Abstract
Background
Septic shock, a leading cause of acute kidney injury, induces release of pro-/anti-inflammatory mediators, leading to increased mortality and poor renal recovery. This is the first in vitro study directly comparing three single-use blood purification devices in terms of removing sepsis-associated mediators and endotoxins.
Methods
In vitro hemoperfusion was performed using oXiris®, CytoSorb®, and Toraymyxin®. Heparinized human plasma from healthy volunteers was pre-incubated with pathologic quantities of inflammatory mediators and filtered in a closed-loop circulation model for 2 h. For each device, the removal of 27 inflammatory mediators was measured over time. Endotoxin removal mediated by oXiris and Toraymyxin was assessed using hemoperfusion over 6 h.
Results
Endotoxin (lipopolysaccharide) removal was most rapid with Toraymyxin; mean adsorptive clearance over the first 30 min was ~ 20 ml/min vs ~ 8 ml/min with oXiris (p < 0.05). There was minimal endotoxin removal with CytoSorb (1 ml/min). At 120 min, there was no significant difference between the endotoxin removal rates using oXiris (mean ± standard deviation, 68.0 ± 4.4%) and Toraymyxin (83.4 ± 3.8%); both were significantly higher vs CytoSorb (− 6.3 ± 4.9%; p < 0.05). Total removal with oXiris was 6.9 μg vs 9.7 μg for Toraymyxin, where the total lipopolysaccharide quantity introduced was approximately 15.8 μg. Removal rates of pro-/anti-inflammatory cytokines and other inflammatory mediators were similar between oXiris and CytoSorb and were higher with CytoSorb and oXiris vs Toraymyxin. Granulocyte colony-stimulating factor was only effectively adsorbed by CytoSorb (99.4%). Differences were detected between the adsorption mechanism of the devices; binding to oXiris was mainly ionic, while CytoSorb was hydrophobic. No specific protein adsorption was found qualitatively with Toraymyxin.
Conclusions
Adsorption rate kinetics varied for individual inflammatory mediators using the three blood purification devices. Mechanisms of adsorption differed between the devices. oXiris was the only device tested that showed both endotoxin and cytokine removal. oXiris showed similar endotoxin adsorption to Toraymyxin and similar adsorption to CytoSorb for the removal of other inflammatory mediators. Differences in device removal capacities could enable treatment to be more tailored to patients.
Electronic supplementary material
The online version of this article (10.1186/s40635-018-0177-2) contains supplementary material, which is available to authorized users.
Keywords: Adsorption, Blood purification, Cytokines, Endotoxins, oXiris, Septic shock, Removal rate
Background
Patients with acute kidney injury (AKI) have raised serum levels of inflammatory mediators, regardless of the cause [1], and are associated with poor outcomes [2]. Septic shock is a leading cause of AKI [2], accounting for half or more of the cases in intensive care units [3]. The pathophysiology of sepsis and septic shock is not completely understood but is known to involve the release of both pro- and anti-inflammatory mediators [4]. Endotoxins are important in the pathogenesis of septic shock, whereby infection triggers a systemic inflammatory response, resulting in release of pro- and anti-inflammatory cytokines [4–6] (Fig. 1). Pro-inflammatory immune responses are thought to be responsible for the tissue damage that occurs in severe sepsis, while anti-inflammatory responses are implicated in the enhanced susceptibility to secondary infections [7]. Sepsis can also involve the activation of coagulation pathways [4], leading to a higher risk of death [8].
Fig. 1.
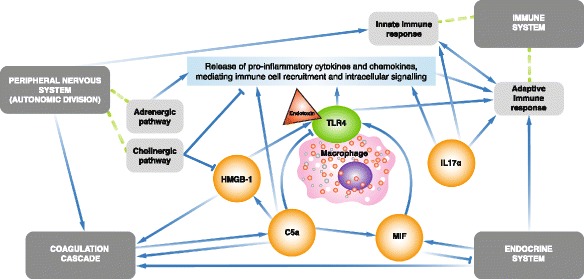
Centers of activity in the inflammatory network driving sepsis. Abbreviations: C5a complement 5a, HMGB-1 high-mobility group box 1 protein, IL-17A interleukin-17A, MIF macrophage migration inhibitory factor, TLR4 Toll-like receptor 4 [6]
Due to the complexity of the immune response, it is unlikely that neutralization of a single indicator would be effective in modulating the inflammatory response, and it is important to look more widely. Several blood purification devices are available that can remove both exogenous and endogenous inflammatory mediators [5]. Such tools include oXiris®, Toraymyxin®, and CytoSorb®. oXiris is a hollow fiber acrylonitrile and methalylsulfonate (AN69) membrane that removes larger molecular weight molecules by membrane binding [5, 9]. Approved first in Europe in 2009, its initial CE-marked indication was extended in 2017 for patients who require blood purification, including those requiring continuous renal replacement therapy (CRRT), and in conditions with excessive endotoxin and inflammatory mediator levels [5, 9]. Approved first in Japan in 1994 and qualifying for CE marking in 1998, Toraymyxin is indicated for use in the treatment of patients with sepsis or septic shock caused by gram-negative bacteria by selectively removing endotoxins from the circulating blood of patients [10]. It consists of a cartridge containing polystyrene-derived woven fibers with the antibiotic polymyxin B immobilized on the surface [5, 10]. Approved first in Europe in 2011 with an indication for use for patients with conditions where excessive cytokine levels exist, CytoSorb is a CE-marked device containing polymer beads to adsorb cytokines that is used in blood pump circuits [5, 11].
