Abstract
Bone is consisted of bone matrix, cells and bioactive factors, and bone matrix is the combination of inorganic minerals and organic polymers. Type I collagen fibril made of five triple-helical collagen chains is the main organic polymer in bone matrix. It plays an important role in the bone formation and remodeling process. Moreover, collagen is one of the most commonly used scaffold materials for bone tissue engineering due to its excellent biocompatibility and biodegradability. However, the low mechanical strength and osteoinductivity of collagen limit its wider applications in bone regeneration field. By incorporating different biomaterials, the properties such as porosity, structural stability, osteoinductivity, osteogenicity of collagen matrixes can be largely improved. This review summarizes and categorizes different kinds of biomaterials including bioceramic, carbon and polymer materials used as components to fabricate collagen based composite scaffolds for bone regeneration. Moreover, the possible directions of future research and development in this field are also proposed.
Keywords: Collagen, Bone regeneration, Composite scaffolds, Biomaterials, Tissue engineering
Graphical abstract
Highlights
-
•
Materials to incorporate collagen scaffolds for bone regeneration are summarized.
-
•
Bioceramics, carbon and polymer materials can increase the mechanical properties and osteogenesis.
-
•
The limitation of collagen based materials is analyzed and the prospects of future research are presented.
1. Introduction
As a natural solid biocomposite, bone has a unique hierarchical structural organization at multi-scales which contributes to the high strength and fracture toughness. It is considered that the excellent mechanical properties of bone come from the well-organized embedding of nano-mineral crystals within the collagen matrix to form the intricately and orderly hierarchical structure [1].
Bone is composed of calcified bone matrix, cells and bioactive factors. Bone matrix contains around 65 wt. % mineral materials, 25 wt. % organic materials, and 10 wt. % water. The hydroxyapatite [Ca10(PO4)6(OH)2, HA] is the main inorganic mineral phase. The Ca/P ratio of HA is less than 1.67. There are some impurities such as CO32−2-, Na+, Mg2+ and so on existing in natural HA. These impurities can lead to the poor crystallization, deficient calcium and carbonation of HA [1]. Besides, many kinds of essential trace elements including silicon (Si), fluorine (F), zinc (Zn), strontium (Sr), magnesium (Mg), boron (B), and copper (Cu), sodium (Na), manganese (Mn), carbonate (CO3), potassium (K), chlorine (Cl) etc. present in biology bone, which play an important role in bone growth or can have an effect on bone metabolism [2], [3], [4]. For instance, the studies have revealed that the silicon ions can induce angiogenesis and osteogenesis while taking too much Na+ may result in osteoporosis [5], [6]. Sr2+ can stimulate the bone-forming function of osteoblasts as well as inhibiting the bone resorbing function of osteoclasts [7], [8], [9]. Mg2+ has an important role in angiogenesis by inducing nitric oxide production and can indirectly influence mineral metabolism [3], [10]. In bone matrix, the organic components consisting of proteins such as collagen are embedded within the calcified matrix. Up to now, over 28 types of collagen have been found in vertebrates and four types of collagen have been verified in bone including type I, III, V and XXIV collagen [11]. Among them, type I collagen is the most abundant protein accounting for 97%. In type I collagen, five triple-helical collagen molecules arrange to form the collagen microsized fibril with a characteristic 67 nm banding feature which is called the D-period [12], [13].
Despite the structure of bone has been reasonably defined, there is not a well-agreed explanation of the mechanism of mineralization [14]. Two theories of mineralization have been reported: direct nucleation of calcium phosphate mineral crystals [15], and matrix vesicle mediated matrix mineralization [16], [17]. Moreover, some bone-related proteins and growth factors such as alkaline phosphatase (ALP), parathyroid hormone (PTH), osteocalcin (OCN), osteonectin (ON), osteopontin (OPN), bone sialoprotein (BSP), bone-morphogenetic protein-2 (BMP-2), and fibroblast growth factor-2 (FGF-2), etc. have been proved to have an effect of regulating essential matrix mineralization [18], [19], [20], [21], [22], [23].
Bone plays an important role in the body by supporting mechanical stress and maintaining ionic balance, while trauma or diseases such as tumor and osteoporosis may lead to bone damage. Bone defects resulted by non-union or mal-union fractures, congenital malformations and surgical resections remain a big challenge in the field of modern medicine. In this situation, finding the proper alternatives for reversing bone defects and regenerating the damage bone seems to be vital.
The most commonly used bone grafts in clinic is autologous and allogeneic bone but there still exists some problems such as infection, limited supply and allograft rejection. Apart from autologous and allogeneic bone, alternative bone graft materials such as metals, polymers and ceramics are applied to not only fill the bone defect but also provide mechanical and structural support.
There are five general therapeutic targets in bone regeneration including vascularization, growth factors, osteogenesis, osteoconductive scaffolds and mechanical environment, in which at least three of them should be completed for successful bone regeneration [24], [25], [26], [27]. Researchers usually focus on the osteoconductive scaffolds, osteogenic cells and the growth factors. Among them, scaffold has the largest renovation potential. As mentioned above, type I collagen is the main organic composition of biological bone and collagen fibrils serve as a template for mineralization. As one of the most commonly used scaffold material, collagens are found to have outstanding biodegradability, weak antigenicity after removal of telopeptides and excellent biocompatibility [28], [29]. Moreover, cells can attach to the surface of collagen via integrin α 2 β 1 [30]. However, the pure collagen scaffold has insufficient mechanical strength for bone regeneration. Culturing the cells on them, the scaffolds will exhibit unstable geometrical properties due to the extensive cell-mediated contraction [31]. In addition, it is considered that the pure collagen materials lack enough bioactivity to stimulate bone formation ability [32]. The strategy of incorporating of bioactive component is still one of the most popular strategies to improve the mechanical strength, bioactivity and osteogenesis of collagen based scaffolds.
To the best of our knowledge, a large number of studies on collagen based composite scaffolds for bone regeneration have been reported. Though some reviews focus on the preparation, properties and applications of collagen based scaffold [33], [34], [35]. While few focus on the materials used to incorporate the collagen scaffold. In view of the sustaining growing interests in the fabrication and application of collagen based composite scaffolds, our goal in this review is to summarize and categorize these composite scaffolds. To accomplish this, the materials used to incorporate are divided into three major categories: bioceramics, carbon-based materials and polymers. The impacts brought by these materials are summarized and the examples are demonstrated. In the end, the limitations and future trends of collagen composite scaffolds for bone regeneration were also summarized.
2. Collagen based composite scaffolds for bone regeneration
Since the regeneration ability of bone and the resource of bone grafts are limited, in order to deal with this problem or accelerate the healing process, a wide variety of collagen-based scaffolds mimicking the native bone tissue microenvironment have been proposed. Diverse materials are applied to modify collagen-based scaffolds for better performances in vitro and in vivo. In this section, they are introduced by separating them into three major categories including bioceramics, carbon-based materials and polymers.
2.1. Collagen based composite scaffolds with bioceramic components
Due to poor mechanical strength of pure collagen scaffold, some kinds of bioceramics with the similar constituent to the intrinsic inorganic components of nature bone are widely used in collagen scaffolds for bone regeneration. Apart from the enhancement of mechanical properties, they can also improve osteoconductive ability, dimensional stability and increase the surface area for cell attachment on the composite scaffolds [36], [37]. Two methods are widely applied to fabricate collagen/bioceramic composite scaffolds: suspension method (direct mixing) [38] and immersion method (co-precipitation) [39], [40]. Moreover, HA crystals can be deposited to form thin HA coatings on the scaffold after soaking in simulated body fluid (SBF). This way is more like a biomimetic process [41], which can mimic the biochemical and biophysical properties of bone matrix. The ‘biomimetic’ scaffolds are expected to take the place of the missing bone. In this section, the related studies about the collagen-based composite scaffolds with incorporation of calcium phosphate (CaP) bioceramics and calcium silicate (CaSi) bioceramics, have been summarized and discussed, respectively.
2.1.1. With calcium phosphate (CaP) based bioceramic components
2.1.1.1. Hydroxyapatite [Ca10(PO4)6(OH)2, HA]
HA is the most commonly used calcium phosphate with the molar ratio of Ca/P = 1.67. Moreover, the biology bone is mainly constructed by the inorganic HA and organic collage components. In addition, these two components possess great biocompatibility, osteoconductivity and bone-bonding ability [42], [43], [44]. Therefore, the collagen/HA composite biomaterial scaffolds have been extensively investigated and used for bone tissue engineering scaffolds. The mechanical strength of pure collagen scaffolds is extraordinarily low, which immensely limits their wider applications in tissue regeneration. The compressive modulus of collagen scaffold can be apparently improved by incorporating HA, and the degree of increase is largely related to the concentration of collagen, the amount of HA, the composite methods and the crosslinking methods [45]. In addition, the surface area of the collagen scaffolds can be increased by combining HA, which will lead to incremental cellular adhesion [46]. Comparing with micro-sized HA component, nano-sized HA particles can be more effectively because of their larger surface area. Moreover, another point that cannot be ignored is that apatite can form direct chemical bonds with the host bone tissue. This characteristic will result in faster and better bone-bonding between the scaffolds and the neighboring host bone tissue with HA component. Cells will proliferate better and show enhanced bioactivity on rough surfaces [47], therefore scaffolds containing HA have higher cellular proliferation than the scaffolds without HA component [46]. Some researchers found micron-sized HA particles might lead to poor resorbability, irregular distribution and brittle constructs of the composite scaffold [48]. Cunniffe et al. [42] raised the proposal of using incorporating nano-sized HA (nHA) particles into collagen scaffolds. The resultant collagen/nHA scaffolds showed highly porous, interconnected structure and the compressive modulus of scaffold increased by 18 times by adding 500 wt.% nHA by the suspension method. Moreover, after seeding the MC3T3-E1 cells for 7 days, the composite scaffold showed deep penetration of cells and cells distribute more evenly comparing with the pure collagen scaffold.
Up to now, three methods have been used to prepare collagen/nHA scaffolds including: direct blending, SBF immersion and co-precipitation. Compared with nonuniform distribution of HA in direct blending and the slow and uncontrollable process of HA formation in SBF, spontaneous co-precipitation of collagen fibrils and nano-HA is considered as a promising way to achieving the same hierarchical structure of bone [49]. The process of nucleation of HA nanocrystals onto collagen fibers is achieved in an aqueous suspension which containing high concentration of Ca2+ and PO43- in a certain ratio and triggered by raising pH of collagen solution to 9–10 at room temperature. The chemical interaction between HA and collagen results in the c-axes of blade-shaped HA nanocrystals aligning along collagen fibers which is similar to bone [50].
