Abstract
Introduction
To compare etanercept (ETN) and placebo (PBO) for maintaining low disease activity (LDA) achieved with ETN in patients with rheumatoid arthritis (RA) from Africa and the Middle East.
Methods
In this subset analysis of the Treat-to-Target trial (ClinicalTrials.gov identifier NCT01981473), 53 adult patients with moderate-to-severe RA nonresponsive to methotrexate were treated with 50 mg ETN/week for 24 weeks (Period 1). Patients achieving LDA were randomized to continue ETN treatment or switched to PBO for an additional 28 weeks (Period 2). The proportion of patients maintaining LDA or remission in each arm at the end of Period 2 was determined. Additional efficacy and patient-reported outcomes (PROs) were also evaluated.
Results
During Period 1, 51 patients achieved LDA according to the disease activity score-28 joints–erythrocyte sedimentation rate (DAS28-ESR LDA) and 30 achieved remission. At week 52, nine of 22 and eight of 29 in the ETN and PBO groups, respectively, remained in DAS28-ESR LDA without experiencing a flare. Additionally, six of 14 and five of 16 in the ETN and PBO groups, respectively, remained in remission. Among patients experiencing a flare during Period 2, 13 of 22 and 21 of 29 received ETN or PBO, respectively. The median time to flare was 193 and 87 days in the ETN and PBO groups, respectively. At week 52, consistently more patients in the ETN group than in the PBO group achieved predetermined efficacy and PRO endpoints.
Conclusions
These data suggest continuing ETN maintenance therapy is beneficial to patients after they have achieved their treatment target. However, this subset analysis is limited by the small patient population and must be interpreted with caution.
Funding
Pfizer.
Trial Registration
ClinicalTrials.gov identifier, NCT0198147.
Keywords: Africa, Efficacy, Etanercept, Maintenance therapy, Middle East, Rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA), a chronic, progressive, systemic inflammatory disease of unknown etiology, is characterized by progressive joint destruction from the distal to proximal joints associated with chronic pain [1]. Worldwide, the annual incidence of RA is estimated at 40 per 100,000 general population [1], and prevalence is estimated at 0.3–1.2% [2]. Although limited, the available data indicate that the prevalence of RA is lower in developing countries, including countries in Africa and the Middle East, than in Western Europe or the USA [3].
Treatment with biologic agents such as tumor necrosis factor (TNF) inhibitors has significantly reduced disease activity and improved the quality of life in patients with RA who have not responded to conventional disease-modifying anti-rheumatic drug (DMARD) therapy [4]. New guidelines, based primarily on data collected on patients from Western Europe and the USA, recommend early treatment of patients to achieve low disease activity (LDA) or clinical remission followed by less intensive treatment to maintain this response [5]. To date, there is little, if any, information on the success of these treatment strategies in RA patients from Africa or the Middle East.
The Treat-to-Target trial (T2T; ClinicalTrials.gov identifier NCT01981473) was conducted in 19 countries worldwide to determine the efficacy of etanercept (ETN) to achieve and maintain LDA and/or remission in patients with active RA. In this post hoc subset analysis, we report our data on patients from six countries in Africa and the Middle East.
Methods
Study Design and Patients
The details of the global T2T trial have been reported elsewhere [6]. Briefly, adult patients with ≥ 1-year history of RA with active moderate-to-severe disease activity (defined as: Disease Activity Score-28 joints [DAS28]–erythrocyte sedimentation rate [ESR] ≥ 3.2; tender joint count [TJC] of ≥ 6 and a swollen joint count [SJC] of ≥ 6 or ESR ≥ 28 mm/h; and C-reactive protein [CRP] level ≥ 3.5 mg/L) who were nonresponsive to prior treatment with methotrexate (MTX) at ≥ 10 mg/week for ≥ 12 weeks were included in the study. Patients were treated weekly with 50 mg ETN (ETN50) + MTX ± other non-biologic DMARDs (nbDMARDs) for 24 weeks (open-label, Period 1); other nbDMARDs included sulfasalazine, hydroxychloroquine and leflunomide administered individually or in combination and optimized during Period 1. Patients achieving low disease activity (LDA), defined as a DAS28-ESR score of < 3.2, at week 24 were randomized into two groups and treated with ETN50 + MTX ± other nbDMARDs or with placebo (PBO) + MTX ± nbDMARDs for another 28 weeks (Period 2); treatments established in Period 1 were maintained during Period 2. Patients in the PBO group who experienced a flare (defined as loss of LDA [DAS28-ESR ≥ 3.2] and worsening of the DAS28-ESR by ≥ 0.6 units) were transferred to treatment with ETN50 (rescue) in a blinded fashion. Patients in the ETN50 group who experienced a flare continued on the assigned therapy treatment. For this post hoc, subset evaluation, data for patients from Africa and the Middle East were extracted from the global study database and analyzed.