Devices such as oXiris can be used on a continuous basis for up to 72 h, although it is recommended that the set is changed every 24 h [9]. Therefore, the ability to retain endotoxins over long periods without the device becoming saturated is of interest. It is possible that differences in the design of these devices may mean that they remove mediators differently (in terms of quantity removed and kinetics). Thus, identifying differences between them could be an important step towards more tailored therapies.
The studies described above have investigated the adsorptive capabilities of these devices for inflammatory mediators such as endotoxins and cytokines. However, we report the first study to directly compare multiple devices across a large spectrum of inflammatory mediators. In this in vitro study, we compared the three single-use blood purification devices in terms of removal of a large panel of sepsis-associated mediators between oXiris, Toraymyxin, and CytoSorb. In addition, we also assessed the removal of endotoxins over a 6-h period by oXiris and Toraymyxin.
Methods
Part 1: Short-term experimental set-up
A total of n = 3 in vitro experimental hemoperfusions were performed comparing oXiris (Baxter, Meyzieu, France), CytoSorb (CytoSorbents Corporation, New Jersey, USA), Toraymyxin (Toray Industries, Tokyo, Japan; Table 1), and a control (tubing circuit without the hemofilter). Devices were primed in accordance with their instructions for use. For each circulation, a total volume of 2-l heparinized fresh frozen human plasma (EFS, Strasbourg, France) was pooled from healthy volunteers and pre-incubated with pathological quantities of inflammatory mediators (Additional file 1: Table S1). The pool was then divided into four reservoirs of 500 ml each and filtered in a closed-loop circulation model for 2 h (Additional file 2: Table S2).
Table 1.
Characteristics of investigated blood purification devices
| Device | Membrane material | Structure | Sterilization mode |
|---|---|---|---|
| oXiris (Baxter) | Copolymer of AN69-coated with PEI and unfractionated heparin | Hollow fiber, symmetric | EtO |
| Toraymyxin (Toray Industries) | PS-based composite woven fiber with immobilized polymyxin B | Woven fibers | Steam |
| CytoSorb (CytoSorbents) | PSDVB copolymer beads, which are highly porous and covered with PVP | Microporous beads | Gamma Irradiation |
Abbreviations: AN69 acrylonitrile and methalylsulfonate, EtO ethylene oxide, PEI polyethylenimine, PSDVB polystyrene divinylbenzene, PS polystyrene, PVP polyvinylpyrrolidone
Measurements (short-term sampling)
Respective concentrations of 27 different inflammatory mediators were measured over time to study the removal capabilities of each device (Additional file 3: Figure S1). Plasma pool samples were taken at times (t) t0, t5, t10, t30, t60, and t120 minutes.
Laboratory analysis
Qualitative evaluation of the proteins adsorbed onto each device was performed by four successive elutions of the membrane with different buffers and subsequent electrophoretic analysis of the elutes. Each device was rinsed with 2 l saline and eluted successively with four different buffers, either 500 ml of an ionic (NaCl 1 M or glycine buffer [HCl] 100 mM [pH 2.25]) or a hydrophobic (glycine buffer [NaOH] 50 mM [pH 11] or SDS 2% in water) desorption mechanism. Devices were rinsed with 1 l of water between the use of each novel desorption media. Samples (> 25 ml) were collected from each desorption circulation and lyophilized at − 80 °C before electrophoretic patterns migration. Samples were diluted (1:2) with a sample buffer (100 M Tris-HCl, pH 6.8, 1% SDS, 4% 2-mercaptoethanol, 0.02% brilliant blue G, and 24% glycerol) (S3047, Sigma) and heated at 100 °C for 4 min. Electrophoretic protein migration was performed using both Tris-tricine (1060–26,000 Da) and Tris-glycine (12,300–78,000 Da) gels, and protein bands were revealed by silver nitrate staining.
Endotoxin (lipopolysaccharide [LPS]) concentrations were measured using the limulus amoebocyte lysate chromogenic method (K-QCL-Lonza assay; LONZA Group Ltd., Basel, Switzerland), concentrations of complement factors C3a and C5a were measured using Singleplex with enzyme-linked immunosorbent assay from commercial kits (Quidel Corporation, San Diego, USA), and high-mobility group box 1 protein (HMGB-1; IBL International GMBH, Hamburg, Germany), and all other markers were measured with MILLIPLEX® Multiplex Assays (Merck Millipore, St-Quentin-en-Yvelines, France) using Luminex® Magpix.
Part 2: Long-term experimental set up
In a separate experiment using the same closed-circuit loop, a hemoperfusion procedure was performed on oXiris, Toraymyxin, and a control tubing over 6 h to document the removal of endotoxins over time. To investigate the saturation of the membrane, three circulations of heparinized human plasma media incubated with LPS were performed successively, using the same device (2 h each), and the LPS concentration was measured in duplicate using the K-QCL-Lonza assay.
Data analysis
Marker removal during the hemofiltration procedure is due exclusively to an adsorption mechanism. The following equation was used to calculate the removal ratio of each marker at the end of the session (i.e., RRAd, %s): RRAds = {CB(0) − CB(120)}/CB(0), where CB(0) is the concentration in the plasma reservoir at baseline (t0), and CB(120) is the concentration at the end of the session (t120).