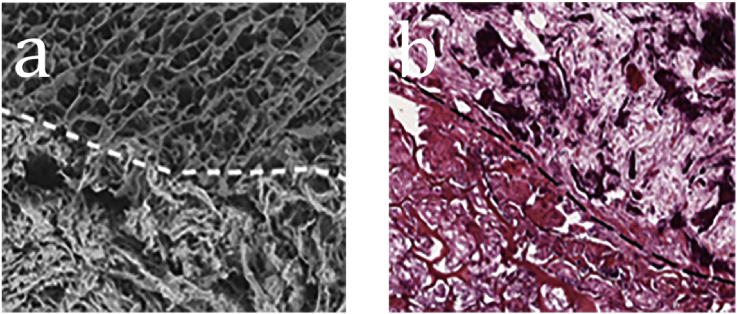
It has mentioned that the HA in nature often has chemical substitutions such as sodium, magnesium, carbonate and citrate ions. Considering Mg2+ has the regulating effect on bone formation process, some researchers prefer choosing magnesium-enriched HA (Mg-HA) as the inorganic component [51]. Calabrese et al. [44] isolated human MSCs from adipose tissue and seeded them on collagen/Mg-HA composite scaffolds. The results of Alizarin red staining and the expression of representative osteogenic markers suggested that collagen/Mg-HA composite scaffolds were capable of inducing loaded MSCs to differentiate toward osteocyte even in the absence of osteoinductive factors. In order to investigate the effect derived from HA component in vivo, Calabrese et al. [52] developed a bilayer scaffold. The first layer was made of pure type I collagen and the second layer was made of collagen/Mg-HA (Fig. 1a). The scaffold was implanted into mice without loading cells and the ability of recruiting host cells could be seen clearly from the result of immunofluorescence staining. Hematoxylin and eosin (H&E) staining at weeks 4 showed the mineralization of the collagen/MHA layer was earlier, faster and completer than that of collagen layer (Fig. 1b). Besides, the result of Alizarin red staining indicated the calcium deposits were mainly in the collagen/Mg-HA layer [52]. Minardi et al. [53] found collagen/Mg-HA can mimic the hypoxic conditions of the osteogenic niche. This condition could inhibit cell growth and maintain the cells in a quiescent state which contributed to an efficient tissue reconstitution.
Fig. 1.
(a) SEM imaging of the bilayer scaffold. (b) H&E staining pictures within the implanted scaffolds at weeks 4. The dotted white line indicates the interface between scaffold layers. The upper half is the pure collagen layer and the bottom half is the collagen/Mg-HA layer [52].
The similar phenomenon also exists in applying Zn-doped HA/collagen scaffold as bone substitution. As mentioned above, Zn has the effect of stimulating cell proliferation and osteogenesis-related gene expression. Yu et al. [54] synthesized Zn-doped mesoporous HA microspheres to modify the collagen scaffold (Zn-MHMs/Collagen scaffold). Compared with MHMs/Collagen scaffold, the Zn-MHMs/Collagen scaffolds with Zn/(Zn + Ca) molar ratio of 0.05 showed significant higher osteogenic activity in vitro and nearly double bone volume to total volume ratio and trabecular number in rat model.
In addition, the biological HA in natural bone contains about 7.4 wt.% carbonate [55]. It is reported that higher carbonated minerals often means higher osteoconductivity and earlier bioresorption due to the increased solubility. Liao et al. [56] fabricated nano-carbonated HA/collagen composites by co-precipitation method. With the increase of the concentration of carbonate, they found the crystal morphologies also changed from plate-like to needle-like, and then to spherical particles.
After years of exploration, collagen/HA composite scaffolds with isotropic equiaxed structures or unidirectionally interconnected pores and controllable micropore structures can be well fabricated [40], [57], [58]. With the development of three-dimensional (3D) printing technology, desired exterior outline can be made to fit the bone defect. But it is still impossible to mimic the intricate intrinsic internal patterns of the nanocrystalline assembly and collagen fibrous scaffold structure of bone matrix. As research continues, the process of bone mineralization is hopeful to be fully understood and this problem may be smoothed out.
2.1.1.2. β-tricalcium phosphate [β-Ca3(PO4)2, β-TCP]
As another representative calcium phosphate bioceramics, β- TCP has been widely used as bone substitutes for many years due to its good osteoconductivity, biocompatibility and biodegradability both in vitro and in vivo. The Ca/P ratio of β-TCP is 1.5. It can not only improve cellular adhesion but accelerate their differentiation and proliferation ability [59]. Some scholars have prepared β-TCP based scaffolds for bone regeneration [60]. Compared with HA, β-TCP has a faster degradation rate and can be completely replaced by newly formed bone tissues [61].
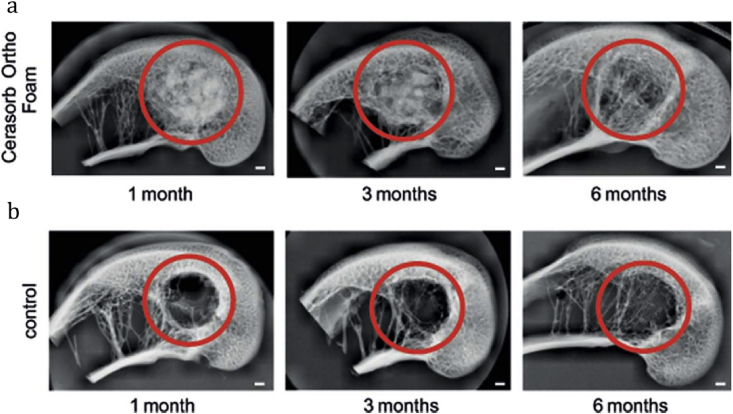
With weight ratio of collagen solution to the β-TCP powder at 9:1, the compressive module of composite scaffold was about three times higher than that of collagen scaffold no matter loading cells or not. By measuring swelling ratio and variation of pore size at different time, they found the composite scaffold almost kept constant while the collagen scaffold obviously increased, suggesting that the β-TCP can enhance the structural stability of collagen scaffold and maintain the cell permeation and proliferation [62]. Moreover, due to the osteoconductive capabilities of β-TCP, the expressions of all osteoblastic markers of the composite scaffolds were much higher than those of the pure collagen scaffold. In vivo, Zheng et al. [63] used Cerasorb Ortho Foam, a kind of collagen/β-TCP composites, as bone void filler in the critical size defects of rabbit distal femoral condyle model. The result showed simultaneous resorption of the void filler and bone formation in the defect without toxic reaction, immunological rejection, and thermal interactions with the bone (Fig. 2). The authors proposed that the incorporation of β-TCP into collagen had a synergistic effect on bone healing. In addition, the composite material supports better contact to the surrounding bone tissue by swelling of the collagen component [63].
Fig. 2.
X-ray pictures of the bone healing process of rabbit distal femoral condyles after surgery. (a) Treated with Cerasorb Ortho Foam. (b) The control group with an empty defect. The red circle indicates drilled holes. Scale bars equal to 1 mm [63].
Murakami et al. [64] investigated the dose effects of β-TCP on the mechanical and biological properties of collagen scaffolds in vitro and in vivo. They found that higher dose of β-TCP nanoparticles resulted in higher mechanical stiffness and larger amount of Ca2+ ions released in vitro and better neovascularization in vivo. But too much Ca2+ ions caused by excessive dose in the bone-forming field might suppress the proliferation of cells and limit bone formation. They recommended the optimal dose of β-TCP ranged from 5 to 10 wt. %.
With different proportions of HA and β-TCP, biphasic calcium phosphate (BCP) is the mixture which combines the stability of HA with the biodegradability of β-TCP. Different biodegradation rates could be achieved by changing the proportion of the composition phases [65]. However, few papers reported about the collagen-based composite scaffold containing BCP for bone regeneration. It is hopeful to use BCP to modify the collagen scaffold.
2.1.2. With calcium silicate (Ca-Si) based bioceramic components
Bioactive glasses (BGs) are a group of silica-based osteoconductive and osteoinductive glass biomaterials containing SiO2-CaO-P2O5 networks. BGs have shown good biocompatibility both in bone and in soft tissue [66]. It is reported that the bioactivity level of BGs is superior to CaP based bioceramics due to the high surface reactivity. When implanted in vivo, they release Na+, Ca2+, as well as soluble silica (presumably Si(OH)4) during the degradation process [67]. These ions stimulate osteogenesis by triggering the cellular proliferation and osteogenic differentiation. Through promoting the expression of gene RCL (a c-Myc responsive growth related gene) and gene CD44, BGs can induce osteoblast proliferation and differentiation. Besides, it can also regulate extracellular matrix remodeling as well as cell-cell and cell matrix attachment [68]. With the formation of silanol functional group (Si-O-H), it can bind directly with living tissues and improve the mineralization process by providing active sites for deposition [66], [69]. Other than osteogenesis, it has been widely accepted that the BG (Bioglass 45S5®) approval by U.S. Food and Drug Administration (FDA) can also induce angiogenesis both in vitro and in vivo [70], [71]. By incorporating BGs into collagen scaffold, it is expected to improve the osteogenic and angiogenesis activity as well as the stiffness when implanted in vivo. It has been found that the release of Ca, P and Si from BG will cause the supersaturation of Ca and P, which will precipitate on the surface of the implants in the form of Ca–P crystals. Through a dehydration process, amorphous crystals will transform into hydroxycarbonate apatite (HCA) [72]. As mentioned above, compared with microsized fillers, the incorporation of nanosized inorganic particles can improve the mechanical properties and osteoconductive ability of the composites to a higher extent and this strategy is also suitable for BGs [73], [74].
