Abstract
Bone grafts have been predominated used to treat bone defects, delayed union or non-union, and spinal fusion in orthopaedic clinically for a period of time, despite the emergency of synthetic bone graft substitutes. Nevertheless, the integration of allogeneic grafts and synthetic substitutes with host bone was found jeopardized in long-term follow-up studies. Hence, the enhancement of osteointegration of these grafts and substitutes with host bone is considerably important. To address this problem, addition of various growth factors, such as bone morphogenetic proteins (BMPs), parathyroid hormone (PTH) and platelet rich plasma (PRP), into structural allografts and synthetic substitutes have been considered. Although clinical applications of these factors have exhibited good bone formation, their further application was limited due to high cost and potential adverse side effects. Alternatively, bioinorganic ions such as magnesium, strontium and zinc are considered as alternative of osteogenic biological factors. Hence, this paper aims to review the currently available bone grafts and bone substitutes as well as the biological and bio-inorganic factors for the treatments of bone defect.
Keywords: Fracture healing, Bone grafts and substitutes, Growth factors, Bioinorganic ions
Graphical abstract
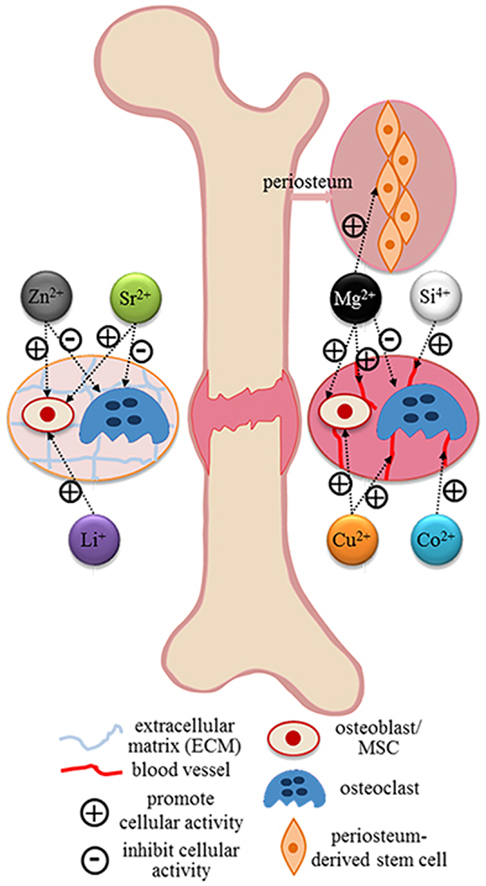
Most common specific targets of relevant bioinorganic ions in their role of therapeutic agents revealed by current researches [273].
Highlights
-
•
Autologous bone graft is the gold standard clinical material for bone regeneration in term of osteoconduction and osteoinduction. However, limited availability and donor site morbidity are concerned.
-
•
Bone allograft becomes to the second higher option for orthopaedic procedures due to the availability in various forms and large quantities. Unfortunately, reduced osteoinductivity may lead to inferior healing as compared with the use of autologous grafts.
-
•
Hence, synthetic bone substitutes and biological factors e.g. calcium phosphate (CaP) cements and ceramics, hydroxyapatite (HAp) and recombinant human bone morphological proteins (rhBMP-2 and rhBMP-7) are considered, either alone or combined, for bone tissue regeneration.
1. Introductions
Bone grafting is one of the most commonly used surgical methods to augment bone regeneration in orthopaedic procedures [3]. Over two million bone grafting procedures were performed annually worldwide, which is the second most frequent tissue transplantation right after blood transfusion [4]. Among all clinical available grafts, autologous bone is still being considered as the gold standard since all necessary properties required in bone regeneration in term of osteoconduction, osteoinduction and osteogenesis are combined [5]. However, the concerns of limited supply and donor site complications are still maintained. Bone allografts dominantly share the second higher option for orthopaedic surgeons and nearly one third of all bone grafts used in North America are allografts [6] since they are available in various forms and large quantities. They are primarily osteoconductive, while reduced osteoinductivity is retained only in demineralized bone matrix (DBM) preparations [7]. Nevertheless, inferior healing was observed compared to the use of autologous grafts and potential for transmission of disease and other infective agents were also reported [8], [9]. More importantly, the amounts of available natural bone grafts traditionally used are still far from meeting the clinical demands, especially when facing the impending global pandemic of aging and obesity [10].
To address these limitations, tremendous alternatives and options have been brought by the emergence of synthetic bone substitutes during the past decades, which made bone grafts and substitutes (BGS) among the most promising market in the orthopaedic industry and it is reported that the revenue from the global market is over two billions in 2013 [11]. Bone grafting procedures are also being gradually shifted from natural grafts to synthetic bone substitutes and biological factors [11]. Among those synthetic bone substitutes and biological factors, calcium phosphate (CaP)-based biomaterials (e.g. hydroxyapatite (HAp), CaP cements and ceramics), and recombinant human bone morphological proteins (rhBMPs, e.g. rhBMP-2 and rhBMP-7) are most widely used, either alone or combined [12]. The former bone substitutes are generally only osteoconductive and mainly being applied in reconstruction of large bone defects, while the rhBMPs are basically osteoinductive with the capability of enhancing the fracture healing [3]. However, the clinical applications of BMPs as off-label drug have been concerned due to supraphysiological dosage, adverse clinical outcomes and cost issue [11], [13], [14]. The applications of stem cell therapy and natural bioinorganic ions as well as the musculoskeletal tissue engineering approach have been extensively investigated [15], [16], [17], [18], [19].
2. Biological structure of bone and its regeneration
2.1. The biology of bone structure
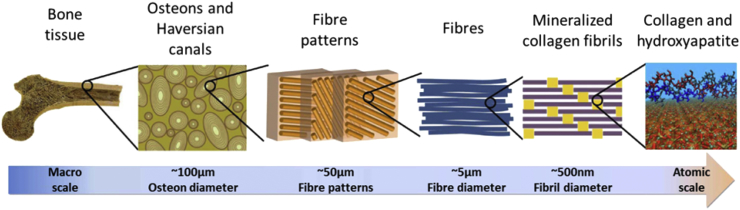
As a rigid organ in body, the bone is able to support and protect various organs but is also able to facilitate mobility [20]. These properties are mainly attributed to the remarkable hierarchical architecture, which is constituted by the soft collagen protein and stiffer apatite mineral, as shown in Fig. 1 [21]. Although the bone structures of different types and species are diverse at the macroscopic level, and the organizations of collagen and minerals are not completely understood, the mineralized fibrils, which is assembled by collagen molecules and mineralized by apatite crystals during the formation of the bone, still acts as the bone's universal elementary building block [22], [23], [24]. In the body, the functionality of bone tissue is related to stiffness, which is directly determined by the natural mineral content within the collagen/mineral composite. For instance, over 80% of mineral content makes the ear vibratable for the purpose of transmitting sound with high fidelity, but it is unable to resorb energy [20]. In contrast, less dense mineral content can enable deer antlers to deform while absorbing energy, but they are non-load bearing [25]. Specific to the long bone, the constitution of mineral content is over 20% [26], which endows the bone to absorb the energy and keeps it light to allow mobility.
Fig. 1.
The hierarchical structure of bone ranging from microscale skeleton to nanoscale collagen and hydroxyapatite. Reprinted by permission from Macmillan Publishers Ltd: Nature Communication [21], copyright (2013).
The bone, once formed, is maintained dynamically through two different processes, modeling and remodeling [27], which are also employed in bone fracture recovery. In bone modeling process, the new bone is formed without prior bone resorption, while in the bone remodeling process, bone formation follows bone resorption [20]. Bone modeling is vigorous during growth, altering the shape and size of the bone. It continues in adulthood, by increasing the ability to resist bending and adapt to functional challenges [28], [29]. On the other hand, bone remodeling is a lifelong process that begins in early fetal life [30], and is in charge of maintaining bone function by continuously replacing damaged bone with new bone [28]. It is reported that about 25% of trabecular bone and 3% of cortical bone are removed and replaced every year [31].
2.2. The biology of bone regeneration
The dynamic equilibrium of the bone efficiently prevents the bone fracture, except for the emergence of a load exceeding the bone strength, or the gradually accumulated damage under cyclic loading (well below bone strength) [32], [33]. Unlike other tissues, bone healing is found to be the recapitulation of the ontological events that take place during embryonic development of the skeleton, which enables the damaged organ to by fully restored to its pre-injury composition, structure and function [34]. Various factors affecting repairing can be applied to classify bone healing, and the extent of tissue loss is among them [35]. Consequently, bone repair can be defined into two categories: primary bone healing and secondary bone healing.
Primary (direct) bone healing mainly happens when the fracture gap is less than 0.1 mm and the fracture site is rigidly stabilized. It is proposed that the bone gap in this process is filled directly by continuous ossification and subsequent Haversian remodeling [36], with the absence of cartilaginous or connective tissue. The formation of callus is also suppressed [35], [37]. However, it must be noted that the concept of direct continuous bone formation is controversial due to the lack of neither histological evidence [35] and clinical cases [38].
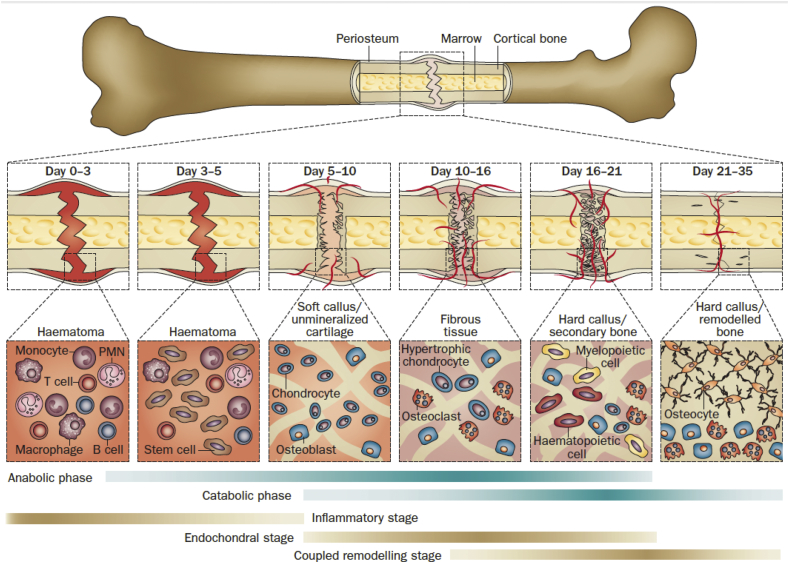
Secondary (indirect) bone healing is the more common form of bone healing and occurs when the fracture edges are less than twice the diameter of the injured bone [35]. In general, multi events, such as blood clotting, inflammatory response, fibrocartilage callus formation, intramembranous and endochondral ossification, and bone remodeling are involved in the secondary bone fracture repair. Specific to the major metabolic activity, anabolism in a bone fracture is activated initially in the form of increasing bone volume by recruiting stem cells differentiation and retardation with chondrocyte apoptosis [39], [40]. The anabolic activity continues in a prolonged phase, which is dominated by catabolic activities. The reduction of callus tissue volume is the symbol of this activity. When the vascular bed increases and vascular flow rate returns to pre-injury level, the catabolic phase reaches the final period [41], [42]. The biological events and activities, as well as the cells involved in typical bone fracture healing at different phases are illustrated in Fig. 2 [34].
Fig. 2.
Illustration of a typical fracture healing process, biological events, and cellular activities at different phases. The primary metabolic phases (blue bars) of fracture healing overlap with biological phases (brown bars). The time scale of healing is equivalent to a mouse closed femur fracture fixed with an intramedullary rod. Abbreviations: PMN, polymorphonuclear leukocyte. Reprinted by permission from Macmillan Publishers Ltd: Nature Reviews Rheumatology [34], copyright (2014).
The large segmental bone defect, or alternatively as critical-sized defect, is an extreme condition in bone healing, which can be caused by high energy trauma, diseases, developmental deformities, revision surgery and tumor resection or osteomyelitis [43], [44], [45], [46]. The extensive bone loss in this defect has been shown to directly affect revascularization and tissue differentiation, and eventually leads to spontaneous bone fracture, which progresses to non-union without interventions [35], [47]. The classical definition of a critically sized segmental bone defect is ‘the smallest osseous defect in a particular bone and species of animal that will not heal spontaneously during the lifetime of the animal’ [48], [49] or ‘shows less than 10% bony regeneration during the lifetime of the animal’ [49], [50]. Even defect size is not the sole parameter to characterize a bone defect as critical [51], it has been found that in most species a length exceeding 2–2.5 times the diameter of the affected bone can be considered the minimum size [35], [52], [53]. The non-union caused by such defects can highly influence the quality of patients' lives due to the prolonged and postoperative treatment costs and also pose a major surgical, socio-economical and research challenges [43].
3. Bone grafts and substitutes for bone defect treatments
Bone substitutes mainly serve as combined functions of mechanical support and osteo-regeneration [54], which involve three important biological properties: osteoconduction, osteoinduction and osteogenesis [55]. Osteoconduction refers to the ability to support the attachment of osteoblast and osteo-progenitor cells, and allow the migration and ingrowth of these cells within the three-dimensional architecture of the graft [55]. Osteoinduction describes that the graft can induce the primitive, undifferentiated and pluripotent cells to develop into the bone-forming cell lineage, by which osteogenesis is induced [55], [56]. Osteogenesis means the osteo-differentiation and subsequently new bone formation by donor cells derived from either the host or grafts [5], [57]. Besides, osteointegration, which defines as the ability of an implant anchoring with the formation of bony tissue around the implant at the bone-implant interface without the formation of fibrous tissue [55], is also an important criterion in evaluating the result of bone healing. The biological properties of some commonly used bone grafts and substitutes are listed in Table 1.
Table 1.
Summary of biological properties of bone grafts and bone substitutes in clinical application (Reprinted from Ref. [1], Copyright (2012), with permission from Elsevier and [2]).
| Osteo-conduction | Osteo-induction | Osteo-genesis | Osteo-integration | Structural support | Disadvantages | Clinical applications | ||
|---|---|---|---|---|---|---|---|---|
| Autologous Bone Grafts | Autologous Cancellous | +++ | +++ | +++ | +++ | – | Limited availability and donor site morbidity | Bone defect, delayed union/non-union, discectomy, arthroplasty |
| Autologous Cortical | + | + | + | + | +++ | Same as above | Bone defects, discectomy | |
| Allogeneic Bone Grafts | Allogeneic Cancellous | + | + | – | ++ | – | Risk of disease transmission and immune reaction | Bone defects, delayed union/non-union, discectomy, arthroplasty |
| Allogeneic Cortical | + | – | – | + | +++ | Sam as above | Bone defects, discectomy | |
| DBM | + | ++ | – | ++ | – | Variable osteoinductivity associated with donors and processing methods | one defects, discectomy, arthroplasty | |
| Synthetic Bone Substitutes | Calcium sulfate | + | – | – | ++ | + | Rapid resorption,osteoconductive only | Bone void filler, bone graft extender |
| HAp | + | – | – | – | ++ | Slow resorption,osteoconductive only | Same as above | |
| Calcium phosphate ceramic | + | – | – | + | ++ | Osteoconductive only | Same as above | |
| Calcium phosphate cement | + | – | – | + | + | Osteoconductive only | Same as above | |
| Bioactive glass | + | – | – | – | Bioactive osteoconductive only | Same as above | ||
| PMMA | – | – | – | – | +++ | Inert, exothermic,monomer-mediate toxic | Segmental bone defect, arthroplasty, vertebroplasty and kyphoplasty | |
Abbreviations: DBM, demineralized bone matrix; HAp, hydroxyapatite; PMMA, poly (methyl methacrylate); IVD, intervertebral disk.
3.1. Natural bone grafts
3.1.1. Autologous bone grafts
An osseous graft harvested from an anatomic site and transplanted to another site within the same individuals is called autologous bone grafting [57], [58]. With the possession of osteoconductive, osteoinductive and osteogenic properties, an autologous bone graft can integrate into the host bone more rapidly and completely [58], therefore being regarded as the gold standard in treating bone defects and the benchmark in evaluating other bone grafts and substitutes. However, the drawbacks of the autograft have been extensively reported, and are related to the harvesting process, including donor site complication and pain, increased blood loss, increased operative time, potential for donor site infection and limited volume of material available [54], [57], [59], [60].
The development of reamer-irrigator-aspirator (RIA) system offers an alternative to other traditional autologous bone graft options such as iliac crest bone graft, with which the graft can be harvested from intramedullary canal of the femur or tibia [61]. In a systematic review covering over 6000 patients, the complication rate with the usage of RIA device is found reduced to 6% as compared to 19.37% from iliac crest bone [62]; while the bone volume is increased from the mean of 15–20 ml with traditional iliac crest bone harvesting to over 40 ml by using RIA [63], [64]. By comparing the bone grafts harvested from different parts of the same patient, higher levels of expression of genes associated with vascular, skeletal and hematopoietic tissues were identified in the RIA samples as compared to that from the iliac crest bone, while stem cells and growth factors in the RIA samples were also more abundant [65]. Nevertheless, the corresponding complications of RIA were also documented, which mainly include iatrogenic fracture, anterior cortical perforation, exsanguination, and heterotopic ossification [62], [66].
Cancellous autografts are the most commonly used form of autologous bone grafting. Few osteoblasts and osteocytes, but abundant mesenchymal stem cells (MSCs) survive as a result of ischemia during transplantation, which helps maintain osteogenic potential and the ability to generate new bone from the graft [1], [67]. Additionally, the large surface area of a cancellous autograft facilitates the superior revascularization and incorporation of the graft locally to the host bone [54]. The graft-derived proteins, which are attributed to the osteoinduction of the graft, are also preserved and present when the autografts are appropriately treated [54], [58]. In the early phase of autograft transplantation, hematoma and inflammation are formed rapidly with the recruitment of MSCs to lay down fibrous granulation tissue. Meanwhile, the necrotic graft tissue is slowly eliminated by macrophages and neovascularization also happens. Next, during the incorporation of the autograft, seams of osteoid are produced by osteoblasts line surrounding the necrotic tissue, this is concurrent with the formation of new bone by accumulated hematopoietic cells within the transplanted bone [54], [57], [58]. This process, which leads to the complete resorption and replacement of the graft, usually takes 6–12 months [68].
Cortical autografts possess excellent structural integrity and are mechanical supportive, due to their limited number of osteoprogenitor cells [57]. Unlike the autologous cancellous graft, the creeping substitution of cortical autograft is mainly mediated by osteoclasts after the rapid hematoma formation and inflammatory response in the early phase of bone regeneration, since the revascularization and remodeling processes are strictly hampered by the dense architecture [58]. Consequently, the appositional bone growth over a necrotic core is the dominant means incorporation of the cortical autograft following osteoclast resorption [69], [70]. This process may take years, depending on the graft size and implantation site [54], [58].
3.1.2. Allogeneic bone grafts
Allogeneic bone graft refers to bony tissue that is harvested from one individual and transplanted to a genetically different individual of the same species [57], [58]. Considering the limitation of autologous bone grafts, bone allograft is considered the best alternative to autografts and has been used effectively in clinical practice in many circumstances, especially for those patients with poor healing potential, established nonunion, and extensive comminution after fractures [1], [58]. The allograft may be machined and customized, and is therefore available in a variety of forms, including cortical, cancellous and highly processed bone derivatives (i.e., demineralized bone matrix) [57]. Compared to autografts, allografts are found to be immunogenic and demonstrate a higher failure rate, which is believed to be caused by activation major histocompatibility complex (MHC) antigens [9]. The initial osteoinduction phase would be destroyed by immune response and inflammatory cells, which could quickly surround the neo-vascular, causing the necrosis of osteo-progenitor cells [71], [72], [73]. Even the exact mechanism of immune response in bone allograft incorporation is not clear; studies have found that allograft acceptance is improved when the immunogenicity is reduced by modifying the allograft to narrow histocompatibility differences [58]. Another issue is the risk of viral transmission, which has been significantly improved by the development of modern tissue banks [54] and improvement in processing technology [74]. Based on these situations, the application of fresh allografts is always limited, and preserved modified allografts are usually preferred in clinical practices [75].
Cancellous allografts are the most common types of commercial allogeneic grafts and are supplied predominately in the form of cuboid blocks [57]. Due to the litter mechanical property they confer and their relative poor healing promoting ability, preserved modified cancellous allografts are mainly applied in scenarios such as spinal fusion augmentation and filler material for cavitary skeletal defects [54], [58]. Compared to autografts, a similar but slower sequence of events happens in the incorporation process of allografts [58]. However, osteointegration may be delayed by a host inflammatory response which leads to the formation of fibrous tissue around the graft, this was found in less than 10% of cases [76]. Meanwhile, the allografts remain entrapped and are never completely resorbed many years after transplantation [54], [59].
Cortical allografts confer rigid mechanical properties and are mainly applied in spinal augmentation for filling large skeletal defects where immediate loading-bearing resistance is required [57]. In consideration of immune responses and for safety, frozen or freeze-dried products that are free of marrow and blood are commonly transplanted [58]. The incorporation of a cortical allograft is also preceded by creeping substitution, which is similar to its autogenous counterpart. In general, the process is initiated by the osteoclastic resorption and followed by sporadic formation of new appositional bone through osteoconduction [1], [58].
Demineralized bone matrix (DBM) is a kind of highly processed allograft derivative with at least 40% of the mineral content of the bone matrix removed by mild acid, while collagens, non-collagenous proteins and growth factors remain [77]. Inferior structural integrity and mechanical properties impart that the DBM is mainly applied for filling bone defects [78]. The osteoconductivity of the DBM is conferred by providing a framework for cell populating and for generating new bone after the demineralization treatment [54]. The osteoinductive property of DBM is mainly determined by the remaining growth factors, which are directly correlated with preparation methods. Much of the commercially available DBM commonly employs 0.5–0.6M of hydrochloric acid as a demineralizing agent. The incorporation of the DBM is similar to that of the autogenous graft, with growth factors triggering an endochondral ossification cascade and culminating in new bone formation at the site of implantation [54].
3.2. Synthetic bone graft substitutes
As mentioned in the previous paragraphs, the serious shortage of natural bone grafts and the little chance of supply meeting the demands in an aging population [79] has triggered the blossom of bone grafts and substitutes (BGS) market [80]. Calcium sulfate, calcium phosphate (CaP) ceramics, CaP cements, bioactive glass or combinations thereof are most commonly synthetic bone substitutes available at present [81].
3.2.1. Calcium sulfate
Calcium sulfate, also known as plaster of Paris, is a kind of osteoconductive and biodegradable ceramics composed of CaSO4 and has been applied in filling void defects since 1892 [82]. It is prepared by heating gypsum with a patented alphahemihydrate crystal structure and can be made in different forms, such as hard pellets or injectable viscous fluids that harden in vivo [57]. Although lacking a macroporous structure, calcium sulfate still has a rapid resorption rate and weak internal strength, which implies that it can only be used to fill small bone defects with rigid internal fixation, the ingrowth of vascular and new bone happens in conjunction with the resorption of the graft [83]. Niu et al., [84] have reported that calcium sulfate was unable to achieve an optimal fusion rate in spinal arthrodesis, mainly because of faster degradation in early phase of bone regeneration than actual bone deposition. However, easy preparation and relative low cost has made calcium sulfate resurgent when combined with other synthetic bone substitutes and/or growth factors [79].
One of the promising approaches is to load antibiotics to this biomaterial. From June 2015 to November 2015, M. Glombitza and E. Steinhausen [85] were using a vancomycin-loaded calcium sulfate/hydroxyapatite to treat the chronic osteomyelitis caused by multi-resistant bacterial for 7 patients, and rapid control of infection was achieved in 6 patients. However, as what can be expected, the replacement of the composite by new bone was not uniform. More recently, Nan Jing et al. [86] modified the traditional Masquelet technique, which has been widely used in treating massive bone defects, by using calcium sulfate to replace the PMMA as cement spacer, so as to make this technique a one-step surgery. In this case report regarding the reconstruction of an open fracture of the calcaneus at right foot, they found the formation of the induced membrane with the implantation of calcium sulfate by X-ray image and a computed tomography scan. But this trial was then stopped by the patient and the calcium sulfate was finally replaced by autologous iliac crest bone grafts, and further characterization on the induced membrane and bone regeneration became impossible [86].
3.2.2. Calcium phosphate ceramics (CaP ceramics)
Calcium phosphate ceramics are constituted by calcium hydroxyapatites, which is a chemical composition similar to the mineral phase of calcified tissues [87]. They are synthetic mineral salts and usually produced by sintering at high temperatures with the exclusion of water vapor and subsequently molded by high-pressure compaction [83]. Porous implants, non-porous dense implants and granular particles with pores are common commercially available forms. As a kind of bioabsorbable ceramic with excellent osteoconductivity, CaP ceramics have received great attention and have been experimented extensively in clinical studies [88], [89], [90], [91], [92]. Unlike the calcium-to-phosphate (Ca/P) ratio of biphasic calcium phosphate (BCP), the ratio of HAp and tricalcium phosphate (TCP), which are most widely used in orthopaedics, can be identified. Several key parameters of CaP ceramics, such as absorption rate and mechanical properties, are strictly related to the Ca/P ratios. In addition, the crystal and porous structure is a highly-considered factor in choosing CaP ceramics.
