound 4 on NaV1.5 channels. Percent blocked current was calculated from peak current measurements from step voltages to −5 mV in the presence of increasing concentrations of compound 4 (n = 22). Data from these experiments were fitted using a Hill equation (IC50 = 5.9 ± 0.9 μM; Hill’s slope = 0.72 ± 0.06). D) Compound 4 slows down the activation but not the inactivation kinetics of NaV1.5 currents. Currents like those illustrated in panel A were fitted with two exponentials functions, and the respective time constants (τ) were plotted. Columns, means from 17 cells before (control) and after a steady-state block (compound 4, at 10 μM). *, statistical significance with a Student’s t test (P < 0.05). E) I–V curves in absence and presence of 10 μM compound 4. The inset shows the normalized current-voltage relationship obtained from same cells, where values were normalized to the maximal current recorded in each condition. F) Activation of peak Na+ conductance (filled circles) and steady-state inactivation (filled squares) of NaV1.5 channels from MDA-MB-231 cells in absence and presence of 10μM compound 4. Smooth lines are fits to Boltzmann functions and V1/2 values were calculated for each condition. V1/2-activation voltage values were significantly different and compound 4 induced a light hyperpolarizing shift from −30.7± 0.9 mV in control condition to − 36.8 ± 2.8 mV in presence of compound 4 (p<0.001, Mann-Whitney Rank Sum test). The V1/2-inactivation voltage was also significantly shifted to hyperpolarized values from − 66.0 ± 2.5 mV in control condition, to − 77.5 ± 3.0 mV in presence of compound 4 (p<0.01, Mann-Whitney Rank Sum test). These data were obtained from 15 cells.
The inhibition of sodium currents (INa inhibition) in individual MDA-MB-231 cells by compounds at 10 μM concentration was initially measured in physiological saline solution (PSS) using whole-cell patch-clamp experiments conducted according to a reported protocol.27 In this study, cells were continuously perifused with PSS control solution (containing the same percentage of DMSO, as used in the solvent for the test compounds) or the test compounds. After 5–10 min perifusion of the compounds, depolarization steps and INa-V protocols were run. At 10 μM, all five compounds showed some reduction in peak sodium currents. Compounds 1 and 4 were most active exhibiting 50 % and 60 % INa inhibition at 10 μM concentration, while compounds 2, 3 and 5 showed relatively less INa inhibition of ~30% (Table 1 and Figs. 2A and 3A). As the compounds 1 and 4 were better blockers, they were further evaluated at a range of concentrations from 0.1 μM to 100 μM to examine the dose response relationship on INa inhibition as well as to determine the IC50 values. Results of these studies are summarized in Figs. 2 and 3.
Both compounds 1 and 4 demonstrated a slow, dose-dependent inhibition of the maximal current elicited for a membrane depolarization from −100 to −5 mV, which was not reversible (for compound 1) or weakly reversible (for compound 4) after wash-out (Figs. 2B and 3B). Compound 1 exhibited an IC50 value of 4.8 ± 1.2 μM (n = 3–6 cells) whereas compound 4 exhibited an IC50 value of 5.9 ± 0.9 μM (n = 17–21 cells), (Figs. 2C and 3C, respectively). In addition, the effect of the two compounds was studied on the activation and inactivation kinetics of the sodium current (Figs. 2D and 3D). Indeed, compound 1 (at 10 μM) significantly slowed down both activation and inactivation kinetics of INa measured for a membrane depolarization from −100 to −5 mV (Figs. 2A and 2D), while compound 4 only reduced kinetics of activation (Figs. 3A and 3D). The effect of Compounds 1 and 4 tested at a concentration of 10 μM on the full INa-Voltage relationship (from −100 to +60 mV) is shown in figures 2E and 3E, respectively. The voltage of half-inactivation (V1/2-inactivation) significantly shifted to hyperpolarized values from −71.6 ± 0.4 mV in control condition, to −88.6 ± 3.4 mV in presence of compound 1 (n = 6 cells, p<0.01, Fig. 2F), while half-voltage of activation (V1/2-activation) was unchanged. Compound 4 also shifted V1/2-inactivation to hyperpolarized values from −66.0 ± 2.5 mV (in control condition) to −77.5 ± 3.0 mV (n= 15 cells, p<0.01, Fig. 3F). In addition, compound 4 also showed a light hyperpolarizing shift in V1/2-activation from −30.7 ± 0.9 mV (in control condition) to −36.8 ± 2.8 mV (n = 15 cells, p<0.001, Fig. 3F).
These results indicate that the lead compounds 1 and 4 exhibited significant blockade of nNav1.5 currents in MDA-MB-231 cells in low micromolar concentrations. These effects were observed to be weakly reversible. Furthermore, these two compounds induced significant leftward shift in the inactivation-voltage relationship suggesting that they could also reduce the window of voltage, and therefore reduce the persistent window current associated with cancer cell invasiveness. The observed nNav1.5 current blockade for compounds 1 and 4 were compared to the positive control tetrodotoxin (TTX), which is a known sodium channel blocker (>95% blockade at 30 μM).22 Compounds 1 and 4 displayed similar activity to TTX with 25 – 35 % blockade at 1 μM, 50–60% blockade at 10 μM. However, it required about 100 μM of compounds 1 and 4 to produce a >95% blockade of channel current. The observed nNav1.5 current blockade by compounds 1 and 4 are unrelated to any effects on cell viability as their maximum cell cytotoxicity concentrations were much higher at 5 μM and 25 μM respectively. Based on these experimental results, compounds 1 and 4 were selected for further evaluation in cell invasion assays.
