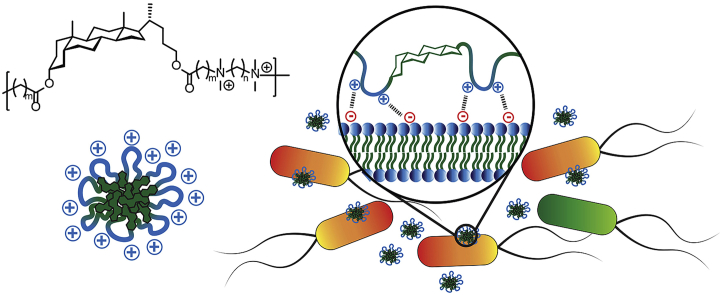
Abstract
Bacterial infections have become a global issue that requires urgent attention, particularly regarding to emergence of multidrug resistant bacteria. We developed quaternary amine-containing antimicrobial poly(bile acid)s that contain a hydrophobic core of lithocholic acid in the main-chain. Interestingly, by choosing appropriate monomers, these cationic polymers can form core-shell micelles. These polymers exhibited biocidal activity against both Gram-positive and Gram-negative bacterial species. It is demonstrated that the micelles can deliver hydrophobic antibiotics that functionally have dual antimicrobial activities. Cytotoxicity assays against HeLa cells showed dosage-dependent toxicity for polymers with longer linkers.
Keywords: Antimicrobial polymers, Bile acid, Quaternary ammonium, Lithocholic acid, Polyionene, Facial amphiphilicity
Graphical abstract
Highlights
-
•
Developed quaternary amine-containing antimicrobial polymers with lithocholic acid in the main-chain.
-
•
These polymers formed cationic micelles in water that showed biocidal activity against bacteria.
-
•
Micelles with antibiotic ampicillin in the core demonstrated extended release kinetics and improved antibacterial activity.
1. Introduction
Microbial infections, especially those caused by resistant bacteria have become problematic due to increasing ineffectiveness of conventional antibiotics [1]. The overuse of antibiotics in health care settings and agriculture, coupled with the slow pace of new antimicrobial discoveries and approvals over the last few decades, have resulted in many new resistant bacterial strains that complicate the well-being of humans, food security, and societal development [2,3]. Therefore, there is an increased interest by the scientific community to explore new avenues to circumvent the problem with resistant bacteria. Bacterial membrane anionic lipids are considered attractive targets to design novel antibacterial agents [4]. In general, antimicrobial polymers are a class of hydrophilic cationic macromolecules that can selectively destroy microorganisms such as bacteria, fungi or protozoans with little or no cytotoxicity to mammalian cells [5,6]. Most antimicrobial polymers contain quaternary ammonium centers as the cations, while others possess cations such as phosphonium, sulfonium or metal centers [[7], [8], [9], [10], [11], [12], [13]].
We have developed several antimicrobial macromolecules utilizing pine tree-derived natural resin acids (or rosins) that have relatively good antimicrobial activities [[14], [15], [16], [17]]. Cationic charges were implemented as pendent groups from polymer backbones. However, antimicrobial agents such as antimicrobial peptides and antimicrobial polymers with facially amphiphilic orientation show better antimicrobial properties due to a local balance of amphiphilicity [18]. We noted bile acids as potential candidates for preparing effective antimicrobial polymers. Bile of mammals and other vertebrates is rich in bile acids, which are amphiphilic steroidal acids. They typically stay conjugated with taurine or glycine in the liver forming bile salts that serve as surfactants to solubilize dietary lipids and fats by the formation of micelles allowing digestion of food. Bile acids have been utilized in many areas including gene delivery [19,20], drug delivery [21], sensing [22], polymeric gels [23], antimicrobial agents [[24], [25], [26]] and other biological applications [27].
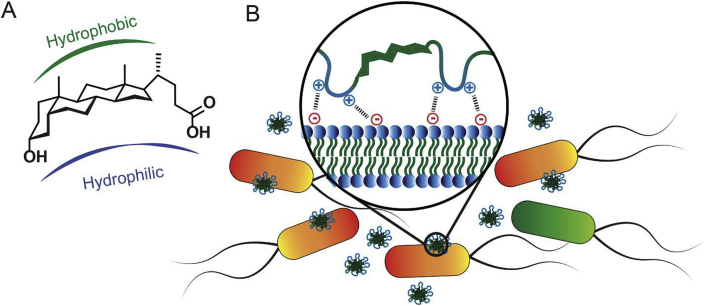
The 5β framework of bile acids or the cis A-B ring junction imparts a curvature to the ring system resulting in two faces with dramatically different properties [28]. Hydroxyl groups of bile acid molecules are positioned in the α-face while their methyl groups are in the β-face, thereby creating facial amphiphilicity (Fig. 1A). The steroidal nucleus with four fused rings provides the hydrophobic core that can preferentially embed into cell membranes. The presence of hydroxyl and carboxylic acid groups offers hydrophilic chemical functionalization to achieve robust molecular designs and architectures to investigate key determinants of its surface activity and the ability to selectively interact with membrane lipids [29,30].
Fig. 1.
(A) Facial amphiphilic structure of lithocholic acid; and (B) the functional importance of this structure in antimicrobial polymers for conferring destructive membrane interactions promoting antimicrobial activity.
Recent advances on bile acid in macromolecular research include a variety of structures having bile acids as repeating units in the polymer backbone, as pendant groups along the polymer chain in block, or statistical polymers and chain end-functional polymers [31]. Notable advances on bile acid polymers have been carried out by Zhu and coworkers [32,33]. Controlled polymerization methods such as ATRP, RAFT and ROMP have been used to make side chain bile acid-containing polymers. Polymers containing bile acids in the main chain have been prepared using step-growth polymerization via incorporating a variety of linkers, such as esters, anhydrides, triazoles, β-amino esters and sulfide [[34], [35], [36], [37], [38]]. Polycondensations are useful to prepare highly efficacious and inexpensive antimicrobial polymers for numerous applications [39]. A unique class of polyelectrolytes known as ammonium polyionenes can be prepared by step-growth polymerizations to have cations in the main chain at regular and specific sites [40]. They are generally prepared from a reaction between ditertiary amines and dihalides via Menschutkin reaction [41,42]. These polymers exhibit strong and fast acting antimicrobial activities [[43], [44], [45]]. However, little is known regarding the preparation of hydrophobic polyionenes that incorporate natural product-derived chemicals targeted for antimicrobial applications.