The study was conducted according to the International Conference on Harmonisation guidelines for good clinical practice, the International Ethical Guidelines for Biomedical Research Involving Human Subjects from the Council for International Organizations of Medical Sciences, and the Declaration of Helsinki. The study protocol and informed consent form were approved by the independent ethics committee or institutional review board at each participating center before patient screening.
Endpoints
The primary endpoint was the proportion of patients who maintained LDA according to the DAS28-ESR (DAS28-ESR LDA) at week 52 without experiencing a flare during Period 2. Secondary endpoints included the proportion of patients (1) maintaining LDA according to the DAS28-CRP (DAS28-CRP LDA) and DAS28-ESR LDA at week 52, (2) maintaining remission based on the DAS28-ESR, (3) achieving 20, 50, 70 and 90% improvement from baseline based on American College of Rheumatology (ACR) criteria (ACR20, ACR50, ACR70 and ACR90, respectively) at week 52 and (4) achieving a Health Activity Questionnaire-Disability Index (HAQ-DI) total score of ≤ 0.5 at week 52. Patient-reported outcomes included change from baseline to week 52 in the TJC based on 28 joints (28TJC) and SJC based on 28 joints (28SJC), moderate or good response based on European League Against Rheumatism (EULAR) and quality of life assessments consisting of physician and patient global assessments (PGA and PtGA, respectively), patient assessment of pain on a visual analog scale (VAS), Euro-Qol 5D (EQ-5D) health state and utility score and work productivity activity impairment (WPAI).
Statistics
Descriptive statistics are presented as the mean and standard deviation for continuous data, and number (N) and percentage for binary data using the last observation carried forward method. For patients who experienced a flare, the last pre-rescue observation was used for all analyses, except where otherwise specified. No comparative statistics were performed because only a subset of the study patient population was included in this analysis and such results could be misinterpreted. The full study had 90% power to detect differences of 17% in the primary endpoint between the ETN and PBO groups with 158 subjects per group. With 22 ETN and 29 PBO subjects in the regions considered in this work, only large differences could be shown to be statistically significant, while smaller, potentially clinically meaningful differences would not reach statistical significance.