Hydroxyapatites (HAp) is a natural occurring mineral form of calcium apatite with the formula of Ca10[PO4]6[OH]2 and comprises about 50% of the weight of the bone, which accounts for its excellent osteoconductive and osteointegrative properties [1], [57]. Meanwhile, HAp has a similar initial mechanical property compared to the cancellous bone--brittle and weak under tension and shear but resistant to compressive loads [87] and may decrease by 30–40% in situ after being implanted for several months [93]. The macroporosity (pore with diameters > 100 μm) and pore interconnectivity of synthetic HAp allow the adhesion, proliferation, and differentiation of osteoprogenitor cells, as well as the revascularization, and subsequently ingrowth of new bone, when implanted in vivo [94], [95]. However, the relatively high Ca/P ratio and crystallinity delay the resorption rate of HAp--a process predetermined by giant cells and macrophages [96]. It has been demonstrated that when porous hydroxyapatite cylinders were implanted in the cancellous bone of rabbits, only 5.4% volume reduction was observed after six months, whereas the number for tricalcium phosphate ceramic was 85.4% under the same conditions [94]. Consequently, the remaining hydroxyapatite grafts within the host bone would compromise the intrinsic strength of the bone at the callus site due to the decreasing of mechanical properties aforementioned [83]. Therefore, HAp alone is more often applied as a coating on implants and external fixator pins or in sites with low mechanical stress [1], [97].
These drawbacks may partially being overcome by the recent development of nanocrystalline HAp, with which larger surface to volume ratio is conferred. This great surface not only significantly reduced the sintering temperature of HAp ceramic, but also led to the increased resorption rate [98]. However, this increasing is not noticeable in clinical observation [99]. On the other hand, efforts were also being made to enhance the mechanical performance of nano-HAp by incorporation of carbon nanotubes (CNTs) [98], [100], [101]. The addition of CNTs, on one hand, increased the open porosity from about 2.52% (pure nano-HAp) to at most 7.93% (with addition of 2 wt.% of CNTs), on the other hand, fracture toughness with the value of 1.88 MPa▪m1/2, which is similar to that of the human cancellous bone, was achieved when the addition amount was 1% weigh percentage [101]. Enhanced bone formation was also observed in rabbit distal femur bone defect model, whereas toxicity was not exhibited in the liver and kidney. Nevertheless, the resorption rate of this nanocomposite was not fully investigated and the enhanced mechanical properties are insufficient to extend the application of HAp in clinic.
Tricalcium phosphate (TCP), especially the rhombohedral β-form, β-tricalcium phosphate (β-TCP), has attracted increased attention since it was first reported in 1920 by Albee [102]. With the chemical formula of Ca3(PO4)2, β-TCP has Ca/P ratio of 1.5 and is thus lower than that of hydroxyapatite that may partially accelerate its degradation and absorption [67]. Like HAp, TCP has even more interconnected porous structures that can directly benefit fibrovascular invasion and bony replacement [1], but at the same time weaken mechanical properties [103]. Due to the thermodynamically unstable physiological pH, a portion of TCP would inevitably convert into hydroxyapatite after implantation and thus partially hamper the degradation of TCP [104], the majority would be resorbed by phagocytosis after 6–24 months with some remaining for years [105]. This makes the TCP effective for filling bone defects caused by trauma and benign tumors but is not favored as a bone-graft substitute owning to an unpredictable biodegradation profile [78].
Recent research started to focus on the enhanced angiogenesis in which the tricalcium phosphate was applying to augment of bone defects [106], [107]. By comparing the in vitro neovascularization capacity of four different types of CaP ceramics, namely HAp, BCP-1 (HAp: β-TCP = 70/30), BCP-2 (HAp: β-TCP = 30/70) and β-TCP, they found human umbilical vein endothelial cells (HUVECs) demonstrated significantly up-regulated proliferation and angiogenesis when cultured with β-TCP and BCP-2, which containing higher amount of β-TCP phase [106]. While in the mice intramuscular implantation model, CaP ceramics containing higher content of β-TCP also induced higher density of microvessels [106]. Variety of hypotheses, such as the porous structure [108], [109], [110], effects of ionic transfer upon degradation of CaP ceramics and homeostasis [111], [112], [113], potential strains imposed on CaP during degradation [114], [115], were proposed to explain the mechanism, nevertheless, crucial investigation is still missing and further studies are necessarily important.
Biphasic calcium phosphate (BCP) is another widely used commercial ceramic obtained by mixing hydroxyapatite and tricalcium phosphate in different concentrations for the purpose of combining the advantages of both calcium salts [116]. By adjusting the formulation, the dissolution rate and mechanical properties can be controlled within ranges and subsequently applied in bulk or as implant coatings [117].
3.2.3. Calcium phosphate cements (CPC)
Calcium phosphate cements, unlike CaP ceramics, usually involve two compounds, one of which is an aqueous curing agent. They were invented by Brown and Chow [118], [119] for the purpose of extending the adaptability and moldability of CaP bone substitutes in the 1980s. They were approved by US Food and Drug Administration (FDA) [87] in 1996. They can be conveniently injected to fill defects with various shapes and subsequently solidified by mixing with an aqueous phase through isothermic reaction. Self-hardened CPCs are generally highly microporous, biocompatibility and mechanical supportive with low bending strength [120]. However, they can only degrade layer by layer as predetermined by the dissolution in physiological conditions and osteoclast resorption activity; subsequently, an ingrowth of neovascular and bone tissue is theoretically hampered compared to the other CaP ceramics that support interconnected macroporosity [87]. According to the composition, apatitic CPCs and brushite CPCs can be identified; properties of CPCs in terms of feasibility, setting reaction and biodegradation rates are highly related to its composition. Apatitic CPCs are viscous, indicating relatively poor injectable ability, but a setting reaction can occur at the physiological pH value and the mechanical properties are slightly stronger than brushite CPCs. Due to the low crystalline structure of the obtained calcium deficient-hydroxyapatite after hardening, a higher degradation rate was demonstrated but still incomplete [121]. The brushite CPCs are feasible for injection and solidify quickly at a low pH value (< 6) [120]. They demonstrate higher degradability, but unpredictable degradation was reported due to the kinetic favorable transformation to hydroxyapatite [104]. Based on its flow behavior before setting, CPCs are clinically favored for bone replacement, especially in percutaneous vertebroplasty [122], [123], [124] and kyphoplasty [125], [126], but not as bone substitutes.
Similar to the CaP ceramics, in order to promote the mechanical properties and biological performances of CPCs, the preparation of nanostructured CaP is developed. Even the up-regulated cell attachment and proliferation, as well as the in vivo bone regeneration was achieved in several investigations [127], [128], [129], the motivation of applying nanostructured CPCs is mainly attributed to the fact that nanosized architecture of native bone tissue and process of bone formation [130], [131], [132], [133], whereas the cellular and molecular mechanism has not been fully elaborated [128]. The incorporation of fibers, which is also inspired by the hierarchical nanostructured of bone, is another approach that being widely investigated to enhance the mechanical strength of CPCs [134]. However, the evidence that these modifications benefit the clinical practice is still missing [135].
Phase separation, which means the separation of powder and liquid components during injection, is another important concern associated with the clinical applications of CPCs [136]. By distracting other crucial properties of CPCs, several methods have achieved clinical success in some applications after the abundant studies over the past two decades [137], [138], [139], [140], [141]. However, recent research tended to gain the relationship of those critical parameters of CPCs by theoretical calculations and analysis [142], [143], [144], [145], [146], [147], [148], due to the extremely difficult optimization by sole experimental work. Since these studies can still hardly being reflected in real experimental practice and thus affect the clinical applications of the CPCs, they will not be highlighted in this review but can be found in other recent review [149].
3.2.4. Bioactive glass
Bioactive glass, also known as bioglass, refers to a group of synthetic silicate-based ceramics and was originally constituted by silicon dioxide (SiO2), sodium oxide (Na2O), calcium oxide (CaO), and phosphorus pentoxide (P2O5) when first developed in 1970s [150]. This was later modified to a more stable composition by addition potassium oxide (K2O), magnesium oxide (MgO) and boric oxide (B2O); the key component, silicate, constitutes 45–52% of its weight [1]. The optimized constitutions lead to a strong physical bonding between bioglass and host bone. This phenomenon, namely bioactivity, was first found on BGS [151]. This bone-binding property is believed to be caused by leaching and the accumulation of silicon ions when exposed to body fluids upon implantation, and the subsequent formation of hydroxyapatite coating on the surface of bioglass [152]. This thin hydroxyapatite coating absorbs proteins and attracts osteo-progenitor cells. In addition, this biological apatite layer is partially replaced by bone through a creep substitution process in long-term implantation [153]. The porosity and relative fast resorption rate in the first two weeks of implantation allows an ingrowth of neo-vascular following deposition of the new bone [12], [67]. One study has demonstrated that bioglass fiber scaffolds can be completely resorbed in six months in vivo with little inflammatory response [154]. Like the other ceramics, the mechanical properties of bioglass were reported to be brittle and weak. Therefore, it has been mainly applied in the reconstruction of facial defects [155], [156] when combined with growth factors [157], [158].
Bioglass 45S5 (46.1 mol.% SiO2, 24.4 mol.% Na2O, 26.9 mol.% CaO and 2.6 mol.% P2O5, now sold by NovaBone Products LLC, US) and S53P4 (53.8 mol.% SiO2, 22.7 mol.% Na2O, 21.8 mol.% CaO and 1.7 mol.% P2O5, now sold by BonAlive Biomaterials, Finland) are two most recognized commercial available bioglasses that can be used as bone graft substitutes in the market. They are made by using the traditional melt-quenching route under high temperature (usually above 1300 °C) and thus unable to be fabricated into amorphous scaffolds due to the crystallization during sintering at that temperature. One of the exception is 13–93, with a composition of 54.6 mol.% SiO2, 6 mol.% Na2O, 22.1 mol.% CaO, 1.7 mol.% P2O5, 7.9 mol.% K2O and 7.7 mol.% MgO, does not crystallize during sintering. However, the bioactivity of 13–93 was significantly reduced in the form of prolonging the formation of hydroxyapatite layer in the stimulated body fluid (SBF) immersion tests, from 8 h on the surface of Bioglass 45S5 to 7 days on the 13–93 [159]. Several clinical trials demonstrated similarity good contact with the host bone when the S53P4 and Bioglass 45S5 was applied to treat bone defect, respectively [160], [161], [162], whereas reduced resorption of S53P4 was exhibited due to the higher silica content [156], [163]. Additionally, inferior healing results were also reported when compared to autologous grafts [164], [165].
The development of sol-gel processing offers another route to produce bioactive glass with porous structure ranging from mesopores to macropores [166], [167], [168], in which 58S (60 mol.% SiO2, 36 mol.% CaO and 4 mol.% P2O5) and 77S (80 mol.% SiO2, 16 mol.% CaO and 4 mol.% P2O5) are representatives. In a research involving the management of critical-sized defect at the femoral condyle of rabbits, the bone regeneration ability and in vivo degradation of melt-derived Bioglass 45S5 and sol-gel-derived bioglass 77S and 58S were compared [169]. Due to the nanoporosity and enhanced surface area, the sol-gel-derived bioglass demonstrated faster degradation speed as compared with Bioglass 45S5 between 4 and 24 weeks after implantation, whereas the bone defect filled with Bioglass 45S5 containing more bone than those filled with 77S or 58S at 8 weeks post-operation but then equalized after implantation for 12 weeks [169]. It seems the fast degradation of bioglass may lead to a higher pH value and accumulated ions in the microenvironment, which is not favored by cells, and thus jeopardized the bone ingrowth [156].
3.2.5. Poly(methyl methacrylate) (PMMA) bone cement
First employed by orthopaedic surgeons 60 years ago [170], poly(methyl methacrylate) (PMMA) remains a key component of modern practice and may be one of the most enduring materials in orthopaedic surgery [171]. It is non-biodegradable and non-resorbable, which makes it more like grout rather than cement, and thus it cannot be considered a bone substitute material even though it is the most commonly used synthetic material used in clinics [172]. Due to its high mechanical property and feasibility for handling, two-part self-polymerizing PMMA bone cement has been widely used in total joint replacement for the fixation of component [173] and percutaneous vertebroplasty [174], [175]. Triggered by infection from prosthetic joints, antibiotic-loaded acrylic cement was developed and has been considered part of antimicrobial prophylaxis in primary arthroplasty [171]. However, the drawbacks of PMMA cement are clear. The polymerization of PMMA is exothermic and may potentially damage adjacent tissues [176], [177]. Moreover, aseptic loosening caused by monomer-mediated bone damage [178], mechanical mismatch and inherent inert property [179], was reportedly inevitable in long-term wearing and thus led to the failure of arthroplasties using PMMA cement [180].
3.3. The adoption of growth factors on bone regeneration
Most bone graft substitutes, especially synthetic ceramics and cements, do not possess any osteoinductive property. The ability to enhance bone healing of those bone substitutes mainly relies on osteoconductive means [57]. In general, the osteoconduction of bone substitute would facilitate the migration and support attachment of progenitor cells, which would then secrete growth factors to stimulate bone formation [1]. However, in situations where the ideal environment for callus formation was disturbed, the secretion of growth factors was missing and was thereby predisposed to delayed union or even non-union [181]. Meanwhile, the requirement of osteoinductive factors presenting at the site of bone injury during bone healing is also critically important. Therefore, directly application of growth factors, some of which are involved in the natural healing process of bone injury, has also been extensively investigated and accepted as a kind of therapeutic strategy in clinics [182]. It must be noticed that only a few biological factors, such as BMPs, fibroblast growth factors (FGF) vascular endothelial growth factors (VEGF), PTH and platelet-rich plasma (PRP), have undergone rigorous preclinical tests and clinical trials (see Table 2) [34].
Table 2.
| Source | Receptors class/target cells | Functions | Clinical applications in orthopaedics | |
|---|---|---|---|---|
| BMPs | Osteoprogenitor cells, osteoblasts, bone extracellular matrix | Serine/threonine kinase receptors, stem cell and chondrocyte | Promotes differentiation of mesenchymal stem cells/ osteoprogenitor cells into chondrocytes and osteoblasts, influences skeletal pattern formation | rhBMP-2 is used for the treatment of anterior lumbar spinal fusion and open tibial fractures, and rhBMP-7 is used for posterolateral lumbar spine fusion |
| FGFs | Macrophage, mesenchymal cells, chondrocytes, osteoblasts | Tyrosine kinase receptors | Mitogenic for mesenchymal stem cells, chondrocytes, and osteoblasts. Increases collagen deposition and angiogenesis [57] |
|
| VEGF | Platelets, chondrocytes in callus | Vascular endothelial cells | Increases angiogenesis and vascular development | |
| PTH | Parathyroid glands | Stem cell, chondrocyte and osteoblast | Increased callus size, bone mass and mineral content | The full length PTH(1–84) and a segment, PTH(1–34), is used to increase the cancellous bone mass and reduce the risk of vertebral and non-vertebral fracture of patients with osteoporosis |
| PRP | blood | Variety cell types | Cocktail of growth factors | Mainly applied in orthopaedics and sports medicine to help hemostasis and musculoskeletal healing [184] |
Abbreviations: BMPs, bone morphogenetic proteins; FGFs, fibroblast growth factors; VEGF, vascular endothelial growth factor; (rh)PTH, (recombinant human)parathyroid hormone; PRP, platelet-rich plasma.
3.4. Bone morphogenetic proteins (BMPs)
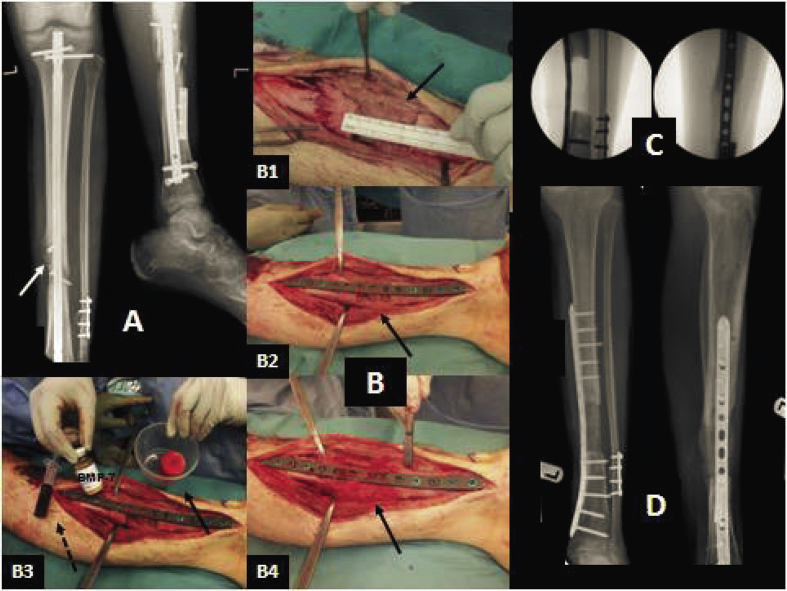
Bone morphogenetic proteins (BMPs), especially BMP-2 (including recombinant human BMP-2, rhBMP-2) and BMP-7 (including recombinant human BMP-7, rhBMP-7), are members of the transforming growth factor beta (TGF-β) superfamily with superior osteoinductive properties and are possibly the most extensively investigated growth factors in treating skeletal defects [34]. BMP-2 is able to induce osteoblastic differentiation from mesenchymal stem cells, and BMP-7 can directly promote angiogenesis. The largest trial in the use of BMPs was in treating open tibial fracture, naming the BMP-2 Evaluation in Surgery for Tibial Trauma (BESTT), which involved multiple clinical centers [185]. In the trial, 450 patients were randomly divided into three groups, one group received BMP-2 at 0.75 mg/ml, the second group received 1.5 mg/ml and the third was the control group. An intramedullary nail was applied universally. Twelve months after surgery, quicker bone callus formation and wound closure with lower infection and less pain were displayed in the patients treated with 1.5 mg/ml rhBMP-2 compared to the control, which indicates the efficiency of BMP-2 in treating tibial open fractures but with a dosage dependent effect. In an earlier study by Friedlaender et al. [186], 124 tibial non-unions were fixed by an intramedullary rod before randomly receiving either rhBMP-7 in a collagen sponge or iliac crest autografting at revision surgery. Nine months later, 81% of patients in the rhBMP-7 group and 85% of those in the autograft group were able to bear full weight without significant pain. At a final follow-up of two years, no statistically significantly differences were observed between these two groups. The use of rhBMP-2 or rhBMP-7 soaked collagen sponge in treating tibial non-unions demonstrated results equivalent to autologous iliac crest grafting, while also reduces persistent donor pain. After being tested in numerous animal models [187] and clinical trials [188], [189], [190], [191], rhBMP-2 (INFUSE™, Medtronic, US) has been approved by FDA and European Medicines Evaluation Agency (EMEA) for the application in anterior lumbar spinal fusion and open tibial fractures [59], [181], while rhBMP-7 (OP-1™, Stryker, US) has received approval in treatment of posterolateral lumbar spine fusion [190], [191]. However, due to the lack of osteoconductivity, commercial available forms of BMPs are always combined with an osteoconductive carrier, such as collagen, allograft or even autologous bone graft, for the purpose of enhancing efficiency (see Fig. 3).
Fig. 3.
Male patient (age 19 years old) with infected non-union after intramedullary nailing of an open tibial fracture. (A). Anteroposterior (AP) and lateral X-rays of the tibia illustrating osteolysis (white arrow) secondary to infection. The patient underwent removal of the nail, extensive debridement and a two-staged reconstruction of the bone defect, using the induced membrane technique for bone regeneration (the Masquelet technique). (B) Intraoperative pictures showing: (1) a 60 mm defect of the tibia (black arrow) at the second stage of the procedure; (2) adequate mechanical stability was provided with internal fixation (locking plate) bridging the defect, while the length was maintained (black arrow); (3) maximum biological stimulation was provided using autologous bone graft harvested from the femoral canal (black arrow, right), bone-marrow mesenchymal stem cells (broken arrow, left) and the osteoinductive factor bone morphogenetic protein-7 (center); (4) the graft was placed to fill the bone defect (black arrow). (C) Intraoperative fluoroscopic images showing the bone defect after fixation. (D) Postoperative AP and lateral X-rays at 3 months, showing the evolution of the bone regeneration process with satisfactory incorporation and mineralization of the graft (photographs courtesy of PVG) [3].
Triggering by the clinical evidence of quicker bone formation stimulated by BMPs, the off-label usage has increased dramatically, which was reported to account for 85% in lumbar fusion procedures in 2008 [192]. Other than the approval application in tibial nonunion, the investigations in treating other long-bone nonunions, such as humerus pseudarthrosis [193], lower limb pseudarthrosis [194], [195], [196], clavicle [197] and ulna [198], have also been reported sporadically, whereas results were found poor or with a very small level of evidence [199]. In a prospective and randomized clinical trial reported by Ekrol et al., in 2008 [200], 30 patients with symptomatic malunion of the distal radius received a corrective osteotomy, either autogenous iliac crest bone grafting (AICBG, 16 patients) or directly application of rhBMP-7 (without any carrier, 14 patients) was conducted randomly. Due to the loss of fixation, an external fixation system was applied in four patients from the rhBMP-7 group and six patients in AICBG group, respectively, before the internal fixation system was used in the remaining patients (10 patients in each group). Although this change makes the result difficult to explain, an inferior healing and union percentage was demonstrated in the group receiving rhBMP-7 treatment and it was believed that their results may have been significantly different if a carrier had been applied. Several researchers have also applied BMPs to improve the foot or ankle arthrodesis fusion in patients with poor surgical healing [201], [202], [203] and the effective adjuvant effect was exhibited [204], whereas randomized controlled trials are still missing.
After all, it is generally accepted that the equivalent clinical outcome would be achieved when using the BMPs to treat some complex bone defects, such as spine fusion and tibial open fracture, as compared to iliac crest autologous bone graft, whereas the high rate of complications is the concern [199]. BMPs are especially soluble proteins and have a tendency to dissipate from their intended locations [57] and lead to several complications. As demonstrated in the previous literature, there tends to be a dose dependent effect in the application of BMPs [205]. The dissipation of proteins would dilute their local concentration, and, in turn, their efficiency. In addition, BMPs can influence several cell types and organs, which subsequently cause heterotopic bone formation. Boraiah et al. reported 10 cases of ectopic bone formation out of 17 complex proximal tibia fracture treated with rhBMP-2; four of them needed extra surgical excision [206]. In some extreme conditions, such as one case reported by Ritting et al., the use of BMP-2 in an ulnar non-union in a nine year old patient led to a persistent inflammatory response and finally caused osteolysis [207]. Additionally, cost effectiveness is another important issue when using BMPs [208].
3.5. Fibroblast growth factors (FGFs)
There are 22 members of fibroblast growth factors family and 4 fibroblast growth factor receptors (FGFRs) being identified and are found secreted by monocytes, macrophages, mesenchymal stem cells, osteoblasts and chondrocytes, starting from the early stages of fracture healing and lasting throughout the whole healing process [209]. The role of FGFs in fracture healing is not well understood, but it has been demonstrated that FGFs not only play a critical role in angiogenesis [210], [211], [212] but also have potent mitogenic effects on mesenchymal progenitor cells [213], all of which are mediated by the FGFs/FGFRs signaling. Among all those FGFs and FGFRs, FGF1, FGF2 and FGFR1-3 were found closely related to bone regeneration by numerous studies [214], [215], [216], [217], in which FGFR1 and FGFR2 have stronger expressions in osteoprogenitors and osteoblasts whereas FGFR3 is more related to chondrogenesis [218]. Hence, the efficiency of FGF2 in treating bone defect was investigated by numerous in vivo animal studies [219], [220], including two non-human primate studies [221], [222], and results also showed promoted fracture healing, whereas this effect is dose and time dependent [218], [223]. One representative clinical trial of rhFGF in treating tibial shaft fracture in 70 patients was reported by Kawaguchi et al. [224]. After fixation by an intramedullary nailing system, patients were randomly injected with either gelatin hydrogel (placebo, 24 patients) or 0.8 mg rhFGF-2 in gelatin hydrogel (low dosage group, 23 patients) or 2.4 mg rhFGF-2 in gelatin hydrogel (high dosage group, 23 patients) at the fracture site. Radiographically analysis demonstrated accelerated fracture healing and higher facture union in both rhFGF treated groups compared to the hydrogel-only treated group, while no difference between the low dosage group and high dosage group was displayed. However, due to our limited understanding on the spatiotemporal expression patterns of FGFs/FGFRs signaling in fracture healing, further studies are still demanded before the clinical trial. In addition, the results of FGFs in treating bone fracture compared to autologous and BMPs are unavailable as well.