We envisioned the possibility to develop dihalides monomers from bile acids such as lithocholic acid that can be used to prepare quaternary ammonium polyionenes. Water soluble cationic polymers with degradable ester linkages can be easily developed using lithocholic acid in the main chain. In this study, we developed difunctional monomers from lithocholic acid, and used them to make cationic polymers that contain quaternary ammonium groups along the polymer backbone. These polyionene antimicrobial polymers formed micelles in water. The antimicrobial activity, cytotoxicity, and drug delivery applications were then demonstrated.
2. Experimental section
2.1. Materials
Lithocholic acid (95%, Aldrich), lithium aluminum hydride (95%, Aldrich), 2-bromoacetyl chloride (95%, Aldrich) and 6-bromohexanoyl chloride (97%, Aldrich) were used as received. N,N,N′,N′-tetramethyl-1,2-ethanediamine (99%, TCI) and N,N,N′,N′-tetramethyl-1,6-hexanediamine (99%, Aldrich) were distilled before use. Tetrahydrofuran (THF) and N, N-dimethylformamide (DMF) were dried over drying columns. Ampicillin was purchased from VWR as the pure form. All other reagents and solvents were from commercial resources and used as received unless otherwise mentioned. Spectrum Spectra/Por® 3 Dialysis Membranes with MWCO 3500 were purchased from VWR. All other reagents used for biological assays were purchased from Thermo Fisher Scientific or Sigma Aldrich and will be mentioned in the respective sections.
2.2. Characterization
1H NMR (300 MHz) spectra were recorded on a Bruker Avance III HD 300 with deuterated chloroform or dimethyl sulfoxide as solvents. The molecular weight of polymers was determined by size exclusion chromatography (SEC) using Agilent 1200 (pump, autosampler), Wyatt DAWN HELEOS-II multiangle light scattering detector (MALS) (λ = 662 nm), Wyatt Optilab T-rEX (dRI) with Viscotek columns (Model- I-MBMMW-3078), exclusion limit (PS)- 200 kDa, max pore size 10,000 Å. The mobile phase was DMF with 0.05 M LiBr. The samples were prepared at 5.0 mg/mL in DMF. The molecular weights were determined using the light scattering detector response with Astra V (Wyatt Technologies, Santa Barbara, CA) software. The polymer dn/dc was estimated from the mass recovery, and values ranged from 0.0062 to 0.0332 mL/g. The morphology of polymer micelles was recorded by Field-Emission Scanning Electron Microscopy (FE-SEM, Zeiss UltraPlus). A solution containing 1.0 mg/mL was prepared in water and 10 μL drop was added on to 1 cm × 1 cm plasma cleaned Si wafers. Drop-cast films were air dried for 12 h and sputtered with gold before imaging. Films were observed using an acceleration voltage of 5.00 kV. Imaging was done under in-lens secondary electron detector with a working distance of 3.00 mm or less during the acquisition of images. The steady-state fluorescence spectra were recorded at room temperature using a PTI QM-400 fluorometer. UV–vis spectra were recorded on a Shimadzu UV 2450 spectrophotometer.
2.3. Synthesis of 3α,5β-cholane-3,24-diol
A suspension of LiAlH4 powder (2.0 g, 0.053 mol, 2.0 equiv.) was added to dry THF 100 mL in a round-bottom flask slowly and carefully in an ice bath. While maintaining cold temperature, lithocholic acid (10 g, 0.027 mol, 1.0 equiv.) was added to the suspension in small quantities under vigorous stirring. After raising to room temperature, and holding for 30 min, the mixture was refluxed for 18 h. Then the reaction mixture was transferred in small amounts to a 10% HCl(aq) 1 L in a conical flask. The product was extracted into diethyl ether 3 times. After drying with anhydrous MgSO4, the solvent was removed by rotary evaporation. The white solid was kept in a vacuum oven at 50 °C overnight to obtain a white crystalline powder (9.6 g, yield = 99%).
2.4. Synthesis of lithocholic dibromide monomers
3α,5β-Cholane-3,24-diol (10.0 g, 0.275 mol, 1 equiv.) was transferred to a solution of 6-bromohexanoyl chloride (23.5 g, 0.11 mol, 4.0 equiv.) dissolved in dry THF that was kept in an ice bath. The flask was sealed and stirred at room temperature for 24 h. Then it was washed with NaHCO3, water, and brine against dichloromethane. The product was concentrated by rotary evaporation and precipitated into methanol three times. The product was filtered, and vacuum dried to obtain a white powder (15.0 g, yield = 75%).
2.5. Synthesis of polymers
Stoichiometric amount of dibromide and ditertiary amine was used for the step-growth polymerization. In a typical procedure, the dibromide (500 mg, 0.698 mmol, 1 equiv.) was transferred to a 25 mL round-bottom flask. It was added with N,N,N′,N′-tetramethyl-1,6-hexanediamine (121.4 mg, 0.698 mmol, 1 equiv.) using dry DMF 5.0 mL. The flask was sealed using a rubber septum and heated to 60 °C for 24 h. After the completion of reaction, the mixture was dialyzed against 3 L of water for 24 h. Then, the remaining solution was freeze-dried for 2 days to obtain a white powder (485 mg, yield = 78%). In a similar manner, a control polymer (Pcontrol) was synthesized using a dibromide monomer made by combining 1,12-dodecanediol with 6-bromohexanoyl chloride and diamine N,N,N′,N′-tetramethyl-1,6-hexanediamine. Polymers are denoted as Pm,n, where m is the number of methylene groups in the dibromide linker and n is the number of methylene groups in the ditertiary amine linker.
2.6. Dynamic light scattering and zeta potential
A Zetasizer Nano ZS (Malvern Instruments, Malvern, UK) with a 4.0 mW 633 nm He−Ne laser and the detector at an angle of 173° was used to measure hydrodynamic diameter (Z-average) and Zeta potential of the micelles. The samples were prepared by dispersing dry polymer in filtered (0.2 μm GHP membrane filter) deionized water with typical micelle concentrations of 0.25 mg/mL. The solutions were at pH 7 and the measurements were carried out at 25 °C. The data processing was done using the general-purpose algorithms provided in the Zetasizer Software (version 7.11). Sample measurements were acquired in duplicate and reported as an average and standard error.
