Abstract
Introduction
Adalimumab (ADA) is a tumor necrosis factor (TNF)-alpha inhibitor indicated for the treatment of inflammatory autoimmune diseases, including ankylosing spondylitis (AS). Patients receiving ADA in Canada are eligible to enroll in the AbbVie Care™ patient support program (AC-PSP), which provides personalized services, including care coach calls (CCCs). We estimated the likelihood of controlled disease in a cohort of AS patients treated with ADA enrolled in the AC-PSP and who received CCCs versus those who did not.
Methods
A longitudinal analysis using de-identified aggregate-level data collected through the AC-PSP was performed. A probabilistic matching algorithm was used to link patient-level records from the AC-PSP database to records from the QuintilesIMS longitudinal prescription transactions database. Patients were indexed on the date of their first prescription of ADA between January 2010 and October 2015. The AC-PSP database included patient assessments of the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), a measure of disease activity. Eligible patients had a baseline BASDAI assessment performed between 90 days before and 30 days after the index date, and a follow-up BASDAI assessment 6–18 months later. Poisson regression was used to estimate the adjusted relative risk (RR) of controlled disease (BASDAI < 4) at the time of follow-up, comparing patients who received CCCs with those who did not.
Results
In total 249 AS patients met eligibility criteria, and 123 (49%) received CCCs. Of the 249 patients, 184 (74%) had controlled disease (BASDAI < 4) at follow-up assessment, 98 (80%) in the CCC group and 86 (68%) in the no CCC group. Multivariable regression analysis demonstrated a 23% increased likelihood of controlled disease in patients who received CCCs relative to those who did not (RR = 1.23; 95% confidence interval, 1.06–1.42; p = 0.0055).
Conclusion
AS patients receiving tailored services through the AC-PSP in the form of CCCs have an increased likelihood of controlled disease within 6–18 months.
Funding
AbbVie
Keywords: Adalimumab, Ankylosing spondylitis, Biologics, Care coach calls, Inflammatory autoimmune diseases, Patient support program
Introduction
Ankylosing spondylitis (AS) is a chronic, progressive inflammatory disease that primarily affects the axial skeleton, leading to inflammation of the sacroiliac joint, spine, and entheses [1]. Long-term functional impairment and disability in patients with AS have been shown to reduce quality of life and increase the risk of comorbid conditions [2]. The onset of AS is typically in the second or third decade of life, imposing an impact on the most productive years of afflicted individuals [3]. In support of this, recent systematic reviews on the economic impact of AS have shown that the indirect costs are much higher than direct healthcare resource use in patients with AS, and the main cost driver in AS is represented by decreased physical function [4, 5]. The physical function impairment in AS is independently determined by patient-reported disease activity and the level of structural damage of the lumbar and cervical spine [6]. The recent AS management guidelines recommend disease monitoring with a broad variety of assessments, including patient-reported outcomes, such as the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) [7]. The BASDAI is used to assess disease activity of patients with AS, which allows a healthcare practitioner to determine the effectiveness of a current drug therapy or the need to institute a new drug therapy for the treatment of AS [8, 9].
Although non-steroidal anti-inflammatory drugs (NSAIDs) remain first-line agents for the treatment of AS, tumor necrosis factor (TNF) antagonists, including adalimumab (ADA), have been highly effective agents for the treatment of patients who have active AS, despite NSAID treatment [10]. Both short-term and long-term clinical studies have demonstrated the efficacy and safety of TNF antagonists in patients with AS [10]. However, poor persistence with and non-adherence to treatment with biologics can undermine the effectiveness of these medications [11, 12]. Considering the high rates (> 40%) of non-adherence observed with chronic TNF antagonist treatment [12], it is necessary to explore approaches to improve patient adherence.
Patient support program (PSP) services, such as nursing services, medication management and counseling, and co-pay assistance, have been shown to have a positive impact on medication adherence, and clinical and humanistic outcomes, and are also instrumental in reducing the healthcare utilization and costs associated with the management of AS [13]. Hence, PSPs have been proposed to educate and empower patients in order to improve adherence, reduce cost, and improve both treatment satisfaction and outcomes by encouraging effective interaction between caregivers and patients [13].
