Abstract
Asthma is characterized by inflammation and architectural changes in the lungs. A number of immune cells and mediators are recognized as initiators of asthma, although therapeutics based on these are not always effective. The multifaceted nature of this syndrome necessitate continued exploration of immunomodulators that may play a role in pathogenesis. We investigated the role of resistin-like molecule-beta (RELM-β), a gut antibacterial, in the development and pathogenesis of Aspergillus-induced allergic airways disease. Age and gender matched C57BL/6J and Retnlb−/− mice rendered allergic to Aspergillus fumigatus were used to measure canonical markers of allergic asthma at early and late time points. Inflammatory cells in airways were similar, although Retnlb−/− mice had reduced tissue inflammation. The absence of RELM-β elevated serum IgA and pro-inflammatory cytokines in the lungs at homeostasis. Markers of chronic disease including goblet cell numbers, Muc genes, airway wall remodelling, and hyperresponsiveness were greater in the absence RELM-β. Specific inflammatory mediators important in antimicrobial defence in allergic asthma were also increased in the absence of RELM-β. These data suggest that while characteristics of allergic asthma develop in the absence of RELM-β, that RELM-β may reduce the development of chronic markers of allergic airways disease.
Introduction
Cellular and molecular composition of the respiratory tract is vast with complicated interplay required for homeostasis. Often times, structural cells and their mediators are altered in response to encounters with innocuous or pathogenic agents leading to dysregulation. Allergen-induced structural and functional changes in airways and parenchyma, while initiated by the bronchial epithelia1, are perpetuated by a misguided immune response2. Allergic asthma is a syndrome that impacts over 235 million people globally3 and approximately 8% of the population in the United States4. Generally considered as an inflammatory disorder with a TH2 bias, structural changes including goblet cell (GC) metaplasia and airway wall remodelling events, contribute to physiologic dysfunction in breathing that can require medical attention.
Airway structural cell- and immune system-derived mediators contribute to asthma pathogenesis. Resistin-like molecules (RELMs) are small (105–114 amino acids) secreted proteins characterized by conserved cysteine rich carboxyl domains5. Family members, RELM-α, RELM-β, resistin, and RELM-γ, found in the gut, lungs, and adipose tissue6, have known functions in the pathophysiology of metabolic7 and inflammatory diseases8. While mice express the entire spectrum of RELM proteins, only resistin and RELM-β, which share sequence homology, have been identified in humans to date5. RELM-β was identified and characterized in the gut5,9,10, but also has ascribed functions in the lung, including participation in inflammation, GC hyperplasia, and fibrosis11,12. RELM-β expression is prominent in alveolar epithelial cells, and its known functions in lung inflammation and proliferation of epithelial cells and fibroblasts have led to its categorization as a marker of allergic asthma in ovalbumin and bleomycin mouse models of asthma and fibrosis11–13. Limitations in mouse models such as rapid reversal of inflammation and lack of remodelling (in ovalbumin models), and excessive inflammation and reversible fibrosis (in bleomycin models)14,15, necessitate the characterization of RELM-β in a robust chronic model of respiratory allergy that utilizes clinically relevant allergen exposures.
Understanding the functions of RELM proteins in the context of the lung may be important in delineating previously unknown mechanisms that drive pulmonary diseases, including asthma. Most asthmatics without co-morbidities or respiratory infections have elevated RELM-β in the bronchoalveolar lavage (BAL) fluid and in bronchial biopsies compared to controls16. We noted elevated RELM-β in the lungs of mice in our model of asthma and influenza (unpublished), which have an altered immune phenotype17,18. Therefore, we hypothesized that RELM-β regulates the development of features of severe asthma with fungal sensitization (SAFS) in response to clinically relevant Aspergillus fumigatus fungal antigens. Our data contradict previous reports that RELM-β promotes macrophagic inflammation, GC metaplasia, and subepithelial fibrosis11–13, and instead, suggest a function for RELM-β as a negative regulator of chronic features in allergen-associated lung disease.
Results
Characteristics of allergic asthma include peribronchovascular (PBV) inflammation, GC metaplasia, and airway wall remodelling events in addition to physiologic changes leading to increased airway hyperresponsiveness (AHR) and an IgE-biased humoral immune response. Pathways that influence the development and pathogenesis of allergic asthma have been elucidated using mouse models, and those that can recapitulate the acute and chronic features of asthma are especially important to delineate functions of cells and their mediators in disease. Herein, using our mouse model of SAFS (Fig. 1), we investigated the role played by RELM-β in the development of characteristics associated with SAFS.
Figure 1.
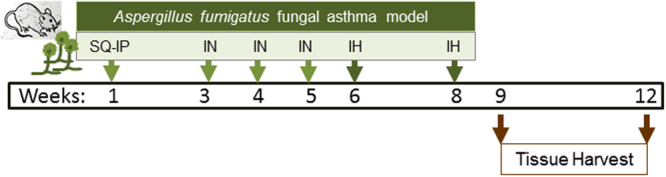
Schematic representation of the timeline in the severe asthma with fungal sensitization model (SAFS). Age and gender-matched mice are administered antigen from Aspergillus fumigatus extract via subcutaneous (SQ) and intraperitoneal (IP) injections. Following a 2 week rest period, antigen is administered via intranasal (IN) route once a week for three weeks. Sensitized mice are then exposed to conidia liberated from live fungal cultures for 10 minutes at a time two weeks apart via inhalation (IH). Samples were harvested at days 7 and 35 after the second fungal challenge.
RELM-β minimally impacts allergic inflammation
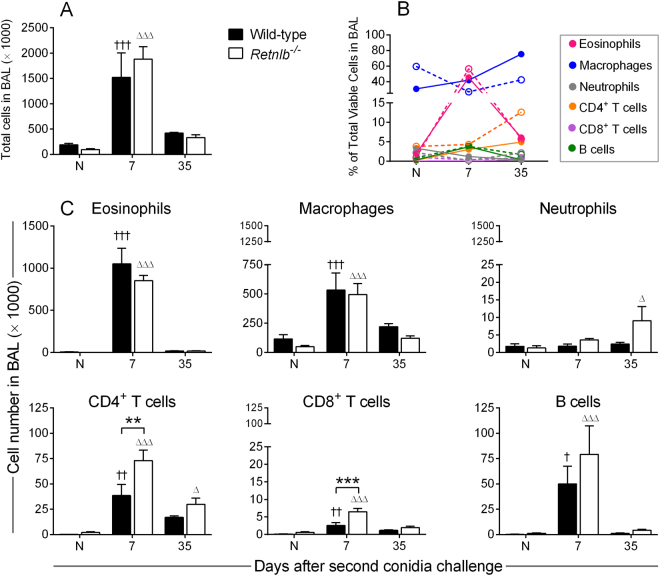
Inflammation is temporally regulated after allergen exposure with waves of infiltrating cells that belong to both innate and adaptive branches of the immune system. BAL was performed, and harvested leukocytes were enumerated and profiled by flow cytometry (Fig. 2) while tissue inflammation was qualitatively determined by histologic analyses (Fig. 3). The total cell population in both WT and Retnlb−/− mice increased after allergen challenge and gradually depleted over time with no major differences between the genotypes (Fig. 2A). Slight differences in cell kinetics were noted when data were normalized to total cell numbers in each group (Fig. 2B). Eosinophils in the airways were similar between the WT and KO mice (Fig. 2B). While macrophage numbers were similar between the genotypes (Fig. 2C), when taken as a percentage of total cells, KO mice had more macrophages at baseline which reduced after allergen challenge. (Fig. 2B). This model of SAFS has minimal neutrophil influx due to eosinophilic dominance19. However, neutrophils were found in the airways of Retnlb−/− mice at the late time point (Fig. 2C). Early recruitment of CD4+ and CD8+ T cells occurred in the absence of RELM-β (Fig. 2C) and KO mice had a larger percentage of CD4+ T cells in the airways at the late timepoint (Fig. 2B).
