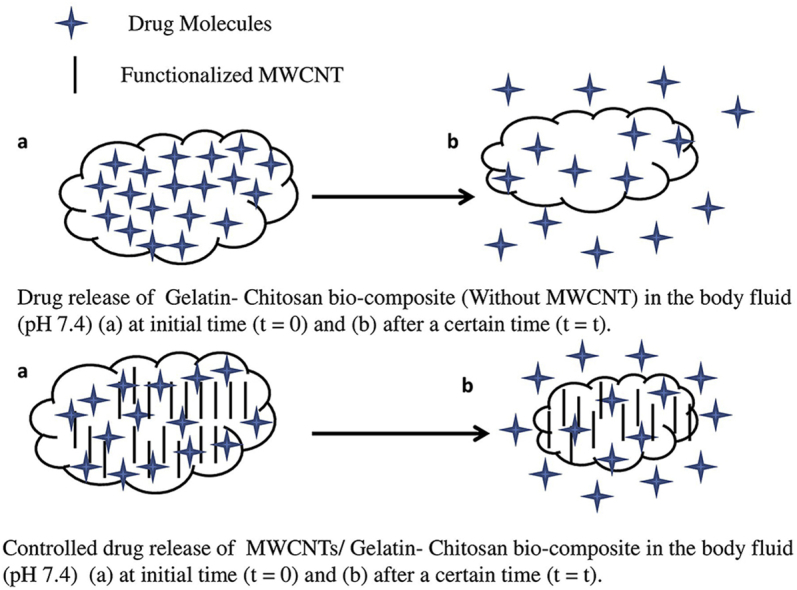
Abstract
This research work blooms the new idea of developing a safe and controlled drug releasing matrix using multi-walled carbon nanotubes (MWCNTs). In aqueous solution, uniform and highly stable dispersion of MWCNTs was obtained after secondary functionalization with polyethylene glycol (PEG) which was studied by Fourier transmission infrared spectroscopy (FTIR) and thermogravimetric analysis (TGA). Solution casting method was used to prepare MWCNTs/gelatin-chitosan nanocomposite films and the effect of MWCNTs on physico-mechanical, thermal and water uptake properties of the nanocomposites were evaluated. Incorporation of MWCNTs into the porous gelatin-chitosan matrix showed interesting stiffness and dampness along with developed microfibrillar structures within the pore walls intended at being used in tissue engineering of bone or cartilage. A common antibiotic drug, ciprofloxacin was incorporated into nanocomposite matrix. The evaluation of the effect of MWCNTs on drug release rate by dissolution test and antimicrobial susceptibility test was performed. Sharp release of the drug was found at early stages (∼1 h), but the rate was reduced afterwards, showing a sustained release. It was observed that for all microorganisms, the antibacterial activities of drug loaded MWCNTs/gelatin-chitosan nanocomposites were higher than that of drug loaded gelatin-chitosan composite films containing no MWCNTs. Comparative statistical studies by ANOVA techniques also showed remarkable difference between the antibacterial activities, exhibited by MWCNTs-incorporated and non-incorporated composite films.
Keywords: Carbon nanotubes, Functionalization, Nanocomposite, Thermo-mechanical properties, Drug dissolution, Antibacterial activities
Graphical abstract
Highlights
-
•
Double functionalization of CNTs in aqueous media.
-
•
Dispersed CNTs were incorporated into gelatine-chitosan blend matrix to prepare nanocomposite.
-
•
Thermal, mechanical, water uptake, cytotoxic property, structural and morphological characterization of that nanocomposite.
-
•
Evaluation of drug dissolution and antibacterial activities of the prepared nanocomposite.
1. Introduction
Carbon nanotubes (CNTs) have been highly attractive and extensively explored by the researchers in various fields such as chemical, physical, materials and biochemical sciences due to their unique and versatile nanostructures with remarkable mechanical, thermal, electrical and optical properties [[1], [2], [3], [4]]. Due to their structural and mechanical properties, CNTs can be used in making composites that are useful in tissue engineering and also suitable for regenerative medicine therapeutics as delivery vehicles for drugs and gene therapy [5]. CNTs have been shown to empower high Young's modulus (∼1 TPa) due to the flexible hexagonal network of carbon atoms which ultimately directed to their excellent mechanical properties [6]. Furthermore, incorporation of CNTs has been recognized as substantial improvement of the mechanical and structural properties of polymer composites [7,8]. These composites can also be used as molecular-level building blocks for the complex and miniaturized medical devices, which have enormous applications in biomedicine [9]. CNTs have positive impacts on cell differentiation and proliferation as well [6,[10], [11], [12], [13]].