2.7. Critical micelle concentration
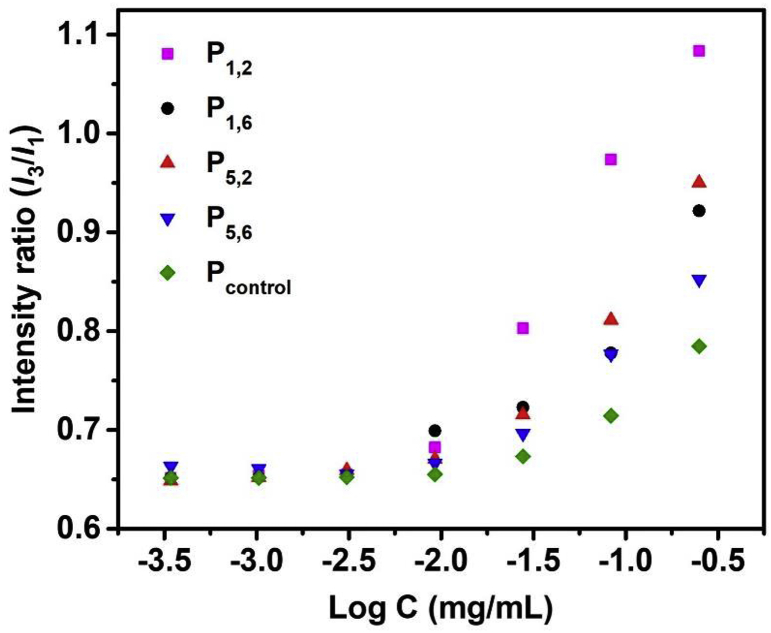
The critical micelle concentration (CMC) value of polymer solutions was determined using pyrene as the probe. A stock solution of pyrene (0.1 mg/mL) in acetone was prepared. Then, 20 μL of the pyrene solution was added to glass vials and left to dry. Next, a series of polymer solutions were made at concentrations ranging from 250 μg/mL to 0.3 μg/mL using serial dilution. Afterwards, 10.0 mL of each polymer solution was added to pyrene containing vials, vortexed for 1 min and left at room temperature for 24 h for the system to reach equilibrium. The polymer solutions were excited using 334 nm excitation wavelength and emission was recorded from 365 nm to 405 nm with a slit width kept at 2 nm. The intensity ratio of the third to first vibronic bands (I3/I1) was calculated by taking the ratio of maximum peak intensity at 373 nm (I1) and 394 nm (I3). Then, plots of I3/I1 as a function of logarithm concentrations of polymers were prepared from which the CMC values were determined at the intersection of the two fitted lines (inflection point) to points of the curves, the horizontal base line at low polymer concentrations and sharply rising I3/I1 line at higher concentrations.
2.8. Antimicrobial assays
Actively-growing cultures of each bacterial strain (Staphylococcus aureus ATCC 25423 and Escherichia coli ATCC 25922) on Mannitol salt agar (MSA) were inoculated on Tryptic Soy Broth (TSB) agar plates. Next, 10 μL of bacterial growth culture (cell concentrations were 1.0 × 106 CFU/mL) was diluted to 1 mL in TSB, and 100 μL of the diluted solution was spread on TSB agar plates to form a bacterial lawn covering the plate surface. Then, 6 mm (diameter) sterile discs were placed on the plate surface, followed by adding polymers and compounds at different concentrations. All experiments were conducted in duplicate. The plates were incubated at 37 °C for 24 h. The development of a clear zone around the disk was indicative of the ability of agents to kill bacteria. The minimum inhibitory concentration (MIC) was determined following established protocols [16].
2.9. Antibiotic loaded micelles and drug release
Antibiotic ampicillin was used to demonstrate the drug encapsulation capability of these polymers. Drug-loaded micelles were prepared by a membrane dialysis method. For this, 10.0 mg polymer P5,6 was mixed with 10.0 mg ampicillin and dissolved in 5 mL of DMSO. Then the solution was dialyzed against 3 L deionized water at room temperature. The water was replaced three times over 24 h. Then the dialysis bag containing the ampicillin loaded micelles was immersed in a graduated glass media bottle containing 150 mL PBS solution at 37 °C. The stirring was kept at 300 rpm. At specific time intervals, 3 mL of the solution was taken out for UV–vis analysis at 204 nm absorption peak. After the measurements, the solution was returned to the bottle. The cumulative release was calculated based on the total release of the contents from the dialysis bag. The drug encapsulation efficiency was determined using a calibration curve made with the UV–vis spectra of ampicillin solutions prepared in PBS buffer at various concentrations. Antibiotic encapsulation efficiency was calculated using the following equation.
2.10. In vitro cytotoxicity against HeLa cells
For the cytotoxicity evaluation assay, HeLa cell viabilities were examined using the Cell Counting Kit-8 (CCK-8) assay. HeLa cells were seeded in a 96-well plate at an initial cell density of 5000 cells/well in 100 μL of DMEM containing 10% Fetal Bovine Serum (FBS). The polymer samples prepared in Opti-MEM were added at varying concentrations and incubated 24 h with 5% CO2 at 37 °C. Then, the CCK-8 reagent was diluted appropriately in PBS and added to the wells. After 2 h incubation, the solution in each well was transferred into a fresh 96 well plate. The absorbance was measured at 490 nm and 600 nm using a Biotek Synergy™ H1 microplate reader. The absorbance at 490 nm was corrected using the O.D. at 600 nm. The percentage cell viability was determined by comparing the cells with only the sample and control wells with only the cell culture medium. Each experiment was conducted in triplicate and the data is presented as the mean and the standard error.