Figure 2.
RELM-β has a minimal impact on airway inflammation. Immune cells were recruited into the airways (collected by bronchoalveolar lavage) in both Retnlb sufficient (wild-type) and deficient (KO) mice (A). Cell populations normalized to cell number showed slight variations in the kinetics between WT (solid circles) and KO (open circles) mice (B). Absolute cell numbers suggest no major differences in innate cell populations at early the early time point although neutrophils were present at the late time point in KO airways (C). RELM-β deficient mice had more T cells, while B cell numbers were similar (C). Data are represented as the mean and SD of n = 4–7 mice/group in one representative study of two independent studies. Data at each time point were compared by two-way ANOVA with Dunnett’s and Sidak’s multiple comparisons test to their naïve controls († and Δ), and between genotypes (*) respectively. One, two, and three symbols represent P < 0.05, P < 0.01, and P < 0.001 correspondingly. N – Naïve.
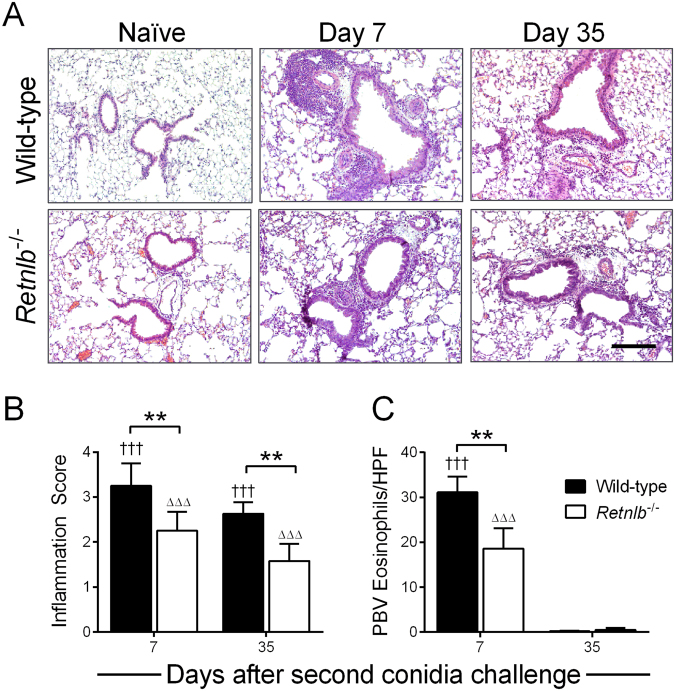
Figure 3.
RELM-β promotes peribronchovascular inflammation. Peribronchovascular inflammation was highest at early time points and gradually decreased over time in both genotypes compared to naïve controls (A), and Retnlb−/− mice had less tissue inflammation compared to WT (B). Tissue eosinophils were lower in KO mice compared to WT (C). Scale bar = 200 µm and applicable to all photo micrographs. Naïve mice in each genotype were scored 0 and did not contain tissue eosinophils. Data are represented as the mean and SD of n = 4–7 mice/group in one representative study of two independent studies. Data at each time point were compared by two-way ANOVA with Dunnett’s and Sidak’s multiple comparisons test to their naïve controls († and Δ), and between genotypes (*) respectively. One, two, and three symbols represent P < 0.05, P < 0.01, and P < 0.001 correspondingly. HPF – high power field.
Tissue inflammation can persist for long periods of time after allergen exposure and considered to participate in airway physiologic responses in asthma20. PBV inflammation was apparent in both genotypes after allergen challenge compared to baseline (Fig. 3). Tissue inflammation was scored through a blinded process and the areas of inflammatory foci were reduced in the Retnlb−/− mice compared to WT controls (Fig. 3B). The number of eosinophils in the PBV areas was reduced in KO mice by one week after allergen challenge (Fig. 3C). While airway inflammation was largely resolved by Day 21 in both genotypes (data not shown), PBV inflammation was diminished but not fully resolved at the final time point tested (Figs 2 and 3). Cumulatively, these data suggest that the absence of RELM-β may only have a minimal impact on allergic inflammation.
RELM-β may inhibit goblet cell metaplasia
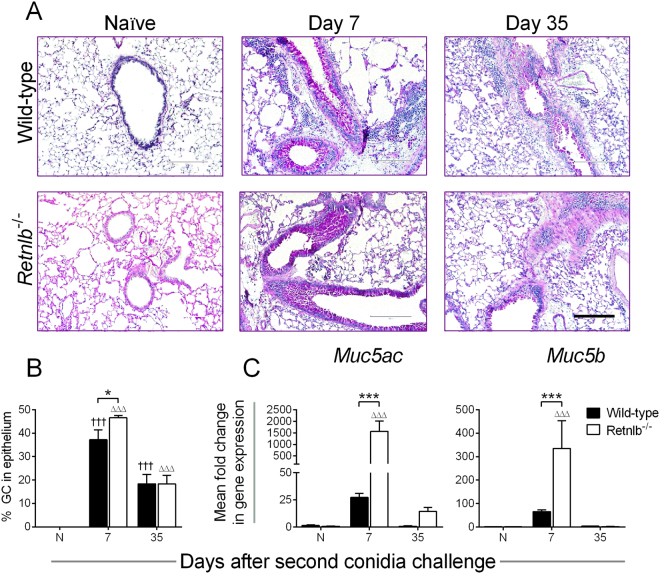
Increased mucus producing cells and mucus hypersecretion are hallmarks of airway inflammation-associated diseases such as allergic asthma. GCs are the primary producers of RELM-β in the gut21 where it is known to be important for anti-parasitic host defence22, barrier integrity10, inflammation23,24, and microbiome maintenance25,26. We investigated GC numbers and mucin genes in both genotypes expecting to find them reduced in the KO mice based on previous reports11. GCs lining the airways were visualized by periodic acid Schiff’s stain (Fig. 4A) and enumerated (Fig. 4B). GCs were elevated early after allergen challenge in both genotypes, but KO mice had noticeably more GCs lining the airways compared to WT, although this trend was not observed at the late time point (Fig. 4B). Corresponding expressions of mucin genes considered as markers of GC metaplasia27, Muc5ac and Muc5b, were markedly elevated in KO mice at early time points (Fig. 4C).
Figure 4.
RELM-β inhibits goblet cell (GC) differentiation and Muc gene expression. Goblet cells were not observed in the naïve (N) controls of either genotype but were abundant in the airway lining after allergen exposure (A). While GCs consisted of approximately 40% of the epithelial lining in both genotypes at the early time point, these cells reduced over time to only about 20% at the late time point (B). More GCs were present in the airways of Retnlb−/− mice at Day 7 (B), corresponding with greater expression of mucus genes, Muc5ac and Muc5b (C). Scale bar = 200 µm applicable to all photo micrographs. Data are represented as the mean and SEM of n = 4–7 mice/group in one representative study of two independent studies. Data at each time point were compared by two-way ANOVA with Dunnett’s and Sidak’s multiple comparisons test to their naïve controls († and Δ), and between genotypes (*) respectively. Three symbols represent P < 0.05 and P < 0.001 correspondingly.