However, CNTs and CNTs/polymer composites represent a set of problems relating to the carcinogenic risk from the exposure and persistency of MWCNTs in human bodies. Some of the researches published that the untreated long MWCNTs which were injected, induced some unwanted responses in the abdominal cavity of mice. So, the treatment of CNTs is mandatory. Not only this, CNTs have a tendency to form a network of agglomeration which ultimately increases the difficulties to disperse them within the polymer matrix during processing due to strong resistance to wetting, high inter-particle van der Waals attractions and high specific area [14,15]. So, preparation of successful CNTs/polymer composites depends on the achievement of stable and well dispersed CNTs in the solvents or polymer matrix. Common techniques widely used for preparing CNTs/polymer composites are solution casting providing numerous advantages such as large area coverage, structural flexibility, low temperature process ability and low cost, although solubility of polymers is essential for this method [[16], [17], [18]]. Both the dispersion and carcinogenic problems of CNTs can be overcome by functionalizing them with surfactants, acids or other agents. Many studies have shown the successful functionalization of CNTs covalently with acid treatment and non-covalently with many other agents [[19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29]].
However, not all chemical treatments alleviate the toxicity risks but only reactions that are able to render carbon nanotubes short and stable suspension without aggregation in the body fluids can provide safe results, risk-free material. Again, the binding affinity by non-covalent interaction between the CNTs surface and the agents is not strong enough, so the dispersion ability of CNTs in aqueous media is still not satisfied for biomedical applications. Therefore, covalent functionalization of the MWCNTs is necessary.
Thus, MWCNTs were functionalized chemically with mix acids, H2SO4/HNO3 (3:1, v/v) followed by addition of polyethylene glycol (PEG). Acid treatment introduces oxygen containing functional groups (−OH, >CO, and —COOH), which furnishes the dispersion ability of MWCNTs. Although oxidized MWCNTs are soluble in water, they have a tendency to show charge screening affect i.e. aggregate in the presence of salts which makes the direct use of MWCNTs limited for biological applications as the salt content is high in most biological solutions, opsonisation [30]. For this reason, hydrophilic PEG is attached to the oxidized CNTs to make more stable CNTs-polymer conjugates in biological environments [[31], [32], [33]]. Nanoparticles, which require long circulation times in blood, usually need particular types of ligand and PEG is exceptionally found to be compatible as a ligand in high concentrations of salts and in extremes of pH [[34], [35], [36]].
In the present work, gelatin and chitosan have been chosen due to their water soluble and film forming properties. The major advantages of these are good cytocompatibility, biodegradability and no surgery is required for removal of polymers [37]. Gelatin is a well characterized gel forming protein fragment obtained by partial degradation of water insoluble collagen fiber commercially available at relatively low cost [38]. Chitosan is a nontoxic, biocompatible, biodegradable polymer [[39], [40], [41]] but has a limitation of being unstable in an aqueous medium. The shortcomings of chitosan film in solution and the easy degradation of rigid gelatin in the swollen state seem to be overcome by using a gelatin-chitosan composite. Gelatin and chitosan are natural polyelectrolytes that may form polyelectrolytic complex via interactions of ammonium ions (-NH3+) of the chitosan and carboxylate ions (-COO-) of the gelatin in the blend system to make it more stable. This might facilitate the delivery of antibiotic drugs such as ciprofloxacin, which were directly loaded on the nanotube surface via π-π stacking and H-bonding. Here we present the formulation of homogeneously dispersed MWCNTs/gelatin-chitosan nanocomposites with high mechanical, thermal and swelling properties as well as their improved drug release capacity.
2. Experimental
2.1. Materials and chemicals
Multi-walled carbon nanotubes (MWCNTs), NC7000 series of 90% purity, showing approximately average diameter of 9.5 nm and 1.5 μm length, produced by the catalytic chemical vapor deposition (CCVD) method were purchased from NANOCYL™, Belgium. Gelatin Type B (Bloom strength-240, Pharmaceutical grade, pyrogen free) was purchased from the OPSO Saline Limited, Bangladesh and chitosan of 90% deacetylation degree having molecular mass of 1100 kDa (medicine grade) was purchased from Yuhuan Ocean Biochemistry Co. Ltd, China. Polyethylene glycol (PEG) was purchased from BASF (Lutrol E 400, Macrogol 400 ph. Eur), Bangladesh. All other chemicals and solvents were of reagent grade.
Seven bacterial strains, including 3 gram-positive (Bacillus subtilis, Staphylococcus aureus, Listeria monocytogenes) and 4 gram-negative (Escherichia coli 0157, Salmonella enteritidis, Salmonella typhi, Klebsiella pneumoniae) were obtained from the Center for Advanced Research in Sciences (CARS), University of Dhaka, Bangladesh.
2.2. Processing
Raw MWCNTs of a definite weight (1.0 gm) were added into 50 ml 3:1 mixture (v/v) of concentrated H2SO4 (98%)/HNO3 (68%) with sonication at 40 °C for 10 h, diluted with water followed by filtration, washed with deionized water and dried overnight in vacuum at 80 °C to obtain primarily functionalized MWCNTs (MWCNTs-COOH) as a dry powdered form.