3.6. Vascular endothelial growth factor (VEGF)
Local vascularity at the fracture site has been recognized as one of the most significant parameters affecting bone regeneration, and the VEGF is a dominant pathway in the two main hormonal pathways controlling angiogenesis, the VEGF pathway and the angiopoietin pathway [225], [226]. Except for angiogenesis, VEGF has also been demonstrated to be osteogenic [227]. In the bone fracture healing process, VEGF is initially released from hematoma and promotes the development of endothelial cells to induce vascular invasion [57] under the hypoxia environment [228]. Consequently, during the endochondral ossification process, VEGF is secreted by hypertrophic chondrocytes in the epiphyseal growth plate to promote the blood vessel invasion of cartilage and blood flow that facilitates new bone formation [229], [230]. Numerous animal studies have shown the effectiveness of exogenous VEGF to promote bone fracture healing [231], [232], [233], [234], [235]. In one research reported by Kaigler et al. [232], rodents with critical sized cranial bone defect were treated by either bioglass alone or VEGF-containing bioglass. Increased vascularization and bone quality was observed in the VEGF-containing group but no significant difference was displayed when comparing the quantity of the newly formed bone. A similar result was documented in other research published from the same laboratory [233], which implied that VEGF tends to contribute to bone maturation but does not enhance the amount of new bone formation [227]. In a rabbit model, either VEGF or autograft, was compared to a carrier-alone group in treating experimental fracture non-unions [234]. Compared to the control group, significant new bone formation and enhanced mechanical properties were observed from a radiological evaluation and bio-mechanical testing, respectively, while no significant difference was demonstrated in the blood flow and vascularity. All the evidence supports the importance of the collaboration of angiogenesis and osteoinductive factors in bone regeneration [236]. Although the cornerstone role of VEGF in angiogenesis during fracture healing has been confirmed and promising bone regeneration outcomes have been demonstrated in preclinical research, VEGF is in fact very unstable and short-lived in vivo, so a gene delivery vehicle is usually employed. Additionally, concerning the risk of haemangiomas or recurrence of tumors stimulated by VEGF, especially for those patients after radiotherapy or tumor excision, the application of VEGF in clinical trials and its direct effect on human fracture healing is strictly limited [235], and the application of VEGF must be very accurate in dosology [227].
3.7. Parathyroid hormone (PTH)
Parathyroid hormone (PTH) is a naturally occurring endocrine containing 84 amino acids and functions as a mediator of calcium and phosphate homeostasis in humans [237]. It has also been demonstrated to increase bone mass, bone strength and reduce bone loss, and the structure-function analysis of PTH has suggested that these activities are mainly attributed to the N-terminal fragment (encompassing amino acids 1–34 and called PTH(1–34)) [238]. Thus, there are two PTH-derived products available nowadays, the full length protein PTH(1–84), with a commercial name of Natpara™ (Shire-NPS Pharmaceuticals, US), and a segment of protein PTH(1–34), which was licensed by FDA in 2002 under the name of Teriparatide (Forteo™, Lilly LLC, US) [237], and they have been developed as drug to increase the cancellous bone mass and reduce the risk of vertebral and non-vertebral fracture of patients with osteoporosis. Although the detailed mechanism is not yet fully understood, it was found that several signaling pathways were involved and the anabolic effect of PTH was exerted mainly through inhibiting the apoptosis of preosteoblasts, increasing osteoblast function and lifespan, thus leading to an increased number of these bone-making cells [239].
Driven by the fact that PTH increases bone mass and prevents fracture in osteoporotic bone [240], a growing number of studies have suggested the ability of PTH to accelerate fracture healing even though most of the studies were focused on animals. In a diaphysial femoral fracture model involving 270 male Sprague Dawley rats, either a placebo or 5 μg/kg or 30 μg/kg PTH(1–34) were injected daily subcutaneously for 35 days [241]. Significantly torsional strength, stiffness, bone mineral content, bone mineral density and cartilage formation were observed in the callus from the group treated with 30 μg/kg PTH compared to that from the control group over 21 days; no difference in osteoclast density was detected. Other animal experiments confirmed the positive effects of PTH on fracture healing in different species, locations and under various pathological conditions [242]. As a summary of these investigations conducted in animal model, it is confirmed that intermittent treatment with PTH has anabolic effects on bone and thus leads to recovery of bone mass and increased mechanical property, whereas continuous exposure to PTH are contrarily cause bone loss [238], [243], [244], [245], [246].
In 2010, Aspenberg et al. conducted a prospective, randomized clinical trial employing 102 postmenopausal women patients with distal radial fractures [247], and they were randomized to receive a placebo, 20 μg PTH daily (ordinary osteoporosis dosage) or 40 μg injection daily (double dosage). No such difference between 20 μg group and 40 μg group was found but a shorter time for the first radiographic evidence of cortical bridging was documented, which were 9.1, 7.4 and 8.8 weeks in the placebo, 20 μg group and 40 μg group, respectively. Further analysis demonstrated that PTH would mainly increase the early callus formation with a dose-dependent pattern, whereas the cortical bridging is not necessarily stimulated by PTH [248]. In another study involving pelvic ramus fractures in 65 osteoporotic women, radiographically bridging of cortical bone was found shortening from 12.6 weeks in the control group to 7.8 weeks in the PTH(1–84)-treated group [249]. More recently, the effect of Teriparatide in treating elderly patients with a pertrochanteric hip fracture was compared to those using risedronate, which is a bisphosphonate drug, in a randomized clinical trial [250], [251]. In 171 patients, 86 received 20 μg Teriparatide every day and others received 35 mg risedronate once per week, started within 2 weeks after surgery. Seventy-eight weeks later, several outcomes, including the BMD at the lumbar spine (LS), femoral neck (FN) and total hip (TH), functionality (through timed up-and-go (TUG) test), hip pain (Charnley score and 100 mm visual analog scale (VAS)), quality of life, radiology outcomes and safety, were comprehensively analyzed. Significantly greater increasing LS and FN BMD, less pain and a faster TUG results were recorded when patients were treated with Teriparatide as compared to those with risedronate [251]. Conclusively, there is little doubt that PTH has positive influence on fracture healing, but it must be noticed that PTH is not a differentiation factor and is unlikely to help if fracture healing is not started properly. Additionally, the robust evidence observed in animal studies has not been demonstrated beyond a reasonable doubt in humans [238].
3.8. Platelet-rich plasma (PRP)
The investigation of platelet-rich plasma (PRP) for bone regeneration represents attempts to harness the power of the cascade of growth factors released by the aggregation and degranulation of platelets in the native fracture haematoma [252]. PRP is mainly produced by isolating and concentrating platelets from peripheral blood with commercially available devices. It is the plasma fraction of autologous blood having a platelet concentration above baseline [253]. It contains various key mitogenic and chemotactic growth factors that include platelet-derived growth factor (PDGF), insulin-like growth factor (IGF), fibroblast growth factors (FGFs), transforming growth factor-beta (TGF-β) and VEGF [254]. For those patients receiving conservative orthopaedic treatment caused by aging and degeneration, such as knee pain and tennis elbow, PRP are frequently used and demonstrate good clinical outcomes [255], [256], [257]. However, when investigating the effect of PRP on bone healing, especially in human bone healing, the clinical results are conflicting and strong supportive evidence is lacking [59]. In 2008, Calori et al. conducted a prospective, randomized trial comparing the treatment effect of rhBMP-7 and PRP in 120 patients with long bone non-unions [258]. They found a union occurring in 68.3% of cases (41 of 60 patients) in the PRP treated group while the number was 86.7% (52 of 60 patients) in the rhBMP-7 group. The mean time to clinical healing was four months in the PRP group compared to 3.5 months in the rhBMP-7 group. These results implied significantly inferior healing when treated with PRP. Another study investigated the efficacy of PRP in the treating 132 patients with delayed union after when the long bone fractures were surgically intervented at the Military Medical Institute in Warsaw between 2009 and 2012 [259]. Bone union was established in 108 patients (81.8%) after PRP administration, whereas 24 patients (18.2%) showed no improvement. They also concluded the location-dependent efficiency of PRP since 100% union (on average of 3.5 months) was exhibited at proximal tibial, whereas the union at proximal humerus was only 63.64% (on average of 3.2 months). The efficacy of PRP in treating nonunion of long bone can also be found in a more recently report involving 94 patients [260]. Autologous PRP (> 2,000,000 platelets/μL) with a dose of 15–20 ml was directly injected to the defect sites and the bridging was radiologically evaluated by X-ray at monthly interval till 4 months. Eighty-two patients (87.23%) had their fracture union at the end of 4 months and no complication was documented. Nonetheless, the negative effect of PRP on bone healing was not rare [261], [262]. Ranly et al. reported that PRP may inhibit bone formation through the prevention of osteoinduction in mice models [263], [264].
In these limited number of human clinical trials involving the usage of PRP in treating orthopaedic defects, faster bone healing was demonstrated, whereas its efficacy was still inferior to BMPs. Nevertheless, it is still insufficient evidence to support its routine use in orthopaedic trauma and well-planed, randomized control trials are still needed [265], [266]. Meanwhile, it must be noticed that the platelet activity is influenced by many factors related to the individual whose blood is being collected [267], and therefore the standardized concentration and biological quantification of PRP in treating bone healing requires further studies.
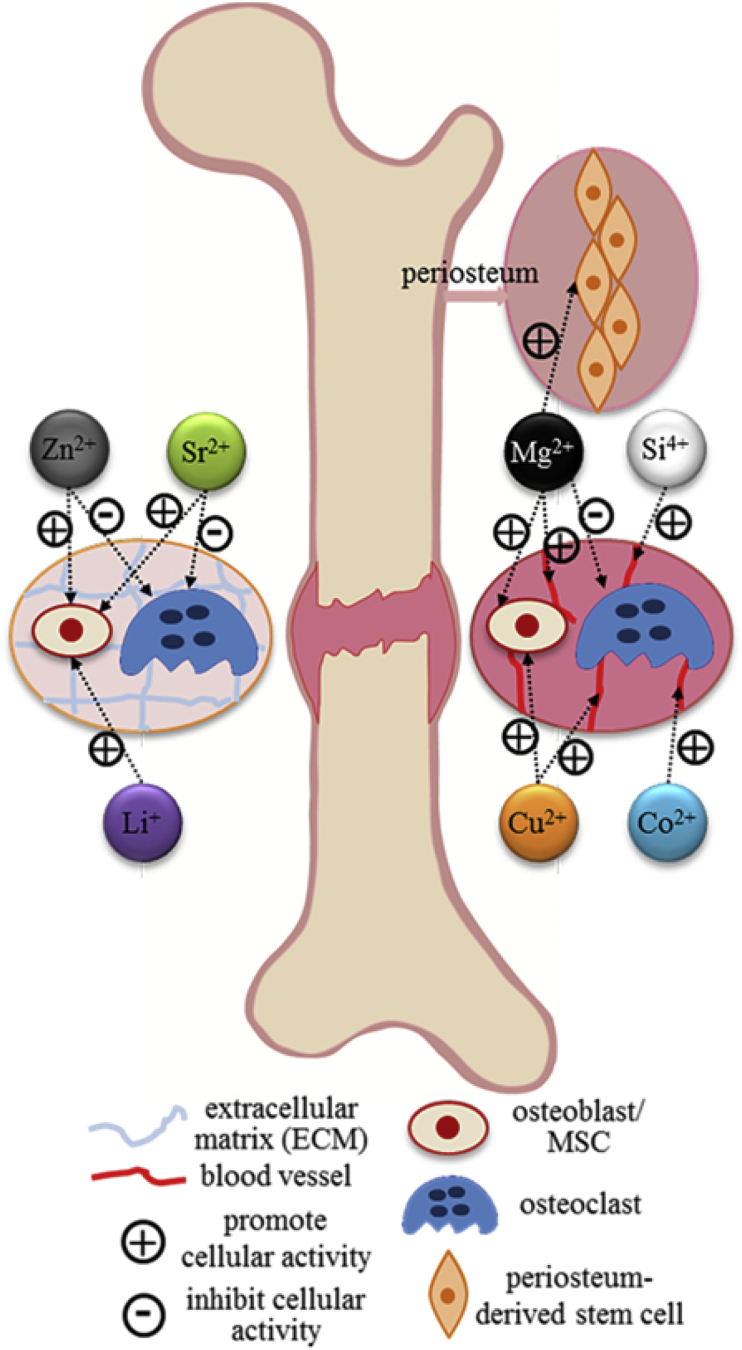
3.9. The adoption of bioinorganic ions on bone regeneration
Triggering by some negative attentions and adverse events regarding the off-label usages of growth factors, safety issue has become a concern [11], [13], [268]. Alternatively, incorporation and/or local delivery of bioinorganic ions, which is also a natural but safer approach, has been highlighted [269]. Inspired by observing nutritional deficiency or excess, bioinorganic ions have long been applied in a variety of therapies even when little was known of their mechanisms [19]. In past few years, the role of metallic ions in the human body had been unraveled gradually (see Table 3 and Fig. 4). Bioinorganic ions, such as silicon, magnesium, strontium, zinc and copper, can still be regarded as essential cofactors of enzymes, coenzymes or prosthetic groups. Additionally, they are actively involved in ion channels or in the process of secondary signaling either on direct stimulation or as an analog [19]. Additionally, incorporation of these ions confers low cost, longer shelf life and perhaps lower risk compared to growth factors [270]. The therapeutic use of bioinorganic ions, especially some heavy metal ions, seems counter-intuitive, but the words of Paracelsus are pertinent: ‘Everything is poisonous and nothing is non-toxic, only the dose makes something not poisonous’ [271]. Consequently, the challenge in using bioinorganic ions in bone healing is also quite clear and has been described succinctly by Ash and Stone: ‘it is indeed a narrow path between poison and nutrition’ [272].
Table 3.
Roles of selected bioinorganic ions and their proposed mechanisms of action (Reprinted from Ref. [270], Copyright (2013), with permission from Elsevier.).
| Role | Mechanism of action | Documented efficiency dosage | |
|---|---|---|---|
| Mg2+ | Osteogenesis, angiogenesis, neural stimulation |
Magnesium induces HIF and activates PGC-1α production in undifferentiated and differentiated hBMSCs, respectively. This stimulates the production of VEGF. Mg2+ enters into DRG neurons and promotes the release of CGPR and then stimulates the PDSCs to express the genes contributing to osteogenic differentiation. |
Mouse pre-osteoblasts and hTMSCs, 50–150 ppm [274], [275], [276]; human BMSCs, 5–10 mM [277], [278] |
| Sr2+ | Osteogenesis | Strontium promotes the activity of bone-forming osteoblastic cells, while inhibiting the bone resorbing osteoclasts. It activates CaSR and downstream signaling pathways. It increases the OPG production and decreases RANKL expression. This promotes osteoblast proliferation, differentiation, and viability and induces the apoptosis of osteoclasts that result to the decrease of bone resorption. |
Rat BMSCs and primary osteoblasts, less than 1 mM [279], [280] |
| Si4+ | Angiogenesis, osteogenesis |
Silicon has been shown to induce angiogenesis by upregulating NOS leading to increased VEGF production at low concentration when cultured with human dermal fibroblasts. Osteogenic mechanism is not well understood. However, Si4+ at higher concentration has been shown to play a vital role in the mineralization process. |
MG-63, HCC1 and human osteoblast-like cells: 10–20 μM [281]; human MSCs: less than 100 μg/mL [282] |
| Zn2+ | Osteogenesis | Zinc is found to get involved in the structural, catalytic or regulation of ALP expression in which it plays an important role in osteogenesis and mineralization. It is also believed that zinc is able to suppress the osteoclastic resorption process. | Mouse Pre-osteoblast: 10−5M [283], [284], [285]; Rat BMSCs: 10−5M [286] |
| Cu+ | Angiogenesis, Osteogenesis |
Copper is reported to be a hypoxia-mimicking factor leading to the induction of angiogenesis. The immune microenvironment induced by Cu2+ may indirectly lead to robust osteogenic differentiation of BMSCs via the activation of Oncostation M (OSM) pathway. | Human BMSCs: less than 50 ppm [287]; mouse pre-osteoblasts: less than 50 ppb [288] |
| Li+ | Osteogenesis | Lithium is able to inhibit the GSK3 expression, which is a negative regulator of the Wnt signaling pathway. Other investigations demonstrated that lithium is able to improve fracture healing by serving as an agonist of Wnt/β-catenin signaling. | Mice: 0.02M daily in drinking water [289] |
| Co2+ | Angiogenesis | Co2+ ion is believed to induce the formation of hypoxia cascade, with which stabilizing HIF-1α. Then, the cells will compensate this hypoxic environment by expressing genes (such as VEGF and EPO) that promote neovascularization and angiogenesis. | Human BMSCs: 100 μM [290], 20 mg/L [291], [292] |
Abbreviations: VEGF, vascular endothelial growth factor; CaSR, calcium sensing receptor; OPG, osteoprotegerin; RANKL, receptor activator of nuclear factor kappa beta ligand; NOS, nitric oxide synthase; ROS, reactive oxygen species; GSK3, glycogen synthase kinase 3; HIF-1α, hypoxia-inducible factor-1α; EPO, erythropoietin; hTMSCs, human TERT-immortalized mesenchymal stem cells; BMSCs, bone marrow stem cells; ALP, alkaline phosphatase.
Fig. 4.
Most common specific targets of relevant bioinorganic ions in their role of therapeutic agents revealed by current researches [273].
3.10. Magnesium (Mg)
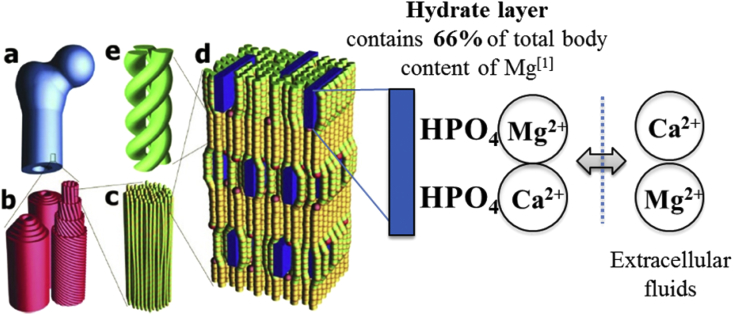
Magnesium is the fourth most abundant cations in the body [293], equal to about 1 mol (24 g) in an adult human body [294] and over 60% is accumulated in bone and teeth [295]. Further studies documented that the majority of Mg that accumulates in bone tissue is concentrated on the hydrated surface layers of apatite crystals instead of incorporated into the lattice structure of bone crystals as showed in Fig. 5. This would allow rapid exchange of Mg2+ between blood and extracellular fluid, leading to ion homeostasis [296], [297], [298]. As an essential element in the human body, magnesium has been found to be cofactor for various enzymatic reactions involved in energy metabolism, protein and nuclei acid synthesis, functional maintenance of parathyroid glands and vitamin D metabolism that are strictly related to bone health [299], [300]. Several researchers studying the effect of a Mg-depleted diet on rats showed decreased systemic bone density [301], inhibition of growth in the proximal end of the tibia [302] and even development of osteoporosis [303]. Meanwhile, a higher intake of magnesium has been proved to efficiently prevent reduction of bone mineral density (BMD) in patients with osteoporosis [304]. On the contrary, toxic symptom induced by magnesium excess is rarely being reported since the Mg concentration is strictly mediated by the kidneys through urine excretion [299].
Fig. 5.
Schematic diagram of hierarchical structure in bone and proposed mechanism of ion-exchange behavior. (a) Macroscopic bone. (b) Haversian osteons in cortical bone, consisting of several concentric lamellar layers that are built from parallel collagen fibers. (c) Fine structure of collagen fiber, consisting of collagen fibrils. (d) Collagen molecular packing with mineral in the fibril. Collagen molecules are shown as green and yellow rods. Mineral crystals are shown as blue tiles. (e) Single molecule triple helix. Reproduced with permission of the International Union of Crystallography [305].
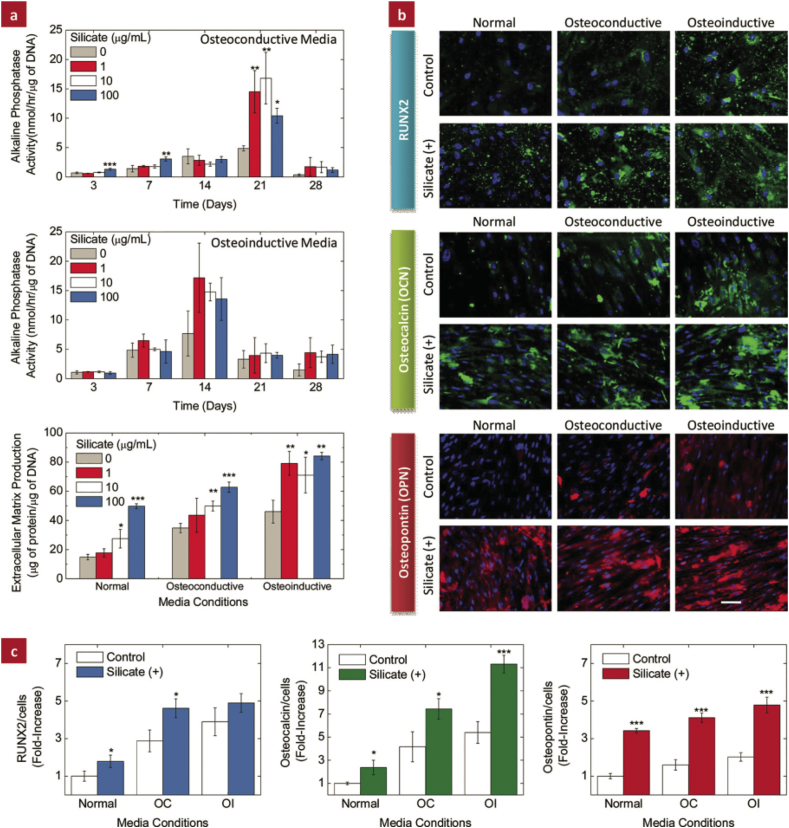
Our previous studies [274], [275] suggested that when magnesium ion concentration fell in an appropriate range (i.e. 50–100 ppm), it was able to up-regulate the viability of mouse pre-osteoblasts. The specific alkaline phosphate (ALP) activity of osteoblasts cultured with Mg ions supplemented media was found to be significantly higher compared with the control. The real-time RT-PCR study also exhibited higher levels of ALP and runt-related transcription factor-2 (Runx2) expression after stimulation with a suitable amount of Mg ions. The highest levels of Type I collagen (Colla 1) and osteopontin (Opn) expression were found on Day 3 from the cells cultured with a conditioned medium. In other research, magnesium was doped into various kinds of materials, including hydroxyapatite, tricalcium phosphate and collage, and the biological activities of those materials were investigated and compared to a non-doped control. Interestingly, when the apatite in the collagen was totally replaced by magnesium, a toxic effect was demonstrated and the formation of extracellular matrix (ECM) was inhibited [306]. However, when the amount of magnesium being doped was controlled in a suitable range [307], densification as well as osteoblastic cellular attachment, proliferation and ALP production improved [277], [278], [308], greater osteogenic properties were also observed in vivo [309]. Meanwhile, the osteoclast formation, polarization, and osteoclast bone resorption was suppressed in vitro [310]. These results are similar to the observations in our previous in vivo studies. High dosage (high-Mg/PCL, 0.6 g Mg in 1 g PCL), low dosage (low-Mg/PCL, 0.1 g Mg in 1 g PCL) Mg/PCL and pure PCL were implanted at the lateral epicondyle of rats. Superior newly formed bone was observed in the low-Mg/PCL group after two months, whereas bone regeneration in the high-Mg/PCL group was even worse than that in control (unpublished data). These phenomena again highlight the importance of dosage when utilizing magnesium in bone healing.