3. Results and discussion
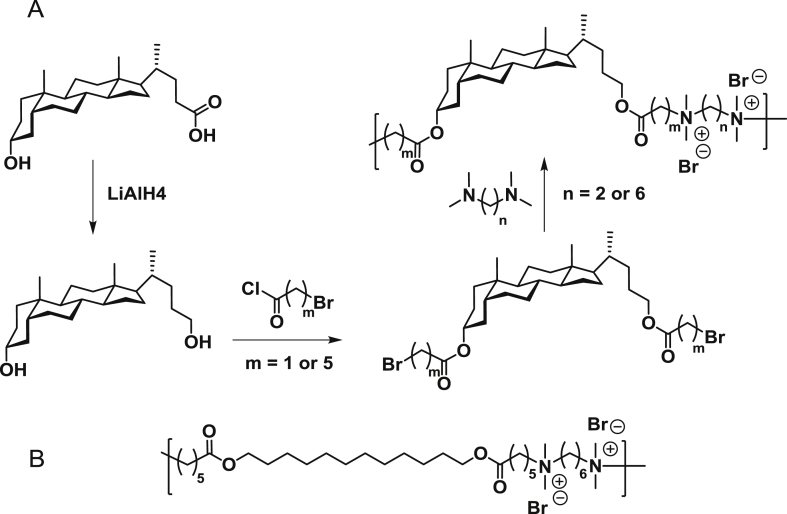
Reduction of lithocholic acid with the aid of LiAlH4 was a robust and high-yield approach to obtain 3α,5β-cholane-3,24-diol. This diol compound was then converted to dibromides using fatty acyl bromides with various lengths of alkyl groups. Cationic lithocholic-containing polymers were synthesized by the stoichiometric copolymerization of dibromocholane derivatives with the corresponding ditertiary amines according to the Menschutkin reaction (Fig. 2) [40].
Fig. 2.
(A) Synthesis of main-chain cationic polymers from lithocholic acid. Polymer labels denoted as Pm,n. where m is the number of methylene groups in the dibromide linker and n is the number of methylene groups in the ditertiary amine linker; (B) The structure of Pcontrol that was synthesized using 1,12-dodecanediol instead of the lithocholic diol.
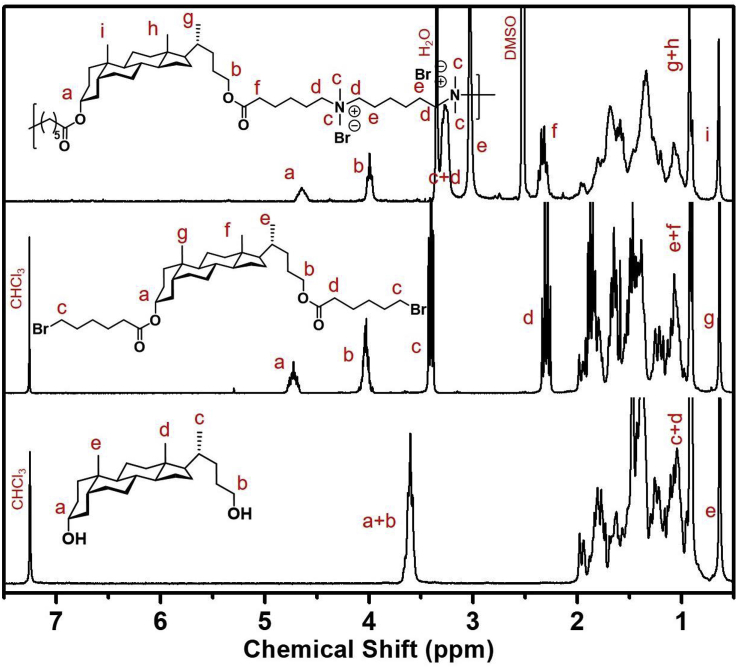
There is a variety of accessible ditertiary amines that can be used for the polymerization allowing us to explore several different polymers for antimicrobial applications as well as antibiotic delivery. The intermediate compounds were purified by simple precipitation because the hydrophobic steroidal structure made the compounds insoluble in methanol. After the polymerization reaction was completed, dialysis against water was employed to remove unreacted amines. The products were characterized by 1H NMR (Fig. 3). The proton peaks next to the alcohol groups overlapped at ∼3.65 ppm. However, after the esterification reaction, they shifted downfield to 4.74 ppm and 4.02 ppm and the methylene group next to the bromine group appeared at 3.40 ppm. After the polymerization reaction, the peaks broadened, and the polymer was no longer soluble in chloroform. 1H NMR of the polymer indicated new peaks relating to the ditertiary amine monomer and also the methylene group at 3.40 ppm shifted upfield to 3.26 ppm due to the formation of quaternary ammonium cations. The molecular weight of the obtained polymers indicated that most of them are short chains except P5,6, which has a molecular weight of 29,000 g/mol. A control polymer (Pcontrol) was made using 1,12-dodecanediol instead of the lithocholic diol. Both diol molecules have comparable contour lengths but differ as multicyclic vs linear structures.
Fig. 3.
1H NMR spectra of lithocholic diol, dibromide monomer (in CDCl3), and polymer P5,6 (in DMSO‑d6) from each step of the synthesis.
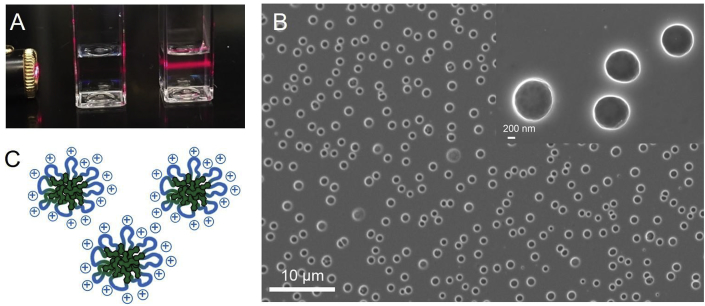
Interestingly, the polymers formed micelles (Fig. 4A) during the dialysis process as observed with a slightly turbid solution having Tindall scattering. These micelles were observed using SEM (Fig. 4B) and they appeared to be spherical in shape. Dynamic light scattering (DLS) was used to measure the size of micelles as summarized in Table 1. The size of micelles depended on the length of the ditertiary amine as well as the dibromide linker. Polymers P5,2 and P5,6 formed smaller micelles while the polymer with shorter dibromide linkers (P1,2 and P1,6) formed larger aggregates. The size difference between SEM and DLS measurements may be due to different processing conditions. Dialysis method produced size populations having low dispersities while those made by redispersing the freeze-dried samples appeared to have broader dispersities. However, the means were similar for the two populations of sizes. In addition, all the polymer micelles had positive zeta potential indicating that they were essentially cationic polymer micelles.
Fig. 4.
