Abstract
As reliable biomarkers of disease activity are lacking, monitoring of therapeutic response in chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) remains a challenge. We sought to determine whether nerve ultrasound and electrophysiology scoring could close this gap. In CIDP patients (fulfilling EFNS/PNS criteria), we performed high-resolution nerve ultrasound to determine ultrasound pattern sum scores (UPSS) and predominant echotexture nerve conduction study scores (NCSS) as well as Medical Research Council sum scores (MRCSS) and inflammatory neuropathy cause and treatment disability scores (INCAT) at baseline and after 12 months of standard treatment. We retrospectively correlated ultrasound morphology with nerve histology when available. 72/80 CIDP patients featured multifocal nerve enlargement, and 35/80 were therapy-naïve. At baseline, clinical scores correlated with NCSS (r2 = 0.397 and r2 = 0.443, p < 0.01), but not or hardly with UPSS (Medical Research Council sum scores MRCSS r2 = 0.013, p = 0.332; inflammatory neuropathy cause and treatment disability scores INCAT r2 = 0.053, p = 0.048). Longitudinal changes in clinical scores, however, correlated significantly with changes in both UPSS and NCSS (r2 = 0.272–0.414, p < 0.0001). Combining nerve/fascicle size with echointensity and histology at baseline, we noted 3 distinct classes: 1) hypoechoic enlargement, reflecting active inflammation and onion bulbs; 2) nerve enlargement with additional hyperechogenic fascicles/perifascicular tissue in > 50% of measured segments, possibly reflecting axonal degeneration; and 3) almost no enlargement, reflecting “burned-out” or “cured” disease without active inflammation. Clinical improvement after 12 months was best in patients with pattern 1 (up to 75% vs up to 43% in pattern 2/3, Fisher’s exact test p < 0.05). Nerve ultrasound has additional value not only for diagnosis, but also for classification of disease state and may predict treatment response.
Electronic supplementary material
The online version of this article (10.1007/s13311-018-0609-4) contains supplementary material, which is available to authorized users.
Keywords: Nerve ultrasonography · CIDP · Polyneuropathy · Ultrasound pattern sum score · Nerve ultrasound and therapy.
Introduction
Diagnosis and guidance of anti-inflammatory therapy are challenging aspects of immune-mediated neuropathy, mainly because reliable biomarkers for disease activity and treatment response are lacking [1]. Currently, nerve conduction studies (NCS) (e.g., the European Federation of Neurological Societies/Peripheral Nerve Society (EFNS/PNS) criteria) have the highest validity of any available method for diagnosis and follow-up; however, quantification of NCS results might be restricted [2]. Clinical scores may be valid tools to determine the clinical status of a patient. However, they are prone to be swayed by the patients’ own interpretation of his or her well-being and do not allow for prediction of treatment response. Subsequently, even thorough neurological assessment may leave the clinician with significant diagnostic and therapeutic uncertainty [3].
As previously shown, high-resolution ultrasound (HRUS) of peripheral nerves represents a promising complementary diagnostic tool in chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) and variants [4–10]. In CIDP, HRUS typically reveals not only multifocal and inhomogeneous nerve enlargement affecting predominantly proximal nerve segments, the brachial plexus, and cervical roots [5], but also distinct echointensity patterns [8] or focal fascicle involvement [7], which may further facilitate differentiation from hereditary or noninflammatory neuropathies [9, 10].
Despite increasing evidence, knowledge about the relationship between sonographic findings and the clinical course is still very limited [11, 12]. Furthermore, the diagnostic value of ultrasonic features other than nerve enlargement (e.g., increased or decreased echosignal) is not yet clear.
Long time observations are operationalized using standardized scores, such as the ultrasound pattern sum score (UPSS)—as recently shown for multifocal motor neuropathy [7, 12, 13].
We have thus decided to evaluate the UPSS as an observational tool and prognostic biomarker for therapeutic response in treatment-naïve and long-term treated CIDP patients and to explore further ultrasonic features of CIDP and variants, particularly nerve echotexture, and their diagnostic and prognostic value. Further, we retrospectively compared histology of the sural nerve with the ultrasound morphology if available. Additionally, we operationalized nerve conduction study data in order to improve comparability with clinical and ultrasound findings.
Methods
Setting and Eligibility
Between May 2014 and May 2016, consecutive patients with CIDP and its variants, who presented to the outpatient clinic of the University Hospitals of Tübingen, Basel and Jena, were prospectively included. The study was registered in the German clinical trial registry (DRKS-ID 00005253) and approved by the local ethics committees (Tübingen 702/2015BO2, Basel 2014-230, and Jena 3663-01/13). Written informed consent to study participation was obtained from all patients.
Correct diagnosis was established according to the EFNS/PNS criteria [14]. Patients were categorized by clinical presentation and treatment status: Previously untreated patients were marked as “untreated neuritis” (UN), whereas already treated patients were marked as “treated neuritis” (TN). Patients in both groups received standard treatment during follow-up.
Neurological Examination
All patients underwent detailed clinical examination including the Medical Research Council sum score (MRCSS, ranging from 0 to 60 points), inflammatory neuropathy cause and treatment disability score (INCAT, ranging from 0 to 10) [15, 16], nerve conduction studies (NCS), and standardized HRUS examination (incl. UPSS) at baseline and after 12 months (UPSSmonth12, MRCSSmonth12, INCATmonth12). Clinical changes of MRCSS and INCAT score were rated as difference of score at months 12 to baseline (named ΔMRCSS, ΔINCAT).
NCS included the median, ulnar, tibial, and fibular nerves on their most involved side including F-waves; for median and ulnar nerve with stimulations from wrist to Erb’s point if tolerated at predefined temperature. In addition, we performed sensory NCS for the sural and the ulnar nerve. NCS findings were categorized as either predominantly demyelinating or axonal. CIDP diagnosis was classified in accordance with EFNS/PNS guidelines as definite, probable, or possible. In patients with additional axonal damage, we added needle electromyography of corresponding muscles. To further quantify NCS data, we devised a scoring system, namely the nerve conduction study score (NCSS): Each pathologic motor nerve was scored with a maximum of 3 points: i) 1 point was assigned for reduced conduction velocity (CV), distal motor latency, prolonged F-wave latency, or possible conduction block, ii) 1 additional point was added if these values reached percentages proposed by EFNS/PNS guidelines, and iii) 1 further point was added for reduced amplitude of the distal compound muscle action potential. Complete lack of motor response was scored with the maximum of 3 points. Each pathologic sensory nerve was scored with a maximum of 2 points: i) 1 point for CV reduction and ii) 1 point for sensory nerve action potential reduction. In analogy to the motor scoring system, 2 points were assigned if no sensory nerve action potential was evocable. The sum of all points of all these nerves yields the NCSS, with a maximum total score of 16 points.