Results
Patients
Of the 489 patients enrolled in the global study and treated in Period 1, 53 were from Africa and Middle East regions and had a mean disease duration of 8.7 years and a baseline DAS28-ESR of 5.9 (Table 1). Patients randomized to ETN (N = 22) and PBO (N = 29) had comparable baseline demographic and disease characteristics (Table 1).
Table 1.
Patient characteristics at baseline (Period 1) and randomization (Period 2)
| Parameter | Period 1 | Period 2 | ||
|---|---|---|---|---|
| Baseline (N = 53) | ETN (N = 22) | PBO (N = 29) | Total (N = 51) | |
| Age, mean (SD), years | 40.6 (10.7) | 36.0 (10.6) | 43.6 (9.8) | 40.3 (10.8) |
| Female, n (%) | 41 (77.4) | 18 (81.8) | 22 (75.9) | 40 (78.4) |
| Race, n (%) | ||||
| White | 40 (75.5) | 17 (77.3) | 23 (79.3) | 40 (78.4) |
| Other | 13 (24.5) | 5 (22.7) | 6 (20.7) | 11 (21.6) |
| Symptom duration, mean (SD), years | 8.7 (5.5) | 8.4 (5.9) | 8.9 (5.5) | 8.7 (5.6) |
| Rheumatoid factor positive, n (%) | 42 (79.2) | 19 (86.4) | 22 (75.9) | 41 (80.4) |
| CCP3 antibody positive, n (%) | 51 (96.2) | 14 (63.6) | 22 (75.9) | 36 (70.6) |
| Prior corticosteroids, n (%) | 39 (73.6) | 17 (77.3) | 20 (69.0) | 37 (72.5) |
| Prior DMARDs, n (%) | 39 (73.6) | 19 (86.4) | 20 (69.0) | 39 (76.5) |
| Prior MTX, n (%) | 53 (100) | 22 (100) | 29 (100) | 51 (100) |
| Prior NSAIDs, n (%) | 26 (49.1) | 11 (50.0) | 14 (48.3) | 25 (49.0) |
| ESR, mean (SD), mm/h | 33.8 (21.2) | 14.5 (12.0)a | 15.1 (10.7)a | |
| CRP, mean (SD), mg/mL | 29.3 (45.2) | 12.1 (14.1)a | 9.2 (11.6)a | |
| DAS28-ESR, mean (SD) | 5.9 (1.0) | 2.5 (0.5)a | 2.5 (0.4)a | |
| DAS28-CRP, mean (SD) | 5.5 (1.0) | 2.5 (0.7)a | 2.4 (0.6)a | |
| CDAI, mean (SD) | 35.3 (11.2) | 5.1 (3.7)a | 5.1 (3.2)a | |
| SDAI, mean (SD) | 38.3 (12.3) | 6.3 (4.1)a | 6.0 (3.6)a | |
| 28SJC score, mean (SD) | 9.4 (4.8) | 1.1 (2.3)a | 0.7 (1.4)a | |
| 28TJC score, mean (SD) | 12.6 (6.4) | 1.3 (1.4)a | 1.1 (1.0)a | |
| HAQ-DI, mean (SD) | 1.5 (0.7) | 0.3 (0.3)a | 0.4 (0.5)a | |
| PGA, mean (SD) | 6.7 (1.6) | 1.4 (0.8)a | 1.7 (1.3)a | |
| PtGA, mean (SD) | 6.6 (1.8) | 1.4 (0.9)a | 1.6 (1.2)a | |
| Patient general health VAS, mean (SD) | 58.4 (22.7) | 11.0 (5.6)a | 13.2 (8.8)a | |
