Abstract
This retrospective cohort study is to investigate the association between herpes simplex virus (HSV) infections and dementia, and the effects of anti-herpetic medications on the risk involved, using Taiwan’s National Health Insurance Research Database (NHIRD). We enrolled a total of 33,448 subjects, and identified 8362 with newly diagnosed HSV infections and 25,086 randomly selected sex- and age-matched controls without HSV infections in a ratio of 1:3, selected from January 1, to December 31, 2000. A multivariable Cox proportional hazards regression model was used to evaluate the risk of developing dementia in the HSV cohort. This analysis revealed an adjusted hazard ratio of 2.564 (95% CI: 2.351-2.795, P < 0.001) for the development of dementia in the HSV-infected cohort relative to the non-HSV cohort. Thus, patients with HSV infections may have a 2.56-fold increased risk of developing dementia. A risk reduction of dementia development in patients affected by HSV infections was found upon treatment with anti-herpetic medications (adjusted HR = 0.092 [95% CI 0.079-0.108], P < 0.001). The usage of anti-herpetic medications in the treatment of HSV infections was associated with a decreased risk of dementia. These findings could be a signal to clinicians caring for patients with HSV infections. Further research is, therefore, necessary to explore the underlying mechanism(s) of these associations.
Electronic supplementary material
The online version of this article (10.1007/s13311-018-0611-x) contains supplementary material, which is available to authorized users.
Key Words: Herpes simplex virus, Dementia, National Health Insurance Research Database, Anti-herpetic medications, Cohort study
Introduction
Dementia is one of the major global health problems. In Taiwan, the prevalence is up to 8% among those aged ≧ 65 in community studies [1, 2]. There are 35.6 million people worldwide who have dementia, with 7.7 million new cases being diagnosed each year, and the incidence continues to rise with the aging population [3]. It is, therefore, considered a heavy burden to family members, caregivers, and society [4–6].
Herpes simplex virus (HSV) infections in non-genital or genital regions are considered as common viral blister diseases [7, 8]. In previous studies, HSV infections have also been reported to be related to Alzheimer dementia [9–11]. Nonetheless, a nationwide, population-based, matched cohort study is still needed to clarify the association between HSV infections and dementia. Furthermore, we have also carried out this study to investigate whether treatment with anti-herpetic medications could attenuate the risk of developing dementia in patients with HSV infections.
Methods
Data Sources and Ethics
The National Health Insurance (NHI) Program was launched in Taiwan in 1995, and as of June 2009, it included contracts with 97% of the medical providers with approximately 23 million beneficiaries, or more than 99% of the entire population [12]. The NHIRD includes comprehensive data on inpatient care, ambulatory care, dental care, and prescription drugs availed by the insured as well as their sex and date of birth. Pursuant to the Personal Information Protection Act, individual identifiers are encrypted before release for research. The diagnoses recorded in the NHIRD are coded according to the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM). All diagnoses of dementia were made by board-certified psychiatrists or neurologists, and HSV infections were confirmed by dermatologists or infection specialists according to the clinical, laboratory, and imaging findings. In Taiwan, the diagnosis, differential diagnosis from herpes zoster, and typing of HSV was based on the results of serum examinations including ELISA, antibody test or PCR in each individual case. The identification of HSV infection using insurance claims dataset was valid and has been used in previous studies [13–15]. Licensed medical records technicians verified the coding before claiming the reimbursements in hospitals and clinics. The NHI Bureau appoints several senior external specialists in neurology, psychiatry, dermatology or infection medicine for randomly reviewing the records of ambulatory care visits and inpatient claims to verify the accuracy of the diagnoses [16]. In this study, we used data from the NHIRD to investigate the association between subjects with HSV infections and dementia over a 10-year period, from the total outpatient and inpatient Longitudinal Health Insurance Database (LHID) in Taiwan (2000-2010). This study was approved by the Institutional Review Board of the Tri-Service General Hospital (TSGH IRB No. 1-104-05-145). Because the patient identifiers were encrypted before their data were used for research purposes to protect confidentiality, the requirement for written or verbal consent from patients for data linkage was waived.
Study Design and Sampled Participants
This study used a matched cohort design. From the LHID, we identified the 8362 subjects as being ≧ 50 years old with newly diagnosed HSV infections, that were selected from January 1, to December 31, 2000, according to ICD-9-CM codes 054: including genital HSV (ICD-9-CM 054.1, HSV-2) and non-genital HSV (ICD-9-CM 054.xx other than 054.1, HSV-1) infections. Each enrolled HSV patient was required to have made at least three outpatient visits within the one-year study period for symptomatic HSV infections according to these ICD-9-CM codes. The date of the HSV infection diagnosis was defined as the index date. For each patient with HSV infection, three controls without a history of HSV infections, which were frequency-matched for the exact age, sex, and index year in the control group (N = 25,086), were enrolled in this study. In addition, patients diagnosed with dementia or HSV infections before 2000 or before the first visit for HSV infections, and all patients aged < 50 were excluded. Data on the usage of anti-herpetic medications, such as acyclovir, famciclovir, ganciclovir, idoxuridine, penciclovir, tromantadine, valaciclovir, and valganciclovir, were collected.
The data of the defined daily dose (DDD) were obtained from the WHO Collaborating Centre for Drug Statistics Methodology (https://www.whocc. no/), and the duration of the usage of anti-herpetic medications was calculated by dividing the cumulative doses by the DDD of the anti-herpetic medications.
While we analyzed the effects on the risk of dementia between the two subgroups with or without anti-herpetic treatment, the sample was divided by the covariates with reference to previous studies using the NHIRD, about the treatment effects of medication associated with the risk of dementia [17–19].
Covariates
The covariates included sex, age group (50-64, ≥ 65 years), geographical area of residence (north, center, south, and east of Taiwan), urbanization level of residence (levels 1 to 4), levels of hospitals as medical centers, regional and local hospitals, and monthly income (in New Taiwan Dollars [NT$]; < 18,000, 18,000-34,999, ≥ 35,000). The urbanization level of residence was defined according to the population, along with various indicators of the level of political, economic, cultural, and metropolitan development. Level 1 was defined as a population of > 1,250,000, and a specific designation as political, economic, cultural, and metropolitan development. Level 2 was defined as a population between 500,000 and 1,249,999, and as playing an important role in the political system, economy, and culture. Urbanization levels 3 and 4 were defined as a population between 149,999 and 499,999, and < 149,999, respectively [20].
The Charlson comorbidity index (CCI) is the most widely used comorbidity index [21, 22]. There are 22 conditions in the CCI [23], and three of these conditions are risk factors for AD: diabetes, cerebrovascular disease, and hemiplegia (stroke) [24]. Comorbidities were assessed using the CCI, which categorizes comorbidities using the ICD-9-CM codes, scores each comorbidity category, and combines all scores to calculate a single comorbidity score. A score of zero indicates that no comorbidities were found, and higher scores indicate higher comorbidity burdens [25].
Study Outcomes
All study participants were followed from the index date until the onset of dementia (ICD-9-CM codes: 290.0, 290.10, 290.11, 290.12, 290.13, 290.20, 290.21, 290.3, 290.41, 290.42, 290.43, 290.8, 290.9, and 331.0), withdrawal from the NHI program, or the end of 2010. These patients with dementia were grouped as Alzheimer dementia (AD, ICD-9-CM 331.0), vascular dementia (VaD, ICD-9-CM 290.41-290.43), and other dementia (ICD-9-CM 290.0, 290.10-290.13, 290.20-290.21, 290.3, 290.8-290.9).
Statistical Analysis
All analyses were performed using the SPSS software version 22 (SPSS Inc., Chicago, Illinois, USA). χ2 and t tests were used to evaluate the distribution of the categorical and continuous variables, respectively. The Fisher exact test for categorical variables was used to statistically examine the differences between the two cohorts. The multivariate Cox proportional hazards regression analysis was used to determine the risk of dementia, and the results were presented as a hazard ratio (HR) with a 95% confidence interval (CI). The difference in the risk of dementia, between HSV-infected subjects and control groups was estimated using the Kaplan-Meier method with the log-rank test. A 2-tailed P value < 0.05 was considered to indicate the statistical significance.