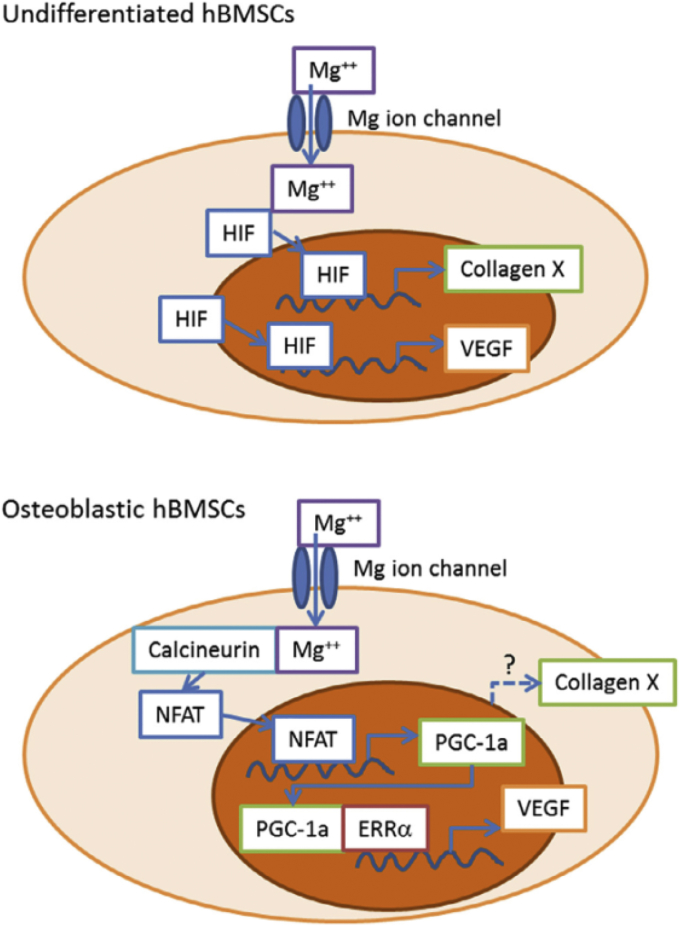
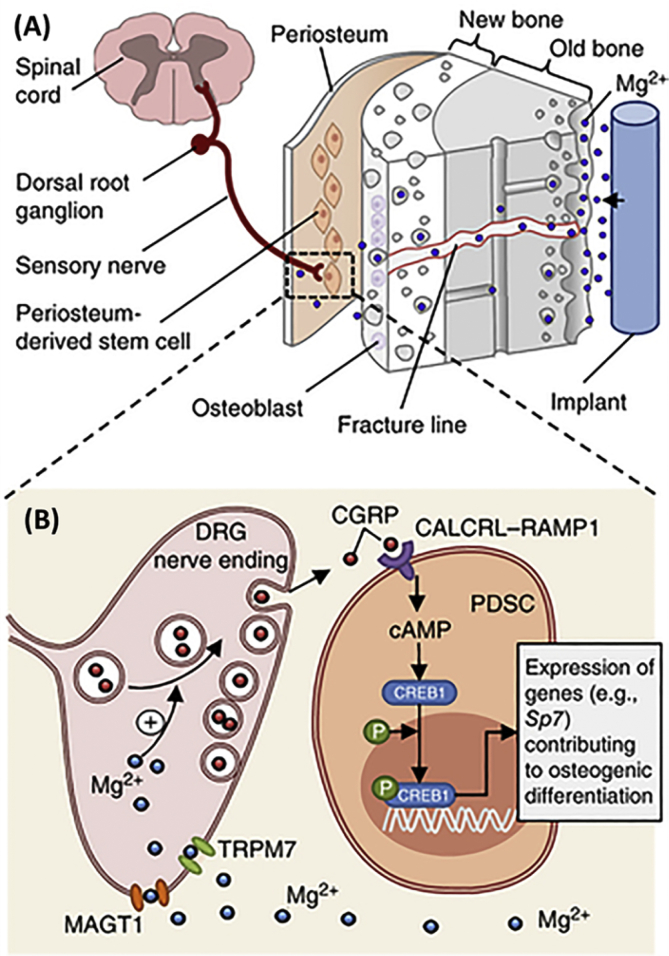
Although the mechanism of magnesium ions on fracture healing is not yet fully explained, recent investigations are bridging this gap. Research conducted by S Yoshizawa et al. [277], [278] conjectured that the osteo-regenerative effect of Mg2+ on undifferentiated human bone marrow stromal cells (hBMSCs) and osteogenic hBMSCs was likely attributed to the subsequent orchestrated responses of activating hypoxia-induced factor 2α (HIF-2α) and peroxisome proliferator-activated receptor gamma coactivator (PGC)-1α, respectively. The hypothesized intracellular signaling cascades were exhibited in Fig. 6. J Zhang et al. found that the rat bone marrow stem cells (BMSCs) depicted significantly up-regulated integrin α5β1 expression when cultured with 5%—Mg-incorporated calcium phosphate cement (5MCPC) and thus promoted the osteogenic differentiation, whereas this effect was not observed when cultured with 10MCPC and 20MCPC [312]. More recently, YF Zhang et al. demonstrated that the magnesium ions may stimulate the accumulation of neuronal calcitonin gene-related polypeptide-α (CGRP) in both the peripheral cortex of the femur and the ipsilateral dorsal root ganglia (DRG) and thus promoted the fracture healing in rat animal model and the mechanism was elucidated in Fig. 7 [311]. This research revealed an undefined role of Mg2+ in CGRP-mediated osteogenic differentiation. In another research, while investigating the long-term in vivo degradation mechanism of Mg alloy, JW Lee et al. found that the existence of Mg may facilitate the crystallization of calcium phosphate in a rabbit femoral condyle defect model [313]. All these recent findings again highlighted the importance of magnesium in fracture healing and suggest the therapeutic potential in orthopaedic clinics.
Fig. 6.
Schematic of hypothesized intracellular signaling cascades by Mg ion stimulation of human bone mesenchymal stem cells (hBMSCs). Addition of MgSO4 will increase intracellular Mg concentration in undifferentiated hBMSCs. HIFs are then translocated into the cell nucleus and induce production of Collagen X-α1 and VEGF. In differentiated hBMSCs, Mg ion activates PGC-1a production, which induces the production of VEGF. Abbreviations: HIF, hypoxia-inducible factor; NFAT, nuclear factor of activated T-cells; PGC-1α, peroxisome proliferation-activated receptor gamma, coactivator 1α; ERRα, estrogen-related receptor α (Reprinted from Ref. [278], Copyright (2014), with permission from Elsevier).
Fig. 7.
Schematic diagram showing (A) diffusion of Mg2+ across the bone toward the periosteum that is innervated by DGR sensory neurons and enriched with PDSCs undergoing osteogenic differentiation into new bone. (B) The released Mg2+ enters DRG neurons via Mg2+ transporters or channels and promotes CGRP-vesicles accumulation and exocytosis. The DRG-released CGRP, in turn, activate the CGRP receptor in PDSCs, which triggers phosphorylation of CREB1 via cAMP and promotes the expression of genes contributing to osteogenic differentiation. Abbreviations: DGR, dorsal root ganglia; PDSCs, periosteum-derived stem cells; CREB1, cAMP-responsive element binding protein 1; cAMP: cyclic adenosine monophosphate (Reprinted by permission from Macmillan Publishers Ltd: Nature Medicine [311], copyright (2016)).
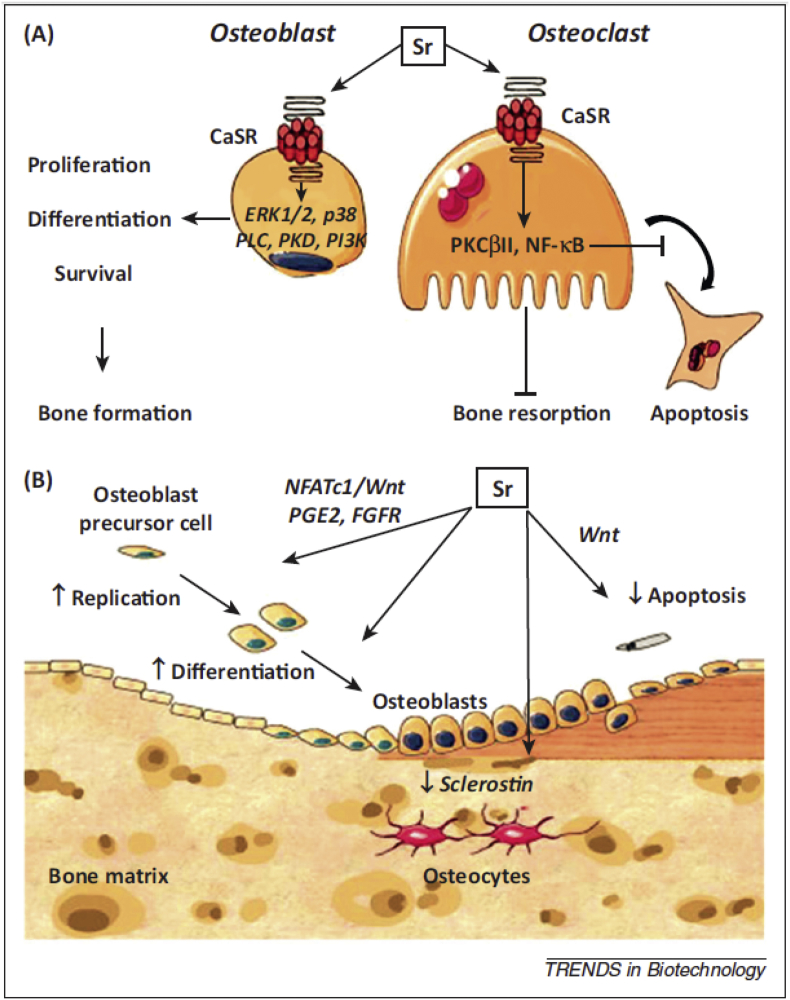
3.11. Strontium (Sr)
Strontium is a bone-seeking element, 98% of which can be found in the skeleton [314]. It accounts for 0.035% of mineral content in the skeleton system [270]. Its size and behavior are similar to calcium as they are in the same periodic group. As a non-essential element, a clinical case regarding the deficiency of strontium is rare but the over feeding of strontium in rats produced rickets by disrupting calcium absorption, vitamin D synthesis and subsequent mineralization [315]. The detailed mechanism is predicted to being attributable to the similarity between strontium and calcium, which allows Sr to share some osteoblast-mediated processes dominated by calcium in bone metabolism as showed in Fig. 8. Briefly, strontium activates the calcium sensing receptor (CaSR) in osteoblast [316], [317] to stimulate the production of osteoprotegerin (OPG) [318], [319], which subsequently suppresses the expression of the receptor activator of nuclear factor kappa beta ligand (RANKL), thus inhibiting RANKL-induced osteocalstogenesis [320]. In one in vitro experiment, rats bone marrow mesenchymal stem cells (BMMCSs) were cultured in an osteogenic medium supplemented with 0.1 or 1 mM Sr2+ (8.7 mg/L or 87 mg/L) for two weeks. Proliferation of BMMCSs was significantly inhibited, while osteoblastic differentiation was promoted dose-dependently [280]. In another cell culture experiment, the dose-dependent effects of strontium on rat primary osteoblasts in terms of nodule formation and mineralization were observed compared to the Sr-depleted control. In the conditioned medium supplemented with a low dosage of Sr (0.5 and 1 μg/mL), nodule formation was reduced, while mineralization was intact. In the conditioned medium supplemented with an intermediate dosage of Sr (2 and 5 μg/mL), neither of these process was affected. In the conditioned medium supplemented with high dosage of Sr (20 and 100 μg/mL), nodule formation was not affected but mineralization was reduced, indicating that the formation of hydroxyapatite was inhibited [279]. Meanwhile, the ability of the strontium to reduce bone resorption [321] and osteoclast activity [322] was also observed when cultured with rat osteoclasts and primary mature rabbit osteoclasts, respectively. Given the dual roles of strontium on bone formation, one strontium salt, strontium ranelate, has been utilized clinically as a prescriptive treatment for postmenopausal women with osteoporosis in Europe [323].
Fig. 8.
(A) A schematic showing the dual mechanism of action of strontium (Sr): the stimulatory role on bone-forming osteoblast cells and the inhibitory role on bone resorbing osteoclast cells. (B) A schematic showing how Sr activates osteoblastogenesis. Abbreviations: CaSR, calcium sensing receptor; ERK1/2, extracellular signal-regulated kinases 1/2; P38, a mitogen-activated protein kinases; PLC, phospholipase C; PKD, protein kinase D; PI3K, phosphatidylinositide 3-kinases; PKCβII, protein kinase C βII; NF-kB, nuclear factor kappa beta; NFATc, nuclear factors of activated T cells; PGE2, prostaglandin, E2; FGFR, fibroblast growth factor receptor (Reprinted from Refs. [270], [324], Copyright (2013, 2012), with permission from Elsevier).
Attempts have also been made to incorporate strontium into synthetic mineral ceramics; mechanisms may involve an exchange with ions on an apatite surface or heteroionic substitution [325]. Data showed that tricalcium phosphate was able to host up to 20 wt.% of strontium [326], [327] and the number was 12 wt.% in hydroxyapatite [328], [329], without provoking rearrangement of the unit cell. The biological activity of those strontium-substituted mineral ceramics has been documented in numerous studies, demonstrating pronounced apatite layer formation [330], increased attachment, proliferation and differentiation when cultured with osteoprecursor cells [331] and human osteoblasts MG-63, and suppressed osteoclasts proliferation [332]. Similar results were displayed in animal studies [333], [334], [335]. Enhanced new bone formation was presented on the surface of strontium-containing mineral ceramics, while resorptive activity of osteoclasts was inhibited. Nevertheless, while analyzing these in vivo characterizations, it must be noted that strontium substitution not only releases Sr2+ into the microenvironment but also alters the other physico-chemical properties, and these effects cannot be isolated from the final results.
Recently, concerns about the adverse side effect of strontium ranelate in patients with established, current or history of cardiovascular events and venous thrombosis has been highlighted [336]. In 2013, increased risk of myocardial infarction in postmenopausal women was reported when using strontium, thus leading to the suspension of this drug [337]. More importantly, this drug has not yet been approved by the FDA so far.
3.12. Silicon (Si)
Silicon is the second most abundant element on earth, and since silicate (a silicate is a silicon-containing anion) is rich in foods and water, deficiency in humans is rare and its pathology is unknown. But in an animal model, chickens on a silicon-depleted diet showed deformed bone development, low collagen formation and stunted growth [338]. Research found that silicon is rich in bone and connective tissue as an integral component of glycosaminoglycan and their protein complexes [339], which may subsequently affect bone formation and maintenance [340]. In research performed by Carlisle, an electron micro-probe was applied to locate silicon in the tibial bones of young mice and rats. Silicon was detected in the early stages of the bio-mineralization process at an active calcification site, increasing in parallel with calcium at low calcium concentrations, and diminishing when the mineral composition approached hydroxyapatite [341]. These observation have confirmed that silicon is associated with calcium in bone metabolism [342].
While testing the effect of aqueous silicon on cellular activity, dose-dependent enhancement of osteoblast proliferation, differentiation and collagen production were observed in vitro [281], [343]. Human osteoblast cells demonstrated 1.8-, 1.5- and 1.2-fold increases in type I collagen, alkaline phosphatase and osteocalcin activity, respectively, when cultured with a conditioned medium supplemented with 0–1.4 ppm (50 μM Si) of orthosilicic acid [281]. In another study, human osteoblast-like cell was incubated with 0.1–100 ppm (3.6 mM) Si for 48 h, and a dose-dependent increase in proliferation and osteogenic differentiation mediated through up-regulation of transforming growth factor beta (TGF-β) was reported [343]. In a recently published paper [282], bioactive silicate nanoplatelets with a concentration of 100 μg/ml triggered osteogenic differentiation of human mesenchymal stem cells (hMSCs) without any osteoinductive factor, and this effect dropped when the concentration exceeded 1 mg/ml (Fig. 9). However, the mechanism of inducing osteogenic differentiation of hMSCs is not fully explained. In numerous bone healing investigations involving the application of Si-substituted CaPs, including Si-HA and Si-TCP, superior biological performance of their stoichiometric counterparts has been documented [344]. However, a critical review pointed out that no direct evidence can link the improved biological performance of Si-substituted CaPs to the released Si [342], since the substitution of silicon not only alters the Si release but may also change the dissolution rate of ceramics [345], [346], grain size in structural composition [347], [348], protein conformation at the material surface [349], or surface topography [348], [350]. As a kind of silica based synthetic bone substitute widely used in orthopaedic applications, bioglass can't be ignored when discussing the effect of silicon on bone regeneration. As mentioned before, it is speculated that the bioactivity of bioglass is attributed to the leaching and accumulating of silicon ions when exposed to body fluids upon implantation, and the subsequently formation of hydroxyapatite coating on the surface [152]. Nevertheless, it is accepted that the hydroxyapatite coating, but not the leaching silicon ions, played an active role in the processes leading to new bone formation [19].
Fig. 9.
Effect of silicate nanoplatelets on hMSCs differentiation. a) The addition of silicate nanoplatelets upregulate alkaline phosphatase (ALP) activity of hMSCs. b) The increase in the RUNX2 (green) and production of bone-related proteins such as osteocalcin (OCN, green), and osteopontin (OPN, red) was observed due to the addition of silicates. Cells in normal media without silicate particles act as a negative control whereas cells in osteoinductive medium serve as a positive control. Cell nuclei were counterstained with DAPI (blue). c) The protein production was quantified using image analysis from the fluorescence images. The intensity of protein per cell was quantified and later normalized by the control (hMSCs in normal growth media with no silicate particles) to obtain a fold-increase in the production of protein (Reprint with permission from Ref. [282]).
3.13. Zinc (Zn)
Zinc is also an essential trace element in the human body with total weight at about 1.4–2.3 g and this number is between 150 and 250 μg/g in bone ash (0.015–0.025 wt.%), which is higher than other tissues [351]. It is involved in the structural, catalytic or regulatory action of several important metalloenzymes and alkaline phosphatase (ALP) is among them. It was found that ALP not only generate phosphates by hydrolyzing pyrophosphates, but they also created an alkaline environment that favored the precipitation and subsequent mineralization of these phosphates onto the extracellular matrix, which were produced by osteoblasts [270]. A far as we know, ALP is a zinc enzyme with three closely spaced metal ions (two Zn ions and one Mg ion) presented at the active center [352]. Further investigations suggested that inactivation of ALP activity is caused by the dissociation of an active center Zn, and preventing the dissociation of this active center Zn can stabilize the enzyme and increase its half-life [353]. Given its important role in skeleton system, zinc deficiency is reported to be associated with decreased bone age [19], whereas high zinc level may lead to hypocupremia by retarding the intestinal absorption of copper [354]. These findings are coincident with some cell culture experiments. Yamaguchi et al. [283] showed significantly increased Runx2, OPG and regucalcin mRNA expressions in osteoblastic MC3T3-E1 cells when zinc supplementation was in the concentration of 10−5 to 10−4 M (0.65 mg/L to 6.5 mg/L). Kwun et al. [284] observed a negative effect of zinc deficiency on the osteogenic activity of the same cell type, bone marker gene transcription and ECM mineralization were reduced through inhibited and delayed Runx2 expression and inhibition of ALP activity in osteoblasts, respectively. However, these regulation effects were not displayed when cultured rat bone marrow stem cells with Zn2+ were supplemented (1*10−5 and 4*10−5 M, eq. to 0.65 mg/L and 2.6 mg/L) osteogenic medium [286]. Suppression effects of zinc on bone resorption and osteoclastogenesis from bone marrow derived osteoclasts were recently shown in the tissue culture system [355].
Zinc has also been found to stabilize the crystal lattice of β-tricalcium phosphate, thus making the dissolution of TCP predictable and complete [326]. In the rabbit femoral defect model, zinc-containing HA/TCP with a high concentration (about 0.6 wt.%, high-Zn-HA/TCP) or low concentration (about 0.3 wt.%, low-Zn-HA/TCP) was applied. Low-Zn-HA/TCP demonstrated increased bone apposition, and high-Zn-HA/TCP led to increased resorption of the host bone [356]. However, in another in vitro study, ceramics containing 0.6 wt.% of zinc displayed inhibited resorptive activity in mature osteoclasts [357]. Still, the boundary between the in vitro studies and in vivo assessments are huge, and controversial results are reported.
3.14. Copper (Cu)
Copper is an essential element, and 90% of copper in plasma is presented in ceruloplasmin [19]. Copper's function in human body was firstly identified in iron metabolism and copper deficiency usually leads to iron overload in brain, liver and other tissues [358]. Later on, copper has been recognized as a cofactor for several other enzymes in body, one of which related to the musculoskeletal system is lysyl oxidase, an enzyme that catalyzes the formation of aldehyde-based crosslinks from lysine residues in collagen and elastin precursors [19]. Consequently, 300% higher collagen solubility was found to lead to copper-deficiency and brittle bone [359]. Recently, being discovered as an essential element in angiogenesis [360] and to initiate endothelia cells towards angiogenesis [361], [362], the application of copper ions as an alternative therapeutic agent in promoting vascularization has attracted increasing attentions [362], [363], [364], [365]. Since vascularization plays a critical role in bone healing [37], it is reasonable to conduct relevant research. Several studies have demonstrated rapid and enhanced vascularization in copper-doped porous scaffolds and increased extracellular matrix (ECM) formation, and the collagen formation in turn supports further blood vessel formation in vivo physically [366], [367]. Interestingly, instead of utilizing the traditional biomaterial-assisted concept, which is to accelerate bone ingrowth from the periphery, copper-doped biomaterials demonstrated a tendency to accelerate bone formation throughout the defect, which is likely attributed to angiogenic effect of Cu2+ [19]. Lately, copper ion has been predicted to positively affect osteogenic differentiation of bone marrow stem cells [287], [368] and mouse MC3T3-E1 preosteoblasts [288] when being doped into mesoporous silica nanosphere and stainless steel, respectively. Nevertheless, excess copper can lead to serious disease in the liver and neurological issues [369], [370]. Again, dosage is critical.
3.15. Other ions
Lithium (Li) has attracted attention due to its role in osteogenesis [270]. A study involving 75 lithium-treated patients reported that the mean bone mineral density in several areas in a treated group was significantly higher than normal participants; this was possibly due to a lower bone turnover in lithium-treated patients [371]. Another case control study compared 231,778 fracture cases and found that the current use of lithium showed a decreased risk of fracture, while an increased risk of fracture was observed among past users [372]. The mechanism of lithium behind osteogenesis is predicted by inhibiting glycogen synthase kinase 3 (GSK3), which is a negative regulator of the Wnt signaling pathway [373], [374]. In addition, Li+ has been shown to activate β-catenin signaling by mediating osteoblast proliferation, differentiation and maturation [375], during bone and cartilage fracture healing [289].
Cobalt (Co) is an integral part of vitamin B12, which stimulates the production of red blood cells [273]. Like copper, cobalt was recently showed to stimulate angiogenesis [270]. Significantly upregulated VEGF was expressed when bone marrow stem cells were treated in a 100 μM CoCl2 supplemented culture medium, and these CoCl2-treated cells subsequently promoted vascularization and osteogenesis when implanted in vivo with a collagen scaffold [290]. This effect was recently found to associate with the hypoxia-mimicking capacity of Co ions. Studies demonstrated that mesoporous bioactive glass (MBG) scaffolds doped with suitable amount of cobalt induce the hypoxia cascade, with which the hypoxia inducible factor-1 (HIF-1) was activated [291] and stabilized [292], and this increased the expression of HIF-α target genes, such as VEGF and erythropoietin (EPO) [376], thus leading to a higher degree of vascularization.
4. Conclusion
Orthopaedics scenarios such as large segmental bone defect may result in delayed union or even non-union if improperly treated clinically. Hence, surgical interventions together with bone grafting techniques are usually necessitated in the treatment process. Even though the emergence of various synthetic bone substitutes offers diversity options, the treatment outcome is still incomparable to the approach of autologous bone graft in terms of bone healing quality and time. One of the reasons leading to inferior bone regeneration when synthetic substitutes used is due to the lack of osteoinductivity. The incorporations of recombinant human growth factors (e.g. rhBMP-2) with bone allograft and other substitutes have been considered and widely applied in clinics. Their clinical outcomes have been extensively reported as well. The treatment efficiency has been approved though. The post-operative complications, the controversy of off-label applications and high application cost have been also documented. The complications may attribute to the uncontrolled release of growth factor that collaterally interfere the un-targeting cells. Alternatively, the incorporation of bioinorganic ions such as magnesium, strontium, silicon, copper and cobalt into bone graft materials provides an economic and feasible solution for bone defect healing. Hopefully, the safety issue of those bioinorganic ions used has been addressed by a number of studies in these years. When therapeutic effect and working mechanism of those ions have been clearly understood, human clinical trial can be possibly implemented. With more new discoveries, bioinorganic ions can be considered to apply in the combination of growth factors for bone defect treatment that may induce synergistic effect in terms of new bone formation.
Competing interests
The authors declare that they have no competing interests.