AbbVie Inc. (St. Laurent, QC, Canada) offers a unique AbbVie Care™ PSP (AC-PSP) (https://www.abbviecare.ca/) for patients across all approved indications for ADA, and was also referred to as PROGRESS when it was initially implemented. Components of AC-PSP include patient education, injection training, delivery and disposal of supplies, financial assistance, patient reminders, and direct contact with trained registered nurses known as wellness case managers (WCMs) [14]. The AC-PSP also records clinical outcome measures, such as BASDAI scores, for reimbursement support, which is provided to insurers if required for initial and/or ongoing approval for ADA. In addition, the AC-PSP services include care coach calls (CCCs) before the start of ADA therapy (initial CCC), as well as during therapy (ongoing CCCs). The AC-PSP services are intended to improve the overall patient experience with ADA treatment, to improve persistence and adherence to therapy, and to ultimately improve treatment outcomes.
A previous study by our group demonstrated increased persistence and adherence to ADA over a 12-month period in patients enrolled in the AC-PSP and receiving CCCs in comparison to patients who did not receive CCCs. The objective of this study was to compare the likelihood of achieving controlled disease state, as defined by the BASDAI score, in a cohort of AS patients enrolled in the AC-PSP who received CCCs versus those who did not receive CCCs.
Methods
Study Design and Study Population
A longitudinal retrospective analysis of patients receiving ADA in Canada and enrolled in the AC-PSP was conducted. Patients were included if they were enrolled in the AC-PSP and (i) were 18 years of age or older; (ii) had a diagnosis of AS, as documented in the AC-PSP; (iii) had their first prescription of ADA filled between January 2010 and October 2015 (index period); (iv) had their initial BASDAI assessment performed between 90 days before and 30 days after the index date; and (v) had a follow-up BASDAI assessment 6–18 months after the initial assessment (Fig. 1).
Fig. 1.
Schematic representation of study design. Patients were selected if they had their first BASDAI assessment made during a period starting 90 days before and ending 30 days after the date of index, and had a subsequent BASDAI assessment made in a period beginning 180 days after and ending 545 days (6–18 months) after the baseline assessment
Data Sources and Data Linkage
The retrospective data collected in the Canadian AC-PSP database as part of regular operations were used for the analysis. The database contains information on patients treated with ADA who were enrolled in the AC-PSP, including patient-level details, such as patient demographics, services rendered, patient diagnoses, and treating physician information. In addition, clinical assessments, including BASDAI scores, were collected, as they are often required by insurers for initial reimbursement approval and ongoing reimbursement.
The AC-PSP database does not capture prescription fill patterns. Therefore, a subcohort of patients with confirmed persistence on ADA was created using the QuintilesIMS Canadian prescription data (LRx). Persistence was defined as not exceeding a gap in days’ supply of ADA > 90 days. Days’ supply refers to the number of days the supply of dispensed medication will last. The LRx database captures de-identified patient-level prescription data collected from retail pharmacies across Canada (with the exception of British Columbia, due to privacy laws), and contains approximately 200 million prescriptions for more than 20 million patients, representing a capture of 75% of prescriptions nationally [15, 16].
A probabilistic matching algorithm was used to link patient-level records from the AC-PSP database to records from the LRx database [17]. This rule-based record linkage retrieves unique patients across the two datasets by finding matches using a combination of multiple common data variables. The data linkage allowed for the study of the association between services and interactions provided through AC-PSP and real-world patient utilization. This methodology was validated externally; the positive predictive value of the algorithms ranged from 95.84% to 99.77%, indicating a low rate of false positives [14].
None of the patients analyzed had their identity or medical records disclosed for the purposes of this study, and only anonymized patient-level data were accessed. Because no identifiable protected health information was extracted or accessed during the course of this study, no institutional review board review or approval was required.