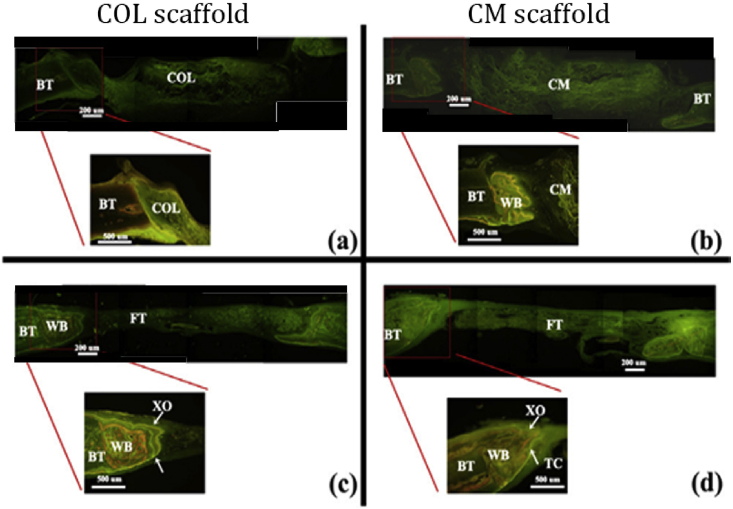
Hsu et al. [75] fabricated collagen/mesoporous bioactive glass nanofibers scaffold (CM) and collagen scaffold (COL) by freeze-drying method. The addition of BG nanofibers limited the shrinkage the scaffold during the cross-linking process and resulted in double the pore size and lower compressive modulus than the COL scaffold. Comparing the two scaffolds soaked in 1.5 × SBF for 7 days, they found the incorporation of mesoporous BG nanofibers can significantly improve its apatite formation ability. However, they found mesoporous bioactive glass nanofibers will inhibit the attachment and proliferation of MG63 cells on CM scaffold. Although CM scaffold had lower compressive strength, Marelli et al. [76] found after conditioning in SBF for 7 days, the compressive modulus of COL/nano-sized BG scaffold was over 16 times than COL scaffold. Moreover, the high concentration of local Ca2+ released by BG could stimulate osteoblasts in the proliferation state to switch to the differentiation state. The higher protein expression of ALP, BSP and OCN on the COL/BG scaffold than COL scaffold also proved that the ionic dissolution products of BGs had the up-regulation effect on cellular osteoblastic differentiation by membrane-mediated ion transfer [77], [78]. Much more newly formed bone could be observed in the scaffolds with BG component after implantation into Sprague–Dawley (SD) rats (Fig. 3), which indicated that the incorporation of BGs into collagen scaffolds could improve the bioactivity and osteogenesis in vivo [75].
Fig. 3.
Fluorescence microscopy images of the bone defect of SD rats after implantation. (a, b): 4 weeks, (c, d): 8 weeks. At week 4, newly formed woven bone had been found beside CM scaffold while no newly formed bone was found in COL scaffold. Larger amount of newly formed bone can be observed in CM scaffold than that in COL scaffold. TC: tetracycline, XO: xylenol orange. WB: woven bone. BT: bone tissue, FT: fibrous tissue [75].
Other than BGs, the silicon containing bioceramics such as wollastonite (CaSiO3, CS) possess excellent bioactivity and biocompatibility, and can rapidly induce the deposition of bone-like apatite on their surface after soaking in SBF [79], [80], [81], [82], [83]. Similar to BGs, CS can also release Si and Ca ions to stimulate cellular proliferation and osteogenic differentiation, and also stimulate the bone regeneration ability [84], [85], [86], [87]. Most importantly, the angiogenesis ability of the scaffold can be promoted as well [88], [89]. In addition, the addition of CS could enhance the mechanical strength of the collagen scaffolds [80], [90]. The similar results were also confirmed by Zhang et al. [91]. The Young's modulus of wet COL/CS nanowire hybrid scaffolds reached nearly three times of Col scaffold. Above all, when hMSCs were cultured on the two scaffolds, the proliferation rate and the osteogenic gene expression level of hybrid scaffold were significantly higher than those of COL scaffold. However, there still needs more investigations about the bioactivity, immunogenicity and osteogenesis of collagen/CS scaffolds when implanted in vivo.
2.2. Collagen based composite scaffolds with carbon-based components
2.2.1. Carbon nanotubes (CNTs)
CNTs are a macromolecular form of allotropes of carbon which possess a cylindrical nanostructure with extremely aspect ratio and low density. The aspect ratios of single-walled carbon nanotubes (SWCNTs) are often over 100 [92]. As one of the strongest and stiffest materials, CNTs have aroused massive attention since they were discovered for the extraordinary mechanical properties, chemical durability, thermal properties and nontoxicity to cells at a low concentration [93], [94], [95]. It has been reported that cells can strongly adhere and proliferate on CNTs [96], [97], [98]. Zanello et al. [97] found neutrally charged CNTs had good bone-tissue compatibility and could sustain osteoblast proliferation and bone-forming functions. Furthermore, Usui et al. [99] has proved that CNTs could closely integrate with bone tissue and accelerate bone repair. They also found this effect is in response to rhBMP-2. Based on these excellent characteristics, it is expected to improve the mechanical strength and osteoinductivity of collagen scaffolds by the incorporating of CNTs. Kim et al. [13] investigated the mechanism of collagen–CNTs interaction and hypothesized CNTs could align along the side of the collagen molecules which would elongate the D-period and decrease the apex. Freeze drying method is widely used to fabricate collagen based composite scaffolds with SWCNT [100]. As expected, the tensile strength and the stress resistance of the collagen/SWCNT composite scaffold were significant higher than pure collagen scaffold. In addition, the bone-like apatite deposition ability and structure stability of the collagen scaffold could be improved after incorporation of SWCNTs. The study of Baktur et al. [101] indicated that the incorporation of MWCNTs into collagen could improve osteogenic differentiation of MSCs by increasing the expression of ALP.
2.2.2. Graphene oxide (GO)
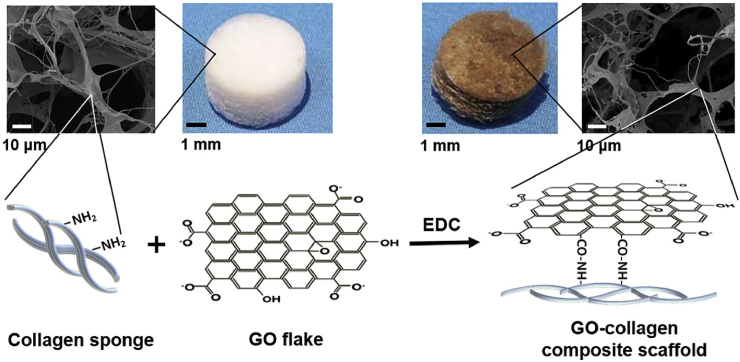
GO is a graphene monolayer with extraordinary elastic modulus, large surface area, great dispersibility and hydrophilicity [102]. It has been reported that GO can promote biological interactions by many surface functional groups such as carboxylic groups which exist at its edges and the phenol hydroxyl and epoxide groups located on its basal plane [103], [104]. Some reports demonstrated that different kinds of mesenchymal stem cells could strongly adhere and proliferate at a fast rate on GO. Moreover, GO could also augment differentiations of these cells, which including stimulating osteogenic differentiation of human hMSCs [105], [106], [107]. GO has been applied as the reinforcement agent in many composite. Moreover, GO has been previously reported to have cytotoxicity, but it did not cause cytotoxicity when incorporated into collagen scaffolds [108], [109]. As for collagen scaffold, GO could be used in a collagen-gelatin film for accelerating wound healing [104]. Inspired by this strategy, Kang et al. [109] fabricated GO-collagen (GO-COL) scaffold crosslinked by 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) (Fig. 4). Compared with pure collagen scaffold and collagen/GO scaffold without EDC, the elastic modulus of GO-COL scaffold was over double. Due to the increased FAK activation and cytoskeletal tension, the growth rate of hMSCs cultured on the GO-COL scaffold nearly doubled. Furthermore, the GO addition significantly promoted cellular osteogenic differentiation through the higher expression of ALP and OP. This was because of the increased focal adhesion formation and more active FAK and ERK pathways caused by the enhancive matrix stiffness [110], [111].
Fig. 4.
A schematic illustrating the covalent conjugation of the carboxyl groups of GO flakes to the amine groups of the collagen scaffolds.
2.3. Collagen based composite scaffolds with polymer components
2.3.1. Natural polymer components
Some naturally derived polymers such as glycosaminoglycans (GAGs) and silk fibroin are often used in collagen based composite scaffolds. GAGs, including chondroitin sulfate (CS), hyaluronan, heparin sulfate, dermatan sulfate, keratan sulfate, etc. are important multifunctional components of the ECM which can bind with growth factors and cytokines [112]. CS is a naturally sulfated GAG existing in cancellous and cortical bone. It can be used as coating material to improve the binding of osteoblasts and osteoclasts to the materials and attract growth factors onto materials and cell surfaces [113]. Hyaluronan is the only non-sulfated glycosaminoglycan and plays an important role in lubrication, water sorption and water retention [114], [115]. In bone, hyaluronan has the effect of recruiting osteoblast precursor cells from the bone marrow as well as regulating bone remodeling by controlling behaviors of osteoclast, osteoblast and osteocyte and being involved in the osteogenic differentiation of MSCs [116], [117]. Collagen/GAGs scaffolds have been widely investigated for various tissue engineering such as skin, peripheral nerve, tendon, and cartilage [118], [119], [120], [121]. It has been found that GAGs attached to the collagen fibrils can increase the proliferation and migration of cells [122]. In addition, the GAG has also been found to reduce the degradation rate and improve the structural stability of the COL/GAG matrix [122], [123]. There still exists a debate that whether the presence of GAGs could inhibit the hydroxyapatite crystal growth, but most studies denied this effect [124], [125], [126], [127], [128].
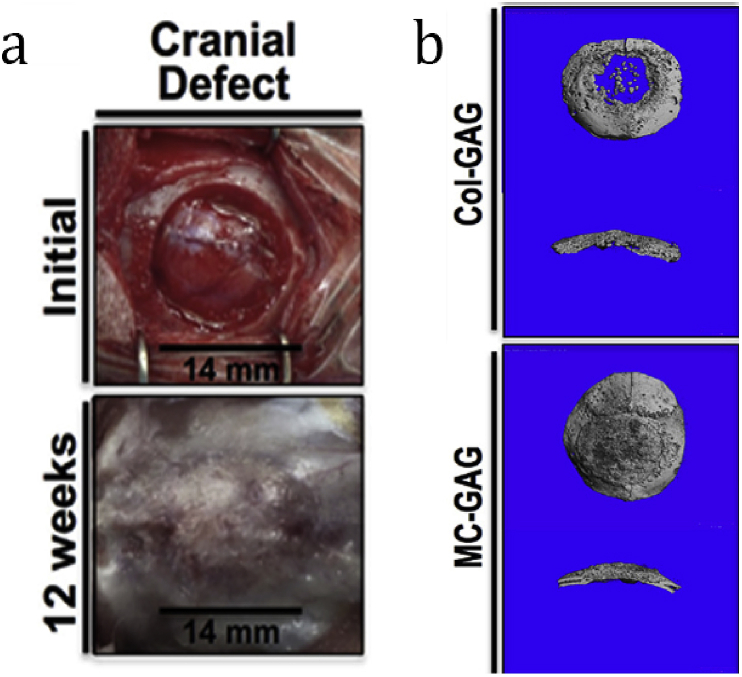
Farrell et al. [129] found COL-GAG (CG) scaffold could provide a suitable 3-D environment for the osteogenic differentiation of MSCs with the stimulating effect of osteogenic factors. Without addition of progenitor stem cells or exogenous growth factors, CG scaffolds can also be used for bone tissue regeneration [130], [131], [132]. Alhag et al. [128] reported the amount of new bone within the 5-mm rat calvarial defect was significantly higher in the cell-free CG scaffolds than that in the cell-seeded scaffolds, which was due to the inflammation reaction and premature scaffold degradation induced by MSC-seeded scaffolds. To achieve greater mechanical stiffness and the presence of the mineral phase, Ren et al. [133] fabricated nanoparticulate mineralized CG scaffold and implanted it in critical sized rabbit cranial defects. The result showed the defects healed after 12 weeks (Fig. 5a) and more mineralized content was observed in nanoparticulate mineralized scaffold (Fig. 5b).