Compounds 1 and 4 were then evaluated for their ability to inhibit invasion of MDA-MB-231 cells using Matrigel-coated inserts in a trans-well invasion assay.41 Matrigel (Becton Dickinson matrix) mimics the extracellular matrix and basement membrane that metastatic cells must cross to enter the blood stream and travel to lymph nodes or invade distant organs. The ability of cancer cells to invade is measured based on the number of cells that can invade the matrigel and migrate through the pores across the membrane. The triple negative MDA-MB-231 cell line is one of the most invasive/metastatic cell lines available to study breast carcinoma. Compounds 1 and 4 were evaluated in the invasion assay at 0.1 μM and 1 μM along with the control compound TTX at 30 μM. The results of the invasion assay are presented in Fig. 4. As shown, both 1 and 4 exhibited 20% inhibition of invasion at 0.1 μM in comparison to the control, whereas, at 1 μM, both compound 1 and 4 showed a significant reduction of invasion (30%), similar level to what is observed for TTX at 30 μM (Fig. 4 and Table 1). These results suggest that our lead compounds 1 and 4 are very effective in inhibiting cell invasion producing an effect similar to TTX at a much lower concentration. Furthermore, the lead compounds 1 and 4 are more drug-like and have simpler chemical structures than TTX. In recent studies, VGSC-blocking antiepileptic drug phenytoin and antiarrhythmic drug ranolazine inhibited the invasion of metastatic MDA-MB-231 cells in vitro and reduced breast cancer metastasis in vivo without much neuro-muscular or cardiac toxicity demonstrating good therapeutic index.42–44 Both these drugs (with Na+ current blockade and invasion inhibition at 50 μM) are considerably less active than our lead compounds (Na+ current blockade and invasion inhibition at 1μM) in vitro. Therefore, we expect our lead compounds to be more potent in vivo and exhibit better therapeutic index than phenytoin and ranolazine.
Figure 4. Effects of compounds 1 and 4 on MDA-MB-231 cancer cells invasive properties.

Effect of compounds 1 and 4 tested at 0.1 and 1 μM concentrations on MDA-MB-231 human breast cancer cell invasiveness, as compared to the effect of the sodium channel inhibitor tetrodotoxin (TTX, 30 μM). Results were obtained from 7 independent experiments, and are expressed as relative invasion (mean ± SD), normalized to the control condition (CTL) obtained without drug but with the maximal solvent dose (DMSO 0.2%). *, indicates a statistical difference at p<0.05 (Mann-Whitney Rank Sum test).
3. Conclusions
The presence of nNav1.5 in aggressive breast cancer cells and their association with invasive behavior is well established. Here, we report the discovery of small, potent drug-like blockers of nNav1.5 channel. A 3D-QSAR model is used to assist the discovery of low micromolar small molecule nNav1.5 blockers. Using this model we have designed, synthesized and evaluated five small molecule compounds as blockers of nNav1.5-dependent inward currents in whole-cell patch-clamp experiments in MDA-MB-231 cells. The identified lead compounds 1 and 4 inhibited peak sodium currents in MDA-MB-231 cells by 35 % and 25 % respectively at 1 μM. These two compounds also produced 30 % reduction of the invasion of MDA-MB-231 cells at 1 μM without affecting the cell viability. The lead compounds are very effective in inhibiting cell invasion producing an effect similar to what is observed for TTX at a much lower concentration. Our lead compounds are much more drug-like and have simpler chemical structures compared to TTX. In addition, our lead compounds are more active in inhibiting breast cancer cell invasion than the known VGSC-blocking drugs such as ranolazine and phenytoin. Compounds 1 and 4, unlike ranolazine and phenytoin, are developed to specifically target the nNav1.5 and represent new and more potent class of compounds that may be further developed in to drugs for the treatment of breast cancer metastasis.
4. Experimental Procedures
4.1. Cell viability assay
Cytotoxicity of the compounds was tested in a MTS assay. Cells were seeded in 96 well plates at 104 cells/well in growth media (5 % FBS in DMEM) and incubated for 24 h at 37 °C in 5 % CO2. Various concentrations of test compounds (1, 5, 10 and 25 μM) or vehicle control (0.25 % DMSO in PBS) were added and the cells were incubated for an additional 24 h. For each test concentration, 5 wells were allotted. After 24 h of incubation proliferation was determined using MTS. 20 μL of MTS were added to each well and the cells were incubated for an additional 30 min. UV absorbance was recorded at 490 nm. The cytotoxicity of the test compounds was compared to the vehicle control (0.25 % DMSO in PBS) and results were compiled as the mean of five repeats using the GraphPad Prism software.
4.2. Cell invasion inhibition assay
Cell invasion experiments were carried out in 24 well plates containing BD Matrigel® invasion chambers (BD Biosciences). The invasion chambers were hydrated for 2 h in the incubator at 37 °C with 200 μL (5 % FBS in DMEM) in the upper insert and 800 μL (10 % FBS in DMEM) in the lower well that acted as a chemoattractant. MDA-MB-231 cells (4 × 104) were seeded in the upper chamber. Various concentrations of the test compounds (0.1 and 1 μM) or vehicle control (0.2% DMSO in PBS) were added in both the lower and upper chambers to maintain uniformity of concentration by 1:100 dilutions. After incubating the cells for 24 h at 37 °C, under 5 % CO2, invaded cells were fixed and stained using a Hema kit. The migrated cells were counted under the microscope by selecting five regions on the whole membrane. Results were compiled as a mean of three repeats using graphPad Prism software. The results are given as a mean ± SD.