The mean adsorption clearance for each mediator was simulated using a model of monoexponential decay (limited to the first 30 min of circulation), employing the two equations: CB(30) = CB(0) e–k t and CL(30) = k × V, where CB(0) and CB(30) were respective concentrations in the plasma reservoir at t0 and t30, respectively. Here, k is the slope of decay, t is the time, V is the plasma reservoir volume, and CL(30) is the mean adsorptive clearance over 30 min of circulation.
The adsorption isotherm was represented using the equation: Quantityadsorbed = RRAds × Quantityintroduced.
Data analyses performed included the removal capabilities of each investigated blood purification device overall and for each tested molecule, as well as kinetic removal profiles of endotoxins, pro-inflammatory cytokines, anti-inflammatory cytokines, and other inflammatory mediators over 120 min. Concentrations of LPS measured at the start of hemofiltration procedure and over time, and the quantity of LPS adsorbed, were calculated following long-term sampling using the above methods.
Statistical analysis
Differences were analyzed using Kruskal-Wallis non-parametric testing using Minitab software (Minitab Inc., State College, USA). The Z value determined how the mean for each group differed from the mean of all observations, with p values < 0.05 indicating a significant difference.
Results
Endotoxin removal
Adsorption of endotoxin was observed with oXiris and Toraymyxin but not with CytoSorb (Fig. 2). Endotoxin removal was most rapid with Toraymyxin (Additional file 3: Figure S1); mean absorptive clearance over the first 30 min was ~ 20 ml/min vs ~ 8 ml/min with oXiris (p < 0.05). Over the first 30 min, the mean adsorption clearance was greatest with CytoSorb, followed by oXiris (Fig. 2). Removal of LPS was not observed with CytoSorb at 1 ml/min. At t120, there was no significant difference between the LPS removal rates (RRs) using oXiris (mean ± standard deviation, 68.0 ± 4.3%) and Toraymyxin (83.4 ± 3.8%); rates with both were significantly higher vs CytoSorb (− 6.3 ± 4.9%; p < 0.05).
Fig. 2.
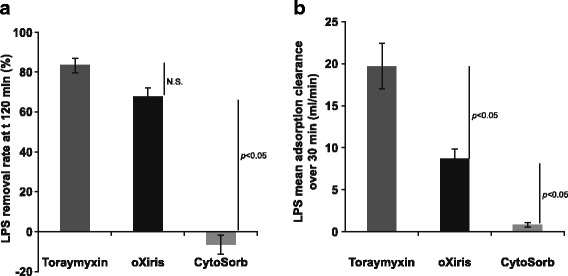
LPS removal with oXiris, Toraymyxin, and CytoSorb. a LPS removal rate at t120 min. b LPS mean adsorption clearance over 30 min. Abbreviations: LPS lipopolysaccharide, N.S. not significant
High endotoxin removal capacities were confirmed with oXiris and Toraymyxin over 6 h. The total removal quantity over 6 h with oXiris was 6.9 vs 9.7 μg for Toraymyxin, where the total LPS quantity introduced was approximately 15.8 μg (Fig. 3). For both oXiris and Toraymyxin, adsorption of LPS followed the Langmuir isotherm model [12]. Faster saturation of the LPS-adsorptive capacity was observed for oXiris than for Toraymyxin (Fig. 3). For oXiris and Toraymyxin, respectively, the calculated RR (%) after circulation 1 was 66.6 vs 75.9%, after circulation 2 was 35.0 vs 51.6%, and after circulation 3 was 30.5 vs 58.2%.
Fig. 3.
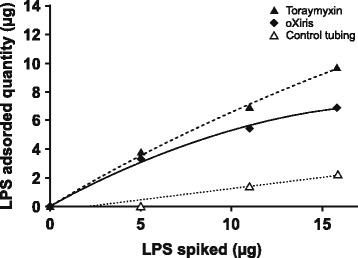
LPS adsorption isotherm obtained with oXiris and Toraymyxin blood purification devices. Abbreviation: LPS lipopolysaccharide
Adsorption of different inflammatory mediators
Adsorption of inflammatory mediators is represented for each device as the mean calculated removal rates at t120 min (Table 2). The mean adsorptive clearances after 30 min of circulation are represented as a function of tested molecules ranked according to their respective molecular weight (Fig. 4).
Table 2.