Finally, the longitudinal change of NCSS was evaluated (NCSS after 12 months—NCSS at baseline visit, ΔNCSS). All patients underwent elaborated serum analysis including ganglioside antibodies, HIV serology, and immunofixation.
Ultrasound Examination
HRUS examination (performed on a Mindray 14 Mhz TE7) included measurements of nerve cross-sectional area (CSA) of the median, ulnar, tibial, and fibular nerves (summarized in the UPSA, 16 points), the diameter of the roots C5 and C6 as well as the CSA of the vagus nerve (UPSB, 3 points), and the sural nerve (UPSC, 1 point) of the most involved side at predefined locations as described before for the UPSS score [12, 13]. Homogeneity of the nerves was evaluated for the median, the ulnar, and the tibial nerve, and a maximum of 9 points was reached if all nerves were enlarged homogenously > 1.5-fold (homogeneity score, HS). The maximum score of the UPSS (UPSA + UPSB + UPSC + HS) is thus 29 points. Finally, longitudinal changes in UPSS were evaluated (UPSS after 12 months—UPSS at baseline (ΔUPSS)).
Additionally, maximum fascicle size was measured at the level of the upper arm (for median and ulnar nerve) and the knee (for fibular nerve) according to previously published boundary values (4.8 mm2 for median, 2.5 mm2 for ulnar, and 3.5 mm2 for fibular nerve; [17]). Nerves were defined as mainly hypoechoic if the fascicular appearance was similar to the lumen of blood vessels (e.g., the brachial artery), as hyperechoic if the grayscale was comparable to lymph node echotexture, and as isoechoic if appearance was comparable to control nerves. Furthermore, we examined whether perifascicular tissue was hyperechoic or not. Using this information, we defined the predominant pattern of echoappearance (> 50% of measured segments) as either hyperechoic or not. Gain, probe frequency, and mechanical index were kept constant during the whole examination. With the help of these findings, we classified distinct ultrasound classes according to Padua et al. [8]. Class 1 was defined as significant nerve enlargement (UPSS ≥ 5) with predominantly hypoechoic fascicle pattern. Class 2 reveals significantly enlarged nerves (UPSS ≥ 5) and increased echointensity of the fascicles or the perifascicular tissue in at least > 50% of all measurement points. Class 3 exhibited no or only slight nerve enlargement (UPSS < 5).
Supportive CIDP Criteria
If available, we analyzed supportive diagnostic criteria stated by the EFNS/PNS guidelines (e.g., magnetic resonance imaging, MRI or histology of the sural nerve biopsy); particularly, if available, histology of the sural nerve was evaluated with regard to signs of acute inflammation (edema, infiltrative cells), chronic demyelination (e.g., onion bulbs), or axonal fiber loss. Nerve biopsies were compared to ultrasound data, when performed not more than 3 months prior to or after baseline ultrasound.
Statistical Analysis
T test with Bonferroni correction was used to evaluate differences in epidemiological data (age, gender, height and weight), clinical scores, ultrasound, and NCS data. To examine the validity of HRUS findings with respect to longitudinal disease development, we correlated ΔUPSS with ΔMRCSS, ΔINCAT, and ΔNCSS as described above (regression analysis and Pearson’s correlation coefficient). Negative values of ΔUPSS, ΔNCSS, and ΔINCAT indicate improvement, whereas a negative ΔMRCSS indicates deterioration and vice versa. Differences in all scores of at least 1 point were rated as improvement or deterioration. The correspondence of cross-sectional UPSS, NCSS, INCAT, and MRCSS as well as their Δ was evaluated by cross-table analysis and Fisher’s exact test. Linear regression analysis was also used to test for a linear correlation among distinct classes, UPSS, MRCSS, INCAT, and NCSS. Predictive values for therapeutic response between all subgroups were evaluated and calculated by Fisher’s exact test. For the ultrasound measurements, intrarater intraclass correlation coefficient (ICC) was measured for each center and examiner (FH and NW for Tuebingen, BD for Basel and BH and CM for Jena) by analyzing 50% of all landmarks online and offline (blinded to the first result); the interrater ICC was measured by reanalyzing every measured landmark by a second examiner (AG) blinded to the first values offline; a mean value of both measurements was used for the UPSS. The NCS scoring and the interpretation of the NCS data with regard on EFNS criteria were done by 2 examiners (AG and NW), evaluating the measurements including curve analyzing, who did not perform data acquisition. Accordingly, mean values were calculated. For all tests, a 2-sided p value < 0.05 was regarded as statistically significant. For statistical analysis, we used IBM SPSS Statistics, version 24 (Chicago, IL, USA).
Results
Baseline Data
80 patients were included, 35 of which presented with UN, whilst 45 were already on treatment (TN). Patients’ baseline characteristics, including mean clinical, ultrasound, and NCS scores are summarized in Table 1. No significant differences were noted between the UN and TN groups, except a higher INCAT in the untreated group. Most patients exhibited classical sensorimotor symptoms.
Table 1.
Baseline data
| Age Weight Height |
UPSS 1st visit |
MRCSS 1st visit |
INCAT 1st visit |
NCSS 1st visit |
Relevant concomitant diseases | Main clinical symptoms | Supportive criteria* [15] | |
|---|---|---|---|---|---|---|---|---|
| Untreated patients (UN) n = 35 | 62.1 (14.8) 80.4 (17.2) 177.3 (9.3) |
12.8 (7.3) | 52.3 (7.9) | 4.4 (2.9) | 8.8 (3.5) | DM n = 4, gammopathy n = 8, malignancy n = 4, hypothyreosis n = 2 | SM n = 13; S n = 11; M n = 5; aSM n = 3; P n = 1; CN n = 2 | 0 n = 1; 1 n = 7; > 1 n = 27 (33 definite, 1 probable, 1 possible) |
| Treated patients (TN) n = 45 | 61.8 (15.1) 80.6 (16.1) 176.9 (8.2) |
12.4 (5.5) | 54.3 (4.7) | 2.6 (1.6) | 7.8 (3.8) | DM n = 3, gammopathy n = 4, malignancy n = 3, hepatitis C n = 1, thyroid gland dysfunction n = 3 | SM n = 27; S n = 6; M n = 3; aSM n = 6; P n = 2; CN n = 1 | 1 n = 7; > 1 n = 38 (45 definite) |
Mean values of baseline data do not differ significantly in both groups except the INCAT, which is higher in the untreated group (UN), p = 0.001. Standard deviations are mentioned in brackets.
