Abstract
High purity Mg was successfully coated on polyetheretherketone (PEEK) by vapor deposition method in order to improve the bioactivity including antibacterial property of PEEK implant. The morphology and elemental composition of the coating were characterized by scanning electron microscopy (SEM) and energy-dispersive spectroscopy (EDS), showing that the coating was mainly composed of Mg at deposition temperature of 175 °C, 185 °C, 200 °C and 230 °C. The higher the substrate temperature was, the larger the Mg particle size was. The coating degraded and gradually peeled off from the surface of PEEK after up to 21 days' immersion. It was found that the degradation of Mg coating could strongly kill Staphylococcus aureus with antibacterial rate reaching to 99%. Mg can be expected to be coated on those bio-inert biomaterials to offer specific bioactivities.
Keywords: PEEK, Mg, Coating, Vapor deposition, Antibacterial activity
Highlights
-
•
High purity Mg was coated on PEEK by vapor deposition method.
-
•
The Mg coating degraded and gradually peeled off from the surface of PEEK after up to 21 days immersion.
-
•
The degradation of Mg coating could strongly kill Staphylococcus aureus with antibacterial rate reaching to 99%.
1. Introduction
Polyetheretherketone (PEEK) with reasonable biocompatibility and suitable Young's modulus to human bone is regarded as one of the most potential candidates for spinal, trauma, oral implantology and orthopedic applications [[1], [2], [3], [4]]. However, PEEK is a kind of bioinert material which induces bad bonding with peripheral tissue, resulting in aseptic loosening even implantation failure [5]. Therefore, some bioactive materials such as HA are usually utilized to form PEEK composites in order to improve their bioactivity [[6], [7], [8], [9]]. Nevertheless, the weak physical bonding between HA and PEEK causes compromised mechanical properties. Meanwhile, various biocompatible materials such as TiO2 [10], Ti [11], Ta [12]and carbon [13] were also employed by plasma treatment [14,15] to improve the biocompatibility. In addition, as serious postoperative complications, bacterial infection after implantation has become an important problem which needs to be solved.
Magnesium (Mg) as a novel biodegradable metal is breaking the current knowledge of corrosion resistant metallic biomaterials. The current application of biodegradable implants is to support the tissue regeneration and heal the specific trauma during degradation. Nowadays, Mg based metals have become most promising candidates for medical applications owing to their character of biodegradation [[16], [17], [18]], and biofunctions of anti-tumor [[19], [20], [21]], antimicrobial [[22], [23], [24], [25], [26]], osteogenesis inductivity [[27], [28], [29], [30]] and others [[31], [32], [33]]. However, the relative low strength has restricted their application in load-bearing position. Mg based metals in form of bulk have been widely investigated, while the study of Mg coating is relatively rare. Since the degradation behavior of Mg is dramatically affected by galvanic corrosion and high purity magnesium possesses superior corrosion resistance, pure Mg was deposited on Mg alloy to improve the corrosion resistance [34,35]. The biological performance is reflected through surface of materials. Therefore, bioactivity of surface becomes very important for biomaterials. As a kind of bioactive material, pure Mg can be coated on the bioinert materials to improve their surface bioactivity. High purity Mg film was deposited on the oxidized Si wafer by physical vapor deposition technique, and both in vitro and in vivo tests demonstrated acceptable foreign body reaction [36]. Li et al. [37] reported that Mg was coated on porous Ti6Al4V scaffolds by arc ion plating, which made them showing better osteogenic properties than that the porous Ti6Al4V scaffolds.
In this study, high purity Mg was deposited on PEEK substrate, and then surface coating was characterized, which would be a new way to improve the bioactivity of PEEK for medical application.
2. Experimental
2.1. Material and coating
A high purity (99.99 wt%) Mg block was employed as evaporation resource. Mg block was placed in a ceramic crucible located inside the furnace at temperature of 175 °C, 185 °C, 200 °C and 230 °C. A commercial PEEK substrate was cut into size of Ф160 mm × 2 mm. The surface was grounded up to 2000 grit sand paper, and then mirror polished with 1 μm diamond grinding paste. The substrates were cleaned ultrasonically in acetone and deionized water, dried by flow N2 gas, then the substrates were placed in the deposition zone at temperature of 150 °C. The working pressure was controlled at about 1Pa. The generated Mg vapor was spontaneously condensed on the surface of the substrate due to the temperature gradient. Scanning electronic microscopy (SEM) was employed to investigate the surface morphology of the coating.
