Abstract
A relatively high number of different medications is currently used for migraine prevention in clinical practice. Although these compounds were initially developed for other indications and differ in their mechanisms of action, some general themes can be identified from the mechanisms at play. Efficacious preventive drugs seem to either suppress excitatory nervous signaling via sodium and/or calcium receptors, facilitate GABAergic inhibition, reduce neuronal sensitization, block cortical spreading depression and/or reduce circulating levels of CGRP. We here review such mechanisms for the different compounds.
Electronic supplementary material
The online version of this article (10.1007/s13311-018-0621-8) contains supplementary material, which is available to authorized users.
Keywords: Migraine prevention, Mechanism of actions, Cortical spreading depression, β blockers, Antiepileptic drugs, Antidepressants, Botulinum toxin
Introduction
Migraine is a disorder characterized by recurrent attacks of headache with moderate to severe intensity, often accompanied by photophobia, phonophobia, and nausea. The headache can aggravate with physical activity, is often unilateral, and may have a throbbing character. The underlying pathophysiology of migraine is only partly understood to date. Malfunctioning of brain areas and channels, which modulate the excitability of nociceptive brain circuits as well as central sensitization are thought to play a role [1], the latter especially in the transformation from episodic to chronic migraine [2]. Migraine aura is thought to relate to cortical spreading depression (CSD), a slow wave of depolarization propagating across the cortex, and some authors have suggested that CSD is also implicated in the pathogenesis of migraine headache [3], although this remains controversial [4]. Calcitonin gene-related peptide (CGRP) is a neuropeptide, which appears firmly connected to migraine pathogenesis. Systemic levels of this strong vasodilator rise during migraine attacks and while they fall thereafter, they appear to remain elevated even interictally as compared to healthy subjects [5]. Interestingly, endogenous CGRP release has been shown also during experimentally induced CSD in cortical rat slices. Intravenous injection of CGRP can trigger migraine attacks.
Patients affected by frequent attacks may need preventive treatment in order to reduce the frequency and severity of attacks. A number of mostly oral pharmacological treatments have been evaluated and are recommended for migraine prevention [6, 7]. Thereof, propranolol, metoprolol, flunarizine, topiramate, and valproate were considered first line treatments in the EFNS guideline for the prevention of migraine [7].
One can generally expect a 50% reduction in attack frequency in every second patient with these compounds, if they are taken regularly. Just as our understanding of migraine pathophysiology is limited in general, the mechanisms of action of such preventive compounds are also understood only in part and for the currently licensed compounds, the clinical development did typically not go from bench to bedside, but rather the opposite route. The majority of available treatments were repurposed for migraine prevention after initial development for other indications such as arterial hypertension, epilepsy, and depression.
In this review, we will discuss potential mechanisms of action of currently available treatment options for migraine prevention and cover agents for both episodic as well as chronic migraine. Because of space limitations, the focus will be on agents that are either licensed for migraine specifically or recommended in international treatment guidelines [7]. Novel compounds specifically targeting the CGRP pathway will be discussed in another dedicated article in this special issue (Reuter et al. Neurotherapeutics). Nonpharmacological migraine prevention strategies are very important in clinical practice [8, 9] but not covered in this article. We refer the interested reader to the article by Puledda and Shields in this issue (Puledda and Shields. Non-pharmacological approaches for migraine. Neurotherapeutics).
General Preventive Treatment Principles and Mechanisms of Action
The recommendations for when to use pharmacological migraine prevention vary greatly between published treatment guidelines. As a general principle, migraine prevention should be considered when attacks affect quality of life and is indicated in roughly one third of migraine patients [10]. Comorbidities should be taken into account when considering and selecting a migraine preventive.
Although the different compounds used for migraine prevention clearly act at different receptor sites or channels, it has been suggested that they may modulate common pathways or mechanisms implicated in migraine pathogenesis. For example, several migraine preventives such as propranolol, topiramate, valproate, amitriptyline [11], and flunarizine [12], but also CGRP antagonists [13] have been shown to suppress CSD. Other compounds have been suggested to reduce neuronal sensitization [14, 15]. Moreover, some of the available migraine preventives may reduce levels of circulating neuropeptides such as CGRP [16] and of “hyperalgesic” cytokines such as tumor necrosis factor α, interleukin-1β, and interleukin 6 [17], although it remains unclear whether such effects directly relate to the medication or indirectly relate to the reduction of attacks.
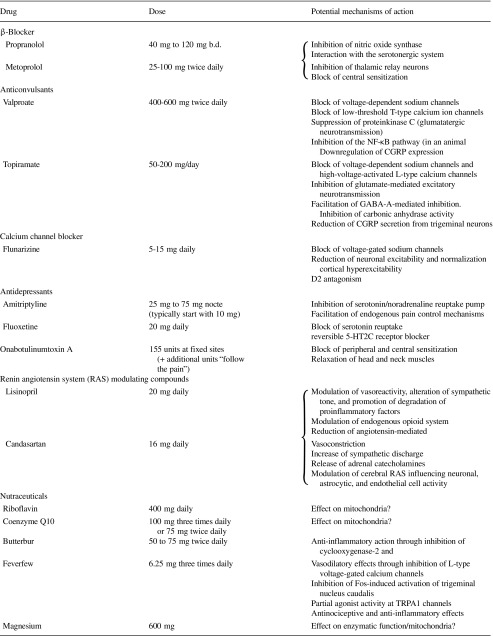
In the following, we will summarize the current understanding of mechanisms of action of specific agents or groups of agents (Table 1).
Table 1.
Preventive treatments used in migraine and their potential mechanisms of action
β-Blockers
Although all beta-adrenergic blockers (BABs) are competitive inhibitors of β-receptors, they may differ with regard to receptor binding (i.e., β1-selectivity and partial agonistic activity) and pharmacokinetic properties [18]. BABs with intrinsic sympathomimetic activity (e.g., acebutolol, alprenolol, oxprenolol, and pindolol) are not effective for migraine prevention [19]. Clinical studies support the efficacy of propranolol (80–240 mg/day), timolol (20–30 mg/day), bisoprolol (5 mg/day), and metoprolol (200 mg/ day) in migraine preventive treatment [20–22]. Atenolol and nadolol also have a moderate effect in reducing migraine attack frequency [19, 23].
The mechanisms of action of BABs in migraine prevention are not completely understood. Inhibition of β1-mediated effects could be considered the main mechanism of action. Indeed, blockade of β1 receptors could inhibit noradrenaline (NA) release and tyrosine hydroxylase activity, the rate-limiting step in NA synthesis [24]. Moreover, propranolol reduces the neuronal firing rate of noradrenergic neurons of the locus coeruleus [25]. Interestingly, BABs also regulate the firing rate of periaqueductal gray matter (PAG) neurons via a GABA-mediated action [26]. Both these effects may contribute to the antimigraine action of BABs. Recent findings in an animal model of trigeminovascular activation showed that propranolol exerts its prophylactic action, at least in part, by interfering with the chronic sensitization processes in the rostral ventromedial medulla and locus coeruleus, and by counteracting the facilitation of trigeminovascular transmission within the trigeminocervical complex [15].
Some BABs may also interact with the serotonergic system by blocking 5-HT2C and 5-HT2B receptors [27–29]. Since 5-HT plays a pivotal role in the pathophysiology of migraine, the BABs-induced effects on this neurotransmitter could account for their prophylactic action on migraine. Moreover, PET studies have supported the possibility that BABs also affect 5-HT synthesis, which is altered in migraine patients [30].
Propranolol inhibits nitric oxide production by blocking inducible nitric oxide synthase (NOS). Propranolol also inhibits kainite-induced currents and is synergistic with N-methyl-D-aspartate blockers, which reduce neuronal activity and have membrane-stabilizing properties [31].
In a double-blind randomized study, it was shown that propranolol and metoprolol decreased VEP amplitude in migraine patients. This action was associated with a better clinical response to the prophylactic treatment [32]. Therefore, it could be hypothesized that BABs have a significant effect on the excitability of the visual system (and probably more generally the cortex) in migraine patients. In migraine patients, the effect of BABs on auditory evoked cortical potentials was correlated with a reduction of migraine attack frequency [33]. This effect of BABs on auditory evoked cortical potential could be due to a modulatory action on serotonergic transmission [34].
Other neurophysiological studies analyzed the contingent negative variation, a slow event-related potential measuring cortical information processing. This potential is increased in amplitude, lacks habituation and is normalized by BABs in association with a clinical improvement in migraine [35]. All these clinical neurophysiological studies support the hypothesis that BABs exert their prophylactic action in migraine by influencing cortical information processing and cortical excitability possibly via NA and 5-HT systems [34, 36, 37].