4.3. Electrophysiology
A whole cell patch clamp technique was used for recording the sodium channel activity in MDA-MB-231 cells. 30,000 cells were seeded into a 35 mm petri dish with the growth media (5% FBS in DMEM). Na+ currents were recorded under voltage-clamp mode using an Axopatch 200B patch clamp amplifier (Axon Instruments, USA), compensating for cell capacitance and series resistance by 60 %. The P/2 sub pulse protocol was applied to correct the linear component of capacitance and cell leak. Borosilicate glass was used to pull the patch pipettes at a resistance of 3–5 MΩ. Analog signals were filtered at 5 kHz and sampled at 10 kHz via a 1440A Digidata converter and analyzed using pCLAMP software (v10.4, Axon Instruments). Cells were studied in ruptured, whole-cell, voltage-clamp mode of the patch-clamp technique. Before starting the experiment growth medium was removed and replaced with physiological saline solution (PSS, composition given below). In this experiment the tip of the micro capillary (perifusion system) delivering the PSS or the test compound was placed almost on top of the cell, so that the cells were continuously perifused with the respective solutions. The stock solution for test compounds was prepared at 10 mM in 100% DMSO and the test concentration used in the assay was 1 μM and 10 μM (0.2 % DSMO in PSS). Sodium currents were recorded by depolarizing the cells from a holding potential of −100 mV to a maximal test pulse of −5 mV for 30 ms every 500 ms. The protocol used to build the current-voltage (INa-V) curve was as follows. From a holding potential of −100 mV, the membrane was stepped to a potential of +60 mV, with 5 mV increments, for 50 ms at a frequency of 2 Hz. The physiological saline solution (PSS) contained (in mM): NaCl 140, KCl 4, MgCl2 1, CaCl2 2, NaH2PO4 0.33, D-Glucose 11.1, and HEPES 10, and was adjusted to pH 7.4. The intrapipette solution (in mM) contained: KCl 130, NaCl 15, CaCl2 0.37, MgCl2 1, Mg-ATP 1, EGTA 1, and HEPES 10, and was adjusted to pH 7.2.
4.4. General Methods for Synthesis
Solvent evaporations were carried out in vacuo using a rotary evaporator. Thin layer chromatography (TLC) was performed on silica gel plates with fluorescent indicator (Dynamic Adsorbents, Inc., Aluminum backed TLC, 20 X 20 cm F-254, 200 μm). Spots were visualized by UV light (254 and 365 nm) or staining agents such as ninhydrin, KMnO4 and iodine. Purification by column and flash chromatography was carried out using silica gel (32–63 μm) from Dynamic Absorbent in the solvent systems indicated. The amount (weight) of silica gel for column chromatography was in the range of 50–100 times the amount (weight) of the crude compounds being separated. The NMR spectra were recorded on a Bruker DPX 300 spectrometer. Chemical shifts are reported in ppm relative to TMS or CDCl3 as internal standard. The values of chemical shifts (δ) and coupling constants J were given in parts per million and in Hz, respectively. Mass spectra were recorded on a MicroMass Platform LCC instrument. HRMS were obtained on a Waters AutoSpec-UltimaTM NT mass spectrometer with an EI source. Anhydrous solvents used for reactions were purchased in Sure-Seal™ bottles from Aldrich chemical company. THF was freshly distilled over sodium/benzophenone. Other reagents were purchased from Aldrich, Alfa Aesar or Acros and used as received.
4.5. Synthesis of compounds 1–3, General procedure
To a solution of alkyl amine (1 equiv) in DMSO (10mL), a solution of alkyl chloride (1 equiv.) in DMSO (5 mL) and Et3N (1 equiv.) were added. The reaction mixture was heated at 80–90°C under a N2 atmosphere for 8 h. TLC examination (MeOH/NH3 saturated CH2Cl2, 1:19) indicated the completion of the reaction. The reaction mixture was diluted with EtOAc (60mL), washed with 1N NaOH (3 × 20 mL), water (2 × 20 mL), brine (20 mL) and dried over Na2SO4. The drying agent was filtered off and the filtrate was concentrated under vacuum to obtain the crude product which was purified by column chromatography over Si gel using MeOH/NH3 saturated CH2Cl2 (1:49) to obtain pure compounds 1–3 in 58 – 60 % yield.
