Abstract
Introduction
Chronic chikungunya (CHIK) arthritis, an inflammatory arthritis, often follows acute CHIK fever (CHIKF), a viral infection. The pathogenesis of chronic CHIK arthritis is poorly characterized, but may resemble other forms of inflammatory arthritis. Clinically, chronic CHIK arthritis sometimes mimics rheumatoid arthritis (RA).
Case Report
We report a patient with well-characterized CHIKF followed 2 months later by chronic CHIK arthritis not only resembling RA clinically, but also associated with RA biomarkers and extra-articular features, including Felty’s syndrome (FS).
Conclusions
We describe this patient’s excellent response to methotrexate and discuss the implications her case provides in understanding this important emerging rheumatic disease.
Keywords: Chikungunya, Chronic chikungunya arthritis, Felty’s syndrome, Methotrexate, Rheumatoid arthritis
Introduction
Chikungunya (CHIK) is an emerging arboviral infection causing acute febrile illness, followed in many patients by chronic inflammatory arthritis. The CHIK epidemic has spread from Africa to Asia and now the entire tropical and sub-tropical world, infecting millions of people and causing major epidemics of disabling arthritis [1]. Both the pathogenesis and the clinical characteristics of chronic CHIK arthritis are incompletely characterized. Acute CHIK fever (CHIKF) is associated with viremia and a strong anti-viral, cytokine response [2]. The pathogenesis of the transition to chronic arthritis, in some, but not all patients, is less clear [3]. The duration and clinical spectrum of chronic CHIK arthritis are also highly variable. Some CHIK arthritis patients clinically resemble rheumatoid arthritis (RA) [4].
We report a 55-year-old woman who developed acute CHIKF, followed 2 months later by symmetric polyarthritis with positive rheumatoid factor (RF) and anti-citrullinated protein (anti-CCP) antibody and 1 year later by Feltys syndrome (FS) with neutropenia and splenomegaly. Her illness suggests pathogenic and clinical overlap between chronic CHIK arthritis and RA. These associations provide insights into the mechanisms of these diseases and for treating inflammatory arthritis patients in CHIK epidemic areas. Fortunately, for this patient, methotrexate (MTX) treatment was very effective. In this case report, we describe this patient’s CHIKF infection, the development of post infectious CHIK arthritis with features of classic RA, including Felty’s syndrome, her successful treatment, and consider more general implications offered by her case.
Case Report
In February 2016, a 55-year-old woman, living in northeastern Brazil in the State of Pernambuco, developed high fever with temperature of 103 °F. She also had arthritis affecting the ankles, wrists, proximal interphalangeal joints (PIP), and knees. She developed a maculopapular rash, retro-orbital headache, and alopecia. In the absence of laboratory testing, her primary care physician diagnosed CHIKF based on her clinical features and the occurrence of a CHIK epidemic. She was treated with dipyrone, paracetamol, and supportive care. Her symptoms resolved after 13 days.
Two months later, she developed recurrent, disabling arthritis in her PIP joints, wrists, knees, and ankles. She also had arthralgias affecting her shoulders, low back pain, and fatigue. These symptoms persisted over the next year. Because of this unrelenting arthritis and severe pain, she returned to her primary care physician in May 2017.
The physician noted the presence of polyarthritis and obtained laboratory tests (Table 1). The white blood cell count (WBC) was decreased (WBC 1800 cell/mm3, normal value 4500–11,000) with decreased neutrophils (neutrophils 414 cell/mm3, normal value 1570–11,000). An abdominal ultrasound demonstrated splenomegaly (uniplanar splenic index 72.6 cm3, normal value 19.8 ± 12.3).
Table 1.