Removal capabilities of investigated blood purification device per mediator. Means expressed ± SD
| Mediators | Removal rates (RR%) at 120 min | ||||
|---|---|---|---|---|---|
| Control tubing | oXiris | CytoSorb | Toraymyxin | ||
| Pro-inflammatory cytokines | IL-3 | 3.9 (± 1.4) | 99.3 (± 0.0) | 99.4 (± 0.0) | 70.2 (± 11.1) |
| IP-10 | 16.6 (± 17.4) | 99.3 (± 0.3) | 99.1 (± 0.2) | 68.6 (± 11.9) | |
| IL-17α | 16.7 (± 7.8) | 98.7 (± 0.4) | 97.6 (± 0.3) | 74.0 (± 6.2) | |
| MIP-1α | 2.9 (± 3.1) | 97.3 (± 0.4) | 97.3 (± 0.4) | 91.0 (± 0.4) | |
| MIP-1β | 4.7 (± 2.9) | 91.5 (± 1.2) | 92.4 (± 0.0) | 70.3 (± 9.0) | |
| HMGB-1 | 8.4 (± 3.2) | 89.5 (± 0.4) | 91.8 (± 0.9) | 61.5 (± 1.9) | |
| IL-8 | 4.6 (± 8.0) | 100 (± 0.0) | 100 (± 0.0) | 34.5 (± 13.1) | |
| IFN-γ | 7.9 (± 8.8) | 99.5 (± 0.3) | 95.7 (± 0.6) | 37.4 (± 8.3) | |
| Eotaxin | 14.3 (± 9.1) | 99.1 (± 0.1) | 99.0 (± 0.0) | 42.2 (± 7.9) | |
| IL-6 | 5.2 (± 9.3) | 93.5 (± 1.4) | 99.6 (± 0.1) | 41.8 (± 14.6) | |
| MIF | 14.3 (± 5.9) | 78.0 (± 24.4) | 83.0 (± 20.2) | 45.1 (± 13.8) | |
| MCP-1 | 6.0 (± 4.1) | 100 (± 0.0) | 100 (± 0.0) | 11.3 (± 4.4) | |
| TNF-α | 11.8 (± 12.5) | 90.1 (± 2.2) | 98.4 (± 0.2) | 17.9 (± 9.2) | |
| IL-1β | 8.2 (± 4.5) | 86.8 (± 1.0) | 97.2 (± 0.0) | 15.0 (± 13.3) | |
| Anti-inflammatory cytokines | IL-4 | 6.1 (± 8.3) | 99.9 (± 0.0) | 99.9 (± 0.0) | 55.9 (± 16.0) |
| IL-2 | 1.8 (± 5.0) | 99.4 (± 0.2) | 99.3 (± 0.3) | 61.6 (± 13.6) | |
| IL-10 | 8.9 (± 7.7) | 99.0 (± 0.4) | 99.8 (± 0.0) | 40.6 (± 14.9) | |
| IL-13 | 12.2 (± 5.9) | 93.5 (± 0.0) | 94.2 (± 0.0) | 73.7 (± 20.6) | |
| IL-1Ra | 11.2 (± 3.6) | 90.2 (± 2.8) | 92.1 (± 0.0) | 35.4 (± 16.2) | |
| IL-12 p70 | 7.0 (± 7.5) | 22.1 (± 4.5) | 76.5 (± 2.5) | 6.9 (± 8.0) | |
| Complement factors | C3a | 14.8 (± 11.5) | 96.4 (± 1.2) | 98.2 (± 0.2) | 67.9 (± 7.5) |
| C5a | 1.3 (± 4.8) | 90.7 (± 0.6) | 95.7 (± 1.2) | 38.8 (± 7.9) | |
| Serine protease | PAI-1 | 8.5 (± 4.9) | 87.9 (± 1.8) | 95.5 (± 0.4) | 30.9 (± 9.6) |
| Growth factors | FGF-23 | 18.3 (± 12.2) | 98.7 (± 1.1) | 99.4 (± 0.8) | 88.4 (± 5.5) |
| FGF-21 | 2.9 (± 0.5) | 96.0 (± 0.9) | 99.9 (± 0.0) | 70.9 (± 10.1) | |
| G-CSF | 8.1 (± 9.0) | 36.0 (± 2.9) | 99.4 (± 0.0) | 16.9 (± 8.0) | |
| Fluid removal | CRRT | – | Yes | No | No |
Abbreviations: C complement, CRRT continuous renal replacement therapy, FGF fibroblast growth factor, G-CSF granulocyte colony-stimulating factor (glycoprotein), HMGB-1 high-mobility group box 1 protein, IL interleukin, IFN interferon, IP interferon-induced protein, LPS lipopolysaccharide, MCP monocyte chemoattractant protein, MIF macrophage migration inhibitory factor, MIP macrophage inflammatory protein, PAI plasminogen activator inhibitor, SD standard deviation, TNF tumor necrosis factor, Ra receptor agonist, α alpha, β beta, γ gamma
Fig. 4.
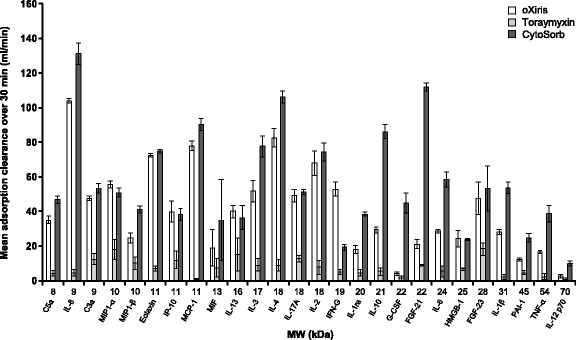
Mean adsorption clearances over 30 min for the inflammatory mediators included in the study. Abbreviations: C3a complement 3a, C5a complement 5a, FGF fibroblast growth factor, G-CSF granulocyte-colony stimulating factor, HMGB-1 high-mobility group box 1 protein, IFN interferon, IL interleukin, IP interferon-induced protein, MCP monocyte chemoattractant protein, MIF macrophage migration inhibitory factor, MIP macrophage inflammatory protein, PAI plasminogen activator inhibitor, TNF tumor necrosis factor, Ra receptor agonist, α alpha, β beta, γ gamma
Cytokines
For oXiris and CytoSorb, RRs of the pro- and anti-inflammatory cytokines studied were in the same ranges for 18 of the 20 mediators investigated (Table 2; Additional file 4: Figure S2). The RRs of > 70% for oXiris and > 80% for CytoSorb were observed for most of the pro- and anti-inflammatory mediators. The only exception was the lower RRs for oXiris vs CytoSorb for interleukin 12 (IL-12 p70; 22.1 ± 4.5% vs 76.5 ± 2.5%, respectively).
