Abstract
Background
In patients with left ventricular systolic dysfunction (LVSD), the rate at which oxygen uptake (VO2) increases upon initiation of exercise is inadequate to match metabolic demands. To gain mechanistic insights into delayed VO2 kinetics in LVSD we simultaneously assessed hemodynamic measurements, ventilatory parameters, and peripheral oxygen utilization during exercise.
Methods and Results
42 patients with symptomatic LVSD (age 59±2 years [mean±SEM], LV ejection fraction (LVEF) 30±1%) and 17 controls (LVEF 68±1%) underwent maximum upright cycle ergometry cardiopulmonary exercise testing (CPET). Hemodynamic monitoring and first-pass radionuclide ventriculography were performed at rest and during exercise. VO2 kinetics were quantified by mean response time (MRT), which was significantly longer in patients with LVSD compared to controls (64±3 vs. 45±5 seconds (s), p =0.004). In LVSD patients, MRT was associated with higher biventricular filling pressures and reduced cardiac output during early exercise. LVSD patients with MRT≥60s, compared to LVSD subjects with MRT<60s, demonstrated greater impairment in right ventricular-pulmonary vascular (RV-PV) function during exercise as evidenced by lower RVEF (35±2 vs. 45±2%, p=0.03), steeper increment in trans-pulmonary gradient relative to cardiac output (3.7 vs. 2.2, p<0.001), and increased ventilatory dead-space fraction (17±1 vs. 12±2%, p=0.03). In contrast, MRT was not associated with LVEF (rest, exercise), PaO2, hemoglobin, or resting pulmonary function test results.
Conclusions
Delayed oxygen uptake upon initiation of exercise (i.e. MRT ≥60s) in LVSD is closely related to impaired RV-PV function and may represent an important surrogate for inability to augment RV performance during physical activity in patients with HF.
Keywords: exercise physiology, exercise testing, heart failure
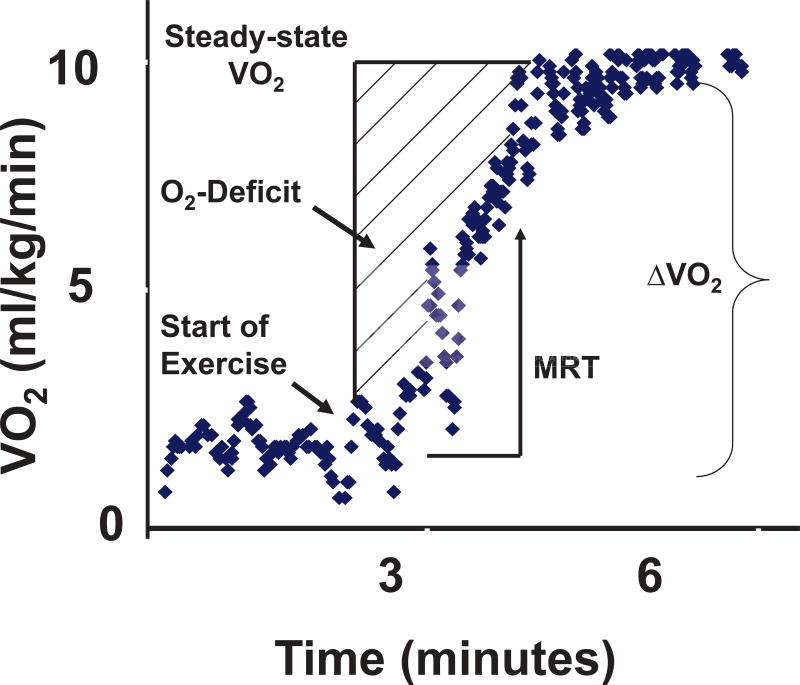
Exercise intolerance is a cardinal manifestation of heart failure (HF). HF patients with left ventricular systolic dysfunction (LVSD) have decreased maximal exercise capacity as measured by peak oxygen uptake (VO2) as well as impaired adaptations to and recovery from submaximal levels of exercise.1–3 At the onset of exercise, oxygen delivery to exercising skeletal muscle must increase in order to maintain cellular homeostasis. The cumulative difference between steady-state oxygen consumption during constant work exercise and actual oxygen uptake from rest until steady-state is referred to as the oxygen deficit (Figure 1).1, 4, 5 The rate at which VO2 reaches steady-state is characterized by the mean response time (MRT), which is the exponential time constant of VO2 rise and approximates the time needed to reach 63% of steady-state VO2.1, 6
Figure 1.
Mean response time (MRT) is the exponential time constant of O2 uptake during exercise and is approximately 63% of the time needed to reach steady-state. MRT was derived during unloaded exercise and calculated as the ratio of oxygen deficit (O2-deficit) and change in oxygen consumption (ΔVO2), defined as the difference between VO2 at rest and steady-state.
Prolonged MRT in patients with LVSD has been independently linked to NYHA HF class7 as well as neurohormonal activation,8 and may be more relevant than peak VO2 to symptoms elicited during activities of daily living.4, 9 In addition, prolonged MRT during early exercise is an independent risk factor for HF mortality and hospitalization.10, 11
Despite the growing recognition of the clinical importance of prolonged MRT in patients with LVSD, its mechanistic basis remains unclear. Previous studies have suggested that impaired cardiac performance and abnormal peripheral oxygen utilization contribute to prolonged MRT, though simultaneous central cardiac and peripheral assessments during exercise have not been performed.2, 6, 12–16 In addition, despite the increasingly recognized role of right ventricular-pulmonary vascular (RV-PV) function in exercise limitation in LVSD,17 the extent to which pulmonary perfusion and RV function are related to MRT remains unknown.
In this study, we hypothesized that slowed VO2 kinetics in LVSD, reflected by a prolonged MRT, were related to an impaired central hemodynamic response during early exercise and, more specifically, to abnormalities in RV-PV function. We therefore sought to determine whether MRT, derived during submaximum exercise, may be a surrogate for impaired RV-PV function in HF. We performed simultaneous assessment of hemodynamic function (i.e. cardiac output and biventricular filling pressures), peripheral muscle oxygen extraction, arterial blood gases, and ventilatory parameters at rest and during submaximal exercise in both HF and control subjects. To further assess abnormalities in RV-PV reserve function, we focused on examination of pressure-flow relationships during early exercise.
Methods
Patient Population and Study Design
Consecutive patients who underwent cardiopulmonary exercise testing (CPET) with invasive hemodynamic monitoring at Massachusetts General Hospital, had LV ejection fraction (LVEF) < 40%, and chronic NYHA class II–IV symptoms despite standard therapy were included in the study population. Exclusion criteria consisted of: 1) incomplete pulmonary arterial catheter pressure measurements; 2) documented intracardiac shunting; 3) steady state exercise of less than 3 min duration or marked oscillatory breathing; or 4) the presence of a pulmonary mechanical limitation to exercise as defined by VE/(forced expiratory volume in 1 second [FEV1] × 35) > 0.7 at the anaerobic threshold.18 Controls consisted of subjects referred for CPET to evaluate dyspnea on exertion during the same period of time as the LVSD group. Controls were required to have normal LV function and normal exercise capacity as reflected by a peak VO2 greater than 80% of that predicted on the basis of age, gender, and height.19 Subjects meeting these inclusion criteria who were similar in age to the LVSD subjects composed the control cohort.