Outpatient investigations
| Date (2017) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | May 23 | June 12 | July 10 | August 7 | August 21 | August 24 | September 20 | October 17 | Normal range |
| White cell count, cell/mm3 | 1800 | – | 2000 | 2500 | 2300 | 2100 | 6800 | 8000 | 4500–11,000 |
| Neutrophils | 414 | – | 420 | 1000 | 529 | 483 | 4094 | 4368 | 1570–7700 |
| Lymphocytes | 1044 | – | 1440 | 1225 | 1495 | 1386 | 2020 | 2816 | 900–3850 |
| Monocytes | 144 | – | 120 | 125 | 230 | 210 | 558 | 696 | 72–1100 |
| Eosinophils | 54 | – | 20 | 100 | 23 | 21 | 82 | 64 | 90–550 |
| Hemoglobin, gm/dl | 11.4 | – | 12.5 | 11.7 | 12 | 12 | 13 | 14.2 | 12–16 |
| Platelet count, per mm3 | 152,000 | – | 146,000 | 151,400 | 128,000 | 139,000 | 178,000 | 192,000 | 150,000–450,000 |
| Mean corpuscular volume, mm3 | 80.38 | – | 80.92 | 90 | 82.7 | 88.9 | 86.06 | 86.7 | 80–100 |
| ESR, mm/h | – | – | 65 | 67 | – | 54 | 31 | 20 | < 20 |
| RF, IU/ml | – | – | 211.3 | – | – | – | – | – | < 30 |
| Anti-CCP antibodies, U/ml | – | – | 274 | – | – | – | – | – | < 17 |
| ANA (Hep 2 cell) | – | Pattern Homogeneous nuclear pattern 1:80 dilution Chromosome metaphase plate reagent |
– | – | – | – | – | – | Non-reactive |
| ELISA chikungunya IgG | – | – | – | – | Reactive | – | – | – | Non-reactive |
| Lactate dehydrogenase U/l | – | 531 | – | – | – | – | – | – | 313–414 |
| Anti-HIV 1 and 2 | – | Non-reactive | – | – | – | – | – | – | Non-reactive |
| Anti-HTLV 1 and 2 | – | Non-reactive | – | – | – | – | – | – | Non-reactive |
| DAS28-ESR | – | – | – | – | 7.39 | – | 2.54 | 2.2 | Remission < 2.6 |
| CDAI | – | – | – | – | 72 | – | 2 | 2 | Remission 0.0–2.8 |
| Bone marrow biopsy | June 1 | ||||||||
| Erythrocyte series: normocellular, nomomaturative, and normoblastic Granulocytic series: discretely hypocellular, with moderate maturative delay. Myoblasts: 6.6%; Promyelocytes: 8.8% Myelocytes: 3%; Rods: 19%; Segmented: 2.2%; Eosinophilic cells: 5%; Signs of dysgranulocitopoiesis Lymphomonoplasmocytic series: lymphocytes: 22%; Plasma cells: 3.6% Megalacariocytic series: normocellular | |||||||||
| Bone marrow aspirate | July 25 | ||||||||
| Normocellular bone marrow, with discreet hypocellularity in the neutrophilic lineage with important maturative delay, discrete increase of blasts and slight eosinophilia | |||||||||
| Abdominal ultrasound | June 20 | ||||||||
| Splenomegaly (uniplanar splenic index = 72.6) | 19.8 ± 12.3 | ||||||||
The patient was referred to a hematologist who obtained a bone marrow examination. The bone marrow aspirate showed “normocellular bone marrow, with discreet hypocellularity in the neutrophilic lineage with significant maturation delay, discrete increase of blasts, and slight eosinophilia.” A bone marrow biopsy demonstrated “reduction in the maturation of the granulocytopenic series”.
The hematologist also obtained an elevated erythrocyte sedimentation rate (ESR) (ESR 65 mm/h, normal value < 20) and RF (RF 211.3 IU/ml, normal value < 30). Other tests obtained by the hematologist are reported in Table 1. The patient continued to have disabling arthritis and was referred to a rheumatologist.
The patient was evaluated by one of us (JKA) in August of 2017. She complained of continuing fatigue, morning stiffness, and painful polyarthritis. Her past medical history was negative. She denied previous arthritis symptoms or known hematologic disease, including leukopenia. Her family history was remarkable in that her mother had RA. She worked as a public employee and denied smoking or drinking.
On physical examination, her blood pressure was 120/80 mmHg, her temperature was 96.8 °C, her respiratory rate was 14/min, and her pulse was 80 beats/min. Examination of the head, eyes, ears, nose, mouth, neck, heart, and lungs was normal. Her spleen was palpable. She had arthritis, including tenderness, swelling, and synovitis in the PIP, metacarpophalangeal joints (MCP), wrists, knees, and ankles (Fig. 1a).
Fig. 1.