4,4-Bis(4-fluorophenyl)-N-(3-(piperdin-1-yl)propyl)butan-1-amine (1)
Prepared from 3-(N-piperidino)propylamine (2.50 g, 17.6 mmol) and 4,4-di-(4-fluorophenyl)butyl chloride 6 (4.93 g, 17.6 mmol) to afford compound 1 (4.00 g, 60%) as a liquid (MeOH/NH3 saturated CH2Cl2, 1:19, Rf = 0.40). 1H-NMR (CDCl3) δ 1.34 – 1.46 (m, 4H), 1.52 (quint, 4H, J = 5.3 Hz), 1.65 (quint, 2H, J = 7.5 Hz), 2.01 (q, 2H, J = 8 Hz), 2.20 – 2.42 (m, 8H), 2.60 (t, 4H, J = 7.5 Hz), 3.85 (t, 1H, J = 7.5 Hz), 6.89 – 6.99 (m, 4H), 7.10 – 7.19 (m, 4H); 13C-NMR (CDCl3) δ 24.4, 26.0, 27.0, 28.5, 33.7, 49.0, 49.7, 49.8, 54.7, 57.9, 115.1 (115.4, F- coupling), 129.0 (129.1, F- coupling), 140.5 (140.6, F- coupling), 159.7 (162.9, F- coupling); MS (ES+) m/z 387 (M+H); HRMS measured for C24H32F2N2: [M+H]+ 386.2535 (found), 386.2534 (calc).
4,4-Bis(4-fluorophenyl)-N-(3-phenylpropyl)butan-1-amine (2)
Prepared from 3-phenylpropylamine (0.240 g, 1.78 mmol), and 4,4-di-(4-fluorophenyl)butyl chloride, 6 (0.500 g, 1.78 mmol) to afford compound 2 (0.230 g, 60.6%) as a thick liquid (MeOH/NH3 saturated CH2Cl2, 1:19, Rf = 0.50). 1H-NMR (CDCl3) δ 1.47 (quint, 3H, J = 7.5 Hz), 1.84 (t, 2H, J = 7.5 Hz), 1.98 (q, 2H, J = 7.8 Hz), 2.63 (quint, 6H, J = 7.1 Hz), 3.82 (t, 1H, J = 7.8 Hz), 3.92 (bs, 1H), 6.90 – 7.10 (m, 4H), 7.08 – 7.21 (m, 7H), 7.22 – 7.30 (m, 2H); 13C-NMR (CDCl3) δ 27.6, 30.7, 33.5 (2C), 49.0, 49.3, 49.7, 115.3, 115.6, 126.1, 128.5, 128.6, 129.2, 129.3, 140.43, 140.5, 141.6, 159.9, 163.1; MS (ES+) m/z 380 (M+H); HRMS measured for C25H27F2N: [M+H]+ 379.2116 (found), 379.2112 (calc).
4,4-Bis(4-fluorophenyl)-N-phenylethylbutan-1-amine (3)
Prepared from 2-phenylethylamine (0.996 g, 8.22 mmol) and 4,4-di-(4-fluorophenyl)butyl chloride 1 (2.31 g, 8.22 mmol) to afford compound 3 (1.74 g, 58.0%) as a liquid (40% ethyl acetate/55% hexanes/5% Et3N, Rf = 0.35). 1H-NMR (CDCl3) δ 1.37–1. 44 (m, 2H), 1.63 (bs, 1H), 1.97 (t, 2H, J = 7.4 Hz), 2.59 – 2.65 (m, 2H), 2.78 (t, 2H, J = 7.0 Hz), 2.83 (q, 2H, J = 7.7 Hz), 3.82 (q, 1H, J = 8.2 Hz), 6.90 – 6.97 (m, 4H), 7.09 – 7.21 (m, 7H), 7.22 – 7.29 (m, 2H); 13C-NMR (CDCl3) δ 28.5, 33.8, 36.4, 49.8, 49.9, 51.2, 115.4 (115.5, F-coupling), 126.4, 128.7, 128.9, 129.2, 129.3, 140.1 (140.7, F-coupling), 160.8 (162.2, F-coupling); MS (ES+) m/z 366 (M+H); HRMS measured for C24H25F2N: [M+H]+ 365.1954 (found), 365.1955 (calc).
4-(4-Fluorophenyl) butan-1-ol (8)
A suspension of AlCl3 (4.00 g, 30.0 mmol) in anhydrous CH2Cl2 (100 mL) was stirred under a N2 atmosphere at 0 °C for 10 min followed by the addition of borane tert-butylamine complex (5.20 g, 60.0 mmol). The reaction mixture was allowed to stir at 0 °C till it gave a clear solution (10–15 mins). To the clear reaction mixture a solution of 4-(4-fluorophenyl)-4-oxobutanoic acid 7 (2.00 g, 10 mmol) in anhydrous CH2Cl2 (10 mL) was added. The resulting mixture was stirred at 0°C for 2h, and TLC examination (EtOAc/hexanes, 1:4, Rf = 0.30) indicated completion of the reaction. The reaction mixture was quenched by the addition of 0.1 N HCl (50 mL). It was extracted with EtOAc (3 × 50 mL); the combined organic layer was washed with 0.1 N HCl (2 × 50 mL), brine (50 mL), and dried over Na2SO4. The drying agent was filtered off and the filtrate was concentrated under vacuum to obtain the crude product. The crude product was purified by column chromatography over Si gel using EtOAc/hexanes (1:4) to afford 4-(4-fluorophenyl)butan-1-ol 4 (0.880 g, 52.3% yield) as a colorless liquid. 1H-NMR (CDCl3) δ 1.56 – 1.73 (m, 4H), 2.14 (bs, 1H), 2.61 (t, 2H, J = 7.4 Hz), 3.65 (t, 2H, J = 6.3 Hz), 6.91– 7.00 (m, 2H), 7.08 – 7.16 (m, 2H); 13C-NMR (CDCl3) δ 27.9, 32.3, 35.0, 62.9, 115.0 (115.3), 129.8 (129.9), 138.0 (138.1), 159.8 (163.0).