Cardiopulmonary Exercise Testing
All patients underwent placement of a pulmonary arterial catheter via the internal jugular vein and placement of a systemic arterial catheter via the radial artery. First-pass radionuclide ventriculography of both ventricles was performed immediately prior to cycle ergometry testing and during the last minute of symptom-limited exercise, as previously described.20 Subjects then underwent maximum incremental upright cycle ergometry CPET (5–15 Watt/min continuous ramp after an initial 3 minute period of unloaded exercise, MedGraphics, St. Paul, MN) with simultaneous hemodynamic monitoring (Witt Biomedical Inc, Melbourne, FL) as previously described.20, 21 Right atrial pressure (RAP), pulmonary arterial pressure (PAP), pulmonary capillary wedge pressure (PCWP), and mean systemic arterial pressure (MAP) were measured in the upright position, at end-expiration, while patients were seated on the cycle, at rest, and at one-minute intervals during exercise. Fick cardiac outputs (CO) were also determined at one minute intervals throughout exercise by measuring VO2 and simultaneous radial arterial and pulmonary arterial oxygen content to calculate the arteriovenous O2 difference (C[a−v]O2). Peak VO2 was defined as the highest O2 uptake, averaged over 30 seconds (s), during the last minute of symptom-limited exercise, as previously described.21
Mean Response Time
VO2 kinetics were assessed during three minutes of unloaded exercise within the exercise protocol described above. The first three minutes were selected for derivation of oxygen kinetics given previous reports indicating that steady-state is achieved for low-to-moderate-work load exercise at approximately three minutes.1, 22–24 As previously reported, oxygen deficit was defined as [t* Δ VO2 − Σ VO2], where t was time from rest to steady-state (i.e. 180 s), ∆VO2 was difference between resting and steady-state VO2 (median highest 30 s in last minute of unloaded exercise) and Σ VO2 was actual cumulative oxygen consumption, as determined by Simpson’s method of multiplying breath-by-breath VO2 values by the number of seconds that transpire between breaths over the 180 s of unloaded exercise (Figure 1). Mean response time, defined as [oxygen deficit]/[ΔVO2], is the exponential time constant of VO2 onset kinetics1, 6 and approximates the time needed to reach 63% of steady-state VO2.
Statistical Methods
SAS 9.3 (SAS Institute Inc., Cary, North Carolina) was used for statistical analysis. All continuous, normally-distributed measurements are presented as the mean±SEM. The Wilk-Shapiro test was used to assess the normality of continuous variables for selection of appropriate comparison testing between subgroups. Categorical data are reported as percentages. Group baseline characteristics were compared using either the Student t test or Fisher’s exact test, as appropriate. For clinical characteristics, comparisons between groups for continuous variables were performed using unpaired two-sample t tests or the Wilcoxon signed rank test, as appropriate. Pearson or Spearman correlation coefficients were calculated, based on whether or not the data was either normally or not normally distributed, respectively. Relationships between MRT, considered as a continuous variable, and other rest and exercise clinical variables were assessed by univariate and multivariate linear regression in LVSD subjects. Subgroup analysis was performed comparing HF patients with MRT greater than or equal to versus less than 60 s. Pressure-flow (PQ) relationships during exercise were analyzed by comparing average PQ value during each minute of exercise between HF subgroups. The selection of the 60 second endpoint for MRT reflected the approximate median of the cohort. Sensitivity analyses were performed using both 55 and 65 s with no significant differences in associations. This study was approved by the institutional review board and all patients provided informed consent. The authors had full access to the data and take responsibility for its integrity and for the manuscript as written.
Results
Population Characteristics
Baseline characteristics for all HF subjects (N = 42), HF subjects stratified by MRT greater than or equal to (N=24) or less than 60 seconds (N=18), and control subjects (N=17), are reported in Table 1. MRT was significantly longer in HF subjects compared to controls (64±3 vs. 45±5s, p=0.004). HF patients with MRT greater than or equal to 60s (HF, MRT ≥60s) did not differ from HF patients with MRT less than 60s (HF, MRT <60s) with respect to age, gender, medication exposures, or LVEF (Table 1).
Table 1.
Clinical Characteristics of Heart Failure Patients Stratified by MRT and Control Subjects
| Characteristic | Heart Failure (n=42) |
HF, MRT ≥60s (n=24) |
HF, MRT <60s (n=18) |
Control (n=17) |
|---|---|---|---|---|
| Age – Years | 59±2 | 57±3 | 61 ± 2 | 61±3 |
| Male Sex - % | 88 | 92 | 84 | 82 |
| Primary Cause of Heart Failure; ischemic - % | 50§ | 50 | 50 | 0 |
| Heart Failure Pharmacotherapy - % | ||||
| Diuretic – Loop | 88§ | 92 | 83 | 6 |
| ACE Inhibitor or ARB | 81§ | 79 | 83 | 41 |
| β-Adrenergic Receptor Antagonist | 95§ | 96 | 94 | 18 |
| Spironolactone – Aldo Blocker | 52§ | 42 | 67 | 0 |
| Digoxin | 50§ | 58 | 39 | 0 |
| Cardiac Resynchronization Therapy - % | 26§ | 25 | 28 | 0 |
| LV Ejection Fraction (rest), % | 30±1§ | 30±1 | 31±1 | 68±1 |
| Hemoglobin (g/dL) | 12.8±0.3 | 12.8±0.5 | 12.8±0.4 | 13.1±0.4 |
| Mean response time, s | 64±3§ | 77±3* | 44±3 | 45±5 |
Continuous variables are presented as mean ± standard error of the mean (SEM) if normally distributed and as median (interquartile range) if not normally distributed.
Indicates P<0.05 for comparison of HF, MRT >60s and HF, MRT <60s.
indicates P<0.05 for comparison of HF and controls.
ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; RV, right ventricular; LV, left ventricular; SVR, systemic vascular resistance; PVR, pulmonary vascular resistance.