Acknowledgements
This work was supported in part by Shenzhen Science and Technology Innovation Funding JCYJ20140414090541811, JCYJ20160429190821781 and JCYJ2016429185449249, Hong Kong Research Grant Council General Research Funds (RGC GRF) (Nos. 718913E, 17214516, N_HKU725/16), HKU Seeding Fund (Nos. 201511160001 and 201411159045), Hong Kong Innovation Technology Fund (No. ITS/147/15), Hong Kong Health and Medical Research Fund (No. 03142446), National Natural Science Foundation of China (NSFC) (Nos. 31370957).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Bhatt R.A., Rozental T.D. Bone graft substitutes. Hand Clin. 2012;28:457–468. doi: 10.1016/j.hcl.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Greenwald A.S., Boden S.D., Goldberg V.M., Yaszemski M., Heim C.S. Bone-graft Substitutes: Facts, Fictions and Applications, AAOS 75th Annual Meeting. 2008. [DOI] [PubMed] [Google Scholar]
- 3.Dimitriou R., Jones E., McGonagle D., Giannoudis P.V. Bone regeneration: current concepts and future directions. BMC Med. 2011;9:66. doi: 10.1186/1741-7015-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campana V., Milano G., Pagano E., Barba M., Cicione C., Salonna G., Lattanzi W., Logroscino G. Bone substitutes in orthopaedic surgery: from basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014;25:2445–2461. doi: 10.1007/s10856-014-5240-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauer T.W., Muschler G.F. Bone graft materials: an overview of the basic science. Clin. Orthop. Relat. Res. 2000;371:10–27. [PubMed] [Google Scholar]
- 6.de Long W.G., Einhorn T.A., Koval K., McKee M., Smith W., Sanders R., Watson T. Bone grafts and bone graft substitutes in orthopaedic trauma surgery: a critical analysis. J. Bone Jt. Surg. Am. 2007;89:649–658. doi: 10.2106/JBJS.F.00465. [DOI] [PubMed] [Google Scholar]
- 7.Fillingham Y., Jacobs J. Bone grafts and their substitutes. Bone Jt. J. 2016;98:6–9. doi: 10.1302/0301-620X.98B.36350. [DOI] [PubMed] [Google Scholar]
- 8.C.F.D. Control, Transmission of HIV through bone transplantation: case report and public health recommendations. MMWR Morb. Mortal. Wkly. Rep. 1988;37:597. [PubMed] [Google Scholar]
- 9.Stevenson S., Horowitz M. The response to bone allografts. J. Bone Jt. Surg. 1992;74:939–950. [PubMed] [Google Scholar]
- 10.Jahangir A.A., Nunley R.M., Mehta S., Sharan A., Fellows t.W.H.P. AAOS; 2008. Bone-graft substitutes in orthopaedic surgery. [Google Scholar]
- 11.GlobalData . 2014. MediPoint: Bone Grafts and Substitutes - Global Analysis and Market Forecasts. [Google Scholar]
- 12.Kurien T., Pearson R.G., Scammell B.E. Bone graft substitutes currently available in orthopaedic practice: the evidence for their use. Bone Jt. J. 2013;95-b:583–597. doi: 10.1302/0301-620X.95B5.30286. [DOI] [PubMed] [Google Scholar]
- 13.FDA . July 1, 2008. Life-threatening Complications Associated with Recombinant Human Bone Morphologenetic Protein in Cervicla Spine Fusion. FDA Public Health Notification. [Google Scholar]
- 14.FDA . July 27, 2010. AMPLIFY™ rhBMP-2 Matrix: Orthopaedic and Rehabilitation Devices Advisory Pannel Presentation. [Google Scholar]
- 15.Hoffman M.D., Xie C., Zhang X., Benoit D.S. The effect of mesenchymal stem cells delivered via hydrogel-based tissue engineered periosteum on bone allograft healing. Biomaterials. 2013;34:8887–8898. doi: 10.1016/j.biomaterials.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Long T., Zhu Z., Awad H.A., Schwarz E.M., Hilton M.J., Dong Y. The effect of mesenchymal stem cell sheets on structural allograft healing of critical sized femoral defects in mice. Biomaterials. 2014;35:2752–2759. doi: 10.1016/j.biomaterials.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffin K.S., Davis K.M., Mckinley T.O., Anglen J.O., Chu T.M.G., Boerckel J.D., Kacena M.A. Evolution of bone grafting: bone grafts and tissue engineering strategies for vascularized bone regeneration. Clin. Rev. Bone Min. Metab. 2015;13:232–244. [Google Scholar]
- 18.Bhumiratana S., Bernhard J.C., Alfi D.M., Yeager K., Eton R.E., Bova J., Gimble J.M., Lopez M.J., Eisig S.B., Vunjak-Novakovic G. Tissue-engineered autologous grafts for facial bone reconstruction. Sci. Transl. Med. 2016;8 doi: 10.1126/scitranslmed.aad5904. 343ra383-343ra383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Habibovic P., Barralet J.E. Bioinorganics and biomaterials: bone repair. Acta Biomater. 2011;7:3013–3026. doi: 10.1016/j.actbio.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 20.Seeman E. Modeling and remodeling. In: Bilezikian J.P., Raisz L.G., Martin T.J., editors. Principles of Bone Biology. Academic Press; 2008. pp. 3–28. [Google Scholar]
- 21.Nair A.K., Gautieri A., Chang S.W., Buehler M.J. Molecular mechanics of mineralized collagen fibrils in bone. Nat. Commun. 2013;4:1724. doi: 10.1038/ncomms2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jäger I., Fratzl P. Mineralized collagen fibrils: a mechanical model with a staggered arrangement of mineral particles. Biophys. J. 2000;79:1737–1746. doi: 10.1016/S0006-3495(00)76426-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta H.S., Seto J., Wagermaier W., Zaslansky P., Boesecke P., Fratzl P. Cooperative deformation of mineral and collagen in bone at the nanoscale. Proc. Natl. Acad. Sci. 2006;103:17741–17746. doi: 10.1073/pnas.0604237103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fratzl P., Weinkamer R. Nature's hierarchical materials. Prog. Mater. Sci. 2007;52:1263–1334. [Google Scholar]
- 25.Currey J.D. The relationship between the stiffness and the mineral content of bone. J. Biomech. 1969;2:477–480. doi: 10.1016/0021-9290(69)90023-2. [DOI] [PubMed] [Google Scholar]
- 26.Agna J.W., Knowles H.C., Jr., Alverson G. The mineral content of normal human bone. J. Clin. Invest. 1958;37:1357. doi: 10.1172/JCI103725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lieberman J.R., Friedlaender G.E. Springer; 2005. Chapter I. Bone Dynamics, Bone Regeneration and Repair; p. 1. [Google Scholar]
- 28.Frost H.M. CRC; 1986. Intermediary Organization of the Skeleton. [Google Scholar]
- 29.Kimmel D.B. A paradigm for skeletal strength homeostasis. J. Bone Min. Res. 1993;8:S515–S522. doi: 10.1002/jbmr.5650081317. [DOI] [PubMed] [Google Scholar]
- 30.Raisz L.G. Physiology and pathophysiology of bone remodeling. Clin. Chem. 1999;45:1353–1358. [PubMed] [Google Scholar]
- 31.Parfitt A. Osteonal and hemi-osteonal remodeling: the spatial and temporal framework for signal traffic in adult human bone. J. Cell. Biochem. 1994;55:273–286. doi: 10.1002/jcb.240550303. [DOI] [PubMed] [Google Scholar]
- 32.Doblaré M., García J.M., Gómez M.J. Modelling bone tissue fracture and healing: a review. Eng. Fract. Mech. 2004;71:1809–1840. [Google Scholar]
- 33.Martin A.D., McCulloch R. Bone dynamics: stress, strain and fracture. J. Sports Sci. 1987;5:155–163. doi: 10.1080/02640418708729773. [DOI] [PubMed] [Google Scholar]
- 34.Einhorn T.A., Gerstenfeld L.C. Fracture healing: mechanisms and interventions. Nat. Rev. Rheumatol. 2015;11:45–54. doi: 10.1038/nrrheum.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seta J.J. Healing of bone fracture: general concepts. In: Sela J.J., Bab I.A., editors. Principles of Bone Regeneration. Springer Science & Business Media; 2012. pp. 1–8. [Google Scholar]
- 36.DeLacure M.D. Physiology of bone healing and bone grafts. Otolaryngol. Clin. North Am. 1994;27:859–874. [PubMed] [Google Scholar]
- 37.Sfeir C., Ho L., Doll B.A., Azari K. Fracture repair. In: Lieberman J.R., Friedlaender G.E., editors. Bone Regeneration and Repair. Springer; 2005. pp. 21–44. [Google Scholar]
- 38.Einhorn T.A. The cell and molecular biology of fracture healing. Clin. Orthop. Relat. Res. 1998;355:S7–S21. doi: 10.1097/00003086-199810001-00003. [DOI] [PubMed] [Google Scholar]
- 39.Lee F.Y.I., Choi Y.W., Behrens F.F., DeFouw D.O., Einhorn T.A. Programmed removal of chondrocytes during endochondral fracture healing. J. Orthop. Res. 1998;16:144–150. doi: 10.1002/jor.1100160124. [DOI] [PubMed] [Google Scholar]
- 40.Gerstenfeld L., Cho T.J., Kon T., Aizawa T., Tsay A., Fitch J., Barnes G., Graves D., Einhorn T. Impaired fracture healing in the absence of TNF-α signaling: the role of TNF-α in endochondral cartilage resorption. J. Bone Min. Res. 2003;18:1584–1592. doi: 10.1359/jbmr.2003.18.9.1584. [DOI] [PubMed] [Google Scholar]
- 41.Melnyk M., Henke T., Claes L., Augat P. Revascularisation during fracture healing with soft tissue injury. Arch. Orthop. Trauma Surg. 2008;128:1159–1165. doi: 10.1007/s00402-007-0543-0. [DOI] [PubMed] [Google Scholar]
- 42.Holstein J.H., Karabin-Kehl B., Scheuer C., Garcia P., Histing T., Meier C., Benninger E., Menger M.D., Pohlemann T. Endostatin inhibits callus remodeling during fracture healing in mice. J. Orthop. Res. 2013;31:1579–1584. doi: 10.1002/jor.22401. [DOI] [PubMed] [Google Scholar]
- 43.Reichert J.C., Saifzadeh S., Wullschleger M.E., Epari D.R., Schütz M.A., Duda G.N., Schell H., van Griensven M., Redl H., Hutmacher D.W. The challenge of establishing preclinical models for segmental bone defect research. Biomaterials. 2009;30:2149–2163. doi: 10.1016/j.biomaterials.2008.12.050. [DOI] [PubMed] [Google Scholar]
- 44.Gugala Z., Gogolewski S. Healing of critical-size segmental bone defects in the sheep tibiae using bioresorbable polylactide membranes. Injury. 2002;33:71–76. doi: 10.1016/s0020-1383(02)00135-3. [DOI] [PubMed] [Google Scholar]
- 45.Laurencin C., Khan Y., El-Amin S.F. Bone graft substitutes. Expert. Rev. Med. devices. 2006;3:49–57. doi: 10.1586/17434440.3.1.49. [DOI] [PubMed] [Google Scholar]
- 46.Wildemann B., Kadow-Romacker A., Pruss A., Haas N., Schmidmaier G. Quantification of growth factors in allogenic bone grafts extracted with three different methods. Cell Tissue Bank. 2007;8:107–114. doi: 10.1007/s10561-006-9021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Claes L., Eckert-Hübner K., Augat P. The fracture gap size influences the local vascularization and tissue differentiation in callus healing. Langenbecks Arch. Surg. 2003;388:316–322. doi: 10.1007/s00423-003-0396-0. [DOI] [PubMed] [Google Scholar]
- 48.Cacchioli A., Spaggiari B., Ravanetti F., Martini F., Borghetti P., Gabbi C. The critical sized bone defect: morphological study of bone healing. Ann. Fac. Medic. Vet. Parma. 2006;26:97. [Google Scholar]
- 49.Spicer P.P., Kretlow J.D., Young S., Jansen J.A., Kasper F.K., Mikos A.G. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat. Protoc. 2012;7:1918–1929. doi: 10.1038/nprot.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gugala Z., Gogolewski S. Regeneration of segmental diaphyseal defects in sheep tibiae using resorbable polymeric membranes: a preliminary study. J. Orthop. Trauma. 1999;13:187–195. doi: 10.1097/00005131-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 51.Lasanianos N.G., Kanakaris N.K., Giannoudis P.V. Current management of long bone large segmental defects. Orthop. Trauma. 2010;24:149–163. [Google Scholar]
- 52.Gugala Z., Lindsey R.W., Gogolewski S. Wiley Online Library; 2007. New approaches in the treatment of critical-size segmental defects in long bones; pp. 147–161. Macromol Symp. [Google Scholar]
- 53.Lindsey R.W., Gugala Z., Milne E., Sun M., Gannon F.H., Latta L.L. The efficacy of cylindrical titanium mesh cage for the reconstruction of a critical-size canine segmental femoral diaphyseal defect. J. Orthop. Res. 2006;24:1438–1453. doi: 10.1002/jor.20154. [DOI] [PubMed] [Google Scholar]
- 54.Khan S.N., Cammisa F.P., Sandhu H.S., Diwan A.D., Girardi F.P., Lane J.M. The biology of bone grafting. J. Am. Acad. Orthop. Surg. 2005;13:77–86. [PubMed] [Google Scholar]
- 55.Albrektsson T., Johansson C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001;10:S96–S101. doi: 10.1007/s005860100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilson-Hench J. Osteoinduction. Prog. Biomed. Eng. 1987;4:29. [Google Scholar]
- 57.Roberts T.T., Rosenbaum A.J. Bone grafts, bone substitutes and orthobiologics: the bridge between basic science and clinical advancements in fracture healing. Organogenesis. 2012;8:114–124. doi: 10.4161/org.23306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goldberg V.M., Akhavan S. Biology of bone grafts. In: Lieberman J.R., Friedlaender G.E., editors. Bone Regeneration and Repair. Springer; 2005. pp. 57–65. [Google Scholar]
- 59.Flynn J. 2011. Fracture Repair and Bone Grafting, OKU 10: Orthopaedic Knowledge Update; pp. 11–21. [Google Scholar]
- 60.Chiarello E., Cadossi M., Tedesco G., Capra P., Calamelli C., Shehu A., Giannini S. Autograft, allograft and bone substitutes in reconstructive orthopedic surgery. Aging Clin. Exp. Res. 2013;25:101–103. doi: 10.1007/s40520-013-0088-8. [DOI] [PubMed] [Google Scholar]
- 61.Kovar F.M., Wozasek G.E. Bone graft harvesting using the RIA (reaming irrigation aspirator) system–a quantitative assessment. Wien. Klin. Wochenschr. 2011;123:285–290. doi: 10.1007/s00508-011-1565-8. [DOI] [PubMed] [Google Scholar]
- 62.Dimitriou R., Mataliotakis G.I., Angoules A.G., Kanakaris N.K., Giannoudis P.V. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: a systematic review. Injury. 2011;42(Suppl 2):S3–S15. doi: 10.1016/j.injury.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 63.Belthur M.V., Conway J.D., Jindal G., Ranade A., Herzenberg J.E. Bone graft harvest using a new intramedullary system. Clin. Orthop. Relat. Res. 2008;466:2973–2980. doi: 10.1007/s11999-008-0538-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pape H.-C., Tarkin I.S. Reamer irrigator aspirator: a new technique for bone graft harvesting from the intramedullary canal. Oper. Tech. Orthop. 2008;18:108–113. [Google Scholar]
- 65.Sagi H.C., Young M.L., Gerstenfeld L., Einhorn T.A., Tornetta P. Qualitative and quantitative differences between bone graft obtained from the medullary canal (with a Reamer/Irrigator/Aspirator) and the iliac crest of the same patient. J. Bone. Jt. Surg. Am. 2012;94:2128–2135. doi: 10.2106/JBJS.L.00159. [DOI] [PubMed] [Google Scholar]
- 66.Mauffrey C., Barlow B.T., Smith W. Management of segmental bone defects. J. Am. Acad. Orthop. Surg. 2015;23:143–153. doi: 10.5435/JAAOS-D-14-00018. [DOI] [PubMed] [Google Scholar]
- 67.Torres J., Tamimi F., Alkhraisat M., Prados-Frutos J.C., Lopez-Cabarcos E. Bone substitutes. In: Turkyilmaz I., editor. Implant Dentistry - the Most Promising Discipline of Dentistry. InTech; 2011. pp. 4–108. [Google Scholar]
- 68.Burchardt H. Biology of bone transplantation. Orthop. Clin. North Am. 1987;18:187–196. [PubMed] [Google Scholar]
- 69.Heiple K.G., Chase S.W., Herndon C.H. A comparative study of the healing process following different types of bone transplantation. J. Bone Jt. Surg. 1963;45:1593–1616. [PubMed] [Google Scholar]
- 70.Abbott L.C., Schottstaedt E.R., Saunders J.B.D.C., Bost F.C. The evaluation of cortical and cancellous bone as grafting material. J. Bone Jt. Surg. 1947;29:381–414. [PubMed] [Google Scholar]
- 71.Stevenson S. The immune response to osteochondral allografts in dogs. J. Bone Jt. Surg. 1987;69:573–582. [PubMed] [Google Scholar]
- 72.Stevenson S., Li X.Q., Martin B. The fate of cancellous and cortical bone after transplantation of fresh and frozen tissue-antigen-matched and mismatched osteochondral allografts in dogs. J. Bone Jt. Surg. 1991;73:1143–1156. [PubMed] [Google Scholar]
- 73.DoNon I. 1984. Studies on the Antigenicity of Bone. [Google Scholar]
- 74.Voggenreiter G., Ascherl R., Blümel G., Schmit-Neuerburg K. Effects of preservation and sterilization on cortical bone grafts. Arch. Orthop. Trauma Surg. 1994;113:294–296. doi: 10.1007/BF00443821. [DOI] [PubMed] [Google Scholar]
- 75.Urist M. Lippincott; 1980. Fundamental and Clinical Bone Physiology. [Google Scholar]
- 76.Kotz R., Poitout D.G. Springer Science & Business Media; 2013. Biomechanics and Biomaterials in Orthopedics. [Google Scholar]
- 77.Boyce T., Edwards J., Scarborough N. Allograft bone: the influence of processing on safety and performance. Orthop. Clin. North Am. 1999;30:571–581. doi: 10.1016/s0030-5898(05)70110-3. [DOI] [PubMed] [Google Scholar]
- 78.Finkemeier C.G. Bone-grafting and bone-graft substitutes. J. Bone Jt. Surg. 2002;84:454–464. doi: 10.2106/00004623-200203000-00020. [DOI] [PubMed] [Google Scholar]
- 79.Greenwald A.S., Boden S.D., Barrack R.L., Bostrom M.P., Goldberg V.M., Yaszemski M., Heim C.S. Proceedings of the American Academy of Orthopaedic Surgeons 77th Annual Meeting. 2010. The evolving role of bone-graft substitutes; p. 6. [Google Scholar]
- 80.Wu S., Liu X., Yeung K.W.K., Liu C., Yang X. Biomimetic porous scaffolds for bone tissue engineering. Mater. Sci. Eng. R. Rep. 2014;80:1–36. [Google Scholar]
- 81.Zwingenberger S., Nich C., Valladares R.D., Yao Z., Stiehler M., Goodman S.B. Recommendations and considerations for the use of biologics in orthopedic surgery. BioDrugs. 2012;26:245–256. doi: 10.2165/11631680-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dreesmann H. Ueber Knochenplombirung1. DMW-Deutsche Med. Wochenschr. 1892;19:445–446. [Google Scholar]
- 83.Carson J.S., Bostrom M.P. Synthetic bone scaffolds and fracture repair. Injury. 2007;38(Suppl 1):S33–S37. doi: 10.1016/j.injury.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 84.Chi-Chien N., Tsung-Ting T., Tsai-Sheng F., Po-Liang L., Lih-Huei C., Wen-Jer C. A comparison of posterolateral lumbar fusion comparing autograft, autogenous laminectomy bone with bone marrow aspirate, and calcium sulphate with bone marrow aspirate: a prospective randomized study. Spine. 2009;34:2715–2719. doi: 10.1097/BRS.0b013e3181b47232. [DOI] [PubMed] [Google Scholar]
- 85.Glombitza M., Steinhausen E. Treatment of chronic osteomyelitis of the lower limb with a new vancomycin-loaded, calcium sulfate/hydroxyapatite composite. Bone Jt. J. 2016;98 39-39. [Google Scholar]
- 86.Jiang N., Qin C.H., Ma Y.F., Wang L., Yu B. Possibility of one-stage surgery to reconstruct bone defects using the modified Masquelet technique with degradable calcium sulfate as a cement spacer: a case report and hypothesis. Biomed. Rep. 2016;4:374–378. doi: 10.3892/br.2016.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zwingenberger S., Nich C., Valladares R.D., Yao Z., Stiehler M., Goodman D.S.B. Recommendations and considerations for the use of biologics in orthopedic surgery. BioDrugs. 2012;26:245–256. doi: 10.2165/11631680-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oonishi H., Iwaki Y., Kin N., Kushitani S., Murata N., Wakitani S., Imoto K. Hydroxyapatite in revision of total hip replacements with massive acetabular defects: 4- to 10-year clinical results. Bone Jt. J. 1997;79:87–92. doi: 10.1302/0301-620x.79b1.1290. [DOI] [PubMed] [Google Scholar]
- 89.Schwartz C., Bordei R. Biphasic phospho-calcium ceramics used as bone substitutes are efficient in the management of severe acetabular bone loss in revision total hip arthroplasties. Eur. J. Orthop. Surg. Traumatol. 2005;15:191–196. [Google Scholar]
- 90.Nich C., Sedel L. Bone substitution in revision hip replacement. Int. Orthop. 2006;30:525–531. doi: 10.1007/s00264-006-0135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gaasbeek R.D.A., Toonen H.G., van Heerwaarden R.J., Buma P. Mechanism of bone incorporation of β -TCP bone substitute in open wedge tibial osteotomy in patients. Biomaterials. 2005;26:6713–6719. doi: 10.1016/j.biomaterials.2005.04.056. [DOI] [PubMed] [Google Scholar]
- 92.Scheer J.H., Adolfsson L.E. Tricalcium phosphate bone substitute in corrective osteotomy of the distal radius. Injury. 2009;40:262–267. doi: 10.1016/j.injury.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 93.Bucholz R.W., Carlton A., Holmes R.E. Hydroxyapatite and tricalcium phosphate bone graft substitutes. Orthop. Clin. North Am. 1987;18:323–334. [PubMed] [Google Scholar]
- 94.Eggli P.S., Müller W., Schenk R.K. Porous hydroxyapatite and tricalcium phosphate cylinders with two different pore size ranges implanted in the cancellous bone of rabbits. A comparative histomorphometric and histologic study of bony ingrowth and implant substitution. Clin. Orthop. Relat. Res. 1988;232 [PubMed] [Google Scholar]
- 95.Huec J.C.L., Schaeverbeke T., Clement D., Faber J., Rebeller A.L. Influence of porosity on the mechanical resistance of hydroxyapatite ceramics under compressive stress. Biomaterials. 1995;16:113–118. doi: 10.1016/0142-9612(95)98272-g. [DOI] [PubMed] [Google Scholar]
- 96.Wenisch S., Stahl J.P., Horas U., Heiss C., Kilian O., Trinkaus K., Hild A., Schnettler R. In vivo mechanisms of hydroxyapatite ceramic degradation by osteoclasts: fine structural microscopy. J. Biomed. Mater. Res. A. 2003;67:713–718. doi: 10.1002/jbm.a.10091. [DOI] [PubMed] [Google Scholar]
- 97.Tonino A.J., van der Wal B.C., Heyligers I.C., Grimm B. Bone remodeling and hydroxyapatite resorption in coated primary hip prostheses. Clin. Orthop. Relat. Res. 2009;467:478–484. doi: 10.1007/s11999-008-0559-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kattimani S., Kondaka K.P. Lingamaneni. 2016. Hydroxyapatite - Past, Present, and Future in Bone Regeneration, Bone and Tissue Regeneration Insights; p. 9. [Google Scholar]
- 99.Dubok V.A. Bioceramics―yesterday, today, tomorrow. Powder Metal Ceram. 2000;39:381–394. [Google Scholar]
- 100.Li H., Zhao Q., Li B., Kang J., Yu Z., Li Y., Song X., Liang C., Wang H. Fabrication and properties of carbon nanotube-reinforced hydroxyapatite composites by a double in situ synthesis process. Carbon N. Y. 2016;101:159–167. [Google Scholar]
- 101.Mukherjee S., Nandi S.K., Kundu B., Chanda A., Sen S., Das P.K. Enhanced bone regeneration with carbon nanotube reinforced hydroxyapatite in animal model. J. Mech. Behav. Biomed. Mater. 2016;60:243–255. doi: 10.1016/j.jmbbm.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 102.Albee F.H. Studies in bone growth triple calcium phosphate as a stimulus to osteogenesis. Ann. Surg. 1920;71:32–39. doi: 10.1097/00000658-192001000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ogose A., Kondo N., Umezu H., Hotta T., Kawashima H., Tokunaga K., Ito T., Kudo N., Hoshino M., Gu W. Histological assessment in grafts of highly purified beta-tricalcium phosphate (OSferion ®; ) in human bones. Biomaterials. 2006;27:1542–1549. doi: 10.1016/j.biomaterials.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 104.Buchanan F.J. Elsevier; 2008. Degradation Rate of Bioresorbable Materials: Prediction and Evaluation. [Google Scholar]