Airway wall remodelling is increased in the absence of RELM-β
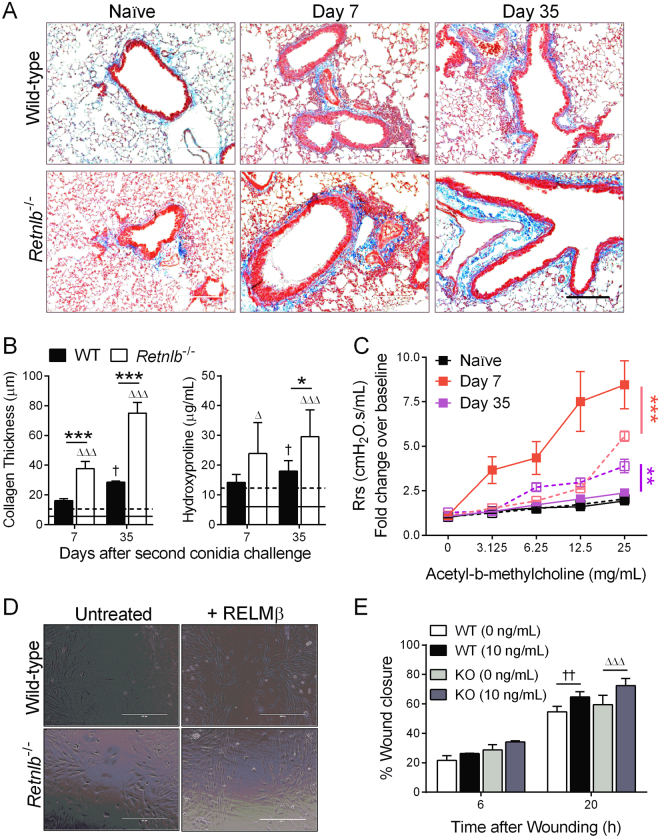
Airway wall remodelling is a characteristic of severe and chronic asthma that can be irreversible. We observed subepithelial fibrosis as a feature of remodelling with trichrome staining (Fig. 5A) and noted that KO mice had more collagen deposition than their WT counterparts especially at late time points (Fig. 5B). Hydroxyproline levels were also elevated in KO mouse lungs compared to WT (Fig. 5B). AHR was measured after methacholine challenge as a physical attribute of inflammation and stiffness of the airways. While airway resistance was higher in WT mice at the early time point, KO mice had elevated responses throughout the time course (data not shown) which remained high at Day 35 (Fig. 5C). Fibroblasts play architecturally important functions in the lungs by providing structural support through matrix deposition, but, can also contribute to lung pathology through cytokine production28 and differentiation into myofibroblasts29. Fibroblast migration and proliferation are initial steps in fibrotic remodelling in allergic asthma30. In order to determine if elevated collagen in the absence of RELM-β was due to altered fibroblast growth/migration/expansion during disease, we measured closure of a scratch wound in the presence/absence of recombinant RELM-β in primary lung fibroblasts from WT and KO mice (Fig. 5D). Wound closure was calculated as a decrease in wound size at each time point. Fibroblasts from KO mice were responsive to exogenous RELM-β, but, our data suggest that while RELM-β may be beneficial for fibroblast outgrowth, it was not necessary (Fig. 5E).
Figure 5.
Airway wall remodelling may impact physiologic responses in the absence of RELM-β. Airway wall remodelling was observed qualitatively with trichrome staining (A) wherein Relnlb KO mice have increased peribronchovascular collagen deposition and hydroxyproline compared to wild-type (WT) controls (B). Resistance of the respiratory system (Rrs) in response to nebulized methacholine was significantly higher in the WT (closed squares) early after allergen challenge, but this increase persisted in the KO (open squares) at the late time point (C). Primary lung fibroblasts isolated from WT and KO mice were wounded and treated/not with 10 ng/mL recombinant RELM-β protein. Wound closure was measured at varying time points based on the initial width of the wound in each replicate, and a significant difference was noted at 20 hours in fibroblasts from both genotypes (D,E). Lines in B represent naïve WT (solid) and KO (dotted). Scale bar = 200 µm (applicable to all in A) and 400 µm (applicable to all in D). Data are represented as the mean and SEM of one representative study with n = 4–7 mice/group (A–C) and as mean and SD of n = 3 wells/group (D-E). Data were analysed by two-way ANOVA with Tukey’s multiple comparisons test. Two and three symbols represent P < 0.01, and P < 0.001 correspondingly. N – Naïve.
RELM-β deficiency affects humoral immune responses to allergen challenge
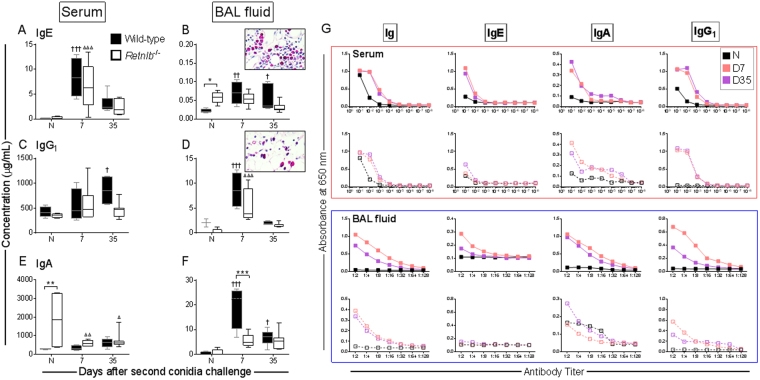
Increased systemic IgE after allergen challenge marked the induction of allergy in both genotypes (Fig. 6A). Local IgE levels were higher in the Retnlb−/− mice at baseline compared to WT controls and did not change as a result of allergen provocation (Fig. 6B). Systemic IgG1 was only increased over baseline at late time points in WT mice and remained equivalent to baseline in the KOs (Fig. 6C). In contrast, local IgG1 levels rapidly increased in both genotypes and returned to baseline levels by Day 35 post-allergen challenge (Fig. 6D). Systemic IgA in naïve Retnlb−/− mice was highly elevated, although allergen challenge caused a rapid reduction (Fig. 6E). The increase in local IgA that occurred after allergen challenge in the WT mice, did not occur in the KO mice where IgA was maintained at a concentration similar to that in the naïve animals throughout the time course (Fig. 6F). We have previously found that antibody producing B cells are dynamically regulated in this model of SAFS18,31. Although B cells were not the focus of this investigation, we did note that there were more Mott cells in the WT lungs (inset Fig. 6B) compared to KO (inset Fig. 6D), which suggests that immunoglobulin production/producing cells may be directly/indirectly regulated by RELM-β. Titres of A. fumigatus-specific isotypes were measured to determine if a difference in specificity of antibodies occurred between the genotypes tested. A. fumigatus-specific immunoglobulin (Ig) titres were generally similar between the two genotypes (Fig. 6G). Although Retnlb−/− mice had elevated serum IgE, the fungal-specific IgE titre in the sera was low in these mice and there was no A. fumigatus-specific IgE in the KO mouse BAL fluid (Fig. 6G).
Figure 6.
Systemic and local antibody responses were induced in both wild-type (WT) and Retnlb null mice (KO). Antibody levels in the serum and bronchoalveolar lavage (BAL) fluid were measured at each time point after the second fungal challenge. Allergen exposure led to an increase in immunoglobulin (Ig) E levels in the serum (A). IgE in the BAL fluid also increased after allergen exposure in WT mice, but did not change over baseline in the KO group which had significantly more IgE at baseline, compared to WT (B). Serum IgG1 levels increased after allergen challenge but did not reach significance until the late time point in the WT mice (C). Changes in the mucosal IgG1 was apparent at early time points in both groups (D). Steady state systemic IgA was high in the KO mice, which reduced after allergen challenge while WT IgA in the serum increased minimally over naïve controls (E). IgA levels in the BAL fluid increased in response to allergen provocation in the WT and this increase was delayed in the KO, but did not reach statistical significance (F). More Mott cells (magenta coloured) were noted in the WT lungs (inset B) than in KO (inset D). Aspergillus fumigatus-specific antibodies were produced in both groups but generally more abundant in the WT mice compared to KO (G). Data are represented as the mean and SD of n = 4–7 mice/group in one representative study of two independent studies. Data shown as range from minimum to maximum where lines and dots represent the median and mean respectively. Data analysed with two-way ANOVA with Sidak’s and Dunnett’s multiple comparisons test to compare data between groups at each time point (*) or to naïve controls within groups († in WT and Δ in KO) respectively where significance values p < 0.05, p < 0.01, and p < 0.001 are denoted by one, two, or three symbols respectively. N – Naïve; D – Day.