AbbVie Care™ Patient Support Program Description and Implementation
AbbVie implemented the AC-PSP to provide comprehensive reimbursement assistance, injection services, and patient educational and adherence support. One of the features of the program is the CCC made to patients before they initiate treatment with ADA, and periodically over the course of therapy. Specifically, some patients received CCCs with a registered and specialized nurse (a WCM). The CCCs were first implemented in 2013. When the CCC service was launched, it was provided to all existing and new patients [14].
Study Outcomes
The primary outcome of interest was the likelihood of controlled disease as defined by the BASDAI score [8], which was categorized as controlled (< 4) and uncontrolled disease (≥ 4) at the time of the follow-up BASDAI assessment (i.e., after 6–18 months). BASDAI assessments were administered independently of the CCC service described above, and did not impact the services provided to the patient. For each patient, the initial BASDAI assessment was made in the period between 90 days before and at most 30 days after the date of index. This time period allowed capture of the initial BASDAI assessment before the full therapeutic effect of ADA was noted while allowing for flexibility in the first BASDAI date. A follow-up BASDAI assessment was made between 6 and 18 months after the baseline assessment for each patient. If multiple BASDAI assessments were collected during this period, the BASDAI assessment closest to the 12-month mark was utilized. The follow-up BASDAI assessment was used as the primary endpoint, and served as both an appropriate time frame for ADA efficacy response in patients with AS, and is commonly used by Canadian insurers to assess continuous biologic therapy efficacy [2, 18]. BASDAI assessments were performed by a healthcare practitioner, usually a nurse, rheumatologist, or a WCM, to determine the effectiveness of ADA therapy for the treatment of AS, as these assessments are often required by insurers for reimbursement purposes.
Statistical Analysis
Poisson regression with robust error variance was used to determine the adjusted relative risk (RR) of controlled disease state (BASDAI score < 4) at the follow-up BASDAI assessment for patients who received CCCs in comparison to patients who did not receive CCCs. All regression analyses were adjusted for confounders, including patient age group, sex, region, prior biologic use, baseline disease severity category (initial BASDAI assessment), and days lapsed between BASDAI assessments. Covariates were selected on the basis of the theoretical plausibility of the variable as a confounder of the association between receiving CCCs/not receiving CCCs and the likelihood of controlled disease [19], and from previous analyses determining confounders [14]. p < 0.05 was considered statistically significant. Data extraction and statistical analyses were conducted using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
Patient Population Characteristics
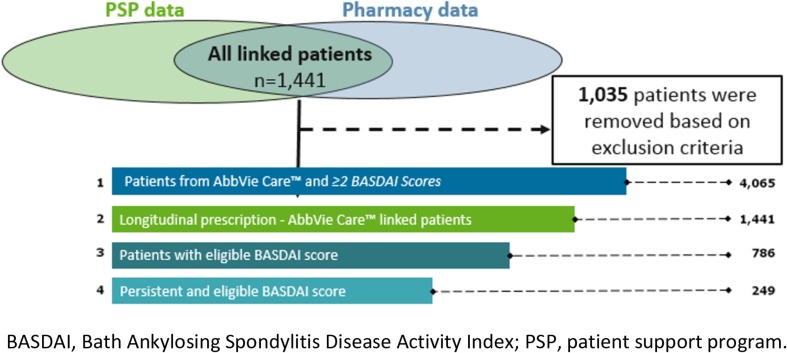
A total of 4065 patients were identified from the AC-PSP database with an AS diagnosis and BASDAI scores on file. Of these patients, 1441 patients were linked with the LRx database. Of the linked patients, 249 were persistent on ADA and had BASDAI scores in the required time frames (Fig. 2). Demographic and clinical characteristics of the linked and persistent cohort are shown in Table 1. Of the 249 patients, 123 (49%) received CCCs and 126 (51%) did not receive CCCs. No significant differences were observed between the groups with respect to sex, age, region, and days lapsed between BASDAI assessments. Most patients were men (59%), were 30 to 39 years of age (26%), and lived in Quebec (57%). Comparison of clinical factors between patients who received CCCs and patients who did not receive CCCs revealed statistically significant differences in biologic-naïve status (p = 0.0421), initial BASDAI assessment category (p = 0.0280), and disease control state at follow-up BASDAI assessment (p = 0.0050). At follow-up BASDAI assessment, 80% of patients (n = 98) who received CCCs experienced disease control state in comparison to 68% of patients (n = 86) who did not receive CCCs (p = 0.0050).