INCAT = inflammatory neuropathy cause and treatment disability score, MRCSS = medical research council sum score, NCSS = nerve conduction studies score, UPSS = ultrasound pattern sum score, (a)SM = (asymmetric) sensorimotor, S = sensory, P = pain, CN = cranial nerve involvement, M = motor. DM = diabetes mellitus type 2.
*Supportive criteria according to the EFNS-guidelines: Nerve biopsy revealed inflammation or chronic demyelination in 11 of 18 cases. Cerebrospinal fluid protein was elevated (> 500 mg/l) in 69 of 80 patients. MRI of the spine was available in 27 patients, 14 of which (13 with definite, 1 with probable CIDP) showed gadolinium enhancement of the cauda equina, whereas 13 patients (7 definite, 5 probable, and 1 possible) did not. 26 of these 27 patients showed ultrasound nerve enlargement with an UPSS of ≥ 5 points
EFNS/PNS electrophysiological diagnostic criteria led to the diagnosis of definite CIDP in 55, probable CIDP in 20, and possible CIDP in 5 patients. When using supportive criteria (Table 1), CIDP was considered as definite in 78 and as probable and possible in 1 each.
Treatment During Observation Period
Overall, therapeutic regimens after baseline did not differ significantly between both groups. During the observation period, 22/35 UN and 32/45 TN patients received intravenous immunoglobulins (IVIG) only at 6 to 12 g per kg of bodyweight over 12 months (according to clinical symptoms). 9 patients received other immunosuppressive treatment, namely rituximab (RTX), azathioprine, or steroids. Eleven patients were treated by immunosuppressants in addition to IVIG or steroids. 6 patients received no therapy due to mild symptoms during observational period.
Cross-Sectional Nerve Conduction Studies at Baseline
No significant differences in NCSS were found between UN and TN (p > 0.05). At baseline, the NCSS correlated significantly with the INCAT (p < 0.001) and inversely with the MRCSS (p < 0.01). In 9 of the UN and 8 of the TN patients, severe axonal damage was noted (i.e., significantly reduced compound muscle action potential amplitude and/or acute denervation in electromyography). Interrater ICC for the NCSS evaluation was 0.96.
Cross-Sectional Ultrasound Data at Baseline
In almost 90% of patients, including all patients with electrophysiologically possible CIDP, mean nerve CSA values were—as expected—significantly increased compared with normal values. UPSS was usually increased to > 8 points and therefore fulfilled the previously suggested ultrasound criteria for CIDP [12]. Eight patients had an UPSS of < 5 points. Mean UPSS did not differ significantly between UN and TN patients (p > 0.05). Further, 67.5% of UN and TN showed at least 2 or more enlarged fascicles at the measured sites without significant differences between both groups (mean fascicle size for all patients 5.9 ± 2.5 mm2 in median nerve, 4.8 ± 2.2 mm2 in ulnar nerve and 3.9 ± 1.8 mm2 in fibular nerve).
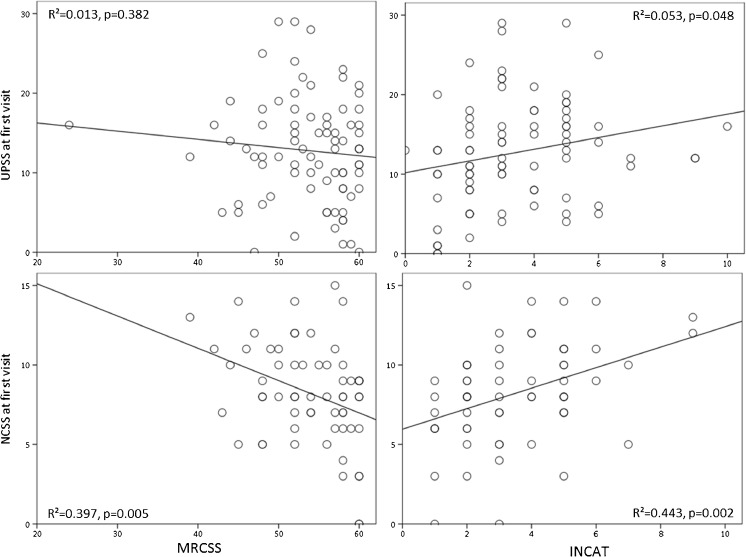
The UPSS showed no significant correlation with the MRCSS (p = 0.332) and a significant but very low correlation with the INCAT overall disability (p = 0.048) (r2 values are shown in Table 2 and Fig. 1). We found no correlation between the ultrasound and the NCS score (p > 0.05). The overall intrarater ICC for the ultrasound measurements and raters was 0.95; the mean inter-rater ICC was 0.90 for 2 raters for each measurement and UPSS.
Table 2.