Relationship Between MRT and Resting Indices of Cardiac, Pulmonary, and Peripheral Function
Resting indices of cardiac performance for all patients are shown in Table 2. When compared to HF patients with MRT<60s, HF patients with MRT≥60s demonstrated higher resting mean PAP (p=0.002) and PCWP (p=0.002), lower RVEF (p=0.004), and a trend toward lower cardiac output (p=0.08) (Table 2). Similarly, linear regression analysis demonstrated that MRT expressed as a continuous variable was related to higher PAP and PCWP, as well as lower resting RVEF (see Supplemental Tables 1 and 2 for full list of resting hemodynamic univariate predictors of MRT). Finally, prolonged MRT was not related to reduced peripheral oxygen extraction; at rest, HF, MRT ≥60s patients demonstrated increased C[a−v]O2 when compared to both HF, MRT <60s (p=0.04) and controls (p=0.02) (Table 2).
Table 2.
Hemodynamic Values, Cardiac Function, and Ventilatory Parameters Measured at Rest
| Variable | HF | HF, MRT ≥60s | HF, MRT <60s | Control |
|---|---|---|---|---|
| RAP, mmHg | 5±1 | 6±1 | 4±1 | 3±0 |
| PAP, mmHg | 25(13)§ | 31(14)* | 20(6) | 15±1†‡ |
| PCWP, mmHg | 15±1§ | 18±2* | 11±2 | 6±1†‡ |
| MAP, mmHg | 83±2§ | 81±2 | 85±3 | 104±2†‡ |
| C[a−v]O2, mL O2/dL | 7.4(2.2)§ | 8.1(2.5)* | 6.9(1.5) | 6.3(2.3)† |
| Cardiac Output, L/min | 3.6(1.1)§ | 3.4(0.9) | 4.1(1.4) | 4.7±0.3†‡ |
| Heart rate, bpm | 71(21) | 69(24) | 73(12) | 74±2 |
| Stroke Volume, mL | 52±3§ | 53±4 | 51±3 | 69±4†‡ |
| SVR, dynes*s/cm5 | 1640(875) | 1706(1082) | 1625(685) | 1733(538) |
| PVR, dynes*s/cm5 | 265(130)§ | 280(145) | 238(114) | 117(121)†‡ |
| PaO2, mmHg | 90±2 | 91±3 | 88±3 | 95±2 |
| PaCO2, mmHg | 38±1 | 37±1 | 39±1 | 36±1 |
| pH, units | 7.45 | 7.45 | 7.44 | 7.43† |
| FEV1, L | 2.30 ± 0.85§ | 2.30 ± 0.97 | 2.29 ± 0.67 | 3.34 ± 0.48†‡ |
| DLCO, ml/min/mmHg | 17.9 ± 6.1§ | 18.0 ± 6.7 | 17.8 ± 5.3 | 23.2 ± 5.5†‡ |
| RV Ejection Fraction, % | 41(18)§ | 35(14) | 48(7) | 56±1†‡ |
Continuous variables are presented as mean ± standard error of the mean (SEM) if normally distributed and as median (interquartile range) if not normally distributed. MAP indicates mean systemic arterial pressure; RAP mean right atrial pressure; PAP mean pulmonary arterial pressure; PVR pulmonary vascular resistance; SVR systemic vascular resistance; C[a−v]O2 indicates arterio-venous difference in oxygen content (i.e. oxygen extraction); FEV1, forced expiratory volume in one second.
Indicates P<0.05 for comparison of HF, MRT >60s and HF, MRT <60s,
indicates P<0.05 for HF, MRT >60s vs. controls,
indicates P<0.05 for comparison of HF, MRT <60s and controls, and
indicates P<0.05 for comparison of HF and controls.
Relationship Between MRT and Indices of Cardiac, Pulmonary, and Peripheral Function During Early Exercise
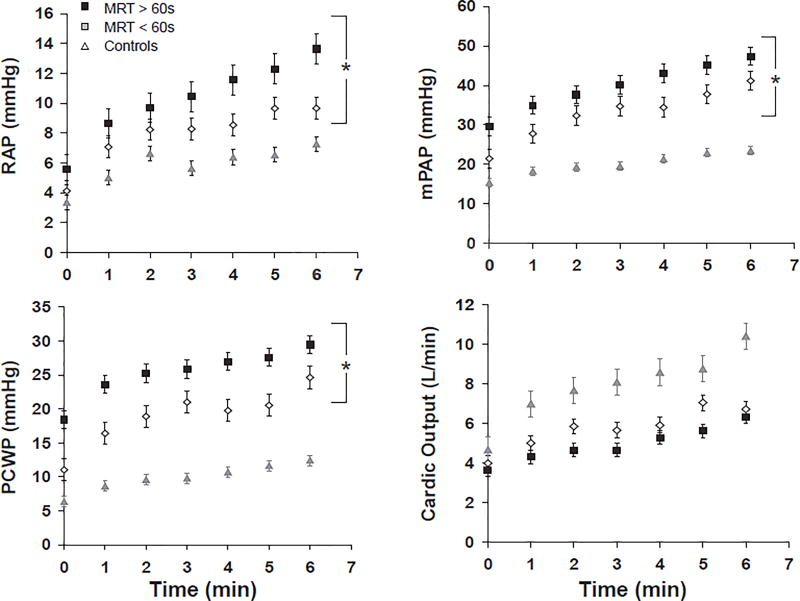
In order the determine the extent to which MRT reflects hemodynamic responses to exercise initiation, we performed hemodynamic measurements during each minute of a standardized period of the initial six minutes of exercise which all subjects were able to complete (Figure 2). Hemodynamic measurements were averaged over the 6-minute period for comparison between groups (Table 3). During exercise, HF patients with MRT ≥60s had elevated intracardiac pressures (RAP, PAP, PCWP) as well as lower exercise cardiac output (median [IQR]) (4.6 [1.9] L/min vs. 5.5 [3.4] L/min, p=0.004) compared to HF patients with MRT <60s despite similar work rates (Figure 2 and Table 3). In addition, HF patients with prolonged MRT had a lower peak exercise RVEF (35±2 vs. 42±2%, p=0.03) and increased dead-space ventilation (17±1 vs. 12±2%, p=0.03) (Table 3), when compared to HF patients with MRT<60s. The average change in RVEF from rest to peak exercise in the HF, MRT <60s vs. HF, MRT ≥60s groups (−2.7±1.8 vs. −0.3±1.6%) was not significantly different (p=0.36). There were no differences in peak exercise LVEF or peak exercise C[a−v]O2 between HF groups stratified by MRT.
Figure 2.
Mean ± SEM hemodynamic measurements at rest (time 0) and during the first 6 minutes of exercise for patients with heart failure, stratified by mean response time (MRT) and control subjects. MRT was derived using hemodynamic measurements corresponding to unloaded exercise, performed at minutes 0 to 3 of exercise. A, Right atrial pressure (RAP); B, mean pulmonary artery pressure (mPAP); C, pulmonary capillary wedge pressure (PCWP); D, Cardiac output. * Denotes p<0.05 for comparison of average hemodynamic measurements during exercise for MRT< 60 sec vs. MRT ≥ 60 sec.
Table 3.