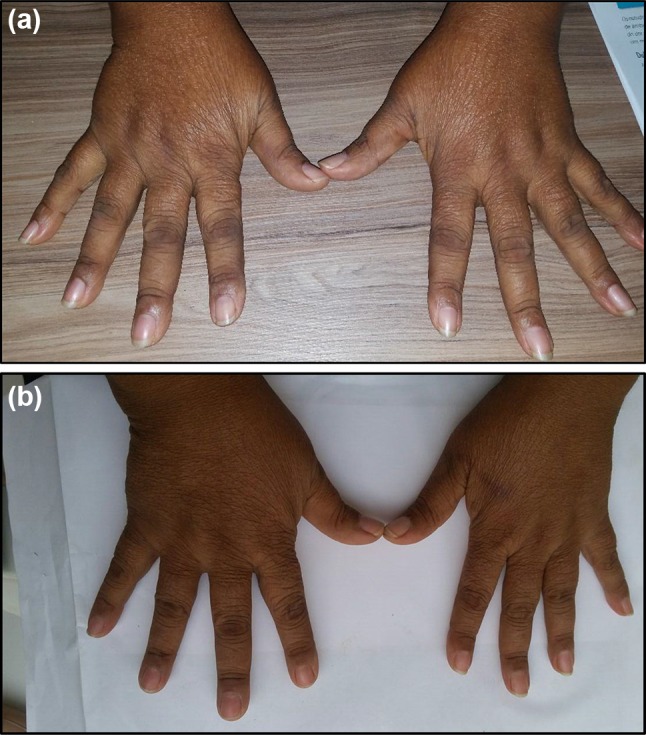
a Arthritis of wrists and metacarpophalangeal joints (pre-treatment with methotrexate. b Decreased arthritis after 5 weeks of treatment with methotrexate 15 mg/week
The rheumatologist obtained a CHIK IgG antibody test by enzyme-linked immunosorbent assay (ELISA) that was positive (IgG 3.15, normal < 0.80) and an anti-CCP antibody test that was elevated (274 U/ml, normal < 17).
The severity of arthritis was quantified. Both disease activity, assessed by Disease Activity Score (DAS28 ESR) (patient 7.39, high disease activity > 5.1) and disease severity, as measured by the Clinical Disease Activity Index (CDAI) (patient 72, high disease activity 22.1–76.0), were markedly elevated at the initial visit. Pain measured by the Visual Analogue Scale (VAS) was 10/10. The patient reported that her quality of life was greatly impaired.
In summary, the patient presented to the rheumatologist with clinical and epidemiologic evidence of recent acute CHIK infection that was confirmed serologically. This illness was followed by a symmetrical polyarthritis, affecting more than ten joints, including the hands, of more than 6 weeks of duration. She had an elevated ESR and positive RF and anti-CCP antibody test. The illness was consistent with American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) 2010 classification criteria for rheumatoid arthritis [5]. The diagnosis of Felty’s syndrome was also established, based on the triad of rheumatoid arthritis, neutropenia, and splenomegaly [6].
To treat her arthritis, our patient was given MTX 15 mg orally every week with folic acid and responded dramatically (Table 1). She was evaluated weekly. After 5 weeks, she reported much decreased fatigue. All RA outcome measures improved (Fig. 1b). DAS28ESR and CDAI decreased to from 7.39 (high disease activity > 5.1) to 2.54, (remission < 2.6) and 72 (high activity 22.1–76.0) to 2.0 (remission 0.0–2.8), respectively. Her VAS pain score decreased from 10/10 to 2/10. In addition, her WBC count returned to normal (increase from 2100 cells/mm3 to 6800 cells/mm3). As of October 2017, she was continuing MTX therapy. She reported resolution of her pain and her WBC count was 8000 cells/mm3. Her DAS28 ESR had fallen further to 2.2 and her CDAI to 2.0.
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Discussion
CHIKF is an emerging viral infection that has spread widely, along with its Aedes mosquito vectors, through tropical and sub-tropical Africa, Asia, and the Americas, causing explosive epidemics of both acute illness and persistent, disabling arthritis [1]. Following acute infection, typically lasting for 1–2 weeks, approximately 35% of patients develop a second phase of illness; disabling, potentially chronic arthritis [7].
Clinically, these patients often resemble RA [8]. Miner and colleagues evaluated a cohort of ten American relief workers who developed CHIK infection in Haiti in 2013. Eight of these individuals developed persistent symmetrical polyarthritis that, like our patient, met ACR/EULAR 2010 criteria for RA. Unlike our patient, however, none of these patients were RF or anti-CCP antibody positive and none had extra-articular disease manifestations [4]. A report from India similarly concluded “what really intrigued us was the propensity of the CHIK alphavirus to cause an RA-like illness”. In this study, 13 of 95 patients and four of 67 patients were RF and anti-CCP antibody positive, respectively [9]. On the other hand, in a Colombian cohort of 109 chronic CHIK arthritis patients, 98.5% had no detectable RF or anti-CCP antibodies [10], and none of the 22 prospectively evaluated La Reunion patients with long-term arthralgia were anti-CCP antibody positive [11].