Cortical spreading depression (CSD) could also represent a target for BABs in migraine. In an experimental model, it was observed that treatment with propranolol suppressed retinal spreading depression [38]. Moreover, treatment with propranolol blocked CSD in rats, without altering regional cerebral blood flow and systemic arterial blood pressure [39].
Finally, it has also been hypothesized that BABs exert some of their therapeutic effects in migraine through an action at the ventroposteromedial thalamic nucleus, which represents a relay of trigeminal sensory input to the primary somatosensory cortex. Considering the complex and widespread nature of the sensory disturbance in migraine, and neurophysiological findings, a possible thalamic involvement in the mechanisms of action of BABs represents a fascinating hypothesis [40, 41].
Antiepileptics
Several antiepileptic drugs (AEDs) acting at different pre- and post-synaptic sites have been studied and proven effective for migraine prevention with the best clinical trial evidence in terms of migraine preventive action being available for topiramate and valproate [7]. However, clearly not all antiepileptics are similarly efficacious in migraine as in epilepsy. Those compounds that possess a rather specific/narrow mechanism of action typically seem to be less efficacious in migraine. As an example, oxcarbazepine was not superior to placebo in reducing the number of migraine attacks in a double-blind trial [42]. Interestingly, oxcarbazepine did also not reduce the frequency of CSD in animal experiments [43], while topiramate and valproate (and also other effective migraine preventives) did [11], suggesting this might be useful as a predictive model for evaluating novel migraine treatments.
Topiramate has been proven efficacious both in episodic as well as chronic migraine and is licensed for migraine prophylaxis in many countries. Topiramate is a “dirty drug” and blocks multiple channels such as voltage-dependent sodium channels and high-voltage-activated L-type calcium channels [44]. It has also been shown to inhibit glutamate-mediated excitatory neurotransmission and facilitate GABA-A-mediated inhibition. Topiramate also inhibits carbonic anhydrase activity [45]. Moreover, it has been shown that topiramate can reduce CGRP secretion from trigeminal neurons in response to depolarizing stimuli [46]. It is currently unclear which of the noted mechanisms is key for topiramate’s effect in migraine prevention. Interestingly, CGRP plasma levels were shown to be unaltered by low-dose (50 mg per day) topiramate treatment in a small migraine trial, providing some preliminary evidence that topiramate effect may be independent from the CGRP pathway in humans [47].
Just like topiramate, there are multiple mechanisms at play by which valproate may improve migraine frequency. Similar to topiramate, valproate also enhances GABAergic inhibition and blocks excitatory ion channels [44]. The exact mechanisms by which this is achieved differ between the two drugs, though. Valproate blocks the degradation of GABA, hence increasing GABA levels in axons and glia cells. It blocks voltage-dependent sodium and low-threshold T-type calcium ion channels [44]. Furthermore, valproate can suppress proteinkinase C, a regulator of the glutamatergic neurotransmission [48]. A more recent study identified the inhibition of the NF-κB pathway (in an animal model of migraine using nitroglycerin triggering) as an additional potential mechanism of action in migraine [49]. In the same preclinical study, valproate also downregulated the expression of CGRP in brain tissue.
Calcium Antagonists
Several calcium antagonists have been used for migraine prevention since the 1980s [50]. In this regard, flunarizine (usually used at daily doses of 5–10 mg [15]) is the best studied compound and licensed for this indication in many countries (although not available in the USA). Verapamil and the antihistaminergic drug cinnarizine are alternatives (e.g., in refractory migraine cases or when flunarizine is not available) that also act at calcium channels. However, both latter drugs are off-label for migraine treatment and the evidence in terms of efficacy is much more scarce than for flunarizine.
Flunarizine is a nonselective calcium antagonist, which has also been shown to block voltage-gated sodium channels [51, 52]. Action at these two sites may reduce neuronal excitability and normalize cortical hyperexcitability in migraine. Furthermore, flunarizine acts as a D2 dopamine antagonist and has in this regard been shown to possess (at high doses) comparable antipsychotic efficacy as haloperidol [53]. It has also been shown that flunarizine can reduce the number and duration of CSD waves [12]. Furthermore, it has been suggested that flunarizine can alleviate CSD-induced mitochondrial injury [12]. Finally, flunarizine has also been shown to increase leptin levels [17], which have been suggested to reflect leptin resistance, which may potentially explain the treatment-related weight gain [54].
Antidepressants
There are more than three placebo-controlled trials each supporting the superiority of the tricyclic antidepressant amitriptyline and of the selective serotonin reuptake inhibitor (SSRI) fluoxetine over placebo for migraine prevention [55]. This effect occurs in the absence of depression and antimigraine effects are typically observed at doses lower than in depression. Less evidence is available for other antidepressants, although at least other tricyclics and the selective serotonin-norepinephrine reuptake inhibitor venlafaxine [56] are probably also effective for migraine prophylaxis.
Amitriptyline is a mixed serotonin-norepinephrine reuptake pump inhibitor and thereby thought to facilitate descending noxious inhibition, i.e., endogenous pain control mechanisms descending from the brainstem to the trigeminal nucleus caudalis and the spinal cord. Alpha2-adrenoceptor blockade has been shown to block the antinociceptive effect of amitriptyline [57] and hence at least part of amitriptyline’s efficacy is thought to be mediated by α2-agonism, but multiple other channel and receptor effects of amitriptyline are known. As such, amitriptyline is thought to act as a sodium channel blocker and also has antimuscarinic and antihistaminic effects. There is also an interaction with the endogenous adenosine system and it has also been shown to suppress cortical spreading depression [11]. As with other preventive migraine medications, it remains unclear which mechanism is key and probably the multiplicity of synergistic effects in multiple pathways explains the clinical efficacy (as well as the broad side-effect profile).
Fluoxetine is a more targeted substance that (relatively) selectively blocks serotonin reuptake from the synaptic cleft leading to increased serotonin levels. Noradrenaline reuptake is inhibited only at higher doses. Furthermore, fluoxetine is a competitive, reversible 5-HT2C receptor blocker [58]. The 5-HT2C mechanism is shared with the old antiheadache drug methysergide and a receptor gene polymorphisms of 5-HT2C has been shown to modulate migraine susceptibility (in a Turkish population) [59]. Hence this receptor blockade may explain (part) of the efficacy of fluoxetine.
Onabotulinumtoxin A
Trials investigating the efficacy of onabotulinumtoxin A in episodic migraine and chronic daily headache have yielded inconclusive results, partly because of methodological limitations regarding the injection paradigm and the dose [60–64]. The efficacy of onabotulinumtoxin A has been demonstrated for chronic migraine in the clinical programme called REsearch Evaluating Migraine Prophylaxis Therapy (PREEMPT) [65–67]. In the two phase III trials of the PREEMPT program (PREEMPT1 and 2), a minimum dose of 155 U (up to a maximum allowed dose of 195 U) of onabotulinumtoxin A was injected into head and neck muscles of each patient. Nowadays, onabotulinumtoxin A is marketed in several countries with an indication for chronic migraine.
Onabotulinum toxin A (BoNT-A) is a protein complex produced by the gram-positive, anaerobic bacterium Clostridium botulinum [68]. There are seven serotypes of botulinum toxin [68], the serotype A has been used in human therapy since 1980 when Scott proposed BoNT-A injection into extraocular muscles as an alternative to strabismus surgery [69]. The first evidence for an effect of BoNT on migraine was the serendipitous observation of an improvement of migraine in patients treated with BoNT for hyperfunctional lines of the face made in the late 1990s by a plastic surgeon [70]. In the subsequent years, several exploratory studies with BoNT-A using heterogeneous doses and injection paradigms were carried out in migraine and tension-type headache and chronic daily headache with inconclusive and mostly negative results [62, 63, 71–74]. A subgroup analysis of 228 CDH patients enrolled in the randomized placebo-controlled study by Mathew et al. showed that the patients without prophylactic medication at the date of study enrolment experienced a significant reduction in the number of headaches in a 30-day period. So the authors concluded that onabotulinumtoxin A was effective in the treatment of patients with CDH, who do not receive other prophylactic medication [75].
Based on these preliminary studies, a series of studies called the Placebo-controlled phase III Research Evaluating Migraine Prophylaxis Therapy (PREEMPT), treated patients with chronic migraine who would receive BoNT-A at fixed sites (procerus, corrugator, frontalis, temporalis, occipitalis, cervical paraspinal muscles and trapezius) in fixed doses (5 units per site for a total of 155 units), including those with or without medication overuse. The studies revealed a significant reduction in headache and migraine days, moderate/severe headache days and cumulative headache hours on headache days as well as in patients’ functioning, vitality, psychological distress, and overall health-related quality of life [65–67].