Results
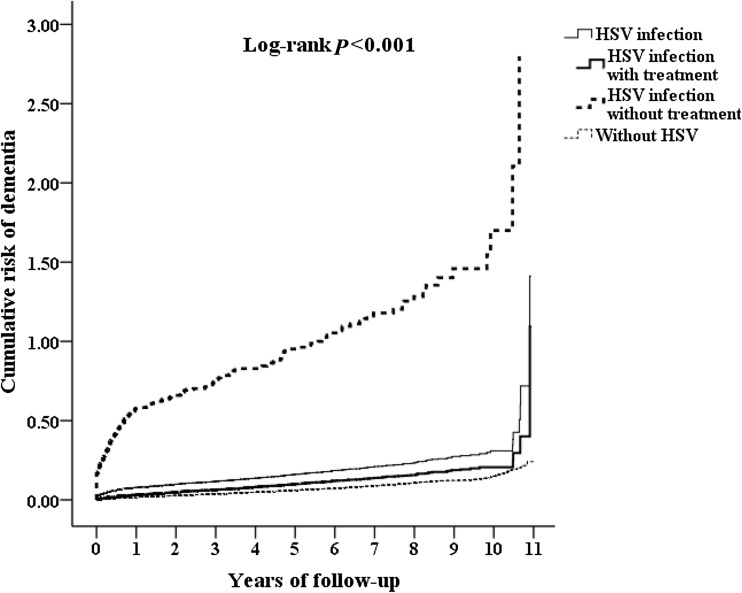
Table 1 shows the sex, age, comorbidities, urbanization, area of residence, income of the HSV patients, and the controls. When compared to controls, HSV patients tended to have higher CCI scores. HSV patients tended to have higher rates in living in urbanization level 1 or 2 areas, and in the north and east of Taiwan, than the control groups. The cumulative incidence of dementia in the HSV-infected subject and control groups, and the difference between the two groups were significant (log-rank test < 0.001, Fig. 1).
Table 1.
Characteristics of study subjects at baseline
| HSV infections | Total n = 33,448 |
With n = 8362 |
Without n = 25,086 |
P | |||
|---|---|---|---|---|---|---|---|
| Characteristics | n | % | n | % | n | % | |
| Gender | 0.999 | ||||||
| Male | 18,896 | 56.49 | 4724 | 56.49 | 14,172 | 56.49 | |
| Female | 14,552 | 43.51 | 3638 | 43.51 | 10,914 | 43.51 | |
| Age group (years) | 0.999 | ||||||
| 50-64 | 10,292 | 30.77 | 2573 | 30.77 | 7719 | 30.77 | |
| ≧ 65 | 23,156 | 69.23 | 5789 | 69.23 | 17,367 | 69.23 | |
| Catastrophic illness card | < 0.001*** | ||||||
| Without | 28,517 | 85.26 | 6307 | 75.42 | 22,210 | 88.54 | |
| With | 4931 | 14.74 | 2055 | 24.58 | 2876 | 11.46 | |
| Insured premium (NT$) | 0.088 | ||||||
| < 18,000 | 33,079 | 98.90 | 8267 | 98.86 | 24,812 | 98.91 | |
| 18,000-34,999 | 335 | 1.00 | 81 | 0.97 | 254 | 1.01 | |
| ≧ 35,000 | 34 | 0.10 | 14 | 0.17 | 20 | 0.08 | |
| CCI_R | 1.30 ± 2.20 | 1.47 ± 2.36 | 1.25 ± 2.13 | < 0.001*** | |||
| Urbanization level | < 0.001*** | ||||||
| 1 (The highest) | 11,082 | 33.13 | 3062 | 36.62 | 8020 | 31.97 | |
| 2 | 15,057 | 45.02 | 3809 | 45.55 | 11,248 | 44.84 | |
| 3 | 2169 | 6.48 | 381 | 4.56 | 1788 | 7.13 | |
| 4 (The lowest) | 5140 | 15.37 | 1110 | 13.27 | 4030 | 16.06 | |
| Location | < 0.001*** | ||||||
| Northern Taiwan | 13,536 | 40.47 | 3739 | 44.71 | 9797 | 39.05 | |
| Middle Taiwan | 8999 | 26.90 | 1955 | 23.38 | 7044 | 28.08 | |
| Southern Taiwan | 8545 | 25.55 | 2028 | 24.25 | 6517 | 25.98 | |
| Eastern Taiwan | 2198 | 6.57 | 615 | 7.35 | 1583 | 6.31 | |
| Outlets islands | 170 | 0.51 | 25 | 0.30 | 145 | 0.58 | |
P: chi-square/Fisher exact test (at category variable), or t test (at continue variable)
CCI_R = Charlson comorbidity index removed dementia
*P < 0.05 for comparison between patients with or without HSV infections
**P < 0.01 for comparison between patients with or without HSV infections
***P < 0.001 for comparison between patients with or without HSV infections
Fig. 1.
Cumulative risk of dementia among subjects aged 50 and over stratified by HSV infections among three groups of controls, HSV infection with anti-herpetic treatment and HSV infection without anti-herpetic treatment in the same plot with log-rank test
Table 2 shows the Cox regression analysis of the factors associated with the risk of developing dementia. The crude HR was 2.542 (2.331-2.771, P < 0.001, data not shown). After adjusting for age, sex, CCI scores, geographical area of residence, urbanization level of residence, and monthly income, the adjusted HR was 2.564 (95% CI 2.351-2.795, P < 0.001). Subjects with either non-genital or genital HSV infections were associated with an increased risk of overall dementia, in comparison to the non-HSV control group. HSV infections were associated with an increased risk in subjects with any type of dementia such as AD, VaD, or other dementia, when compared to the control group. The HSV-infected subjects were associated with overall increased risk in dementia even after we excluded individuals with a diagnosis of dementia within the first year and the first 5 years.
Table 2.