As in our patient, acute CHIK infection is treated symptomatically as a viral infection. The pathogenesis and the treatment of chronic CHIK arthritis are less clear. There are high levels of viremia in acute CHIK fever [12], but persistent viral infection has not been demonstrated in synovial fluid in chronic CHIK arthritis patients [13]. This finding suggests that the mechanism of chronic CHIK arthritis may be a post-infectious inflammatory process. In addition, although RA autoantibodies are usually not present in chronic CHIK arthritis, the cytokine profile in CHIK chronic infection, including IFN-α, IL-5, IL-6, IL-10, and particularly IL-7 and IL-15, is similar to the cytokine signature seen in RA [2].
CHIK patients such as the individual presented here have painful and persistent arthritis. Their clinical illness often mimics RA, if not to the striking degree seen in our case. In our initial evaluation of this patient, we found many clinical similarities to other chronic CHIK arthritis patients that we have treated during a widespread Brazilian CHIK epidemic. We also considered other diseases that can mimic RA [14] (Table 2), but these conditions do not demonstrate the classical symmetrical polyarthritis with synovitis of the MCP and PIP joints seen in this patient. In addition, both RF and anti-CCP antibodies were present in this case. These autoantibodies are detected in some diseases other than RA [15] (Table 3), but this patient did not have clinical features of these disorders. We diagnosed chronic CHIK arthritis mimicking RA.
Table 2.
Diseases that can mimic rheumatoid arthritis
| Viral polyarthritis |
| Rubella |
| Parvovirus |
| Hepatitis C |
| Hepatitis B |
| Alphaviruses |
| Human T lymphotrophic virus type 1) |
| Systemic rheumatic diseases |
| Systemic lupus erythematosus |
| Sjögren’s syndrome, dermatomyositis) |
| Palindromic rheumatism |
| Hypermobility syndrome |
| Fibromyalgia |
| Spondyloarthropathy |
| Reactive arthritis |
| Arthritis of inflammatory bowel disease |
| Psoriatic arthritis |
| Polymyalgia rheumatic |
| Infectious arthritis |
| Lyme arthritis |
| Crystalline arthritis |
| Osteoarthritis |
| Paraneoplastic disease |
| Sarcoid arthritis |
Table 3.
Diseases other than rheumatoid arthritis associated with positive rheumatoid factor or anti-CCP antibody
| Positive rheumatoid factor |
| Autoimmune disease |
| Rheumatoid arthritis |
| Primary Sjögren’s syndrome |
| Mixed cryoglobulinemia (hepatitis C) |
| Systemic lupus erythematosus |
| Mixed connective tissue disease |
| Polymyositis/dermatomyositis |
| Systemic sclerosis |
| Infection |
| Subacute bacterial endocarditis |
| Hepatitis (A, B, and C) |
| Epstein–Barr virus and cytomegalovirus infections |
| Tuberculosis |
| Syphilis |
| Miscellaneous |
| Sarcoidosis 28 |
| Waldenström’s macroglobulinemia 25 |
| Liver cirrhosis 25 |
| Interstitial lung diseases |
| Positive anti-CCP antibody |
| Systemic lupus erythematosusa |
| Sjögren’s syndromea |
| Psoriatic arthritis |
| Tuberculosis |
| Hepatitis C |
| Chronic obstructive pulmonary disease |
| Alpha 1 anti-trypsin deficiency |
aMore likely in erosive disease (possible RA overlap syndrome)
This patient also had Felty’s syndrome (FS). As described by Felty in 1924, this syndrome consists of a triad of RA, neutropenia, and splenomegaly [16]. In addition to the presence of these features, the diagnosis of FS requires exclusion of other disorders that might cause similar symptoms [17] (Table 4). None of these conditions were present in this patient. Thus, the finding of FS in this patient, a rare but classical extra-articular manifestation, strengthens the diagnosis of RA.
Table 4.