The exact mechanism by which BoNT-A reduces migraine days in chronic migraineurs is not yet fully understood, although an increasing body of evidence suggests the dominant hypothesis that the toxin exerts its antinociceptive action via peripheral mechanisms [76]. While it was initially thought that BoNT-A may exert positive effects in primary headache disorders simply by the relaxation of muscles in the head and neck region, it turned out that BoNT-A is rather ineffective in disorders, where muscle tension may play a key role such as tension-type headache. It is today believed that BoNT-A acts in migraine by directly inhibiting peripheral sensitization by attenuating neuropeptide and neurotransmitter exocytosis from peripheral sensory neurons, thereby indirectly reducing central sensitization, the hallmark of chronic migraine [76]. The effectiveness of BoNT-A in chronic migraine, as opposed to negative findings in episodic migraine, may relate to the fact that sensitization phenomena play a more important role in chronic versus episodic migraine.
Neuronal stimulation initiates a series of intracellular events that leads to the fusion of a neuropeptide and/or neurotransmitter containing vesicle with the nerve cell membrane. This process is facilitated by interaction between proteins on the vesicle, the so-called vesicle-associated membrane protein (VAMP/synaptobrevin) and on the internal membrane surface, the synaptosomal associated protein (SNAP-25), which together form the soluble N-ethylmaleimide–sensitive factor attachment protein receptor (SNARE) complex [77–79]. The SNARE complex is instrumental to vesicular trafficking and to vesicle fusion with the membrane. BoNT-A adheres to the nerve cells and enters inside through the endocytosis mechanism and inhibits fusion of intracellular vesicles with the nerve membrane [80] by cleaving SNAP-25 [81]. Once intraneuronal vesicular fusion is impaired because of BONT-A, neuropeptide release is inhibited and receptors are downregulated [77, 79]. A large number of experiments in vitro and in vivo showed that [82] botulinum toxin could also inhibit the release of a variety of neurotransmitters, including glutamate, GABA, aspartate, catecholamines, dopamine, and monoamine neurotransmitters [83–85]. All these neurotransmitters and neuropeptides are key signaling molecules in chronic migraine and it is therefore likely that, altogether, these mechanisms are important in the current notion of migraine prevention because they are capable of disrupting the cascade of events that leads to peripheral and central sensitization, mainly via the block of release of inflammatory neuropeptides from stimulated trigeminal sensory neurons [76–78, 86]. This mechanism of action is supported by clinical studies demonstrating suppression of cutaneous allodynia, an indicator of central sensitization, after onabotulinumtoxin A injection in the periorbital skin [87, 88].
In this framework, it is relevant to note that, in an animal model, administration of BoNT-A in close proximity of sensory fibers that bifurcate from parent axons of intracranial meningeal nociceptors and reach extracranial tissues by crossing the calvarial bones through the sutures [89–91] proved more effective in inhibiting the responses of nociceptors to topical capsaicin than when it was injected into muscles and sutures [92].
Other Compounds
ACE and ARB Agents
There are two randomized controlled studies suggesting an effect of candesartan at a dose of 16 mg per day in migraine prevention with comparable efficacy to propranolol. Candesartan is an angiotensin II receptor, type 1 blocking (ARB) agent and thus it reduces the effect of angiotensin, which has several effects that may be relevant to migraine, such as increased sympathetic discharge, and adrenal medullary catecholamine release. Molecular biology confirmed the components of the cerebral renin–angiotensin system (RAS) and their location in the brain [93]. RAS may play a role also in migraine pathogenesis. Brain RAS could act independently of the peripheral RAS, influencing neuronal, astrocytic, and endothelial cell activity [94].
Lisinopril, an angiotensin-converting enzyme inhibitor (ACEI), has been shown to be prophylactically effective at a dose of 10 mg twice daily in a small, double-blind, placebo-controlled crossover trial [95]. ACEIs modulate vasoreactivity, alter sympathetic tone, and promote degradation of proinflammatory factors, such as substance P, enkephalin, and bradykinin [95, 96]. An additional mechanism of action for ACEI in migraine could be modulation of the endogenous opioid system. As a matter of fact, the effect of this class of drugs has been reported to be blocked by antagonizing opioid receptors [97]. In addition to its traditional role as a circulating hormone, angiotensin is also involved in local functions through activity of tissue renin-angiotensin system that occur in many organs, including the brain (both systemic and presumptive neurally derived angiotensin) [98]. Angiotensin II receptors are located on neurones, astrocytes, and endothelium, and the hormonal effect is mainly mediated through the angiotensin II type 1 receptor. Brain angiotensin II type 2 receptors are located in areas predominantly involved in sensory processing, but their function remains to be clarified [99]. The RAS modulates cerebrovascular flow [100], and influences fluid and electrolyte homeostasis, autonomic pathways, and neuroendocrine systems. Angiotensin II modulates potassium channels and calcium activity in cells [98], increases the concentration of dopamine and of the main serotonin metabolite, 5-hydroxyindoleacetic acid, and activates nuclear factor kB, which is associated with increased expression of inducible nitric oxide synthase [101, 102].
Feverfew (Tanacetum parthenium L.)
Double-blind placebo-controlled studies suggested efficacy of feverfew in migraine prophylaxis, although it is usually not considered first choice as antimigraine preventive drug for tolerability (and safety) reasons [103–105]. The most frequent adverse events reported were sore mouth and tongue for the presence of ulcers, swollen lips, loss of taste, abdominal pain, and gastrointestinal disturbances [105]. Moreover, the safety of long-term use of feverfew needs to be established [106].
Feverfew is a member of the Asteracee family, empirically used as herbal remedy for migraine. Parthenolide, a sesquiterpene lactone, appears to be feverfew’s active ingredient and has multiple actions in the central nervous system. Several of its properties suggest a potential mechanism of action in migraine prevention, including evidence to suggest that parthenolide inhibits Fos-induced activation of the nucleus trigeminalis caudalis [107], a nucleus central to migraine pathogenesis, and evidence for partial agonist activity of parthenolide at TRPA1 channels [108], which have been implicated in migraine pathogenesis [109]. It has also been demonstrated that oral administration of feverfew extract leads to significant antinociceptive and anti-inflammatory effects in experimental models [110]. This action could be explained by the regulation of degradation of the inhibitory protein of NF-kB and of expression of inducible NOS by parthenolide, the active constituent of feverfew [101]. Moreover, there is evidence that feverfew inhibits human blood platelet aggregation in vitro, probably through the protein kinase C pathway [111]. Feverfew could also exert modulation of 5-HT neurotransmission, antagonizing the effects mediated by 5-HT2B and 5-HT2A receptors implicated in the plasticity changes underlying chronic headache [112–114].
Butterbur (Petasites hybridus)
Some promising results support the efficacy of this extract in reducing migraine attacks [115–117]. Nevertheless, a recent systematic review shows that there is only moderate evidence for its effectiveness as an antimigraine drug [118].
Butterbur is a shrub with a long history of use for medicinal purposes. It demonstrates a variety of properties that render it a candidate for migraine prophylaxis, including anti-inflammatory action through inhibition of cyclooxygenase-2 [119]. Moreover, the co-active ingredients petasins could exert a block of Ca2+ channels [120–123].
Riboflavin (Vitamin B2)
A placebo-controlled, double-blind trial showed that a high dose of riboflavin can be effective in migraine prophylaxis [124]. This positive result was confirmed by an open-label study [125]. Riboflavin was comparable to propranolol after 3 and 6 months of administration in an RCT assessing its efficacy for adult migraine prophylaxis [126].
Riboflavin is a vitamin that plays an important role in cellular energy production through its two active coenzyme forms that are involved in oxidation-reduction reactions during a variety of cellular processes [127]. Brain energy metabolism has been found to be abnormal in migraine headache [128]. Riboflavin catalyzes the activity of flavoenzymes in the mitochondrial respiratory chain and improves the clinical and biochemical abnormalities in patients with inborn errors of mitochondrial metabolism [129, 130]. As mitochondrial deficits are suspected in some patients with migraine headache, this could reflect a mechanism of action in migraine prevention.
Magnesium
While a role for magnesium-related mechanisms can be hypothesized in migraine pathogenesis, limited evidence has indicated efficacy in migraine prophylaxis. In fact, several studies suggested a deficiency of Mg2+ in the central nervous system of migraineurs [131–133]. A Mg2+ deficit in migraine was also described at a peripheral level, in serum and blood cells [134, 135].
Clinical trials evaluated the potential usefulness of oral Mg2+ in migraine prevention, showing conflicting results [136, 137]. Three separate RCTs with varying methodologies, magnesium dosages, and formulations have found oral magnesium to be effective for migraine prophylaxis in adults [138–140]. Another RCT contradicted these findings and found oral magnesium to be no different than placebo on interim analysis in a sample of refractory migraine patients and therefore halted recruitment prior to achieving the planned sample size [141]. A possible effect of Mg2+ supplementation was demonstrated for short-term prophylaxis of menstrual migraine [142].