4-(4-Fluorophenyl)butan-1-al (9)
PCC (7.13 g, 33.1 mmol) was ground with silica gel (7.13 g) and suspended in anhydrous CH2Cl2 (100 mL) under a N2 atmosphere at room temperature. After stirring for 10–15 mins, a solution of 4-(4-fluorophenyl)butan-1-ol (8) (0.857 g, 5.09 mmol) in anhydrous CH2Cl2 (10 mL) was added to the suspension. After 6 h of stirring at room temperature, TLC examination (EtOAc/hexanes, 1:4, Rf = 0.50) indicated completion of the reaction. The reaction mixture was filtered through celite and washed with CH2Cl2 (200 mL). The filtrate was concentrated under vacuum to obtain the crude product. The crude product was purified by column chromatography over silica gel, using EtOAc/hexanes (1:4) to afford 4-(4-fluorophenyl)butan-1-al (9) (0.444 g, 52.5% yield) as a colorless liquid. 1H-NMR (CDCl3) δ 1.92 (pent, 2H, J = 7.7 Hz), 2.44 (td, 2H, J1 = 7.2 Hz, J2 = 1.5 Hz), 2.62 (t, 2H, J = 7.7 Hz), 6.91 – 7.01 (m, 2H), 7.08 – 7.16 (m, 2H), 9.74 (t, 1H, J = 1.5 Hz); 13C-NMR (CDCl3) δ 23.9, 34.3, 43.1, 115.1 (115.4, F-coupling), 129.9, 137.0, 159.9 (163.1, F-coupling), 202.2.
4-(4-Fluorophenyl)-N-(3-(piperdin-1-yl)propyl)butan-1-amine (4)
3-(N-Piperidino)propyl amine (0.380 g, 2.67 mmol) was dissolved in anhydrous CH2Cl2 (25 mL) under a N2 atmosphere. 4-(4-Fluorophenyl)butan-1-al (9) (0.444 g, 2.67 mmol) was added to the reaction mixture. After stirring the reaction mixture for 30 mins, NaBH(OAc)3 (1.13 g, 5.34 mmol) was added. After stirring the reaction mixture for 5 h at room temperature, TLC examination (MeOH/NH3 saturated CH2Cl2, 1:19, Rf = 0.40) indicated completion of the reaction. The reaction mixture was concentrated under vacuum and taken up into EtOAc (60 mL); the organic layer was washed with 1 N NaOH (3 × 30 mL), water (30 mL), brine (30 mL) and dried over Na2SO4. The drying agent was filtered off and the filtrate was concentrated under vacuum to obtain the crude product. The crude product was purified by column chromatography over Si gel, using MeOH/NH3 saturated CH2Cl2 (3:97) to afford 4-(4-fluorophenyl)-N-(3-(piperdin-1-yl)propyl)butan-1-amine (4) (0.224 g, 30.0% yield). 1H-NMR (CDCl3) δ 1.35 – 1.45 (m, 2H), 1.46 – 1.61 (m, 7H), 1.62 – 1.73 (m, 3H), 2.27 – 2.43 (m, 6H), 2.60 (quint, 6H, J = 7.4 Hz), 6.89 – 6.98 (m, 2H), 7.06 – 7.14 (m, 2H); 13C-NMR (CDCl3) δ 24.6, 26.2, 27.0, 29.5, 29.7, 35.1, 49.2, 49.9, 54.8, 58.1, 115.0 (115.2, F-coupling), 129.8 (129.9, F-coupling), 138.1 (138.2, F-coupling), 159.7 (163.0, F-coupling); MS (ES+) m/z 293 (M+H); HRMS measured for C18H29FN2: [M+H]+ 292.2324 (found), 292.2315 (calc).
Neopentyl magnesium bromide (11)
In an oven dried three-neck flask connected to a condenser and dropping funnel was placed dry magnesium turnings (1.60 g, 66.0 mmol) under a N2 atmosphere. The magnesium was further flame dried for half an hour under N2 before adding a crystal of I2 and anhydrous THF (20 mL). A solution of neopentyl bromide 10 (10.0 g, 66.0 mmol) in THF (20 mL) was slowly added to the reaction mixture through a dropping funnel. Disappearance of the coloration and beginning of effervescence indicated the onset of the reaction. No external heating was applied until the complete addition of neopentyl bromide. After refluxing for 3 h, the heating was stopped and the concentration of Grignard reagent generated was determined to be 1.48 M by titration against a solution of isopropanol in xylene. This Grignard reagent was used in the next step.