Mediators of allergy and immunity are dynamically impacted by the absence of RELM-β
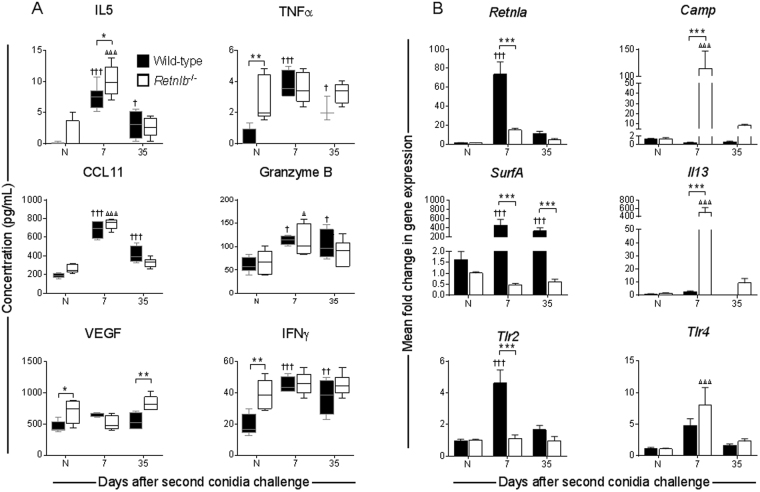
Since asthma is an inflammatory disease with multiple leukocyte involvement, cytokines and growth factors in the microenvironment are important to activate/deactivate both immune and structural cells during disease pathogenesis. Mediators that are of known importance to allergy and host defence in the lungs were selected for comparative analysis (Fig. 7). Interestingly, naïve Retnlb−/− mice had elevated pro-inflammatory mediators, TNFα, VEGF, and IFNγ, compared to naïve WT controls (Fig. 7A). As expected, IL-5 and CCL11 were elevated soon after allergen exposure in both genotypes (Fig. 7A). Emphasizing the complexity of asthma, markers of TH1 responses, such as TNFα and IFNγ were also elevated in mice of both genotypes (Fig. 7A). IL-13 is a key TH2 cytokine known to have functions in AHR and airway remodelling, among others, during allergy. IL-13 promotes RELM-β in the gut32 possibly through enhancement of GC metaplasia and also induces RELM-β production by airway epithelial cells16. However, we found that Il13 was highly expressed in mice deficient in Retnlb compared to WT controls (Fig. 7B).
Figure 7.
Biomarkers of inflammation increased in response to allergen challenge. Cytokines in the bronchoalveolar lavage fluid were measured in each genotype and found to be elevated in mice subjected to the fungal asthma model. Notably, Retnlb deficient mice had elevated TNFα, VEGF, and IFNγ at steady state compared to WT controls (A). Wild-type mice had rapid increase in cytokines at early time points with gradual depletion over time whereas Retnlb KO followed similar trends in cytokine production, albeit at slightly lower levels at early time points (A). Wild-type mice had markedly elevated expression of Retnla and SurfA in the lung while Retnlb null mice had markedly elevated Camp and Il13 (B). Expression of Tlr2 dominated at the early time point in the wild-type mice while Trl4 took precedence in the Retnlb null mice (B). Data shown as range from minimum to maximum where lines and dots represent the median and mean respectively (A) and as the mean and SD (B) of n = 4–7 mice/group and representative of one independent study of two. Data analysed with two-way ANOVA with Sidak’s and Dunnett’s multiple comparisons test to compare data between groups at each time point (*) or to naïve controls within groups († in WT and Δ in KO) respectively, where significance values p < 0.05, p < 0.01, and p < 0.001 are denoted by one, two, or three symbols respectively. N – Naïve.
Granzyme B, a serine protease secreted by various leukocytes including T cells33 and elevated in asthmatics34, has diverse functions including regaining homeostasis by killing TH2 cells35. Since we noted an increase in T cells in the BAL of KO mice (Fig. 2C), we quantified granzyme B as a marker of T cell activation in the airways and found that allergen exposure resulted in an increase in granzyme B levels in both genotypes (Fig. 7A). Vascular endothelial growth factor (VEGF), also known to be increased in asthmatics36, was increased in KO mice at baseline but not further induced by allergen exposure (Fig. 7A).
Additional mediators that rapidly turn over or are not secreted were measured as changes in gene expression. Differences in expression of selected genes between the two genotypes reached significance at early time points but were similar by the late time point (Fig. 7B). The expression of Retnla was measured to determine if there may be a compensatory mechanism in the KO mice. While Retnla was rapidly increased in WT mice after allergen challenge, its increase in the KO mice was comparatively less (Fig. 7B). Pulmonary surfactants line the airways and reduce the surface tension between the tissue and air, and surfactant A has been suggested to have protective and detrimental roles in asthma37,38. While WT mice had a significant increase in SurfA expression, this gene was not affected by allergen exposure in KO animals (Fig. 7B). Cathelicidin antimicrobial peptide (CAMP) is a bactericidal39 agent that activates eosinophils40, and is upregulated during virus infection41, but, its role in asthma is unclear. Since RELM-β also has antimicrobial functions, we investigated how its absence may impact Camp after allergen challenge and found that Retnlb KO mice had rapid upregulation of Camp which did not occur in the WT (Fig. 7B). Since our chosen allergen is known to regulate toll-like receptor (TLR)-responses to activate innate immune responses42, we investigated Tlr2 and Tlr4 expression in the lungs and found that WT mice had high expression of Tlr2 while KO upregulated Tlr4 (Fig. 7B).
Discussion
Lack of understanding in disease aetiology, treatment insensitivity, and complications in the pathogenesis and genetic-environmental interactions are only some reasons for the delay in identifying a cure to asthma. While various mediators from immune, endocrine43, and neural systems44 have been demonstrated to function in the pathogenesis of allergic asthma, complex interactions between systems and their products decrease the likelihood that a single molecule can be targeted as the initiator/perpetuator of this condition. A number of studies have implicated RELM-β as a pro-fibrogenic mediator during airways disease11–13,16. We noted that RELM-β was dynamically regulated upon virus infection in our model of asthma and influenza morbidity (unpublished). Since its role had not been previously determined in a mouse model that utilizes a clinical allergen in its natural route of exposure, we investigated inflammatory and molecular profiles in Retnlb−/− mice subjected to an A. fumigatus-induced model of SAFS to determine its role in pathogenesis. We report that the absence of RELM-β influenced inflammation, increased GC metaplasia, worsened subepithelial fibrosis, and sustained airway resistance. Based on these data, we suggest that RELM-β mitigates the development of characteristics associated with SAFS, which warrants further investigation into the mechanism by which RELM-β may influence features of chronicity in airways disease.