Hemodynamic values, ventilatory parameters, and cardiac function measured during submaximal and peak exercise
| Variable | HF | HF, MRT ≥60s |
HF, MRT <60s |
Control | |
|---|---|---|---|---|---|
| Submaximal Exercise | |||||
| RAP, mmHg | 10(8)§ | 11(9)* | 8(5) | 6(4)†‡ | |
| PAP, mmHg | 40(18)§ | 43(13)* | 33(20) | 21±1†‡ | |
| PCWP, mmHg | 25(15)§ | 27(13)* | 19(14) | 10(6)†‡ | |
| MAP, mmHg | 90±1§ | 88±1 | 88±6 | 109±1†‡ | |
| C[a−v]O2, mL O2/dL | 10.7(3.4)§ | 11.2(3.3) | 10.2(2.8) | 8.9(2.2)†‡ | |
| Cardiac Output, L/min | 4.9(2.6)§ | 4.6(1.9)* | 5.5(3.4) | 8.1(2.8)†‡ | |
| Heart rate, bpm | 84(25) | 83(26) | 85(25) | 79(20) | |
| PaO2, mmHg | 92±1 | 92±2 | 91±1 | 92(21)‡ | |
| PaCO2, mmHg | 38(7) | 37(6)* | 40(6) | 36±1‡ | |
| pH, units | 7.44§ | 7.44 | 7.44 | 7.43†‡ | |
| Vd/Vt,% | 15±1§ | 17±1* | 12±2 | 11±1† | |
| Work, watts | 51±4 | 52±4 | 50±4 | 57±6 | |
| Peak Exercise | |||||
| RV Ejection Fraction (peak), % | 39(16)§ | 37(21)* | 45(11) | 58±1†‡ | |
| LV Ejection Fraction (peak), % | 33±1§ | 32±2 | 36±2 | 71±1†‡ | |
| Peak C[a−v]O2, mL O2/dL | 13.6±0.4 | 13.7±0.7 | 13.6±0.5 | 12.8±0.3 | |
| Peak VO2, ml/kg/min | 12.4±0.6§ | 11.5±0.9* | 13.5±0.9 | 23.0±1.5†‡ | |
| Work, Watts | 79±7 | 76±8 | 84±8 | 132±10†‡ | |
Continuous variables are presented as mean ± standard error of the mean (SEM) if normally distributed and as median (interquartile range) if not normally distributed. MAP indicates mean systemic arterial pressure; RAP mean right atrial pressure; PAP mean pulmonary arterial pressure; C[a−v]O2 indicates arterio-venous difference in oxygen content (i.e. oxygen extraction); Vd indicates dead-space ventilation. Submaximal exercise values were averaged over the first 6 minutes of exercise, with the exception of work, which refers to the work rate at 6 minutes.
Indicates P<0.05 for comparison of HF, MRT >60s and HF, MRT <60s,
indicates P<0.05 for HF, MRT >60s vs. controls,
indicates P<0.05 for comparison of HF, MRT <60s and controls, and
indicates P<0.05 for comparison of HF and controls.
Univariate regression analysis similarly demonstrated that MRT, expressed as a continuous variable, was directly related to elevated cardiac filling pressures during submaximal exercise (Supplemental Tables 1 and 2), reduced RVEF (ρ= −0.54, p=0.0007), and a trend towards reduced LVEF (β= −0.32, p=0.06). With respect to ventilatory parameters, MRT was positively associated with the dead-space to tidal volume ratio (Vd/Vt, p=0.04) and VE/VCO2 (p=0.03), and negatively related to exercise arterial pCO2 (p=0.02) averaged over the initial 6 minutes of exercise. Finally, MRT was associated with increased peripheral oxygen extraction (C[a−v]O2) during submaximal exercise (p=0.04), but showed no association with exercise heart rate (absolute or relative increase from resting).
Right Ventricular-Pulmonary Vascular Unit Function and MRT
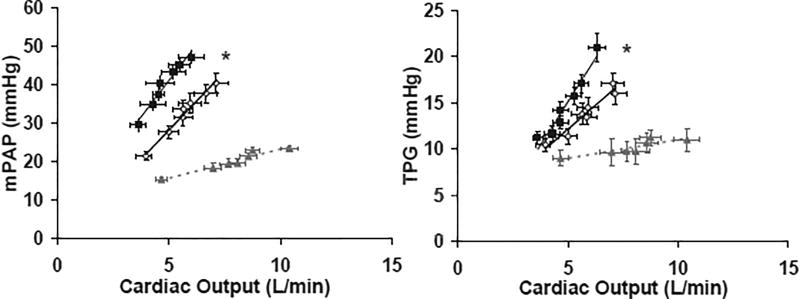
The ability of the RV-PV unit to accommodate increased flow requisite for exercise is best examined by measuring pressure-flow relationships in the pulmonary circulation. To further assess the relationship between MRT and RV-PV function during exercise we compared PAP vs. CO and trans-pulmonary gradient [TPG=mPAP-PCWP] vs. CO during the first 6-minutes of exercise in HF patients stratified by MRT (<60s vs. ≥60s) and control subjects (Figure 3). The overall group of HF patients, compared to controls, showed a higher PAP vs. CO slope (7.0 mmHg/L*min−1 vs. 1.5 mmHg/L*min−1, p<0.001) and TPG vs. CO slope (3.3 mmHg/L*min−1 vs. 1.9 mmHg/L*min−1, respectively, both p<0.001). Compared to HF patients with MRT < 60s, HF patients with MRT ≥60s demonstrated increased PAP relative to CO (slope, 6.8 vs. 6.0 mmHg/L*min−1, p<0.001) and increased incremental pulmonary vascular resistance (TPG vs. CO slope, 3.75 vs. 2.23 mmHg/L*min−1, p<0.001) during submaximal exercise.
Figure 3.
Pressure-flow relationship of right ventricular-pulmonary vascular (RV-PV) unit (A) and incremental pulmonary vascular resistance (B) during exercise for patients with HF, stratified by mean response time (MRT) and control subjects. The pressure-flow relationship (pulmonary artery pressure vs. cardiac output [A] and trans-pulmonary gradient vs. cardiac output [B]) were assessed at rest and each minute of a standardized 6-minute exercise protocol. Shown are averages across all subjects within each group. PAP, pulmonary artery pressure; TPG, trans-pulmonary gradient. * Indicates p<0.05 for comparison of average hemodynamic measurements during exercise for MRT < 60 sec vs. MRT ≥ 60 sec.
Predictive Value of MRT and Peak VO2 in Early Exercise Hemodynamics and Cardiac Function
Peak VO2 is a standard exercise parameter that is widely used in the evaluation of patients with LVSD. However, peak VO2 does not necessarily reflect cardiovascular performance during low-level exercise representative of activities of daily living. We hypothesized that MRT would be more closely associated with hemodynamic dysfunction during low-level exercise compared to peak VO2.