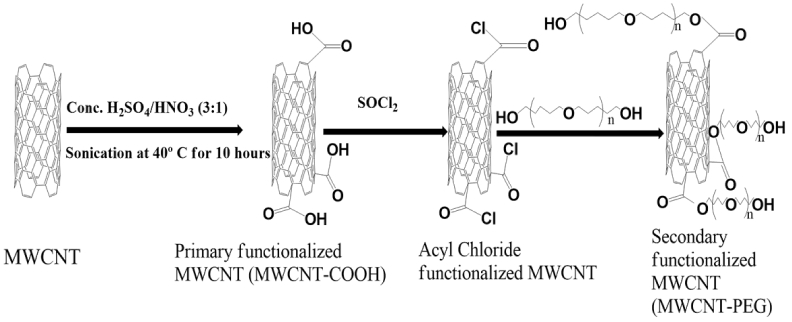
The secondary functionalization of MWCNTs was carried out by transforming the carboxyl groups of the oxidized MWCNTs into acyl chloride groups by stirring the dispersion with SOCl2 for 24 h at 65 °C. After that the dispersion was washed off with THF, filtered and dried overnight at ambient temperature. The acyl chloride-functionalized MWCNTs were continuously stirred in PEG (400 g/mol) at 120 °C for 48 h. Then, the resultant mixture was washed using THF and filtered through a Teflon W membrane, followed by drying at ambient temperature for 48 h to get secondary functionalized MWCNTs (MWCNTs-PEG). The schematic diagrams of the functionalization reactions (primary and secondary) are shown in Fig. 1.
Fig. 1.
Reaction scheme for primary and secondary functionalization of MWCNTs.
The films were prepared by blending chitosan (1%) and gelatin (10%) solutions thoroughly for 1 h followed by casted on the silicon cloth covered frame mounted on flat glass plate for film formulation. The film was dried in laminar air flow overnight and peeled off. Dispersed MWCNTs were added into the solution of chitosan (1%)-gelatin (10%) mixture , which was then stirred for 30 min to homogenize and sonicated for 1 h to remove air bubbles before film casting.
Stock solution of ciprofloxacin lactate was prepared in the concentration of 1% (w/v). The prepared drug solution was then added to 0.25% (wt/wt) MWCNTs-COOH/gelatin-chitosan nanocomposite and 0.25% (wt/wt) MWCNTs-PEG/gelatin-chitosan nanocomposite solutions respectively such that 10 μg of the drug was present in 0.28 cm2 area of the nanocomposite films (this area is equal to the area of a standard antibiotic disc).
2.3. Characterization
The attachment of the functional groups on the surface of MWCNTs was identified by the Fourier transform infrared (FTIR) spectrophotometer, Imprestige-21, Shimadzu Corporation, JAPAN (wave number range of 400–4000 cm−1), equipped with an attenuated total reflectance (ATR) device (wave number range of 700–4000 cm−1 with 20 scanning rate and resolution of 4 cm−1) for the confirmation of gelatin-chitosan interaction in the composites.
The mechanical properties of the composites were determined by universal testing machine (UTM), Hounsfield Series S Testing Machine (UK), conducting load of 500 N at room temperature. The gauge length was 110 mm having crosshead speed of 10 mm/s throughout the experiment. The test specimens were cut into a dumbbell or dog bone shaped following the standard ISO 3167 (specimen size: 150 mm long with the center section having 80 mm × 10 mm dimensions).
Thermogravimetric analysis (TGA) was carried out in a TGA-50H SHIMADZU thermo-gravimetric analyzer, Japan, under nitrogen atmosphere (10 ml/min) and with a rate of heating of 10 °C/min from room temperature to 600 °C.
The water sorption resistance of the nanocomposites was evaluated by weighing square pieces of dry samples (Wd), immersing these in distilled water with pH = 7.0 for 48 h at 25 °C. The samples were then removed from water periodically, blot dried and weighed (Ww) again and thus the swelling percentage was calculated by using the equation: Swelling (%) = [Ww–Wd/Wd] × 100.
As the body temperature of humans and several other mammals is 37 °C thus every chemical reaction that occurs has its optimal rate at that temperature. Therefore, the swelling behavior of composites was also measured in phosphate buffer solution showing pH 7.4 at 37 °C. Each point presented here was the mean value of triplicate measurements and the results were expressed as percentage of swelling calculated from upper equation.
The surface morphology of the MWCNTs/gelatin-chitosan nanocomposites (sputter-coated with platinum) was studied with scanning electron microscopy (SEM) (before & after tensile tests, respectively) and elemental analysis of pristine CNTs and functionalized CNTs were done with energy dispersive X-ray (EDX) spectroscopy using a JEOL 6400 SEM at an accelerating voltage of 25 keV.
The dissolution studies were conducted with universal dissolution tester, UDT-804, LOGAN INTRUMENTS CORP, USA, using phosphate buffer (pH = 7.4) at constant temperature of 37 °C. The films (4 cm2 with a thickness of 0.3 mm) were placed such that they remain dipped in buffer solution and rotated in 100 rpm paddle speed. 10 ml of the sample was withdrawn in every 10 min and the concentration of drug in each portion was estimated using UV/Visible spectrophotometer at the respective wavelength. Equal volume of fresh buffer solution was replenished with each portion of the solution withdrawal.