Airways inflammation, eosinophilia, and serum IgE are hallmarks of fungal asthma that can be modelled effectively in mice19,45. Activation of recruited inflammatory cells at the barrier can lead to hyperactivation of epithelia and fibroblasts46 via immune-epithelial cross-talk. Inflammation in this SAFS model may be initiated by TLR activation in bronchial epithelia leading to pro-inflammatory cytokine and cell recruitment that continues through positive feedback. Recombinant RELM-β activates gut macrophages23 and its administration into the airways leads to macrophage dominant inflammation in the airways11, suggesting a pro-inflammatory function for this molecule. Although allergen exposure resulted in an increase in airway macrophages, since there was no difference between the groups, RELM-β may not be important in macrophage recruitment or egression. However, when data were normalized to the cell numbers in the airways, we noted that naïve KO mice had more macrophages. Since airway macrophages are a source of RELM-β13, it would be of interest to determine the phenotypic and functional characteristics of these macrophages.
Eosinophils and TH cells typically take centre stage in asthma studies. Eosinophils in the airways were similar between the genotypes although PBV eosinophils were lower in the KO mice suggesting that RELM-β may play a role in eosinophil recruitment into the lungs, but once there, their egression into the airways may not be affected. Since matrix metalloproteinase (MMP)-2 promotes eosinophil migration across the epithelium47, it would be worth investigating MMP-2 expression in the Retnlb null mice. Contrary to a previously identified role for RELM-β as a CD4+ T cell chemoattractant48, we found more T cells in the airways of RELM-β null mice similar to findings by Liu et al. in the bleomycin model12. The impact of RELM-β on leukocyte populations in the lungs after allergen exposure may only be minimal, however, it may be important to determine functional responses of each cell type, especially T cells, to RELM-β in order to fully elucidate its role in inflammation.
Cytokines are major contributors to asthma pathology. While we did not measure all mediators in the allergic lungs, a persistent cytokine storm occurred in both genotypes suggesting that RELM-β may not directly regulate these. The absence of RELM-β led to increased expression of Tlr4 and Il13, although there was no major impact on airways or PBV inflammation in Retnlb−/− compared to WT. However, increased baseline levels of TNFα, VEGF, and IFNγ in the Retnlb−/− mice suggest that RELM-β may be necessary to prevent the host from being in a heightened TH1 inflammatory state at homeostasis. As such, it would be of interest to determine if the absence of RELM-β may enhance resistance to intracellular pathogens, similar to its role against worms9. Naïve KO mice also had more macrophages as a percentage of total compared to naïve WT mice suggesting a function for RELM-β in immune homeostasis in the lungs. Related molecule, RELM-α, is most notably known for its function as a mediator of TH2 responses including allergic asthma49. Interestingly, Retnla expression was significantly lower in the KO mice compared to WT controls suggesting that RELM-α may not be performing a compensatory role in this model. It is possible that the differences observed between the genotypes were due to the low expression of RELM-α thereby essentially removing both RELMs from immunoregulation during allergen exposure in the KO animals. Alternatively, our data may indicate that RELM-β does not play a direct role in regulating inflammation in response to allergen provocation.
Mucus hypersecretion through GC hyperplasia and metaplasia after allergen exposure can alter lung physiology by slowing ciliary beat and narrowing the airway lumen. Similar to inflammation, GC metaplasia is a feature that is effectively recapitulated in our SAFS model19,50. A previous report demonstrated that intranasal administration of A. fumigatus antigens to Retnlb−/− mice for three weeks caused a ~20% reduction in GCs11. Here, we report the contradictory finding that KO mice had increased bronchial GCs and mucin gene expression in response to A. fumigatus allergen exposure suggesting that RELM-β may negatively regulate mucus production in the lung during allergy. These data also indicate that Retnlb−/− mice may have a different mucin composition, which in turn can have a physiologic impact especially when taken together with the lack of SurfA expression. Surfactants are important regulators of fluid balance in the lungs37, while mucins can provide nutrients to sustain bacterial colonization51. Since asthmatics are considered to be at high risk for bacterial infections52, it may be that dysregulated surfactant and mucus in airways during asthma exacerbations can increase susceptibility to subsequent bacterial infections. Gut colonization of germ-free mice leads to an increase in RELM-β secretion in the gut21, suggesting that RELM-β is dynamically regulated by host-pathogen interactions. Antimicrobial peptides such as CAMP may be of extreme importance in such instances, and the hyperexpression of Camp in Retnlb−/− mouse lungs may indicate heightened antimicrobial defences as CAMP is both antibacterial53 and antiviral41. Additionally, RELM-β is considered a bactericidal agent that controls microbial populations26. Since asthmatics have elevated RELM-β16 and mucus54, investigating the role of RELM-β as a regulator of mucin composition and antimicrobial defences in the lung is of interest especially from the perspective of cause and effect relationship between the microbiome and asthma.
Airway wall remodelling events, including subepithelial fibrosis and smooth muscle cell hyperplasia, can occur in chronic lung diseases such as asthma and chronic obstructive pulmonary disease. The inability to recapitulate this clinical feature is often a criticism of mouse models55. Our SAFS model however, results in robust remodelling that continues for months after the fungal challenge19,56 allowing us to investigate this phenomenon. Inflammation, subepithelial fibrosis, and mediators such as IL-13 all lead to AHR and we show that the absence of RELM-β results in increased subepithelial fibrosis, AHR, and Il13 expression. Interestingly, PBV smooth muscle hyperplasia was equivalent between WT and RELM-β null mice (data not shown) indicating that sustained AHR in KO mice may not be due to smooth muscle cell hyperplasia. Whilst IL-13 has been shown to induce RELM-β production32, a possible feedback regulation on IL-13 by RELM-β has not been previously demonstrated. Since IL-13 can influence airway inflammation, hyperresponsiveness, fibrosis, and fibroblast functions57–59, these characteristics may have been heightened due to Il13 overexpression in Retnlb−/− mice, thereby suggesting a novel function for RELM-β as a negative regulator of IL-13 in asthma.
Collagen is an important extracellular matrix component, that can be detrimental to lungs when dysregulated. We found that PBV collagen deposition was highly elevated in the absence of RELM-β, even at baseline, suggesting that RELM-β may be anti-fibrogenic thereby contradicting several other studies11,12,16. While others have noted that RELM-β increases mito- and moto-genic properties11 and collagen production13 in fibroblasts, our findings suggest that although RELM-β promotes primary lung fibroblast growth, it is not required. Therefore, altered collagen in the lungs may not be due to aberrant fibroblast growth in Retnlb null mice. These findings support another study that demonstrated a protective role for RELM-β as an inhibitor non-alcoholic steatohepatitis60. We previously demonstrated that the nature of the allergen and the route of exposure causes dynamic changes in asthma pathogenesis19. Additionally, the function of RELM-β in the gut may be through dynamic feedback regulation with the microbiome25,26. Therefore, it is possible that differences in background strains of founders and the variations in models, together with housing conditions and their possible influence on the microbiome, may also have contributed to differences between our data and that of others.
While the expression and functions of RELM-β may be dependent on a variety of factors, including the immune system and microbiome, elucidating its role in lung and gut diseases during various insults is important to distinguish positive/negative feedback mechanisms that may be beneficial to identify targets for therapy. As the incidence of asthma increases worldwide, understanding the function of immunomodulators in different endotypes of asthma is important to identify novel approaches to personalized therapeutics. Herein, we show that resistin family member, RELM-β, regulates immune responses to allergens and propose that it inhibits the development of chronic features of allergic asthma.
Materials and Methods
Ethics Statement
All animal work described herein were performed in strict accordance with protocols approved by the Institutional Animal Care and Use Committee (IACUC, approval number 15-003.0) at the University of Tennessee Health Science Center in Memphis.
Mouse Model of SAFS
Animals were maintained in micro-isolator cages with alpha-dri bedding and ad libitum access to food and water, and housed in a temperature and humidity controlled room with purified air at a 12 hour light-dark cycles. Mice with a targeted deletion in Retnlb23 backcrossed to C57BL/6 strain were received as a kind gift from Drs. Gary Wu and Angela Haczku. C57BL/6 mice from Jackson Laboratories (Bar Harbor, ME) were used as wild-type (WT) controls. Seven-week old mice of both genders were used as we determined no gender-based differences in the development of SAFS in pilot studies.