| Patient pain assessment VAS, mean (SD) | 64.7 (19.9) | 9.9 (5.6)a | 12.1 (8.7)a | |
CCP3 Cyclic citrullinated peptide, CDAI clinical disease activity index, CRP C-reactive protein, DAS28 Disease Activity Score in 28 joints, DMARD disease-modifying anti-rheumatic drug, ESR erythrocyte sedimentation rate, ETN etanercept, HAQ-DI health assessment questionnaire-disability index, MTX methotrexate, NSAID non-steroidal, anti-inflammatory drug, PBO placebo, PGA physician global assessment, PtGA patient global assessment, SD standard deviation, SDAI Simplified Disease Activity Index, 28SJC swollen joint count based on 28 joints, TJC tender joint count based on 28 joints, VAS visual analogue scale
aData at week 24 prior to entry into Period 2
Efficacy
In Period 1, 51 of 53 (96.2%) patients achieved DAS28-ESR LDA (cutoff of < 3.2) at week 24 and entered Period 2. Of these 51 patients, 30 (58.8%) also achieved remission according to the DAS28-ESR (< 2.6; DAS28-ESR remission).
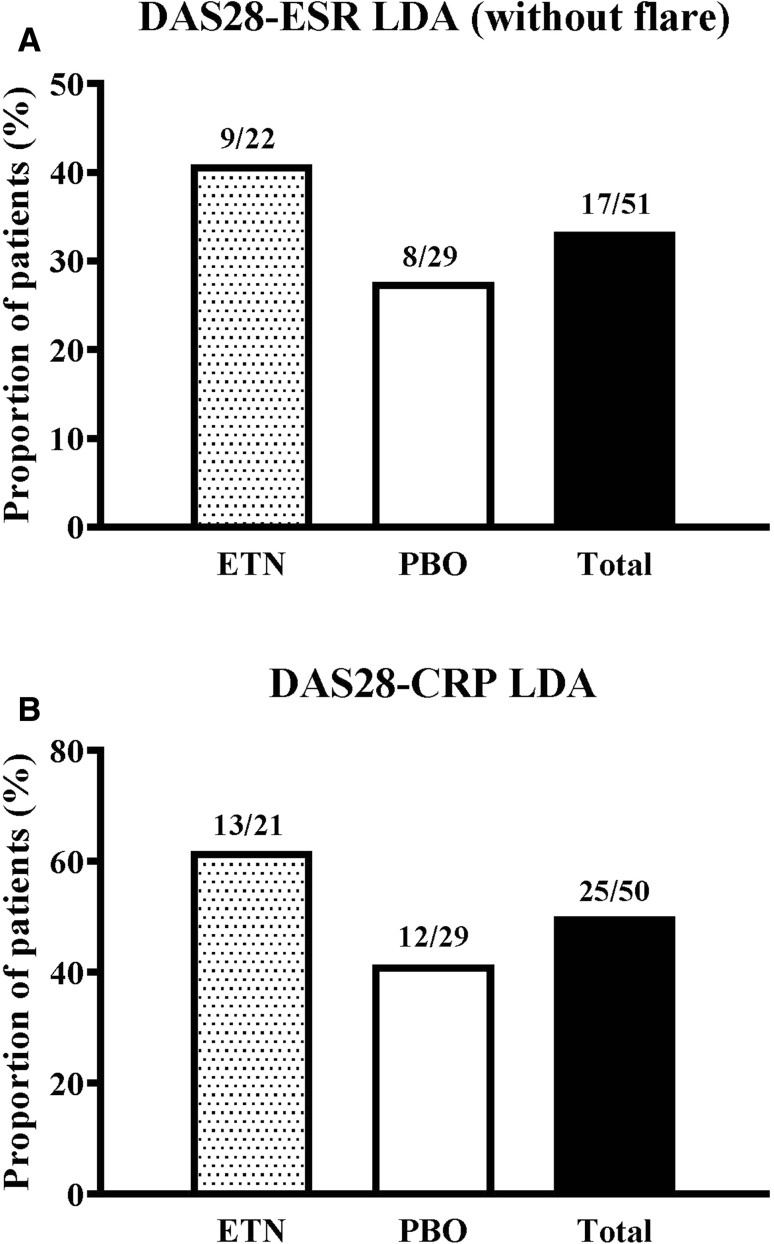
In Period 2, nine of 22 (40.9%) patients in the ETN group and eight of 29 (27.6%) patients in the PBO group remained with DAS28-ESR LDA at week 52 without experiencing a flare (Fig. 1a). At week 52, 39 of 51 (76.4%) patients remained with DAS28-ESR LDA using the last observation carried forward imputation of data, including post-flare data from patients on rescue therapy: 16 of 22 (72.7%) in the ETN group and 23 of 29 (79.3%) in the PBO group, respectively. Also based on the last pre-rescue observation carried forward imputation of data, 25 of 50 patients (50.0%) remained with DAS28-CRP LDA (< 3.2) at week 52; 13 of 21 (61.9%) in the ETN group and 12 of 29 (41.4%) in the PBO group, respectively (Fig. 1b).
Fig. 1.
Proportion of patients at week 52 remaining with (a) Disease Activity Score in 28 joints-erythrocyte sedimentation rate (DAS28-ESR) low disease activity (LDA) without experiencing a flare, and (b) DAS28-C-reactive protein (CRP) LDA. ETN Etanercept, PBO placebo
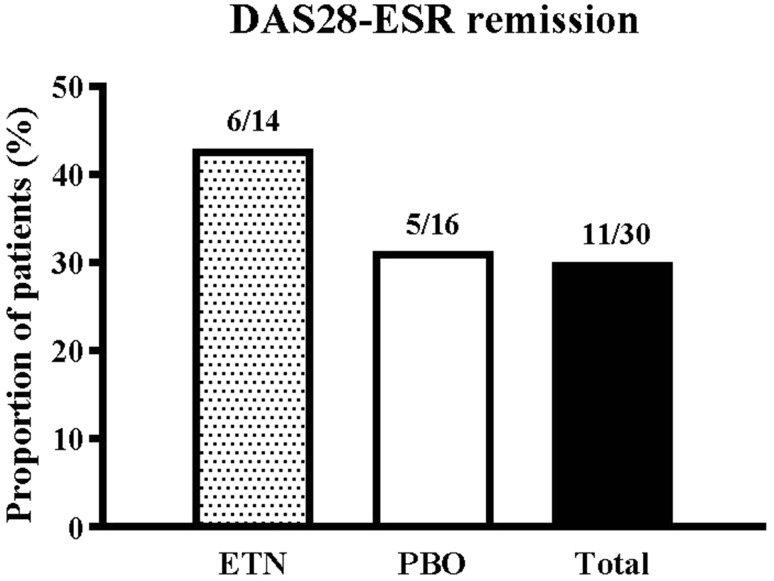
Among patients who had achieved DAS28-ESR remission in Period 1, 11 of 30 (36.7%) patients remained in remission at week 52, the end of Period 2: six of 14 (42.9%) patients in the ETN group and five of 16 (31.3%) patients in the PBO group, respectively (Fig. 2).