The amount of drug ciprofloxacin released in the buffer solution was determined by UV-1800 UV/Vis spectrophotometer, SHIMADZU, Japan. Ciprofloxacin absorbed the light at 273 nm.
In case of cytotoxicity, brine shrimp lethality bioassay technique of Meyer [42] was applied for the determination of cytotoxic property of the aqueous solution of raw MWCNTs and MWCNTs-PEG/gelatin-chitosan nanocomposite. Stock solution of 100 mg/100 ml was prepared and then it was dissolved in DMSO to prepare solutions of varying concentrations such as 400, 200, 100, 50, 25, 12.5, 6.25, 3.125, 1.563 and 0.78125 μg/ml by serial dilution technique.
The controls used in this work were vincristine sulphate (positive) and DMSO (negative). The solutions were added to the vials which were marked previously and contained 10 brines shrimp nauplii (alive) in 5 ml simulated sea water and after 24 h the vials were inspected using a magnifying glass and counted the number of survived nauplii in each vial.
Antibacterial activity test of composite films was performed using agar diffusion method with selected gram-positive (Bacillus subtilis, Staphylococcus aureus and Listeria monocytogenes) and gram-negative (Escherichia coli 0157, Salmonella enteritidis, Salmonella typhi and Klebsiella pneumoniae) bacterial species. The zones of inhibition were determined by placing a definite size of film into discs in inoculated solidified nutrient agar medium in a petri dish which was incubated for 24 h at 37 ± 1 °C. This was done in triplicate manner with each film for each organism and an average diameter of zone of inhibition was recorded in millimeters.
3. Results and discussion
3.1. Infrared spectroscopy
3.1.1. FTIR analysis of functionalized MWCNTs
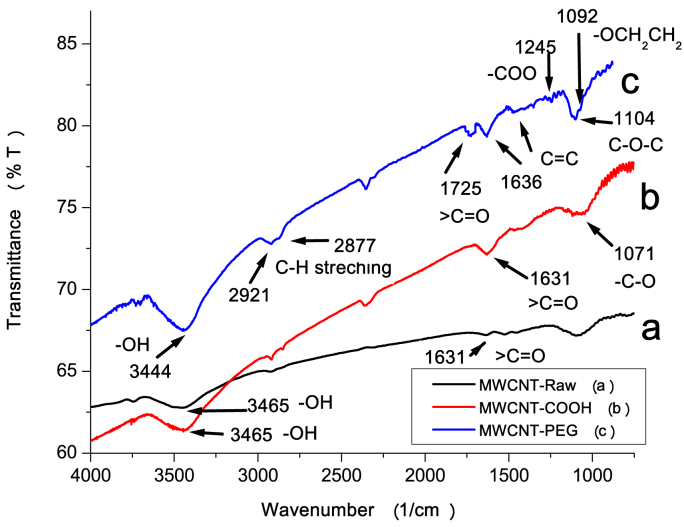
The FTIR spectra of MWCNTs-Raw, MWCNTs-COOH and MWCNTs-PEG are shown in Fig. 2. From the IR absorption spectra, it was found that the IR absorption spectrum of MWCNTs-COOH mainly consisted of -OH stretch at 3465 cm−1 and carboxyl >C O stretch at 1631 cm−1 which are the characteristic peaks and could also be found in the IR spectrum of the raw MWCNTs. However, the intensity of the two peaks in MWCNTs-Raw spectrum was much lower than that in MWCNTs-COOH spectrum. In addition, MWCNTs-COOH has a new peak of small intensity at 1725 cm−1, which may be stretching vibrations of carbonyl groups (C O) as carboxylic groups were formed during the oxidation of hydroxyl compounds. The bands in 1071 cm−1 region demonstrate the presence of C–O bonds in MWCNTs-COOH.
Fig. 2.
FTIR spectra of raw and functionalized MWCNTs.
The appearance of -OH and >C O in MWCNTs-Raw is mainly attributed to the non-stoichiometric crystal water in KBr and the original defects on MWCNTs, and the appearance of carboxyl and hydroxyl groups in MWCNTs-COOH suggests that during the chemical-oxidation process, part of the sidewall carbons were oxidized to these groups.
From the FTIR spectrum of MWCNTs-PEG, C C stretching mode of the aromatic ring was observed between 1636 cm−1 and 1540 cm−1. The stretching vibration of >C O in ester at 1725 cm−1, characteristic absorption peak of C—O—C at 1104 cm−1, asymmetric and symmetric stretching of C—H deformation at 2921 and 2877 cm−1 were also found in the spectrum, which were not found in the spectrum of the other two samples. It could also be observed that the stretching vibrations of the repeating -OCH2CH2 units of PEG and -COO bonds were at 1092 cm−1 and 1245 cm−1 respectively, which is the proof of a successful secondary (PEGylation) functionalization reaction.