Fig. 5.
a) Cranial defects with 14 mm in diameter were created in the parietal bones by a hand powered trephine. Initial defect before implantation (top) and 12 weeks after implantation of nanoparticulate mineralized collagen/GAG scaffolds (bottom) cranial defects are shown. b) Representative three-dimensional reconstructions of the CT scans of two kinds of scaffold with cross sections are shown [133].
As a model candidate material for tissue engineering scaffold, silk fibroin is mass-reproducible and possesses desirable mechanical properties. Wang et al. [134] fabricated the collagen-silk fibroin/hydroxyapatite (COL-SF/HA) scaffolds and implanted the BMSCs-seeded scaffolds into bone tunnels of Wistar male rat. The histological analysis results showed the percentage of new bone formation of COL-SF/HA scaffolds after 8 weeks of implantation was almost two times of the COL/HA scaffold, which indicated that silk fibroin can largely improve the osteogenesis ability of COL/HA scaffold.
2.3.2. Artificial polymer materials
Apart from these natural polymer materials mentioned above, there are a lot of biodegradable polymers such as polycaprolactone (PCL), poly (glycolic acid) (PGA), poly (lactic acid) (PLA), poly (vinyl alcohol), poly (ethylene terephthalate), etc. have been widely applied to fabricate collagen based scaffolds for bone regeneration [135], [136], [137], [138], [139], [140], [141]. These materials can be used as the base component of scaffolds alone just like collagen as well as the enforcement component of collagen scaffold. For example, Weisgerber et al. [142] prepared a multi-scale composite with the mineralized COL/glycosaminoglycan (CGCaP) scaffold and a PCL support frame. The CGCaP scaffold provided micron-scale porosity while the PCL support frame provided the millimeter-scale porosity. With the addition of PCL working as the load bearing component, the elastic modulu of CGCaP-PCL scaffold was 6000-fold in comparison of CGCaP scaffold and reached to 6.8 ± 0.4 MPa.
Similarly, Wang et al. [143] developed macroporous PCL framework by rapid prototyping technology and incorporated collagen scaffold with microporous networks. After the deposition of HA by biomimetic mineralization, HA-Col-PCL composite scaffold with unique nano−micro−macro hierarchical structure was formed. The composite construct with custom-made shape had similar compressive modulus similar to cancellous bone and outstanding osteogenesis to promote rapid bone regeneration in rabbit radius.
Hiraoka et al. [144] found that incorporation of PGA fiber allowed the compression modulus of collagen sponge to increase over six-fold with the weight ratio of collagen to PGA fiber at 0.2. Moreover, PGA incorporation could suppress the shrinkage of collagen sponge when submerged in SBF, therefore the number of mouse fibroblast L929 cells attached increased and cells could infiltrate more deeply. Due to the inflammatory response induced by the high level of PGA fiber, it is suggested the best collagen to PGA weight ratio is 0.8. Fujita et al. [138] reported the ALP activity of rat bone marrow stromal cells (RBMSCs) seeded on collagen/PGA scaffold irradiated by ultraviolet rays was higher than the composite scaffold without irradiation. Furthermore, the addition of basic fibroblast growth factor (bFGF) and dexamethasone (Dex) in primary medium could respectively promote the proliferation and differentiation of RBMSCs seeded on the composite scaffold.
Most artificial polymers are hydrophobic while collagen is hydrophilic, which lead to the weak interaction between them. To deal with this problem, Liu et al. [145] treated PLA fiber with diamine and glutaraldehyde to prepare surface-activated PLA fiber. The compression modulus of the collagen/surface-activated PLA scaffold was much higher than the collagen/untreated PLA scaffold, which was due to the chemically bonding between surface-activated PLA fiber and collagen macromolecule. Moreover, the hydrophilicity of the activated surface of PLA fiber also increased the cellular attachment and dispersion.
3. Conclusions and future perspectives
As we have mentioned above, collagen scaffolds can be modified by diverse materials in order to improve the mechanical and biological properties in bone regeneration field. As the basic component, collagen contribute to the biocompatibility, hydrophilicity, porosity, biodegradability and osteoconduction. Bioceramics working as the mineral component can improve the osteoinduction, osteointegration and mechanical properties. CNT, GO and polymers are expected to improve the mechanical properties and structural stability.
In recent years, aimed at achieving the synthetic property of multiple compound modifications, more and more researchers have begun to apply more than one material to combine with the collagen scaffold. In addition, there are a lot of scaffolds loading with various cells such as osteoblasts, MSCs, embryonic stem cells (ESCs), pre-osteoblasts (MC3T3), and adipose derived stem cells (ADSCs) as well as biofactors such as BMP, transforming growth factor beta (TGF-β), insulin-like growth factors (IGF), vascular endothelial growth factor (VEGF), insulin, platelet-derived growth factors (PDGF), fibroblast growth factors (FGF), bisphosphonates, denosumab and nucleic acids (DNA, mRNA), etc. being investigated for bone regeneration. The cells can be separated into osteoblasts, preosteoblasts and stem cells. The biofactors can be divided into chemical drugs, proteins (growth factors and cytokines), and nucleic acids, etc. These chemical drugs and proteins can promote osteogenesis and angiogenesis of the scaffold by many signal pathways. For example, TGF-β induces osteogenic differentiation via PI3K, SMAD 2/3 and RhoA pathways and FGF-2 and BMP-2 can provide synergistic effects of differentiating MSCs into osteoblasts by activating RAS/RAF/MEK/MAPK and SMAD pathways.
The injectable collagen hydrogel scaffolds are often used as the delivery platform because collagen hydrogel can swell without disintegrating and the hydrophilic biofactors can incorporated into the hydrophobic collagen molecules. The degradation rate of collagen composite scaffolds can be changed by altering the proportion of different components, thereby the release rate can be controlled. Unlike chemical drugs and proteins, ncleic acids can encode these growth factors that promote bone growth. The strategy of combining the tissue engineering scaffolds with gene therapy provides a powerful and useful tool to enhance the bone regenerative process and helps people to understand the biology of bone tissue regeneration.
Up to now, a variety of collagen composite scaffolds for bone regeneration have been investigated in vitro. But there still lack of thorough experiments in vivo to validate the utility of these scaffolds. Moreover, fabricating composite scaffold which can meet all the desired properties including porosity, pore size, biocompatibility, mechanical integrity, structure stability, osteoconductivity and osteoinductivity to achieve optimal bone regeneration is still a challenge. Besides, no method can be used to fibracate collagen scaffold which completely mimic the complex internal and external multi-level hierarchical structures of nature bone. With the development of bioprinting technology, gene therapy and biomimetic mineralization, the successful clinical use of composite collagen scaffold for bone regeneration is just around the corner.
Acknowledgements
The authors gratefully acknowledge the support of the National Natural Science Foundation of China (No. 81672134), Science and Technology Commission of Shanghai Municipality (No. 15441905300, 17510710800, 16DZ0503800).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Jingdi Chen, Email: ibptcjd@fzu.edu.cn.
Kaili Lin, Email: linkaili@tongji.edu.cn, lklecnu@aliyun.com.