Fig. 2.
Patient selection criteria for linked cohort
Table 1.
Patients’ demographics and clinical characteristics
| Patient characteristics | All patients | CCCs | No CCCs | p value |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Total patients | ||||
| N (%) | 249 (100) | 123 (49) | 126 (51) | |
| Sex | 0.32 | |||
| Male | 146 (59) | 71 (58) | 75 (60) | |
| Female | 103 (41) | 52 (42) | 51 (40) | |
| Age group (years) | 0.29 | |||
| 18–29 | 52 (21) | 26 (21) | 26 (21) | |
| 30–39 | 65 (26) | 30 (24) | 35 (28) | |
| 40–49 | 58 (23) | 29 (24) | 29 (23) | |
| 50–59 | 45 (18) | 25 (20) | 20 (16) | |
| 60–69 | 22 (9) | 10 (8) | 12 (10) | |
| ≥ 70 | 7 (3) | 3 (2) | 4 (3) | |
| Biologic history | 0.04 | |||
| Yes | 49 (20) | 22 (18) | 27 (21) | |
| No | 200 (80) | 101 (82) | 99 (79) | |
| Region | 0.23 | |||
| Ontario | 84 (34) | 54 (44) | 30 (24) | |
| Québec | 142 (57) | 62 (50) | 80 (63) | |
| East | 15 (6) | 7 (6) | 8 (6) | |
| Unknown | 8 (3) | 0 (0) | 8 (6) | |
| Disease control at baseline (initial BASDAI) | 0.02 | |||
| Controlled | 5 (2) | 1 (1) | 4 (3) | |
| Not controlled | 244 (98) | 122 (99) | 122 (97) | |
| Initial BASDAI score | Mean (SD) | |||
| 6.9 (1.6) | 6.9 (1.5) | 6.8 (1.6) | < 0.0001 | |
| Days Lapsed between BASDAI assessments | Median (IQR) | 0.40 | ||
| 345 (138) | 340 (121) | 361 (170) | ||
| Disease control (follow-up BASDAI) | 0.0050 | |||
| Controlled | 184 (74) | 98 (80) | 86 (68) | |
| Not controlled | 65 (26) | 25 (20) | 40 (32) | |
| Follow-up BASDAI score | Mean (SD) | |||
| 2.8 (2.0) | 2.6 (1.9) | 3.0 (2.0) | < 0.0001 | |
Comparison of characteristics between groups was performed using the t test for normally distributed values and the Wilcoxon rank sum test for non-normally distributed data
The χ2 test was used for comparison of categorical data, unless expected cell counts were < 5, in which case the Fisher exact test was used
BASDAI Bath Ankylosing Spondylitis Disease Activity Index, CCC care coach call, IQR interquartile range, PSP patient support program
Multivariable Regression Analysis
After adjustment for age, sex, region, biologics-naïve status, disease control status at baseline, and number of days between assessments, CCCs were associated with an increased probability of controlled disease state (RR, 1.23; 95% CI 1.06–1.42; p = 0.0055). Patients who received CCCs had a 23% increase in the probability of controlled disease state at follow-up BASDAI assessment, between 6 and 18 months after the baseline assessment (Table 2).
Table 2.