- 105.Bohner M. Physical and chemical aspects of calcium phosphates used in spinal surgery. Eur. Spine J. 2001;10:S114–S121. doi: 10.1007/s005860100276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen Y., Wang J., Zhu X.D., Tang Z.R., Yang X., Tan Y.F., Fan Y.J., Zhang X.D. Enhanced effect of beta-tricalcium phosphate phase on neovascularization of porous calcium phosphate ceramics: in vitro and in vivo evidence. Acta Biomater. 2015;11:435–448. doi: 10.1016/j.actbio.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 107.Malhotra A., Habibovic P. Calcium phosphates and angiogenesis: implications and advances for bone regeneration. Trends Biotechnol. 2016;34:983–992. doi: 10.1016/j.tibtech.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 108.Klenke F.M., Liu Y., Yuan H., Hunziker E.B., Siebenrock K.A., Hofstetter W. Impact of pore size on the vascularization and osseointegration of ceramic bone substitutes in vivo. J. Biomed. Mater. Res. A. 2008;85:777–786. doi: 10.1002/jbm.a.31559. [DOI] [PubMed] [Google Scholar]
- 109.Bai F., Wang Z., Lu J., Liu J., Chen G., Lv R., Wang J., Lin K., Zhang J., Huang X. The correlation between the internal structure and vascularization of controllable porous bioceramic materials in vivo: a quantitative study. Tissue Eng. Part A. 2010;16:3791–3803. doi: 10.1089/ten.TEA.2010.0148. [DOI] [PubMed] [Google Scholar]
- 110.Xiao X., Wang W., Liu D., Zhang H., Gao P., Geng L., Yuan Y., Lu J., Wang Z. The promotion of angiogenesis induced by three-dimensional porous beta-tricalcium phosphate scaffold with different interconnection sizes via activation of PI3K/Akt pathways. Sci. Rep. 2015;5:9409. doi: 10.1038/srep09409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Barrère F., van Blitterswijk C.A., de Groot K. Bone regeneration: molecular and cellular interactions with calcium phosphate ceramics. Int. J. Nanomed. 2006;1:317. [PMC free article] [PubMed] [Google Scholar]
- 112.Shanahan C.M., Crouthamel M.H., Kapustin A., Giachelli C.M. Arterial calcification in chronic kidney disease: key roles for calcium and phosphate. Circ. Res. 2011;109:697–711. doi: 10.1161/CIRCRESAHA.110.234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Di Marco G.S., König M., Stock C., Wiesinger A., Hillebrand U., Reiermann S., Reuter S., Amler S., Köhler G., Buck F. High phosphate directly affects endothelial function by downregulating annexin II. Kidney Int. 2013;83:213–222. doi: 10.1038/ki.2012.300. [DOI] [PubMed] [Google Scholar]
- 114.Sandino C., Checa S., Prendergast P.J., Lacroix D. Simulation of angiogenesis and cell differentiation in a CaP scaffold subjected to compressive strains using a lattice modeling approach. Biomaterials. 2010;31:2446–2452. doi: 10.1016/j.biomaterials.2009.11.063. [DOI] [PubMed] [Google Scholar]
- 115.Jufri N.F., Mohamedali A., Avolio A., Baker M.S. Mechanical stretch: physiological and pathological implications for human vascular endothelial cells. Vasc. Cell. 2015;7:8. doi: 10.1186/s13221-015-0033-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Daculsi G., LeGeros R., Nery E., Lynch K., Kerebel B. Transformation of biphasic calcium phosphate ceramics in vivo: ultrastructural and physicochemical characterization. J. Biomed. Mater. Res. 1989;23:883–894. doi: 10.1002/jbm.820230806. [DOI] [PubMed] [Google Scholar]
- 117.Williams D.F. There is no such thing as a biocompatible material. Biomaterials. 2014;35:10009–10014. doi: 10.1016/j.biomaterials.2014.08.035. [DOI] [PubMed] [Google Scholar]
- 118.Brown W., Chow L. A new calcium-phosphate setting cement. J. Dent. Res. 1983;62 doi: 10.1177/00220345900690121201. 672-672. [DOI] [PubMed] [Google Scholar]
- 119.Brown W.E. 1987. A New Calcium Phosphate, Water-setting Cement; pp. 351–379. Cements research progress. [Google Scholar]
- 120.Alkhraisat M.H., Rueda C., Jerez L.B., Tamimi Marino F., Torres J., Gbureck U., Lopez Cabarcos E. Effect of silica gel on the cohesion, properties and biological performance of brushite cement. Acta Biomater. 2010;6:257–265. doi: 10.1016/j.actbio.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 121.Ishikawa K. Calcium phosphate cement. In: Ben-Nissan B., editor. Advances in Calcium Phosphate Biomaterials. Springer; 2014. pp. 199–227. [Google Scholar]
- 122.Verron E., Pissonnier M.L., Lesoeur J., Schnitzler V., Fellah B.H., Pascal-Moussellard H., Pilet P., Gauthier O., Bouler J.M. Vertebroplasty using bisphosphonate-loaded calcium phosphate cement in a standardized vertebral body bone defect in an osteoporotic sheep model. Acta Biomater. 2014;10:4887–4895. doi: 10.1016/j.actbio.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 123.Nakano M., Kawaguchi Y., Kimura T., Hirano N. Transpedicular vertebroplasty after intervertebral cavity formation versus conservative treatment for osteoporotic burst fractures. Spine J. 2014;14:39–48. doi: 10.1016/j.spinee.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 124.Tarsuslugil S.M., O'Hara R.M., Dunne N.J., Buchanan F.J., Orr J.F., Barton D.C., Wilcox R.K. Development of calcium phosphate cement for the augmentation of traumatically fractured porcine specimens using vertebroplasty. J. Biomech. 2013;46:711–715. doi: 10.1016/j.jbiomech.2012.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Maestretti G., Sutter P., Monnard E., Ciarpaglini R., Wahl P., Hoogewoud H., Gautier E. A prospective study of percutaneous balloon kyphoplasty with calcium phosphate cement in traumatic vertebral fractures: 10-year results. Eur. Spine J. 2014;23:1354–1360. doi: 10.1007/s00586-014-3206-1. [DOI] [PubMed] [Google Scholar]
- 126.Zaryanov A.V., Park D.K., Khalil J.G., Baker K.C., Fischgrund J.S. Cement augmentation in vertebral burst fractures. Neurosurg. Focus. 2014;37 doi: 10.3171/2014.5.FOCUS1495. E5-E5. [DOI] [PubMed] [Google Scholar]
- 127.Dorozhkin S.V. Nanosized and nanocrystalline calcium orthophosphates. Acta Biomater. 2010;6:715–734. doi: 10.1016/j.actbio.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 128.Zhou C., Hong Y., Zhang X. Applications of nanostructured calcium phosphate in tissue engineering. Biomater. Sci. 2013;1:1012. doi: 10.1039/c3bm60058k. [DOI] [PubMed] [Google Scholar]
- 129.Wang P., Zhao L., Liu J., Weir M.D., Zhou X., Xu H.H. Bone tissue engineering via nanostructured calcium phosphate biomaterials and stem cells. Bone Res. 2014;2:14017. doi: 10.1038/boneres.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Alivisatos A. Naturally aligned nanocrystals. Science. 2000;289:736–737. doi: 10.1126/science.289.5480.736. [DOI] [PubMed] [Google Scholar]
- 131.Tang R., Wang L., Orme C.A., Bonstein T., Bush P.J., Nancollas G.H. Dissolution at the nanoscale: self-preservation of biominerals. Angew. Chem. 2004;116:2751–2755. doi: 10.1002/anie.200353652. [DOI] [PubMed] [Google Scholar]
- 132.Olszta M.J., Cheng X., Jee S.S., Kumar R., Kim Y.-Y., Kaufman M.J., Douglas E.P., Gower L.B. Bone structure and formation: a new perspective. Mater. Sci. Eng. R. Rep. 2007;58:77–116. [Google Scholar]
- 133.Kim D.W., Cho I.-S., Kim J.Y., Jang H.L., Han G.S., Ryu H.-S., Shin H., Jung H.S., Kim H., Hong K.S. Simple large-scale synthesis of hydroxyapatite nanoparticles: in situ observation of crystallization process. Langmuir. 2009;26:384–388. doi: 10.1021/la902157z. [DOI] [PubMed] [Google Scholar]
- 134.Geffers M., Groll J., Gbureck U. Reinforcement strategies for load-bearing calcium phosphate biocements. Mater. (Basel) 2015;8:2700–2717. [Google Scholar]
- 135.O'Hara R.M., Orr J.F., Buchanan F.J., Wilcox R.K., Barton D.C., Dunne N.J. Development of a bovine collagen–apatitic calcium phosphate cement for potential fracture treatment through vertebroplasty. Acta Biomater. 2012;8:4043–4052. doi: 10.1016/j.actbio.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 136.Sugawara A., Asaoka K., Ding S.-J. Calcium phosphate-based cements: clinical needs and recent progress. J. Mater. Chem. B. 2013;1:1081–1089. doi: 10.1039/c2tb00061j. [DOI] [PubMed] [Google Scholar]
- 137.Keating J., Hajducka C., Harper J. Minimal internal fixation and calcium-phosphate cement in the treatment of fractures of the tibial plateau. Bone Jt. J. 2003;85:68–73. doi: 10.1302/0301-620x.85b1.12575. [DOI] [PubMed] [Google Scholar]
- 138.Ishiguro S., Kasai Y., Sudo A., Iida K., Uchida A. Percutaneous vertebroplasty for osteoporotic compression fractures using calcium phosphate cement. J. Orthop. Surg. 2010;18:346–351. doi: 10.1177/230949901001800318. [DOI] [PubMed] [Google Scholar]
- 139.Nakano M., Hirano N., Zukawa M., Suzuki K., Hirose J., Kimura T., Kawaguchi Y. Vertebroplasty using calcium phosphate cement for osteoporotic vertebral fractures: study of outcomes at a minimum follow-up of two years. Asian Spine J. 2012;6:34–42. doi: 10.4184/asj.2012.6.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yin X., Li J., Xu J., Huang Z., Rong K., Fan C. Clinical assessment of calcium phosphate cement to treat tibial plateau fractures. J. Biomater. Appl. 2013;28:199–206. doi: 10.1177/0885328212443295. [DOI] [PubMed] [Google Scholar]
- 141.Nakamura T., Matsumine A., Asanuma K., Matsubara T., Sudo A. Treatment of bone defect with calcium phosphate cement subsequent to tumor curettage in pediatric patients. Oncol. Lett. 2016;11:247–252. doi: 10.3892/ol.2015.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Benbow J., Bridgwater J. 1993. Paste Flow and Extrusion. Oxford. [Google Scholar]
- 143.Yaras P., Kalyon D., Yilmazer U. Flow instabilities in capillary flow of concentrated suspensions. Rheol. Acta. 1994;33:48–59. [Google Scholar]
- 144.Coussot P., Ancey C. Rheophysical classification of concentrated suspensions and granular pastes. Phys. Rev. E. 1999;59:4445. [Google Scholar]
- 145.Rough S., Wilson D., Bridgwater J. A model describing liquid phase migration within an extruding microcrystalline cellulose paste. Chem. Eng. Res. Des. 2002;80:701–714. [Google Scholar]
- 146.Bohner M., Baroud G. Injectability of calcium phosphate pastes. Biomaterials. 2005;26:1553–1563. doi: 10.1016/j.biomaterials.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 147.Patel M.J. University of Cambridge; 2008. Theoretical Aspects of Paste Formulation for Extrusion. [Google Scholar]
- 148.Habib M.A.M. Université de Sherbrooke; 2010. Investigation and electromechanical solution for the limited injectability of the hydraulic calcium phosphate paste. PhD Thesis. ISBN: 9780494750629. [Google Scholar]
- 149.O'Neill R., McCarthy H.O., Montufar E.B., Ginebra M.P., Wilson D.I., Lennon A., Dunne N. Critical review: injectability of calcium phosphate pastes and cements. Acta Biomater. 2017;50:1–19. doi: 10.1016/j.actbio.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 150.Hench L.L., Splinter R.J., Allen W.C., Greenlee T.K. Bonding mechanism at interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 1971;5:117–141. [Google Scholar]
- 151.Hench L.L., Paschall H.A. Direct chemical bond of bioactive glass-ceramic materials to bone and muscle. J. Biomed. Mater. Res. 1973;7:25–42. doi: 10.1002/jbm.820070304. [DOI] [PubMed] [Google Scholar]
- 152.Välimäki V.V., Aro H. Molecular basis for action of bioactive glasses as bone graft substitute. Scand. J. Surg. 2006;95:95–102. doi: 10.1177/145749690609500204. [DOI] [PubMed] [Google Scholar]
- 153.Neo M., Nakamura T., Ohtsuki C., Kasai R., Kokubo T., Yamamuro T. Ultrastructural study of the A-W GC-bone interface after long-term implantation in rat and human bone. J. Biomed. Mater. Res. 1994;28:365–372. doi: 10.1002/jbm.820280311. [DOI] [PubMed] [Google Scholar]
- 154.Moimas L., Biasotto M., Lenarda R.D., Olivo A., Schmid C. Rabbit pilot study on the resorbability of three-dimensional bioactive glass fibre scaffolds. Acta Biomater. 2006;2:191–199. doi: 10.1016/j.actbio.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 155.Azenha M.R., Lacerda S.A.D., Marão H.F., Filho O.P., Filho O.M. Evaluation of crystallized biosilicate in the reconstruction of calvarial defects. J. Maxillofac. Oral Surg. 2015;14:1–7. doi: 10.1007/s12663-015-0755-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jones J.R. Review of bioactive glass: from Hench to hybrids. Acta Biomater. 2013;9:4457–4486. doi: 10.1016/j.actbio.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 157.Liu W.C., Robu I.S., Patel R., Leu M.C., Velez M., Chu T.M.G. The effects of 3D bioactive glass scaffolds and BMP-2 on bone formation in rat femoral critical size defects and adjacent bones. Biomed. Mater. 2014;9:045013. doi: 10.1088/1748-6041/9/4/045013. [DOI] [PubMed] [Google Scholar]
- 158.Chu T.M.G., Leu M.C., Robu I.S., Liu W.-C., Valez M. 2013. Effects of Bioactive Glass Scaffold and BMP-2 in Segmental Defects. [Google Scholar]
- 159.Watts S., Hill R., O'Donnell M., Law R. Influence of magnesia on the structure and properties of bioactive glasses. J. Non Cryst. Solids. 2010;356:517–524. [Google Scholar]
- 160.Lindfors N., Hyvönen P., Nyyssönen M., Kirjavainen M., Kankare J., Gullichsen E., Salo J. Bioactive glass S53P4 as bone graft substitute in treatment of osteomyelitis. Bone. 2010;47:212–218. doi: 10.1016/j.bone.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 161.Gaisser D.M., Hench L.L. Clinical applications of bioactive glass: orthopaedics. In: Hench L.L., editor. An Introduction to Bioceramics. World Scientific Publishing Co Inc.; 2013. pp. 151–158. [Google Scholar]
- 162.Lindfors N.C., Koski I., Heikkilä J.T., Mattila K., Aho A.J. A prospective randomized 14-year follow-up study of bioactive glass and autogenous bone as bone graft substitutes in benign bone tumors. J. Biomed. Mater. Res. B Appl. Biomater. 2010;94:157–164. doi: 10.1002/jbm.b.31636. [DOI] [PubMed] [Google Scholar]
- 163.Hupa L., Karlsson K.H., Hupa M., Aro H.T. Comparison of bioactive glasses in vitro and in vivo. Glass Technol. Part A. 2010;51:89–92. [Google Scholar]
- 164.Frantzén J., Rantakokko J., Aro H.T., Hein J., Kajander S., Gullichsen E., Kotilainen E., Lindfors N.C. Instrumented spondylodesis in degenerative spondylolisthesis with bioactive glass and autologous bone: a prospective 11-year follow-up. Clin. Spine Surg. 2011;24:455–461. doi: 10.1097/BSD.0b013e31822a20c6. [DOI] [PubMed] [Google Scholar]
- 165.Pernaa K., Koski I., Mattila K., Gullichsen E., Heikkila J., Aho A., Lindfors N. Bioactive glass S53P4 and autograft bone in treatment of depressed tibial plateau fractures-a prospective randomized 11-year follow-up. J. Long. Term. Eff. Med. Implants. 2011;21 doi: 10.1615/jlongtermeffmedimplants.v21.i2.40. [DOI] [PubMed] [Google Scholar]
- 166.Li R., Clark A., Hench L. An investigation of bioactive glass powders by sol-gel processing. J. Appl. Biomater. 1991;2:231–239. doi: 10.1002/jab.770020403. [DOI] [PubMed] [Google Scholar]
- 167.Brinker C.J., Scherer G.W. Academic press; 2013. Sol-gel science: the physics and chemistry of sol-gel processing. [Google Scholar]
- 168.Hench L.L. The story of Bioglass. J. Mater. Sci. Mater. Med. 2006;17:967–978. doi: 10.1007/s10856-006-0432-z. [DOI] [PubMed] [Google Scholar]
- 169.Wheeler D., Eschbach E., Hoellrich R., Montfort M., Chamberland D. Assessment of resorbable bioactive material for grafting of critical-size cancellous defects. J. Orthop. Res. 2000;18:140–148. doi: 10.1002/jor.1100180120. [DOI] [PubMed] [Google Scholar]
- 170.Judet J., Judet R. The use of an artificial femoral head for arthroplasty of the hip joint. J. Bone Jt. Sur. Br. 1950;32:166–173. doi: 10.1302/0301-620X.32B2.166. [DOI] [PubMed] [Google Scholar]
- 171.Webb J., Spencer R. The role of polymethylmethacrylate bone cement in modern orthopaedic surgery. J. Bone Jt. Surg. Br. 2007;89:851–857. doi: 10.1302/0301-620X.89B7.19148. [DOI] [PubMed] [Google Scholar]
- 172.Hernández L., Gurruchaga M., Goni I. Injectable acrylic bone cements for vertebroplasty based on a radiopaque hydroxyapatite. Formulation and rheological behaviour. J. Mater. Sci. Mater. Med. 2009;20:89–97. doi: 10.1007/s10856-008-3542-y. [DOI] [PubMed] [Google Scholar]
- 173.Kühn K.-D., Breusch S., Malchau H. Springer Medizin Verlag; Berlin: 2005. Properties of Bone Cement: what Is Bone Cement? [Google Scholar]
- 174.Galibert P., Deramond H., Rosat P., Le Gars D. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty. Neurochirurgie. 1986;33:166–168. [PubMed] [Google Scholar]
- 175.McGraw J.K., Lippert J.A., Minkus K.D., Rami P.M., Davis T.M., Budzik R.F. Prospective evaluation of pain relief in 100 patients undergoing percutaneous vertebroplasty: results and follow-up. J. Vasc. Interv. Radiol. 2002;13:883–886. doi: 10.1016/s1051-0443(07)61770-9. [DOI] [PubMed] [Google Scholar]
- 176.Larsson S. Cement augmentation in fracture treatment. Scand. J. Surg. 2006;95:111–118. doi: 10.1177/145749690609500206. [DOI] [PubMed] [Google Scholar]
- 177.Ahn D.K., Choi D.J., Lee S., Kim K.S., Kim T.W., Chun T.H. Spinal cord injury caused by bone cement after percutaneous vertebroplasty-one case of long-term follow-up and the result of delayed removal. J. Korean Orthop. Assoc. 2009;44:386–390. [Google Scholar]
- 178.Kindt-Larsen T., Smith D.B., Jensen J.S. Innovations in acrylic bone cement and application equipment. J. Appl. Biomater. 1995;6:75–83. doi: 10.1002/jab.770060111. [DOI] [PubMed] [Google Scholar]
- 179.Charnley J. Springer Science & Business Media; 2012. Low Friction Arthroplasty of the Hip: Theory and Practice. [Google Scholar]
- 180.Kenny S., Buggy M. Bone cements and fillers: a review. J. Mater. Sci. Mater. Med. 2003;14:923–938. doi: 10.1023/a:1026394530192. [DOI] [PubMed] [Google Scholar]
- 181.Harwood P.J., Ferguson D.O. (ii) an update on fracture healing and non-union. Orthop. Trauma. 2015;29:228–242. [Google Scholar]
- 182.Daniel Mark F., James Min-Leong W., Conor C., Khan W.S. Preclinical and clinical studies on the use of growth factors for bone repair: a systematic review. Curr. Stem Cell Res. Ther. 2013;8:260–268. doi: 10.2174/1574888x11308030011. (269) [DOI] [PubMed] [Google Scholar]
- 183.Lieberman J.R., Daluiski A., Einhorn T.A. The role of growth factors in the repair of bone. J. Bone. Jt. Surg. Am. 2002;84:1032–1044. doi: 10.2106/00004623-200206000-00022. [DOI] [PubMed] [Google Scholar]
- 184.Foster T.E., Puskas B.L., Mandelbaum B.R., Gerhardt M.B., Rodeo S.A. Platelet-rich plasma from basic science to clinical applications. Am. J. Sports Med. 2009;37:2259–2272. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- 185.Govender S., Csimma C., Genant H.K., Valentin-Opran A., Amit Y., Arbel R., Aro H., Atar D., Bishay M., Börner M.G. Recombinant human bone morphogenetic Protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J. Bone Jt. Surg. 2002;84:2123–2134. doi: 10.2106/00004623-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 186.Friedlaender G.E., Perry C.R., Cole J.D., Cook S.D., Cierny G., Muschler G.F., Zych G.A., Calhoun J.H., Laforte A.J., Yin S. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J. Bone Jt. Surg. 2001;83-a(suppl 1):S151–S158. [PMC free article] [PubMed] [Google Scholar]
- 187.Mauffrey C., Seligson D., Lichte P., Pape H., Al-Rayyan M. Bone graft substitutes for articular support and metaphyseal comminution: what are the options? Injury. 2011;42:S35–S39. doi: 10.1016/j.injury.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 188.Dimar J.R., Glassman S.D., Burkus K.J., Carreon L.Y. Clinical outcomes and fusion success at 2 years of single-level instrumented posterolateral fusions with recombinant human bone morphogenetic protein-2/compression resistant matrix versus iliac crest bone graft. Spine. 2006;31:2534–2539. doi: 10.1097/01.brs.0000240715.78657.81. [DOI] [PubMed] [Google Scholar]
- 189.Jones A.L., Bucholz R.W., Bosse M.J., Mirza S.K., Lyon T.R., Webb L.X., Pollak A.N., Golden J.D., Valentin-Opran A. Recombinant human BMP-2 and allograft compared with autogenous bone graft for reconstruction of diaphyseal tibial fractures with cortical defects. J. Bone Jt. Surg. 2006;88:1431–1441. doi: 10.2106/JBJS.E.00381. [DOI] [PubMed] [Google Scholar]
- 190.Kanakaris N.K., Calori G.M., Verdonk R., Burssens P., De Biase P., Capanna R., Vangosa L.B., Cherubino P., Baldo F., Ristiniemi J. Application of BMP-7 to tibial non-unions: a 3-year multicenter experience. Injury. 2008;39:S83–S90. doi: 10.1016/S0020-1383(08)70019-6. [DOI] [PubMed] [Google Scholar]
- 191.Vaccaro A.R., Lawrence J.P., Patel T., Katz L.D., Anderson D.G., Fischgrund J.S., Krop J., Fehlings M.G., Wong D. The safety and efficacy of OP-1 (rhBMP-7) as a replacement for iliac crest autograft in posterolateral lumbar arthrodesis: a long-term (> 4 years) pivotal study. Spine. 2008;33:2850–2862. doi: 10.1097/BRS.0b013e31818a314d. [DOI] [PubMed] [Google Scholar]
- 192.Kaito T. Biologic enhancement of spinal fusion with bone morphogenetic proteins: current position based on clinical evidence and future perspective. J. Spine Surg. 2016;2:357–358. doi: 10.21037/jss.2016.12.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Bong M.R., Capla E.L., Egol K.A., Sorkin A.T., Distefano M., Buckle R., Chandler R.W., Koval K.J. Osteogenic protein-1 (bone morphogenic protein-7) combined with various adjuncts in the treatment of humeral diaphyseal nonunions. Bull. Hosp. Jt. Dis. 2005;63:20. [PubMed] [Google Scholar]
- 194.Dimitriou R., Dahabreh Z., Katsoulis E., Matthews S., Branfoot T., Giannoudis P. Application of recombinant BMP-7 on persistent upper and lower limb non-unions. Injury. 2005;36:S51–S59. doi: 10.1016/j.injury.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 195.Moghaddam A., Elleser C., Biglari B., Wentzensen A., Zimmermann G. Clinical application of BMP 7 in long bone non-unions. Arch. Orthop. Trauma Surg. 2010;130:71–76. doi: 10.1007/s00402-009-0982-x. [DOI] [PubMed] [Google Scholar]
- 196.Ronga M., Baldo F., Zappalà G., Cherubino P., Group B.-I.O.S. Recombinant human bone morphogenetic protein-7 for treatment of long bone non-union: an observational, retrospective, non-randomized study of 105 patients. Injury. 2006;37:S51–S56. doi: 10.1016/j.injury.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 197.Evans R.O., Goldberg J.A., Bruce W.J., Walsh W. Reoperated clavicular nonunion treated with osteogenic protein 1 and electrical stimulation. J. Shoulder Elb. Surg. 2004;13:573–575. doi: 10.1016/j.jse.2004.01.031. [DOI] [PubMed] [Google Scholar]
- 198.Clark R.R., McKinley T.O. Bilateral olecranon epiphyseal fracture non-union in a competitive athlete. Iowa Orthop. J. 2010;30:179. [PMC free article] [PubMed] [Google Scholar]
- 199.Courvoisier A., Sailhan F., Laffenetre O., Obert L. B.M.P.i.O.S. French Study Group of, Bone morphogenetic protein and orthopaedic surgery: can we legitimate its off-label use? Int. Orthop. 2014;38:2601–2605. doi: 10.1007/s00264-014-2534-4. [DOI] [PubMed] [Google Scholar]