Magnesium is found ubiquitous in the human body and is an essential cofactor for more than 350 enzymes. It plays an important role in a multitude of biological processes, some of which might be linked to migraine pathogenesis [143]. It could modulate mitochondrial oxidative phosphorylation, 5-HT neurotransmission, and the NO system [144]. Moreover, it could regulate the uptake of glutamate into astrocytes and interfere with NMDA receptor function [144] and thereby improve migraine.
Conclusions
Several compounds with heterogenous mechanisms of action have been studied for the prevention of migraine during the past decades with some of them being licensed for this indication. Potential mechanisms of action include a reduction of excitatory glutamatergic neurotransmission by inhibition of sodium and calcium channels, facilitation of inhibitory GABAergic neurotransmission, reduction of central sensitization, and suppression of cortical spreading depression, but a number of other mechanism may be at play. While the migraine preventive properties of the available compounds were mostly discovered post hoc by circumstantial evidence, the advent of CGRP antibodies will for the first time allow a treatment tailored to the disease after systematic drug development from bench to bedside (Reuter et al. Neurotherapeutics, in this issue).
Electronic supplementary material
(PDF 511 kb)
Acknowledgments
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
References
- 1.Sprenger T, Goadsby PJ. Migraine pathogenesis and state of pharmacological treatment options. BMC Med. 2009;7:71. doi: 10.1186/1741-7015-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aurora SK, Brin MF. Chronic migraine: an update on physiology, imaging, and the mechanism of action of two available pharmacologic therapies. Headache. 2017;57(1):109–25. doi: 10.1111/head.12999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolay H, Reuter U, Dunn AK, Huang Z, Boas DA, Moskowitz MA. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med. 2002;8(2):136–42. doi: 10.1038/nm0202-136. [DOI] [PubMed] [Google Scholar]
- 4.Wolthausen J, Sternberg S, Gerloff C, May A. Are cortical spreading depression and headache in migraine causally linked? Cephalalgia. 2009;29(2):244–9. doi: 10.1111/j.1468-2982.2008.01713.x. [DOI] [PubMed] [Google Scholar]
- 5.Niazi AK, Andelova M, Sprenger T. Is the migrainous brain normal outside of acute attacks? Lessons learned from psychophysical, neurochemical and functional neuroimaging studies. Expert Rev Neurother. 2013;13(9):1061–7. doi: 10.1586/14737175.2013.835587. [DOI] [PubMed] [Google Scholar]
- 6.Goadsby PJ, Sprenger T. Current practice and future directions in the prevention and acute management of migraine. Lancet Neurol. 2010;9(3):285–98. doi: 10.1016/S1474-4422(10)70005-3. [DOI] [PubMed] [Google Scholar]
- 7.Evers S, Afra J, Frese A, Goadsby PJ, Linde M, May A, et al. EFNS guideline on the drug treatment of migraine--revised report of an EFNS task force. Eur J Neurol. 2009;16(9):968–81. doi: 10.1111/j.1468-1331.2009.02748.x. [DOI] [PubMed] [Google Scholar]
- 8.Kropp P, Meyer B, Dresler T, Fritsche G, Gaul C, Niederberger U, et al. [Relaxation techniques and behavioural therapy for the treatment of migraine : guidelines from the German Migraine and Headache Society]. Schmerz 2017. [DOI] [PubMed]
- 9.Puledda F, Goadsby PJ. An update on non-pharmacological neuromodulation for the acute and preventive treatment of migraine. Headache. 2017;57(4):685–91. doi: 10.1111/head.13069. [DOI] [PubMed] [Google Scholar]
- 10.Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68(5):343–9. doi: 10.1212/01.wnl.0000252808.97649.21. [DOI] [PubMed] [Google Scholar]
- 11.Ayata C, Jin H, Kudo C, Dalkara T, Moskowitz MA. Suppression of cortical spreading depression in migraine prophylaxis. Ann Neurol. 2006;59(4):652–61. doi: 10.1002/ana.20778. [DOI] [PubMed] [Google Scholar]
- 12.Li F, Qiu E, Dong Z, Liu R, Wu S, Yu S. Protection of flunarizine on cerebral mitochondria injury induced by cortical spreading depression under hypoxic conditions. J Headache Pain. 2011;12(1):47–53. doi: 10.1007/s10194-011-0300-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tozzi A, de Iure A, Di Filippo M, Costa C, Caproni S, Pisani A, et al. Critical role of calcitonin gene-related peptide receptors in cortical spreading depression. Proc Natl Acad Sci U S A. 2012;109(46):18985–90. doi: 10.1073/pnas.1215435109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dolly JO, Aoki KR. The structure and mode of action of different botulinum toxins. Eur J Neurol. 2006;13(Suppl 4):1–9. doi: 10.1111/j.1468-1331.2006.01648.x. [DOI] [PubMed] [Google Scholar]
- 15.Boyer N, Signoret-Genest J, Artola A, Dallel R, Monconduit L. Propranolol treatment prevents chronic central sensitization induced by repeated dural stimulation. Pain. 2017;158(10):2025–34. doi: 10.1097/j.pain.0000000000001007. [DOI] [PubMed] [Google Scholar]
- 16.Cernuda-Morollon E, Ramon C, Martinez-Camblor P, Serrano-Pertierra E, Larrosa D, Pascual J. OnabotulinumtoxinA decreases interictal CGRP plasma levels in patients with chronic migraine. Pain. 2015;156(5):820–4. doi: 10.1097/j.pain.0000000000000119. [DOI] [PubMed] [Google Scholar]
- 17.Hirfanoglu T, Serdaroglu A, Gulbahar O, Cansu A. Prophylactic drugs and cytokine and leptin levels in children with migraine. Pediatr Neurol. 2009;41(4):281–7. doi: 10.1016/j.pediatrneurol.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 18.Bangalore S, Messerli FH, Kostis JB, Pepine CJ. Cardiovascular protection using beta-blockers: a critical review of the evidence. J Am Coll Cardiol. 2007;50(7):563–72. doi: 10.1016/j.jacc.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 19.Limmroth V, Michel MC. The prevention of migraine: a critical review with special emphasis on beta-adrenoceptor blockers. Br J Clin Pharmacol. 2001;52(3):237–43. doi: 10.1046/j.0306-5251.2001.01459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tfelt-Hansen P. Efficacy of beta-blockers in migraine. A critical review. Cephalalgia. 1986;6(Suppl 5):15–24. doi: 10.1177/03331024860060S502. [DOI] [PubMed] [Google Scholar]
- 21.Schellenberg R, Lichtenthal A, Wohling H, Graf C, Brixius K. Nebivolol and metoprolol for treating migraine: an advance on beta-blocker treatment? Headache 2008;48(1):118–25. [DOI] [PubMed]
- 22.van de Ven LL, Franke CL, Koehler PJ. Prophylactic treatment of migraine with bisoprolol: a placebo-controlled study. Cephalalgia. 1997;17(5):596–9. doi: 10.1046/j.1468-2982.1997.1705596.x. [DOI] [PubMed] [Google Scholar]
- 23.Buchanan TM, Ramadan NM. Prophylactic pharmacotherapy for migraine headaches. Semin Neurol. 2006;26(2):188–98. doi: 10.1055/s-2006-939919. [DOI] [PubMed] [Google Scholar]
- 24.Hanbauer I, Kopin IJ, Guidotti A, Costa E. Induction of tyrosine hydroxylase elicited by beta adrenergic receptor agonists in normal and decentralized sympathetic ganglia: role of cyclic 3′,5′ - adenosine monophosphate. J Pharmacol Exp Ther. 1975;193(1):95–104. [PubMed] [Google Scholar]
- 25.Hieble JP. Adrenoceptor subclassification: an approach to improved cardiovascular therapeutics. Pharm Acta Helv. 2000;74(2–3):163–71. doi: 10.1016/S0031-6865(99)00030-8. [DOI] [PubMed] [Google Scholar]