Multivariable regression analyses
| Patient characteristics | Relative risk | 95% CI | p value |
|---|---|---|---|
| Sex | |||
| Male | Reference | ||
| Female | 0.33 | 0.93 | 0.32 |
| Age group (years) | |||
| 18–29 | 0.91 | 1.03 | 0.91 |
| 30–39 | 0.76 | 1.07 | 0.75 |
| 40–49 | 0.77 | 0.93 | 0.77 |
| 50–59 | 0.39 | 0.80 | 0.39 |
| 60–69 | 0.80 | 1.06 | 0.80 |
| ≥ 70 | Reference | ||
| Biologic history | |||
| Yes | Reference | ||
| No | 1.26 | 1.00–1.60 | 0.05 |
| Region | |||
| Ontario | 0.05 | 0.83 | 0.04 |
| Québec | Reference | ||
| East | 0.66 | 0.93 | 0.66 |
| Unknown | 0.61 | 0.87 | 0.61 |
| Disease control at baseline (initial BASDAI) | |||
| Controlled | 1.82 | 1.26–2.62 | 0.0013 |
| Not controlled | Reference | ||
| Days lapsed, median days (IQR) | 1.00 | 1.00–1.00 | 0.39 |
| Disease control (follow-up BASDAI) | |||
| Controlled | 1.23 | 1.06–1.42 | 0.0055 |
| Not controlled | Reference | ||
The comparison of cohort characteristics was performed using Poisson regression, with robust error variance adjusting for age, sex, region, biologics-naïve status, disease control status at baseline, and number of days between assessments
BASDAI Bath Ankylosing Spondylitis Disease Activity Index, CCC care coach call, CI confidence interval, IQR interquartile range, PSP patient support program
Discussion
This retrospective real-word analysis demonstrated that patients with AS who were persistent on ADA and received coaching consultations as part of the AC-PSP had increased likelihood of controlled disease as determined by the BASDAI assessment versus those patients who were persistent on ADA, but did not receive this coaching service. Specifically, multivariable regression analysis demonstrated that patients with AS who received coaching had a 23% increased likelihood of controlled disease within 6–18 months in a persistent cohort of patients.
Our results are in line with those of Van den Bosch and colleagues [20] who previously evaluated the effectiveness of ADA in the context of PSP utilization in the PASSION study among patients with rheumatoid arthritis, which showed that patients with rheumatoid arthritis who participated in the PSP achieved an overall significantly greater improvement in clinical, functional, and patient-reported outcomes when compared with patients who did not participate in the PSP. Similarly, Marshall and colleagues assessed a cohort of patients with Crohn’s disease treated with ADA who were enrolled in an ADA PSP and compared the likelihood of achieving a Harvey–Bradshaw index remission between those who received CCCs versus those who did not receive CCCs [21]. This study demonstrated a 17% increased likelihood of achieving remission in a cohort of patients with Crohn’s disease known to be persistent to therapy receiving CCCs relative to those patients who did not (RR, 1.17; 95% CI 1.03–1.34; p = 0.0192).
Our study has several implications. The study results showed significant associations in improvements in patient-reported outcomes in patients with AS treated with ADA who participated in the AC-PSP, and who received tailored services in the form of continuous CCCs within 18 months. Improved disease control suggests that ADA therapy, in addition to AC-PSP participation, may lead to meaningful improvements in longer-term health outcomes of patients with AS through better medication adherence. This is in-line with previous findings from our group which demonstrated a positive association between ongoing CCCs and patient persistence and adherence to ADA over the first 12 months of observation. Specifically, patients receiving CCCs were found to have 72% decreased risk of therapy discontinuation (HR = 0.282, p < 0.0001), and greater odds of being adherent (OR = 1.483, p < 0.0001) when compared to those patients who did not receive CCCs. Treatment initiation abandonment rate was also significantly higher in patients who did not receive initial CCCs (p < 0.0001) [14]. Second, in view of these significant associations in improved clinical and functional outcomes, further refining PSPs can help patients and healthcare professionals better manage disease and optimize treatment regimens. These data should also encourage physicians to enroll patients in PSPs for such inflammatory conditions, and should encourage developers to further invest in and refine PSPs to help patients, beyond providing medications. There are also associated economic implications of improved disease control status, including healthcare utilization and use of healthcare professionals’ time and work productivity [22, 23]. To our knowledge, the CCC is the first example of a patient-centric service that was associated with improved clinical outcomes: patients with AS who received CCCs had a 23% increased likelihood of achieving a controlled disease state. Consequently, more patients with AS with controlled disease may equate to reduced healthcare utilization, thereby reducing costs for providers, insurers, employers, and patients themselves. In addition, ADA treatment and AC-PSP participation may lead to improved productivity and reduce workday loss among employed patients with AS [22, 23]. This study adds to the literature by providing quantitative evidence to inform policy makers and funders that PSPs may be associated with generating meaningful improvements in the health outcomes of patients with AS.