Cross-sectional data for UPSS and correlation with MRCSS and INCAT and longitudinal change according to classes and groups
| ΔUPSS (12 months–baseline) | ΔMRCSS (12 months–baseline) | ΔINCAT (12 months–baseline) | ΔNCSS (12 months–baseline) | Concordant changes (correspondence in %) | Correlation (baseline) | Correlation (12 months) | |
|---|---|---|---|---|---|---|---|
| All patients | − 1.6 (3.2) | + 2.1 (7.7) | − 0.9 (1.2) | − 0.4 (0.7) | UPSS/MRCSS: 69.8 UPSS/INCAT: 73.6 NCSS/MRCSS: 63.1 NCSS/INCAT: 65.8 |
UPSS/MRCSS: r2 = 0.013, p = 0.332 UPSS/INCAT: r2 = 0.053, p = 0.048 NCSS/MRCSS: r2 = 0.397, p = 0.005 NCSS/INCAT: r2 = 0.443, p = 0.002 NCSS/UPSS: r2 = 0.040, p = 0.168 |
UPSS/MRCSS: r2 = 0.042, p = 0.216 UPSS/INCAT: r2 = 0.141, p = 0.056 NCSS/MRCSS: r2 = 0.261, p = 0.005 NCSS/INCAT: r2 = 0.423, p = 0.043 NCSS/UPSS: r2 = 0.056, p = 0.123 |
| UN all patients | − 1.8 (4.0) | + 4.4 (8.2) | − 2.3 (1.5) | − 1.0 (2.6) | UPSS/MRCSS: 66.7 UPSS/INCAT: 66.7 NCSS/MRCSS 61.3 NCSS/INCAT: 67.1 |
||
| TN all patients | + 0.1 (7.8) | + 0.1 (7.8) | − 0.2 (1.7) | + 0.1 (3.3) | UPSS/MRCSS: 75.5 UPSS/INCAT: 72.2 NCSS/MRCSS 65.6 NCSS/INCAT: 62.1 |
||
| Class 1 n = 30 (65.5% UN) | − 2.3 (4.5) | + 5.5 (9.3) | − 1.9 (1.5) | − 1.0 (2.9) | |||
| Class 2 n = 42 (66.6% TN) | − 1.2 (3.7) | + 0.2 (6.4) | − 0.4 (1.7) | 0.1 (1.3) | |||
| Class 3 n = 8 (87.5% TN) | + 0.1 (1.2) | − 0.4 (1.5) | − 0.1 (1.8) | 0.3 (1.1) | |||
| Subgroups: | |||||||
| Cranial nerve involvement n = 3 | − 8.0 (2.5) | + 12.5 (7.6) | − 3.3 (2.5) | − 1.3 (0.1) | |||
| Pain predominant n = 3 | + 1.0 (2.9) | − 2.0 (2.3) | − 1.0 (1.2) | + 1.5 (0.7) | |||
Concordances were evaluated by cross-tab analysis, correlations by regression analysis. Significant correlations (p < 0.05) are shown in bold print. Significant differences between the distinct ultrasound classes (t test with Bonferroni p < 0.05) are shown in italic
INCAT = inflammatory neuropathy cause and treatment disability score, MRCSS = medical research council sum score, NCSS = nerve conduction studies score, UN = untreated neuritis, UPSS = ultrasound pattern sum score, TN = treated neuritis
Fig. 1.
Linear regression analysis between the UPSS or NCSS and the MRCSS or INCAT scores at baseline. Whereas NCSS correlates significantly with clinical impairment (p < 0.001), ultrasound only does insufficiently, particularly with motor impairment, MRCSS (p = 0.332). INCAT = inflammatory neuropathy cause and treatment disability score, MRCSS = medical research council sum score, NCSS = nerve conduction studies score, UPSS = ultrasound pattern sum score
Classification of Ultrasound Findings
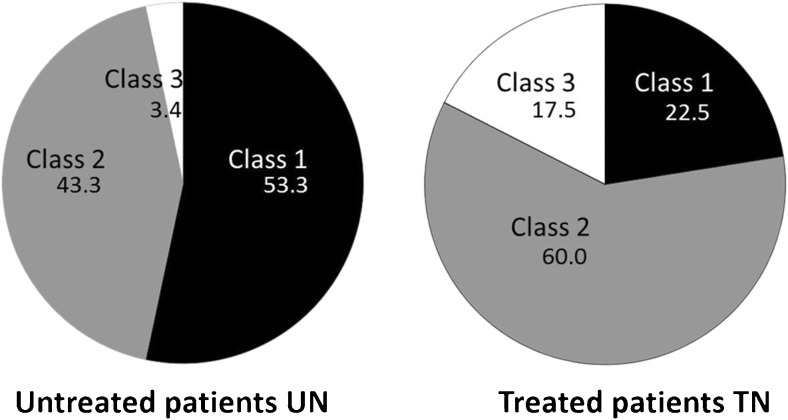
Combining UPSS, echointensity, fascicle size, and perifascicular tissue increase, we defined 3 classes of nerve morphology in a posthoc analysis (compare the algorithm of Fig. 2). This data analysis and classification is in line with previously published findings of Padua et al. [8]. We found a different distribution of ultrasound patterns within UN and TN patients (Table 2; Fig. 3). Class 1 (significant nerve enlargement (UPSS ≥ 5; Fig. 2A) with predominantly hypoechoic fascicle pattern and without prominent perifascicular tissue increase) was the predominant pattern in UN (65.5%; compared to TN with 34.5%), whereas in the TN group, class 2 (featuring significantly enlarged nerves (UPSS ≥ 5; Fig. 2B) and increased echointensity of the fascicles or the perifascicular tissue at > 50% of all measurement points) was more frequent (66.6 vs 33.4% in the UN group). Class 3 (Fig. 2C) exhibited no or only slight nerve enlargement (UPSS < 5), sometimes with increased echointensity and was mostly found in treated patients (87.5; compared to 12.5% in UN). Fascicles were significantly more often enlarged in class 1 patients (96.2%) compared with those in class 2 (48.6%) and class 3 (25%), (Fisher’s exact test, p < 0.0001). No significant differences were found between classes for motor impairment MRCSS, concomitant diseases, therapeutic regime, or supportive diagnostics (p > 0.05). The mean INCAT score, however, was significantly higher in class 1 and 2 compared to class 3 patients (p < 0.01 for both).
Fig. 2.
The distinct ultrasound pattern classes (based on previously published data from Padua et al. [8]) of immune-mediated neuropathies. If UPSS does not reach ≥ 5 points, the patient is classified class 3, whereas an UPSS ≥ 5 points with increased perifascicular tissue (single asterisk) or increased fascicle echosignal (number sign) is defined class 2 and patients with enlarged nerves without increased echosignal or tissue are defined class 1 (Fascicles can be hypo- and isoechoic). Corresponding histology images are shown for class 1 (A, with many onion bulbs, black arrows, Patient 3) and 2 (B, with signs of additional axonal damage, arrow and slight edema, asterisk, Patient 7). (C) The nerve biopsy of patient 9 with severe axonal damage and no signs of acute inflammation. The 9 patients’ biopsy results are summarized concerning onion bulbs, infiltration/edema and axonal damage (− = not found, + = found with mild +, moderate ++, or high amount +++, (+) = only slight findings). Of note, in untreated class 1 neuropathy patients (UN) inflammation and edema predominated, whereas in treated ones (TN), onion bulbs were the catchiest finding. UN = untreated neuritis, UPSS = ultrasound pattern sum score, TN = treated neuritis
Fig. 3.