References
- 1.Olszta M.J., Cheng X.G., Jee S.S., Kumar R., Kim Y.Y., Kaufman M.J., Douglas E.P., Gower L.B. Bone structure and formation: a new perspective. Mater. Sci. Eng. R Rep. 2007;58(3–5):77–116. [Google Scholar]
- 2.Ma Z.J., Yamaguchi M. Alternation in bone components with increasing age of newborn rats: role of zinc in bone growth. J. Bone Miner. Metabol. 2000;18(5):264–270. doi: 10.1007/pl00010640. [DOI] [PubMed] [Google Scholar]
- 3.Lin K., Zhou Y., Zhou Y., Qu H., Chen F., Zhu Y., Chang J. Biomimetic hydroxyapatite porous microspheres with co-substituted essential trace elements: surfactant-free hydrothermal synthesis, enhanced degradation and drug release. J. Mater. Chem. 2011;21(41):16558–16565. [Google Scholar]
- 4.Lin K., Chang J., Liu X., Chen L., Zhou Y. Synthesis of element-substituted hydroxyapatite with controllable morphology and chemical composition using calcium silicate as precursor. Crystengcomm. 2011;13(15):4850–4855. [Google Scholar]
- 5.Li H., Chang J. Bioactive silicate materials stimulate angiogenesis in fibroblast and endothelial cell co-culture system through paracrine effect. Acta Biomater. 2013;9(6):6981–6991. doi: 10.1016/j.actbio.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Itoh R., Suyama Y. Sodium excretion in relation to calcium and hydroxyproline excretion in a healthy Japanese population. Am. J. Clin. Nutr. 1996;63(5):735–740. doi: 10.1093/ajcn/63.5.735. [DOI] [PubMed] [Google Scholar]
- 7.Lin K., Liu P., Wei L., Zou Z., Zhang W., Qian Y., Shen Y., Chang J. Strontium substituted hydroxyapatite porous microspheres: surfactant-free hydrothermal synthesis, enhanced biological response and sustained drug release. Chem. Eng. J. 2013;222(15):49–59. [Google Scholar]
- 8.Saidak Z., Marie P.J. Strontium signaling: molecular mechanisms and therapeutic implications in osteoporosis. Pharmacol. Ther. 2012;136(2):216–226. doi: 10.1016/j.pharmthera.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Yang F., Yang D., Tu J., Zheng Q., Cai L., Wang L. Strontium enhances osteogenic differentiation of mesenchymal stem cells and in vivo bone formation by activating Wnt/catenin signaling. Stem Cells. 2011;29(6):981–991. doi: 10.1002/stem.646. [DOI] [PubMed] [Google Scholar]
- 10.Maier J.A.M., Bernardini D., Rayssiguier Y., Mazur A. High concentrations of magnesium modulate vascular endothelial cell behaviour in vitro. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2004;1689(1):6–12. doi: 10.1016/j.bbadis.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Kadler K.E., Baldock C., Bella J., Boothandford R.P. Collagens at a glance. J. Cell Sci. 2007;120(Pt 12):1955. doi: 10.1242/jcs.03453. [DOI] [PubMed] [Google Scholar]
- 12.Kukreti U., Belkoff S.M. Collagen fibril D-period may change as a function of strain and location in ligament. J. Biomech. 2000;33(12):1569–1574. doi: 10.1016/s0021-9290(00)00150-0. [DOI] [PubMed] [Google Scholar]
- 13.Kim T., Sridharan I., Zhu B., Orgel J., Rong W. Effect of CNT on collagen fiber structure, stiffness assembly kinetics and stem cell differentiation. Mater. Sci. Eng. C. 2015;49:281–289. doi: 10.1016/j.msec.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y., Luo D., Wang T. Hierarchical structures of bone and bioinspired bone tissue engineering. Small. 2016;12(34):4611–4632. doi: 10.1002/smll.201600626. [DOI] [PubMed] [Google Scholar]
- 15.Cölfen H. Biomineralization: a crystal-clear view. Nat. Mater. 2010;9(12):960. doi: 10.1038/nmat2911. [DOI] [PubMed] [Google Scholar]
- 16.Stewart A.J., Roberts S.J., Seawright E., Davey M.G., Fleming R.H., Farquharson C. The presence of PHOSPHO1 in matrix vesicles and its developmental expression prior to skeletal mineralization. Bone. 2006;39(5):1000. doi: 10.1016/j.bone.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 17.Anderson H.C., Garimella R., Tague S.E. The role of matrix vesicles in growth plate development and biomineralization. Front. Biosci. A J. Virtual Libr. 2005;10(1–3):822. doi: 10.2741/1576. [DOI] [PubMed] [Google Scholar]
- 18.Chai Y.C., Carlier A., Bolander J., Roberts S.J., Geris L., Schrooten J., Van O.H., Luyten F.P. Current views on calcium phosphate osteogenicity and the translation into effective bone regeneration strategies. Acta Biomater. 2012;8(11):3876–3887. doi: 10.1016/j.actbio.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Mochida Y., Parisuthiman D., Pornprasertsuk-Damrongsri S., Atsawasuwan P., Sricholpech M., Boskey A.L., Yamauchi M. Decorin modulates collagen matrix assembly and mineralization. Matrix Biol. J. Int. Soc. Matrix Biol. 2009;28(1):44–52. doi: 10.1016/j.matbio.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyer U., Meyer T., Vosshans J., Joos U. Decreased expression of osteocalcin and osteonectin in relation to high strains and decreased mineralization in mandibular distraction osteogenesis. J. Cranio Maxillofac. Surg. 1999;27(4):222–227. doi: 10.1016/s1010-5182(99)80033-x. [DOI] [PubMed] [Google Scholar]
- 21.H I. Why does bone matrix contain non-collagenous proteins? The possible roles of osteocalcin, osteonectin, osteopontin and bone sialoprotein in bone mineralisation and resorption. Cell Biol. Int. 1994;18(6):617–628. doi: 10.1006/cbir.1994.1088. [DOI] [PubMed] [Google Scholar]
- 22.Rosenthal A.K., Gohr C.M., Uzuki M., Masuda I. Osteopontin promotes pathologic mineralization in articular cartilage. Matrix Biol. 2007;26(2):96–105. doi: 10.1016/j.matbio.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monfoulet L., Malaval L., Aubin J.E., Rittling S.R., Gadeau A.P., Fricain J.-C., Chassande O. Bone sialoprotein, but not osteopontin, deficiency impairs the mineralization of regenerating bone during cortical defect healing. Bone. 2010;46(2):447–452. doi: 10.1016/j.bone.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Yunus B.R., Sampath Kumar T.S., Doble M. Design of biocomposite materials for bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2015;57:452. doi: 10.1016/j.msec.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Jahan K., Tabrizian M. Composite biopolymers for bone regeneration enhancement in bony defects. Biomater. Sci. 2015;4(1):25. doi: 10.1039/c5bm00163c. [DOI] [PubMed] [Google Scholar]
- 26.Giannoudis P.V., Einhorn T.A., Marsh D. Fracture healing: a harmony of optimal biology and optimal fixation? Inj. Int. J. Care Inj. 2007;38(4):S1–S2. doi: 10.1016/s0020-1383(08)70002-0. [DOI] [PubMed] [Google Scholar]
- 27.Giannoudis P.V., Einhorn T.A., Schmidmaier G., Marsh D. The diamond concept – open questions. Inj. Int. J. Care Inj. 2008;39(2):S5–S8. doi: 10.1016/S0020-1383(08)70010-X. [DOI] [PubMed] [Google Scholar]
- 28.Wang W., Yeung K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: a review. Bioact. Mater. 2017 doi: 10.1016/j.bioactmat.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chvapil M., Droegemueller W. Collagen sponge in gynecologic use. Obstet. Gynecol. Annu. 1981;10:363. [PubMed] [Google Scholar]
- 30.Sayin E., Rashid R.H., Rodríguez-Cabello J.C., Elsheikh A., Baran E.T., Hasirci V. Human adipose derived stem cells are superior to human osteoblasts (HOB) in bone tissue engineering on a collagen-fibroin-ELR blend. Bioact. Mater. 2017;2(2):71–81. doi: 10.1016/j.bioactmat.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mizuno M., Fujisawa R., Kuboki Y. Type I collagen-induced osteoblastic differentiation of bone-marrow cells mediated by collagen-alpha 2 beta 1 integrin interaction. J. Cell Physiol. 2000;184(2):207–213. doi: 10.1002/1097-4652(200008)184:2<207::AID-JCP8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 32.Otsuka M., Nakagawa H., Otsuka K., Ito A., Higuchi W.I. Effect of geometrical structure on the in vivo quality change of a three-dimensionally perforated porous bone cell scaffold made of apatite/collagen composite. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013;101(2):338. doi: 10.1002/jbm.b.32844. [DOI] [PubMed] [Google Scholar]
- 33.Jing Z., Wu Y., Su W., Tian M., Jiang W., Cao L., Zhao L., Zhao Z. Carbon nanotube reinforced collagen/hydroxyapatite scaffolds improve bone tissue formation in vitro and in vivo. Ann. Biomed. Eng. 2017:1–13. doi: 10.1007/s10439-017-1866-9. [DOI] [PubMed] [Google Scholar]
- 34.Fullana M.J., Wnek G.E. Electrospun collagen and its applications in regenerative medicine. Drug Deliv. Transl. Res. 2012;2(5):313–322. doi: 10.1007/s13346-012-0087-x. [DOI] [PubMed] [Google Scholar]
- 35.Ferreira A.M., Gentile P., Chiono V., Ciardelli G. Collagen for bone tissue regeneration. Acta Biomater. 2012;8(9):3191–3200. doi: 10.1016/j.actbio.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 36.Geiger M., Li R.H., Friess W. Collagen sponges for bone regeneration with rhBMP-2. Adv. Drug Deliv. Rev. 2003;55(12):1613. doi: 10.1016/j.addr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 37.Yunus B.R., Sampath Kumar T.S., Doble M. Design of biocomposite materials for bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2015;57:452–463. doi: 10.1016/j.msec.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 38.Xia L., Yin Z., Mao L., Wang X., Liu J., Jiang X., Zhang Z., Lin K., Chang J., Fang B. Akermanite bioceramics promote osteogenesis, angiogenesis and suppress osteoclastogenesis for osteoporotic bone regeneration. Sci. Rep. 2016;6:22005. doi: 10.1038/srep22005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kikuchi M., Ikoma T., Syoji D., Matsumoto H., Koyama Y., Itoh S., Takakuda K., Shinomiya K., Tanaka M. Porous body preparation of hydroxyapatite/collagen nanocomposites for bone tissue regeneration. Key Eng. Mater. 2003;254–256:561–564. [Google Scholar]