- 200.Ekrol I., Hajducka C., Court-Brown C., McQueen M.M. A comparison of RhBMP-7 (OP-1) and autogenous graft for metaphyseal defects after osteotomy of the distal radius. Injury. 2008;39:S73–S82. doi: 10.1016/S0020-1383(08)70018-4. [DOI] [PubMed] [Google Scholar]
- 201.Bibbo C., Patel D.V., Haskell M.D. Recombinant bone morphogenetic protein-2 (rhBMP-2) in high-risk ankle and hindfoot fusions. Foot Ankle Int. 2009;30:597–603. doi: 10.3113/FAI.2009.0597. [DOI] [PubMed] [Google Scholar]
- 202.Schuberth J.M., DiDomenico L.A., Mendicino R.W. The utility and effectiveness of bone morphogenetic protein in foot and ankle surgery. J. Foot Ankle Surg. 2009;48:309–314. doi: 10.1053/j.jfas.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 203.El-Amin S.F., Hogan M.V., Allen A.A., Hinds J., Laurencin C.T. The indications and use of bone morphogenetic proteins in foot, ankle, and tibia surgery. Foot Ankle Clin. 2010;15:543–551. doi: 10.1016/j.fcl.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 204.Fourman M.S., Borst E.W., Bogner E., Rozbruch S.R., Fragomen A.T. Recombinant human BMP-2 increases the incidence and rate of healing in complex ankle arthrodesis. Clin. Orthop. Relat. Res. 2014;472:732–739. doi: 10.1007/s11999-013-3261-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Cowan C.M., Aghaloo T., Chou Y.-F., Walder B., Zhang X., Soo C., Ting K., Wu B. MicroCT evaluation of three-dimensional mineralization in response to BMP-2 doses in vitro and in critical sized rat calvarial defects. Tissue Eng. 2007;13:501–512. doi: 10.1089/ten.2006.0141. [DOI] [PubMed] [Google Scholar]
- 206.Boraiah S., Ohawkes P. Complications of recombinant human BMP-2 for treating complex tibial plateau fractures: a preliminary report. Clin. Orthop. Relat. Res. 2009;467:3257–3262. doi: 10.1007/s11999-009-1039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ritting A.W., Weber E.W., Lee M.C. Exaggerated inflammatory response and bony resorption from BMP-2 use in a pediatric forearm nonunion. J. Hand Surg. 2012;37:316–321. doi: 10.1016/j.jhsa.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 208.Tannoury C., An H.S. Complications with use of bone morphogenetic Protein-2 (BMP-2) in spine surgery. Spine J. 2014;3:552–559. doi: 10.1016/j.spinee.2013.08.060. [DOI] [PubMed] [Google Scholar]
- 209.Dimitriou R., Tsiridis E., Giannoudis P.V. Current concepts of molecular aspects of bone healing. Injury. 2005;36:1392–1404. doi: 10.1016/j.injury.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 210.Collin-Osdoby P., Rothe L., Bekker S., Anderson F., Huang Y., Osdoby P. Basic fibroblast growth factor stimulates osteoclast recruitment, development, and bone pit resorption in association with angiogenesis in vivo on the chick chorioallantoic membrane and activates isolated avian osteoclast resorption in vitro. J. Bone Min. Res. 2002;17:1859–1871. doi: 10.1359/jbmr.2002.17.10.1859. [DOI] [PubMed] [Google Scholar]
- 211.Hayek A., Culler F.L., Beattie G.M., Lopez A.D., Cuevas P., Baird A. An in vivo model for study of the angiogenic effects of basic fibroblast growth factor. Biochem. Biophys. Res. Commun. 1987;147:876–880. doi: 10.1016/0006-291x(87)91011-4. [DOI] [PubMed] [Google Scholar]
- 212.Montesano R., Vassalli J.D., Baird A., Guillemin R., Orci L. Basic fibroblast growth factor induces angiogenesis in vitro. Proc. Natl. Acad. Sci. 1986;83:7297–7301. doi: 10.1073/pnas.83.19.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Coutu D.L., Ra F.O. Mo, Jacques G. Inhibition of cellular senescence by developmentally regulated FGF receptors in mesenchymal stem cells. Blood. 2011;117:6801–6812. doi: 10.1182/blood-2010-12-321539. [DOI] [PubMed] [Google Scholar]
- 214.Pacicca D., Patel N., Lee C., Salisbury K., Lehmann W., Carvalho R., Gerstenfeld L., Einhorn T. Expression of angiogenic factors during distraction osteogenesis. Bone. 2003;33:889–898. doi: 10.1016/j.bone.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 215.Haque T., Amako M., Nakada S., Lauzier D., Hamdy R. An immunohistochemical analysis of the temporal and spatial expression of growth factors FGF 1, 2 and 18, IGF 1 and 2, and TGFß1 during distraction osteogenesis. Histol. Histopathol. 2007:119–128. doi: 10.14670/HH-22.119. [DOI] [PubMed] [Google Scholar]
- 216.Schmid G.J., Kobayashi C., Sandell L.J., Ornitz D.M. Fibroblast growth factor expression during skeletal fracture healing in mice. Dev. Dyn. 2009;238:766–774. doi: 10.1002/dvdy.21882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Su N., Sun Q., Li C., Lu X., Qi H., Chen S., Yang J., Du X., Zhao L., He Q. Gain-of-function mutation in FGFR3 in mice leads to decreased bone mass by affecting both osteoblastogenesis and osteoclastogenesis. Hum. Mol. Genet. 2010:1199–1210. doi: 10.1093/hmg/ddp590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Du X., Xie Y., Xian C.J., Chen L. Role of FGFs/FGFRs in skeletal development and bone regeneration. J. Cell. Physiol. 2012;227:3731–3743. doi: 10.1002/jcp.24083. [DOI] [PubMed] [Google Scholar]
- 219.Chen W.J., Jingushi S., Aoyama I., Anzai J., Hirata G., Tamura M., Iwamoto Y. Effects of FGF-2 on metaphyseal fracture repair in rabbit tibiae. J. Bone Min. Metab. 2004;22:303–309. doi: 10.1007/s00774-003-0487-6. [DOI] [PubMed] [Google Scholar]
- 220.Nakamura T., Hara Y., Tagawa M., Tamura M., Yuge T., Fukuda H., Nigi H. Recombinant human basic fibroblast growth factor accelerates fracture healing by enhancing callus remodeling in experimental dog tibial fracture. J. Bone Min. Res. 1998;13:942–949. doi: 10.1359/jbmr.1998.13.6.942. [DOI] [PubMed] [Google Scholar]
- 221.Kawaguchi H., Nakamura K., Tabata Y., Ikada Y., Aoyama I., Anzai J., Nakamura T., Hiyama Y., Tamura M. Acceleration of fracture healing in nonhuman primates by fibroblast growth factor-2. J. Clin. Endocrinol. Metab. 2001;86:875–880. doi: 10.1210/jcem.86.2.7199. [DOI] [PubMed] [Google Scholar]
- 222.Radomsky M.L., Aufdemorte T.B., Swain L.D., Fox W.C., Spiro R.C., Poser J.W. Novel formulation of fibroblast growth factor-2 in a hyaluronan gel accelerates fracture healing in nonhuman primates. J. Orthop. Res. 1999;17:607–614. doi: 10.1002/jor.1100170422. [DOI] [PubMed] [Google Scholar]
- 223.Fei Y., Gronowicz G., Hurley M.M. Fibroblast growth factor-2, bone homeostasis and fracture repair. Curr. Pharm. Des. 2013;19:3354–3363. doi: 10.2174/1381612811319190002. [DOI] [PubMed] [Google Scholar]
- 224.Kawaguchi H., Oka H., Jingushi S., Izumi T., Fukunaga M., Sato K., Matsushita T., Nakamura K. A local application of recombinant human fibroblast growth factor 2 for tibial shaft fractures: a randomized, placebo-controlled trial. J. Bone Min. Res. 2010;25:2735–2743. doi: 10.1002/jbmr.146. [DOI] [PubMed] [Google Scholar]
- 225.Tsiridis E., Upadhyay N., Giannoudis P. Molecular aspects of fracture healing: which are the important molecules? Injury. 2007;38(Suppl 1):S11–S25. doi: 10.1016/j.injury.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 226.Gerstenfeld L.C., Cullinane D.M., Barnes G.L., Graves D.T., Einhorn T.A. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J. Cell. Biochem. 2003;88:873–884. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- 227.Keramaris N.C., Calori G.M., Nikolaou V.S., Schemitsch E.H., Giannoudis P.V. Fracture vascularity and bone healing: a systematic review of the role of VEGF. Injury. 2008;39:S45–S57. doi: 10.1016/S0020-1383(08)70015-9. [DOI] [PubMed] [Google Scholar]
- 228.Wan C., Gilbert S.R., Wang Y., Cao X., Shen X., Ramaswamy G., Jacobsen K.A., Alaql Z.S., Eberhardt A.W., Gerstenfeld L.C., Einhorn T.A., Deng L., Clemens T.L. Activation of the hypoxia-inducible factor-1alpha pathway accelerates bone regeneration. Proc. Natl. Acad. Sci. 2008;105:686–691. doi: 10.1073/pnas.0708474105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Keramaris N.C., Calori G.M., Nikolaou V.S., Schemitsch E.H., Giannoudis P.V. Fracture vascularity and bone healing: a systematic review of the role of VEGF. Injury. 2008;39:S45–S57. doi: 10.1016/S0020-1383(08)70015-9. [DOI] [PubMed] [Google Scholar]
- 230.Eckardt H., Bundgaard K.G., Christensen K.S., Lind M., Hansen E.S., Hvid I. Effects of locally applied vascular endothelial growth factor (VEGF) and VEGF-inhibitor to the rabbit tibia during distraction osteogenesis. J. Orthop. Res. 2003;21:335–340. doi: 10.1016/S0736-0266(02)00159-6. [DOI] [PubMed] [Google Scholar]
- 231.Geiger F., Lorenz H., Xu W., Szalay K., Kasten P., Claes L., Augat P., Richter W. VEGF producing bone marrow stromal cells (BMSC) enhance vascularization and resorption of a natural coral bone substitute. Bone. 2007;41:516–522. doi: 10.1016/j.bone.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 232.Kaigler D., Wang Z., Horger K., Mooney D.J. VEGF scaffolds enhance angiogenesis and bone regeneration in irradiated osseous defects. J. Bone Min. Res. 2006;21:735–744. doi: 10.1359/jbmr.060120. [DOI] [PubMed] [Google Scholar]
- 233.Kent L.J., Darnell K., Zhuo W., Krebsbach P.H., Mooney D.J. Coating of VEGF-releasing scaffolds with bioactive glass for angiogenesis and bone regeneration. Biomaterials. 2006;27:3249–3255. doi: 10.1016/j.biomaterials.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 234.Eckardt H., Ding M., Lind M., Hansen E.S., Christensen K.S., Hvid I. Recombinant human vascular endothelial growth factor enhances bone healing in an experimental nonunion model. J. Bone Jt. Surg. Br. 2005;87:1434–1438. doi: 10.1302/0301-620X.87B10.16226. [DOI] [PubMed] [Google Scholar]
- 235.García J.R., Clark A.Y., García A.J. Integrin-specific hydrogels functionalized with VEGF for vascularization and bone regeneration of critical-size bone defects. J. Biomed. Mater. Res. A. 2016;104 doi: 10.1002/jbm.a.35777. 1845. [DOI] [PubMed] [Google Scholar]
- 236.Hankenson K.D., Dishowitz M., Gray C., Schenker M. Angiogenesis in bone regeneration. Injury. 2011;42:556–561. doi: 10.1016/j.injury.2011.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Babu S., Sandiford N.A., Vrahas M. Use of Teriparatide to improve fracture healing: what is the evidence? World J. Orthop. 2015;6:457–461. doi: 10.5312/wjo.v6.i6.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Per A. Annotation: parathyroid hormone and fracture healing. Acta Orthop. 2013;84:4–6. doi: 10.3109/17453674.2013.771301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Jilka R.L. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone. 2007;40:1434–1446. doi: 10.1016/j.bone.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Neer R.M., Arnaud C.D., Zanchetta J.R., Prince R., Gaich G.A., Reginster J.Y., Hodsman A.B., Eriksen E.F., Ish-Shalom S., Genant H.K. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N. Engl. J. Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 241.Alkhiary Y.M., Gerstenfeld L.C., Elizabeth K., Michael W., Masahiko S., Mitlak B.H., Einhorn T.A. Enhancement of experimental fracture-healing by systemic administration of recombinant human parathyroid hormone (PTH 1-34) J. Bone Jt. Surg. Am. 2005;87:731–741. doi: 10.2106/JBJS.D.02115. [DOI] [PubMed] [Google Scholar]
- 242.Ralf S., Per A. Parathyroid hormone–a drug for orthopedic surgery? Acta. Orthop. Scand. 2004;75:654–662. doi: 10.1080/00016470410004012. [DOI] [PubMed] [Google Scholar]
- 243.Wronski T., Yen C.-F., Qi H., Dann L. Parathyroid hormone is more effective than estrogen or bisphosphonates for restoration of lost bone mass in ovariectomized rats. Endocrinology. 1993;132:823–831. doi: 10.1210/endo.132.2.8425497. [DOI] [PubMed] [Google Scholar]
- 244.Jerome C., Burr D., Van Bibber T., Hock J., Brommage R. Treatment with human parathyroid hormone (1-34) for 18 months increases cancellous bone volume and improves trabecular architecture in ovariectomized cynomolgus monkeys (Macaca fascicularis) Bone. 2001;28:150–159. doi: 10.1016/s8756-3282(00)00430-0. [DOI] [PubMed] [Google Scholar]
- 245.Sato M., Westmore M., Ma Y.L., Schmidt A., Zeng Q.Q., Glass E.V., Vahle J., Brommage R., Jerome C.P., Turner C.H. Teriparatide [PTH (1–34)] strengthens the proximal femur of ovariectomized nonhuman primates despite increasing porosity. J. Bone Min. Res. 2004;19:623–629. doi: 10.1359/JBMR.040112. [DOI] [PubMed] [Google Scholar]
- 246.Ellegaard M., Jorgensen N.R., Schwarz P. Parathyroid hormone and bone healing. Calcif. Tissue Int. 2010;87:1–13. doi: 10.1007/s00223-010-9360-5. [DOI] [PubMed] [Google Scholar]
- 247.Aspenberg P., Genant H.K., Johansson T., Nino A.J., See K., Krohn K., Garcia-Hernandez P.A., Recknor C.P., Einhorn T.A., Dalsky G.P., Mitlak B.H., Fierlinger A., Lakshmanan M.C. Teriparatide for acceleration of fracture repair in humans: a prospective, randomized, double-blind study of 102 postmenopausal women with distal radial fractures. J. Bone Min. Res. 2010;25:404–414. doi: 10.1359/jbmr.090731. [DOI] [PubMed] [Google Scholar]
- 248.Aspenberg P., Johansson T. Teriparatide improves early callus formation in distal radial fractures: analysis of a subgroup of patients within a randomized trial. Acta Orthop. 2010;81:234–236. doi: 10.3109/17453671003761946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Peichl P., Holzer L.A., Maier R., Holzer G. Parathyroid hormone 1-84 accelerates fracture-healing in pubic bones of elderly osteoporotic women. J. Bone Jt. Surg. 2011;93:1583–1587. doi: 10.2106/JBJS.J.01379. [DOI] [PubMed] [Google Scholar]
- 250.Aspenberg P., Malouf J., Tarantino U., García-Hernández P.A., Corradini C., Overgaard S., Stepan J.J., Borris L., Lespessailles E., Frihagen F. Effects of Teriparatide compared with risedronate on recovery after pertrochanteric hip fracture. J. Bone. Jt. Surg. Am. 2016;98:1868–1878. doi: 10.2106/JBJS.15.01217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Malouf-Sierra J., Tarantino U., Garcia-Hernandez P.A., Corradini C., Overgaard S., Stepan J.J., Borris L., Lespessailles E., Frihagen F., Papavasiliou K., Petto H., Aspenberg P., Caeiro J.R., Marin F. Effect of Teriparatide or risedronate in elderly patients with a recent pertrochanteric hip fracture: final results of a 78-week randomized clinical trial. J. Bone Min. Res. 2017;32(5):1040–1051. doi: 10.1002/jbmr.3067. [DOI] [PubMed] [Google Scholar]
- 252.Nauth A., Ristevski B., Li R., Schemitsch E.H. Growth factors and bone regeneration: how much bone can we expect? Injury. 2011;42:574–579. doi: 10.1016/j.injury.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 253.Marx R.E. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10:225–228. doi: 10.1097/00008505-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 254.Sampson S., Gerhardt M., Mandelbaum B. Platelet rich plasma injection grafts for musculoskeletal injuries: a review. Curr. Rev. Musculoskelet. Med. 2008;1:165–174. doi: 10.1007/s12178-008-9032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Mandeep D., Sandeep P., Kamal B. Platelet-rich plasma intra-articular knee injections for the treatment of degenerative cartilage lesions and osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2011;19:528–535. doi: 10.1007/s00167-010-1238-6. [DOI] [PubMed] [Google Scholar]
- 256.Mishra A., Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am. J. Sports Med. 2006;34:1774–1778. doi: 10.1177/0363546506288850. [DOI] [PubMed] [Google Scholar]
- 257.Gosens T., Peerbooms J.C., Van L.W., den Oudsten B.L. Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with 2-year follow-up. Am. J. Sports Med. 2011;39:1200–1208. doi: 10.1177/0363546510397173. [DOI] [PubMed] [Google Scholar]
- 258.Calori G.M., Tagliabue L., Gala L., D'Imporzano M., Peretti G., Albisetti W. Application of rhBMP-7 and platelet-rich plasma in the treatment of long bone non-unions. Injury. 2008;39:1391–1402. doi: 10.1016/j.injury.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 259.Gołos J., Waliński T., Piekarczyk P., Kwiatkowski K. Results of the use of platelet rich plasma in the treatment of delayed union of long bones. Ortop. Traumatol. Rehabil. 2013;16:397–406. doi: 10.5604/15093492.1119617. [DOI] [PubMed] [Google Scholar]
- 260.Malhotra R., Kumar V., Garg B., Singh R., Jain V., Coshic P., Chatterjee K. Role of autologous platelet-rich plasma in treatment of long-bone nonunions: a prospective study. Musculoskelet. Surg. 2015;99:243–248. doi: 10.1007/s12306-015-0378-8. [DOI] [PubMed] [Google Scholar]
- 261.Oryan A., Alidadi S., Moshiri A. Platelet-rich plasma for bone healing and regeneration. Expert Opin. Biol. Ther. 2016;16:213–232. doi: 10.1517/14712598.2016.1118458. [DOI] [PubMed] [Google Scholar]
- 262.Roffi A., Di Matteo B., Krishnakumar G.S., Kon E., Filardo G. Platelet-rich plasma for the treatment of bone defects: from pre-clinical rational to evidence in the clinical practice. A systematic review. Int. Orthop. 2016;41:221–237. doi: 10.1007/s00264-016-3342-9. [DOI] [PubMed] [Google Scholar]
- 263.Ranly D.M., Jacquelyn M.M., Todd K., Lohmann C.H., Timothy M., Cochran D.L., Zvi S., Boyan B.D. Platelet-derived growth factor inhibits demineralized bone matrix-induced intramuscular cartilage and bone formation. A study of immunocompromised mice. J. Bone Jt. Surg. Am. 2005;87:2052–2064. doi: 10.2106/JBJS.D.02752. [DOI] [PubMed] [Google Scholar]
- 264.Ranly D., Lohmann C., Boyan D.,B., Schwartz Z. Platelet-rich plasma inhibits demineralized bone matrix-induced bone formation in nude mice. J. Bone & Jt. Surg. 2007;89:139–147. doi: 10.2106/JBJS.F.00388. [DOI] [PubMed] [Google Scholar]
- 265.Alsousou J., Thompson M., Hulley P., Noble A., Willett K. The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery. Bone Jt. J. 2009;91:987–996. doi: 10.1302/0301-620X.91B8.22546. [DOI] [PubMed] [Google Scholar]
- 266.Griffin X.L., Smith C.M., Costa M.L. The clinical use of platelet-rich plasma in the promotion of bone healing: a systematic review. Injury. 2009;40:158–162. doi: 10.1016/j.injury.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 267.Han B., Woodell-May J., Ponticiello M., Yang Z., Nimni M. The effect of thrombin activation of platelet-rich plasma on demineralized bone matrix osteoinductivity. J. Bone Jt. Surg. 2009;91:1459–1470. doi: 10.2106/JBJS.H.00246. [DOI] [PubMed] [Google Scholar]
- 268.Beitzel K., Allen D., Apostolakos J., Russell R.P., McCarthy M.B., Gallo G.J., Cote M.P., Mazzocca A.D. US definitions, current use, and FDA stance on use of platelet-rich plasma in sports medicine. J. Knee Surg. 2015;28:29–34. doi: 10.1055/s-0034-1390030. [DOI] [PubMed] [Google Scholar]
- 269.Thompson K.H., Chris O. Boon and bane of metal ions in medicine. Science. 2003;300:936–939. doi: 10.1126/science.1083004. [DOI] [PubMed] [Google Scholar]
- 270.Bose S., Fielding G., Tarafder S., Bandyopadhyay A. Understanding of dopant-induced osteogenesis and angiogenesis in calcium phosphate ceramics. Trends Biotechnol. 2013;31:594–605. doi: 10.1016/j.tibtech.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.MADEA, Verkehrsmedizin . 2007. Fahreignung, Fahrsicherheit, Unfallrekonstruktion, Blood Alcohol Levels. [Google Scholar]
- 272.Stone R., Ash C. A question of dose. Science. 2003;300:925. [Google Scholar]
- 273.Mourino V., Cattalini J.P., Boccaccini A.R. Metallic ions as therapeutic agents in tissue engineering scaffolds: an overview of their biological applications and strategies for new developments. J. R. Soc. Interface. 2012;9:401–419. doi: 10.1098/rsif.2011.0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Wong H.M., Yeung K.W., Lam K.O., Tam V., Chu P.K., Luk K.D., Cheung K. A biodegradable polymer-based coating to control the performance of magnesium alloy orthopaedic implants. Biomaterials. 2010;31:2084–2096. doi: 10.1016/j.biomaterials.2009.11.111. [DOI] [PubMed] [Google Scholar]
- 275.Wong H.M., Wu S., Chu P.K., Cheng S.H., Luk K.D., Cheung K.M., Yeung K.W. Low-modulus Mg/PCL hybrid bone substitute for osteoporotic fracture fixation. Biomaterials. 2013;34:7016–7032. doi: 10.1016/j.biomaterials.2013.05.062. [DOI] [PubMed] [Google Scholar]
- 276.Wang W., Wong H., Leung F., Cheung K., Yeung K. Magnesium ions enriched decellularized bone allografts for bone tissue engineering. Tissue Eng. Part A. 2015 S232-S232. [Google Scholar]
- 277.Yoshizawa S., Brown A., Barchowsky A., Sfeir C. Role of magnesium ions on osteogenic response in bone marrow stromal cells. Connect. Tissue Res. 2014;55(Suppl 1):155–159. doi: 10.3109/03008207.2014.923877. [DOI] [PubMed] [Google Scholar]
- 278.Yoshizawa S., Brown A., Barchowsky A., Sfeir C. Magnesium ion stimulation of bone marrow stromal cells enhances osteogenic activity, simulating the effect of magnesium alloy degradation. Acta Biomater. 2014;10:2834–2842. doi: 10.1016/j.actbio.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 279.Verberckmoes S.C., Broe M.E., D'Haese De P.C. Dose-dependent effects of strontium on osteoblast function and mineralization. Kidney Int. 2003;64:534–543. doi: 10.1046/j.1523-1755.2003.00123.x. [DOI] [PubMed] [Google Scholar]
- 280.Li Y., Li J., Zhu S., Luo E., Feng G., Chen Q., Hu J. Effects of strontium on proliferation and differentiation of rat bone marrow mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2012;418:725–730. doi: 10.1016/j.bbrc.2012.01.088. [DOI] [PubMed] [Google Scholar]
- 281.Reffitt D.M., Ogston N., Jugdaohsingh R., Cheung H.F.J., Evans B.A.J., Thompson R.P.H., Powell J.J., Hampson G.N. Orthosilicic acid stimulates collagen type 1 synthesis and osteoblastic differentiation in human osteoblast-like cells in vitro. Bone. 2003;32:127–135. doi: 10.1016/s8756-3282(02)00950-x. [DOI] [PubMed] [Google Scholar]
- 282.Gaharwar A.K., Mihaila S.M., Swami A., Patel A., Sant S., Reis R.L., Marques A.P., Gomes M.E., Khademhosseini A. Bioactive silicate nanoplatelets for osteogenic differentiation of human mesenchymal stem cells. Adv. Mater. 2013;25:3329–3336. doi: 10.1002/adma.201300584. [DOI] [PubMed] [Google Scholar]
- 283.Yamaguchi M., Goto M., Uchiyama S., Nakagawa T. Effect of zinc on gene expression in osteoblastic MC3T3-E1 cells: enhancement of Runx2, OPG, and regucalcin mRNA expressions. Mol. Cell. Biomech. 2008;312:157–166. doi: 10.1007/s11010-008-9731-7. [DOI] [PubMed] [Google Scholar]
- 284.Kwun I.S., Cho YELomeda R.A., Shin H.I., Choi J.Y., Kang Y.H., Beattie J.H. Zinc deficiency suppresses matrix mineralization and retards osteogenesis transiently with catch-up possibly through Runx 2 modulation. Bone. 2010;46:732–741. doi: 10.1016/j.bone.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 285.Wang W., Li T.L., Wong H.M., Chu P.K., Kao R.Y., Wu S., Leung F.K., Wong T.M., To M.K., Cheung K.M., Yeung K.W. Development of novel implants with self-antibacterial performance through in-situ growth of 1D ZnO nanowire. Colloids Surf. B Biointerfaces. 2016;141:623–633. doi: 10.1016/j.colsurfb.2016.02.036. [DOI] [PubMed] [Google Scholar]