Fig. 2.
Proportion of patients at week 52 remaining in DAS28-ESR remission
During Period 2, 34 of 51 (66.7%) patients experienced a flare (Table 2) of whom 13 of 22 (59.1%) were in the ETN group and 21 of 29 (72.4%) were in the PBO group. Of the patients who experienced a flare, seven of 13 (53.8%) patients in the ETN group and 15 of 21 (71.4%) patients in the PBO group achieved DAS28-ESR LDA at week 52 with rescue therapy. The median time to flare was 193 (95% confidence interval [CI] 86.0, not available) days in the ETN group and 87 (95% CI 85.0, 141.0) days in the PBO group.
Table 2.
Proportion of patients with low disease activity according to DAS28-ESR cutoff at week 52 without post-flare censoring
| Patient group | ETN (N = 22) | PBO (N = 29) |
|---|---|---|
| Overall | 16/22 (72.7%) | 23/29 (79.3%) |
| Did not flare | 9/9 (100%) | 8/8 (100%) |
| Did flarea | 7/13 (53.8%) | 15/21 (71.4%) |
LDA Low disease activity
aAll patients who experienced a flare were administered etanercept rescue treatment
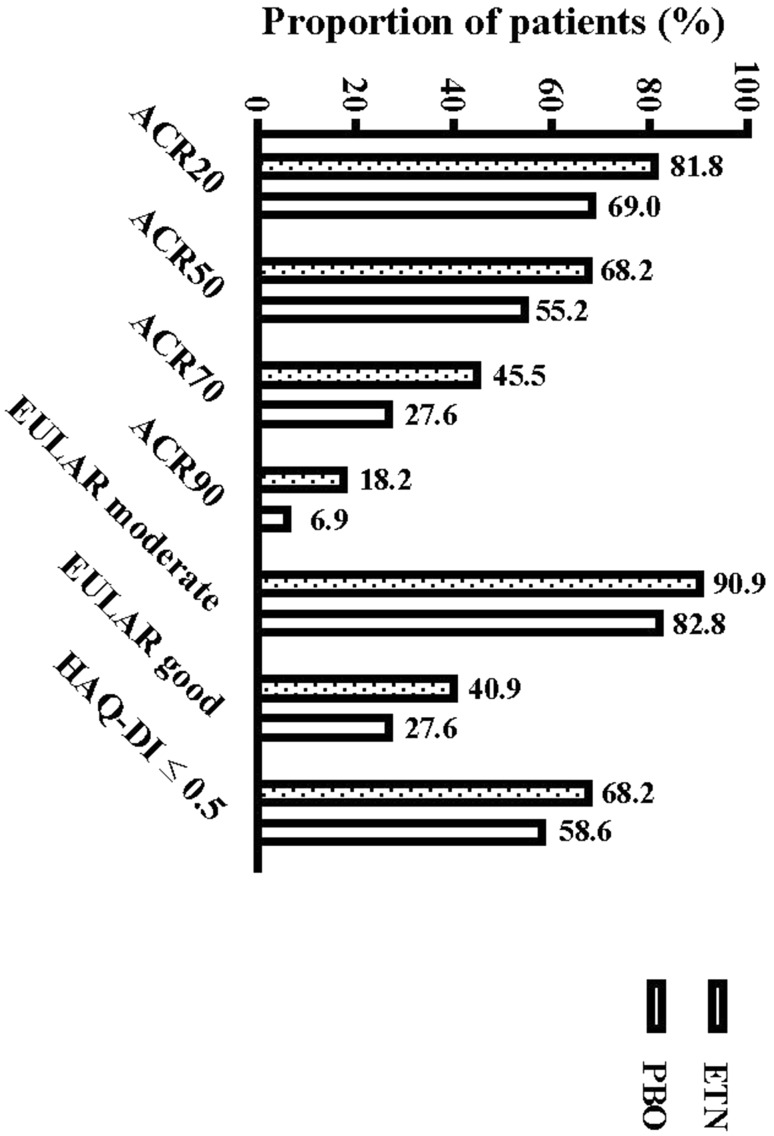
At week 52, consistently greater proportions of patients in the ETN group than in the PBO group achieved ACR20, ACR50, ACR70 and ACR90 (Fig. 3). Greater improvement from baseline to week 52 was observed in patients administered ETN than in those administered PBO for predetermined efficacy endpoints that included DAS28-ESR, DAS28-CRP, ESR, CRP, 28TJC, and 28SJC (Table 3). At week 52, consistently greater proportions of patients in the ETN group than in the PBO group achieved HAQ-DI ≤ 0.5 and EULAR moderate or good response (Fig. 3). Greater improvement from baseline to week 52 was observed in patients administered ETN than in those administered PBO for predetermined quality of life measures that included PGA, PtGA, pain VAS, EQ-5D health state and utility score and WPAI assessments (Table 3).