Our study was a retrospective, observational study performed with real-world data and is subject to several limitations. First, there are potential selection biases for subjects who received the CCCs based on the date that they enrolled into the AC-PSP. While the CCC service was introduced in 2013, it was offered to all existing patients enrolled in the program and not only new patients. In addition, patients were not randomized to receive CCCs. Second, although all patients were persistent on ADA for the duration of the study period, the study did not objectively consider adherence to treatment. Third, only a small proportion of patients treated with ADA were eligible for this analysis and, therefore, this study had a relatively small sample size. On a similar note is that several patients had controlled disease at the onset of the observation period and were included in the analysis. This is an artifact of a real-world evidence study that accepts all patients for analysis. In our study, we were limited to the profile of patients in the AC-PSP who may have had controlled disease at the onset of the observation period. However, the number of patients in controlled disease state at baseline was small in both groups: 1% (CCCs cohort) and 3% (no CCCs cohort), as such, the number of patients in controlled disease state at baseline could not have driven the incremental benefit associated with CCCs, and regression analyses were used to address and control baseline imbalances. Fourth, limited data on baseline disease characteristics were available, including inflammatory markers, X-ray data, peripheral involvement (synovitis, enthesitis), and extra-articular manifestations, which could all have potentially influenced treatment response. Similarly, data on factors such as healthier lifestyles in general, including better nutrition, receipt of ancillary services such as physical therapy, engaging in exercise, and so forth, were not available as part of the AC-PSP or the LRx database, which also could have potentially influenced treatment response. Therefore, the results of the study should be interpreted with the caution of unobserved confounding factors.
Future indirect measures of these additional factors associated with medication adherence may be conducted through various forms of self-reporting by the patient, including in-depth patient interviews. Participation in the PSP has been associated with significantly reduced total cost, all-cause medical cost, and disease-related medical cost in patients with Crohn’s disease, ulcerative colitis, rheumatoid arthritis, psoriasis, psoriatic arthritis, and AS treated with ADA [11]. However, a formal economic evaluation was not within the scope of the current study, and real-world studies in the future can focus on examining the economic impact of PSP strategies, specifically in patients with AS treated with ADA.
Conclusions
After adjusting for confounders, this study found that patients with AS receiving tailored services from the Canadian AC-PSP in the form of CCCs were associated with an increased likelihood of controlled disease within 6–18 months from the initial BASDAI assessment compared with those patients who did not receive CCCs. These results may be instrumental in further helping to refine disease management and interventions aimed at improving clinical outcomes in patients with AS.
Acknowledgements
Poster presented at the Annual Congress of European League Against Rheumatism (EULAR), 14–17 June 2017, Madrid, Spain.
Funding
Financial support for the study and article processing charges was provided by AbbVie. AbbVie participated in the design of the study, interpretation of data, review, and approval of this publication. All authors contributed to the development of the publication and maintained control over the final content. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Medical Writing, Editorial, and Other Assistance
Project management support for this study was provided by Dr. Jennifer Glass, PhD, from QuintilesIMS. Statistical support was provided by Marie-Josée Brabant, MSc. Analytical support was provided by Ali Tehrani from QuintilesIMS. Medical writing support was provided by Paranjoy Saharia, MSc, and Dr. Nidhi Chada, PhD, from QuintilesIMS. Publication planning support was provided by Dr. Jelena Ivanovic, PhD, from QuintilesIMS. Project management, statistical and analytical support, medical writing, and publication planning were funded by AbbVie.