The RRs of all 14 pro-inflammatory mediators were statistically lower with Toraymyxin vs oXiris and CytoSorb, except for MIF (p = 0.103) and MIP-1A (p = 0.87; RR of the six inflammatory mediators were statistically lower with Toraymyxin vs oXiris and CytoSorb, except for IL-13 (p = 0.055) (Table 2; Additional file 4: Figure S2). Adsorption clearances and kinetics for each of the pro- and anti-inflammatory mediators investigated are shown in Fig. 4 and Additional file 3: Figure S1. In general, adsorption kinetics were faster with CytoSorb vs oXiris. However, for most mediators, t120 levels were comparable, and no significant differences were seen between CytoSorb and oXiris. The only exceptions were for IL-12 p70 and IL-1β, which were lower (indicating greater removal) with CytoSorb than with oXiris at t120 (69.0 ± 9.6 vs 238.4 ± 36.1 pg/ml and 0.8 ± 0 vs 3.8 ± 0.2 pg/ml, respectively).
Other inflammatory mediators
The RRs of complement factors (C3a and C5a), serine proteases (plasminogen activator inhibitor; PAI-1), fibroblast growth factors (FGF-23 and FGF-21), and glycoproteins (G-CSF) were higher with CytoSorb and oXiris vs Toraymyxin (Table 2; Additional file 4: Figure S2). RRs were in the same range for oXiris and CytoSorb for three of the six mediators but were slightly lower with oXiris for C5a (90.7 ± 0.6% vs 95.7 ± 1.2%, respectively) and PAI-1 (87.9 ± 1.8% vs 95.5 ± 0.4%) and significantly lower with oXiris for G-CSF (36.0 ± 2.9% vs 99.4 ± 0.0%).
RRs of all six factors (C3a, C5a, PAI-1, FGF-23, FGF-21, and G-CSF) were lower with Toraymyxin vs oXiris and CytoSorb. Differences were significant for all markers except for G-CSF which was only effectively adsorbed by CytoSorb (RR 99.4%; Table 2; Additional file 4: Figure S2). The kinetic removal profiles of the complement factors (C3a and C5a) and FGF-23 were comparable between oXiris and CytoSorb. FGF-21 and PAI-1 were initially removed more rapidly with CytoSorb, but the levels were comparable by 120 min (Fig. 4; Additional file 3: Figure S1).
Mechanism of adsorption
Figure 5 shows the pattern of proteins eluted from the devices using different patterns (Tris-glycine on top and Tris-tricine on bottom) and buffers to differentiate ionic (NaCl 1 M and glycine pH 2.25 buffers) from hydrophobic (glycine pH 11 and SDS 2% buffer) mechanisms. Strong bands can be seen with the NaCl 1 M and pH 2.25 glycine buffers in the eluted fraction from oXiris and in the eluted fraction from the SDS buffer for CytoSorb. Strong bands were not detected with the eluted fractions from any of the four buffers for Toraymyxin.
Fig. 5.
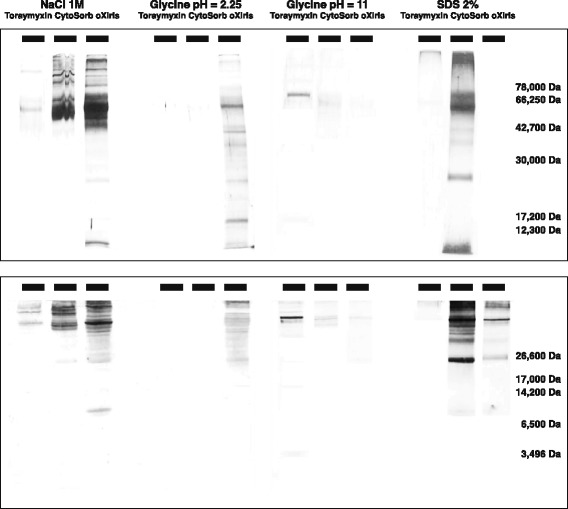
Electrophoretic patterns of membrane elutes. Upper panel is a Tris-glycine gel; lower panel is a Tris-tricine gel
Discussion
In this study, we have shown that the three most widely available sorbent devices have very different spectrums of solute removal. While Toraymyxin is efficient in removing endotoxins, it does not effectively remove inflammatory mediators. Conversely, while Cytosorb removes a wide range of inflammatory mediators, it does not remove endotoxins. oXiris, by contrast, has similar adsorbent characteristics to Toraymyxin for endotoxins and to Cytosorb for most inflammatory mediators.