MRT was inversely associated with peak VO2 (β= −0.36, p=0.02). Univariate associations between peak VO2 and other hemodynamic and ventriculographic measurements are summarized in Supplemental Table 3. Similar to MRT, peak VO2 showed univariate association with indices of reduced cardiac reserve (i.e. lower exercise cardiac output) and associated elevations of intracardiac pressures (RAP, PAP) (univariate p <0.05, for all) during early exercise. Peak VO2 also showed association with rest (β=0.37, p=0.02) and peak exercise RVEF (β=0.40, p=0.02) but neither rest nor peak exercise LVEF. To better assess the relative value of MRT versus peak VO2 in predicting invasive indices of cardiac performance during early exercise a bivariate analysis was performed. After adjusting for peak VO2, MRT remained an independent predictor of submaximal exercise PAP (p=0.007) and PCWP (p=0.024), as well as peak exercise RVEF (p=0.003), but not submaximal exercise cardiac output.
Discussion
The ability to rapidly increase oxygen delivery to skeletal muscle during early exercise is critical for maintenance of cellular homeostasis. In this study, through comprehensive physiologic assessments during early exercise, we determined that delayed oxygen uptake kinetics, quantified by MRT, were closely related to reduced exercise cardiac output and elevated biventricular filling pressures. Specifically, prolonged MRT was related to three independent metrics of abnormal right ventricular-pulmonary vascular function: (i) reduced resting and exercise RVEF, (ii) increased dead-space ventilation, and (iii) a steep PAP-flow relationship during early exercise. In contrast, MRT was not associated with systemic oxygen levels (exercise PaO2), spirometry indices, or impaired peripheral oxygen utilization, providing further support for a central cardiac etiology of delayed VO2 kinetics. In aggregate, these findings indicate that prolonged MRT in patients with LVSD signals an impaired hemodynamic response to exercise characterized by impaired RV-PV reserve.
Previous studies assessing the delayed VO2 kinetics of LVSD have been limited to isolated assessments of central and peripheral mechanisms, without integration of parameters of pulmonary, peripheral, and cardiac function. Analysis of central mechanisms have been limited to one study relating MRT to peak cardiac index,25 and another that relied on serial estimates of cardiac output using radionuclide counts.2 Our study is the first to examine the relationship between MRT and serial Fick cardiac output measurements, with simultaneous assessment of biventricular filling pressures during early exercise. It is also the first study to disaggregate right versus left ventricular function in the assessment of prolonged MRT. With respect to pulmonary mechanisms of delayed VO2 kinetics, while previous work26 has identified a relative increase in dead-space ventilation in LVSD compared to controls, and others13 have linked abnormal ventilation-perfusion matching to peak VO2 in HF, no previous studies have examined the relationship between pulmonary function or dead-space ventilation and oxygen kinetics. Similarly, studies assessing the contribution of peripheral mechanisms to abnormal oxygen uptake in HF have been limited to correlations of skeletal muscle function to peak VO2,27, 28 or correlations of histologic assessments to VO2 kinetics,29 without simultaneous assessment of central hemodynamics.
The time constant of VO2 kinetics for patients with LVSD in this study (64±3s) was similar to that described in previous studies that examined subjects with comparable degrees of systolic impairment including reports from Koike et al.2 (MRT of 58±8s), Schalcher et al. (50.1±14.2s)11 and Seitsema et al. (67±26s).3 MRTs previously reported in healthy subjects by Lewalter et al. (35.1±9.9s)30 and Sietsema et al. (37±25s)3 were also qualitatively similar to the MRT of control subjects reported here (45±5s).
Relationship between MRT, Cardiac Output and Peripheral Oxygen Extraction
Augmentation of VO2 in response to exercise, according to the Fick equation, is related to either an increase in cardiac output, an increase in peripheral muscle oxygen extraction (C[a−v]O2), or both. Our study is the first to integrate assessment of both the cardiac output and peripheral extraction components of oxygen uptake during measurement of MRT. Previously, Meyer and colleagues25 demonstrated correlation between MRT and peak cardiac index in LVSD, while Koike et al.2 extended these findings by dynamically assessing cardiac output using radionuclide counts in patients with recent myocardial infarction. The latter group demonstrated correlation between MRT, exercise cardiac output, and LVEF, inferring a relationship between MRT and LV systolic function.
Our study, in contrast to that of Koike and colleagues,2 was confined to HF patients with reduced LVEF and employed Fick measurements of cardiac output which tend to be more reliable than radionuclide volumetric measurements in LVSD, where mitral regurgitation is common. Similar to Koike et al,2 we found that HF patients with prolonged MRT (≥60s) demonstrated relatively impaired exercise cardiac output (4.6 [1.9] L/min) compared to both HF patients with MRT <60s (5.5 [3.4] L/min) and control patients (8.1 [2.8] L/min). Notably, there was no significant difference in LVEF between groups of HF patients stratified by MRT in this study, suggesting that the link between impaired exercise cardiac output and prolonged VO2 kinetics is not necessarily linked to degree of LV systolic dysfunction.
Potential peripheral contributions to delayed VO2 augmentation in LVSD include exercise arterial hypoxemia, reduced local oxygen delivery,28 and impaired oxygen extraction by skeletal muscle.29, 31 The limited evidence available in healthy controls suggests that oxygen transport within exercising muscle is not a limiting factor in oxygen extraction.32,33 We found no differences in arterial oxygen concentration between HF groups stratified by MRT or control patients, suggesting that exercise arterial hypoxemia was not responsible for delayed VO2 kinetics during early exercise. In contrast, we found independent association between MRT and increased peripheral oxygen extraction both at rest and during early exercise, which likely reflects a compensatory mechanism for reduced cardiac output during submaximal exercise.34, 35 The finding of no significant difference in peripheral oxygen extraction at peak exercise between HF groups stratified by MRT further argues against a relationship between prolonged MRT and blunted peripheral oxygen extraction. In aggregate, our findings argue against abnormal peripheral oxygen extraction as a primary mechanism for delayed VO2 kinetics in LVSD during submaximal exercise.