Disclosures
Louis Bessette has served as a speaker for AbbVie, Amgen, BMS, Janssen, Roche, UCB, Pfizer, Merck, Celgene, Lilly, and Novartis and as a consultant for AbbVie, Amgen, BMS, Janssen, Roche, UCB, Pfizer, Celgene, Lilly, and Novartis. He also collaborated on research with AbbVie, Amgen, BMS, Janssen, Roche, UCB, Pfizer, Merck, Celgene, Sanofi, Lilly, and Novartis. Carter Thorne has served as a speaker for Medexus/Medac and as a consultant for AbbVie, Amgen, Celgene, Centocor, Genzyme, Hospira, Janssen, Lilly, Medexus/Medac, Merck, Novartis, Pfizer, and Sanofi. He also collaborated on research with AbbVie, Amgen, Celgene, CaREBiodam, Lilly, Novartis, and Pfizer. Brad Millson is an employee of QuintilesIMS and has collaborated on this study as a consultant paid by AbbVie. Katia Charland is an employee of QuintilesIMS and has collaborated on this study as a consultant paid by AbbVie. Krishna Donepudi is an employee of QuintilesIMS and has collaborated on this study as a consultant paid by AbbVie. Tania Gaetano is an employee of AbbVie and owns AbbVie shares. Valencia Remple is an employee of AbbVie and owns AbbVie shares. Martin Latour is an employee of AbbVie and owns AbbVie shares. Sandra Gazel is an employee of AbbVie and owns AbbVie shares. Marie-Claude Laliberté is an employee of AbbVie and owns AbbVie shares. Gerald Lebovic has nothing to disclose.
Compliance with Ethics Guidelines
None of the patients analyzed had their identity or medical records disclosed for the purposes of this study, and only anonymized patient-level data were accessed. Because no identifiable protected health information was extracted or accessed during the course of this study, no institutional review board review or approval was required.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to 10.6084/m9.figshare.6011627.
References
- 1.Lee JW, Kang JH, Yim YR, et al. Predictors of switching anti-tumor necrosis factor therapy in patients with ankylosing spondylitis. PLoS One. 2015;10(7):e0131864. doi: 10.1371/journal.pone.0131864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Heijde D, Schiff MH, Sieper J, et al. Adalimumab effectiveness for the treatment of ankylosing spondylitis is maintained for up to 2 years: long-term results from the ATLAS trial. Ann Rheum Dis. 2009;68(6):922–929. doi: 10.1136/ard.2007.087270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gouveia EB, Elmann D, Morales MS. Ankylosing spondylitis and uveitis: overview. Rev Bras Reumatol. 2012;52(5):742–756. doi: 10.1590/S0482-50042012000500009. [DOI] [PubMed] [Google Scholar]
- 4.Palla I, Trieste L, Tani C, et al. A systematic literature review of the economic impact of ankylosing spondylitis. Clin Exp Rheumatol. 2012;30(4 Suppl 73):S136–S141. [PubMed] [Google Scholar]
- 5.Boonen A, Mau W. The economic burden of disease: comparison between rheumatoid arthritis and ankylosing spondylitis. Clin Exp Rheumatol. 2009;27(4 Suppl 55):S112–S117. [PubMed] [Google Scholar]
- 6.Landewé R, Dougados M, Mielants H, van der Tempel H, van der Heijde D. Physical function in ankylosing spondylitis is independently determined by both disease activity and radiographic damage of the spine. Ann Rheum Dis. 2009;68(6):863–867. doi: 10.1136/ard.2008.091793. [DOI] [PubMed] [Google Scholar]
- 7.van der Heijde D, Ramiro S, Landewé R, et al. 2016 Update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017;76(6):978–991. doi: 10.1136/annrheumdis-2016-210770. [DOI] [PubMed] [Google Scholar]
- 8.Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol. 1994;21(12):2286–2291. [PubMed] [Google Scholar]
- 9.Calin A, Nakache JP, Gueguen A, Zeidler H, Mielants H, Dougados M. Defining disease activity in ankylosing spondylitis: is a combination of variables (Bath Ankylosing Spondylitis Disease Activity Index) an appropriate instrument? Rheumatology (Oxford) 1999;38(9):878–882. doi: 10.1093/rheumatology/38.9.878. [DOI] [PubMed] [Google Scholar]