- 40.Yunoki S., Ikoma T., Monkawa A., Marukawa E., Sotome S., Shinomiya K., Tanaka J. Three-dimensional porous hydroxyapatite/collagen composite with rubber-like elasticity. Mater. Sci. Eng. C. 2007;18(4):393–409. doi: 10.1163/156856207780425077. [DOI] [PubMed] [Google Scholar]
- 41.Shen X., Chen L., Cai X., Tong T., Tong H., Hu J. A novel method for the fabrication of homogeneous hydroxyapatite/collagen nanocomposite and nanocomposite scaffold with hierarchical porosity. J. Mater. Sci. Mater. Med. 2011;22(2):299–305. doi: 10.1007/s10856-010-4199-x. [DOI] [PubMed] [Google Scholar]
- 42.Lickorish D., Ramshaw J.A.M., Werkmeister J.A., Glattauer V., Howlett C.R. Collagen-hydroxyapatite composite prepared by biomimetic process. J. Biomed. Mater Res. A. 2004;68(1):19–27. doi: 10.1002/jbm.a.20031. [DOI] [PubMed] [Google Scholar]
- 43.Cunniffe G.M., Dickson G.R., Partap S., Stanton K.T., O'Brien F.J. Development and characterisation of a collagen nano-hydroxyapatite composite scaffold for bone tissue engineering. J. Mater Sci. Mater Med. 2010;21(8):2293–2298. doi: 10.1007/s10856-009-3964-1. [DOI] [PubMed] [Google Scholar]
- 44.Andronescu E., Voicu G., Ficai M., Mohora I.A., Trusca R., Ficai A. Collagen/hydroxyapatite composite materials with desired ceramic properties. J. Electron Microsc. 2011;60(3):253–259. doi: 10.1093/jmicro/dfr010. [DOI] [PubMed] [Google Scholar]
- 45.Calabrese G., Giuffrida R., Fabbi C., Figallo E., Furno D.L., Gulino R., Colarossi C., Fullone F., Giuffrida R., Parenti R. Collagen-hydroxyapatite scaffolds induce human adipose derived stem cells osteogenic differentiation In vitro. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0151181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sionkowska A., Kozlowska J. Properties and modification of porous 3-D collagen/hydroxyapatite composites. Int. J. Biol. Macromol. 2013;52:250–259. doi: 10.1016/j.ijbiomac.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 47.Pasqui D., Torricelli P., De C.M., Fini M., Barbucci R. Carboxymethyl cellulose-hydroxyapatite hybrid hydrogel as a composite material for bone tissue engineering applications. J. Biomed. Mater. Res. Part A. 2014;102(5):1568–1579. doi: 10.1002/jbm.a.34810. [DOI] [PubMed] [Google Scholar]
- 48.Kunzler T.P., Drobek T., Schuler M., Spencer N.D. Systematic study of osteoblast and fibroblast response to roughness by means of surface-morphology gradients. Biomaterials. 2007;28(13):2175. doi: 10.1016/j.biomaterials.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 49.Wei G., Ma P.X. Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials. 2004;25(19):4749–4757. doi: 10.1016/j.biomaterials.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 50.Cui F.-Z., Li Y., Ge J. Self-assembly of mineralized collagen composites. Mater. Sci. Eng. R Rep. 2007;57(1):1–27. [Google Scholar]
- 51.Kikuchi M., Itoh S., Ichinose S., Shinomiya K., Tanaka J. Self-organization mechanism in a bone-like hydroxyapatite/collagen nanocomposite synthesized in vitro and its biological reaction in vivo. Biomaterials. 2001;22(13):1705–1711. doi: 10.1016/s0142-9612(00)00305-7. [DOI] [PubMed] [Google Scholar]
- 52.Sartori M., Pagani S., Ferrari A., Costa V., Carina V., Figallo E., Maltarello M.C., Martini L., Fini M., Giavaresi G. A new bi-layered scaffold for osteochondral tissue regeneration: In vitro and in vivo preclinical investigations. Mater. Sci. Eng. C. 2017;70(Part 1):101–111. doi: 10.1016/j.msec.2016.08.027. [DOI] [PubMed] [Google Scholar]
- 53.Calabrese G., Giuffrida R., Forte S., Salvatorelli L., Fabbi C., Figallo E., Gulisano M., Parenti R., Magro G., Colarossi C. Bone augmentation after ectopic implantation of a cell-free collagen-hydroxyapatite scaffold in the mouse. Sci. Rep. 2016;6:36399. doi: 10.1038/srep36399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Minardi S., Corradetti B., Taraballi F., Sandri M., Van E.J., Cabrera F.J., Weiner B.K., Tampieri A., Tasciotti E. Evaluation of the osteoinductive potential of a bio-inspired scaffold mimicking the osteogenic niche for bone augmentation. Biomaterials. 2015;62:128. doi: 10.1016/j.biomaterials.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 55.Yu W., Sun T.W., Qi C., Ding Z., Zhao H., Zhao S., Shi Z., Zhu Y.J., Chen D., He Y. Evaluation of zinc-doped mesoporous hydroxyapatite microspheres for the construction of a novel biomimetic scaffold optimized for bone augmentation. Int. J. Nanomed. 2017;12:2293. doi: 10.2147/IJN.S126505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Legeros R.Z. Calcium phosphates in oral biology and medicine. Monogr. Oral Sci. 1991;15(15):1. [PubMed] [Google Scholar]
- 57.Liao S., Ngiam M., Watari F., Ramakrishna S., Chan C.K. Systematic fabrication of nano-carbonated hydroxyapatite/collagen composites for biomimetic bone grafts. Bioinspir. Biomim. 2007;2(3):37. doi: 10.1088/1748-3182/2/3/001. [DOI] [PubMed] [Google Scholar]
- 58.Xia Z., Yu X., Jiang X., Brody H.D., Rowe D.W., Wei M. Fabrication and characterization of biomimetic collagen-apatite scaffolds with tunable structures for bone tissue engineering. Acta Biomater. 2013;9(7):7308–7319. doi: 10.1016/j.actbio.2013.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yunoki S., Ikoma T., Monkawa A., Ohta K., Kikuchi M., Sotome S., Shinomiya K., Tanaka J. Control of pore structure and mechanical property in hydroxyapatite/collagen composite using unidirectional ice growth. Mater. Lett. 2006;60(8):999–1002. [Google Scholar]
- 60.Lohfeld S., Cahill S., Barron V., McHugh P., Dürselen L., Kreja L., Bausewein C., Ignatius A. Fabrication, mechanical and in vivo performance of polycaprolactone/tricalcium phosphate composite scaffolds. Acta Biomater. 2012;8(9):3446–3456. doi: 10.1016/j.actbio.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 61.Arahira T., Todo M. Variation of mechanical behavior of β-TCP/collagen two phase composite scaffold with mesenchymal stem cell in vitro. J. Mech. Behav. Biomed. Mater. 2016;61:464. doi: 10.1016/j.jmbbm.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 62.Cao H., Kuboyama N. A biodegradable porous composite scaffold of PGA/β-TCP for bone tissue engineering. Bone. 2010;46(2):386–395. doi: 10.1016/j.bone.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 63.Arahira T., Todo M. Effects of proliferation and differentiation of mesenchymal stem cells on compressive mechanical behavior of collagen/β-TCP composite scaffold. J. Mech. Behav. Biomed. Mater. 2014;39(3):218. doi: 10.1016/j.jmbbm.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 64.Zheng H., Bai Y., Shih M.S., Hoffmann C., Peters F., Waldner C., Hübner W.D. Effect of a β-TCP collagen composite bone substitute on healing of drilled bone voids in the distal femoral condyle of rabbits. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014;102(2):376–383. doi: 10.1002/jbm.b.33016. [DOI] [PubMed] [Google Scholar]
- 65.Murakami S., Miyaji H., Nishida E., Kawamoto K., Miyata S., Takita H., Akasaka T., Fugetsu B., Iwanaga T., Hongo H., Amizuka N., Sugaya T., Kawanami M. Dose effects of beta-tricalcium phosphate nanoparticles on biocompatibility and bone conductive ability of three-dimensional collagen scaffolds. Dent. Mater J. 2017 doi: 10.4012/dmj.2016-295. [DOI] [PubMed] [Google Scholar]
- 66.Ebrahimi M., Botelho M.G., Dorozhkin S.V. Biphasic calcium phosphates bioceramics (HA/TCP): concept, physicochemical properties and the impact of standardization of study protocols in biomaterials research. Mater Sci. Eng. C Mater Biol. Appl. 2017;71:1293–1312. doi: 10.1016/j.msec.2016.11.039. [DOI] [PubMed] [Google Scholar]
- 67.Bosetti M., Cannas M. The effect of bioactive glasses on bone marrow stromal cells differentiation. Biomaterials. 2005;26(18):3873–3879. doi: 10.1016/j.biomaterials.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 68.Hench L.L. Bioactive glasses and glass-ceramics. Mater. Sci. Forum. 1998:155–179. [Google Scholar]
- 69.Xynos I.D., Edgar A.J., Buttery L.D., Hench L.L., Polak J.M. Gene-expression profiling of human osteoblasts following treatment with the ionic products of Bioglass 45S5 dissolution. J. Biomed. Mater Res. 2001;55(2):151. doi: 10.1002/1097-4636(200105)55:2<151::aid-jbm1001>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 70.Wu C., Chang J. A review of bioactive silicate ceramics. Biomed. Mater. 2013;8(3):032001. doi: 10.1088/1748-6041/8/3/032001. [DOI] [PubMed] [Google Scholar]
- 71.Leu A., Leach J.K. Proangiogenic potential of a collagen/bioactive glass substrate. Pharm. Res. 2008;25(5):1222–1229. doi: 10.1007/s11095-007-9508-9. [DOI] [PubMed] [Google Scholar]
- 72.Gorustovich A.A., Roether J.A., Boccaccini A.R. Effect of bioactive glasses on angiogenesis: a review of in vitro and in vivo evidences. Tissue Eng. Part B Rev. 2010;16(2):199. doi: 10.1089/ten.TEB.2009.0416. [DOI] [PubMed] [Google Scholar]
- 73.Izquierdobarba I., Arcos D., Sakamoto Y., Terasaki O., Lópeznoriega A., Valletregí M. High-performance mesoporous bioceramics mimicking bone mineralization. Chem. Mater. 2012;20(9):3191–3198. [Google Scholar]
- 74.Boccaccini A.R., Erol M., Stark W.J., Mohn D., Hong Z., Mano J.F. Polymer/bioactive glass nanocomposites for biomedical applications: a review. Compos. Sci. Technol. 2010;70(13):1764–1776. [Google Scholar]