The risk of all dementias between HSV-infected cohort and comparison cohort
| Model | All dementia cases | Dementia in the first year excluded | Dementia in the first 5 years excluded | |||
|---|---|---|---|---|---|---|
| Characteristics | Adjusted HR (95% CI) | P | Adjusted HR (95% CI) | P | Adjusted HR (95% CI) | P |
| HSV infection (reference without) | 2.564 (2.351-2.795) | < 0.001*** | 1.649 (1.443-1.869) | < 0.001*** | 1.646 (1.304-2.078) | < 0.001*** |
| HSV-1 infection | 2.588 (2.379-2.826) | <0.001*** | 1.679 (1.474-1.912) | < 0.001*** | 1.709 (1.354-5.159) | < 0.001*** |
| HSV-2 infection | 2.003 (1.832-2.197) | < 0.001*** | 0.751 (0.312-1.807) | 0.522 | 0 | 0.890 |
| Male (reference: female) | 1.103 (1.023-1.189) | 0.010* | 1.083 (0.989-1.187) | 0.086 | 1.066 (0.931-1.220) | 0.356 |
| ≧ 65 years (reference: 50–64 years) | 1.644 (1.45-1.859) | < 0.001*** | 1.789 (1.519-2.107) | < 0.001*** | 2.287 (1.708-3.062) | < 0.001*** |
| Catastrophic illness card (reference: without) | 1.193 (1.082-1.315) | < 0.001*** | 1.345 (1.193-1.516) | < 0.001*** | 1.324 (1.101-1.592) | 0.003** |
| Insured premium (NT$) (reference < 18,000) | ||||||
| 18,000-34,999 | 0.443 (0.271-0.725) | 0.001** | 0.471 (0.273-0.814) | 0.007** | 0.802 (0.442-1.457) | 0.470 |
| ≧ 35,000 | 0 | 0.829 | 0 | 0.862 | 0 | 0.911 |
| Diabetes mellitus (DM) (reference: without) | 0.923 (0.839-1.015) | 0.098 | 0.950 (0.849-1.064) | 0.378 | 0.961 (0.812-1.137) | 0.642 |
| Hypertension (reference: without) | 0.864 (0.794-0.939) | 0.001** | 0.843 (0.763-0.931) | 0.001** | 0.828 (0.717-0.956) | 0.010* |
| Hyperlipidemia (reference: without) | 0.687 (0.524-0.900) | 0.006** | 0.728 (0.536-0.989) | 0.043* | 0.642 (0.405-1.017) | 0.059 |
| Coronary artery disease (CAD) (reference: without) | 0.752 (0.665-0.852) | < 0.001*** | 0.709 (0.610-0.824) | < 0.001*** | 0.669 (0.534-0.837) | < 0.001*** |
| Obesity (reference: without) | 1.045 (0.147-7.425) | 0.956 | 1.602 (0.225-11.394) | 0.638 | 0 | 0.934 |
| Cancer (reference: without) | 0.380 (0.311-0.465) | < 0.001*** | 0.310 (0.238-0.403) | < 0.001*** | 0.392 (0.267-0.576) | < 0.001*** |
| CCI_R | 0.889 (0.870-0.908) | < 0.001*** | 0.981 (0.950-1.013) | 0.248 | 0.948 (0.899-0.999) | 0.046* |
| Urbanization level (reference: 4, the lowest) | ||||||
| 1 (The highest) | 0.825 (0.742-0.917) | < 0.001*** | 0.818 (0.719-0.930) | 0.002** | 0.847 (0.700-1.025) | 0.087 |
| 2 | 0.786 (0.713-0.866) | < 0.001*** | 0.790 (0.703-0.897) | < 0.001*** | 0.786 (0.661-0.936) | 0.007** |
| 3 | 0.855 (0.725-1.008) | 0.062 | 0.846 (0.696-1.028) | 0.092 | 0.901 (0.680-1.196) | 0.472 |
HR = hazard ratio; CI = confidence intervals; Adjusted HR = adjusted for all variables in the table
Comparison between patients with or without HSV infections: *P < 0.05, **P < 0.01, ***P < 0.001
We have also analyzed the individual outcomes, as AD, VaD, and other dementia in Tables 3, 4, and 5, respectively. In general, the HSV subjects were associated with an increased risk in individual outcomes as AD, VaD, and other dementia. Furthermore, excluding those individuals with a diagnosis of dementia within the first year and the first 5 years, the HSV subjects were still associated with increased risk of individual types of dementia.
Table 3.
The risk of Alzheimer dementia between HSV-infected cohort and comparison cohort
| Model | All dementia cases | Dementia in the first year excluded | Dementia in the first 5 years excluded | |||
|---|---|---|---|---|---|---|
| Characteristics | Adjusted HR (95% CI) | P | Adjusted HR (95% CI) | P | Adjusted HR (95% CI) | P |
| HSV infection (reference without) | 2.739 (2.511-2.985) | < 0.001*** | 1.670 (1.009-2.233) | 0.029* | 1.648 (1.048-5.218) | 0.044* |
| HSV-1 infection | 2.799 (2.610–3.012) | < 0.001*** | 1.770 (1.053-2.323) | 0.001** | 1.709 (1.354-2.158) | < 0.001*** |
| HSV-2 infection | 0 | 0.961 | 0 | 0.980 | 0 | 0.890 |
| Male (reference: female) | 1.449 (0.730-2.879) | 0.289 | 1.192 (0.759-1.870) | 0.446 | 1.449 (0.730-2.879) | 0.289 |
| ≧ 65 years (reference: 50–64 years) | 2.158 (0.516-9.018) | 0.292 | 1.431 (0.687-2.983) | 0.338 | 2.158 (0.516-9.018) | 0.292 |
| Catastrophic illness card (reference: without) | 0.783 (0.276-2.220) | 0.645 | 1.068 (0.570-2.003) | 0.837 | 0.783 (0.276-2.220) | 0.645 |
| Insured premium (NT$) (reference < 18,000) | ||||||
| 18,000-34,999 | 0 | 0.975 | 0 | 0.963 | 0 | 0.975 |
| ≧ 35,000 | 0 | 0.994 | 0 | 0.991 | 0 | 0.994 |
| Diabetes mellitus (DM) (reference: without) | 0.718 (0.316-1.633) | 0.429 | 0.686 (0.376-1.250) | 0.218 | 0.718 (0.316-1.633) | 0.429 |
| Hypertension (reference: without) | 1.331 (0.678-2.612) | 0.407 | 0.836 (0.512-1.364) | 0.473 | 1.331 (0.678-2.612) | 0.407 |
| Hyperlipidemia (reference: without) | 1.576 (0.362-6.856) | 0.545 | 1.405 (0.432-4.574) | 0.572 | 1.576 (0.362-6.856) | 0.545 |
| Coronary artery disease (CAD) (reference: without) | 0.614 (0.211-1.784) | 0.370 | 0.460 (0.197-1.073) | 0.072 | 0.614 (0.211-1.784) | 0.370 |
| Obesity (reference: without) | 0 | 0.996 | 0 | 0.994 | 0 | 0.996 |
| Cancer (reference: without) | 0.249 (0.032-1.915) | 0.182 | 0.113 (0.019-0.667) | 0.016* | 0.249 (0.032-1.915) | 0.182 |
| CCI_R | 1.079 (0.891-1.306) | 0.437 | 1.025 (0.862-1.219) | 0.777 | 1.079 (0.891-1.306) | 0.437 |
| Urbanization level (reference: 4, the lowest) | ||||||
| 1 (The highest) | 1.522 (0.576-4.017) | 0.397 | 0.820 (0.443-1.517) | 0.527 | 1.552 (0.576-4.017) | 0.397 |
| 2 | 0.988 (0.382-2.555) | 0.980 | 0.786 (0.450-1.372) | 0.397 | 0.988 (0.382-2.555) | 0.980 |
| 3 | 0.902 (0.182-4.474) | 0.899 | 0.431 (0.127-1.456) | 0.175 | 0.902 (0.182-4.474) | 0.899 |
AD = Alzheimer dementia; HR = hazard ratio; CI = confidence intervals; Adjusted HR: adjusted for all variables in the table
Comparison between patients with or without HSV infections: *P < 0.05, **P < 0.01, ***P < 0.001
Table 4.