Allergen sensitization and challenge were performed as previously described in detail19,56,61. Briefly, A. fumigatus extract (Greer Labs, Lenoir, NC) -sensitized mice were exposed via inhalation to live unmanipulated conidia liberated from an 8-day old A. fumigatus culture (NIH strain 5233, ATCC, Manassas, VA) for 10 minutes, rested for two weeks, and repeated. Untreated mice were used as naïve controls that were euthanized at the equivalence of week 8 in the treated mice. Tissue were collected at predetermined time points after the second fungal inhalation challenge to capture the early and late phases of the allergic response. The timeline of the treatments is schematically represented in Fig. 1.
AHR Measurement
Anesthetized mice were intubated with an 18-g metal catheter, attached to a computer-controlled small animal ventilator (flexiVent FX1, SCIREQ, Quebec, Canada) and a methacholine dose-response curve was performed as previously described62. Peak values for each dose was used to calculate the mean and standard deviation for independent doses at each time point.
Tissue Harvest and Processing
Animals were euthanized by CO2 asphyxiation per approved protocols and BAL was performed. Lavage contents were centrifuged at 1,500 × g for 10 minutes to separate the fluid from cells. The BAL fluid was stored at −80 °C until use, while the cells were stained for flow cytometric analyses as described below.
As we have not noted lobular discrepancies in inflammation, GC metaplasia, and airway wall remodelling events in this natural inhalation-induced model of allergic asthma during time when the model was developed and characterized, we divided the lungs for various analyses. Cardiac, middle, and lower half of the proximal right lung lobe were snap frozen in liquid nitrogen, and stored at −80 °C for RNA analyses. The remaining half of the proximal right lobe and the distal lobe were collected for protein analyses. Left lung lobes were inflated ex vivo with 10% normal buffered formalin for histology. Pooled blood in the thoracic cavity was centrifuged at 12,000 × g for 10 minutes to separate serum which was stored at −80 °C until use.
Flow Cytometric Analyses
Following red blood cell lysis, BAL cells were incubated in human gamma globulin for 30 min and stained with fluorescently-tagged antibodies for 30 minutes on ice, Mac-3-biotin was probed for using streptavidin-BV605, and cells were subsequently fixed with 1 × stabilizing fixative (BD Biosciences, San Jose, CA). Data from filtered fixed cells were acquired using a LSR Fortessa (BD) cytometer. The following antibodies (clones in parentheses) were purchased from BD Biosciences and used at 1:50 dilution unless stated otherwise: CD8α-FITC (53–6.7), CD19-PerCP/Cy5.5 (1D3), Ly6G-V450 (1A8), CD193 (CCR3)-Alexa Fluor 647 (83103), CD4-Alexa Fluor 700 (RM4-5), CD3ε-PE/Cy7 (145-2C11), NK1.1-APC/Cy7 (PK136), Siglec-F-PE/CF594 (E50-2440), CD107b (Mac-3)-biotin (M3/84, BioLegend, 1:500), Streptavidin-BV 605 (BioLegend, 1:200). Matched isotype antibodies were used at the same concentrations. FCS files were analysed using FlowJo v10.3 (Flowjo, LLC, Ashland, OR) and the Supplemental Figure shows the gating strategy.
Histological Analyses
Left lungs were sectioned at 4 µm and glass slide affixed sections were stained with haematoxylin and eosin, periodic acid Schiff’s, and Masson’s trichrome stains for analyses of inflammation, GCs, and collagen deposition respectively. All slides were scored by an investigator blinded to the genotypes and study time points.
Ten equivalent areas at 100x magnification along the large airways were scored in one lung section for each mouse in the group. A score of ‘0’ indicates no inflammatory foci in H&E while a score of ‘3’ indicates the most inflammation observed around blood vessels and airways.
Eosinophils in inflammatory foci around the large airways and blood vessels were counted in each section based on the nuclear morphometry and pink staining in the cytoplasm at 1000x magnification. Fifty fields were counted in each sample. GCs were enumerated along 100 µm of large bronchi and represented as the percentage of epithelia lining the airway.
The thickness of trichrome stained collagen fibrils from the basement membrane of the bronchial epithelia toward the parenchyma was measured in ten randomly selected airways in each lung using a calibrated Nikon microscope with NIS-Elements software v4.2. The average in ten fields were calculated for each mouse lung and the mean and standard error of the mean were calculated and reported for each group.
Quantification of Antibodies
IgA and IgG1 (Bethyl Labs, Montgomery, TX) and IgE (BD Biosciences) were quantified in serum and BAL fluid by ELISA. Serum samples were diluted at 1:5000 for IgA and IgG1, and 1:500 for IgE while BAL fluid samples were diluted at 1:100 for IgA and IgG1, and used neat for IgE. A. fumigatus-specific titres of total Ig, IgA, IgG1, and IgE in serum and BAL fluid were measured as previously described63,64.
Quantitative real-time PCR
RNA in right lung lobes were extracted with Trizol reagent (Invitrogen, Carlsbad, CA), and 1 µg of RNA was used to generate cDNA with iSCRIPT™ reagents (BioRad, Hercules, CA). Diluted cDNA was used with RNA-specific Quantitect primer sets for Hprt-1, Muc5ac, Muc5b, Retnla, Camp, SurfA, Il13, Tlr2, and Tlr4, with SYBR® Green master mix (all from Qiagen, Hilden, Germany) to determine changes in gene expression using the ABI 7500 (Applied Biosystems, Foster City, CA). Gene expression changes were calculated with the 2−ΔΔCt method relative to Hprt-1 housekeeping gene and standardized to naïve controls.
Multiplex Cytokine Assay
Lung lobes were homogenized in 1 mL of Roche cOmplete Protease Inhibitor (Basel, Switzerland) and centrifuged to remove debris. Supernatants were diluted two-fold and quantified using the R&D Systems’ (Minneapolis, MN) Mouse Magnetic Luminex Assays with a Luminex MAGPIX running xPONENT 4.2 software.
Primary Fibroblast Wound Healing Assay
Fibroblasts were isolated from lungs of WT and Retnlb−/− mice following a standard protocol65. Cultured fibroblasts were seeded in 24-well plates at 4 × 105 cells/mL and incubated for 24 hrs. Confluent monolayers were scratched with sterile pipet tips, and wounded cells were incubated in media containing 5% of FBS with/without 10 ng/mL of recombinant murine RELM-β (PeproTech, NJ). Cultures were photographed at 20× magnification with an EVOS cell imaging system (ThermoFisher, Waltham, MA) and wound area measured at different time intervals with ImageJ software v1.48.
Statistical Analyses
Each genotype consisted of 4–7 mice at each time point. The entire study was repeated independently for reproducibility. Data were analysed by two-way ANOVA with Dunnett’s, Sidak’s, or Tukey’s multiple comparisons tests as appropriate using GraphPad Prism software v6.01 (La Jolla, CA) as noted in each Figure Legend.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Acknowledgements
The authors thank Brandi Livingston (St. Jude Children’s Research Hospital), Ghana Gurung, and Stacey Barnett (UTHSC) in the Laboratory Animal Care Units for husbandry and care of animals during the study. Special thanks to Drs Gary Wu (University of Pennsylvania) and Angela Hackzu (University of California, Davis) for providing us with Retnlb−/− mice. We also thank John Snyder at UTHSC for assistance in quantifying goblet cells. These studies were partially supported by grants from the American Lung Association (Biomedical Research Grant, RG-350980), Le Bonheur Associate Board Young Investigator Award, and the National Institutes of Health (R01-AI125481) all to AES.