- 75.Misra S.K., Mohn D., Brunner T.J., Stark W.J., Philip S.E., Roy I., Salih V., Knowles J.C., Boccaccini A.R. Comparison of nanoscale and microscale bioactive glass on the properties of P(3HB)/Bioglass composites. Biomaterials. 2008;29(12):1750–1761. doi: 10.1016/j.biomaterials.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 76.Hsu F.Y., Lu M.R., Weng R.C., Lin H.M. Hierarchically biomimetic scaffold of a collagen-mesoporous bioactive glass nanofiber composite for bone tissue engineering. Biomed. Mater. 2015;10(2):025007. doi: 10.1088/1748-6041/10/2/025007. [DOI] [PubMed] [Google Scholar]
- 77.Marelli B., Ghezzi C.E., Mohn D., Stark W.J., Barralet J.E., Boccaccini A.R., Nazhat S.N. Accelerated mineralization of dense collagen-nano bioactive glass hybrid gels increases scaffold stiffness and regulates osteoblastic function. Biomaterials. 2011;32(34):8915–8926. doi: 10.1016/j.biomaterials.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 78.Christodoulou I., Buttery L.D., Saravanapavan P., Tai G., Hench L.L., Polak J.M. Dose- and time-dependent effect of bioactive gel-glass ionic-dissolution products on human fetal osteoblast-specific gene expression. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005;74B(1):529–537. doi: 10.1002/jbm.b.30249. [DOI] [PubMed] [Google Scholar]
- 79.Christodoulou I., Tai B.G. Characterization of humen fetal osteoblasts by microarray analysis following stimulation with 58S bioactive gel-glass ionic dissolution products. J. Biomed. Mater Res. 2006;77(2):431–446. doi: 10.1002/jbm.b.30455. [DOI] [PubMed] [Google Scholar]
- 80.Griciuc A., Popescunegreanu T., Grecu R., Simon V. Corem composite matrix behaviour in simulated body fluid. J. Optoelectron. Adv. Mater. 2005:2827–2830. [Google Scholar]
- 81.Li X., Chang J. Preparation and characterization of bioactive collagen/wollastonite composite scaffolds. J. Mater. Sci. Mater. Med. 2005;16(4):361–365. doi: 10.1007/s10856-005-0636-7. [DOI] [PubMed] [Google Scholar]
- 82.Shen Y., Liu W., Lin K., Pan H., Darvell B.W., Peng S., Wen C., Deng L., Lu W.W., Chang J. Interfacial pH: a critical factor for osteoporotic bone regeneration. Langmuir Acs J. Surf. Colloids. 2011;27(6):2701–2708. doi: 10.1021/la104876w. [DOI] [PubMed] [Google Scholar]
- 83.Xu S., Lin K., Wang Z., Chang J., Wang L., Lu J., Ning C. Reconstruction of calvarial defect of rabbits using porous calcium silicate bioactive ceramics. Biomaterials. 2008;29(17):2588–2596. doi: 10.1016/j.biomaterials.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 84.Wang X., Zhou Y., Xia L., Zhao C., Chen L., Yi D., Chang J., Huang L., Zheng X., Zhu H. Fabrication of nano-structured calcium silicate coatings with enhanced stability, bioactivity and osteogenic and angiogenic activity. Colloids Surf. B Biointerfaces. 2015;126:358–366. doi: 10.1016/j.colsurfb.2014.11.044. [DOI] [PubMed] [Google Scholar]
- 85.Li H., Zhai W., Chang J. Effects of wollastonite on proliferation and differentiation of human bone marrow-derived stromal cells in PHBV/wollastonite composite scaffolds. J. Biomater. Appl. 2008;24(3):231–246. doi: 10.1177/0885328208096043. [DOI] [PubMed] [Google Scholar]
- 86.Zhao L., Chang J. Preparation and characterization of macroporous chitosan/wollastonite composite scaffolds for tissue engineering. J. Mater. Sci. Mater. Med. 2004;15(5):625–629. doi: 10.1023/b:jmsm.0000026103.44687.d0. [DOI] [PubMed] [Google Scholar]
- 87.Lin K., Yong L., Hai H., Lei C., Zhen W., Jiang C. Degradation and silicon excretion of the calcium silicate bioactive ceramics during bone regeneration using rabbit femur defect model. J. Mater. Sci. Mater. Med. 2015;26(6):1–8. doi: 10.1007/s10856-015-5523-2. [DOI] [PubMed] [Google Scholar]
- 88.Lin K., Chang J., Cheng R. In vitro hydroxyapatite forming ability and dissolution of tobermorite nanofibers. Acta Biomater. 2007;3(2):271–276. doi: 10.1016/j.actbio.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 89.Li H., Chang J. Stimulation of proangiogenesis by calcium silicate bioactive ceramic. Acta Biomater. 2013;9(2):5379–5389. doi: 10.1016/j.actbio.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 90.Wang C., Lin K., Chang J., Sun J. Osteogenesis and angiogenesis induced by porous β-CaSiO(3)/PDLGA composite scaffold via activation of AMPK/ERK1/2 and PI3K/Akt pathways. Biomaterials. 2013;34(1):64–77. doi: 10.1016/j.biomaterials.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 91.Lin K., Zhai W., Ni S., Chang J., Zeng Y., Qian W. Study of the mechanical property and in vitro biocompatibility of CaSiO ceramics. Ceram. Int. 2005;31(2):323–326. [Google Scholar]
- 92.Zhang Q., Nakamoto T., Chen S., Kawazoe N., Lin K., Chang J., Chen G. Collagen/wollastonite nanowire hybrid scaffolds promoting osteogenic differentiation and angiogenic factor expression of mesenchymal stem cells. J. Nanosci. Nanotechnol. 2014;14(4):3221–3227. doi: 10.1166/jnn.2014.8607. [DOI] [PubMed] [Google Scholar]
- 93.Ajayan P.M. Nanotubes from carbon. Chem. Rev. 1999;99(7):1787. doi: 10.1021/cr970102g. [DOI] [PubMed] [Google Scholar]
- 94.Ruoff R.S., Lorents D.C. Mechanical and thermal properties of carbon nanotubes. Carbon. 1995;33(7):925–930. [Google Scholar]
- 95.Miyagawa H., Misra M., Mohanty A.K. Mechanical properties of carbon nanotubes and their polymer nanocomposites. J. Nanosci. Nanotechnol. 2005;5(10):1593–1615. doi: 10.1166/jnn.2005.181. [DOI] [PubMed] [Google Scholar]
- 96.Macdonald R., Laurenzi B., Ajayan G.P., Stegemann J. Collagen-carbon nanotube composite materials as scaffolds in tissue engineering. J. Biomed. Mater. Res. Part A. 2005;74A(3):489–496. doi: 10.1002/jbm.a.30386. [DOI] [PubMed] [Google Scholar]
- 97.Price R.L., Waid M.C., Haberstroh K.M., Webster T.J. Selective bone cell adhesion on formulations containing carbon nanofibers. Biomaterials. 2003;24(11):1877. doi: 10.1016/s0142-9612(02)00609-9. [DOI] [PubMed] [Google Scholar]
- 98.Zanello L.P., Zhao B., Hu H., Haddon R.C. Bone cell proliferation on carbon nanotubes. Nano Lett. 2006;6(3):562. doi: 10.1021/nl051861e. [DOI] [PubMed] [Google Scholar]
- 99.Aoki N., Yokoyama A., Nodasaka Y., Akasaka T., Uo M., Sato Y., Tohji K., Watari F. Cell culture on a carbon nanotube scaffold. J. Biomed. Nanotechnol. 2005;1(4):402–405. [Google Scholar]
- 100.Usui Y., Aoki K., Narita N., Murakami N., Nakamura I., Nakamura K., Ishigaki N., Yamazaki H., Horiuchi H., Kato H. Carbon nanotubes with high bone-tissue compatibility and bone-formation acceleration effects. Small. 2008;4(2):240–246. doi: 10.1002/smll.200700670. [DOI] [PubMed] [Google Scholar]
- 101.Silva E.E.D., Colleta H.H.M.D., Ferlauto A.S., Moreira R.L., Resende R.R., Oliveira S., Kitten G.T., Lacerda R.G., Ladeira L.O. Nanostructured 3-D collagen/nanotube biocomposites for future bone regeneration scaffolds. Nano Res. 2009;2(6):462–473. [Google Scholar]
- 102.Baktur R., Yoon S.H., Kwon S. Effects of multiwalled carbon nanotube reinforced collagen scaffolds on the osteogenic differentiation of mesenchymal stem cells. J. Nanomater. 2013,(2013-11-24) 2013;2013(3):7. [Google Scholar]
- 103.Gao W. The chemistry of graphene oxide. Graphene Oxide. 2015:61–95. [Google Scholar]
- 104.Zhu Y., Murali S., Cai W., Li X., Suk J.W., Potts J.R., Ruoff R.S. Graphene and graphene oxide: synthesis, properties, and applications. Adv. Mater. 2010;22(35):3906–3924. doi: 10.1002/adma.201001068. [DOI] [PubMed] [Google Scholar]
- 105.Mitra T., Manna P.J., Raja S.T.K., Gnanamani A., Kundu P.P. Curcumin loaded nano graphene oxide reinforced fish scale collagen – a 3D scaffold biomaterial for wound healing applications. Rsc Adv. 2015;5(119):98653–98665. [Google Scholar]
- 106.Chen G.Y., Pang D.W.P., Hwang S.M., Tuan H.Y., Hu Y.C. A graphene-based platform for induced pluripotent stem cells culture and differentiation. Biomaterials. 2012;33(2):418–427. doi: 10.1016/j.biomaterials.2011.09.071. [DOI] [PubMed] [Google Scholar]
- 107.Nayak T.R., Andersen H., Makam V.S., Khaw C., Bae S., Xu X., Ee P.-L.R., Ahn J.-H., Hong B.H., Pastorin G., Özyilmaz B. Graphene for controlled and accelerated osteogenic differentiation of human mesenchymal stem cells. ACS Nano. 2011;5(6):4670–4678. doi: 10.1021/nn200500h. [DOI] [PubMed] [Google Scholar]
- 108.Lee W.C., Lim C.H.Y.X., Shi H., Tang L.A.L., Wang Y., Lim C.T., Loh K.P. Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide. ACS Nano. 2011;5(9):7334–7341. doi: 10.1021/nn202190c. [DOI] [PubMed] [Google Scholar]
- 109.Wang A., Pu K., Dong B., Liu Y., Zhang L., Zhang Z., Duan W., Zhu Y. Role of surface charge and oxidative stress in cytotoxicity and genotoxicity of graphene oxide towards human lung fibroblast cells. J. Appl. Toxicol. Jat. 2013;33(10):1156–1164. doi: 10.1002/jat.2877. [DOI] [PubMed] [Google Scholar]
- 110.Kang S., Park J.B., Lee T.J., Ryu S., Bhang S.H., La W.G., Noh M.K., Hong B.H., Kim B.S. Covalent conjugation of mechanically stiff graphene oxide flakes to three-dimensional collagen scaffolds for osteogenic differentiation of human mesenchymal stem cells. Carbon. 2015;83:162–172. [Google Scholar]
- 111.Ge C., Xiao G., Jiang D., Franceschi R.T. Critical role of the extracellular signal–regulated kinase–MAPK pathway in osteoblast differentiation and skeletal development. J. Cell Biol. 2007;176(5):709. doi: 10.1083/jcb.200610046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Namgung S., Baik K.Y., Park J., Hong S. Controlling the growth and differentiation of human mesenchymal stem cells by the arrangement of individual carbon nanotubes. ACS Nano. 2011;5(9):7383–7390. doi: 10.1021/nn2023057. [DOI] [PubMed] [Google Scholar]