2.2. Immersion test
The Mg coated PEEK samples were subjected to an immersion test in Hank's solution at 37 ± 0.5 °C with an extraction ratio of 1.25 cm2/mL up to 21 days. The pH values of the Hank's solution were monitored and the Hank's solution was refreshed every day.
2.3. Antibacterial test
Each experimental sample was placed in a centrifuge tube immerging in a (1–2) × 105 cfu/mL Staphylococcus aureus (S. aureus) suspension with a ratio of 1.25 cm2/mL, and then samples were incubated at 37 ± 0.5 °C for 6 h, 12 h and 24 h, respectively. The co-cultured bacterial suspensions were diluted to (1–2) × 103 cfu/mL. 0.1 mL of the diluted bacterial suspension was added onto the nutrition agar plate and spread evenly, which was incubated again at 37 ± 0.5 °C for another 24 h, and then the bacterial colonies were counted. The antibacterial rate was determined by the following equation:
| C (%) = (A−B)/A × 100% | (1) |
where C is the antibacterial rate, A is the number of bacterial colonies for the uncoated PEEK sample, and B denotes the number of bacterial colonies for the Mg coated PEEK sample.
3. Results and discussion
3.1. Surface morphology
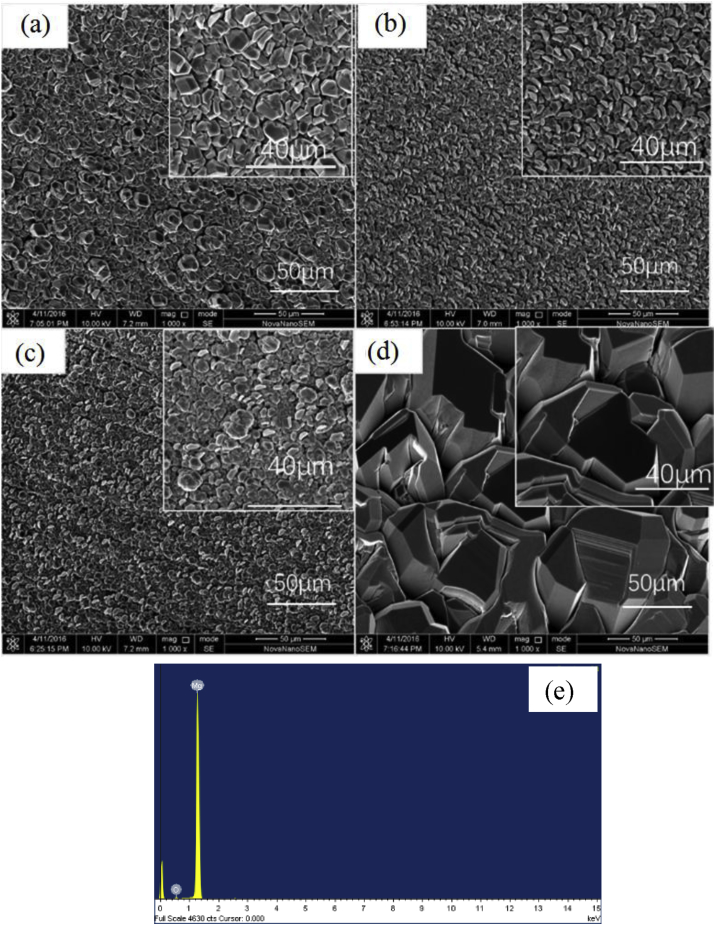
The surface morphologies of Mg coating on PEEK substrate at different temperature of 175 °C, 185 °C, 200 °C and 230 °C are shown in Fig. 1 showing that the PEEK surface was fully covered by Mg particles. It can be seen that the coating is uniform and dense. The EDS analysis showed that the coating was pure Mg (Fig. 1e). The size of particle was changed with the temperature of substrate. The particle size was 5–15 μm on the substrate at 175 °C. Increasing the temperature to 200 °C, there was no obvious change on the particle size. However, the particle size was increased to 30–50 μm when the substrate temperature reached to 230 °C. Atsushi et al. [38] obtained a similar result. The different particle size could affect the degradation rate of Mg coating.
Fig. 1.
Surface morphologies of Mg coating fabricated at different temperatures, (a) 175 °C; (b) 185 °C; (c) 200 °C; (d) 230 °C; (e) EDS analysis.