Author Contributions
K.S.L. performed sample harvest, flow cytometry, multiplex, and data analyses. M.P. performed animal modelling, AHR and data analysis, and sample harvest. M.T. performed qPCR and data analysis, wound healing assay, and measurements of collagen thickness. B.G. performed mouse genotyping. A.E.S. designed the study, performed ELISA, analysed data, and wrote the manuscript. All authors edited and approved the final version of the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-25321-y.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Erle DJ, Sheppard D. The cell biology of asthma. The Journal of cell biology. 2014;205:621–631. doi: 10.1083/jcb.201401050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murdoch JR, Lloyd CM. Chronic inflammation and asthma. Mutation research. 2010;690:24–39. doi: 10.1016/j.mrfmmm.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. Asthma, http://www.who.int/mediacentre/factsheets/fs307/en/index.html (2017).
- 4.CDC. Asthma in the US, http://www.cdc.gov/vitalsigns/asthma/ (2011).
- 5.Steppan CM, et al. A family of tissue-specific resistin-like molecules. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:502–506. doi: 10.1073/pnas.98.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holcomb IN, et al. FIZZ1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family. The EMBO journal. 2000;19:4046–4055. doi: 10.1093/emboj/19.15.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asano T, et al. Physiological significance of resistin and resistin-like molecules in the inflammatory process and insulin resistance. Current diabetes reviews. 2006;2:449–454. doi: 10.2174/1573399810602040449. [DOI] [PubMed] [Google Scholar]
- 8.Al Hannan F, Culligan KG. Human resistin and the RELM of Inflammation in diabesity. Diabetology & metabolic syndrome. 2015;7:54. doi: 10.1186/s13098-015-0050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Artis D, et al. RELMbeta/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:13596–13600. doi: 10.1073/pnas.0404034101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hogan SP, et al. Resistin-like molecule beta regulates innate colonic function: barrier integrity and inflammation susceptibility. The Journal of allergy and clinical immunology. 2006;118:257–268. doi: 10.1016/j.jaci.2006.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mishra A, et al. Resistin-like molecule-beta is an allergen-induced cytokine with inflammatory and remodeling activity in the murine lung. American journal of physiology. Lung cellular and molecular physiology. 2007;293:L305–313. doi: 10.1152/ajplung.00147.2007. [DOI] [PubMed] [Google Scholar]
- 12.Liu T, et al. FIZZ2/RELM-beta induction and role in pulmonary fibrosis. Journal of immunology. 2011;187:450–461. doi: 10.4049/jimmunol.1000964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang CL, et al. Resistin-like molecule-beta (RELM-beta) targets airways fibroblasts to effect remodelling in asthma: from mouse to man. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2015;45:940–952. doi: 10.1111/cea.12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zosky GR, et al. Ovalbumin-sensitized mice are good models for airway hyperresponsiveness but not acute physiological responses to allergen inhalation. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2008;38:829–838. doi: 10.1111/j.1365-2222.2007.02884.x. [DOI] [PubMed] [Google Scholar]
- 15.Degryse AL, Lawson WE. Progress toward improving animal models for idiopathic pulmonary fibrosis. The American journal of the medical sciences. 2011;341:444–449. doi: 10.1097/MAJ.0b013e31821aa000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang C, et al. Resistin-like molecule-beta is a human airway remodelling mediator. The European respiratory journal. 2012;39:458–466. doi: 10.1183/09031936.00107811. [DOI] [PubMed] [Google Scholar]
- 17.Samarasinghe AE, et al. The immune profile associated with acute allergic asthma accelerates clearance of influenza virus. Immunology and cell biology. 2014;92:449–459. doi: 10.1038/icb.2013.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doorley, L. A., LeMessurier, K. S., Iverson, A. R., Palipane, M. & Samarasinghe, A. E. Humoral immune responses during asthma and influenza co-morbidity in mice. Immunobiology, 10.1016/j.imbio.2017.08.002 (2017). [DOI] [PMC free article] [PubMed]
- 19.Samarasinghe AE, Hoselton SA, Schuh JM. A comparison between intratracheal and inhalation delivery of Aspergillus fumigatus conidia in the development of fungal allergic asthma in C57BL/6 mice. Fungal Biol. 2011;115:21–29. doi: 10.1016/j.funbio.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manni ML, et al. The complex relationship between inflammation and lung function in severe asthma. Mucosal immunology. 2014;7:1186–1198. doi: 10.1038/mi.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He W, et al. Bacterial colonization leads to the colonic secretion of RELMbeta/FIZZ2, a novel goblet cell-specific protein. Gastroenterology. 2003;125:1388–1397. doi: 10.1016/j.gastro.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Herbert DR, et al. Intestinal epithelial cell secretion of RELM-beta protects against gastrointestinal worm infection. The Journal of experimental medicine. 2009;206:2947–2957. doi: 10.1084/jem.20091268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McVay LD, et al. Absence of bacterially induced RELMbeta reduces injury in the dextran sodium sulfate model of colitis. J Clin Invest. 2006;116:2914–2923. doi: 10.1172/JCI28121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nair MG, et al. Goblet cell-derived resistin-like molecule beta augments CD4+ T cell production of IFN-gamma and infection-induced intestinal inflammation. Journal of immunology. 2008;181:4709–4715. doi: 10.4049/jimmunol.181.7.4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morampudi V, et al. The goblet cell-derived mediator RELM-beta drives spontaneous colitis in Muc2-deficient mice by promoting commensal microbial dysbiosis. Mucosal immunology. 2016;9:1218–1233. doi: 10.1038/mi.2015.140. [DOI] [PubMed] [Google Scholar]
- 26.Propheter DC, Chara AL, Harris TA, Ruhn KA, Hooper LV. Resistin-like molecule beta is a bactericidal protein that promotes spatial segregation of the microbiota and the colonic epithelium. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:11027–11033. doi: 10.1073/pnas.1711395114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams OW, Sharafkhaneh A, Kim V, Dickey BF, Evans CM. Airway mucus: From production to secretion. American journal of respiratory cell and molecular biology. 2006;34:527–536. doi: 10.1165/rcmb.2005-0436SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kendall RT, Feghali-Bostwick CA. Fibroblasts in fibrosis: novel roles and mediators. Frontiers in pharmacology. 2014;5:123. doi: 10.3389/fphar.2014.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burgess JK, Mauad T, Tjin G, Karlsson JC, Westergren-Thorsson G. The extracellular matrix - the under-recognized element in lung disease? The Journal of pathology. 2016;240:397–409. doi: 10.1002/path.4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sugiura H, et al. Cultured lung fibroblasts from ovalbumin-challenged “asthmatic” mice differ functionally from normal. American journal of respiratory cell and molecular biology. 2007;37:424–430. doi: 10.1165/rcmb.2007-0089OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samarasinghe AE, Hoselton SA, Schuh JM. The absence of VPAC2 leads to aberrant antibody production in Aspergillus fumigatus sensitized and challenged mice. Peptides. 2011;32:131–137. doi: 10.1016/j.peptides.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun R, et al. Interleukin-13 Receptor alpha1-Dependent Responses in the Intestine Are Critical to Parasite Clearance. Infection and immunity. 2016;84:1032–1044. doi: 10.1128/IAI.00990-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grossman WJ, et al. Differential expression of granzymes A and B in human cytotoxic lymphocyte subsets and T regulatory cells. Blood. 2004;104:2840–2848. doi: 10.1182/blood-2004-03-0859. [DOI] [PubMed] [Google Scholar]