Prolonged MRT is Related to Impaired Right Ventricular-Pulmonary Vascular Function
This is the first study to associate delayed oxygen kinetics with abnormal RV-PV function during submaximal exercise. Using three independent evaluative tools, we demonstrate that a prolonged MRT was associated with reduced RVEF (rest, exercise), increased dead-space ventilation during early exercise, and abnormal RV-PV coupling as reflected by a steeper pressure-flow slope and increased pulmonary vascular resistance during exercise. In normal individuals initiation of exercise is associated with a rapid increase in cardiac output as well as improved efficiency of gas exchange through pulmonary vascular recruitment and improved ventilation-perfusion matching. Our group recently demonstrated that exercise imposes a greater relative load on the RV compared to the LV.17
Therefore, the importance of RV function in increasing pulmonary perfusion, coupled with the known inefficiency of gas exchange that arises with pulmonary vascular dysfunction and abnormal ventricular-vascular coupling, likely explains why blunted oxygen uptake augmentation during early exercise closely reflects impaired RV-PV function in HF. Potential mechanisms of abnormal RV-PV coupling in patients with prolonged MRT include reduced pulmonary vascular recruitment,36 blunted reduction in pulmonary vascular tone during exercise,37 structural changes to pulmonary vasculature in the setting of chronically elevated left-sided pressures,38 and dynamic mitral regurgitation (MR) during exercise.39 Indeed, while prolonged MRT was associated with increased left-sided filling pressures (rest and exercise PCWP), the finding of an increased TPG-CO slope in HF patients with prolonged MRT highlights the role of abnormal pre-capillary pulmonary vascular function in the delayed oxygen kinetics of LVSD.
Clinical Implications
Exercise limitation is a hallmark of LVSD and represents a major source of HF morbidity. Underlying this clinical limitation is a central pathophysiologic feature of heart failure, namely the inability of the heart to deliver oxygen at a rate commensurate with metabolic need. Peak VO2 remains the gold-standard barometer of maximum exercise capacity and is a well-established determinant of survival in LVSD.40, 41 Nonetheless, peak VO2 is limited as an assessment tool given its reliance on patient motivation,42 the influence of non-cardiac comorbidities on ability to perform high workloads, and the fact that it reflects a physiologic state rarely achieved by HF patients.43 Determination of VO2 kinetics during submaximal testing represents an easily measurable and reproducible parameter that may have more relevance to daily morbidity than peak VO2. Here we find in bivariate analysis that MRT and peak VO2 provide complementary insight into the hemodynamic derangements during early exercise in LVSD. While peak VO2 was independently associated with submaximal exercise cardiac output, MRT showed unique association with elevated biventricular filling pressures in early exercise as well as reduced RV function at peak exercise. These data, in concert with the previously documented complementary roles of MRT and peak VO2 in predicting survival in LVSD,7, 11 provide further rationale to adopt routine derivation of oxygen kinetics during CPET in patients with LVSD.
By highlighting the central role of aberrant RV-PV function in the delayed VO2 augmentation of HF, our findings suggest that MRT is a non-invasive surrogate for RV-PV function which is technically difficult to assess at rest and particularly difficult to assess during exercise.44 MRT, coupled with other non-invasive submaximum CPET parameters, represents a potentially attractive endpoint for trials that are increasingly being performed to evaluate therapies directed at RV-PV function in HF.45 This work further solidifies the role of aberrant right ventricular-pulmonary vascular coupling in the exercise intolerance of systolic HF and potentially identifies a subset of HF patients who may benefit from therapy targeting the RV-PV unit, such as phosphodiesterase 5 inhibition.20
Limitations
Our study has several limitations. First, the control group was limited in size given the relative infrequency of referral for patients who are found to have no significant cardiopulmonary disease upon undergoing CPET with invasive hemodynamic monitoring. The relatively small overall sample size without correction for multiple hypothesis testing may have increased the possibility of type I error. However, the validity of our findings are supported by their internal consistency during ascertainment by separate independent measurement techniques (for example, MRT was related to impaired RV-PV function reflected by (i) reduced RVEF measured by ventriculography, (ii) increased PAP/CO slope measured by pulmonary arterial catheterization, and (iii) increased dead-space ventilation measured by concurrent mixed expired gas and arterial blood analyses of carbon dioxide). Second, there is no widely accepted standardized time window for calculation of MRT and previous studies have utilized a range of three to six minutes of exercise.2, 3, 25, 46 Given that MRT was derived during an initial three minutes of unloaded exercise, we cannot rule out the possibility that alternative mechanisms are responsible for VO2 augmentation during later phases of exercise. While a late phase of VO2 augmentation has been described in HF,24 this has only been previously characterized in the setting of heavy exercise22 or after achievement of the anaerobic threshold,47 neither of which was the case for any patient in this study. The advantage of our approach is that a three minute period of steady state exercise can be incorporated into an incremental CPET, avoiding the need for repeated tests on subjects. Furthermore, MRT values derived from our study were highly consistent with values derived from other studies in similar HF populations,2, 3, 11 suggesting that methodological variations yield similar MRTs. Third, given that a substantial percentage of patients with HF were exposed to β-blockade, we cannot fully rule out a contribution of chronotropic incompetence to prolonged MRT. Fourth, the overall size of the study limited our power to detect significant differences in clinical outcome between LVSD groups stratified by MRT. Finally, the relatively depressed LVEF and peak VO2 of our study population reflects a relatively advanced HF population and these findings may not be necessarily generalizeable to patients with milder systolic impairment.
Conclusion
In patients with systolic heart failure, delayed augmentation of oxygen uptake during early exercise is closely related to impaired cardiac reserve, characterized by abnormal right ventricular-pulmonary vascular function. Our findings support the potential role of using MRT, which is easily derived from standard CPET, as a non-invasive surrogate for RV-PV function in LVSD.
Supplementary Material
Acknowledgments
We thank the staff of the cardiopulmonary exercise laboratory for helping with data collection.