The risk of vascular dementia between HSV-infected cohort and comparison cohort
| Model | All dementia cases | Dementia in the first year excluded | Dementia in the first 5 years excluded | |||
|---|---|---|---|---|---|---|
| Characteristics | Adjusted HR (95% CI) | P | Adjusted HR (95% CI) | P | Adjusted HR (95% CI) | P |
| HSV infection (reference: without) | 1.878 (1.344-2.624) | < 0.001*** | 1.694 (1.113-2.580) | 0.014* | 2.194 (1.163-4.137) | 0.015* |
| HSV-1 infection | 1.823 (1.293–2.571) | < 0.001*** | 1.624 (1.051-2.509) | 0.029* | 2.265 (1.201-4.272) | 0.012* |
| HSV-2 infection | 3.209 (1.022–10.080) | 0.046* | 3.574 (0.882-14.473) | 0.074 | 0 | 0.996 |
| Male (reference: female) | 1.064 (0.822-1.378) | 0.637 | 1.115 (0.830-1.498) | 0.469 | 1.290 (0.845-1.970) | 0.238 |
| ≧ 65 years (reference: 50-64 years) | 3.527 (1.923–6.469) | < 0.001*** | 4.044 (1.897-8.621) | < 0.001*** | 3.977 (1.256-12.589) | 0.019* |
| Catastrophic illness card (reference: without) | 1.759 (1.290-2.398) | < 0.001*** | 1.773 (1.238-2.538) | 0.002** | 1.211 (0.675-2.172) | 0.520 |
| Insured premium (NT$) (reference < 18,000) | ||||||
| 18,000-34,999 | 0.635 (0.158-2.560) | 0.523 | 0.762 (0.189-3.079) | 0.703 | 1.383 (0.338-5.659) | 0.652 |
| ≧ 35,000 | 0 | 0.957 | 0 | 0.962 | 0 | 0.973 |
| Diabetes mellitus (DM) (reference: without) | 1.153 (0.852-1.560) | 0.356 | 1.134 (0.801-1.608) | 0.478 | 0.941 (0.562-1.577) | 0.819 |
| Hypertension (reference: without) | 1.174 (0.897-1.536) | 0.242 | 1.054 (0.776-1.432) | 0.737 | 0.844 (0.544-1.308) | 0.448 |
| Hyperlipidemia (reference: without) | 1.465 (0.789-2.722) | 0.226 | 1.854 (0.990–3.472) | 0.054 | 1.640 (0.649-4.415) | 0.292 |
| Coronary artery disease (CAD) (reference: without) | 0.558 (0.358-0.872) | 0.010** | 0.613 (0.377-0.996) | 0.048* | 0.646 (0.330-1.264) | 0.202 |
| Obesity (reference: without) | 0 | 0.986 | 0 | 0.971 | 0 | 0.979 |
| Cancer (reference: without) | 0.034 (0.009-0.132) | < 0.001*** | 0.060 (0.015-0.234) | < 0.001*** | 0.115 (0.021-0.640) | 0.014* |
| CCI_R | 1.069 (0.966-1.184) | 0.198 | 1.033 (0.915-1.165) | 0.602 | .1.008 (0.847-1.200) | 0.928 |
| Urbanization level (reference: 4, the lowest) | ||||||
| 1 (The highest) | 1.195 (0.828-1.726) | 0.341 | 1.089 (0.728-1.631) | 0.678 | 0.849 (0.485-1.487) | 0.567 |
| 2 | 0.945 (0.665-1.343) | 0.751 | 0.799 (0.541-1.178) | 0.257 | 0.648 (0.385-1.098) | 0.107 |
| 3 | 0.606 (0.305-1.202) | 0.152 | 0.559 (0.261-1.196) | 0.134 | 0.727 (0.295-1.788) | 0.487 |
VaD = Vascular dementia; HR = hazard ratio; CI = confidence intervals; Adjusted HR = adjusted for all variables in the table
Comparison between patients with or without HSV infections: *P < 0.05, **P < 0.01, ***P < 0.001
Table 5.
The risk of other dementia between HSV-infected cohort and comparison cohort
| Model | All dementia cases | Dementia in the first year excluded | Dementia in the first 5 years excluded | |||
|---|---|---|---|---|---|---|
| Characteristics | Adjusted HR (95% CI) | P | Adjusted HR (95% CI) | P | Adjusted HR (95% CI) | P |
| HSV infection (reference: without) | 1.974 (1.810-2.152) | < 0.001*** | 1.674 (1.458-1.922) | < 0.001*** | 1.607 (1.246-2.073) | < 0.001*** |
| HSV-1 infection | 2.760 (2.518-3.025) | < 0.001*** | 1.722 (1.499-1.978) | < 0.001*** | 1.670 (1.295-2.155) | < 0.001*** |
| HSV-2 infection | 1.808 (1.150-2.844) | 0.010* | 0.517 (0.166-1.605) | 0.254 | 0 | 0.898 |
| Male (reference: female) | 1.109 (1.023-1.202) | 0.012* | 1.076 (0.975-1.187) | 0.144 | 1.028 (0.899-1.190) | 0.706 |
| ≧ 65 years (reference: 50-64 years) | 1.556 (1.370-1.768) | < 0.001*** | 1.699 (1.430-2.018) | < 0.001*** | 2.172 (1.600–2.966) | < 0.001*** |
| Catastrophic illness card (reference: without) | 1.270 (1.143-1.410) | < 0.001*** | 1.311 (1.152-1.493) | < 0.001*** | 1.368 (1.122-1.667) | 0.002** |
| Insured premium (NT$) (reference < 18,000) | ||||||
| 18,000-34,999 | 0.412 (0.239-0.711) | 0.001** | 0.463 (0.256-0.838) | 0.011* | 0.766 (0.396-1.482) | 0.429 |
| ≧ 35,000 | 0 | 0.841 | 0 | 0.874 | 0 | 0.917 |
| Diabetes mellitus (DM) (reference: without) | 0.906 (0.818-1.004) | 0.059 | 0.940 (0.832-1.063) | 0.323 | 0.974 (0.811-1.170) | 0.782 |
| Hypertension (reference: without) | 0.840 (0.768-0.919) | < 0.001*** | 0.820 (0.736-0.913) | < 0.001*** | 0.804 (0.687-0.940) | 0.006** |
| Hyperlipidemia (reference: without) | 0.601 (0.441-0.819) | 0.001** | 0.588 (0.408-0.848) | 0.004** | 0.514 (0.295-0.894) | 0.019* |
| Coronary artery disease (CAD) (reference: without) | 0.786 (0.690-0.896) | < 0.001*** | 0.739 (0.629-0.867) | < 0.001*** | 0.682 (0.353-0.870) | 0.002** |
| Obesity (reference: without) | 1.194 (0.180-8.484) | 0.860 | 1.902 (0.267-13.524) | 0.521 | 0 | 0.938 |
| Cancer (reference: without) | 0.441 (0.359-0.542 | < 0.001*** | 0.359 (0.273-0.472) | < 0.001*** | 0.444 (0.297-0.668) | < 0.001*** |
| CCI_R | 0.960 (0.953-0.987) | 0.004** | 0.975 (0.942-1.010) | 0.157 | 0.934 (0.882-0.990) | 0.021* |
| Urbanization level (reference 4, the lowest) | ||||||
| 1 (The highest) | 0.835 (0.745-0.935) | 0.002** | 0.790 (0.688-0.908) | 0.001** | 0.823 (0.668-1.012) | 0.065 |
| 2 | 0.800 (0.721-0.888) | < 0.001*** | 0.789 (0.696-0.894) | < 0.001*** | 0.798 (0.661-0.963) | 0.018* |
| 3 | 0.909 (0.765-1.079) | 0.275 | 0.905 (0.738-1.110) | 0.340 | 0.943 (0.698-1.274) | 0.702 |
HR = hazard ratio; CI = confidence intervals; Adjusted HR = adjusted for all variables in the table.
Comparison between patients with or without HSV infections: *P < 0.05, **P < 0.01, ***P < 0.001
Supplementary Table 1 shows the sex, age, comorbidities, urbanization, area of residence, and the income of the HSV subjects with and without anti-herpetic medication treatment. Compared to the subjects without anti-herpetic medication treatment, the treated subjects tended to have a higher male to female ratio, CCI scores, residence in higher urbanization level, and living in northern Taiwan.