3.2. Immersion test
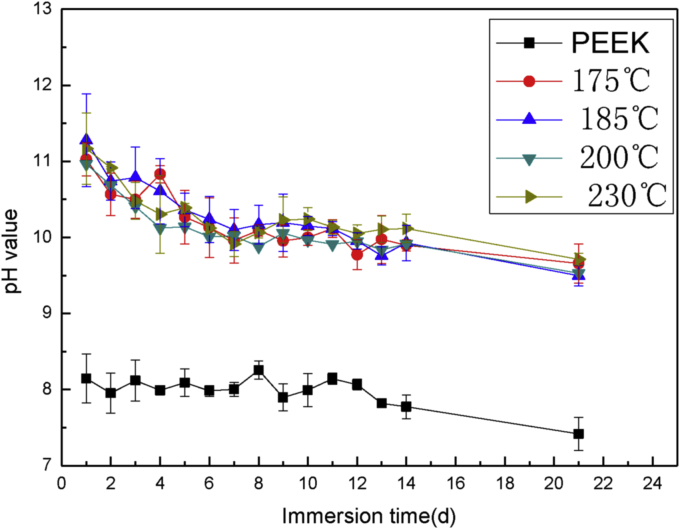
Fig. 2 shows the pH changes of Hanks' solution immersed with Mg coated samples at different temperatures. Higher pH value represents higher degradation rate. At the first day, the pH rapidly reached to about 11 for all the samples. The pH slowly decreased down to 10 from day 2 to day 7, and then was steady at around 10. After 14 days, the coating began to peel off from the surface of PEEK, and pH dropped to 9.5. From day 14 to day 21, the Mg coating broke into pieces. The Mg coating completely vanished after 21 days. At day 1, the pH of coating deposited at 230 °C and 185 °C was higher than that of other samples. The Mg particles deposited at 230 °C were larger and the distance between particles was also larger, as shown in Fig. 1d, thus the contact area with solution was large, which increased the degradation rate. For the sample deposited at 185 °C, particles were small but the surface was relatively loose, so it was easy to degrade. The drop of pH was derived from the protection of degradation product which decreased the degradation rate of Mg coating. The surface of the sample deposited at 200 °C was more dense than the others, so the pH was the lowest. From day 4, the pH of sample deposited at 230 °C was steady at around 10 and it was the last one to completely peel off, so 230 °C was an optimal deposition temperature for the relatively long time implant.
Fig. 2.
pH changes of Hanks' solution immersed with Mg coated samples at different temperatures.
Compared with the Mg coated Ti6Al4V alloy by arc ion plating method [37], the degradation period of Mg coating on PEEK was obviously longer. The pH of Mg coated Ti6Al4V alloy rapidly dropped to 8 at day 4, while the pH of Mg coated PEEK was still around 10 on the last stage. Besides the different intrinsic morphology for different deposition, galvanic corrosion would not take place for the Mg coated PEEK, so the degradation rate was much lower.
3.3. Antibacterial activity
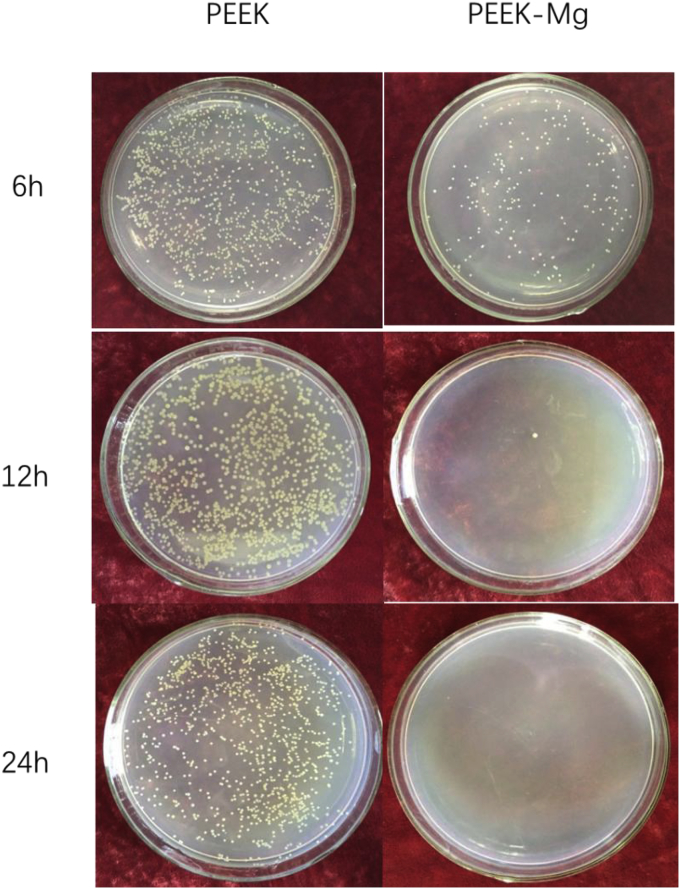
Over fast degradation rate is still the main problem of Mg. Therefore, the sample with the deposition temperature of 230 °C which possess relative good anticorrosion property is adopted for antibacterial test. Fig. 3 shows the antibacterial effects of PEEK with and without Mg coating co-cultured with S. aureus at 37 °C for 6 h, 12 h and 24 h. After co-culturing for 6 h, there was much bacteria on the medium cultured with uncoated PEEK, while there was less bacterial colony for the Mg coated PEEK. After co-culturing for 12 h, the number of bacterial colonies was increasing, while there was only one bacterial colony for the Mg coated sample. As for 24 h, there was no obvious change for PEEK, and there was no bacterial for Mg coated PEEK. PEEK is a bio-inert material without antibacterial function. With the degradation of Mg coating, the strong alkaline environment could kill most bacteria. The antibacterial rate Mg coated sample was 78% for 6 h. When co-cultured for 12 h and 24 h, the antibacterial rate reached to 99%. At the whole degradation period of Mg coating, the pH was kept at relatively high level. Therefore, the antibacterial activity would be maintained until the complete degradation of Mg coating.
Fig. 3.
Antibacterial effects of PEEK with and without Mg coating co-cultured with S. aureus at 37 °C for 6 h, 12 h and 24 h
4. Conclusion