((5,5-Dimethylhex-1-en-1-yl)oxy)trimethylsilane (12)
1.48 M Neopentyl magnesium bromide (7) (40 mL) was dissolved in anhydrous THF (150 mL) and was cooled to −100 °C followed by the addition of CuBr.Me2S (0.510 g, 2.50 mmol) and HMPA (21.1 g, 118 mmol) under a N2 atmosphere. After stirring for 10 mins, a solution of acrolein (2.76 g, 49.2 mmol) and chlorotrimethylsilane (10.7 g, 98.4 mmol) in THF (50 mL) were added to the reaction mixture via a cannula. After stirring the reaction mixture for 4 h at −100 °C under a N2 atmosphere, Et3N (9.95 g, 98.4 mmol) and silica gel (30.0 g, 4 times the expected weight of the product) was added. After stirring for 10 min at room temperature, hexanes (400 mL) was added. The slurry was filtered off and the filtrate was concentrated under vacuum to obtain the crude product. The crude product was purified by vacuum distillation (5 T, 66 °C) and (5,5-dimethylhex-1-enyloxy)trimethylsilane (12) (6.00 g, 61.0%) was obtained as colorless liquid (hexanes, Rf = 0.50); 1H-NMR (CDCl3) δ 0.16 (s, 9H), 0.86 (s, 9H), 1.14 – 1.23 (m, 2H), 1.78 – 1.88 (m, 2H), 4.96 (dt, 1H, J1 = 12Hz, J2 = 7.4 Hz), 6.18 (dt, J1 = 12 Hz, J2 = 1.2 Hz); 13C-NMR (CDCl3) δ 0.2, 22.9, 29.6, 30.4, 45.3, 113.0, 139.2.
5,5-Dimethyl-N-(3-(piperidin-1-yl)propyl)hexan-1-amine (5)
Compound 12 (0.782 g, 3.97 mmol) was dissolved in anhydrous MeOH (15 mL) under a N2 atmosphere. The reaction mixture was cooled to 0 °C and KF (0.396 g, 6.82 mmol) was added. After stirring the reaction mixture for 15 min, TLC examination (EtOAc/hexanes, 1:19, Rf = 0.60) indicated a complete conversion to 5,5-dimethylhexanal (13), which was directly added via cannula to the solution of 3-(N-piperidino)propylamine (0.566 g, 3.97 mmol) in anhydrous MeOH (70 mL) under a N2 atmosphere. After stirring the reaction mixture for 30 min, NaBH(OAc)3 (1.68 g, 7.94 mmol) was added to the reaction mixture. After 5 h of stirring at room temperature, TLC examination (MeOH/NH3 saturated CH2Cl2, 1:19, Rf = 0.40) indicated the completion of reaction. The reaction mixture was concentrated under vacuum, the residue taken up in EtOAc (50 mL) and washed with 1 N NaOH (3 × 30 mL). The organic layer was washed with water (30 mL) followed by brine (30mL) and dried over Na2SO4. The drying agent was filtered off and the filtrate was concentrated under vacuum to obtain the crude product. The crude product was purified by column chromatography over silica gel using MeOH/NH3 saturated CH2Cl2 (3:97) to afford 5,5-dimethyl-N-(3-(piperidin-1-yl)propyl)hexan-1-amine (5) (0.500 g, 49.5%) as a clear light yellow liquid. 1H-NMR (CDCl3) δ 0.84 (s, 9H), 1.10 – 1.31 (m, 4H), 1.35 – 1.49 (m, 4H), 1.55 (quint, 4H, J = 5.4 Hz), 1.68 (quint, 2H, J = 7.2 Hz), 2.27 – 2.39 (m, 6H), 2.53 – 2.65 (m, 4H); 13C-NMR (CDCl3) δ 22.6, 24.7, 26.2, 27.3, 29.6, 30.5, 31.3, 44.4, 49.2, 50.3, 54.9, 58; HRMS measured for C16H34N2: [M+H]+ 254.2731 (found), 254.2722 (calc).
Supplementary Material
Highlights.
Identified novel nNav1.5 blockers that inhibit breast cancer cell invasion.
Most active compound blocked nNav1.5 currents by 50% at 1 μM.
Most active compound displayed 30% reduction in cell invasion at 0.1 μM.
Acknowledgments
Funding from NIH (1UL1RR025777, R03DE025058-0) is acknowledged. The authors wish to acknowledge the financial support by the Collaborative Programmatic Development Grant from the UAB Comprehensive Cancer Center. SD would like to acknowledge the graduate fellowship from the UAB chemistry department. SR was supported by the INSERM, the Région Centre-Val de Loire, the “Ligue Nationale contre le Cancer”, the “Association CANCEN”, and the “Prix Ruban Rose Avenir 2017”.