The distribution of the 3 distinct classes as pie charts in the therapy naïve (untreated) and the chronic treated group in percentages
Supportive Criteria, Comparison of Nerve Biopsy, and Ultrasound Morphology
Supportive criteria are summarized in Table 1. Nerve biopsy was performed in 18 patients. In 11 of these 18 biopsies, we found signs of acute inflammation (e.g., macrophages, T-lymphocytes, perineural edema) or chronic demyelination (e.g., onion bulbs). 9 of these were performed at baseline visit and therefore concurrent with baseline ultrasound data (4 patients [2 UN and 2 TN] revealed class 1 pattern, 4 [2 UN and TN each] class 2 pattern and 1 TN class 3 pattern); overall results are summarized in Fig. 2. Taken together, all patients with class 1 presentation featured signs of acute inflammation or demyelination, i.e., onion blubs (Fig. 2A), whereas in class 2 presentation, at least moderate to severe (additional or solely) axonal damage was found with only slight signs of inflammation or demyelination (Fig. 2B). In class 3 (Fig. 2C), severe axonal damage or no inflammation was found. Histology of untreated class 1 patients predominantly exhibited infiltration and edema, whereas in treated class 1 patients, onion bulbs were the most frequent finding.
Longitudinal Ultrasound, Electrophysiological, and Clinical Data After 12 months Follow-Up
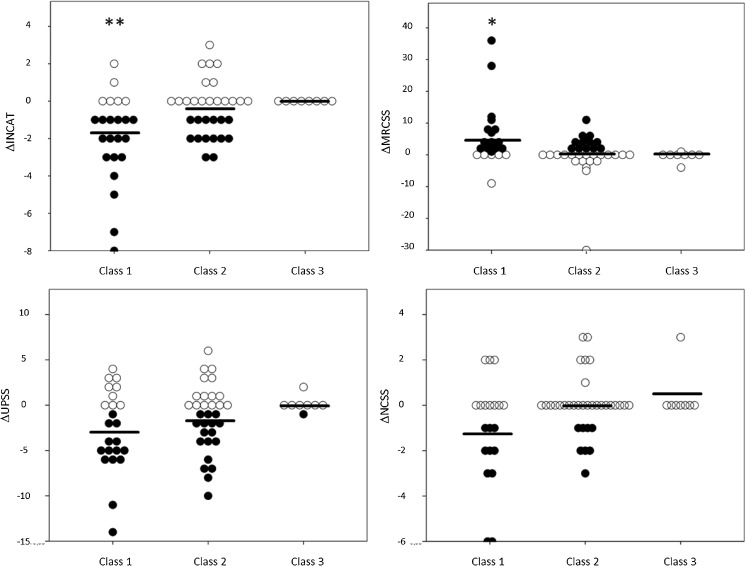
The development of clinical scores, NCSS, and ultrasound parameters is shown for all patients as dot plots with improved, deteriorated or stable conditions in Fig. 4. This overall development correlated well not only for the UPSS with MRCSS and INCAT scores (between 70 and 75% concordance), but also for the NCSS with MRCSS and INCAT (63 and 65% concordance) (Table 2). The relative concordances were similar between UN and TN patients (Table 2). In contrast to the overall findings, 3 UN and 2 TN patients developed progressive nerve enlargement (with an increase in UPSS) despite clinical improvement or at least stabilization. In these cases, therapy was either delayed (initiation > 12 months from symptom onset) or applied with an inconsistent therapeutic regime. 12 patients (6 UN and 6 TN) were lost to follow-up at 12 months.
Fig. 4.
Intraindividual (dots) and mean (black lines) change (Δ) of the UPSS, the MRCSS, the INCAT overall disability score, and the NCSS for all 3 classes. There is significant higher number of patients with improvement (black dots) in class 1 for MRCSS compared to class 2 (single asterisk) and for INCAT compared to classes 2 and 3 (double asterisk), although intraindividual course can be distinct, independently from morphological pattern. Patients with unchanged or deteriorated scores compared to baseline are marked with white dots. INCAT = inflammatory neuropathy cause and treatment disability score, MRCSS = medical research council sum score, NCSS = nerve conduction studies score, UPSS = ultrasound pattern sum score
UPSS and NCSS as Longitudinal Biomarkers
ΔUPSS correlated significantly with the ΔINCAT (p < 0.0001) and inversely with the ΔMRCSS (p < 0.0001) for both UN and TN patients (Fig. 5 including r2 values). Also, the ΔNCSS correlated significantly with ΔMRCSS or ΔINCAT (p < 0.0001 for both). Changes in UPSS and NCSS also correlated significantly (r2 = 0.165, p < 0.0001).
Fig. 5.
The linear regression between the changes of the ultrasound pattern sum score (UPSS) and nerve conduction study score (NCSS) and MRC sum score or INCAT disability score in all patients. MRC = Medical Research Council, INCAT = inflammatory neuropathy cause and treatment disability score
Ultrasound Morphology as a Prognostic Biomarker
Both patient groups with nerve enlargement (hypoechoic class 1 and hyperechoic class 2) did not show any significant differences concerning mean baseline UPSS (p = 0.188), NCSS (p = 0.213), or baseline clinical scores (p = 0.842 and p = 0.986), indicating that patients in both groups were evenly clinically affected. None of the patients showed essential morphological switch from 1 to another class within the 12-month period. However, as shown in Fig. 4, class 1 patients showed a significantly better improvement of MRCSS and INCAT compared to those in class 2 (t test p < 0.03 for MRCSS and p < 0.01 for INCAT) and class 3 (p < 0.05 for INCAT).
Cross-table analyses indicated that presence of class 1 had a positive predictive value (PPV) for good therapeutic response of 0.69 for MRCSS improvement and 0.75 for INCAT improvement, whereas class 2 presence only revealed a PPV for therapeutic response of 0.40 for MRCSS and 0.43 for INCAT improvement, and class 3 pattern a PPV of 0 for both scores. Fisher’s exact revealed significance in favor of class 1 for PPVs of MRCSS (9.354, p = 0.008) and INCAT improvement (11.100, p < 0.001). Consequently, mean NCSS and UPSS improvement was significantly higher in class 1 compared to classes 2 and 3 (t test with Bonferroni correction p < 0.01). Figure 6 shows an example of regression of nerve enlargement in a patient with class 1 pattern and clinical improvement.
Fig. 6.
Example of ultrasound results (class 1) of a patient with CIDP before and 12 months after treatment. Significant reduction of the cross-section areas in ulnar and median nerve (FA = forearm, UA = upper arm) and of the diameter of the cervical root 6 (C6) is shown. CIDP = chronic inflammatory demyelinating polyradiculoneuropathy
Particular Clinical Features and Therapeutic Regimes
Patients with cranial nerve involvement experienced better improvement when compared to other patients, whereas patients with pain showed a higher risk for deterioration, which was also shown for UPSS and NCSS change (t test with Bonferroni correction p < 0.05; Table 2). Other CIDP variants did not show significant differences (p > 0.05).