3.1.2. ATR–IR analysis of MWCNTs/gelatin-chitosan composites
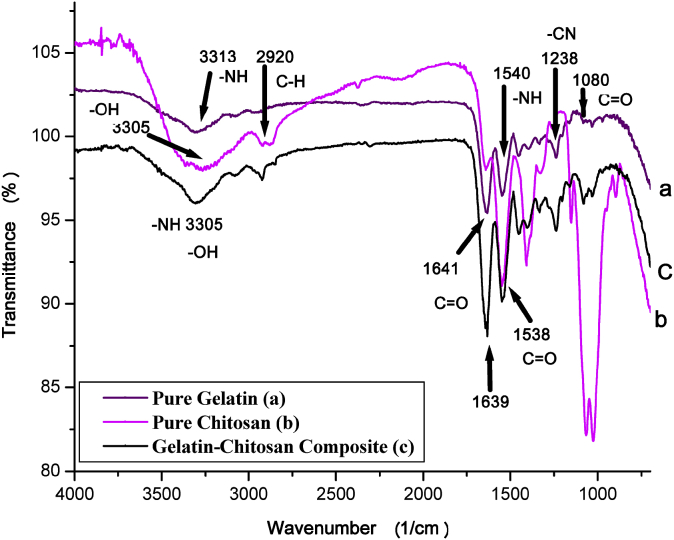
ATR-IR was measured for gelatin, chitosan and gelatin-chitosan composite and their corresponding spectra are shown in Fig. 3. Gelatin and chitosan showed their typical ATR-IR spectrum however, from the ATR-IR spectrum of gelatin-chitosan blend, it is seen that the peak at 1641 cm−1 was shifted to 1639 cm−1 with same relative intensity because of the degree of amidization between the ammonium (-NH3+) ions of the chitosan and the carboxylate (-COO−) ions of the gelatin through partial conversion of electrostatic bonds into chemical bonds. The broader peak at 3305 cm−1 indicates the presence of a polymeric associated hydroxyl group.
Fig. 3.
ATR spectra of (a) pure gelatin, (b) pure chitosan and (c) gelatin-chitosan blend.
3.2. Mechanical properties
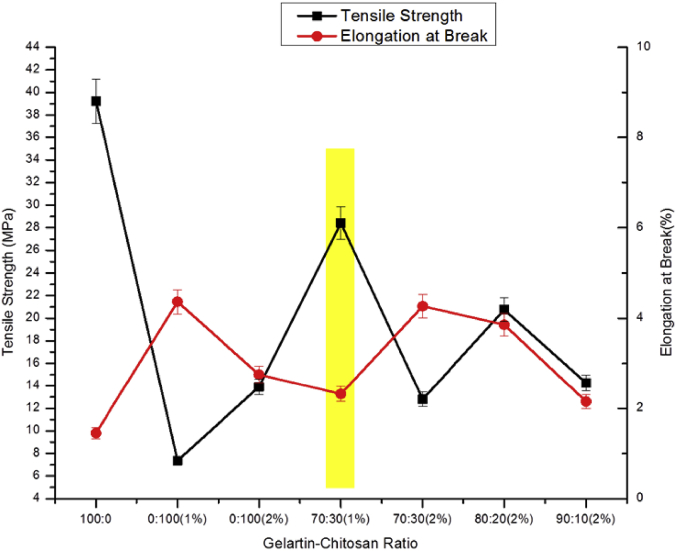
The physico-mechanical properties i.e., tensile strength (TS), elongation at break (EB) and tensile modulus (TM) of the pure gelatin, pure chitosan, gelatin-chitosan composite and MWCNTs/gelatin-chitosan nanocomposites were investigated. It is revealed from Fig. 4 that the TS was highest for pure gelatin and the EB was lowest where it was reciprocal for pure chitosan (1%). However, 2% pure chitosan showed higher tensile strength than 1% pure chitosan with lower elongation at break. Suitable films of gelatin-chitosan blends were prepared to expiate both the TS and EB. From Fig. 4 it has been observed that introducing flexible chitosan into comparatively rigid gelatin increases the TS as intermolecular interactions of gelatin was weakening coupling with the chitosan.
Fig. 4.
Effect of gelatin and chitosan loading on TS and EB of the composites.
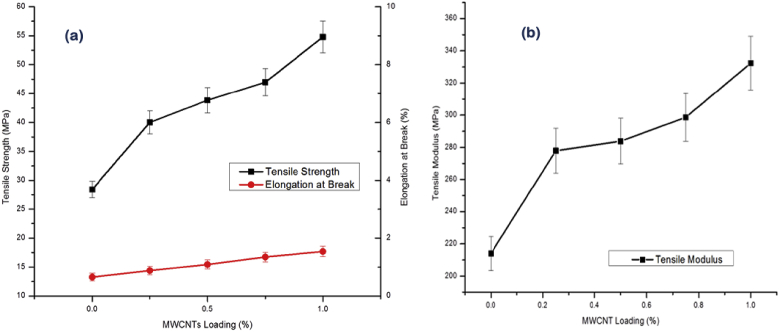
From Fig. 5a, it is observed that the successive incorporation of the MWCNTs (up to 1.0 wt %) in gelatin-chitosan composite increased the TS up to 92.82% and EB up to 33.21% compared to the gelatin-chitosan composite having no MWCNTs.