List of abbreviations
- CDCl3
Deuterated chloroform
- CH2Cl2
Methylene dichloride
- 13C-NMR
Carbon-13 nuclear magnetic resonance
- CoMFA
Comparative molecular field analysis
- CuBr.Me2S
Copper bromide dimethyl sulfide
- DMEM
Dulbecco’s modified eagle medium
- DMSO
Dimethyl sulfoxide
- 3D-QSAR
3-Dimensional quantitative structure activity relationship
- Et3N
Triethylamine
- EtOAc
Ethyl acetate
- ER
Estrogen receptor
- FBS
fetal bovine serum
- HER-2
Human epidermal growth receptor 2
- 1H-NMR
Hydrogen-1 nuclear magnetic resonance
- IC50
Inhibition concentration at 50%
- INa
Sodium currents
- MeOH
Methanol
- MTS
[3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium]
- NaBH(OAc)3
Sodium triacetoxyborohydride
- nNav1.5
Neonatal Nav1.5 isoform
- Na2SO4
Sodium sulfate
- NH3
Ammonia
- NHE1
Na+/H+ exchanger type 1
- NMR
Nuclear magnetic resonance
- PBS
Phosphate buffered saline
- pHi
Intracellular pH
- ppm
Parts per million
- PR
Progesterone receptor
- PSS
Physiological saline solution
- Rf
Retention factor
- THF
Tetrahydrofuran
- TMS
Tetramethylsilane
- TLC
Thin layer chromatography
- TTX
Tetrodotoxin
- UV
Ultraviolet
- VGSC
Voltage-gated sodium channels
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.DeSantis CE, Ma J, Goding Sauer A, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin. 2017;67(6):439–448. doi: 10.3322/caac.21412. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 3.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331(6024):1559–64. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 4.Weigelt B, Peterse JL, van‘t Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005;5(8):591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- 5.Guan X. Cancer metastases: challenges and opportunities. Acta Pharmaceutica Sinica B. 2015;5(5):402–418. doi: 10.1016/j.apsb.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehlen P, Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer. 2006;6(6):449–58. doi: 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- 7.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 8.Weber GF. Why does cancer therapy lack effective anti-metastasis drugs? Cancer Lett. 2013;328(2):207–11. doi: 10.1016/j.canlet.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 9.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 10.Roger S, Potier M, Vandier C, Besson P, Le Guennec JY. Voltage-gated sodium channels: new targets in cancer therapy? Curr Pharm Des. 2006;12(28):3681–95. doi: 10.2174/138161206778522047. [DOI] [PubMed] [Google Scholar]
- 11.Brackenbury WJ. Voltage-gated sodium channels and metastatic disease. Channels (Austin) 2012;6(5):352–61. doi: 10.4161/chan.21910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lastraioli E, Iorio J, Arcangeli A. Ion channel expression as promising cancer biomarker. Biochim Biophys Acta. 2015;1848(10 Pt B):2685–702. doi: 10.1016/j.bbamem.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 13.Chahine M, Desaphy JF. Editorial: Recent Advances in Voltage-Gated Sodium Channels, their Pharmacology and Related Diseases. Front Pharmacol. 2016;7:20. doi: 10.3389/fphar.2016.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel F, Brackenbury WJ. Dual roles of voltage-gated sodium channels in development and cancer. Int J Dev Biol. 2015;59(7–9):357–66. doi: 10.1387/ijdb.150171wb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Besson P, Driffort V, Bon E, Gradek F, Chevalier S, Roger S. How do voltage-gated sodium channels enhance migration and invasiveness in cancer cells? Biochim Biophys Acta. 2015;1848(10 Pt B):2493–501. doi: 10.1016/j.bbamem.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 16.Catterall WA. Voltage-gated sodium channels at 60: structure, function and pathophysiology. J Physiol. 2012;590(11):2577–89. doi: 10.1113/jphysiol.2011.224204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roger S, Gillet L, Le Guennec JY, Besson P. Voltage-gated sodium channels and cancer: is excitability their primary role? Front Pharmacol. 2015;6:152. doi: 10.3389/fphar.2015.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.House CD, Vaske CJ, Schwartz AM, Obias V, Frank B, Luu T, Sarvazyan N, Irby R, Strausberg RL, Hales TG, Stuart JM, Lee NH. Voltage-gated Na+ channel SCN5A is a key regulator of a gene transcriptional network that controls colon cancer invasion. Cancer Res. 2010;70(17):6957–67. doi: 10.1158/0008-5472.CAN-10-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blandino JK, Viglione MP, Bradley WA, Oie HK, Kim YI. Voltage-dependent sodium channels in human small-cell lung cancer cells: role in action potentials and inhibition by Lambert-Eaton syndrome IgG. J Membr Biol. 1995;143(2):153–63. doi: 10.1007/BF00234661. [DOI] [PubMed] [Google Scholar]
- 20.Grimes JA, Fraser SP, Stephens GJ, Downing JE, Laniado ME, Foster CS, Abel PD, Djamgoz MB. Differential expression of voltage-activated Na+ currents in two prostatic tumour cell lines: contribution to invasiveness in vitro. FEBS letters. 1995;369(2–3):290–4. doi: 10.1016/0014-5793(95)00772-2. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita N, Hamada H, Tsuruo T, Ogata E. Enhancement of voltage-gated Na+ channel current associated with multidrug resistance in human leukemia cells. Cancer Res. 1987;47(14):3736–41. [PubMed] [Google Scholar]