- 286.Popp J.R., Love B.J., Goldstein A.S. Effect of soluble zinc on differentiation of osteoprogenitor cells. J. Biomed. Mater. Res. A. 2007;81A:766–769. doi: 10.1002/jbm.a.31214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Wu C., Zhou Y., Xu M., Han P., Chen L., Chang J., Xiao Y. Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity. Biomaterials. 2013;34:422–433. doi: 10.1016/j.biomaterials.2012.09.066. [DOI] [PubMed] [Google Scholar]
- 288.Ren L., Wong H.M., Yan C.H., Yeung K.W., Yang K. Osteogenic ability of Cu-bearing stainless steel. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015;103:1433–1444. doi: 10.1002/jbm.b.33318. [DOI] [PubMed] [Google Scholar]
- 289.Chen Y., Whetstone H.C., Lin A.C., Nadesan P., Wei Q., Poon R., Alman B.A. Beta-catenin signaling plays a disparate role in different phases of fracture repair: implications for therapy to improve bone healing. PLoS Med. 2007;4:1216–1229. doi: 10.1371/journal.pmed.0040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Wei F., Crawford R., Yin X. Enhancing in vivo vascularized bone formation by cobalt chloride-treated bone marrow stromal cells in a tissue engineered periosteum model. Biomaterials. 2010;31:3580–3589. doi: 10.1016/j.biomaterials.2010.01.083. [DOI] [PubMed] [Google Scholar]
- 291.Wu C., Zhou Y., Fan W., Han P., Chang J., Yuen J., Zhang M., Xiao Y. Hypoxia-mimicking mesoporous bioactive glass scaffolds with controllable cobalt ion release for bone tissue engineering. Biomaterials. 2012;33:2076–2085. doi: 10.1016/j.biomaterials.2011.11.042. [DOI] [PubMed] [Google Scholar]
- 292.Quinlan E., Partap S., Azevedo M.M., Jell G., Stevens M.M., O'Brien F.J. Hypoxia-mimicking bioactive glass/collagen glycosaminoglycan composite scaffolds to enhance angiogenesis and bone repair. Biomaterials. 2015;52:358–366. doi: 10.1016/j.biomaterials.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 293.Wolf F.I., Cittadini A. Chemistry and biochemistry of magnesium. Mol. Asp. Med. 2003;24:3–9. doi: 10.1016/s0098-2997(02)00087-0. [DOI] [PubMed] [Google Scholar]
- 294.Wallach S. Magnesium:Its biologic significance. Med. Phys. 1982;9:588–589. [Google Scholar]
- 295.Vormann J. Magnesium: nutrition and metabolism. Mol. Asp. Med. 2003;24:27–37. doi: 10.1016/s0098-2997(02)00089-4. [DOI] [PubMed] [Google Scholar]
- 296.Neuman W.F., Neuman M.W. The nature of the mineral phase of bone. Chem. Rev. 1953;53:1–45. [Google Scholar]
- 297.Neuman W.F., Mulryan B.J. Synthetic hydroxyapatite crystals. IV. Magnesium incorporation. Calcif. Tissue Res. 1971;7 doi: 10.1007/BF02062601. [DOI] [PubMed] [Google Scholar]
- 298.Glimcher M. The nature of the mineral phase in bone: biological and clinical implications. In: Aviloi L., SM K., editors. Metabolic Bone Disease & Clinically Related Disorders. Academic Press; 1998. pp. 23–50. [Google Scholar]
- 299.Saris N.E.L., Mervaala E., Karppanen H., Khawaja J.A., Lewenstam A. Magnesium: an update on physiological, clinical and analytical aspects. Clin. Chim. Acta. 2000;294:1–26. doi: 10.1016/s0009-8981(99)00258-2. [DOI] [PubMed] [Google Scholar]
- 300.Classen H.G., Baier S., Schimatschek H.F., Classen C.U. Clinically relevant interactions between hormones and magnesium metabolism - a review. Magnes. B. 1995;17 [Google Scholar]
- 301.Del Barrio R.A., Giro G., Belluci M.M., Pereira R.M., Orrico S.R. Effect of severe dietary magnesium deficiency on systemic bone density and removal torque of osseointegrated implants. Int. J. Oral Maxillofac. Surg. 2010;25:1125–1130. [PubMed] [Google Scholar]
- 302.Bernick S., Hungerford G.F. Effect of dietary magnesium deficiency on bones and teeth of rats. J. Dent. Res. 1965;44:1317–1324. [Google Scholar]
- 303.Stendig-Lindberg G., Koeller W., Bauer A., Rob P.M. Experimentally induced prolonged magnesium deficiency causes osteoporosis in the rat. Cell. Mol. Biol. Lett. 2004;15:97–107. doi: 10.1016/j.ejim.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 304.Velazguez J., Jimenez A., Chomon B., Villa T. Magnesium supplementation and bone turnover. Nutr. Rev. 1999;57:227. doi: 10.1111/j.1753-4887.1999.tb06948.x. [DOI] [PubMed] [Google Scholar]
- 305.Zhou H., Burger C., Sics I., Hsiao B.S., Chu B., Graham L., Glimcher M.J. Small-angle X-ray study of the three-dimensional collagen/mineral superstructure in intramuscular fish bone. J. Appl. Crystallogr. 2007:666–668. [Google Scholar]
- 306.Serre C., Papillard M., Chavassieux P., Voegel J., Boivin G. Influence of magnesium substitution on a collagen–apatite biomaterial on the production of a calcifying matrix by human osteoblasts. J. Biomed. Mater. Res. 1998;42:626–633. doi: 10.1002/(sici)1097-4636(19981215)42:4<626::aid-jbm20>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 307.Suchanek W.L., Byrappa K., Shuk P., Riman R.E., Janas V.F., TenHuisen K.S. Preparation of magnesium-substituted hydroxyapatite powders by the mechanochemical–hydrothermal method. Biomaterials. 2004;25:4647–4657. doi: 10.1016/j.biomaterials.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 308.Xue W., Dahlquist K., Banerjee A., Bandyopadhyay A., Bose S. Synthesis and characterization of tricalcium phosphate with Zn and Mg based dopants. J. Mater. Sci. Mater. Med. 2008;19:2669–2677. doi: 10.1007/s10856-008-3395-4. [DOI] [PubMed] [Google Scholar]
- 309.Landi E., Logroscino G., Proietti L., Tampieri A., Sandri M., Sprio S. Biomimetic Mg-substituted hydroxyapatite: from synthesis to in vivo behaviour. J. Mater. Sci. Mater. Med. 2008;19:239–247. doi: 10.1007/s10856-006-0032-y. [DOI] [PubMed] [Google Scholar]
- 310.Zhai Z., Qu X., Li H., Yang K., Wan P., Tan L., Ouyang Z., Liu X., Tian B., Xiao F., Wang W., Jiang C., Tang T., Fan Q., Qin A., Dai K. The effect of metallic magnesium degradation products on osteoclast-induced osteolysis and attenuation of NF-kappaB and NFATc1 signaling. Biomaterials. 2014;35:6299–6310. doi: 10.1016/j.biomaterials.2014.04.044. [DOI] [PubMed] [Google Scholar]
- 311.Zhang Y., Xu J., Ruan Y.C., Yu M.K., O'Laughlin M., Wise H., Chen D., Tian L., Shi D., Wang J., Chen S., Feng J.Q., Chow D.H., Xie X., Zheng L., Huang L., Huang S., Leung K., Lu N., Zhao L., Li H., Zhao D., Guo X., Chan K., Witte F., Chan H.C., Zheng Y., Qin L. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat. Med. 2016;22:1160–1169. doi: 10.1038/nm.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Zhang J., Ma X., Lin D., Shi H., Yuan Y., Tang W., Zhou H., Guo H., Qian J., Liu C. Magnesium modification of a calcium phosphate cement alters bone marrow stromal cell behavior via an integrin-mediated mechanism. Biomaterials. 2015;53:251–264. doi: 10.1016/j.biomaterials.2015.02.097. [DOI] [PubMed] [Google Scholar]
- 313.Lee J.W., Han H.S., Han K.J., Park J., Jeon H., Ok M.R., Seok H.-K., Ahn J.P., Lee K.E., Lee D.H. Long-term clinical study and multiscale analysis of in vivo biodegradation mechanism of Mg alloy. Proc. Natl. Acad. Sci. 2016;113:716–721. doi: 10.1073/pnas.1518238113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Skoryna S.C. Metabolic aspects of the pharmacologic use of trace elements in human subjects with specific reference to stable strontium. Trace Subst. Environ. Health. 1984;18:23. [Google Scholar]
- 315.Jung C., Jung J. The nature of the injury to the calcifying mechanism in rickets due to strontium. Biochem. J. 1935;29:2640–2645. doi: 10.1042/bj0292640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Coulombe J., Faure H., Robin B. In vitro effects of strontium ranelate on the extracellular calcium-sensing receptor. Biochem. Biophys. Res. Commun. 2004;323:1184–1190. doi: 10.1016/j.bbrc.2004.08.209. [DOI] [PubMed] [Google Scholar]
- 317.Brown E.M. Is the calcium receptor a molecular target for the actions of strontium on bone? Osteoporos. Int. 2003;14(suppl 3):S25–S34. doi: 10.1007/s00198-002-1343-6. [DOI] [PubMed] [Google Scholar]
- 318.Kostenuik P.J., Shalhoub V. Osteoprotegerin: a physiological and pharmacological inhibitor of bone resorption. Curr. Pharm. Des. 2001;7:613–635. doi: 10.2174/1381612013397807. [DOI] [PubMed] [Google Scholar]
- 319.Peng S., Liu X.S., Zhou G., Li Z., Luk K.D., Guo X.E., Lu W.W. Osteoprotegerin deficiency attenuates strontium-mediated inhibition of osteoclastogenesis and bone resorption. J. Bone Min. Res. 2011;26:1272–1282. doi: 10.1002/jbmr.325. [DOI] [PubMed] [Google Scholar]
- 320.Steeve Kwan T., Jean-Pierre P., Fran?Ois M., Judith C., Johanne M.P. Strontium ranelate inhibits key factors affecting bone remodeling in human osteoarthritic subchondral bone osteoblasts. Bone. 2011;49:559–567. doi: 10.1016/j.bone.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 321.Baron R., Tsouderos Y. In vitro effects of S12911-2 on osteoclast function and bone marrow macrophage differentiation. Eur. J. Pharmacol. 2002;450:11–17. doi: 10.1016/s0014-2999(02)02040-x. [DOI] [PubMed] [Google Scholar]
- 322.Hurtel-Lemaire A.S., Mentaverri R., Caudrillier A., Cournarie F., Wattel A., Kamel S., Terwilliger E.F., Brown E.M., Brazier M. The calcium-sensing receptor is involved in strontium ranelate-induced osteoclast apoptosis. J. Biol. Chem. 2009;284:575–584. doi: 10.1074/jbc.M801668200. [DOI] [PubMed] [Google Scholar]
- 323.Meunier P.J., Christian R., Ego S., Sergio O., Badurski J.E., Spector T.D., Jorge C., Adam B., Ernst-Martin L., Stig P.N. The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N. Engl. J. Med. 2004;350:459–468. doi: 10.1056/NEJMoa022436. [DOI] [PubMed] [Google Scholar]
- 324.Saidak Z., Marie P.J. Strontium signaling: molecular mechanisms and therapeutic implications in osteoporosis. Pharmacol. Ther. 2012;136:216–226. doi: 10.1016/j.pharmthera.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 325.Boivin G., Deloffre P., Perrat B., Panczer G., Boudeulle M., Mauras Y., Allain P., Tsouderos Y., Meunier P.J. Strontium distribution and interactions with bone mineral in monkey iliac bone after strontium salt (S 12911) administration. J. Bone Min. Res. 1996;11:1302–1311. doi: 10.1002/jbmr.5650110915. [DOI] [PubMed] [Google Scholar]
- 326.Bigi A., Foresti E., Gandolfi M., Gazzano M., Roveri N. Isomorphous substitutions in β-tricalcium phosphate: the different effects of zinc and strontium. J. Inorg. Biochem. 1997;66:259–265. [Google Scholar]
- 327.Saint-Jean S.J., Camiré C.L., Nevsten P., Hansen S., Ginebra M.P. Study of the reactivity and in vitro bioactivity of Sr-substituted alpha-TCP cements. J. Mater. Sci. Mater. Med. 2005;16:993–1001. doi: 10.1007/s10856-005-4754-z. [DOI] [PubMed] [Google Scholar]
- 328.Verberckmoes S.C., Behets G.J., Oste L., Bervoets A.R., Lamberts L.V., Drakopoulos M., Somogyi A., Cool P., Dorriné W., Broe M.E.D. Effects of strontium on the physicochemical characteristics of hydroxyapatite. Calcif. Tissue Int. 2004;75:405–415. doi: 10.1007/s00223-004-0260-4. [DOI] [PubMed] [Google Scholar]
- 329.Wang X., Ye J. Variation of crystal structure of hydroxyapatite in calcium phosphate cement by the substitution of strontium ions. J. Mater. Sci. Mater. Med. 2008;19:1183–1186. doi: 10.1007/s10856-007-3209-0. [DOI] [PubMed] [Google Scholar]
- 330.Christoffersen J., Christoffersen M.R., Kolthoff N., Bärenholdt O. Effects of strontium ions on growth and dissolution of hydroxyapatite and on bone mineral detection. Bone. 1997;20:47–54. doi: 10.1016/s8756-3282(96)00316-x. [DOI] [PubMed] [Google Scholar]
- 331.Xue W., Moore J.L., Hosick H.L., Bose S., Bandyopadhyay A., Lu W.W., Cheung K.M.C., Luk K.D.K. Osteoprecursor cell response to strontium-containing hydroxyapatite ceramics. J. Biomed. Mater. Res. A. 2006;79:804–814. doi: 10.1002/jbm.a.30815. [DOI] [PubMed] [Google Scholar]
- 332.Capuccini C., Torricelli P., Sima F., Boanini E., Ristoscu C., Bracci B., Socol G., Fini M., Mihailescu I.N., Bigi A. Strontium-substituted hydroxyapatite coatings synthesized by pulsed-laser deposition: in vitro osteoblast and osteoclast response. Acta Biomater. 2008;4:1885–1893. doi: 10.1016/j.actbio.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 333.Wong C.T., Lu W.W., Chan W.K., Cheung K.M.C., Luk K.D.K., Lu D.S., Rabie A.B.M., Deng L.F., Leong J.C.Y. In vivo cancellous bone remodeling on a strontium-containing hydroxyapatite (sr-HA) bioactive cement. J. Biomed. Mater. Res. A. 2004;68A:513–521. doi: 10.1002/jbm.a.20089. [DOI] [PubMed] [Google Scholar]
- 334.Gorustovich A.A., Steimetz T., Cabrini R.L., López J.M.P. Osteoconductivity of strontium-doped bioactive glass particles: a histomorphometric study in rats. J. Biomed. Mater. Res. A. 2009;92:232–237. doi: 10.1002/jbm.a.32355. [DOI] [PubMed] [Google Scholar]
- 335.Luo X., Barbieri D., Zhang Y., Yan Y., Bruijn J.D., Yuan H. Strontium-containing apatite/poly lactide composites favoring osteogenic differentiation and in vivo bone formation. ACS Biomater. Sci. Eng. 2015;1:85–93. [Google Scholar]
- 336.Strontium Ranelate (Protos) and Risk of Adverse Events. A.G. Department of Health; 2014. [Google Scholar]
- 337.Bolland M.J., Grey A. A comparison of adverse event and fracture efficacy data for strontium ranelate in regulatory documents and the publication record. BMJ Open. 2014;4:1–8. doi: 10.1136/bmjopen-2014-005787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Carlisle E.M. Biochemical and morphological changes associated with long bone abnormalities in silicon deficiency. J. Nutr. 1980;110:1046–1056. doi: 10.1093/jn/110.5.1046. [DOI] [PubMed] [Google Scholar]
- 339.Sc W.G.B. Academic Press; 1986. Trace Elements in Human and Animal Nutrition. [Google Scholar]
- 340.Nielsen F.H. Micronutrients in parenteral nutrition: boron, silicon, and fluoride. Gastroenterology. 2009;137:S55–S60. doi: 10.1053/j.gastro.2009.07.072. [DOI] [PubMed] [Google Scholar]
- 341.Carlisle E.M. Silicon: a possible factor in bone calcification. Science. 1970;167:279–280. doi: 10.1126/science.167.3916.279. [DOI] [PubMed] [Google Scholar]
- 342.Bohner M. Silicon-substituted calcium phosphates - a critical view. Biomaterials. 2009;30:6403–6406. doi: 10.1016/j.biomaterials.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 343.Keeting P.E., Oursler M.J., Wiegand K.E., Bonde S.K., Spelsberg T.C., Riggs B.L. Zeolite A increases proliferation, differentiation and TGF-beta production in normal adult human osteoblast-like cells in vitro. J. Bone Min. Res. 1992;7:1281–1289. doi: 10.1002/jbmr.5650071107. [DOI] [PubMed] [Google Scholar]
- 344.Pietak A.M., Reid J.W., Stott M.J., Sayer M. Silicon substitution in the calcium phosphate bioceramics. Biomaterials. 2007;28:4023–4032. doi: 10.1016/j.biomaterials.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 345.Botelho C.M., Brooks R.A., Spence G., Mcfarlane I., Lopes M.A., Best S.M., Santos J.D., Rushton N., Bonfield W. Differentiation of mononuclear precursors into osteoclasts on the surface of Si-substituted hydroxyapatite. J. Biomed. Mater. Res. A. 2006;78:709–720. doi: 10.1002/jbm.a.30726. [DOI] [PubMed] [Google Scholar]
- 346.Guth K., Buckland T., Hing K.A. Silicon dissolution from microporous silicon substituted hydroxyapatite and its effect on osteoblast behaviour. Key Eng. Mater. 2006;309–311:117–120. [Google Scholar]
- 347.Porter A.E., Best S.M., William B. Ultrastructural comparison of hydroxyapatite and silicon-substituted hydroxyapatite for biomedical applications. J. Biomed. Mater. Res. A. 2004;68A:133–141. doi: 10.1002/jbm.a.20064. [DOI] [PubMed] [Google Scholar]
- 348.Porter A.E., Patel N., Skepper J.N., Best S.M., Bonfield W. Effect of sintered silicate-substituted hydroxyapatite on remodelling processes at the bone-implant interface. Biomaterials. 2004;25:3303–3314. doi: 10.1016/j.biomaterials.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 349.Kawai T., Ogata S., Bonfield W., Best S., Ohtsuki C., Santos J.D., Lopes M.A., Brooks R.A., Rushton N., Botelho C.M. In Vitro analysis of protein adhesion to phase pure hydroxyapatite and silicon substituted hydroxyapatite. key Eng. Mater. 2005;284–286:461–464. [Google Scholar]
- 350.Curtis A., Wilkinson C. Topographical control of cells. Biomaterials. 1997;18:1573–1583. doi: 10.1016/s0142-9612(97)00144-0. [DOI] [PubMed] [Google Scholar]
- 351.Calhoun N.R., S.J., Becker K.L. The role of zinc in bone metabolism. Clin. Orthop. Relat. Res. 1974;103:212–234. doi: 10.1097/00003086-197409000-00084. [DOI] [PubMed] [Google Scholar]
- 352.Coleman J.E. Structure and mechanism of alkaline phosphatase. Annu. Rev. Biophys. Biomol. Struct. 1992;21:441–483. doi: 10.1146/annurev.bb.21.060192.002301. [DOI] [PubMed] [Google Scholar]
- 353.Hall S.L., Dimai H.P., Farley J.R. Effects of zinc on human skeletal alkaline phosphatase activity in vitro. Calcif. Tissue Int. 1999;64:163–172. doi: 10.1007/s002239900597. [DOI] [PubMed] [Google Scholar]
- 354.Peter H., Amanda P., Fink J.K., Sandy W., Zachary L., Brewer G.J. Myelopolyneuropathy and pancytopenia due to copper deficiency and high zinc levels of unknown origin II. The denture cream is a primary source of excessive zinc. Neurotoxicology. 2009;30:996–999. doi: 10.1016/j.neuro.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 355.Masayoshi Y. Role of nutritional zinc in the prevention of osteoporosis. Mol. Cell. Biomech. 2010;338:241–254. doi: 10.1007/s11010-009-0358-0. [DOI] [PubMed] [Google Scholar]
- 356.Kawamura H., Ito A., Miyakawa S., Layrolle P., Ojima K., Ichinose N., Tateishi T. Stimulatory effect of zinc-releasing calcium phosphate implant on bone formation in rabbit femora. J. Biomed. Mater. Res. 2000;50:184–190. doi: 10.1002/(sici)1097-4636(200005)50:2<184::aid-jbm13>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 357.Yamada Y., Ito A., Kojima H., Sakane M., Miyakawa S., Uemura T., Legeros R.Z. Inhibitory effect of Zn2+ in zinc-containing beta-tricalcium phosphate on resorbing activity of mature osteoclasts. J. Biomed. Mater. Res. A. 2008;84:344–352. doi: 10.1002/jbm.a.31265. [DOI] [PubMed] [Google Scholar]
- 358.Lee G.R., Nacht S., Lukens J.N., Cartwright G.E. Iron metabolism in copper-deficient swine. J. Clin. Invest. 1968;47:2058–2069. doi: 10.1172/JCI105891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Rucker R.B., Riggins R.S., Laughlin R., Chan M.M., Chen M., Tom K. Effects of nutritional copper deficiency on the biomechanical properties of bone and arterial elastin metabolism in the chick. J. Nutr. 1975;105:1062–1070. doi: 10.1093/jn/105.8.1062. [DOI] [PubMed] [Google Scholar]
- 360.Harris E.D. A requirement for copper in angiogenesis. Nutr. Rev. 2004;62:60–64. doi: 10.1111/j.1753-4887.2004.tb00025.x. [DOI] [PubMed] [Google Scholar]
- 361.Hu G.F. Copper stimulates proliferation of human endothelial cells under culture. J. Cell. Biochem. 1998;69:326–335. doi: 10.1002/(sici)1097-4644(19980601)69:3<326::aid-jcb10>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 362.Gerard C., Ljbarralet B. The stimulation of angiogenesis and collagen deposition by copper. Biomaterials. 2010;31:824–831. doi: 10.1016/j.biomaterials.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 363.Jake B., Uwe G., Pamela H., Elke V., Catherine G., Doillon C.J. Angiogenesis in calcium phosphate scaffolds by inorganic copper ion release. Tissue Eng. Part A. 2009;15:1601–1609. doi: 10.1089/ten.tea.2007.0370. [DOI] [PubMed] [Google Scholar]
- 364.Li Q.F., Ding X.Q., Kang Y.J. Copper promotion of angiogenesis in isolated rat aortic ring: role of vascular endothelial growth factor. J. Nutr. Biochem. 2014;25:44–49. doi: 10.1016/j.jnutbio.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 365.Natalia M.S., Ewa S., Krishna Prasad V., Monika U., Jan N., Mateusz W., Marta K., Awomir J.S., André C. Nanoparticles of copper stimulate angiogenesis at systemic and molecular level. Int. J. Mol. Sci. 2015;16:4838–4849. doi: 10.3390/ijms16034838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Lydia F., Suneeta M., Lyann U., Wen Z., Diane R., Stefan V., Daniel L., Jorg M., Francis I., Olopade O.I. X-ray fluorescence microscopy reveals large-scale relocalization and extracellular translocation of cellular copper during angiogenesis. Proc. Natl. Acad. Sci. 2007;104:2247–2252. doi: 10.1073/pnas.0607238104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Lüthen F., Bergemann C., Bulnheim U., Prinz C., Neumann H.G., Podbielski A., Bader R., Rychly J. A dual role of copper on the surface of bone implants. Mater. Sci. Forum. 2010:600–605. [Google Scholar]
- 368.Shi M., Chen Z., Farnaghi S., Friis T., Mao X., Xiao Y., Wu C. Copper-doped mesoporous silica nanospheres, a promising immunomodulatory agent for inducing osteogenesis. Acta Biomater. 2016;30:334–344. doi: 10.1016/j.actbio.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 369.Ferenci P. Review article: diagnosis and current therapy of Wilson's disease. Aliment. Pharmacol. Ther. 2004;19:157–165. doi: 10.1046/j.1365-2036.2003.01813.x. [DOI] [PubMed] [Google Scholar]
- 370.Ricardo U., Alejandro M., Magdalena A. Estimating risk from copper excess in human populations. Am. J. Clin. Nutr. 2008;88:867S–871S. doi: 10.1093/ajcn/88.3.867S. [DOI] [PubMed] [Google Scholar]
- 371.Ali Z., Omrani G.R., Masoud Mousavi N. Lithium's effect on bone mineral density. Bone. 2009;44:331–334. doi: 10.1016/j.bone.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 372.Wilting I., Vries F.D., Thio B.M.K.S., Cooper C., Heerdink E.R., Leufkens H.G.M., Nolen W.A., Egberts A.C.G., Staa T.P.V. Lithium use and the risk of fractures. Bone. 2007;40:1252–1258. doi: 10.1016/j.bone.2006.12.055. [DOI] [PubMed] [Google Scholar]
- 373.Hedgepeth C.M., Conrad L.J., Zhang J., Huang H.C., Lee V.M., Klein P.S. Activation of the Wnt signaling pathway: a molecular mechanism for lithium action. Dev. Biol. 1997;185:82–91. doi: 10.1006/dbio.1997.8552. [DOI] [PubMed] [Google Scholar]
- 374.Chalecka-Franaszek E., Chuang D.M. Lithium activates the serine/threonine kinase Akt-1 and suppresses glutamate-induced inhibition of Akt-1 activity in neurons. Proc. Natl. Acad. Sci. 1999;96:8745–8750. doi: 10.1073/pnas.96.15.8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Wei W., Lingzhou Z., Kaimin W., Qianli M., Shenglin M., Paul C., Qintao W., Yumei Z. The role of integrin-linked kinase/β-catenin pathway in the enhanced MG63 differentiation by micro/nano-textured topography. Biomaterials. 2013;34:631–640. doi: 10.1016/j.biomaterials.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 376.Emilie P., Hélène L., Samuel V., Pascal D., Myriam L., Edwige P., Olivier N., Myriam B. Synergistic effects of CoCl(2) and ROCK inhibition on mesenchymal stem cell differentiation into neuron-like cells. J. Cell. Sci. 2006;8:2667–2678. doi: 10.1242/jcs.03004. [DOI] [PubMed] [Google Scholar]