We could not find any significant differences concerning the development of MRCSS, INCAT, NCSS, and UPSS or the effect of morphology classes with regard to different therapeutic regimens (t test with Bonferroni correction p > 0.05 for Δs between patients with IVIG, patients with steroids/immunosuppressants, patients with a mixture of several therapies, and patients without treatment). Therapeutic response was not different between patients with definite, probable or possible UN or TN.
Cross-Sectional Ultrasound and NCS Data at 12 months
Just like at baseline, the cross-sectional UPSSmonth12 did not show any significant correlation with the MRCSSmonth12 and INCATmonth12, neither in UN nor in TN, whilst the NCSSmonth12 did (with MRCSSmonth12 p < 0.01 and with INCATmonth12 p < 0.05, r2 values in Table 2). NCSSmonth12 and UPSSmonth12 did not correlate (p > 0.05).
Discussion
Multifocal nerve and fascicle enlargement, quantified by the ultrasound pattern sum score (UPSS), is notable in about 90% of untreated and treated CIDP variants, but the amount of nerve enlargement does not consistently correlate with clinical impairment. Over a 12-month follow-up period, however, UPSS changes correlate significantly with clinical development under treatment, showing a decrease of nerve enlargement in case of improvement and vice versa. Similarly, a quantification of nerve conduction studies, as realized by the nerve conduction study score (NCSS), facilitated the interpretation of the electrophysiology data. This score correlated significantly with the clinical development, the ultrasound behavior, and the current clinical status according to clinical scores.
Additionally, ultrasound provided important information about nerve morphology. Our data suggest that therapeutic response is better in patients with hypoechoic nerve enlargement (PPV up to 0.75), which might reflect acute inflammation, than in those with hyperechoic fascicles or perifascicular tissue proliferation and those without nerve enlargement (PPV up to 0.43), which may correspond to predominantly axonal damage or non inflammatory stages. This assumption was confirmed in a small retrospective comparison with sural nerve biopsy results. We therefore conclude that ultrasound studies may significantly enrich nerve conduction data and that the findings of both of these noninvasive techniques could—if summarized in scores and classes—represent promising biomarkers for therapy response.
Cross-Sectional Baseline Data
In our cohort, all patients fulfilled the criteria of definite, probable, or possible CIDP or its variants. Accordingly, most of them featured proximal and predominantly multifocal nerve enlargement. Both treatment-naïve and treated patients featured nerve enlargement to a similar amount—as described in the literature—however, with different intraindividual variability (Fig. 1).
Correlation between nerve enlargement, as seen in HRUS, and clinical disability has been discussed controversially [8, 12, 18]. In our study, there were no consistent direct correlations of nerve enlargement (UPSS) and clinical scores or the NCSS, neither at baseline nor at follow-up and neither in the therapy-naïve nor in the treated group. Our NCS score, however, significantly reflected clinical impairment at first baseline and follow-up (Table 2; Fig. 1).
Ultrasound Morphology
Differences concerning echointensity depending on disease duration have been previously described [8, 12, 19]. We summarized findings and classified 3 distinct classes of ultrasound morphology. The 3 HRUS morphology classes are in line with previous data from Padua et al. [8] and are consistent with the finding of several histopathological CIDP types. In our cohort with 9 chronologically comparable nerve biopsies, we found infiltration of inflammatory cells, edema, or onion bulbs in all patients with class 1, whereas in patients with classes 2 and 3 (additive or solely), axonal damage without significant inflammation or prominent onion bulbs was seen. In conclusion, our classification likely reflects histopathophysiological features which may represent the major advantage of ultrasound over NCS data.
Until now, different clinical aspects and distinct therapeutic responses have been described [20, 21]. Ultrasound might help closing this gap of diagnostic uncertainty.
Longitudinal Data over 12 months
Impact of anti-inflammatory therapy on ultrasonic nerve structure remains unclear, although decrease of nerve enlargement with successful treatment has been discussed [18, 22]. In our cohort, therapeutic response was significantly better in patients with enlarged nerves and hypoechoic signal (class 1) compared to those with predominant hyperechoic fascicles or perifascicular tissue (p < 0.03; Fig. 4). Patients without nerve enlargement revealed almost unchanged ultrasonic presentation at follow-up, regardless of treatment. There was no switch from one to another class during the observation period. As shown in our comparison with nerve histopathology, this emphasizes the suggestion that hypoechoic nerve enlargement corresponds to reversible inflammatory changes like edema or infiltration, whereas hyperechoic changes might indicate chronic irreversible changes with predominantly axonal damage, increased connective/scar tissue or fibrosis (Fig. 2A, B). This consideration, however, must be interpreted with caution as nerve biopsy was only performed in few patients of our cohort. Nevertheless, this is, to the best of our knowledge, the first comparison of ultrasound morphology and nerve histology in CIDP as prognostic and therapeutic marker.
In case of clinical improvement, decrease of nerve enlargement was notable in most patients, regardless of onset morphology. In contrast, when clinical disability increased, nerve enlargement usually progressed, in both UN and in TN. Significant correlations were found between ΔUPSS, ΔNCSS, and ΔMRCSS as well as ΔINCAT overall disability scores (p < 0.0001) (Table 2; Fig. 5). Even changes of electrophysiological and ultrasonic findings correlated significantly (p < 0.0001), in contrast to the cross-sectional data at baseline and at 12 months. The implementation of a quantitative NCS score facilitated the comparison of the follow-up and the baseline data.
HRUS in general and the UPSS in particular, together with the NCSS, could represent promising therapeutic response markers in therapy-naïve as well as in chronic/treated CIDP and could improve the guidance of immunomodulatory regimens. The changes of clinical, ultrasonic, and electrophysiological scores are useful to document patients’ development. Distinct ultrasound morphologies in CIDP reflecting distinct histopathology and distinct therapeutic susceptibility emphasize the additive role of ultrasound in immune-mediated neuropathies in addition to NCS. As an advantage, ultrasound might even be of diagnostic value in case of marked axonal damage, in which NCS validity may be limited. In analogy to disease surveillance concepts in multiple sclerosis (MS), imaging modalities might enrich the future in CIDP therapy, comparable to the “no evidence of disease activity” (NEDA) in MS [23].