Fig. 5.
Effect of MWCNTs loading on (a) TS and EB and (b) tensile modulus of the nanocomposites.
This increase in TS of the CNTs loaded gelatin-chitosan composites hints that the mobility of the molecular chains of the blends was hindered due to the presence of MWCNTs in the matrix. Because of the resilient properties of MWCNTs, homogeneously dispersed MWCNTs might add rigidity and hardness via interfacial interaction with the gelatin-chitosan matrix. The stress that build-up at this interface during extension, has been transferred from the matrix to the nanotubes and the transfer of the load depends on the interfacial shear stress between the matrix and the nanotubes. Again, by the high degree orientation of MWCNTs in the matrix induce the domains of crystallinity which ultimately absorb energy through their highly flexible elastic behavior during deformation [43].
The effect of MWCNTs on EB is inconsistent to the established relationship between EB and TS. Although EB is known to response inversely with the change in TS, but Fig. 5a showed the increase in EB with the successive addition of MWCNTs due to one special type of property entitled as tunneling effect of nanomaterials [44,45]. As a filler of a matrix, generally nanomaterials create channels through which they dissipate energy and impart toughness to the matrix. Thus tunneling effect of MWCNTs is considered to increase the EB of the nanotube reinforced matrix. Similarly, TM of the composites was also increased by 55.33% for 1% (wt/wt) loading of MWCNTs as shown in Fig. 5b.
3.3. Thermal property analysis
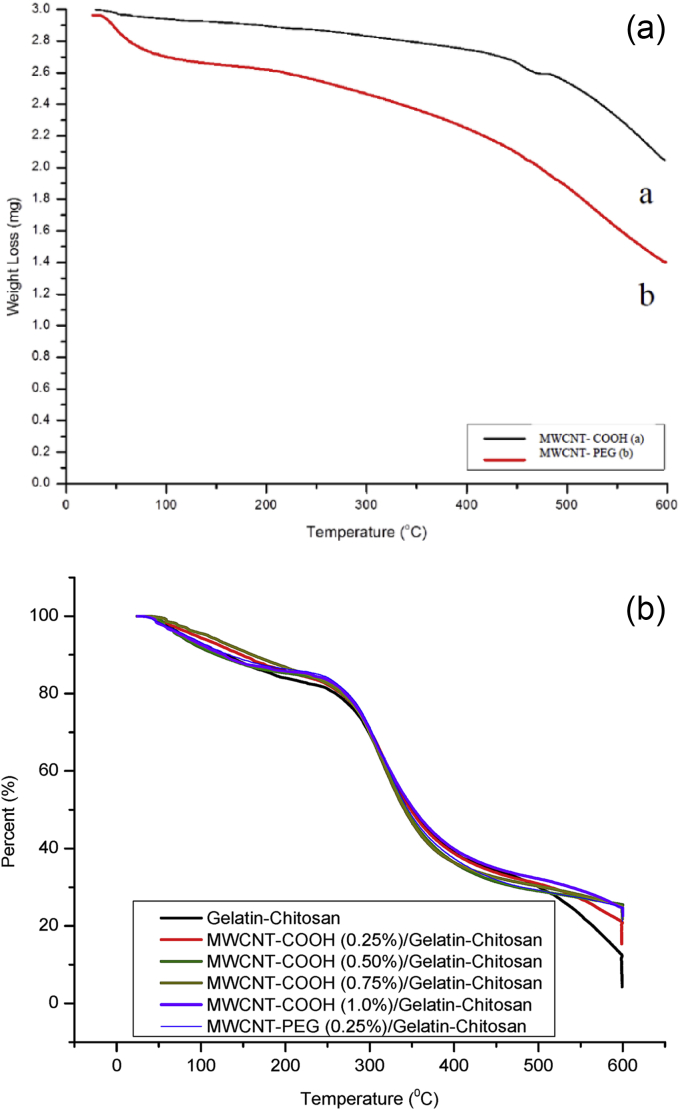
Thermal property analyses are considered in determining the functional groups in recent times, and TGA is the technique that used widely for this purpose as it is simple and the information can be presented by a simple thermogram. Fig. 6a displays the thermograms of primary and secondary functionalized MWCNTs.
Fig. 6a.
TGA analysis of (a) carboxylated MWCNTs, and (b) PEG-grafted MWCNTs; Fig. 6b. TGA analysis of different composite films.