Fig. 3.
Proportion of patients achieving various endpoints at week 52. ACR20, -50, -70, -90 American College of Rheumatology-based criteria for 20, 50, 70 and 90% improvement from baseline, respectively, EULAR European League Against Rheumatism, HAQ-DI Health Activity Questionnaire-Disability Index,
Table 3.
Mean data for efficacy and health-related quality of life scores
| Parameter | Period 1 | Period 2 | Period 1 | Period 2 | ||
|---|---|---|---|---|---|---|
| ETN50 + MTX ± nbDMARDs (N = 34) | PBO + MTX ± nbDMARDs (N = 27) | |||||
| At baseline (N = 64) | At week 24 | At week 52 | At baseline (N = 64) | At week 24 | At week 52 | |
| DAS28-ESR | 6.0 (0.9) | 2.5 (0.5) | 3.3 (1.3) | 5.8 (1.1) | 2.5 (0.4) | 4.0 (1.3) |
| DAS28-CRP | 5.8 (0.8) | 2.5 (0.7) | 3.0 (1.4) | 5.4 (1.1) | 2.4 (0.6) | 3.5 (1.2) |
| CDAI | 36.0 (9.8) | 5.1 (3.7) | 10.1 (10.9) | 35.0 (12.5) | 5.1 (3.2) | 14.0 (11.5) |
| SDAI | 40.6 (10.8) | 6.3 (4.1) | 11.0 (11.7) | 36.6 (13.7) | 6.0 (3.6) | 15.3 (11.6) |
| ESR | 38.5 (27.1) | 14.5 (12.0) | 25.5 (17.3) | 29.2 (14.1) | 15.1 (10.7) | 27.7 (15.3) |
| CRP | 45.7 (62.1) | 12.1 (14.1) | 10.0 (15.0) | 16.2 (21.8) | 9.2 (11.6) | 12.4 (14.2) |
| 28TJC | 12.6 (5.7) | 1.3 (1.4) | 2.7 (3.8) | 12.7 (6.8) | 1.1 (1.0) | 4.6 (5.7) |
| 28SJC | 9.5 (5.1) | 1.1 (2.3) | 2.8 (4.3) | 9.4 (4.7) | 0.7 (1.4) | 2.4 (3.7) |
| PGA | 6.9 (1.1) | 1.4 (0.8) | 2.1 (2.0) | 6.6 (1.9) | 1.7 (1.3) | 3.5 (2.2) |
| HAQ-DI | 1.6 (0.5) | 0.3 (0.3) | 0.4 (0.5) | 1.3 (0.8) | 0.4 (0.5) | 0.6 (0.7) |
| PtGA | 7.0 (1.4) | 1.4 (0.9) | 2.5 (2.2) | 6.3 (2.1) | 1.6 (1.2) | 3.6 (2.3) |
| Patient general health | 63.6 (20.1) | 11.0 (5.6) | 22.1 (24.8) | 55.0 (24.9) | 13.2 (8.8) | 34.3 (25.4) |
| Pain VAS | 68.4 (15.4) | 9.9 (5.6) | 21.6 (24.5) | 63.1 (22.8) | 12.1 (8.7) | 32.5 (22.2) |
| EQ-5D Health State | 43.7 (17.6) | 87.4 (6.7) | 80.6 (18.7) | 43.6 (25.4) | 86.8 (9.4) | 72.2 (19.8) |
| EQ-5D Utility Score | 0.3 (0.4) | 0.9 (0.1) | 0.8 (0.2) | 0.4 (0.4) | 0.8 (0.2) | 0.7 (0.2) |
| WPAI: % time missed due to health | 10.4 (12.3) | 3.6 (9.1) | 1.0 (2.6) | 7.5 (12.9) | 10.0 (16.5) | 2.4 (4.1) |
| WPAI: % impairment while working due to health | 45.5 (25.1) | 13.0 (19.5) | 6.7 (12.1) | 40.0 (19.5) | 15.0 (21.2) | 16.7 (15.3) |
| WPAI: % overall work impairment due to health | 4.9 (4.9) | 2.2 (4.6) | 1.1 (2.3) | 4.0 (4.2) | 6.5 (10.4) | 2.0 (2.8) |
| WPAI: % activity impairment due to health | 60.5 (17.6) | 13.6 (12.9) | 9.2 (13.8) | 60.0 (22.0) | 16.4 (16.4) | 13.8 (17.7) |
Data are presented as the mean with the SD in parenthesis
EQ-5D Euro-Qol 5D, nbDMARD non-biological DMARD, WPAI work productivity activity impairment
Safety
Since the study was not designed for randomization based on geographical region, the number of patients in this subset analysis was too small for meaningful characterization of safety parameters. In the global population in Period 2, adverse events were reported in 37% of patients in the ETN group and 43% of patients in the PBO group. Serious adverse events were reported in 1 and 4% of patients in the ETN and PBO groups, respectively.
Discussion
The global Phase 4 T2T study was designed to evaluate and demonstrate the effectiveness of an ETN-free regimen on maintenance of LDA or remission in patients with moderate-to-severe RA who had achieved LDA or remission on 24 weeks of ETN treatment [6]. The subset analysis reported here was undertaken to evaluate how patients from Africa and the Middle East responded under these test conditions.