Endotoxin removal
We observed RRs for endotoxins comparable with previous in vitro and clinical studies [13, 14]. Harm and colleagues found a reduction in LPS activity of 60 ± 14% with Toraymyxin using fresh human whole blood in vitro [14], while Romaschin and colleagues found a reduction in LPS activity of 88% with Toraymyxin using bovine serum or plasma in vitro [13]. In a systematic review of 28 studies of direct hemoperfusion with Toraymyxin, endotoxin levels decreased by 33–80% after Toraymyxin hemoperfusion in the 17 studies measuring such levels [15]. Overall, this review concluded that selective LPS-adsorption was associated with improvements in clinical outcomes (significant increases in mean arterial blood pressure, + 19 mmHg; reductions in vasoactive dopamine/dobutamine dose, − 1.8 μg/kg/min; and increases in gas exchange, mean arterial partial pressure of oxygen/fraction of inspired oxygen ratio, + 32 units) and in mortality risk (risk ratio, 0.53; 95% CI, 0.43–0.65). More recently, improvements in hemodynamic indices and survival rates have also been shown following Toraymyxin hemoperfusion of patients with severe sepsis or septic shock in the randomized controlled EUPHAS trial vs conventional therapy [15], the EUPHAS 2 registry [16], and in a prospective study [17]. However, no improvement was seen in either mortality or organ failure with Toraymyxin in a study enrolling 243 patients from 18 French intensive care units [18].
There is a paucity of data on the clinical effects of oXiris [19], and studies are ongoing [20]. However, the clinical benefits from the use of oXiris have been reported in a small study that found expedited improvement in organ function with oXiris vs conventional continuous venovenous hemofiltration only [21]. The total Sequential Organ Failure Assessment score improved significantly from baseline to 48 h in the oXiris group (p = 0.011; control p = 0.515), decreasing by 37% vs an increase of 3% in the control group (p = 0.013) [21].
We confirmed high endotoxin removal capacity with both oXiris and Toraymyxin over 6 h (oXiris 6.9 μg vs Toraymyxin 9.7 μg; total LPS quantity introduced was approximately 15.8 μg). High endotoxin plasma load in septic human blood has been previously reported in the range of 3–30 μg [13], supporting the clinically relevant removal capabilities of both devices.
Pro- and anti-inflammatory mediators
Increased circulating levels of both pro-inflammatory (IL-6) and anti-inflammatory (IL-10) mediators have been associated with poor survival in numerous studies in patients with sepsis, including patients with septic shock randomized to different fluid resuscitation strategies [4]. Both IL-6 and IL-10 were effectively removed by oXiris (RR > 90%) and CytoSorb (RR > 90%), but RRs were lower for Toraymyxin (< 50%). RRs observed here for IL-10 (RR > 95% with CytoSorb) differ from the review by Honore and colleagues, who noted that CytoSorb was unable to capture IL-10 in humans [22], but are consistent with preclinical studies by Kellum and colleagues [23].
Elevated levels of C3a and C5a have been reported in sepsis, and high levels of C3a are associated with a higher risk of death [8, 24]. In our study, oXiris and CytoSorb displayed a significantly higher reduction of C3a and C5a (p < 0.05) compared with Toraymyxin. Additionally, greater removal of PAI-1 was found with oXiris and CytoSorb vs Toraymyxin. Levels of PAI-1, a marker of vascular endothelial cell activation and a major regulator of fibrinolysis, are increased by endotoxins, altering the normal balance between blood coagulation and fibrinolysis [5]. Following hemoperfusion, particularly with oXiris and CytoSorb, PAI-1 levels are decreased, modulating fibrinolysis and reducing the risk of sepsis-associated thrombosis formation. A recent study also indicated that the role of oXiris in fibrinolysis modulation may warrant further investigation [25]. Significant heterogeneity of inflammatory mediator levels in patients with sepsis/septic shock numerous investigators [4, 26]. Differential removal characteristics might therefore be advantageous and lead to a more personalized approach to medicine.
We observed that the results are consistent with the nature of the devices investigated; oXiris is a highly electrically charged hydrogel, while CytoSorb is a hydrophobic resin. However, previous studies using molecular mechanic simulations have shown that the binding of polymyxin B to endotoxins occurs through hydrophobic bonds between fatty acid chains of the lipid A in endotoxin and hydrophobic amino acids of polymyxin B and through ionic interactions between the endotoxin’s negatively charged phosphate groups in lipid A and the positively charged amino groups of polymyxin B [5, 27]. Toraymyxin is a device made of rolled woven fibers, whereby the blood circulates at the external side of the fibers [10]. From a design perspective, the accessible surface area for adsorption is most probably much lower as compared with CytoSorb, which is made of small polymer beads [11]. The oXiris device also benefits from a large surface area available for adsorption [9], the bulk membrane being completely dense and isotropic. In the case of Toraymyxin, the proportion of charged groups at the surface to accessible remaining hydrophobic residues is also unknown, and both chemical groups may compete.
Our study has limitations that should be acknowledged. First, as an in vitro study, the outcomes observed here may not be representative of the clinical setting. Second, the experimental setup used human plasma that had been preincubated with the mediators. This system does not allow us to examine the impact of each device on the inflammatory response itself, i.e., the generation and release of the inflammatory mediators during and after the treatment, as well as potential interactions with blood cells. Furthermore, we cannot exclude the possibility of competition between the different mediators for adsorption, given they were added simultaneously in each experiment. However, the high reproducibility observed between experiments, combined with the relatively low quantities of soluble toxins introduced, suggests that limited competition occurred in the experimental setup. Finally, the concentrations used were pathological, and our data can only be used to comment on the adsorption of these mediators at these concentrations.