From Fig. 6a it is found that both oxidized and PEG-grafted MWCNTs show weight loss between 90 °C and 600 °C. Significant weight loss in case of MWCNTs-PEG indicates that amino functional groups are more stable and they could participate in some thermal reactions at temperatures more than 600 °C. As the reaction had been carried out in the absence of a solvent with high concentration of low molecular weight PEG, it is prospective that during the washing process unreacted PEG was completely washed out. Thus, the mass loss occurred only from the covalently attached PEG on the surface of the MWCNTs.
Fig. 6b represents TGA thermograms of gelatin, chitosan, gelatin-chitosan and MWCNTs/gelatin-chitosan nanocomposites. Except chitosan, all the samples exhibited a small weight loss (6–8%) at 80–90 °C as water molecules had been evaporated. Due to the hydrophilic nature of chitosan, the greater weight loss was observed at this temperature. The second weight loss was found to begin at about 210 °C and the corresponding weight loss of about 57% is attributed to the decomposition of chitosan. The total weight loss was 59% at about 500 °C .
The TGA plot of pure gelatin along with all composites showed three thermal stages. The first stage indicates the loss of water as well as NH3 and CO2 gases from the biopolymers between 25 °C and 220 °C followed by gelatin decomposition between 250 °C and 450 °C because of the disintegration of intermolecular and partial breaking of the molecular structure and finally combustion of the remaining material between 450 °C and 750 °C.
The presence of MWCNTs (both primary and secondary functionalized) had little effect on thermal property of gelatin-chitosan composite. The decomposition temperature at the end was abated with the addition of MWCNTs. The TGA curve of 1.0 wt% MWCNTs loaded composite shifted towards the higher temperatures compared to that of others. At above 380 °C, chitosan completely decomposed to residues whereas the composites displayed the best thermal stability. At the temperatures above 480 °C, gelatin underwent weight loss which indicates the chemical degradation resulting from bond scission in the polymeric backbone. The reason of thermal stability of nanocomposites may be due to physico-chemical adsorption of gelatin-chitosan polymer chains onto the MWCNTs surface. MWCNTs can easily arrange themselves within the helical structure of gelatin-chitosan protein polymer chain due to their nanosize and establish strong hydrophobic attraction with hydrophobic ends of amino acids chain.
3.4. Swelling behavior
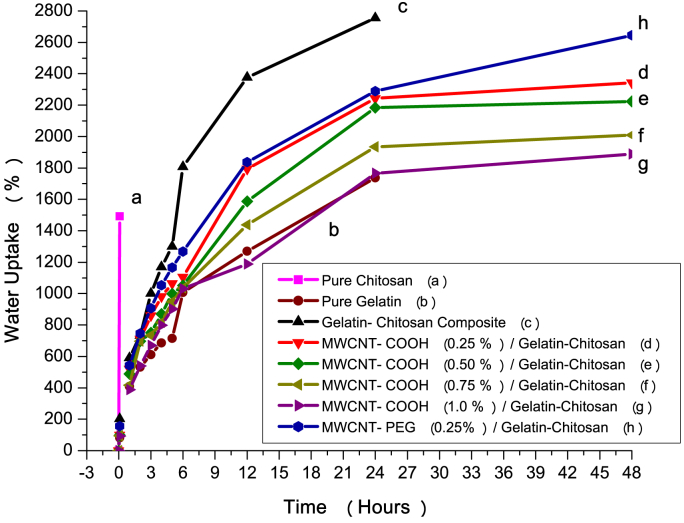
Degree of swelling (calculated from the average of three independent trials of nanocomposites) was investigated to comprehend the influence of MWCNTs on the swelling behavior of the nanocomposites. It is evident from Fig. 7 that the uptake of water was notably higher for pure chitosan, moderate for gelatin-chitosan blends and least in case of pure gelatin. Fig. 7 also indicates that chitosan dissolved after 10 min. This is because chitosan has a shortcoming as it becomes unstable in aqueous medium due to the salt formation between ammonium (-NH3+) ions of the chitosan chains and the carboxylate (-COO-) ions of its solvent, acetic acid. It was observed that gelatin-chitosan film showed much higher water uptake than the pure gelatin film. The swelling of the gelatin-chitosan film would increase by inducing free -OH, -NH2, and -NHOCOCH3 groups due to the increasing chitosan content in the blend. However, network structure (through the electrostatic interaction between the ammonium -NH3+ ions of the chitosan and -COO- ions of the gelatin) might be generated by increasing the content of chitosan which decreases the swelling due to stiffness of complex chains. This combination of opposite effects thus produced swelling of the gelatin-chitosan films, which was more than the pure gelatin film.
Fig. 7.
Swelling behavior of pure chitosan, pure gelatin, gelatin-chitosan composite and different MWCNTs/gelatin-chitosan nanocomposites.