The baseline disease characteristics of this subset were similar to those of the global population, with patients having a long-standing (mean of 8.7 vs. 8.1 years) and high level of disease activity (mean DAS28-ESR 5.9 vs. 6.4) [6]. The efficacy of ETN treatment was demonstrated in Period 1 when 96.2% of the regional patients achieved DAS28-ESR LDA compared with 67.7% of patients achieving it in the global population. The reasons for this difference are unclear. One possible explanation is that the patients in this subpopulation were young and, consequently, may respond better. Also, since these patients had no prior exposure to TNF inhibitors and this region is generally underserved, these patients may have been unusually responsive to treatment. Another possibility is that the result is related to ethnic composition of this subset population. However, this result could also be random as the numbers are too few for meaningful statistical analyses. Additional targeted studies would be needed to investigate this issue further.
A greater proportion of patients in the ETN group than in the PBO group maintained DAS28-ESR LDA, DAS28-ESR remission and DAS28-CRP LDA at week 52. A greater proportion of patients in the ETN group compared with those in the PBO group also maintained the responses they achieved by week 24 through to week 52 for all efficacy endpoints and quality of life measures. These data are consistent with those observed in the global study [6]. Other studies have also reported similar results showing that although decreasing the dose of ETN after achieving LDA or remission may be sufficient to maintain this status, discontinuing ETN resulted in worsening of disease state and patient-reported outcomes [7–11].
Although in this subset analysis we attempted to address a medical need in an underserved region of the world, the patient population included was not ethnically diverse. This is a limitation of this analysis and reflects where clinical trials can be undertaken in this region. Importantly, our analysis underscores the dearth of clinical data on other ethnicities in this region and the need for more studies on this patient population. Given the limited information available on RA patients from this region of the world, it would have been useful to have obtained radiographic evidence of disease level and progression/regression in response to treatment. However, this was not in the global study protocol.
Conclusions
The data reported here suggest that it would be beneficial to patients to continue ETN as maintenance therapy after they have achieved the treatment target, LDA or remission. However, this subset analysis is limited by the small patient population and must be interpreted with caution. In all cases the decision on whether or not to continue ETN therapy should be left to the treating physician and the patient. Additional studies with more patients and including predictors of response may be needed to elucidate these issues further.
Acknowledgements
Funding
The study and article processing charges was sponsored by Pfizer. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship of this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Medical Writing and/or Editorial Assistance
Medical writing support was provided by Mukund Nori, PhD, MBA, CMPP, of Engage Scientific Solutions and was funded by Pfizer.
Author Contributions
All authors are responsible for the content of this manuscript. The authors together conceptualized the manuscript content, interpreted the data, reviewed multiple drafts, and approved the final version for submission.