- 34.Bratke K, et al. Increase in granzyme B+ lymphocytes and soluble granzyme B in bronchoalveolar lavage of allergen challenged patients with atopic asthma. Clinical and experimental immunology. 2004;136:542–548. doi: 10.1111/j.1365-2249.2004.02468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Devadas S, et al. Granzyme B is critical for T cell receptor-induced cell death of type 2 helper T cells. Immunity. 2006;25:237–247. doi: 10.1016/j.immuni.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 36.Asai K, et al. Increased levels of vascular endothelial growth factor in induced sputum in asthmatic patients. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2003;33:595–599. doi: 10.1046/j.1365-2222.2003.01576.x. [DOI] [PubMed] [Google Scholar]
- 37.Hohlfeld JM. The role of surfactant in asthma. Respiratory research. 2002;3:4. doi: 10.1186/rr176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baritussio A. Lung surfactant, asthma, and allergens: a story in evolution. American journal of respiratory and critical care medicine. 2004;169:550–551. doi: 10.1164/rccm.2312019. [DOI] [PubMed] [Google Scholar]
- 39.Kosciuczuk EM, et al. Cathelicidins: family of antimicrobial peptides. A review. Molecular biology reports. 2012;39:10957–10970. doi: 10.1007/s11033-012-1997-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun J, Dahlen B, Agerberth B, Haeggstrom JZ. The antimicrobial peptide LL-37 induces synthesis and release of cysteinyl leukotrienes from human eosinophils–implications for asthma. Allergy. 2013;68:304–311. doi: 10.1111/all.12087. [DOI] [PubMed] [Google Scholar]
- 41.LeMessurier KS, Lin Y, McCullers JA, Samarasinghe AE. Antimicrobial peptides alter early immune response to influenza A virus infection in C57BL/6 mice. Antiviral research. 2016;133:208–217. doi: 10.1016/j.antiviral.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chai LY, et al. Modulation of Toll-like receptor 2 (TLR2) and TLR4 responses by Aspergillus fumigatus. Infection and immunity. 2009;77:2184–2192. doi: 10.1128/IAI.01455-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Forbes L. Asthma and atopy: endocrine or metabolic conditions? Thorax. 2005;60:793–794. doi: 10.1136/thx.2005.047050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trankner D, Hahne N, Sugino K, Hoon MA, Zuker C. Population of sensory neurons essential for asthmatic hyperreactivity of inflamed airways. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:11515–11520. doi: 10.1073/pnas.1411032111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Agarwal R, Gupta D. Severe asthma and fungi: current evidence. Medical mycology. 2011;49(Suppl 1):S150–157. doi: 10.3109/13693786.2010.504752. [DOI] [PubMed] [Google Scholar]
- 46.Vignola AM, et al. Assessment of airway inflammation in asthma. American journal of respiratory and critical care medicine. 1998;157:S184–187. doi: 10.1164/ajrccm.157.5.rsaa-3. [DOI] [PubMed] [Google Scholar]
- 47.Corry DB, et al. Decreased allergic lung inflammatory cell egression and increased susceptibility to asphyxiation in MMP2-deficiency. Nature immunology. 2002;3:347–353. doi: 10.1038/ni773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bergstrom KS, et al. Goblet Cell Derived RELM-beta Recruits CD4+ T Cells during Infectious Colitis to Promote Protective Intestinal Epithelial Cell Proliferation. PLoS pathogens. 2015;11:e1005108. doi: 10.1371/journal.ppat.1005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Munitz A, Cole ET, Karo-Atar D, Finkelman FD, Rothenberg ME. Resistin-like molecule-alpha regulates IL-13-induced chemokine production but not allergen-induced airway responses. American journal of respiratory cell and molecular biology. 2012;46:703–713. doi: 10.1165/rcmb.2011-0391OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Samarasinghe AE, Hoselton SA, Schuh JM. The absence of the VPAC(2) receptor does not protect mice from Aspergillus induced allergic asthma. Peptides. 2010;31:1068–1075. doi: 10.1016/j.peptides.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Falk PG, Hooper LV, Midtvedt T, Gordon JI. Creating and maintaining the gastrointestinal ecosystem: what we know and need to know from gnotobiology. Microbiology and molecular biology reviews: MMBR. 1998;62:1157–1170. doi: 10.1128/mmbr.62.4.1157-1170.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Juhn, Y. J. Risks for infection in patients with asthma (or other atopic conditions): is asthma more than a chronic airway disease? The Journal of allergy and clinical immunology134, 247–257, quiz 258–249, 10.1016/j.jaci.2014.04.024 (2014). [DOI] [PMC free article] [PubMed]
- 53.Nizet V, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 54.Evans CM, Kim K, Tuvim MJ, Dickey BF. Mucus hypersecretion in asthma: causes and effects. Current opinion in pulmonary medicine. 2009;15:4–11. doi: 10.1097/MCP.0b013e32831da8d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kumar RK, Foster PS. Are mouse models of asthma appropriate for investigating the pathogenesis of airway hyper-responsiveness? Frontiers in physiology. 2012;3:312. doi: 10.3389/fphys.2012.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoselton SA, Samarasinghe AE, Seydel JM, Schuh JM. An inhalation model of airway allergic response to inhalation of environmental Aspergillus fumigatus conidia in sensitized BALB/c mice. Medical mycology. 2010;48:1056–1065. doi: 10.3109/13693786.2010.485582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wills-Karp M. Interleukin-13 in asthma pathogenesis. Immunological reviews. 2004;202:175–190. doi: 10.1111/j.0105-2896.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- 58.Saito A, Okazaki H, Sugawara I, Yamamoto K, Takizawa H. Potential action of IL-4 and IL-13 as fibrogenic factors on lung fibroblasts in vitro. International archives of allergy and immunology. 2003;132:168–176. doi: 10.1159/000073718. [DOI] [PubMed] [Google Scholar]
- 59.Hashimoto S, Gon Y, Takeshita I, Maruoka S, Horie T. IL-4 and IL-13 induce myofibroblastic phenotype of human lung fibroblasts through c-Jun NH2-terminal kinase-dependent pathway. The Journal of allergy and clinical immunology. 2001;107:1001–1008. doi: 10.1067/mai.2001.114702. [DOI] [PubMed] [Google Scholar]
- 60.Okubo H, et al. Involvement of resistin-like molecule beta in the development of methionine-choline deficient diet-induced non-alcoholic steatohepatitis in mice. Scientific reports. 2016;6:20157. doi: 10.1038/srep20157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schuh JM, Hoselton SA. An inhalation model of allergic fungal asthma: Aspergillus fumigatus-induced inflammation and remodeling in allergic airway disease. Methods Mol Biol. 2013;1032:173–184. doi: 10.1007/978-1-62703-496-8_14. [DOI] [PubMed] [Google Scholar]
- 62.Samarasinghe AE, et al. Eosinophils Promote Antiviral Immunity in Mice Infected with Influenza A Virus. Journal of immunology. 2017;198:3214–3226. doi: 10.4049/jimmunol.1600787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ghosh S, Hoselton SA, Schuh JM. mu-chain-deficient mice possess B-1 cells and produce IgG and IgE, but not IgA, following systemic sensitization and inhalational challenge in a fungal asthma model. Journal of immunology. 2012;189:1322–1329. doi: 10.4049/jimmunol.1200138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Apiwattanakul N, Palipane M, Samarasinghe AE. Immune responses to fungal aeroallergen in Heligmosomoides polygyrus-infected mice vary by age. Cellular immunology. 2017;317:26–36. doi: 10.1016/j.cellimm.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 65.Seluanov, A., Vaidya, A. & Gorbunova, V. Establishing primary adult fibroblast cultures from rodents. Journal of visualized experiments: JoVE, 10.3791/2033 (2010). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.