Sources of Funding
The authors gratefully acknowledge support from the National Heart Lung and Blood Institute (NIH-K23HL091106, GDL), the American Heart Association (11FTF7290032 RM), the National Heart Lung and Blood Institute Heart Failure Network training grant (GDL), and the Heart Failure Research Innovation Fund (GDL, RMM, NC, BD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Arena R, Humphrey R, Peberdy MA. Measurement of oxygen consumption on-kinetics during exercise: Implications for patients with heart failure. J Card Fail. 2001;7:302–310. doi: 10.1054/jcaf.2001.27666. [DOI] [PubMed] [Google Scholar]
- 2.Koike A, Hiroe M, Adachi H, Yajima T, Yamauchi Y, Nogami A, Ito H, Miyahara Y, Korenaga M, Marumo F. Oxygen uptake kinetics are determined by cardiac function at onset of exercise rather than peak exercise in patients with prior myocardial infarction. Circulation. 1994;90:2324–2332. doi: 10.1161/01.cir.90.5.2324. [DOI] [PubMed] [Google Scholar]
- 3.Sietsema KE, Ben-Dov I, Zhang YY, Sullivan C, Wasserman K. Dynamics of oxygen uptake for submaximal exercise and recovery in patients with chronic heart failure. Chest. 1994;105:1693–1700. doi: 10.1378/chest.105.6.1693. [DOI] [PubMed] [Google Scholar]
- 4.Chelimsky-Fallick C, Stevenson LW, Lem V, Whipp BJ. Excessive oxygen deficit during low-level exercise in heart failure. Am J Cardiol. 1995;76:799–802. doi: 10.1016/s0002-9149(99)80230-6. [DOI] [PubMed] [Google Scholar]
- 5.Cross AM, Jr, Higginbotham MB. Oxygen deficit during exercise testing in heart failure. Relation to submaximal exercise tolerance. Chest. 1995;107:904–908. doi: 10.1378/chest.107.4.904. [DOI] [PubMed] [Google Scholar]
- 6.Whipp BJ, Ward SA. Physiological determinants of pulmonary gas exchange kinetics during exercise. Med Sci Sports Exerc. 1990;22:62–71. [PubMed] [Google Scholar]
- 7.Brunner-La Rocca HP, Weilenmann D, Follath F, Schlumpf M, Rickli H, Schalcher C, Maly FE, Candinas R, Kiowski W. Oxygen uptake kinetics during low level exercise in patients with heart failure: Relation to neurohormones, peak oxygen consumption, and clinical findings. Heart. 1999;81:121–127. doi: 10.1136/hrt.81.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang YY, Wasserman K, Sietsema KE, Ben-Dov I, Barstow TJ, Mizumoto G, Sullivan CS. O2 uptake kinetics in response to exercise. A measure of tissue anaerobiosis in heart failure. Chest. 1993;103:735–741. doi: 10.1378/chest.103.3.735. [DOI] [PubMed] [Google Scholar]
- 9.Metra M, Nodari S, Raccagni D, Garbellini M, Boldi E, Bontempi L, Gaiti M, Dei Cas L. Maximal and submaximal exercise testing in heart failure. J Cardiovasc Pharmacol. 1998;32(Suppl 1):S36–45. doi: 10.1097/00005344-199800003-00007. [DOI] [PubMed] [Google Scholar]
- 10.Brunner-La Rocca HP, Weilenmann D, Schalcher C, Schlumpf M, Follath F, Candinas R, Kiowski W. Prognostic significance of oxygen uptake kinetics during low level exercise in patients with heart failure. Am J Cardiol. 1999;84:741–744. A749. doi: 10.1016/s0002-9149(99)00426-9. [DOI] [PubMed] [Google Scholar]
- 11.Schalcher C, Rickli H, Brehm M, Weilenmann D, Oechslin E, Kiowski W, Brunner-La Rocca HP. Prolonged oxygen uptake kinetics during low-intensity exercise are related to poor prognosis in patients with mild-to-moderate congestive heart failure. Chest. 2003;124:580–586. doi: 10.1378/chest.124.2.580. [DOI] [PubMed] [Google Scholar]
- 12.Lewis NP, Banning AP, Cooper JP, Sundar AS, Facey PE, Evans WD, Henderson AH. Impaired matching of perfusion and ventilation in heart failure detected by 133xenon. Basic Res Cardiol. 1996;91(Suppl 1):45–49. doi: 10.1007/BF00810523. [DOI] [PubMed] [Google Scholar]
- 13.Uren NG, Davies SW, Agnew JE, Irwin AG, Jordan SL, Hilson AJ, Lipkin DP. Reduction of mismatch of global ventilation and perfusion on exercise is related to exercise capacity in chronic heart failure. Br Heart J. 1993;70:241–246. doi: 10.1136/hrt.70.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kay GN, Ashar MS, Bubien RS, Dailey SM. Relationship between heart rate and oxygen kinetics during constant workload exercise. Pacing Clin Electrophysiol. 1995;18:1853–1860. doi: 10.1111/j.1540-8159.1995.tb03832.x. [DOI] [PubMed] [Google Scholar]
- 15.Krum H, Goldsmith R, Wilshire-Clement M, Miller M, Packer M. Role of endothelin in the exercise intolerance of chronic heart failure. Am J Cardiol. 1995;75:1282–1283. doi: 10.1016/s0002-9149(99)80783-8. [DOI] [PubMed] [Google Scholar]
- 16.Shoemaker JK, Naylor HL, Hogeman CS, Sinoway LI. Blood flow dynamics in heart failure. Circulation. 1999;99:3002–3008. doi: 10.1161/01.cir.99.23.3002. [DOI] [PubMed] [Google Scholar]
- 17.Lewis GD, Murphy RM, Shah RV, Pappagianopoulos PP, Malhotra R, Bloch KD, Systrom DM, Semigran MJ. Pulmonary vascular response patterns during exercise in left ventricular systolic dysfunction predict exercise capacity and outcomes. Circ Heart Fail. 2011;4:276–285. doi: 10.1161/CIRCHEARTFAILURE.110.959437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansen JE, Sue DY, Wasserman K. Predicted values for clinical exercise testing. Am Rev Respir Dis. 1984;129:S49–55. doi: 10.1164/arrd.1984.129.2P2.S49. [DOI] [PubMed] [Google Scholar]
- 19.Jones NL, Makrides L, Hitchcock C, Chypchar T, McCartney N. Normal standards for an incremental progressive cycle ergometer test. Am Rev Respir Dis. 1985;131:700–708. doi: 10.1164/arrd.1985.131.5.700. [DOI] [PubMed] [Google Scholar]
- 20.Lewis GD, Shah R, Shahzad K, Camuso JM, Pappagianopoulos PP, Hung J, Tawakol A, Gerszten RE, Systrom DM, Bloch KD, Semigran MJ. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation. 2007;116:1555–1562. doi: 10.1161/CIRCULATIONAHA.107.716373. [DOI] [PubMed] [Google Scholar]
- 21.Lewis GD, Lachmann J, Camuso J, Lepore JJ, Shin J, Martinovic ME, Systrom DM, Bloch KD, Semigran MJ. Sildenafil improves exercise hemodynamics and oxygen uptake in patients with systolic heart failure. Circulation. 2007;115:59–66. doi: 10.1161/CIRCULATIONAHA.106.626226. [DOI] [PubMed] [Google Scholar]
- 22.Barstow TJ. Characterization of vo2 kinetics during heavy exercise. Med Sci Sports Exerc. 1994;26:1327–1334. [PubMed] [Google Scholar]
- 23.Whipp BJ. Dynamics of pulmonary gas exchange. Circulation. 1987;76:VI18–28. [PubMed] [Google Scholar]