Limitations
Some nerves in CIDP patients can progressively enlarge despite improving symptoms [19], as it was the case in 3 UN patients as well as 2 TN patients of our cohort despite clinical improvement (or at least stability). An explanation for this may be a delay in treatment initiation or an inconsistent therapeutic regime due to lack of patient compliance, as it was the case in these patients. It must be kept in mind, that some patients show hypertrophic nerve enlargement progression independent from clinical development. Obviously, prolonged longitudinal data from larger cohorts will be necessary to understand ultrasound pathomorphology in CIDP. Our small group of comparable histology and ultrasound data must be interpreted with caution, e.g., missing nerve enlargement (class 3) could be a sign of curation (no evidence of disease activity) as well as “burned-out” disease stage. Further, additional testing—e.g., paranodal antibodies—must be bared in mind for further sonomorphology studies.
The NCSS, which was developed by our group in order to facilitate follow-up interpretation of electrophysiological findings, represents a new tool to operationalize NCS data. This score currently lacks validation in large prospective multicenter studies; however, it helped in this study to accurately compare UPSS and NCS data.
Of note, the screening examination of all patients by UPSS and NCSS might take more than 1 h depending on examiner’s experience, which might be difficult to realize in daily routine, although the follow-up exams are easier to perform and NCSS validation only enriches the daily NCS routine and does not waste much time. Trainee programs would be helpful to facilitate examination. Furthermore, the UPSS could be shortened in the future by defining the most sensitive measurement points as Goedee and colleagues already tried to do [5]. More multicenter data or meta-analyses will be necessary to find out the most sensitive landmarks for diagnosis and standardization of ultrasound in CIDP.
With regard on distinct therapeutic procedures, we could not evaluate significant differences, which might be surprising with regard on the anti-edematous effect of steroids. However, we must consider that i) our study population had no predefined therapeutic regime and therefore many of them had combination of distinct substances or at least other medication during observation period than before. ii) In some patients, switch of medication was necessary during observation period and therefore the distinct subgroups were too small for reliable data analysis. Clearly, further studies with homogenous patient groups and prospectively defined therapies are warranted. The results of our study can help to design such studies. Nevertheless, this therapeutic heterogeneity reflects the daily routine in our centers.
Conclusion
The severity of NCS pathologies as evaluated by a scoring system correlates well with clinical disability in cross-sectional observation of both treatment-naïve and treated CIDP patients and operates as an accurate longitudinal biomarker of disease severity. Similarly, the ultrasound pattern sum score (UPSS) was found to be a suitable longitudinal observation marker with changes in ultrasonic parameters correlating significantly with the change in clinical disability, independently of disease stage or therapeutic protocol.
Additionally and most importantly, ultrasound morphology may represent a marker for therapeutic susceptibility: patients with hypoechoic fascicles and enlarged nerves (representing inflammation and onion bulbs) experienced a significantly improved clinical course during treatment than those with increased fascicular or perifascicular echointensity (associated with axonal damage). Patients without nerve enlargement remain almost unchanged in ultrasound, electrophysiological, and clinical scores. Those patients might be candidates for therapeutic release.
Electronic Supplementary Material
(PDF 1224 kb)
Acknowledgments
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Abbreviations
- Anti-MAG
myelin-associated glycopeptide antibodies
- C5 and 6
cervical roots 5 and 6
- CIDP
chronic inflammatory demyelinating polyradiculoneuritis
- TN
treated chronic neuritis
- CSA
cross-sectional area
- DADS
distal acquired demyelinating sensory neuropathy
- EFNS
European Federation of Neurological Societies
- HRUS
high-resolution ultrasound
- INCAT
inflammatory neuropathy cause and treatment disability score
- IVIG
intravenous immunoglobulin
- MADSAM
multifocal acquired demyelinating sensory and motor neuropathy
- MGUS
monoclonal gammopathy of unknown significance
- MRCSS
medical research council sum score
- NCS
nerve conduction studies
- ON
onset neuritis
- PPV
positive predictive value
- RTX
rituximab
- UPSS
ultrasound pattern sum score
Author Contributions
Florian Härtig, Marlene Ross, Natalie Winter, and Alexander Grimm designed the study and participated in data acquisition, analysis, interpretation, and drafted the manuscript. Nele M. Dammeier, Bianka Heiling, Nadine Fedtke, Antje Bornemann, and Tim W. Rattay participated in data acquisition and were involved in drafting the manuscript. Bernhard F. Décard participated in the acquisition of data. Hubertus Axer and Holger Lerche participated in the design of the study and acquisition of data, were involved in the analysis and interpretation of data, and helped to draft the manuscript. All authors read, critically revised, and approved the final manuscript.
Compliance with Ethical Standards
The study was registered in the German clinical trial registry (DRKS-ID 00005253) and approved by the local ethics committees (Tübingen 702/2015BO2, Basel 2014-230, and Jena 3663-01/13). Written informed consent to study participation was obtained from all patients.
Conflict of Interest
T.W.R. received travel and accommodation costs from Baxalta for a symposium on multifocal motor neuropathy and once from Merz for a botulinum toxin class, both unrelated to this work. A.G. received reimbursement for travel and accommodation costs from Pfizer for a polyneuropathy symposium. F.H. received reimbursement for travel and accommodation costs from Bayer AG. MK reports nonfinancial support from Biogen, personal fees from Roche, grants, personal fees and nonfinancial support from Novartis, and personal fees from Genzyme. H. L. reports grants from German Federal Ministry of Education and Research and grants from Deutsche Forschungsgemeinschaft during the conduct of the study, personal fees and other from UCB, personal fees from Desitin, personal fees and other from BioMarin, personal fees and other from Telethon, personal fees and other from Eisai, and grants, personal fees, and other from Bial, outside the submitted work. There are no conflicts of interest.
Footnotes
Statistical analysis was conducted by Dr. Alexander Grimm, MD, Tübingen University
Florian Härtig, Marlene Ross, Natalie Winter and Alexander Grimm contributed equally to this work.