- 113.Macri L., Silverstein D., Clark R.A.F. Growth factor binding to the pericellular matrix and its importance in tissue engineering. Adv. Drug Deliv. Rev. 2007;59(13):1366–1381. doi: 10.1016/j.addr.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 114.Ruoslahti E., Yamaguchi Y. Proteoglycans as modulators of growth factor activities. Cell. 1991;64(5):867–869. doi: 10.1016/0092-8674(91)90308-l. [DOI] [PubMed] [Google Scholar]
- 115.Segura T., Anderson B.C., Chung P.H., Webber R.E., Shull K.R., Shea L.D. Crosslinked hyaluronic acid hydrogels: a strategy to functionalize and pattern. Biomaterials. 2005;26(4):359–371. doi: 10.1016/j.biomaterials.2004.02.067. [DOI] [PubMed] [Google Scholar]
- 116.Zhou Z., Yang Z., Huang T., Liu L., Liu Q., Zhao Y., Zeng W., Yi Q., Cao D. Effect of chemical cross-linking on properties of gelatin/hyaluronic acid composite hydrogels. Polym. - Plast. Technol. Eng. 2013;52(1):45–50. [Google Scholar]
- 117.Bastow E.R., Byers S., Golub S.B., Clarkin C.E., Pitsillides A.A., Fosang A.J. Hyaluronan synthesis and degradation in cartilage and bone. Cell. Mol. Life Sci. 2008;65(3):395–413. doi: 10.1007/s00018-007-7360-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Astachov L., Vago R., Aviv M., Nevo Z. Hyaluronan and mesenchymal stem cells: from germ layer to cartilage and bone. Front. Biosci. 2011;16(1):261–276. doi: 10.2741/3687. [DOI] [PubMed] [Google Scholar]
- 119.Chamberlain L.J., Yannas I.V., Hsu H.P., Strichartz G., Spector M. Collagen-GAG substrate enhances the quality of nerve regeneration through collagen tubes up to Level of autograft. Exp. Neurol. 1998;154(2):315–329. doi: 10.1006/exnr.1998.6955. [DOI] [PubMed] [Google Scholar]
- 120.Yannas I.V., Lee E., Orgill D.P., Skrabut E.M., Murphy G.F. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc. Natl. Acad. Sci. U. S. A. 1989;86(3):933. doi: 10.1073/pnas.86.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Levingstone T.J., Thompson E., Matsiko A., Schepens A., Gleeson J.P., O'Brien F.J. Multi-layered collagen-based scaffolds for osteochondral defect repair in rabbits. Acta Biomater. 2016;32:149. doi: 10.1016/j.actbio.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 122.Caliari S.R., Weisgerber D.W., Ramirez M.A., Kelkhoff D.O., Harley B.A. The influence of collagen-glycosaminoglycan scaffold relative density and microstructural anisotropy on tenocyte bioactivity and transcriptomic stability. J. Mech. Behav. Biomed. Mater. 2012;11(7):27. doi: 10.1016/j.jmbbm.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pieper J.S., van Wachem P.B., Mja V.L., Brouwer L.A., Hafmans T., Veerkamp J.H., van Kuppevelt T.H. Attachment of glycosaminoglycans to collagenous matrices modulates the tissue response in rats. Biomaterials. 2000;21(16):1689. doi: 10.1016/s0142-9612(00)00052-1. [DOI] [PubMed] [Google Scholar]
- 124.Pieper J., Oosterhof A., Veerkamp Pj, J., Van K.T. Preparation and characterization of porous crosslinked collagenous matrices containing bioavailable chondroitin sulphate. Biomaterials. 1999;20(9):847–858. doi: 10.1016/s0142-9612(98)00240-3. [DOI] [PubMed] [Google Scholar]
- 125.Rees S.G., Hughes W.D.S.R., Embery G. Effect of serum albumin on glycosaminoglycan inhibition of hydroxyapatite formation. Biomaterials. 2004;25(6):971–977. doi: 10.1016/s0142-9612(03)00618-5. [DOI] [PubMed] [Google Scholar]
- 126.Balasundaram G., Sato M., Webster T. Using hydroxyapatite nanoparticles and decreased crystallinity to promote osteoblast adhesion similar to functionalizing with RGD. Biomaterials. 2006;27(14):2798. doi: 10.1016/j.biomaterials.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 127.Rees S.G., Shellis R.P., Embery G. Inhibition of hydroxyapatite crystal growth by bone proteoglycans and proteoglycan components. Biochem. Biophys. Res. Commun. 2002;292(3):727–733. doi: 10.1006/bbrc.2002.6699. [DOI] [PubMed] [Google Scholar]
- 128.Tierney C.M., Jaasma M.J., O'Brien F.J. Osteoblast activity on collagen-GAG scaffolds is affected by collagen and GAG concentrations. J. Biomed. Mater. Res. Part A. 2009;91A(1):92–101. doi: 10.1002/jbm.a.32207. [DOI] [PubMed] [Google Scholar]
- 129.Alhag M., Farrell E., Toner M., Lee T.C., O'Brien F.J., Claffey N. Evaluation of the ability of collagen–glycosaminoglycan scaffolds with or without mesenchymal stem cells to heal bone defects in Wistar rats. Oral Maxillofac. Surg. 2012;16(1):47–55. doi: 10.1007/s10006-011-0299-0. [DOI] [PubMed] [Google Scholar]
- 130.Farrell E., O'Brien F.J., Doyle P., Fischer J., Yannas I., Harley B.A., O'Connell B., Prendergast P.J., Campbell V.A. A collagen-glycosaminoglycan scaffold supports adult rat mesenchymal stem cell differentiation along osteogenic and chondrogenic routes. Tissue Eng. 2006;12(3):459. doi: 10.1089/ten.2006.12.459. [DOI] [PubMed] [Google Scholar]
- 131.Kruger E.A., Im D.D., Bischoff D.S., Pereira C.T., Huang W., Rudkin G.H., Yamaguchi D.T., Miller T.A. In vitro mineralization of human mesenchymal stem cells on three-dimensional type I collagen versus PLGA scaffolds: a comparative analysis. Plast. Reconstr. Surg. 2011;127(6):2301–2311. doi: 10.1097/PRS.0b013e318213a004. [DOI] [PubMed] [Google Scholar]
- 132.Ren X., Bischoff D., Weisgerber D.W., Lewis M.S., Tu V., Yamaguchi D.T., Miller T.A., Harley B.A.C., Lee J.C. Osteogenesis on nanoparticulate mineralized collagen scaffolds via autogenous activation of the canonical BMP receptor signaling pathway. Biomaterials. 2015;50:107–114. doi: 10.1016/j.biomaterials.2015.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lee J.C., Pereira C.T., Ren X., Huang W., Bischoff D., Weisgerber D.W., Yamaguchi D.T., Harley B.A., Miller T.A. Optimizing collagen scaffolds for bone engineering: effects of cross-linking and mineral content on structural contraction and osteogenesis. J. Craniofac. Surg. 2015;26(6):1992–1996. doi: 10.1097/SCS.0000000000001918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ren X., Tu V., Bischoff D., Weisgerber D.W., Lewis M.S., Yamaguchi D.T., Miller T.A., Harley B.A., Lee J.C. Nanoparticulate mineralized collagen scaffolds induce in vivo bone regeneration independent of progenitor cell loading or exogenous growth factor stimulation. Biomaterials. 2016;89:67. doi: 10.1016/j.biomaterials.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang J., Yang Q., Mao C., Zhang S. Osteogenic differentiation of bone marrow mesenchymal stem cells on the collagen/silk fibroin bi-template-induced biomimetic bone substitutes. J. Biomed. Mater. Res. Part A. 2012;100(11):2929. doi: 10.1002/jbm.a.34236. [DOI] [PubMed] [Google Scholar]
- 136.Degirmenbasi N., Kalyon D.M., Birinci E. Biocomposites of nanohydroxyapatite with collagen and poly(vinyl alcohol) Colloids Surf. B Biointerfaces. 2006;48(1):42–49. doi: 10.1016/j.colsurfb.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 137.Tang Z.B., Cao J.K., Wen N., Wang H.B., Zhang Z.W., Liu Z.Q., Zhou J., Duan C.M., Cui F.Z., Wang C.Y. Posterolateral spinal fusion with nano-hydroxyapatite-collagen/PLA composite and autologous adipose-derived mesenchymal stem cells in a rabbit model. J. Tissue Eng. Regen. Med. 2012;6(4):325–336. doi: 10.1002/term.445. [DOI] [PubMed] [Google Scholar]
- 138.Takamoto T., Hiraoka Y., Tabata Y. Enhanced proliferation and osteogenic differentiation of rat mesenchymal stem cells in collagen sponge reinforced with different poly(ethylene terephthalate) fibers. J. Biomater. Sci. Polym. Ed. 2007;18(7):865–881. doi: 10.1163/156856207781367738. [DOI] [PubMed] [Google Scholar]
- 139.Fujita M., Kinoshita Y., Sato E., Maeda H., Ozono S., Negishi H., Kawase T., Hiraoka Y., Takamoto T., Tabata Y. Proliferation and differentiation of rat bone marrow stromal cells on poly(glycolic acid)-collagen sponge. Tissue Eng. 2005;11(9–10):1346. doi: 10.1089/ten.2005.11.1346. [DOI] [PubMed] [Google Scholar]
- 140.Ghezzi C.E., Marelli B., Donelli I., Alessandrino A., Freddi G., Nazhat S.N. Multilayered dense collagen-silk fibroin hybrid: a platform for mesenchymal stem cell differentiation towards chondrogenic and osteogenic lineages. J. Tissue Eng. Regen. Med. 2017;11(7):2046–2059. doi: 10.1002/term.2100. [DOI] [PubMed] [Google Scholar]
- 141.Wu J., Hong Y. Enhancing cell infiltration of electrospun fibrous scaffolds in tissue regeneration. Bioact. Mater. 2016;1(1):56–64. doi: 10.1016/j.bioactmat.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Stratton S., Shelke N.B., Hoshino K., Rudraiah S., Kumbar S.G. Bioactive polymeric scaffolds for tissue engineering. Bioact. Mater. 2016;1(2):93–108. doi: 10.1016/j.bioactmat.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Weisgerber D.W., Erning K., Flanagan C.L., Hollister S.J., Harley B.A. Evaluation of multi-scale mineralized collagen-polycaprolactone composites for bone tissue engineering. J. Mech. Behav. Biomed. Mater. 2016;61:318–327. doi: 10.1016/j.jmbbm.2016.03.032. [DOI] [PubMed] [Google Scholar]
- 144.Wang J., Wu D., Zhang Z., Li J., Shen Y., Wang Z., Li Y., Zhang Z., Sun J. Biomimetically ornamented rapid prototyping fabrication of an apatite-collagen-polycaprolactone composite construct with nano-micro-macro hierarchical structure for large bone defect treatment. ACS Appl. Mater Interfaces. 2015;7(47):26244–26256. doi: 10.1021/acsami.5b08534. [DOI] [PubMed] [Google Scholar]
- 145.Hiraoka Y., Kimura Y.H., Tabata Y. Fabrication and biocompatibility of collagen sponge reinforced with poly(glycolic acid) fiber. Tissue Eng. 2003;9(6):1101–1112. doi: 10.1089/10763270360728017. [DOI] [PubMed] [Google Scholar]