Polymer particle characterization: (A) An image of polymer P5,6 solution in DI water (right) and DI water (left) against a laser; (B) SEM image of the polymer P5,6 micelles; (C) An illustration of polymer micelles where the hydrophobic core is made of the steroidal units and the cationic groups produce the hydrophilic outer layer.
Table 1.
Polymer characterization and antimicrobial test results.a Bacteria used in tests were Staphylococcus aureus ATCC 25423 and Escherichia coli ATCC 25922.
| Polymer | Molecular Weight (Mnb, g/mol) | Dispersity |
Micelle Sizec (nm) | Zeta Potential (mV) | CMC (μg/mL) | MIC (μg/mL) |
|
|---|---|---|---|---|---|---|---|
| (Ð) | S. aureus | E. coli | |||||
| P1,2 | 9100 | 1.12 | >1000 | +43 ± 1 | 7.7 | 16.6 | 28.0 |
| P1,6 | 9500 | 1.09 | 600 ± 48 | +60 ± 6 | 7.0 | 14.5 | 21.5 |
| P5,2 | 8000 | 1.08 | 194 ± 3 | +67 ± 1 | 11.6 | 21.5 | 29.3 |
| P5,6 | 29,800 | 1.45 | 267 ± 12 | +74 ± 2 | 11.0 | 23.0 | 24.8 |
| P5,6-Ampicillin | – | – | 166 ± 1d | +59 ± 1 | – | 2.7 | 11.8 |
| Pcontrol | 7300 | 1.07 | 464 ± 41 | +82 ± 1 | 12.0 | 14.1 | 15.9 |
Data represented as the average and standard error.
Number-average molecular weight determined by SEC.
The mean hydrodynamic diameter (Z-average) of particles determined by DLS.
Ampicillin loaded micelles were prepared by dialysis method.
Amphiphilic block copolymers that contain hydrophobic and cationic regions can self-assemble to form core-shell structures in aqueous solutions [46]. Although in this study homopolymers are developed, it can be proposed that in these lithocholic polymer micelles, the hydrophobic interior may be mostly composed of steroidal core structures while the cationic linkers form the outer surface (Fig. 4C). This is also supported by the fact that the cationic outer layer prevents the formation of larger aggregates by electrostatic repulsions. According to the zeta potential values, the surface charge increased when the cationic quaternary ammonium center is further away from the steroidal core.
The CMC of polymers was measured using pyrene as a spatially sensitive fluorescent probe. The ratio of intensities of the third to first vibronic bands (I3/I1) increases with decreasing polarity around pyrene microenvironment. The I3/I1 increased sharply with increasing polymer concentrations indicating the partition of pyrene molecules into the hydrophobic core of the polymer micelles (Fig. 5). The CMC for the polymers was determined to be in the range of 7.0–12.0 μg/mL. The micelles made of polymers with shorter dibromide linkers had lower CMC values. Overall, these results indicate that the micelles prepared from lithocholic polyionenes contain a hydrophobic region.
Fig. 5.
Change of pyrene intensity ratio (I3/I1) versus the concentration of polymers in aqueous solution.
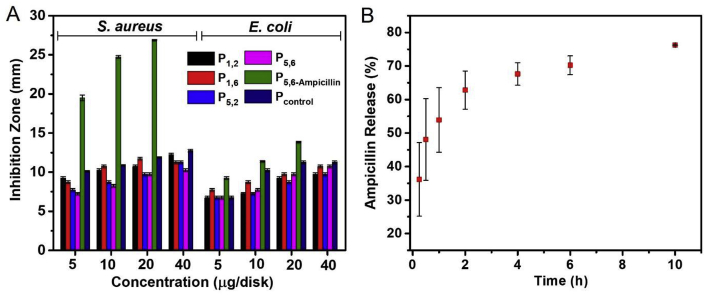
The antimicrobial activity was evaluated using disk diffusion assays against Gram-positive S. aureus and Gram-negative E. coli (Table 1 and Fig. 6A). The starting lithocholic acid or the dibromide did not show significant antibacterial activity against either bacterium. However, cationic lithocholic-containing polymers showed strong activities against both bacteria at quite low concentrations demonstrating their broad-spectrum activity. As observed for most cationic antimicrobial polymers, our polymers also showed better inhibition towards Gram-positive bacteria. Bile acid-based amphiphiles have been demonstrated to have strong interactions with cell membranes [30,47]. Therefore, the mechanism of action for these novel polymers may be largely associated with membrane damage to bacterial cells. There is a possibility to enhance antibacterial activity by incorporating hydrophobic antibiotics in the hydrophobic core of these micelles. In such a system, it can be envisioned that the cationic micelles get electrostatically attracted toward bacterial membranes and disrupt them, and simultaneously release the antibiotic cargo that can further attack its specific site causing further damage to the bacterial cell.
Fig. 6.
(A) Antimicrobial activities of polymers as demonstrated by disk diffusion assays; (B) Cumulative release profiles of the antibiotic ampicillin using micelles of P5,6-Ampicillin.
In addition, low biocompatibility, poor bacterial cell penetration, undesirable side effects, and susceptibility to microbial resistance mechanisms are some of the causes leading to the clinical failure of many antibiotics [48]. Polymeric micelles and other nanostructures, which can incorporate antibiotics as carriers, are considered as a promising therapeutic approach [[49], [50], [51]]. Bile acids and their derivatives show promise as drug delivery systems and as therapeutic agents [52]. In this study, the β-lactam antibiotic ampicillin was successfully loaded onto polymer micelles (P5,6) via a dialysis method with initial loading of ampicillin and polymer in equal weights. After dialysis, the polymer micelles were able to retain 0.289 ± 0.036 mg/mg of the antibiotic based on the weight of ampicillin incorporated versus the total weight of the polymer. The encapsulation efficiency was at 28.9%. Fig. 6B shows the release profile of ampicillin in PBS at 37 °C. The antibiotic release profile indicated a sustained release over several hours. After 10 h period, the cumulative release reached 76.4%. Such behavior shows that the hydrophobic drug molecules were encapsulated inside the hydrophobic core of polymer micelles. These antibiotic-loaded micelles showed enhanced activity against bacteria compared to the polymer alone (Fig. 6A).