- 24.Xu F, Rhodes EC. Oxygen uptake kinetics during exercise. Sports Med. 1999;27:313–327. doi: 10.2165/00007256-199927050-00003. [DOI] [PubMed] [Google Scholar]
- 25.Meyer K, Schwaibold M, Hajric R, Westbrook S, Ebfeld D, Leyk D, Roskamm H. Delayed vo2 kinetics during ramp exercise: A criterion for cardiopulmonary exercise capacity in chronic heart failure. Med Sci Sports Exerc. 1998;30:643–648. doi: 10.1097/00005768-199805000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Wasserman K, Zhang YY, Gitt A, Belardinelli R, Koike A, Lubarsky L, Agostoni PG. Lung function and exercise gas exchange in chronic heart failure. Circulation. 1997;96:2221–2227. doi: 10.1161/01.cir.96.7.2221. [DOI] [PubMed] [Google Scholar]
- 27.Sullivan MJ, Duscha BD, Klitgaard H, Kraus WE, Cobb FR, Saltin B. Altered expression of myosin heavy chain in human skeletal muscle in chronic heart failure. Med Sci Sports Exerc. 1997;29:860–866. doi: 10.1097/00005768-199707000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Duscha BD, Kraus WE, Keteyian SJ, Sullivan MJ, Green HJ, Schachat FH, Pippen AM, Brawner CA, Blank JM, Annex BH. Capillary density of skeletal muscle: A contributing mechanism for exercise intolerance in class ii–iii chronic heart failure independent of other peripheral alterations. J Am Coll Cardiol. 1999;33:1956–1963. doi: 10.1016/s0735-1097(99)00101-1. [DOI] [PubMed] [Google Scholar]
- 29.Barstow TJ, Jones AM, Nguyen PH, Casaburi R. Influence of muscle fiber type and pedal frequency on oxygen uptake kinetics of heavy exercise. J Appl Physiol. 1996;81:1642–1650. doi: 10.1152/jappl.1996.81.4.1642. [DOI] [PubMed] [Google Scholar]
- 30.Lewalter T, Rickli H, MacCarter D, Jung W, Schimpf R, Schwartze P, Candinas R, Luderitz B. Oxygen uptake kinetics during low intensity exercise: Relevance for rate adaptive pacemaker programming. Heart. 1997;77:168–172. doi: 10.1136/hrt.77.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Massie BM, Conway M, Yonge R, Frostick S, Sleight P, Ledingham J, Radda G, Rajagopalan B. 31p nuclear magnetic resonance evidence of abnormal skeletal muscle metabolism in patients with congestive heart failure. Am J Cardiol. 1987;60:309–315. doi: 10.1016/0002-9149(87)90233-5. [DOI] [PubMed] [Google Scholar]
- 32.Grassi B, Poole DC, Richardson RS, Knight DR, Erickson BK, Wagner PD. Muscle o2 uptake kinetics in humans: Implications for metabolic control. J Appl Physiol. 1996;80:988–998. doi: 10.1152/jappl.1996.80.3.988. [DOI] [PubMed] [Google Scholar]
- 33.Williamson JW, Raven PB, Whipp BJ. Unaltered oxygen uptake kinetics at exercise onset with lower-body positive pressure in humans. Exp Physiol. 1996;81:695–705. doi: 10.1113/expphysiol.1996.sp003970. [DOI] [PubMed] [Google Scholar]
- 34.Agostoni PG, Wasserman K, Perego GB, Guazzi M, Cattadori G, Palermo P, Lauri G, Marenzi G. Non-invasive measurement of stroke volume during exercise in heart failure patients. Clin Sci (Lond) 2000;98:545–551. [PubMed] [Google Scholar]
- 35.Perego GB, Marenzi GC, Guazzi M, Sganzerla P, Assanelli E, Palermo P, Conconi B, Lauri G, Agostoni PG. Contribution of po2, p50, and hb to changes in arteriovenous o2 content during exercise in heart failure. J Appl Physiol. 1996;80:623–631. doi: 10.1152/jappl.1996.80.2.623. [DOI] [PubMed] [Google Scholar]
- 36.West JB, Dollery CT, Naimark A. Distribution of blood flow in isolated lung; relation to vascular and alveolar pressures. J Appl Physiol. 1964;19:713–724. doi: 10.1152/jappl.1964.19.4.713. [DOI] [PubMed] [Google Scholar]
- 37.Moraes DL, Colucci WS, Givertz MM. Secondary pulmonary hypertension in chronic heart failure: The role of the endothelium in pathophysiology and management. Circulation. 2000;102:1718–1723. doi: 10.1161/01.cir.102.14.1718. [DOI] [PubMed] [Google Scholar]
- 38.Randhawa PS, Chopra P, Tandon HD. Autopsy study of pulmonary vascular changes in patients of rheumatic mitral stenosis. Indian J Med Res. 1983;78:681–688. [PubMed] [Google Scholar]
- 39.Tumminello G, Lancellotti P, Lempereur M, D'Orio V, Pierard LA. Determinants of pulmonary artery hypertension at rest and during exercise in patients with heart failure. Eur Heart J. 2007;28:569–574. doi: 10.1093/eurheartj/ehl561. [DOI] [PubMed] [Google Scholar]
- 40.Aaronson KD, Schwartz JS, Chen TM, Wong KL, Goin JE, Mancini DM. Development and prospective validation of a clinical index to predict survival in ambulatory patients referred for cardiac transplant evaluation. Circulation. 1997;95:2660–2667. doi: 10.1161/01.cir.95.12.2660. [DOI] [PubMed] [Google Scholar]
- 41.Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH, Jr, Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83:778–786. doi: 10.1161/01.cir.83.3.778. [DOI] [PubMed] [Google Scholar]
- 42.Janicki JS, Gupta S, Ferris ST, McElroy PA. Long-term reproducibility of respiratory gas exchange measurements during exercise in patients with stable cardiac failure. Chest. 1990;97:12–17. doi: 10.1378/chest.97.1.12. [DOI] [PubMed] [Google Scholar]
- 43.Larsen AI, Aarsland T, Kristiansen M, Haugland A, Dickstein K. Assessing the effect of exercise training in men with heart failure; comparison of maximal, submaximal and endurance exercise protocols. Eur Heart J. 2001;22:684–692. doi: 10.1053/euhj.2000.2286. [DOI] [PubMed] [Google Scholar]
- 44.Champion HC, Michelakis ED, Hassoun PM. Comprehensive invasive and noninvasive approach to the right ventricle-pulmonary circulation unit: State of the art and clinical and research implications. Circulation. 2009;120:992–1007. doi: 10.1161/CIRCULATIONAHA.106.674028. [DOI] [PubMed] [Google Scholar]
- 45.Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction: A target of phosphodiesterase-5 inhibition in a 1-year study. Circulation. 2011;124:164–174. doi: 10.1161/CIRCULATIONAHA.110.983866. [DOI] [PubMed] [Google Scholar]
- 46.Belardinelli R, Zhang YY, Wasserman K, Purcaro A, Agostoni PG. A four-minute submaximal constant work rate exercise test to assess cardiovascular functional class in chronic heart failure. Am J Cardiol. 1998;81:1210–1214. doi: 10.1016/s0002-9149(98)00093-9. [DOI] [PubMed] [Google Scholar]
- 47.Whipp BJ, Wasserman K. Oxygen uptake kinetics for various intensities of constant-load work. J Appl Physiol. 1972;33:351–356. doi: 10.1152/jappl.1972.33.3.351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.