In this preliminary study, pure Mg was coated on polyetheretherketone (PEEK) substrate by vapor deposition method. The optimal substrate temperature was 230 °C, and the Mg coating on PEEK would sustain at least for 14 days in Hank's solution. Mg coated PEEK exhibited excellent antibacterial property with antibacterial rate reaching to 99%. By vapor deposition method, biofunctional Mg may be coated on various bio-inert medical materials to improve their bioactivities.
Acknowledgements
The authors gratefully acknowledge the financial support from Key Program of China on Biomedical Materials Research and Tissue and Organ Replacement (2016YFC1101804); National Natural Science Foundation of China (51631009; 81500897; 81400528). The deposition system was donated by Shenyang Kejing Auto-Instrument Co., Ltd.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Lili Tan, Email: lltan@imr.ac.cn.
Ke Yang, Email: kyang@imr.ac.cn.
References
- 1.Kurtz S.M., Devine J.N. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials. 2007;28:4845–4869. doi: 10.1016/j.biomaterials.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Panayotov I.V., Orti V., Cuisinier F., Yachouh J. Polyetheretherketone (PEEK) for medical applications. J. Mater. Sci. Mater. Med. 2016;27 doi: 10.1007/s10856-016-5731-4. [DOI] [PubMed] [Google Scholar]
- 3.Najeeb S., Zafar M.S., Khurshid Z., Siddiqui F. Applications of polyetheretherketone (PEEK) in oral implantology and prosthodontics. J. Prosthodont. Res. 2016;60:12–19. doi: 10.1016/j.jpor.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Du Y.-W., Zhang L.-N., Hou Z.-T., Ye X., Gu H.-S., Yan G.-P. Physical modification of polyetheretherketone for orthopedic implants. Front. Mater. Sci. 2014;8:313–324. [Google Scholar]
- 5.Wu X., Liu X., Wei J., Ma J., Deng F., Wei S. Nano-TiO2/PEEK bioactive composite as a bone substitute material: in vitro and in vivo studies. Int. J. Nanomed. 2012;7:1215. doi: 10.2147/IJN.S28101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu S., Hariram K.P., Kumar R., Cheang P., Aik K.K. In vitro apatite formation and its growth kinetics on hydroxyapatite/polyetheretherketone biocomposites. Biomaterials. 2005;26:2343–2352. doi: 10.1016/j.biomaterials.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 7.Converse G.L., Yue W., Roeder R.K. Processing and tensile properties of hydroxyapatite-whisker-reinforced polyetheretherketone. Biomaterials. 2007;28:927–935. doi: 10.1016/j.biomaterials.2006.10.031. [DOI] [PubMed] [Google Scholar]
- 8.Abu Bakar M.S., Cheng M.H.W., Tang S.M., Yu S.C., Liao K., Tan C.T. Tensile properties, tension–tension fatigue and biological response of polyetheretherketone–hydroxyapatite composites for load-bearing orthopedic implants. Biomaterials. 2003;24:2245–2250. doi: 10.1016/s0142-9612(03)00028-0. [DOI] [PubMed] [Google Scholar]
- 9.Wong K.L., Wong C.T., Liu W.C., Pan H.B., Fong M.K., Lam W.M. Mechanical properties and in vitro response of strontium-containing hydroxyapatite/polyetheretherketone composites. Biomaterials. 2009;30:3810–3817. doi: 10.1016/j.biomaterials.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 10.Lu T., Liu X., Qian S., Cao H., Qiao Y., Mei Y. Multilevel surface engineering of nanostructured TiO2 on carbon-fiber-reinforced polyetheretherketone. Biomaterials. 2014;35:5731–5740. doi: 10.1016/j.biomaterials.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Suska F., Omar O., Emanuelsson L., Taylor M., Gruner P., Kinbrum A. Enhancement of CRF-PEEK osseointegration by plasma-sprayed hydroxyapatite: a rabbit model. J. Biomater. Appl. 2014;29:234. doi: 10.1177/0885328214521669. [DOI] [PubMed] [Google Scholar]