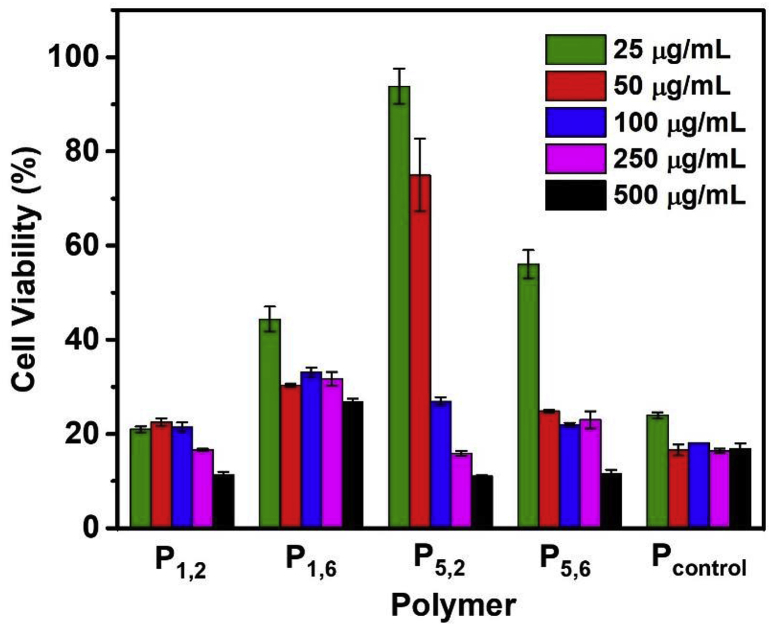
Next, cell viability tests were carried out using a CCK-8 assay against HeLa cells (Fig. 7). In DMEM media containing FBS, flocculation was observed for polymers P1,2 and P5,2. The presence of quaternary ammonium centers in a close proximity, only separated by two methylene groups may have a major influence for this phenomenon. Therefore, the micellar dispersions were made in Opti-MEM reduced serum media. In general, the polymers appeared to be cytotoxic to HeLa cells. Interestingly, polymer P5,2 caused less toxicity relative to the other polymers at low dosages. The control polymer with linear alkyl chains and P1,2 had the highest toxicity. Given the low MIC values as well as the high toxicity, Pcontrol appeared to be the most biocidal polymer.
Fig. 7.
Cell viability assay of various polymers against HeLa cells.
For a broad range of applications of biocidal polymers, it is necessary to have efficacy as well as biocompatibility [53]. The cytotoxicity and antimicrobial activity of such polymers are influenced by the balance of hydrophobic content relative to the charged and hydrophilic groups. Incorporation of hydrophilic moieties such as PEG [[54], [55], [56]] and carbohydrates [57] can dramatically improve the biocompatibility of polycations without significant loss of antimicrobial activity. Such modifications can be used to improve selectivity of the antimicrobial agent towards bacteria over mammalian cells. Although not investigated in this study, PEG containing dibromide can be incorporated into the step-growth polymerization reaction to obtain more hydrophilic systems that may have better cytocompatibility.
4. Conclusions
In conclusion, main-chain quaternary ammonium containing antimicrobial polymers were developed using lithocholic acid as the hydrophobic structure. The synthesis involved only three simple steps that have the potential for scalability. These novel polyionenes formed micelles in aqueous media with a cationic surface and a hydrophobic core. The size of micelles can be controlled by varying the length of the dibromide and amine linkers. These polyionenes showed prominent broad-spectrum antimicrobial activities. A dual functional antimicrobial property was demonstrated by encapsulating the antibiotic ampicillin into the hydrophobic core of the polymer micelles. Cytotoxicity against HeLa cells indicated dose dependent cytotoxicity at higher dosages that may require further optimization to achieve better selectivity towards bacteria over mammalian cells.
Acknowledgements
The authors would like to thank National Science Foundation (DMR-1608151). The assistance of H. N. Lokupitiya (USC) with FE-SEM imaging and A. Purchel (UMN) with SEC measurements is highly appreciated. Authors thank Marpe Bam (USC) for initial investigations with biocompatibility studies.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Luepke K.H., Suda K.J., Boucher H., Russo R.L., Bonney M.W., Hunt T.D. Past, present, and future of antibacterial economics: increasing bacterial resistance, limited antibiotic pipeline, and societal implications. Pharmacother. 2017;37:71–84. doi: 10.1002/phar.1868. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . WHO; 2014. Antimicrobial Resistance: Global Report on Surveillance. [Google Scholar]
- 3.Kumar A., Chordia N. Bacterial resistance against antibiotics. In: Arora G., Sajid A., Kalia V.C., editors. Drug Resistance in Bacteria, Fungi, Malaria, and Cancer. Springer International Publishing; Cham: 2017. pp. 171–192. [Google Scholar]
- 4.Mingeot-Leclercq M.-P., Decout J.-L. Bacterial lipid membranes as promising targets to fight antimicrobial resistance, molecular foundations and illustration through the renewal of aminoglycoside antibiotics and emergence of amphiphilic aminoglycosides. MedChemComm. 2016;7:586–611. [Google Scholar]
- 5.Ganewatta M.S., Tang C. Controlling macromolecular structures towards effective antimicrobial polymers. Polymer. 2015;63:A1–A29. [Google Scholar]
- 6.Krumm C., Tiller J.C. Antimicrobial polymers and surfaces–natural mimics or surpassing nature? Bio-inspired Polym. 2016:490–522. [Google Scholar]
- 7.Muñoz-Bonilla A., Fernández-García M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012;37:281–339. [Google Scholar]
- 8.Zhang J., Chen Y.P., Miller K.P., Ganewatta M.S., Bam M., Yan Y. Antimicrobial metallopolymers and their bioconjugates with conventional antibiotics against multidrug-resistant bacteria. J. Am. Chem. Soc. 2014;136:4873–4876. doi: 10.1021/ja5011338. [DOI] [PubMed] [Google Scholar]