Conclusions
oXiris was shown to have the broadest adsorption capacity of the devices tested. oXiris displayed similar adsorption to Toraymyxin for endotoxin removal and similar adsorption to CytoSorb for the removal of most cytokines and other inflammatory mediators. Further investigation is warranted as to whether a more continuous blood purification with oXiris may improve outcomes in terms of reducing immune cell activation and organ damage compared to short-term intended use (e.g., Toraymyxin use is limited to 2 h).
Additional files
Table S1. Pathological concentrations of mediators included in the study. (DOCX 25 kb)
Table S2. Inflammatory mediators included in the study. (DOCX 30 kb)
Figure S1. Kinetic removal profiles of a) endotoxin, b) pro-inflammatory cytokines, c) anti-inflammatory cytokines, and d) other inflammatory mediators. (DOCX 867 kb)
Figure S2. Removal ratios of a) pro-inflammatory cytokines, b) anti-inflammatory cytokines, and c) other inflammatory mediators. (DOCX 148 kb)
Acknowledgements
Editorial support for the development of this manuscript was provided by SciMentum, with funding provided by Baxter. The authors thank Emmanuelle Blanc and Thierry Crost for their technical contribution during the data collection.
Funding
This study was sponsored by Baxter.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AKI
Acute kidney injury
- C
Complement
- CRRT
Continuous renal replacement therapy
- FGF
Fibroblast growth factor
- G-CSF
Granulocyte colony-stimulating factor
- HMGB
High-mobility group box 1 protein
- IFN
Interferon
- IL
Interleukin
- IP
Interferon-induced protein
- LPS
Lipopolysaccharide
- MCP
Monocyte chemoattractant protein
- MIF
Macrophage migration inhibitory factor
- MIP
Macrophage inflammatory protein
- PAI
Plasminogen activator inhibitor
- RR
Removal rates
- TNF
Tumor necrosis factor
- α
Alpha
- β
Beta
- γ
Gamma
Authors’ contributions
BM contributed to the conception and design of the work, data analysis and interpretation, and drafting of this manuscript. CL contributed to the design of the work and data collection, JAK contributed to the data interpretation and drafting of the article. All authors were accountable for all aspects of the work, working and critically revising the manuscript, and approved the final version to be published.
Ethics approval and consent to participate
Not applicable.
Competing interests
BM and CL are Baxter employees. JAK has a consulting contract with Baxter.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s40635-018-0177-2) contains supplementary material, which is available to authorized users.
Contributor Information
Benjamin Malard, Phone: +33 4 72 45 39 01, Email: benjamin_malard@baxter.com.
Corine Lambert, Email: corine_lambert@baxter.com.
John A. Kellum, Email: kellum@pitt.edu
References
- 1.Quinto BM, Iizuka IJ, Monte JC, Santos BF, Pereira V, Durão MS, Dalboni MA, Cendoroglo M, OF S, Batista MC. TNF-α depuration is a predictor of mortality in critically ill patients under continuous veno-venous hemodiafiltration treatment. Cytokine. 2015;71:255–260. doi: 10.1016/j.cyto.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 2.Zarbock A, Gomez H, Kellum JA. Sepsis-induced AKI revisited: pathophysiology, prevention and future therapies. Curr Opin Crit Care. 2014;20:588–595. doi: 10.1097/MCC.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zarjou A, Agarwal A. Sepsis and acute kidney injury. J Am Soc Nephrol. 2011;22:999–1006. doi: 10.1681/ASN.2010050484. [DOI] [PubMed] [Google Scholar]
- 4.Kellum JA, Pike F, Yealy DM, Huang DT, Shapiro NI, Angus DC. Relationship between alternative resuscitation strategies, host response and injury biomarkers, and outcome in septic shock: analysis of the protocol-based care for early septic shock study. Crit Care Med. 2017;45:438–445. doi: 10.1097/CCM.0000000000002206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esteban E, Ferrer R, Alsina L, Artigas A. Immunomodulation in sepsis: the role of endotoxin removal by polymyxin B-immobilized cartridge. Mediat Inflamm. 2013;2013:507539. doi: 10.1155/2013/507539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. Nat Rev Immunol. 2008;8:776–787. doi: 10.1038/nri2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 8.Ward PA. The dark side of C5a in sepsis. Nat Rev Immunol. 2004;4:133–142. doi: 10.1038/nri1269. [DOI] [PubMed] [Google Scholar]
- 9.Baxter (2017) oXiris. Instructions for Use
- 10.Toray Industries (2016) Toraymyxin PMX 20-R. Instructions for Use
- 11.CytoSorbents Inc. (2012) CytoSorb 300mL device. Instructions for Use
- 12.Patiha HE, Hidayat Y, Firdaus M. The langmuir isotherm adsorption equation: the monolayer approach. IOP Conf Ser: Mater Sci Eng. 2016;107:012067. doi: 10.1088/1757-899X/107/1/012067. [DOI] [Google Scholar]