Disclosures
Bonnie Vlahos is an employee of Pfizer and owns stock in Pfizer. Heather E. Jones is an employee of Pfizer and owns stock in Pfizer. Ron Pedersen is an employee of Pfizer and owns stock in Pfizer. Khalid Shirazy is an employee of Pfizer and owns stock in Pfizer. Hassan Bassiouni and Catherine Elizabeth Spargo have nothing to disclose.
Compliance with Ethics Guidelines
The study was conducted according to the International Conference on Harmonisation guidelines for Good Clinical Practice (GCP), the International Ethical Guidelines for Biomedical Research Involving Human Subjects from the Council for International Organizations of Medical Sciences, and the Declaration of Helsinki. The study protocol and informed consent form were approved by the independent ethics committee or institutional review board at each participating center before patient screening.
Data Availability
The datasets generated during the global study are available at: https://clinicaltrials.gov/ct2/show/NCT01981473.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to 10.6084/m9.figshare.5817519.
References
- 1.Kourilovitch M, Galarza-Maldonado C, Ortiz-Prado E. Diagnosis and classification of rheumatoid arthritis. J Autoimmun. 2014;48–49:26–30. doi: 10.1016/j.jaut.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 2.Halabi H, Alarfaj A, Alawneh K, et al. Challenges and opportunities in the early diagnosis and optimal management of rheumatoid arthritis in Africa and the Middle East. Int J Rheum Dis. 2015;18(3):268–275. doi: 10.1111/1756-185X.12320. [DOI] [PubMed] [Google Scholar]
- 3.Mody GM, Cardiel MH. Challenges in the management of rheumatoid arthritis in developing countries. Best Pract Res Clin Rheumatol. 2008;22(4):621–641. doi: 10.1016/j.berh.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Conti F, Ceccarelli F, Massaro L, et al. Biological therapies in rheumatic diseases. Clin Ter. 2013;164(5):e413–e428. doi: 10.7417/CT.2013.1622. [DOI] [PubMed] [Google Scholar]
- 5.Singh JA, Saag KG, Bridges SL, Jr, et al. 2015 American College of Rheumatology Guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68(1):1–25. doi: 10.1002/acr.22783. [DOI] [PubMed] [Google Scholar]
- 6.Pavelka K, Akkoc N, Al-Maini M, et al. Maintenance of remission with combination etanercept-DMARD therapy versus DMARDs alone in active rheumatoid arthritis: results of an international treat-to-target study conducted in regions with limited biologic access. Rheumatol Int. 2017 doi: 10.1007/s00296-017-3749-7. [DOI] [PubMed] [Google Scholar]
- 7.Wiland P, Dudler J, Veale D, et al. The effect of reduced or withdrawn etanercept-methotrexate therapy on patient-reported outcomes in patients with early rheumatoid arthritis. J Rheumatol. 2016;43(7):1268–1277. doi: 10.3899/jrheum.151179. [DOI] [PubMed] [Google Scholar]
- 8.van Herwaarden N, den Broeder AA, Jacobs W, et al. Down-titration and discontinuation strategies of tumor necrosis factor–blocking agents for rheumatoid arthritis in patients with low disease activity. Cochrane Database Syst Rev. 2014 doi: 10.1002/14651858.CD010455.pub2. [DOI] [PubMed] [Google Scholar]
- 9.van Vollenhoven RF, Ostergaard M, Leirisalo-Repo M, et al. Full dose, reduced dose or discontinuation of etanercept in rheumatoid arthritis. Ann Rheum Dis. 2016;75(1):52–58. doi: 10.1136/annrheumdis-2014-205726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emery P, Hammoudeh M, FitzGerald O, et al. Sustained remission with etanercept tapering in early rheumatoid arthritis. N Engl J Med. 2014;371(19):1781–1792. doi: 10.1056/NEJMoa1316133. [DOI] [PubMed] [Google Scholar]
- 11.Smolen JS, Nash P, Durez P, et al. Maintenance, reduction, or withdrawal of etanercept after treatment with etanercept and methotrexate in patients with moderate rheumatoid arthritis (PRESERVE): a randomised controlled trial. Lancet. 2013;381(9870):918–929. doi: 10.1016/S0140-6736(12)61811-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during the global study are available at: https://clinicaltrials.gov/ct2/show/NCT01981473.