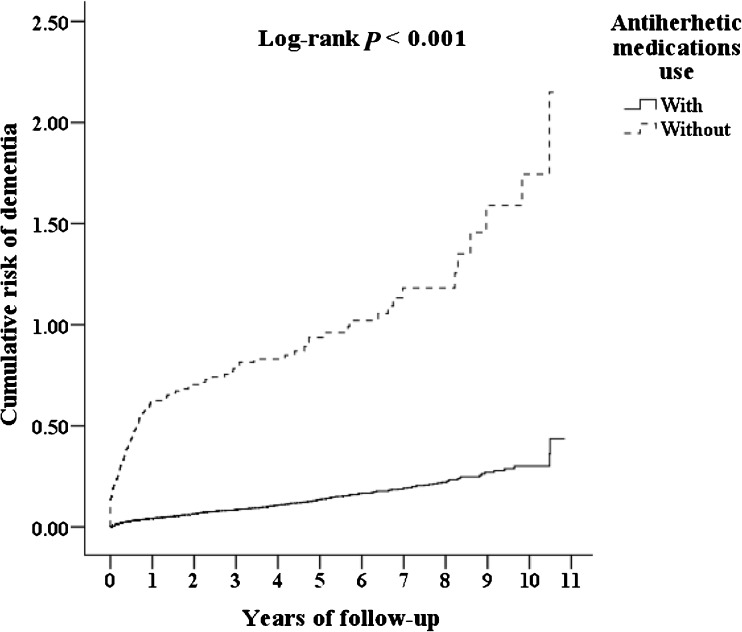
Subjects with HSV infections taking anti-herpetic medications (N = 7215) were compared to those patients not taking them (N = 1147). In the subgroup with anti-herpetic medications treatment, 419 (5.80%) developed dementia in the longitudinal follow-up within 10 years. In the subgroup without anti-herpetic medications treatment, 325 (28.33%) developed dementia in the same follow-up period. Anti-herpetic medications, either over-all (adjusted HR 0.092, 0.079-0.108, P < 0.001) or individual antivirals, were associated with decreased risk of developing dementia (Table 6). Table 6 also shows the percentages of these medications, the mean durations of medications, and the adjusted hazard ratios, percentage of the decrease of risk in each day of usage of antivirals, and the differences between the durations of antiviral treatment and the associations with the decrease of risk of dementia, by the over-all or individual anti-herpetic medications. Cumulative risk of dementia among patients with HSV infections aged 50 and over was stratified by anti-herpetic medication usage with log-rank test (log-rank test < 0.001, Fig. 2). Subjects with anti-herpetic treatment were associated with a decreased risk of overall dementia, AD, VaD, and other dementia. In addition, either genital or non-genital HSV infection in the subjects with anti-herpetic medications showed a decreased risk of dementia when compared to the group without anti-herpetic medications.
Table 6.
Percentage, duration, percentage of decrease of risk by days, and adjusted hazard ratio of anti-herpetic medications
| Anti-herpetic medications | With versus without | ||||
|---|---|---|---|---|---|
| Type | n (%) | Duration (days) | n % decrease of risk/day | Adjusted HR (95% CI) | P |
| Over-all | 7215 | 90.01 ± 121.78 | 1.009 | 0.092 (0.079-0.108) | < 0.001*** |
| Acyclovir | 1835 (25.43%) | 91.32 ± 127.62 | 1.025 | 0.064 (0.028-0.083) | < 0.001*** |
| 1-29 days | 1125 (15.59%) | 26.54 ± 35.42 | 1.616 | 0.571 (0.254-0.895) | < 0.001*** |
| ≧ 30 days | 710 (9.84%) | 193.93 ± 273.71 | 0.500 | 0.031 (0.012-0.104) | 0.007** |
| Famciclovir | 1799 (24.94%) | 86.25 ± 116.40 | 1.054 | 0.091 (0.075-0.100) | 0.003** |
| 1–29 days | 997 (13.82%) | 22.45 ± 32.45 | 1.804 | 0.595 (0.157-0.912) | 0.014* |
| ≧30 days | 802 (11.12%) | 165.56 ± 220.76 | 0.579 | 0.042 (0.022-0.089) | 0.001** |
| Ganciclovir | 698 (9.68%) | 95.44 ± 129.08 | 0.810 | 0.227 (0.107-0.545) | 0.015* |
| 1–29 days | 401 (5.56%) | 27.72 ± 39.56 | 1.652 | 0.542 (0.264-0.988) | 0.040* |
| ≧ 30 days | 297 (4.12%) | 186.87 ± 249.95 | 0.506 | 0.055 (0.024-0.167) | 0.007** |
| Valciclovir | 842 (11.67%) | 79.28 ± 118.53 | 1.068 | 0.153 (0.096-0.317) | 0.001** |
| 1-29 days | 459 (6.36%) | 20.79 ± 31.12 | 1.837 | 0.618 (0.154-0.997) | 0.048* |
| ≧ 30 days | 383 (5.31%) | 149.38 ± 223.29 | 0.603 | 0.099 (0.045-0.268) | < 0.001*** |
| Valganciclovir | 1977 (27.40%) | 88.46 ± 120.74 | 1.034 | 0.085 (0.053-0.098) | < 0.001*** |
| 1-29 days | 1013 (14.04%) | 25.31 ± 36.78 | 1.671 | 0.577 (0.256-0.752) | < 0.001*** |
| ≧ 30 days | 964 (13.36%) | 154.82 ± 208.97 | 0.596 | 0.077 (0.032-0.091) | < 0.001*** |
*P < 0.05, **P < 0.01, ***P < 0.001
Fig. 2.
Cumulative risk of dementia among HSV-infected patients aged 50 and over stratified by anti-herpetic medication use with log-rank test
Discussion
In our study, HSV-infected subjects had an almost 3-fold increased risk of developing any type of dementia, including AD, VaD, or other dementia, in comparison to the control group. The Kaplan-Meier analysis revealed that HSV-infected patients had a significantly lower 10-year psychiatric disorders-free survival rate than the controls. In addition, it took 1 year to achieve a significantly adjusted HR, and therefore, 10 years appear to be a reasonable period to follow-up patients with HSV infections. Even though the individuals with a diagnosis of dementia within the first year and the first 5 years were excluded, the HSV-infected subjects were still associated with increased risk of individual types of dementia. Several previous local cohort studies revealed that seropositivity of HSV increased the risk of Alzheimer disease [26]. However, this is the first nationwide, matched cohort study for the association between both HSV types 1 or 2 infections and the risk of all types of dementia.
In this study, we defined at least three outpatient visits within the one-year study period for HSV infections according to these ICD-9-CM codes as the study cases. However, this probably means that only those with significant clinically visible symptoms of reactivations of HSV were selected. This is not a weakness of the method but probably a strength, which could in some way correspond to those of previous cohort studies indicating an increased risk with HSV IgM-antibodies as a measure of reactivated HSV infection, as previous researches have already pointed out [26, 27]. Furthermore, at least three crucial points validate the diagnostic specificity in this claims dataset based study: first, all clinicians used the ICD-9-CM codes in Taiwan; second, licensed medical records technicians verified the coding before claiming the reimbursements; and third, the National Health Insurance Administration authority verified the audit.
The underlying mechanisms of the association between HSV infections and dementia remain unclear. Nonetheless, inflammatory changes in the brain have been reported in studies on the pathogenesis of dementia [28, 29], and previous researchers found that infectious diseases, such as hepatitis C viral infection, Helicobacter pylori infection, cytomegalovirus infections, chronic osteomyelitis, or even sepsis, were associated with an increased risk of dementia [30–34]. In 1982, Ball first suggested that HSV-1 might be involved in the pathogenesis of AD by finding that the brain regions damaged in HSV encephalitis, the limbic system, are the same as those affected the earliest in AD [35]. This is reinforced by the finding that receptors for HSV-1 are selectively expressed in the limbic system [36]. Several other studies have shown that HSV-1 and being a carrier of the apolipoprotein E allele 4 (ApoE e4) together confer risk for AD [9, 11, 27, 37–39]. Some studies have found that amyloid-β (Aβ) peptides have antiviral activity or protective effects against HSV infections in the brain by preventing the virus from fusing with the plasma membrane [40], and further suggested HSV infections as a possible risk factor for AD [41–43]. β-amyloid peptides have been found to have antimicrobial activity, including against HSV-1 [40, 44]. Previous studies have also shown that HSV-1 DNA is detected in AD plaques [45], HSV-1 infection-induced synaptic dysfunction via glycogen synthase kinase 3 (GSK-3) activation and intraneuronal amyloid-β protein accumulation [46], as well as the presence of intrathecal antibodies to HSV-1 [47]. Nevertheless, the potential pathogenetic link between HSV infections and the risk of other types of dementia, as shown in this study, remain unclear. Pro-inflammatory factors or oxidative stress might induce neuroinflammation and neurodegeneration and, thus, contribute to the pathogenesis of dementia [9, 48].