Conditions to be excluded in the diagnosis of Felty’s syndrome
| Systemic lupus erythematosusa |
| Large granular lymphocyte syndromea,b |
| Drug-induced neutropeniac |
| Amyloidosisb,c |
| Hematologic malignancyc |
| Sarcoidosisb |
| Tuberculosisb |
| HIV infectionb |
| Epstein–Barr virus infectionb |
| Malariab |
| Cirrhosis |
aNeutropenia
bSplenomegaly
cRheumatoid arthritis patients
There are, however, aspects of this patient’s inflammatory arthritis that are not typical of classical rheumatoid arthritis. First, she lived in Pernambuco, Brazil, and her arthritis began 2 months after an acute illness with fever, maculopapular rash, and arthralgia. The clinical features of her acute illness and the epidemiology of a widespread CHIK epidemic in northeastern Brazil suggested CHIKF that was confirmed serologically [18]. Secondly, the intensity of her arthritic pain was severe, intractable, and disabling, even for a patient with RA and more typical of pain that we have observed with chronic CHIK arthritis. Finally, the development of FS was surprising for a patient with RA of only 1 year’s duration. Most reported Felty’s RA patients have disease duration of greater than 10 years [19].
We next decided on the treatment of her arthritis. Considering the safety and effectiveness of MTX in the treatment of RA and related disorders and its widespread availability and relative cost-effectiveness, it is not surprising that MTX has been used in the treatment of persistent CHIK arthritis. However, available studies evaluating MTX treatment of chronic CHIK arthritis are limited [20, 21]. Ravindran and Alias evaluated two regimens in 62 patients, triple therapy of MTX (15 mg/week), HCQ (400 mg/day), and SSZ (1 g/day) compared to HCQ (400 mg/day) monotherapy. At 24 weeks, using DAS28-ESR good clinical response as the primary outcome measure, MTX triple therapy was markedly superior to HCQ (DAS28-ESR < 3.2, 84 vs. 14%, respectively) [22]. Treatment was well tolerated. There are no well-controlled studies of MTX monotherapy in CHIK arthritis, but we chose this agent as a promising option for our patient’s arthritis, covering both chronic CHIK arthritis and RA. We also relied on data supporting the use of MTX in the treatment of FS [23]. We were gratified by the patient’s excellent response.
With all the available data, how should we characterize our patient’s arthritis? Did the patient have two illnesses, first acute CHIKF and then separately RA? This possibility cannot be excluded, but the timing of her illness, her severe pain, and the rapid development of FS suggest that CHIK infection contributed to her arthritis. Given the current limitations in the understanding of the pathogenesis of CHIK arthritis and for that matter, the pathogenesis of RA, it is impossible to determine whether our patient had CHIK-induced RA or CHIK arthritis that strikingly resembled RA. We will let the reader decide.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
José Kennedy Amaral and Robert T. Schoen have nothing to disclose.
Compliance with Ethics Guidelines
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to 10.6084/m9.figshare.5909191.
References
- 1.Yactoyo S, Staples JE, Millot V, Cibrelus L, Pardo PR. Epidemiology of chikungunya in the Americas. J Infect Dis. 2016;214(5):441–445. doi: 10.1093/infdis/jiw390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng LF, Chow A, Sun Y, Kwek DJ, Lim P, Dimatatac F, et al. IL-1β, IL-6, and RANTES as biomarkers of chikungunya severity. PLoS One. 2009;4(1):e4261. doi: 10.1371/journal.pone.0004261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amaral Pereira J, Kennedy Schoen RT. Management of chikungunya arthritis. Clin Rheumatol. 2017;36:2179–2186. doi: 10.1007/s10067-017-3766-7. [DOI] [PubMed] [Google Scholar]
- 4.Miner JJ, Aw Yeang HX, Fox JM, Taffner S, Malkova ON, Oh ST, et al. Brief report: chikungunya viral arthritis in the united states: a mimic of seronegative rheumatoid arthritis. Arthritis Rheumatol. 2015;67:1214–1220. doi: 10.1002/art.39027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kay J, Upchurch KS. ACR/EULAR 2010 rheumatoid arthritis classification criteria. Rheumatology. 2010;51(suppl 6):vi5–vi9. doi: 10.1093/rheumatology/kes279. [DOI] [PubMed] [Google Scholar]