- 26.Xiao C, Zhou C, Atlas G, Delphin E, Ye JH. Labetalol facilitates GABAergic transmission to rat periaqueductal gray neurons via antagonizing beta1-adrenergic receptors--a possible mechanism underlying labetalol-induced analgesia. Brain Res. 2008;1198:34–43. doi: 10.1016/j.brainres.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koella WP. CNS-related (side-)effects of beta-blockers with special reference to mechanisms of action. Eur J Clin Pharmacol. 1985;28(Suppl):55–63. doi: 10.1007/BF00543711. [DOI] [PubMed] [Google Scholar]
- 28.Ablad B, Dahlof C. Migraine and beta-blockade: modulation of sympathetic neurotransmission. Cephalalgia 1986;6 Suppl 5:7–13. [DOI] [PubMed]
- 29.Kalkman HO. Is migraine prophylactic activity caused by 5-HT2B or 5-HT2C receptor blockade? Life Sci. 1994;54(10):641–4. doi: 10.1016/0024-3205(94)00546-X. [DOI] [PubMed] [Google Scholar]
- 30.Chugani DC, Niimura K, Chaturvedi S, Muzik O, Fakhouri M, Lee ML, et al. Increased brain serotonin synthesis in migraine. Neurology. 1999;53(7):1473–9. doi: 10.1212/WNL.53.7.1473. [DOI] [PubMed] [Google Scholar]
- 31.Ramadan NM. Prophylactic migraine therapy: mechanisms and evidence. Curr Pain Headache Rep. 2004;8(2):91–5. doi: 10.1007/s11916-004-0022-z. [DOI] [PubMed] [Google Scholar]
- 32.Diener HC, Scholz E, Dichgans J, Gerber WD, Jack A, Bille A, et al. Central effects of drugs used in migraine prophylaxis evaluated by visual evoked potentials. Ann Neurol. 1989;25(2):125–30. doi: 10.1002/ana.410250204. [DOI] [PubMed] [Google Scholar]
- 33.Sandor PS, Afra J, Ambrosini A, Schoenen J. Prophylactic treatment of migraine with beta-blockers and riboflavin: differential effects on the intensity dependence of auditory evoked cortical potentials. Headache. 2000;40(1):30–5. doi: 10.1046/j.1526-4610.2000.00005.x. [DOI] [PubMed] [Google Scholar]
- 34.Hegerl U, Juckel G. Intensity dependence of auditory evoked potentials as an indicator of central serotonergic neurotransmission: a new hypothesis. Biol Psychiatry. 1993;33(3):173–87. doi: 10.1016/0006-3223(93)90137-3. [DOI] [PubMed] [Google Scholar]
- 35.Siniatchkin M, Andrasik F, Kropp P, Niederberger U, Strenge H, Averkina N, et al. Central mechanisms of controlled-release metoprolol in migraine: a double-blind, placebo-controlled study. Cephalalgia. 2007;27(9):1024–32. doi: 10.1111/j.1468-2982.2007.01377.x. [DOI] [PubMed] [Google Scholar]
- 36.Aston-Jones G, Chen S, Zhu Y, Oshinsky ML. A neural circuit for circadian regulation of arousal. Nat Neurosci. 2001;4(7):732–8. doi: 10.1038/89522. [DOI] [PubMed] [Google Scholar]
- 37.Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain research. Brain Res Rev. 2003;42(1):33–84. doi: 10.1016/S0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- 38.Wiedemann M, de Lima VM, Hanke W. Effects of antimigraine drugs on retinal spreading depression. Naunyn Schmiedeberg's Arch Pharmacol. 1996;353(5):552–6. doi: 10.1007/BF00169175. [DOI] [PubMed] [Google Scholar]
- 39.Richter F, Mikulik O, Ebersberger A, Schaible HG. Noradrenergic agonists and antagonists influence migration of cortical spreading depression in rat-a possible mechanism of migraine prophylaxis and prevention of postischemic neuronal damage. J Cereb Blood Flow Metab. 2005;25(9):1225–35. doi: 10.1038/sj.jcbfm.9600120. [DOI] [PubMed] [Google Scholar]
- 40.Kaube H, Goadsby PJ. Anti-migraine compounds fail to modulate the propagation of cortical spreading depression in the cat. Eur Neurol. 1994;34(1):30–5. doi: 10.1159/000117004. [DOI] [PubMed] [Google Scholar]
- 41.Shields KG, Goadsby PJ. Propranolol modulates trigeminovascular responses in thalamic ventroposteromedial nucleus: a role in migraine? Brain J Neurol. 2005;128(Pt 1):86–97. doi: 10.1093/brain/awh298. [DOI] [PubMed] [Google Scholar]
- 42.Silberstein S, Saper J, Berenson F, Somogyi M, McCague K, D'Souza J. Oxcarbazepine in migraine headache: a double-blind, randomized, placebo-controlled study. Neurology. 2008;70(7):548–55. doi: 10.1212/01.wnl.0000297551.27191.70. [DOI] [PubMed] [Google Scholar]
- 43.Hoffmann U, Dilekoz E, Kudo C, Ayata C. Oxcarbazepine does not suppress cortical spreading depression. Cephalalgia. 2011;31(5):537–42. doi: 10.1177/0333102410388433. [DOI] [PubMed] [Google Scholar]
- 44.Cutrer FM. Antiepileptic drugs: how they work in headache. Headache. 2001;41(Suppl 1):S3–10. doi: 10.1046/j.1526-4610.2001.01154-2.x. [DOI] [PubMed] [Google Scholar]
- 45.Shank RP, Doose DR, Streeter AJ, Bialer M. Plasma and whole blood pharmacokinetics of topiramate: the role of carbonic anhydrase. Epilepsy Res. 2005;63(2–3):103–12. doi: 10.1016/j.eplepsyres.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 46.Durham PL, Niemann C, Cady R. Repression of stimulated calcitonin gene-related peptide secretion by topiramate. Headache. 2006;46(8):1291–5. doi: 10.1111/j.1526-4610.2006.00538.x. [DOI] [PubMed] [Google Scholar]
- 47.Garcia-Estevez DA, Pardo-Parrado M, Silvarrey-Rodriguez S. Frequent episodic migraine and calcitonin gene-related peptide. Influence of treatment with topiramate and zonisamide on levels of the peptide. Rev Neurol. 2017;65(4):153–6. [PubMed] [Google Scholar]
- 48.Chen G, Manji HK, Hawver DB, Wright CB, Potter WZ. Chronic sodium valproate selectively decreases protein kinase C alpha and epsilon in vitro. J Neurochem. 1994;63(6):2361–4. doi: 10.1046/j.1471-4159.1994.63062361.x. [DOI] [PubMed] [Google Scholar]
- 49.Li Y, Zhang Q, Qi D, Zhang L, Yi L, Li Q, et al. Valproate ameliorates nitroglycerin-induced migraine in trigeminal nucleus caudalis in rats through inhibition of NF-small ka, CyrillicB. J Headache Pain. 2016;17:49. doi: 10.1186/s10194-016-0631-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Solomon GD. Comparative efficacy of calcium antagonist drugs in the prophylaxis of migraine. Headache. 1985;25(7):368–71. doi: 10.1111/j.1526-4610.1985.hed2507368.x. [DOI] [PubMed] [Google Scholar]
- 51.Ye Q, Yan LY, Xue LJ, Wang Q, Zhou ZK, Xiao H, et al. Flunarizine blocks voltage-gated Na(+) and Ca(2+) currents in cultured rat cortical neurons: a possible locus of action in the prevention of migraine. Neurosci Lett. 2011;487(3):394–9. doi: 10.1016/j.neulet.2010.10.064. [DOI] [PubMed] [Google Scholar]
- 52.Ye Q, Wang Q, Yan LY, Wu WH, Liu S, Xiao H, et al. Flunarizine inhibits sensory neuron excitability by blocking voltage-gated Na+ and Ca2+ currents in trigeminal ganglion neurons. Chin Med J 2011;124(17):2649–55. [PubMed]
- 53.Bisol LW, Brunstein MG, Ottoni GL, Ramos FL, Borba DL, Daltio CS, et al. Is flunarizine a long-acting oral atypical antipsychotic? A randomized clinical trial versus haloperidol for the treatment of schizophrenia. J Clin Psychiatry. 2008;69(10):1572–9. doi: 10.4088/JCP.v69n1007. [DOI] [PubMed] [Google Scholar]
- 54.Berilgen MS, Bulut S, Gonen M, Tekatas A, Dag E, Mungen B. Comparison of the effects of amitriptyline and flunarizine on weight gain and serum leptin, C peptide and insulin levels when used as migraine preventive treatment. Cephalalgia. 2005;25(11):1048–53. doi: 10.1111/j.1468-2982.2005.00956.x. [DOI] [PubMed] [Google Scholar]
- 55.Jackson JL, Cogbill E, Santana-Davila R, Eldredge C, Collier W, Gradall A, et al. A comparative effectiveness meta-analysis of drugs for the prophylaxis of migraine headache. PLoS One. 2015;10(7):e0130733. doi: 10.1371/journal.pone.0130733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ozyalcin SN, Talu GK, Kiziltan E, Yucel B, Ertas M, Disci R. The efficacy and safety of venlafaxine in the prophylaxis of migraine. Headache. 2005;45(2):144–52. doi: 10.1111/j.1526-4610.2005.05029.x. [DOI] [PubMed] [Google Scholar]