- 12.Lu T., Wen J., Qian S., Cao H., Ning C., Pan X. Enhanced osteointegration on tantalum-implanted polyetheretherketone surface with bone-like elastic modulus. Biomaterials. 2015;51:173–183. doi: 10.1016/j.biomaterials.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 13.Wang H., Xu M., Zhang W., Kwok D.T.K., Jiang J., Wu Z. Mechanical and biological characteristics of diamond-like carbon coated poly aryl-ether-ether-ketone. Biomaterials. 2010;31:8181–8187. doi: 10.1016/j.biomaterials.2010.07.054. [DOI] [PubMed] [Google Scholar]
- 14.Briem D., Strametz S., Schröoder K., Meenen N.M., Lehmann W., Linhart W. Response of primary fibroblasts and osteoblasts to plasma treated polyetheretherketone (PEEK) surfaces. J. Mater. Sci. Mater. Med. 2005;16:671–677. doi: 10.1007/s10856-005-2539-z. [DOI] [PubMed] [Google Scholar]
- 15.Schröder K., Meyer-Plath A., Keller D., Ohl A. On the applicability of plasma assisted chemical micropatterning to different polymeric biomaterials. Plasma Polym. 2002;7:103–125. [Google Scholar]
- 16.Zhao D., Witte F., Lu F., Wang J., Li J., Qin L. Current status on clinical applications of magnesium-based orthopaedic implants: a review from clinical translational perspective. Biomaterials. 2016:287–302. doi: 10.1016/j.biomaterials.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 17.Tan L., Yu X., Wan P., Yang K. Biodegradable materials for bone repairs: a review. J. Mater. Sci. Technol. 2013;29:503–513. [Google Scholar]
- 18.Cui L.-Y., Wei G.-B., Zeng R.-C., Li S.-Q., Zou Y.-H., Han E.-H. Corrosion resistance of a novel SnO2-doped dicalcium phosphate coating on AZ31 magnesium alloy. Bioact. Mater. 2017 doi: 10.1016/j.bioactmat.2017.11.001. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Q., Jin S., Lin X., Zhang Y., Ren L., Yang K. Cytotoxic effects of biodegradation of pure Mg and MAO-Mg on tumor cells of MG63 and KB. J. Mater. Sci. Technol. 2014;30:487–492. [Google Scholar]
- 20.Qu X., Jin F., Hao Y., Zhu Z., Li H., Tang T. Nonlinear association between magnesium intake and the risk of colorectal cancer. Eur. J. Gastroenterol. Hepatol. 2013;25:309–318. doi: 10.1097/MEG.0b013e32835c073c. [DOI] [PubMed] [Google Scholar]
- 21.Li M., Ren L., Li L.H., He P., Lan G.B., Zhang Y. Cytotoxic effect on osteosarcoma MG-63 cells by degradation of magnesium. J. Mater. Sci. Technol. 2014;30:888–893. [Google Scholar]
- 22.Robinson D.A., Griffith R.W., Dan S., Evans R.B., Conzemius M.G. In vitro antibacterial properties of magnesium metal against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. Acta Biomater. 2009;6:1869–1877. doi: 10.1016/j.actbio.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Ren L., Lin X., Tan L., Yang K. Effect of surface coating on antibacterial behavior of magnesium based metals. Mater. Lett. 2011;65:3509–3511. [Google Scholar]
- 24.Li Y., Liu G., Zhai Z., Liu L., Li H., Yang K. Antibacterial properties of magnesium in an in vitro and in vivo model of implant-associated MRSA infection. Antimicrob. Agents Chemother. 2014;58:7586–7591. doi: 10.1128/AAC.03936-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui L.Y., Ji X.U., Na L.U., Zeng R.C., Zou Y.H., Shuo-Qi L.I. In vitro corrosion resistance and antibacterial properties of layer-by-layer assembled chitosan/poly-L-glutamic acid coating on AZ31 magnesium alloys. Trans. Nonferr. Metals Soc. China. 2017;27:1081–1086. [Google Scholar]