Previous studies have shown that the reactivation of HSV increases the risk for AD [26, 27]. In one study from Taiwan, the seroprevalence rate for HSV-1 infection reached 95.0% for those over 30 years of age, and for HSV-2, the rate was 31.2% for those over 60 years old [49]. Therefore, considering the high prevalence of latent HSV-1 in the population aged over 30, many of the “newly diagnosed” HSV-1 infections may represent reactivation of the virus, with positive immunoglobulin (IG) G and IgM, rather than a newly acquired primary HSV infection (IgG−, IgM+). One study also pointed out that cold sores, or herpes labialis, only occur in 20 to 40% of the population infected by HSV-1, and the other 60 to 80% of subjects might have been infected but not affected, that is, they were not symptomatic [50]. Furthermore, in our study, the adjusted HR was 2.564 for the risk of developing dementia in the HSV infection group, which is very close to the finding of the HR of 2.55 in the risk of developing AD in a previous study [27]. In our study, serology data was not available. Therefore, a future study using HSV serology is needed to confirm the association between re-activation of HSV and the risk of AD.
Moreover, we also found that anti-herpetic medications were associated with a lower risk of dementia in patients with HSV infections, and the adjusted HR was 0.092. This means that treatment with anti-herpetic medications could reduce nearly 90.8% of the risk of developing dementia in patients with HSV infections. The HSV-infected subjects treated with anti-herpetic medications showed a decreased risk in all types of dementia such as AD, VaD, or other dementia, when compared to the group without anti-herpetic medications. In general, patients with shorter (< 30 days) or longer (≧ 30 days) durations of anti-herpetic medications were associated with a decreased risk of dementia, and the treatment duration of ≧ 30 days was associated with a lower risk of dementia than those of a duration of < 30 days. In the patients with ophthalmic or other specified complications, oral and intravenous anti-herpetic medications also showed the effects of reducing the risk of dementia in these patients.
The role of the anti-herpetic medication treatment for the prevention of AD have not been studied in the past, even though one author argued that antiviral agents in neurodegenerative disorders could be a new paradigm targeting AD [35]. Our study found that anti-herpetic medications could attenuate the risk of developing dementia (adjusted HR 0.092, 0.079-0.108, P < 0.001). One previous study demonstrated improvement in cognition of HSV-1 IgG seropositive schizophrenia patients with valacyclovir treatment for 18 weeks and compared it to a HSV-1 IgG seronegative control group in a clinical trial [51]. Our present study might be the first report on the role of anti-herpetic medication treatment in attenuating the risk of developing dementia for patients with HSV infections in a nationwide, population-based study. However, in the HSV-group, only 13.7% (1147 in 8362) did not receive anti-herpetic medications. This suggests that further study is needed to clarify whether anti-herpetic medication usage plays a role in reducing the risk of dementia for HSV-infected patients. Meanwhile, a clinical trial using valacyclovir for patients with early AD is ongoing in Sweden [52].
In addition, recent studies have reported trends in decreasing incidence of Alzheimer disease and other cognitive impairments since the late twentieth century, and researchers suggest that this results from increasing level of education and decreasing prevalence of cardiovascular comorbidities [53–57]. We also speculate that the introduction of anti-herpetic medications, for example, acyclovir [58], since the 1980s, could also contribute to this trend by decreasing the risk of dementia in HSV-infected patients.
In this study, we found that males are over-represented in the HSV-infected cohort (4724 males and 3638 females), a finding that is different from results seen in seroprevalence studies in Europe and North America, in which far more females than males are reported to have either HSV-1 or HSV-2 infections [59, 60]. However, a seroprevalence study of HSV-1 and HSV-2 in Taiwan found no significant association between sex and HSV-1 seropositivity, but females had higher rates of HSV-2 seropositivity, and overall age-weighted seropositive rate of HSV-1 and HSV-2 was 63.2% (95% CI, 60.6-65.7%), and 7.7% (95% CI, 6.2-9.5%), respectively [49]. We, therefore, speculate that the HSV-1 predominance in this study might result in the divergence of the sex ratio in this study in comparison to studies in Europe and North America. Furthermore, different study designs using claims dataset or seroprevalence data might also play an important role. However, the reasons for the difference among these studies remain unknown and need further investigation.
Limitations
There are several limitations to this study. First, patients with dementia could be identified using insurance claims data. However, data on the severity, stage, or impact on their caregivers were not available, and in such a study based on a claims dataset, we could only estimate treatment durations of each anti-herpetic medication by dividing the cumulative doses of individual medications by defined daily dose (DDD). The National Health Insurance Research Database (NHIRD) does not contain HSV serology data, either. Second, other residual confounding factors, such as education, genetics or dietary factors, are also not included in the NHIRD. Since there are no imaging findings or other laboratory data included in the NHIRD, we could only rely on the professional diagnosis of dementia by board-certified psychiatrists or neurologists as aforementioned. Furthermore, several community studies have revealed that Alzheimer dementia is the most common cause of dementia (40-60% in all dementias), followed by vascular dementia (20-30% in all dementias), and mixed or other dementias (7-15%) in Taiwan [1, 61, 62], whereas most of the dementias in our study were other types. One possible hypothesis is that some of the other dementias might actually be AD cases. Another possibility is that clinicians might put these types of dementia with a progressive course and no evidence of previous cerebrovascular events into this category instead of AD. In addition, the NHIRD does not have data on APOE-e4 genotype, which is not only a risk for AD in Taiwan and other countries [63–65], but also increases the frequency of symptomatic oral herpetic lesions [38] without viral shedding [38, 66]. Further studies are, therefore, warranted to determine the APOE genotypes in HSV-infected patients and controls.
Conclusions
Patients with HSV infections may have a 2.56-fold increased risk of developing dementia. The usage of anti-herpetic medications in the treatment of HSV infections was associated with a decreased risk of dementia. These findings might well be a signal to the clinicians caring for patients with HSV infections. Further research is necessary to explore the underlying mechanism(s) of this association.
Electronic supplementary material
(PDF 1224 kb)
(DOCX 20 kb)
Acknowledgments
This study was supported by grants from Tri-Service Hospital Research Foundation (TSGH-C105-130, TSGH-C106-002, and TSGH-C107-004), and the sponsor has no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Liu CK, Lai CL, Tai CT, et al. Incidence and Subtypes of Dementia in Southern Taiwan: Impact of Socio-Demographic Factors. Neurology. 1998;506:1572–1579. doi: 10.1212/WNL.50.6.1572. [DOI] [PubMed] [Google Scholar]
- 2.Sun Y, Lee HJ, Yang SC, et al. A Nationwide Survey of Mild Cognitive Impairment and Dementia, Including Very Mild Dementia, in Taiwan. PLoS One. 2014;96:e100303. doi: 10.1371/journal.pone.0100303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Dementia Cases Set to Triple by 2050 but Still Largely Ignored. Available from: http://www.who.int/mediacentre/news/releases/2012/dementia_20120411/en/index.html. Accesed 17 Feb 2018.