- 13.Romaschin AD, Obiezu-Forster CV, Shoji H, Klein DJ. Novel insights into the direct removal of endotoxin by polymyxin B hemoperfusion. Blood Purif. 2017;44:193–197. doi: 10.1159/000475982. [DOI] [PubMed] [Google Scholar]
- 14.Harm S, Falkenhagen D, Hartmann J. Endotoxin adsorbents in extracorporeal blood purification: do they fulfill expectations? Int J Artif Organs. 2014;37:222–232. doi: 10.5301/ijao.5000304. [DOI] [PubMed] [Google Scholar]
- 15.Cruz DN, Perazella MA, Bellomo R, de Cal M, Polanco N, Corradi V, Lentini P, Nalesso F, Ueno T, Ranieri VM, Ronco C. Effectiveness of polymyxin B-immobilized fiber column in sepsis: a systematic review. Crit Care. 2007;11:R47. doi: 10.1186/cc5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cutuli SL, Artigas A, Fumagalli R, Monti G, Ranieri VM, Ronco C, Antonelli M, The EUPHAS 2 Collaborative Group Polymyxin-B hemoperfusion in septic patients: analysis of a multicenter registry. Ann Intensive Care. 2016;6:77. doi: 10.1186/s13613-016-0178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yaroustovsky M, Abramyan M, Krotenko N, Popov D, Plyushch M, Popok Z. Endotoxin adsorption using polymyxin B immobilized fiber cartridges in severe sepsis patients following cardiac surgery. Int J Artif Organs. 2014;37:299–307. doi: 10.5301/ijao.5000322. [DOI] [PubMed] [Google Scholar]
- 18.Payen DM, Guilhot J, Launey Y, Lukaszewicz AC, Kaaki M, Veber B, Pottecher J, Joannes-Boyau O, Martin-Lefevre L, Jabaudon M, Mimoz O, Coudroy R, Ferrandière M, Kipnis E, Vela C, Chevallier S, Mallat J, Robert R, The ABDOMIX Group Early use of polymyxin B hemoperfusion in patients with septic shock due to peritonitis: a multicenter randomized control trial. Intensive Care Med. 2015;41:975–984. doi: 10.1007/s00134-015-3751-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hattori N, Oda S. Cytokine-adsorbing hemofilter: old but new modality for septic acute kidney injury. Ren Replace Ther. 2016;2:41. doi: 10.1186/s41100-016-0051-1. [DOI] [Google Scholar]
- 20.Broman M (2017) Comparing cytokines, toxins adsorbing oXiris filter to ST150 filter during CRRT in patients with septic shock (oXiris). https://www.clinicaltrials.gov/ct2/show/NCT02600312. Accessed 1 Nov 2017
- 21.Shum HP, Chan KC, Kwan MC, Yan WW. Application of endotoxin and cytokine adsorption haemofilter in septic acute kidney injury due to Gram-negative bacterial infection. Hong Kong Med J. 2013;19:491–497. doi: 10.12809/hkmj133910. [DOI] [PubMed] [Google Scholar]
- 22.Honore PM, Jacobs R, Joannes-Boyau O, De Regt J, De Waele E, van Gorp V, Boer W, Verfaillie L, Spapen HD. Newly designed CRRT membranes for Sepsis and SIRS—a pragmatic approach for bedside intensivists summarizing the more recent advances: a systematic structured review. ASAIO J. 2013;59:99–106. doi: 10.1097/MAT.0b013e3182816a75. [DOI] [PubMed] [Google Scholar]
- 23.Kellum JA, Song M, Venkataraman R. Hemoadsorption removes tumor necrosis factor, interleukin-6, and interleukin-10, reduces nuclear factor-kappaB DNA binding, and improves short-term survival in lethal endotoxemia. Crit Care Med. 2004;32:801–805. doi: 10.1097/01.CCM.0000114997.39857.69. [DOI] [PubMed] [Google Scholar]
- 24.Nakae H, Endo S, Inada K, Takakuwa T, Kasai T, Yoshida M. Serum complement levels and severity of sepsis. Res Commun Chem Pathol Pharmacol. 1994;84:189–195. [PubMed] [Google Scholar]
- 25.Turani F, Busatti S, Martini S, Barchetta R, Belli A, Falco M. Tromboelastography (TEG) may detect hypercoagulation in early sepsis and improves anticoagulation during extracorporeal treatments. Crit Care. 2015;19(Suppl 1):P341. doi: 10.1186/cc14421. [DOI] [Google Scholar]
- 26.Pierrakos C, Vincent JL. Sepsis biomarkers: a review. Crit Care. 2010;14:R15. doi: 10.1186/cc8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vesentini S, Soncini M, Zaupa A, Silvestri V, Fiore GB, Redaelli A. Multi-scale analysis of the Toraymyxin adsoprtion cartridge. Part I: molecular interaction of polymyxin B with endotoxins. Int J Artif Organs. 2006;29:239–250. doi: 10.1177/039139880602900210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Pathological concentrations of mediators included in the study. (DOCX 25 kb)
Table S2. Inflammatory mediators included in the study. (DOCX 30 kb)
Figure S1. Kinetic removal profiles of a) endotoxin, b) pro-inflammatory cytokines, c) anti-inflammatory cytokines, and d) other inflammatory mediators. (DOCX 867 kb)
Figure S2. Removal ratios of a) pro-inflammatory cytokines, b) anti-inflammatory cytokines, and c) other inflammatory mediators. (DOCX 148 kb)
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