- 9.Xue Y., Xiao H., Zhang Y. Antimicrobial polymeric materials with quaternary ammonium and phosphonium salts. Int. J. Mol. Sci. 2015;16:3626–3655. doi: 10.3390/ijms16023626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J., Yan J., Pageni P., Yan Y., Wirth A., Chen Y.-P. Anion-responsive metallopolymer hydrogels for healthcare applications. Sci. Rep. 2015;5:11914. doi: 10.1038/srep11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santos M., Fonseca A., Mendonça P., Branco R., Serra A., Morais P. Recent developments in antimicrobial polymers: a review. Materials. 2016;9:599. doi: 10.3390/ma9070599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang P., Bam M., Pageni P., Zhu T., Chen Y.P., Nagarkatti M. Trio act of boronolectin with antibiotic-metal complexed macromolecules toward broad-spectrum antimicrobial efficacy. ACS Infect. Dis. 2017;3:845–853. doi: 10.1021/acsinfecdis.7b00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan Y., Zhang J., Ren L., Tang C. Metal-containing and related polymers for biomedical applications. Chem. Soc. Rev. 2016;45:5232–5263. doi: 10.1039/c6cs00026f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J., Chen Y.P., Yao K., Wilbon P.A., Zhang W., Ren L. Robust antimicrobial compounds and polymers derived from natural resin acids. Chem. Commun. 2012;48:916–918. doi: 10.1039/c1cc16432e. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y., Wilbon P.A., Chen Y.P., Zhou J., Nagarkatti M., Wang C. Amphipathic antibacterial agents using cationic methacrylic polymers with natural rosin as pendant group. RSC Adv. 2012;2:10275–10282. [Google Scholar]
- 16.Ganewatta M.S., Chen Y.P., Wang J., Zhou J., Ebalunode J., Nagarkatti M. Bio-inspired resin acid-derived materials as anti-bacterial resistance agents with unexpected activities. Chem. Sci. 2014;5:2011–2016. [Google Scholar]
- 17.Ganewatta M.S., Miller K.P., Singleton S.P., Mehrpouya-Bahrami P., Chen Y.P., Yan Y. Antibacterial and biofilm-disrupting coatings from resin acid-derived materials. Biomacromolecules. 2015;16:3336–3344. doi: 10.1021/acs.biomac.5b01005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gabriel G.J., Maegerlein J.A., Nelson C.F., Dabkowski J.M., Eren T., Nüsslein K. Comparison of facially amphiphilic versus segregated monomers in the design of antibacterial copolymers. Chem. Eur J. 2009;15:433–439. doi: 10.1002/chem.200801233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker S., Sofia M.J., Kakarla R., Kogan N.A., Wierichs L., Longley C.B. Cationic facial amphiphiles: a promising class of transfection agents. Proc. Natl. Acad. Sci. U.S.A. 1996;93:1585–1590. doi: 10.1073/pnas.93.4.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moon H.-H., Joo M.K., Mok H., Lee M., Hwang K.-C., Kim S.W. MSC-based VEGF gene therapy in rat myocardial infarction model using facial amphipathic bile acid-conjugated polyethyleneimine. Biomaterials. 2014;35:1744–1754. doi: 10.1016/j.biomaterials.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 21.Patil S., Patil S., Gawali S., Shende S., Jadhav S., Basu S. Novel self-assembled lithocholic acid nanoparticles for drug delivery in cancer. RSC Adv. 2013;3:19760–19764. [Google Scholar]
- 22.Khatri V.K., Chahar M., Pavani K., Pandey P.S. Bile acid-based cyclic bisbenzimidazolium receptors for anion recognition: Highly improved receptors for fluoride and chloride ions. J. Org. Chem. 2007;72:10224–10226. doi: 10.1021/jo701341r. [DOI] [PubMed] [Google Scholar]
- 23.Pal A., Basit H., Sen S., Aswal V.K., Bhattacharya S. Structure and properties of two component hydrogels comprising lithocholic acid and organic amines. J. Mater. Chem. 2009;19:4325–4334. [Google Scholar]
- 24.Ye W., Li Y., Zhou Z., Wang X., Yao J., Liu J. Synthesis and antibacterial activity of new long-chain-alkyl bile acid-based amphiphiles. Bioorg. Chem. 2013;51:1–7. doi: 10.1016/j.bioorg.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 25.do Nascimento P.G.G., Lemos T.L.G., Almeida M.C.S., de Souza J.M.O., Bizerra A.M.C., Santiago G.M.P. Lithocholic acid and derivatives: antibacterial activity. Steroids. 2015;104:8–15. doi: 10.1016/j.steroids.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Lai X.-Z., Feng Y., Pollard J., Chin J.N., Rybak M.J., Bucki R. Ceragenins: cholic acid-based mimics of antimicrobial peptides. Acc. Chem. Res. 2008;41:1233–1240. doi: 10.1021/ar700270t. [DOI] [PubMed] [Google Scholar]
- 27.Hofmann A.F. Bile acids: trying to understand their chemistry and biology with the hope of helping patients. Hepatology. 2009;49:1403–1418. doi: 10.1002/hep.22789. [DOI] [PubMed] [Google Scholar]
- 28.Li Y., Dias J.R. Dimeric and oligomeric steroids. Chem. Rev. 1997;97:283–304. doi: 10.1021/cr9600565. [DOI] [PubMed] [Google Scholar]
- 29.Singh M., Singh A., Kundu S., Bansal S., Bajaj A. Deciphering the role of charge, hydration, and hydrophobicity for cytotoxic activities and membrane interactions of bile acid based facial amphiphiles. Biochim. Biophys. Acta. 2013;1828:1926–1937. doi: 10.1016/j.bbamem.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Bansal S., Singh M., Kidwai S., Bhargava P., Singh A., Sreekanth V. Bile acid amphiphiles with tunable head groups as highly selective antitubercular agents. MedChemComm. 2014;5:1761–1768. [Google Scholar]
- 31.Cunningham A.J., Zhu X.X. Polymers made of bile acids: from soft to hard biomaterials. Can. J. Chem. 2016;94:659–666. [Google Scholar]
- 32.Zhang J., Zhu X. Biomaterials made of bile acids. Sci. China Ser. B Chem. 2009;52:849–861. [Google Scholar]
- 33.Zhu X.-X., Nichifor M. Polymeric materials containing bile acids. Acc. Chem. Res. 2002;35:539–546. doi: 10.1021/ar0101180. [DOI] [PubMed] [Google Scholar]
- 34.Zuluaga F., Valderruten N.E., Wagener K.B. The ambient temperature synthesis and characterization of bile acid polymers. Polym. Bull. 1999;42:41–46. [Google Scholar]