- 4.Tzeng NS, Chang CW, Hsu JY, et al. Caregiver Burden for Patients with Dementia with or without Hiring Foreign Health Aides: A Cross-Sectional Study in a Northern Taiwan Memory Clinic. J Med Sci. 2015;356:239–247. doi: 10.4103/1011-4564.172999. [DOI] [Google Scholar]
- 5.Tzeng NS, Chiang WS, Chen SY, et al. The Impact of Pharmacological Treatments on Cognitive Function and Severity of Behavioral Symptoms in Geriatric Elder Patients with Dementia: Pharmacological Treatments on in Elder Patients with Dementia. Taiwanese J Psychiatry. 2017;311:69–79. [Google Scholar]
- 6.Wang HY, Chen JH, Huang SY, et al. Forensic Evaluations for Offenders with Dementia in Taiwan’s Criminal Courts. J Am Acad Psychiatry Law. in press. [PubMed]
- 7.Gupta R, Warren T, Wald A. Genital Herpes. Lancet. 2007;3709605:2127–2137. doi: 10.1016/S0140-6736(07)61908-4. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert S, Corey L, Cunningham A, et al. An Update on Short-Course Intermittent and Prevention Therapies for Herpes Labialis. Herpes. 2007;14(Suppl 1):13A–18A. [PubMed] [Google Scholar]
- 9.Harris SA, Harris EA. Herpes Simplex Virus Type 1 and Other Pathogens Are Key Causative Factors in Sporadic Alzheimer’s Disease. J Alzheimers Dis. 2015;482:319–353. doi: 10.3233/JAD-142853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Itzhaki RF, Lathe R, Balin BJ, et al. Microbes and Alzheimer’s Disease. Journal of Alzheimer’s disease : JAD. 2016;514:979–984. doi: 10.3233/JAD-160152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Itzhaki RF. Herpes and Alzheimer’s Disease: Subversion in the Central Nervous System and How It Might Be Halted. J Alzheimers Dis. 2016;544:1273–1281. doi: 10.3233/JAD-160607. [DOI] [PubMed] [Google Scholar]
- 12.Ho Chan WS. Taiwan’s Healthcare Report 2010. EPMA J. 2010;14:563–585. doi: 10.1007/s13167-010-0056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang JH, Ho JD, Chen YH, Lin HC. Increased Risk of Stroke after a Herpes Zoster Attack: A Population-Based Follow-up Study. Stroke. 2009;4011:3443–3448. doi: 10.1161/STROKEAHA.109.562017. [DOI] [PubMed] [Google Scholar]
- 14.Chiang CH, Huang CC, Chan WL, et al. Herpes Simplex Virus Infection and Risk of Atrial Fibrillation: A Nationwide Study. Int J Cardiol. 1642;2013:201–204. doi: 10.1016/j.ijcard.2011.06.126. [DOI] [PubMed] [Google Scholar]
- 15.Huang CC, Chan WL, Chen YC, et al. Herpes Simplex Virus Infection and Erectile Dysfunction: A Nationwide Population-Based Study. Andrology. 2013;12:240–244. doi: 10.1111/j.2047-2927.2012.00037.x. [DOI] [PubMed] [Google Scholar]
- 16.Ministry of Justice. National Health Insurance Reimbursement Regulations. 2014 Available from: http://law.moj.gov.tw/LawClass/LawAllIf.aspx?PCode=L0060006. Accesed 17 Feb 2018.
- 17.Chao PC, Chien WC, Chung CH, et al. Cognitive Enhancers Associated with Decreased Risk of Injury in Patients with Dementia: A Nationwide Cohort Study in Taiwan. J Investig Med. 2017; 10.1136/jim-2017-000595. [DOI] [PubMed]
- 18.Tzeng NS, Chung CH, Lin FH, et al. Magnesium Oxide Use and Reduced Risk of Dementia: A Retrospective, Nationwide Cohort Study in Taiwan. Curr Med Res Opin. 2018;341:163–169. doi: 10.1080/03007995.2017.1385449. [DOI] [PubMed] [Google Scholar]
- 19.Tai SY, Chien CY, Chang YH, Yang YH. Cilostazol Use Is Associated with Reduced Risk of Dementia: A Nationwide Cohort Study. Neurotherapeutics. 2017;143:784–791. doi: 10.1007/s13311-017-0512-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang CY, Chen WL, Liou YF, et al. Increased Risk of Major Depression in the Three Years Following a Femoral Neck Fracture--a National Population-Based Follow-up Study. PLoS One. 2014;93:e89867. doi: 10.1371/journal.pone.0089867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J Chronic Dis. 1987;405:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 22.de Groot V, Beckerman H, Lankhorst GJ, Bouter LM. How to Measure Comorbidity. A Critical Review of Available Methods. J Clin Epidemiol. 2003;563:221–229. doi: 10.1016/s0895-4356(02)00585-1. [DOI] [PubMed] [Google Scholar]
- 23.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a Combined Comorbidity Index. J Clin Epidemiol. 1994;4711:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 24.Mayeux R, Stern Y. Epidemiology of Alzheimer Disease. Cold Spring Harb Perspect Med. 2012;28. [DOI] [PMC free article] [PubMed]
- 25.Needham DM, Scales DC, Laupacis A, Pronovost PJ. A Systematic Review of the Charlson Comorbidity Index Using Canadian Administrative Databases: A Perspective on Risk Adjustment in Critical Care Research. Journal of Critical Care. 2005;201:12–19. doi: 10.1016/j.jcrc.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Lovheim H, Gilthorpe J, Johansson A, et al. Herpes Simplex Infection and the Risk of Alzheimer’s Disease: A Nested Case-Control Study. Alzheimers Dement. 2015;116:587–592. doi: 10.1016/j.jalz.2014.07.157. [DOI] [PubMed] [Google Scholar]
- 27.Letenneur L, Peres K, Fleury H, et al. Seropositivity to Herpes Simplex Virus Antibodies and Risk of Alzheimer’s Disease: A Population-Based Cohort Study. PLoS One. 2008;311:e3637. doi: 10.1371/journal.pone.0003637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daulatzai MA. Fundamental Role of Pan-Inflammation and Oxidative-Nitrosative Pathways in Neuropathogenesis of Alzheimer’s Disease. Am J Neurodegener Dis. 2016;51:1–28. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Stefaniak J, O'Brien J. Imaging of Neuroinflammation in Dementia: A Review. J Neurol Neurosurg Psychiatry. 2016;871:21–28. doi: 10.1136/jnnp-2015-311336. [DOI] [PubMed] [Google Scholar]
- 30.Kao LT, Sheu JJ, Lin HC, Tsai MC, Chung SD. Association between Sepsis and Dementia. J Clin Neurosci. 2015;229:1430–1433. doi: 10.1016/j.jocn.2015.02.035. [DOI] [PubMed] [Google Scholar]
- 31.Chiu WC, Tsan YT, Tsai SL, et al. Hepatitis C Viral Infection and the Risk of Dementia. Eur J Neurol. 2014;218:1068–e1059. doi: 10.1111/ene.12317. [DOI] [PubMed] [Google Scholar]
- 32.Huang WS, Yang TY, Shen WC, et al. Association between Helicobacter Pylori Infection and Dementia. J Clin Neurosci. 2014;218:1355–1358. doi: 10.1016/j.jocn.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 33.Tseng CH, Huang WS, Muo CH, Kao CH. Increased Risk of Dementia among Chronic Osteomyelitis Patients. Eur J Clin Microbiol Infect Dis. 2015;341:153–159. doi: 10.1007/s10096-014-2200-1. [DOI] [PubMed] [Google Scholar]
- 34.Barnes LL, Capuano AW, Aiello AE, et al. Cytomegalovirus Infection and Risk of Alzheimer Disease in Older Black and White Individuals. J Infect Dis. 2015;2112:230–237. doi: 10.1093/infdis/jiu437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ball MJ. Limbic Predilection in Alzheimer Dementia: Is Reactivated Herpesvirus Involved? Can J Neurol Sci. 1982;93:303–306. doi: 10.1017/S0317167100044115. [DOI] [PubMed] [Google Scholar]
- 36.Lathe R, Haas JG. Distribution of Cellular Hsv-1 Receptor Expression in Human Brain. J Neurovirol. 2017;233:376–384. doi: 10.1007/s13365-016-0504-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Itzhaki RF, Lin WR, Shang D, et al. Herpes Simplex Virus Type 1 in Brain and Risk of Alzheimer’s Disease. Lancet. 1997;3499047:241–244. doi: 10.1016/S0140-6736(96)10149-5. [DOI] [PubMed] [Google Scholar]