- 57.Gray AM, Pache DM, Sewell RD. Do alpha2-adrenoceptors play an integral role in the antinociceptive mechanism of action of antidepressant compounds? Eur J Pharmacol. 1999;378(2):161–8. doi: 10.1016/S0014-2999(99)00464-1. [DOI] [PubMed] [Google Scholar]
- 58.Ni YG, Miledi R. Blockage of 5HT2C serotonin receptors by fluoxetine (Prozac) Proc Natl Acad Sci U S A. 1997;94(5):2036–40. doi: 10.1073/pnas.94.5.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yucel Y, Coskun S, Cengiz B, Ozdemir HH, Uzar E, Cim A, et al. Association of polymorphisms within the serotonin receptor genes 5-HTR1A, 5-HTR1B, 5-HTR2A and 5-HTR2C and migraine susceptibility in a Turkish population. Clin Psychopharmacol Neurosci. 2016;14(3):250–5. doi: 10.9758/cpn.2016.14.3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aurora SK, Gawel M, Brandes JL, Pokta S, Vandenburgh AM, Group BNAEMS Botulinum toxin type a prophylactic treatment of episodic migraine: a randomized, double-blind, placebo-controlled exploratory study. Headache. 2007;47(4):486–99. doi: 10.1111/j.1526-4610.2006.00624.x. [DOI] [PubMed] [Google Scholar]
- 61.Freitag FG, Diamond S, Diamond M, Urban G. Botulinum toxin type A in the treatment of chronic migraine without medication overuse. Headache. 2008;48(2):201–9. doi: 10.1111/j.1526-4610.2007.00963.x. [DOI] [PubMed] [Google Scholar]
- 62.Mathew NT, Frishberg BM, Gawel M, Dimitrova R, Gibson J, Turkel C, et al. Botulinum toxin type A (BOTOX) for the prophylactic treatment of chronic daily headache: a randomized, double-blind, placebo-controlled trial. Headache. 2005;45(4):293–307. doi: 10.1111/j.1526-4610.2005.05066.x. [DOI] [PubMed] [Google Scholar]
- 63.Silberstein S, Mathew N, Saper J, Jenkins S. Botulinum toxin type A as a migraine preventive treatment. For the BOTOX Migraine Clinical Research Group. Headache 2000;40(6):445–50. [DOI] [PubMed]
- 64.Silberstein SD, Stark SR, Lucas SM, Christie SN, Degryse RE, Turkel CC, et al. Botulinum toxin type A for the prophylactic treatment of chronic daily headache: a randomized, double-blind, placebo-controlled trial. Mayo Clin Proc. 2005;80(9):1126–37. doi: 10.4065/80.9.1126. [DOI] [PubMed] [Google Scholar]
- 65.Aurora SK, Dodick DW, Turkel CC, DeGryse RE, Silberstein SD, Lipton RB, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia. 2010;30(7):793–803. doi: 10.1177/0333102410364676. [DOI] [PubMed] [Google Scholar]
- 66.Diener HC, Dodick DW, Aurora SK, Turkel CC, DeGryse RE, Lipton RB, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010;30(7):804–14. doi: 10.1177/0333102410364677. [DOI] [PubMed] [Google Scholar]
- 67.Dodick DW, Turkel CC, DeGryse RE, Aurora SK, Silberstein SD, Lipton RB, et al. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache. 2010;50(6):921–36. doi: 10.1111/j.1526-4610.2010.01678.x. [DOI] [PubMed] [Google Scholar]
- 68.Erbguth FJ. From poison to remedy: the chequered history of botulinum toxin. J Neural Transm. 2008;115(4):559–65. doi: 10.1007/s00702-007-0728-2. [DOI] [PubMed] [Google Scholar]
- 69.Scott AB. Botulinum toxin injection into extraocular muscles as an alternative to strabismus surgery. Ophthalmology. 1980;87(10):1044–9. doi: 10.1016/S0161-6420(80)35127-0. [DOI] [PubMed] [Google Scholar]
- 70.Binder WJ, Blitzer A, Brin MF. Treatment of hyperfunctional lines of the face with botulinum toxin A. Dermatol Surg. 1998;24(11):1198–205. doi: 10.1111/j.1524-4725.1998.tb04098.x. [DOI] [PubMed] [Google Scholar]
- 71.Silberstein SD, Gobel H, Jensen R, Elkind AH, Degryse R, Walcott JM, et al. Botulinum toxin type A in the prophylactic treatment of chronic tension-type headache: a multicentre, double-blind, randomized, placebo-controlled, parallel-group study. Cephalalgia. 2006;26(7):790–800. doi: 10.1111/j.1468-2982.2006.01114.x. [DOI] [PubMed] [Google Scholar]
- 72.Ondo WG, Vuong KD, Derman HS. Botulinum toxin A for chronic daily headache: a randomized, placebo-controlled, parallel design study. Cephalalgia. 2004;24(1):60–5. doi: 10.1111/j.1468-2982.2004.00641.x. [DOI] [PubMed] [Google Scholar]
- 73.Jackson JL, Kuriyama A, Hayashino Y. Botulinum toxin A for prophylactic treatment of migraine and tension headaches in adults: a meta-analysis. JAMA. 2012;307(16):1736–45. doi: 10.1001/jama.2012.505. [DOI] [PubMed] [Google Scholar]
- 74.Gaul C, Holle-Lee D, Straube A. Botulinum toxin type A in headache treatment: established and experimental indications. Nervenarzt. 2016;87(8):853–9. doi: 10.1007/s00115-016-0138-5. [DOI] [PubMed] [Google Scholar]
- 75.Escher CM, Paracka L, Dressler D, Kollewe K. Botulinum toxin in the management of chronic migraine: clinical evidence and experience. Ther Adv Neurol Disord. 2017;10(2):127–35. doi: 10.1177/1756285616677005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aoki KR. Review of a proposed mechanism for the antinociceptive action of botulinum toxin type A. Neurotoxicology. 2005;26(5):785–93. doi: 10.1016/j.neuro.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 77.Burstein R, Zhang X, Levy D, Aoki KR, Brin MF. Selective inhibition of meningeal nociceptors by botulinum neurotoxin type A: therapeutic implications for migraine and other pains. Cephalalgia. 2014;34(11):853–69. doi: 10.1177/0333102414527648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aoki KR. Evidence for antinociceptive activity of botulinum toxin type A in pain management. Headache. 2003;43(Suppl 1):S9–15. doi: 10.1046/j.1526-4610.43.7s.3.x. [DOI] [PubMed] [Google Scholar]
- 79.Whitcup SM, Turkel CC, DeGryse RE, Brin MF. Development of onabotulinumtoxinA for chronic migraine. Ann N Y Acad Sci. 2014;1329:67–80. doi: 10.1111/nyas.12488. [DOI] [PubMed] [Google Scholar]
- 80.Simpson LL. The origin, structure, and pharmacological activity of botulinum toxin. Pharmacol Rev. 1981;33(3):155–88. [PubMed] [Google Scholar]
- 81.Blasi J, Chapman ER, Link E, Binz T, Yamasaki S, De Camilli P, et al. Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature. 1993;365(6442):160–3. doi: 10.1038/365160a0. [DOI] [PubMed] [Google Scholar]
- 82.Mustafa G, Anderson EM, Bokrand-Donatelli Y, Neubert JK, Caudle RM. Anti-nociceptive effect of a conjugate of substance P and light chain of botulinum neurotoxin type A. Pain. 2013;154(11):2547–53. doi: 10.1016/j.pain.2013.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Verderio C, Grumelli C, Raiteri L, Coco S, Paluzzi S, Caccin P, et al. Traffic of botulinum toxins A and E in excitatory and inhibitory neurons. Traffic. 2007;8(2):142–53. doi: 10.1111/j.1600-0854.2006.00520.x. [DOI] [PubMed] [Google Scholar]
- 84.Drinovac V, Bach-Rojecky L, Lackovic Z. Association of antinociceptive action of botulinum toxin type A with GABA-A receptor. J Neural Transm. 2014;121(6):665–9. doi: 10.1007/s00702-013-1150-6. [DOI] [PubMed] [Google Scholar]
- 85.Habermann E. Inhibition by tetanus and botulinum A toxin of the release of [3H]noradrenaline and [3H]GABA from rat brain homogenate. Experientia. 1988;44(3):224–6. doi: 10.1007/BF01941714. [DOI] [PubMed] [Google Scholar]