- 26.Liu L., Li P., Zou Y., Luo K., Zhang F., Zeng R.C. In vitro corrosion and antibacterial performance of polysiloxane and poly(acrylic acid)/gentamicin sulfate composite coatings on AZ31 alloy. Surf. Coat. Technol. 2016;291:7–14. [Google Scholar]
- 27.Chen L., Fu X., Pan H., Peng W., Lei W., Tan L. Biodegradable Mg-Cu alloys with enhanced osteogenesis, angiogenesis, and long-lasting antibacterial effects. Sci. Rep. 2016;6 doi: 10.1038/srep27374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Z., Mao X., Tan L., Friis T., Wu C., Crawford R. Osteoimmunomodulatory properties of magnesium scaffolds coated with β-tricalcium phosphate. Biomaterials. 2014;35:8553–8565. doi: 10.1016/j.biomaterials.2014.06.038. [DOI] [PubMed] [Google Scholar]
- 29.Zhai Z., Qu X., Li H., Yang K., Wan P., Tan L. The effect of metallic magnesium degradation products on osteoclast-induced osteolysis and attenuation of NF-κB and NFATc1 signaling. Biomaterials. 2014;35:6299–6310. doi: 10.1016/j.biomaterials.2014.04.044. [DOI] [PubMed] [Google Scholar]
- 30.Cheng M.Q., Wahafu T., Jiang G.F., Liu W., Qiao Y.Q., Peng X.C. A novel open-porous magnesium scaffold with controllable microstructures and properties for bone regeneration. Sci. Rep. 2016;6:24134. doi: 10.1038/srep24134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo Y., Ren L., Liu C., Yuan Y., Lin X., Tan L. Effect of implantation of biodegradable magnesium alloy on BMP-2 expression in bone of ovariectomized osteoporosis rats. Mater. Sci. Eng. C. 2013;33:4470–4474. doi: 10.1016/j.msec.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 32.Zeng J., Ren L., Yuan Y., Wang Y., Zhao J., Zeng R. Short-term effect of magnesium implantation on the osteomyelitis modeled animals induced by Staphylococcus aureus. J. Mater. Sci. Mater. Med. 2013;24:2405–2416. doi: 10.1007/s10856-013-4982-6. [DOI] [PubMed] [Google Scholar]
- 33.Wan P., Wu J., Tan L., Zhang B., Yang K. Research on super-hydrophobic surface of biodegradable magnesium alloys used for vascular stents. Mater. Sci. Eng. C. 2013;33:2885–2890. doi: 10.1016/j.msec.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 34.Fukumoto S., Sugahara K., Yamamoto A., Tsubakino H. Improvement of corrosion resistance and adhesion of coating layer for magnesium alloy coated with high purity magnesium. Mater. Trans. 2003;44:518–523. [Google Scholar]
- 35.Tsubakino H., Yamamoto A., Fukumoto S., Watanabe A., Sugahara K., Inoue H. High-purity magnesium coating on magnesium alloys by vapor deposition technique for improving corrosion resistance. Mater. Trans. 2003;44:504–510. [Google Scholar]
- 36.Salunke P., Shanov V., Witte F. High purity biodegradable magnesium coating for implant application. Mater. Sci. Eng. B. 2011;176:1711–1717. [Google Scholar]
- 37.Li X., Gao P., Wan P., Pei Y., Shi L., Fan B. Novel bio-functional magnesium coating on porous Ti6Al4V orthopaedic implants: in vitro and in vivo study. Sci. Rep. 2017;7 doi: 10.1038/srep40755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamamoto A., Watanabe A., Sugahara K., Fukumoto S., Tsubakino H. Platform science and technology for advanced magnesium alloys. Deposition coating of magnesium alloys with pure magnesium. Mater. Trans. 2001;42:1237–1242. [Google Scholar]