- 38.Strandberg TE, Pitkala K, Eerola J, Tilvis R, Tienari PJ. Interaction of Herpesviridae, Apoe Gene, and Education in Cognitive Impairment. Neurobiol Aging. 2005;267:1001–1004. doi: 10.1016/j.neurobiolaging.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 39.Feart C, Helmer C, Fleury H, et al. Association between Igm Anti-Herpes Simplex Virus and Plasma Amyloid-Beta Levels. PLoS One. 2011;612:e29480. doi: 10.1371/journal.pone.0029480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bourgade K, Le Page A, Bocti C, et al. Protective Effect of Amyloid-Beta Peptides against Herpes Simplex Virus-1 Infection in a Neuronal Cell Culture Model. J Alzheimers Dis. 2016;504:1227–1241. doi: 10.3233/JAD-150652. [DOI] [PubMed] [Google Scholar]
- 41.Wozniak MA, Itzhaki RF, Shipley SJ, Dobson CB. Herpes Simplex Virus Infection Causes Cellular Beta-Amyloid Accumulation and Secretase Upregulation. Neurosci Lett. 2007;4292-3:95–100. doi: 10.1016/j.neulet.2007.09.077. [DOI] [PubMed] [Google Scholar]
- 42.Zambrano A, Solis L, Salvadores N, et al. Neuronal Cytoskeletal Dynamic Modification and Neurodegeneration Induced by Infection with Herpes Simplex Virus Type 1. J Alzheimers Dis. 2008;143:259–269. doi: 10.3233/JAD-2008-14301. [DOI] [PubMed] [Google Scholar]
- 43.Civitelli L, Marcocci ME, Celestino I, et al. Herpes Simplex Virus Type 1 Infection in Neurons Leads to Production and Nuclear Localization of App Intracellular Domain (Aicd): Implications for Alzheimer’s Disease Pathogenesis. J Neurovirol. 2015;215:480–490. doi: 10.1007/s13365-015-0344-0. [DOI] [PubMed] [Google Scholar]
- 44.Soscia SJ, Kirby JE, Washicosky KJ, et al. The Alzheimer's Disease-Associated Amyloid Beta-Protein Is an Antimicrobial Peptide. PLoS One. 2010;53:e9505. doi: 10.1371/journal.pone.0009505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wozniak MA, Mee AP, Itzhaki RF. Herpes Simplex Virus Type 1 DNA Is Located within Alzheimer’s Disease Amyloid Plaques. J Pathol. 2009;2171:131–138. doi: 10.1002/path.2449. [DOI] [PubMed] [Google Scholar]
- 46.Piacentini R, Li Puma DD, Ripoli C, et al. Herpes Simplex Virus Type-1 Infection Induces Synaptic Dysfunction in Cultured Cortical Neurons Via Gsk-3 Activation and Intraneuronal Amyloid-Beta Protein Accumulation. Sci Rep. 2015;5:15444. doi: 10.1038/srep15444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wozniak MA, Shipley SJ, Combrinck M, Wilcock GK, Itzhaki RF. Productive Herpes Simplex Virus in Brain of Elderly Normal Subjects and Alzheimer’s Disease Patients. J Med Virol. 2005;752:300–306. doi: 10.1002/jmv.20271. [DOI] [PubMed] [Google Scholar]
- 48.Tzeng NS, Chung CH, Yeh CB, et al. Are Chronic Periodontitis and Gingivitis Associated with Dementia? A Nationwide, Retrospective, Matched-Cohort Study in Taiwan. Neuroepidemiology. 2016;472:82–93. doi: 10.1159/000449166. [DOI] [PubMed] [Google Scholar]
- 49.Shen JH, Huang KY, Chao-Yu C, et al. Seroprevalence of Herpes Simplex Virus Type 1 and 2 in Taiwan and Risk Factor Analysis, 2007. PLoS One. 2015;108:e0134178. doi: 10.1371/journal.pone.0134178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Itzhaki RF. Herpes Simplex Virus Type 1 and Alzheimer's Disease: Increasing Evidence for a Major Role of the Virus. Front Aging Neurosci. 2014;6:202. doi: 10.3389/fnagi.2014.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prasad KM, Eack SM, Keshavan MS, et al. Antiherpes Virus-Specific Treatment and Cognition in Schizophrenia: A Test-of-Concept Randomized Double-Blind Placebo-Controlled Trial. Schizophr Bull. 2013;394:857–866. doi: 10.1093/schbul/sbs040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lovheim H. Feasibility and Effects of Valaciclovir Treatment in Persons with Early Alzheimer’s Disease (Valz-Pilot). Available from: https://clinicaltrials.gov/ct2/show/record/NCT02997982. Accesed 21 Feb 2018.
- 53.Derby CA, Katz MJ, Lipton RB, Hall CB. Trends in Dementia Incidence in a Birth Cohort Analysis of the Einstein Aging Study. JAMA Neurol. 2017;7411:1345–1351. doi: 10.1001/jamaneurol.2017.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gao M, Kuang W, Qiu P, et al. The Time Trends of Cognitive Impairment Incidence among Older Chinese People in the Community: Based on the Clhls Cohorts from 1998 to 2014. Age Ageing. 2017;465:787–793. doi: 10.1093/ageing/afx038. [DOI] [PubMed] [Google Scholar]
- 55.Langa KM. Is the Risk of Alzheimer's Disease and Dementia Declining? Alzheimers Res Ther. 2015;71:34. doi: 10.1186/s13195-015-0118-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee Y. The Recent Decline in Prevalence of Dementia in Developed Countries: Implications for Prevention in the Republic of Korea. J Korean Med Sci. 2014;297:913–918. doi: 10.3346/jkms.2014.29.7.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu YT, Fratiglioni L, Matthews FE, et al. Dementia in Western Europe: Epidemiological Evidence and Implications for Policy Making. Lancet Neurol. 2016;151:116–124. doi: 10.1016/S1474-4422(15)00092-7. [DOI] [PubMed] [Google Scholar]
- 58.Brigden D, Fiddian P, Rosling AE, Ravenscroft T. Acyclovir - a Review of the Preclinical and Early Clinical Data of a New Antiherpes Drug. Antiviral Res. 1981;14:203–212. doi: 10.1016/0166-3542(81)90011-5. [DOI] [PubMed] [Google Scholar]
- 59.Pebody RG, Andrews N, Brown D, et al. The Seroepidemiology of Herpes Simplex Virus Type 1 and 2 in Europe. Sex Transm Infect. 2004;803:185–191. doi: 10.1136/sti.2003.005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bradley H, Markowitz LE, Gibson T, McQuillan GM. Seroprevalence of Herpes Simplex Virus Types 1 and 2--United States, 1999-2010. J Infect Dis. 2014;2093:325–333. doi: 10.1093/infdis/jit458. [DOI] [PubMed] [Google Scholar]
- 61.Liu HC, Lin KN, Teng EL, et al. Prevalence and Subtypes of Dementia in Taiwan: A Community Survey of 5297 Individuals. J Am Geriatr Soc. 1995;432:144–149. doi: 10.1111/j.1532-5415.1995.tb06379.x. [DOI] [PubMed] [Google Scholar]
- 62.Lin RT, Lai CL, Tai CT, et al. Prevalence and Subtypes of Dementia in Southern Taiwan: Impact of Age, Sex, Education, and Urbanization. J Neurol Sci. 1601;1998:67–75. doi: 10.1016/s0022-510x(98)00225-1. [DOI] [PubMed] [Google Scholar]
- 63.Chuang WL, Hsieh YC, Wang CY, Kuo HC, Huang CC. Association of Apolipoproteins E4 and C1 with Onset Age and Memory: A Study of Sporadic Alzheimer Disease in Taiwan. J Geriatr Psychiatry Neurol. 2010;231:42–48. doi: 10.1177/0891988709351804. [DOI] [PubMed] [Google Scholar]
- 64.Kamboh MI, Apolipoprotein E. Polymorphism and Susceptibility to Alzheimer’s Disease. Hum Biol. 1995;672:195–215. [PubMed] [Google Scholar]
- 65.Ward A, Crean S, Mercaldi CJ, et al. Prevalence of Apolipoprotein E4 Genotype and Homozygotes (Apoe E4/4) among Patients Diagnosed with Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Neuroepidemiology. 2012;381:1–17. doi: 10.1159/000334607. [DOI] [PubMed] [Google Scholar]
- 66.Koelle DM, Magaret A, Warren T, Schellenberg GD, Wald A. Apoe Genotype Is Associated with Oral Herpetic Lesions but Not Genital or Oral Herpes Simplex Virus Shedding. Sex Transm Infect. 2010;863:202–206. doi: 10.1136/sti.2009.039735. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1224 kb)
(DOCX 20 kb)