- 10.Rudwaleit M, Claudepierre P, Kron M, Kary S, Wong R, Kupper H. Effectiveness of adalimumab in treating patients with ankylosing spondylitis associated with enthesitis and peripheral arthritis. Arthritis Res Ther. 2010;12(2):R43. doi: 10.1186/ar2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubin DT, Mittal M, Davis M, Johnson S, Chao J, Skup M. Impact of a patient support program on patient adherence to adalimumab and direct medical costs in Crohn’s disease, ulcerative colitis, rheumatoid arthritis, psoriasis, psoriatic arthritis, and ankylosing spondylitis. J Manag Care Spec Pharm. 2017;23(8):859–867. doi: 10.18553/jmcp.2017.16272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrold LR, Andrade SE. Medication adherence of patients with selected rheumatic conditions: a systematic review of the literature. Semin Arthritis Rheum. 2009;38(5):396–402. doi: 10.1016/j.semarthrit.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganguli A, Clewell J, Shillington AC. The impact of patient support programs on adherence, clinical, humanistic, and economic patient outcomes: a targeted systematic review. Patient Prefer Adherence. 2016;10:711–725. doi: 10.2147/PPA.S101175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marshall JK, Bessette L, Thorne C, et al. Impact of the adalimumab patient support program’s care coach calls on persistence and adherence in Canada: an observational retrospective cohort study. Clin Ther. 2018;40(3):415–429.e6. [DOI] [PubMed]
- 15.Iczkovitz S, Dhalla D, Terres JAR. Rosiglitazone use and associated adverse event rates in Canada: an updated analysis. BMC Res Notes. 2015;8(1):505. doi: 10.1186/s13104-015-1448-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weitz JI, Semchuk W, Turpie AG, et al. Trends in prescribing oral anticoagulants in Canada, 2008–2014. Clin Ther. 2015;37(11):2506-14.e4. doi: 10.1016/j.clinthera.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Blakely T, Salmond C. Probabilistic record linkage and a method to calculate the positive predictive value. Int J Epidemiol. 2002;31(6):1246–1252. doi: 10.1093/ije/31.6.1246. [DOI] [PubMed] [Google Scholar]
- 18.Revicki DA, Luo MP, Wordsworth P, Wong RL, Chen N, Davis JC. Adalimumab reduces pain, fatigue, and stiffness in patients with ankylosing spondylitis: results from the adalimumab trial evaluating long-term safety and efficacy for ankylosing spondylitis (ATLAS) J Rheumatol. 2008;35(7):1346–1353. [PubMed] [Google Scholar]
- 19.Lee PH. Should we adjust for a confounder if empirical and theoretical criteria yield contradictory results? A simulation study. Sci Rep. 2014;4:6085. doi: 10.1038/srep06085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van den Bosch F, Ostor AJK, Wassenberg S, et al. Impact of participation in the adalimumab (Humira) patient support program on rheumatoid arthritis treatment course: results from the PASSION study. Rheumatol Ther. 2017;4(1):85–96. doi: 10.1007/s40744-017-0061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marshall JK, Narula N, Lebovic G, et al. P699 Impact of adalimumab’s patient support program on clinical outcomes in inflammatory bowel diseases: results from the COMPANION study. J Crohns Colitis. 2017;11(1):S438–S439. doi: 10.1093/ecco-jcc/jjx002.823. [DOI] [Google Scholar]
- 22.Krueger KP, Berger BA, Felkey B. Medication adherence and persistence: a comprehensive review. Adv Ther. 2005;22(4):313–356. doi: 10.1007/BF02850081. [DOI] [PubMed] [Google Scholar]
- 23.De Vera MA, Mailman J, Galo JS. Economics of non-adherence to biologic therapies in rheumatoid arthritis. Curr Rheumatol Rep. 2014;16(11):460. doi: 10.1007/s11926-014-0460-5. [DOI] [PubMed] [Google Scholar]