- 86.Aoki KR, Francis J. Updates on the antinociceptive mechanism hypothesis of botulinum toxin A. Parkinsonism & related disorders. 2011;17 Suppl 1:S28–33. [DOI] [PubMed]
- 87.Gazerani P, Pedersen NS, Staahl C, Drewes AM, Arendt-Nielsen L. Subcutaneous Botulinum toxin type A reduces capsaicin-induced trigeminal pain and vasomotor reactions in human skin. Pain. 2009;141(1–2):60–9. doi: 10.1016/j.pain.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 88.Gazerani P, Staahl C, Drewes AM, Arendt-Nielsen L. The effects of Botulinum toxin type A on capsaicin-evoked pain, flare, and secondary hyperalgesia in an experimental human model of trigeminal sensitization. Pain. 2006;122(3):315–25. doi: 10.1016/j.pain.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 89.Kosaras B, Jakubowski M, Kainz V, Burstein R. Sensory innervation of the calvarial bones of the mouse. J Comp Neurol. 2009;515(3):331–48. doi: 10.1002/cne.22049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schueler M, Messlinger K, Dux M, Neuhuber WL, De Col R. Extracranial projections of meningeal afferents and their impact on meningeal nociception and headache. Pain. 2013;154(9):1622–31. doi: 10.1016/j.pain.2013.04.040. [DOI] [PubMed] [Google Scholar]
- 91.Schueler M, Neuhuber WL, De Col R, Messlinger K. Innervation of rat and human dura mater and pericranial tissues in the parieto-temporal region by meningeal afferents. Headache. 2014;54(6):996–1009. doi: 10.1111/head.12371. [DOI] [PubMed] [Google Scholar]
- 92.Zhang X, Strassman AM, Novack V, Brin MF, Burstein R. Extracranial injections of botulinum neurotoxin type A inhibit intracranial meningeal nociceptors’ responses to stimulation of TRPV1 and TRPA1 channels: are we getting closer to solving this puzzle? Cephalalgia. 2016;36(9):875–86. doi: 10.1177/0333102416636843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Phillips MI, de Oliveira EM. Brain renin angiotensin in disease. J Mol Med. 2008;86(6):715–22. doi: 10.1007/s00109-008-0331-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tronvik E, Stovner LJ, Schrader H, Bovim G. Involvement of the renin-angiotensin system in migraine. Journal of hypertension supplement : official journal of the International Society of Hypertension. 2006;24(1):S139–43. [DOI] [PubMed]
- 95.Schrader H, Stovner LJ, Helde G, Sand T, Bovim G. Prophylactic treatment of migraine with angiotensin converting enzyme inhibitor (lisinopril): randomised, placebo controlled, crossover study. BMJ. 2001;322(7277):19–22. doi: 10.1136/bmj.322.7277.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ito H, Takemori K, Suzuki T. Role of angiotensin II type 1 receptor in the leucocytes and endothelial cells of brain microvessels in the pathogenesis of hypertensive cerebral injury. J Hypertens. 2001;19(3 Pt 2):591–7. doi: 10.1097/00004872-200103001-00011. [DOI] [PubMed] [Google Scholar]
- 97.Montastruc P, Dang-Tran L, Carvajal A, Rostin M, Montastruc JL. Naloxone reverses the effects of enalapril and enalaprilic acid on the pressor responses to afferent vagal stimulation. Neuropeptides. 1985;6(6):537–42. doi: 10.1016/0143-4179(85)90116-7. [DOI] [PubMed] [Google Scholar]
- 98.Allen AM, Moeller I, Jenkins TA, Zhuo J, Aldred GP, Chai SY, et al. Angiotensin receptors in the nervous system. Brain Res Bull. 1998;47(1):17–28. doi: 10.1016/S0361-9230(98)00039-2. [DOI] [PubMed] [Google Scholar]
- 99.Saavedra JM. Brain angiotensin II: new developments, unanswered questions and therapeutic opportunities. Cell Mol Neurobiol. 2005;25(3–4):485–512. doi: 10.1007/s10571-005-4011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nishimura Y, Ito T, Saavedra JM. Angiotensin II AT(1) blockade normalizes cerebrovascular autoregulation and reduces cerebral ischemia in spontaneously hypertensive rats. Stroke. 2000;31(10):2478–86. doi: 10.1161/01.STR.31.10.2478. [DOI] [PubMed] [Google Scholar]
- 101.Reuter U, Chiarugi A, Bolay H, Moskowitz MA. Nuclear factor-kappaB as a molecular target for migraine therapy. Ann Neurol. 2002;51(4):507–16. doi: 10.1002/ana.10159. [DOI] [PubMed] [Google Scholar]
- 102.Lorenzo O, Ruiz-Ortega M, Suzuki Y, Ruperez M, Esteban V, Sugaya T, et al. Angiotensin III activates nuclear transcription factor-kappaB in cultured mesangial cells mainly via AT(2) receptors: studies with AT(1) receptor-knockout mice. J Am Soc Nephrol. 2002;13(5):1162–71. doi: 10.1681/ASN.V1351162. [DOI] [PubMed] [Google Scholar]
- 103.Pfaffenrath V, Diener HC, Fischer M, Friede M, Henneicke-von Zepelin HH, Investigators The efficacy and safety of Tanacetum parthenium (feverfew) in migraine prophylaxis--a double-blind, multicentre, randomized placebo-controlled dose-response study. Cephalalgia. 2002;22(7):523–32. doi: 10.1046/j.1468-2982.2002.00396.x. [DOI] [PubMed] [Google Scholar]
- 104.Pittler MH, Ernst E. Feverfew for preventing migraine. Cochrane Database Syst Rev. 2004;1:CD002286. doi: 10.1002/14651858.CD002286.pub2. [DOI] [PubMed] [Google Scholar]
- 105.Diener HC, Pfaffenrath V, Schnitker J, Friede M, Henneicke-von Zepelin HH. Efficacy and safety of 6.25 mg t.i.d. feverfew CO2-extract (MIG-99) in migraine prevention—a randomized, double-blind, multicentre, placebo-controlled study. Cephalalgia. 2005;25(11):1031–41. doi: 10.1111/j.1468-2982.2005.00950.x. [DOI] [PubMed] [Google Scholar]
- 106.Johnson ES, Kadam NP, Hylands DM, Hylands PJ. Efficacy of feverfew as prophylactic treatment of migraine. Br Med J. 1985;291(6495):569–73. doi: 10.1136/bmj.291.6495.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tassorelli C, Greco R, Morazzoni P, Riva A, Sandrini G, Nappi G. Parthenolide is the component of tanacetum parthenium that inhibits nitroglycerin-induced Fos activation: studies in an animal model of migraine. Cephalalgia. 2005;25(8):612–21. doi: 10.1111/j.1468-2982.2005.00915.x. [DOI] [PubMed] [Google Scholar]
- 108.Materazzi S, Benemei S, Fusi C, Gualdani R, De Siena G, Vastani N, et al. Parthenolide inhibits nociception and neurogenic vasodilatation in the trigeminovascular system by targeting the TRPA1 channel. Pain. 2013;154(12):2750–8. doi: 10.1016/j.pain.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Benemei S, Fusi C, Trevisan G, Geppetti P. The TRPA1 channel in migraine mechanism and treatment. Br J Pharmacol. 2014;171(10):2552–67. doi: 10.1111/bph.12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jain NK, Kulkarni SK. Antinociceptive and anti-inflammatory effects of Tanacetum parthenium L. extract in mice and rats. J Ethnopharmacol. 1999;68(1–3):251–9. doi: 10.1016/S0378-8741(99)00115-4. [DOI] [PubMed] [Google Scholar]
- 111.Groenewegen WA, Heptinstall S. A comparison of the effects of an extract of feverfew and parthenolide, a component of feverfew, on human platelet activity in-vitro. J Pharm Pharmacol. 1990;42(8):553–7. doi: 10.1111/j.2042-7158.1990.tb07057.x. [DOI] [PubMed] [Google Scholar]
- 112.Mittra S, Datta A, Singh SK, Singh A. 5-Hydroxytryptamine-inhibiting property of Feverfew: role of parthenolide content. Acta Pharmacol Sin. 2000;21(12):1106–14. [PubMed] [Google Scholar]
- 113.Sommer C. Is serotonin hyperalgesic or analgesic? Curr Pain Headache Rep. 2006;10(2):101–6. doi: 10.1007/s11916-006-0020-4. [DOI] [PubMed] [Google Scholar]