- 35.Gouin S., Zhu X.X., Lehnert S. New polyanhydrides made from a bile acid dimer and sebacic acid: Synthesis, characterization, and degradation. Macromolecules. 2000;33:5379–5383. [Google Scholar]
- 36.Gangwal J.J., Kulkarni M.G. Synthesis and characterization of bile acid-based poly β amino esters for paclitaxel delivery. J. Appl. Polym. Sci. 2011;122:220–232. [Google Scholar]
- 37.Li W., Tian T., Lan Y., Zhu W., Li J., Zhang M. Self-assembled main-chain poly (bile acid) membranes that wrinkle. Polym. Chem. 2014;5:743–751. [Google Scholar]
- 38.Sun J., Li W., Xiao L., Yu G., Shi J. Main chain poly(bile acid) directed plasmonic nanospheres with amphiphilic binding pockets and photo-triggered destruction. RSC Adv. 2016;6:62200–62207. [Google Scholar]
- 39.Zhang M., Teo J.J., Liu S., Liang Z.C., Ding X., Ono R.J. Simple and cost-effective polycondensation routes to antimicrobial consumer products. Polym. Chem. 2016;7:3923–3932. [Google Scholar]
- 40.Williams S.R., Long T.E. Recent advances in the synthesis and structure–property relationships of ammonium ionenes. Prog. Polym. Sci. 2009;34:762–782. [Google Scholar]
- 41.Casson D., Rembaum A. Solution properties of novel polyelectrolytes. Macromolecules. 1972;5:75–81. [Google Scholar]
- 42.Abboud J-uM., Notario R., Bertrán J., Solà M. One century of physical organic chemistry: the Menshutkin Reaction. Prog. Phys. Org. Chem. 2007:1–182. John Wiley & Sons, Inc. [Google Scholar]
- 43.Mattheis C., Zheng M., Agarwal S. Closing one of the last gaps in polyionene compositions: alkyloxyethylammonium Ionenes as fast-acting biocides. Macromol. Biosci. 2012;12:341–349. doi: 10.1002/mabi.201100316. [DOI] [PubMed] [Google Scholar]
- 44.Strassburg A., Kracke F., Wenners J., Jemeljanova A., Kuepper J., Petersen H. Nontoxic, hydrophilic cationic polymers–identified as class of antimicrobial polymers. Macromol. Biosci. 2015;15:1710–1723. doi: 10.1002/mabi.201500207. [DOI] [PubMed] [Google Scholar]
- 45.Liu S., Ono R.J., Wu H., Teo J.Y., Liang Z.C., Xu K. Highly potent antimicrobial polyionenes with rapid killing kinetics, skin biocompatibility and in vivo bactericidal activity. Biomaterials. 2017;127:36–48. doi: 10.1016/j.biomaterials.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 46.Lam S.J., Wong E.H.H., Boyer C., Qiao G.G. Antimicrobial polymeric nanoparticles. Prog. Polym. Sci. 2018;76:40–64. [Google Scholar]
- 47.Li Z.-C., Li F.-M., Arase S., Takeoka S., Tsuchida E. Intermolecular interactions of lithocholic acid derivatives with phospholipid as bilayer membrane. Langmuir. 1995;11:3161–3166. [Google Scholar]
- 48.Wang Y., Ke X., Voo Z.X., Yap S.S.L., Yang C., Gao S. Biodegradable functional polycarbonate micelles for controlled release of amphotericin B. Acta Biomater. 2016;46:211–220. doi: 10.1016/j.actbio.2016.09.036. [DOI] [PubMed] [Google Scholar]
- 49.Liu L., Venkatraman S.S., Yang Y.-Y., Guo K., Lu J., He B. Polymeric micelles anchored with TAT for delivery of antibiotics across the blood–brain barrier. Biopolymers. 2008;90:617–623. doi: 10.1002/bip.20998. [DOI] [PubMed] [Google Scholar]
- 50.Xiong M.-H., Bao Y., Yang X.-Z., Zhu Y.-H., Wang J. Delivery of antibiotics with polymeric particles. Adv. Drug Deliv. Rev. 2014;78:63–76. doi: 10.1016/j.addr.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 51.Stebbins N.D., Ouimet M.A., Uhrich K.E. Antibiotic-containing polymers for localized, sustained drug delivery. Adv. Drug Deliv. Rev. 2014;78:77–87. doi: 10.1016/j.addr.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Faustino C., Serafim C., Rijo P., Reis C.P. Bile acids and bile acid derivatives: use in drug delivery systems and as therapeutic agents. Expet Opin. Drug Deliv. 2016;13:1133–1148. doi: 10.1080/17425247.2016.1178233. [DOI] [PubMed] [Google Scholar]
- 53.Timofeeva L., Kleshcheva N. Antimicrobial polymers: mechanism of action, factors of activity, and applications. Appl. Microbiol. Biotechnol. 2011;89:475–492. doi: 10.1007/s00253-010-2920-9. [DOI] [PubMed] [Google Scholar]
- 54.Venkataraman S., Zhang Y., Liu L., Yang Y.-Y. Design, syntheses and evaluation of hemocompatible pegylated-antimicrobial polymers with well-controlled molecular structures. Biomaterials. 2010;31:1751–1756. doi: 10.1016/j.biomaterials.2009.11.030. [DOI] [PubMed] [Google Scholar]
- 55.King A., Chakrabarty S., Zhang W., Zeng X., Ohman D.E., Wood L.F. High antimicrobial effectiveness with low hemolytic and cytotoxic activity for PEG/quaternary copolyoxetanes. Biomacromolecules. 2014;15:456–467. doi: 10.1021/bm401794p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Uppu D.S.S.M., Akkapeddi P., Manjunath G.B., Yarlagadda V., Hoque J., Haldar J. Polymers with tunable side-chain amphiphilicity as non-hemolytic antibacterial agents. Chem. Commun. 2013;49:9389–9391. doi: 10.1039/c3cc43751e. [DOI] [PubMed] [Google Scholar]
- 57.Álvarez-Paino M., Muñoz-Bonilla A., López-Fabal F., Gómez-Garcés J.L., Heuts J.P.A., Fernández-García M. Effect of glycounits on the antimicrobial properties and toxicity behavior of polymers based on quaternized DMAEMA. Biomacromolecules. 2015;16:295–303. doi: 10.1021/bm5014876. [DOI] [PubMed] [Google Scholar]