References
- 1.Allen JA, Lewis RA. CIDP diagnostic pitfalls and perception of treatment benefit. Neurology. 2015;85:498–504. doi: 10.1212/WNL.0000000000001833. [DOI] [PubMed] [Google Scholar]
- 2.Chin RL, Deng C, Bril V, Hartung H-P, Merkies ISJ, Donofrio PD, et al. Follow-up nerve conduction studies in CIDP after treatment with IGIV-C: Comparison of patients with and without subsequent relapse. Muscle Nerve. 2015;52:498–502. doi: 10.1002/mus.24624. [DOI] [PubMed] [Google Scholar]
- 3.Katzberg HD, Latov N, Walker FO. Measuring disease activity and clinical response during maintenance therapy in CIDP: from ICE trial outcome measures to future clinical biomarkers. Neurodegener Dis Manag 2017 Jan 23. [DOI] [PubMed]
- 4.Gasparotti R, Padua L, Briani C, Lauria G. New technologies for the assessment of neuropathies. Nat Rev Neurol. 2017 Mar 17. doi: 10.1038/nrneurol.2017.31 [DOI] [PubMed]
- 5.Goedee HS, van der Pol WL, van Asseldonk JH, Franssen H, Notermans NC, Vrancken AJ, et al. Diagnostic value of sonography in treatment-naïve chronic inflammatory neuropathies. Neurology. 2017;88(2):143–151. doi: 10.1212/WNL.0000000000003483. [DOI] [PubMed] [Google Scholar]
- 6.Rattay TW, Winter N, Décard B, Dammeier N, Härtig F, Ceanga M, et al. Nerve ultrasound as follow-up tool in treated multifocal motor neuropathy. Eur J Neurology (accepted) [DOI] [PubMed]
- 7.Zaidman CM, Harms MB, Pestronk A. Ultrasound of inherited vs. acquired demyelinating polyneuropathies. J Neurol. 2013;260(12):3115–3121. doi: 10.1007/s00415-013-7123-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Padua L, Granata G, Sabatelli M, Inghilleri M, Lucchetta M, Luigetti M, et al. Heterogeneity of root and nerve ultrasound pattern in CIDP patients. Clin Neurophysiol. 2014;125(1):160–165. doi: 10.1016/j.clinph.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 9.Grimm A, Vittore D, Schubert V, Lipski C, Heiling B, Decard BF, et al. Ultrasound pattern sum score, homogeneity score and regional nerve enlargement index for differentiation of demyelinating inflammatory and hereditary neuropathies. Clin Neurophysiol. 2016;127(7):2618–2624. doi: 10.1016/j.clinph.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Grimm A, Rattay TW, Winter N, Axer H. Peripheral nerve ultrasound scoring systems: benchmarking and comparative analysis. J Neurol. 2017;264(2):243–253. doi: 10.1007/s00415-016-8305-y. [DOI] [PubMed] [Google Scholar]
- 11.Di Pasquale A, Morino S, Loreti S, Bucci E, Vanacore N, Antonini G. Peripheral nerve ultrasound changes in CIDP and correlations with nerve conduction velocity. Neurology. 2015;84(8):803–809. doi: 10.1212/WNL.0000000000001291. [DOI] [PubMed] [Google Scholar]
- 12.Grimm A, Vittore D, Schubert V, Rasenack M, Decard BF, Heiling B, et al. Ultrasound aspects in therapy-naive CIDP compared to long-term treated CIDP. J Neurol. 2016;263(6):1074–1082. doi: 10.1007/s00415-016-8100-9. [DOI] [PubMed] [Google Scholar]
- 13.Grimm A, Decard BF, Axer H, Fuhr P. The Ultrasound pattern sum score—UPSS. A new method to differentiate acute and subacute neuropathies using ultrasound of the peripheral nerves. Clin Neurophysiol. 2015;126(11):2216–2225. doi: 10.1016/j.clinph.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Joint Task Force of the EFNS and the PNS European Federation of Neurological Societies/Peripheral Nerve Society Guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society—first revision. J Peripher Nerv Syst. 2010;15:1–9. doi: 10.1111/j.1529-8027.2010.00245.x. [DOI] [PubMed] [Google Scholar]
- 15.Merkies IS, Schmitz PI, van der Meché FG, Samijn JP, van Doorn PA. Clinimetric evaluation of a new overall disability scale in immune mediated polyneuropathies. Inflammatory Neuropathy Cause and Treatment (INCAT) group. J Neurol Neurosurg Psychiatry. 2002;72(5):596–601. doi: 10.1136/jnnp.72.5.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panaite PA, Renaud S, Kraftsik R, Steck AJ, Kuntzer T. Impairment and disability in 20 CIDP patients according to disease activity status. J Peripher Nerv Syst. 2013;18(3):241–6. doi: 10.1111/jns5.12038. [DOI] [PubMed] [Google Scholar]
- 17.Grimm A, Winter N, Rattay TW, Härtig F, Dammeier N, Auffenberg E, et al A look inside the nerve –morphology of nerve fascicles in healthy controls and patients with polyneuropathy. Clin Neurophysiol 2017 (accepted) [DOI] [PubMed]
- 18.Zaidman CM, Pestronk A. Nerve size in chronic inflammatory demyelinating neuropathy varies with disease activity and therapy response over time: a retrospective ultrasound study. Muscle Nerve. 2014;50(5):733–738. doi: 10.1002/mus.24227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grimm A, Schubert V, Axer H, Ziemann U. Giant nerves in chronic inflammatory polyradiculoneuropathy. Muscle Nerve 2017b Feb;55(2):285–289. [DOI] [PubMed]
- 20.Doppler K, Appeltshauser L, Villmann C, Martin C, Peles E, Krämer HH, et al. Auto-antibodies to contactin-associated protein 1 (Caspr) in two patients with painful inflammatory neuropathy. Brain. 2016;139(Pt 10):2617–2630. doi: 10.1093/brain/aww189. [DOI] [PubMed] [Google Scholar]
- 21.Mathey EK, Park SB, Hughes RA, Pollard JD, Armati PJ, Barnett MH, et al. Chronic inflammatory demyelinating polyradiculoneuropathy: from pathology to phenotype. J Neurol Neurosurg Psychiatry. 2015;86(9):973–85. doi: 10.1136/jnnp-2014-309697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerasnoudis A, Pitarokoili K, Gold R, Yoon MS. Nerve ultrasound and electrophysiology for therapy monitoring in chronic inflammatory demyelinating polyneuropathy. J Neuroimaging. 2015;25(6):931–9. doi: 10.1111/jon.12279. [DOI] [PubMed] [Google Scholar]
- 23.Kappos L, De Stefano N, Freedman MS, Cree BA, Radue E-W, Sprenger T, et al. Inclusion of brain volume loss in a revised measure of “no evidence of disease activity” (NEDA-4) in relapsing-remitting multiple sclerosis. Mult Scler Houndmills Basingstoke Engl. 2016;22:1297–305. doi: 10.1177/1352458515616701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1224 kb)