- 114.Srikiatkhachorn A, Puangniyom S, Govitrapong P. Plasticity of 5-HT serotonin receptor in patients with analgesic-induced transformed migraine. Headache. 1998;38(7):534–9. doi: 10.1046/j.1526-4610.1998.3807534.x. [DOI] [PubMed] [Google Scholar]
- 115.Grossman W, Schmidramsl H. An extract of Petasites hybridus is effective in the prophylaxis of migraine. Alternative medicine review: a journal of clinical therapeutic. 2001;6(3):303–10. [PubMed] [Google Scholar]
- 116.Diener HC, Rahlfs VW, Danesch U. The first placebo-controlled trial of a special butterbur root extract for the prevention of migraine: reanalysis of efficacy criteria. Eur Neurol. 2004;51(2):89–97. doi: 10.1159/000076535. [DOI] [PubMed] [Google Scholar]
- 117.Lipton RB, Gobel H, Einhaupl KM, Wilks K, Mauskop A. Petasites hybridus root (butterbur) is an effective preventive treatment for migraine. Neurology. 2004;63(12):2240–4. doi: 10.1212/01.WNL.0000147290.68260.11. [DOI] [PubMed] [Google Scholar]
- 118.Agosti R, Duke RK, Chrubasik JE, Chrubasik S. Effectiveness of Petasites hybridus preparations in the prophylaxis of migraine: a systematic review. Phytomedicine. 2006;13(9–10):743–6. doi: 10.1016/j.phymed.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 119.Fiebich BL, Grozdeva M, Hess S, Hull M, Danesch U, Bodensieck A, et al. Petasites hybridus extracts in vitro inhibit COX-2 and PGE2 release by direct interaction with the enzyme and by preventing p42/44 MAP kinase activation in rat primary microglial cells. Planta Med. 2005;71(1):12–9. doi: 10.1055/s-2005-837744. [DOI] [PubMed] [Google Scholar]
- 120.Thomet OA, Wiesmann UN, Schapowal A, Bizer C, Simon HU. Role of petasin in the potential anti-inflammatory activity of a plant extract of petasites hybridus. Biochem Pharmacol. 2001;61(8):1041–7. doi: 10.1016/S0006-2952(01)00552-4. [DOI] [PubMed] [Google Scholar]
- 121.Wang GJ, Wu XC, Lin YL, Ren J, Shum AY, Wu YY, et al. Ca2+ channel blocking effect of iso-S-petasin in rat aortic smooth muscle cells. Eur J Pharmacol. 2002;445(3):239–45. doi: 10.1016/S0014-2999(02)01764-8. [DOI] [PubMed] [Google Scholar]
- 122.Wu SN, Chen H, Lin YL. The mechanism of inhibitory actions of S-petasin, a sesquiterpene of Petasites formosanus, on L-type calcium current in NG108-15 neuronal cells. Planta Med. 2003;69(2):118–24. doi: 10.1055/s-2003-37711. [DOI] [PubMed] [Google Scholar]
- 123.Wang GJ, Shum AY, Lin YL, Liao JF, Wu XC, Ren J, et al. Calcium channel blockade in vascular smooth muscle cells: major hypotensive mechanism of S-petasin, a hypotensive sesquiterpene from Petasites formosanus. J Pharmacol Exp Ther 2001;297(1):240–6. [PubMed]
- 124.Schoenen J, Jacquy J, Lenaerts M. Effectiveness of high-dose riboflavin in migraine prophylaxis. A randomized controlled trial. Neurology. 1998;50(2):466–70. doi: 10.1212/WNL.50.2.466. [DOI] [PubMed] [Google Scholar]
- 125.Boehnke C, Reuter U, Flach U, Schuh-Hofer S, Einhaupl KM, Arnold G. High-dose riboflavin treatment is efficacious in migraine prophylaxis: an open study in a tertiary care centre. Eur J Neurol. 2004;11(7):475–7. doi: 10.1111/j.1468-1331.2004.00813.x. [DOI] [PubMed] [Google Scholar]
- 126.Nambiar N, Aiyappa C, Srinivasa R. Oral riboflavin versus oral propranolol in migraine prophylaxis: an open label randomized controlled trial. Neurol Asia. 2011;16:223–9. [Google Scholar]
- 127.Riboflavin. Monograph. Altern Med Rev. 2008;13(4):334–40. [PubMed]
- 128.Meissner K, Fassler M, Rucker G, Kleijnen J, Hrobjartsson A, Schneider A, et al. Differential effectiveness of placebo treatments: a systematic review of migraine prophylaxis. JAMA Intern Med. 2013;173(21):1941–51. doi: 10.1001/jamainternmed.2013.10391. [DOI] [PubMed] [Google Scholar]
- 129.Gasparini CF, Sutherland HG, Griffiths LR. Studies on the pathophysiology and genetic basis of migraine. Curr Genomics. 2013;14(5):300–15. doi: 10.2174/13892029113149990007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Goadsby PJ, Holland PR, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev. 2017;97(2):553–622. doi: 10.1152/physrev.00034.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Welch KM, Ramadan NM. Mitochondria, magnesium and migraine. J Neurol Sci. 1995;134(1–2):9–14. doi: 10.1016/0022-510X(95)00196-1. [DOI] [PubMed] [Google Scholar]
- 132.Lodi R, Iotti S, Cortelli P, Pierangeli G, Cevoli S, Clementi V, et al. Deficient energy metabolism is associated with low free magnesium in the brains of patients with migraine and cluster headache. Brain Res Bull. 2001;54(4):437–41. doi: 10.1016/S0361-9230(01)00440-3. [DOI] [PubMed] [Google Scholar]
- 133.Boska MD, Welch KM, Barker PB, Nelson JA, Schultz L. Contrasts in cortical magnesium, phospholipid and energy metabolism between migraine syndromes. Neurology. 2002;58(8):1227–33. doi: 10.1212/WNL.58.8.1227. [DOI] [PubMed] [Google Scholar]
- 134.Mauskop A, Altura BT, Cracco RQ, Altura BM. Deficiency in serum ionized magnesium but not total magnesium in patients with migraines. Possible role of ICa2+/IMg2+ ratio. Headache. 1993;33(3):135–8. doi: 10.1111/j.1526-4610.1993.hed3303135.x. [DOI] [PubMed] [Google Scholar]
- 135.Sarchielli P, Coata G, Firenze C, Morucci P, Abbritti G, Gallai V. Serum and salivary magnesium levels in migraine and tension-type headache. Results in a group of adult patients. Cephalalgia 1992;12(1):21–7. [DOI] [PubMed]
- 136.Gupta VK. Magnesium therapy for migraine: do we need more trials or more reflection? Headache. 2004;44(5):445–6. doi: 10.1111/j.1526-4610.2004.04098_2.x. [DOI] [PubMed] [Google Scholar]
- 137.Wang F, Van Den Eeden SK, Ackerson LM, Salk SE, Reince RH, Elin RJ. Oral magnesium oxide prophylaxis of frequent migrainous headache in children: a randomized, double-blind, placebo-controlled trial. Headache. 2003;43(6):601–10. doi: 10.1046/j.1526-4610.2003.03102.x. [DOI] [PubMed] [Google Scholar]
- 138.Koseoglu E, Talaslioglu A, Gonul AS, Kula M. The effects of magnesium prophylaxis in migraine without aura. Magnes Res. 2008;21(2):101–8. [PubMed] [Google Scholar]
- 139.Tarighat Esfanjani A, Mahdavi R, Ebrahimi Mameghani M, Talebi M, Nikniaz Z, Safaiyan A. The effects of magnesium, L-carnitine, and concurrent magnesium-L-carnitine supplementation in migraine prophylaxis. Biol Trace Elem Res. 2012;150(1–3):42–8. doi: 10.1007/s12011-012-9487-5. [DOI] [PubMed] [Google Scholar]
- 140.Peikert A, Wilimzig C, Kohne-Volland R. Prophylaxis of migraine with oral magnesium: results from a prospective, multi-center, placebo-controlled and double-blind randomized study. Cephalalgia. 1996;16(4):257–63. doi: 10.1046/j.1468-2982.1996.1604257.x. [DOI] [PubMed] [Google Scholar]
- 141.Pfaffenrath V, Wessely P, Meyer C, Isler HR, Evers S, Grotemeyer KH, et al. Magnesium in the prophylaxis of migraine—a double-blind placebo-controlled study. Cephalalgia. 1996;16(6):436–40. doi: 10.1046/j.1468-2982.1996.1606436.x. [DOI] [PubMed] [Google Scholar]
- 142.Silberstein SD, Goldberg J. Menstrually related migraine: breaking the cycle in your clinical practice. J Reprod Med. 2007;52(10):888–95. [PubMed] [Google Scholar]
- 143.Taylor FR. Nutraceuticals and headache: the biological basis. Headache. 2011;51(3):484–501. doi: 10.1111/j.1526-4610.2011.01847.x. [DOI] [PubMed] [Google Scholar]
- 144.Mauskop A, Altura BM. Role of magnesium in the pathogenesis and treatment of migraines. Clin Neurosci. 1998;5(1):24–7. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 511 kb)