- 6.Bagher Owlia M, Newman K, Akhtari M. Felty’s syndrome, insights and updates. Open Rheumatol J. 2014;8:129–136. doi: 10.2174/1874312901408010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodríguez-Morales AJ, Cardona-Ospina JA, Fernanda Urbano-Garzón S, Sebastian Hurtado-Zapata J. Prevalence of post-chikungunya infection chronic inflammatory arthritis: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2016;68:1849–1858. doi: 10.1002/acr.22900. [DOI] [PubMed] [Google Scholar]
- 8.Tanay A. Chikungunya virus and autoimmunity. Curr Opin Rheumatol. 2017;29:389–393. doi: 10.1097/BOR.0000000000000396. [DOI] [PubMed] [Google Scholar]
- 9.Chopra A, Anuradha V, Lagoo-Joshi V, Kunjir V, Salvi S, Saluja M. Chikungunya virus aches and pains: an emerging challenge. Arthritis Rheumatol. 2008;58:2921–2922. doi: 10.1002/art.23753. [DOI] [PubMed] [Google Scholar]
- 10.Jaller Raad J, Segura Rosero A, Vidal Martínez J, Parody A, Jaller Raad R, Caballero Tovar D, et al. Immunological response of a population from the Caribbean region of Colombia infected with the chikungunya virus. Revista Colombiana de Reumatología (English Edition) 2016;23:85–91. [Google Scholar]
- 11.Schilte C, Staikowsky Couderc T, Madec Y, Carpentier F, Kassab S, et al. Chikungunya virus-associated long-term arthralgia: a 36-month prospective longitudinal study. PLoS Negl Trop Dis. 2013;7:e2137. doi: 10.1371/journal.pntd.0002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaffar-Bandjee MC, Das T, Hoarau JJ, Krejbich Trotot P, Denizot M, Ribera A, et al. Chikungunya virus takes centre stage in virally induced arthritis: possible cellular and molecular mechanisms to pathogenesis. Microbes Infect. 2009;11:1206–1218. doi: 10.1016/j.micinf.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Chang A, Martins K, Encinales L, Reid SP, Acuña M, Encinales C, et al. (2017) Cross-sectional analysis of chikungunya arthritis patients 22-month post-infection demonstrates a lack of viral persistence in synovial fluid [abstract]. Arthritis Rheumatol 69(suppl 10) (Chang A, Martins K. Contributed equally). [DOI] [PMC free article] [PubMed]
- 14.Venebles PJW, Maini RN. Diagnosis and differential diagnosis of rheumatoid arthritis. In: Romain PL editor. UptoDate. 2017.
- 15.Taylor PC, Maini RN. Biomarkers in the diagnosis and assessment of rheumatoid arthritis. In: Romain PL editor. UptoDate. 2018.
- 16.Felty AR. Chronic arthritis in the adult associated with splenomegaly and leukopenia. Bull Johns Hopkins Hosp. 1924;35:16. [Google Scholar]
- 17.Kay J. Clinical manifestations and diagnosis of Felty’s syndrome. In: Romain PL editors. UptoDate. 2018.
- 18.Cavalcanti LP, Freitas AR, Brasil P, Cunha RV. Surveillance of deaths caused by arboviruses in Brazil: from dengue to chikungunya. Memórias do Instituto Oswaldo Cruz. 2017;112:583–585. doi: 10.1590/0074-02760160537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldberg J, Pinals RS. Felty’s syndrome. Semin Arthritis Rheumatol. 1980;10:52–65. doi: 10.1016/0049-0172(80)90014-1. [DOI] [PubMed] [Google Scholar]
- 20.Martí-Carvajal A, Ramon-Pardo P, Javelle E, Simon F, Aldighieri S, Horvath H, et al. Interventions for treating patients with chikungunya virus infection-related rheumatic and musculoskeletal disorders: a systematic review. PLoS One. 2017;12:e0179028. doi: 10.1371/journal.pone.0179028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amaral JK, Sutaria R, Schoen RT. Treatment of chronic chikungunya arthritis with methotrexate: a systematic review. Arthritis Care Res. 2018 doi: 10.1002/acr.23519. [DOI] [PubMed] [Google Scholar]
- 22.Ravindran V, Alias G. Efficacy of combination DMARD therapy vs. hydroxychloroquine monotherapy in chronic persistent chikungunya arthritis: a 24 week randomized controlled open label study. Clin Rheumatol. 2017;36:1335–1340. doi: 10.1007/s10067-016-3429-0. [DOI] [PubMed] [Google Scholar]
- 23.Wassenberg S, Herborn G, Rau R. Methotrexate treatment in Felty’s syndrome. Br J Rheumatol. 1998;37:908–911. doi: 10.1093/rheumatology/37.8.908. [DOI] [PubMed] [Google Scholar]