- 22.Roger S, Besson P, Le Guennec JY. Involvement of a novel fast inward sodium current in the invasion capacity of a breast cancer cell line. Biochim Biophys Acta. 2003;1616(2):107–11. doi: 10.1016/j.bbamem.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Brackenbury WJ, Chioni AM, Diss JK, Djamgoz MB. The neonatal splice variant of Nav1. 5 potentiates in vitro invasive behaviour of MDA-MB-231 human breast cancer cells. Breast Cancer Res and Trea. 2007;101(2):149–60. doi: 10.1007/s10549-006-9281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Catterall WA. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- 25.Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev. 2005;57(4):397–409. doi: 10.1124/pr.57.4.4. [DOI] [PubMed] [Google Scholar]
- 26.Fraser SP, Diss JK, Chioni AM, Mycielska ME, Pan H, Yamaci RF, Pani F, Siwy Z, Krasowska M, Grzywna Z, Brackenbury WJ, Theodorou D, Koyuturk M, Kaya H, Battaloglu E, De Bella MT, Slade MJ, Tolhurst R, Palmieri C, Jiang J, Latchman DS, Coombes RC, Djamgoz MB. Voltage-gated sodium channel expression and potentiation of human breast cancer metastasis. Clin Cancer Res. 2005;11(15):5381–89. doi: 10.1158/1078-0432.CCR-05-0327. [DOI] [PubMed] [Google Scholar]
- 27.Gillet L, Roger S, Besson P, Lecaille F, Gore J, Bougnoux P, Lalmanach G, Le Guennec JY. Voltage-gated Sodium Channel Activity Promotes Cysteine Cathepsin-dependent Invasiveness and Colony Growth of Human Cancer Cells. J Biol Chem. 2009;284(13):8680–91. doi: 10.1074/jbc.M806891200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brisson L, Gillet L, Calaghan S, Besson P, Le Guennec JY, Roger S, Gore J. Na(V)1. 5 enhances breast cancer cell invasiveness by increasing NHE1-dependent H(+) efflux in caveolae. Oncogene. 2011;30(17):2070–6. doi: 10.1038/onc.2010.574. [DOI] [PubMed] [Google Scholar]
- 29.Bon E, Driffort V, Gradek F, Martinez-Caceres C, Anchelin M, Pelegrin P, Cayuela ML, Marionneau-Lambot S, Oullier T, Guibon R, Fromont G, Gutierrez-Pajares JL, Domingo I, Piver E, Moreau A, Burlaud-Gaillard J, Frank PG, Chevalier S, Besson P, Roger S. SCN4B acts as a metastasis-suppressor gene preventing hyperactivation of cell migration in breast cancer. Nat Commun. 2016;7:13648. doi: 10.1038/ncomms13648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127(4):679–95. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Mohamed MM, Sloane BF. Cysteine cathepsins: multifunctional enzymes in cancer. Nat Rev Cancer. 2006;6(10):764–75. doi: 10.1038/nrc1949. [DOI] [PubMed] [Google Scholar]
- 32.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2(3):161–74. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 33.Busco G, Cardone RA, Greco MR, Bellizzi A, Colella M, Antelmi E, Mancini MT, Dell’Aquila ME, Casavola V, Paradiso A, Reshkin SJ. NHE1 promotes invadopodial ECM proteolysis through acidification of the peri-invadopodial space. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2010;24(10):3903–15. doi: 10.1096/fj.09-149518. [DOI] [PubMed] [Google Scholar]
- 34.Nelson M, Yang M, Millican-Slater R, Brackenbury WJ. Nav1. 5 regulates breast tumor growth and metastatic dissemination in vivo. Oncotarget. 2015;6(32):32914–29. doi: 10.18632/oncotarget.5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Camerino DC, Desaphy JF, Tricarico D, Pierno S, Liantonio A. Therapeutic approaches to ion channel diseases. Adv Genet. 2008;64:81–145. doi: 10.1016/S0065-2660(08)00804-3. [DOI] [PubMed] [Google Scholar]
- 36.Fozzard HA, Sheets MF, Hanck DA. The sodium channel as a target for local anesthetic drugs. Front Pharmacol. 2011;2:68. doi: 10.3389/fphar.2011.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zha C, Brown GB, Brouillette WJ. A highly predictive 3D-QSAR model for binding to the voltage-gated sodium channel: design of potent new ligands. Bioorganic Med Chem. 2014;22(1):95–104. doi: 10.1016/j.bmc.2013.11.049. [DOI] [PubMed] [Google Scholar]
- 38.Cramer RD, III, Patterson DE, Bunce JD. Comparative molecular field analysis (CoMFA) 1. Effect of shape on binding of steroids to carrier proteins. J Am Chem Soc. 1988;110:5959–67. doi: 10.1021/ja00226a005. [DOI] [PubMed] [Google Scholar]
- 39.Lau CKDC, Scheigetz J. Reductive deoxygenation of aryl aldehydes and ketones by tert-butylamine-borane and aluminium chloride. J Org Chem. 1989;54:491–494. [Google Scholar]
- 40.Satoshi Matsuzawa YH. Eiichi Nakamura, Isao Kuwajima, Chlorosilane-accelerated conjugate addition of catalytic and stoichiometric organocopper reagents. Tetrahedron. 1989;45(2):349–362. [Google Scholar]
- 41.Welch DR, Krizman DB, Nicolson GL. Multiple phenotypic divergence of mammary adenocarcinoma cell clones. I. In vitro and in vivo properties. Clin Exp Metastasis. 1984;2(4):333–55. doi: 10.1007/BF00135172. [DOI] [PubMed] [Google Scholar]
- 42.Driffort V, Gillet L, Bon E, Marionneau-Lambot S, Oullier T, Joulin V, Collin C, Pages JC, Jourdan ML, Chevalier S, Bougnoux P, Le Guennec JY, Besson P, Roger S. Ranolazine inhibits NaV1.5-mediated breast cancer cell invasiveness and lung colonization. Mol Cancer. 2014;13:264. doi: 10.1186/1476-4598-13-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nelson M, Yang M, Dowle AA, Thomas JR, Brackenbury WJ. The sodium channel-blocking antiepileptic drug phenytoin inhibits breast tumour growth and metastasis. Mol Cancer. 2015;14:13. doi: 10.1186/s12943-014-0277-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang M, Kozminski DJ, Wold LA, Modak R, Calhoun JD, Isom LL, Brackenbury WJ. Therapeutic potential for phenytoin: targeting Na(v)1.5 sodium channels to reduce migration and invasion in metastatic breast cancer. Breast Cancer Res Treat. 2012;134(2):603–15. doi: 10.1007/s10549-012-2102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.