Addition of small amount (0.25 wt%) of MWCNTs in gelatin-chitosan polymer matrix enhanced the hydrophobicity to the films. So, the swelling of the MWCNTs/gelatin-chitosan composites became lower than that of gelatin-chitosan composites. This effect of MWCNTs can be attributed to shielding effect which is due to the fact that nanotubes were distributed irregularly in the gelatin-chitosan matrix and timbered a well-developed three dimensional network [46]. As a result of which MWCNTs could prevent solvent diffusion into gelatin-chitosan polymer matrix. At 1.0% (wt/wt) MWCNTs loading in the nanocomposite, lowest swelling was observed when compared with that of gelatin-chitosan blend. From Fig. 7 it is also observed that the swelling behavior of the nanocomposite with secondary functionalized MWCNTs (MWCNTs-PEG) is slightly higher, which might be due to the capillarity of MWCNTs-PEG.
Moreover, after 24 h pure gelatin underwent complete disintegration. On the contrary, it was still possible to handle gelatin-chitosan blend and MWCNTs incorporated nanocomposites. However, after 48 h it was only possible to stretch the nanocomposites without any rapture but could not sustain any stress. The hydrophobic nature of the nanocomposites was enhanced as the interactions between nanotubes and the blends impeded the penetration of the water molecules so that they took longer time to compete for the same hydrophilic sites along the molecular skeleton of blends. Statistical significance was determined among the three composites using ANOVA for their swelling behavior but there is no significance difference was observed as shown in Tables S1 and S2 (Supporting Information).
3.5. In vitro degradation
Buffer uptake of the films was investigated as the time to degrade will affect the condition of cell growth. From the water uptake results, swelling behavior of different composites were obtained and thus selective films were used in this test in order to compare the in vitro degradation result. It was found that with the increase of swelling time buffer uptake was also enhanced and gelatin-chitosan composite dissolved after 40 min in the buffer solution which represents simulated body fluid. As the swelling time in the buffer was increased, the concentration of bond charges within the composite became higher than the concentration of salt in the external solution; thus the film was expanded by large ion-swelling pressure and the composite showed increasing sensitivity to ionic solution.
From Fig. 8 it was also observed that the MWCNTs-COOH/gelatin-chitosan composite dissolved more rapidly (30 min) than the gelatin-chitosan composite, due to the charge screening effect as -COOH aggregate in the emergence of salts. The sensitivity to ionic solution increased as bond charge concentration within the composites became higher than the concentration of salt in the external solution. The MWCNTs-PEG/gelatin-chitosan composite kept swelling up to 45 min and afterwards dissolved slowly. In this way, MWCNTs-PEG maintained the structure of the composite and thus suitable for drug delivery system.
Fig. 8.
In vitro degradation of different composite films.
3.6. Morphology of MWCNTs/gelatin-chitosan composite
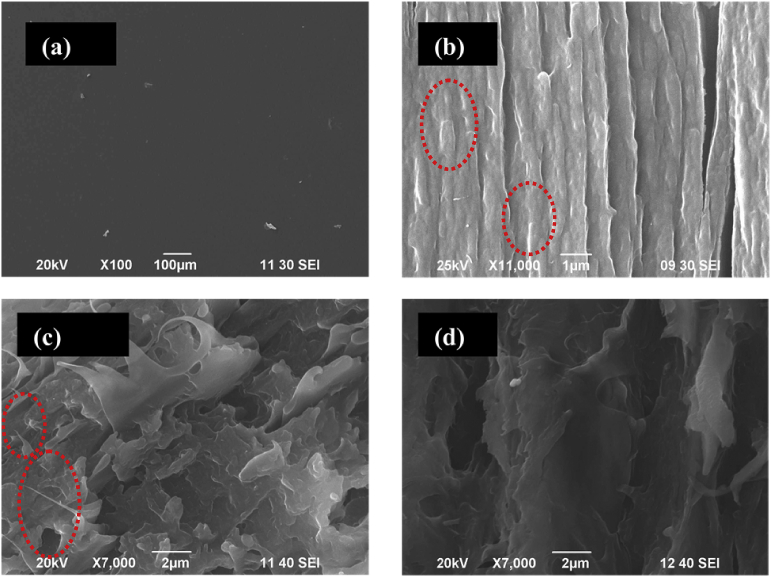
The surface and interface of the MWCNTs-COOH/gelatin-chitosan nanocomposite (0.25 wt% MWCNTs) were investigated by scanning electron microscopy (SEM) as shown in Fig. 9a and b, respectively. It is conspicuous that MWCNTs were not seen on the surface of the composite and clean surface of nanocomposite is attributed to the fact that MWCNTs penetrated through the gelatin-chitosan matrix ensuring their homogeneous distribution. The tips of the MWCNTs, which were punctured and visible on the interface of the surface, were liable for conferring higher mechanical properties and thermal stability to the composites. Resembling SEM images for the interface of CNTs reinforced polymer-based nanocomposites have been reported and the result of this research is similar like them. [ 44]
Fig. 9.
SEM images of (a) surface and (b) interface of MWCNTs-COOH/gelatin-chitosan composite (punctured tips of the MWCNTs is visible inside the dashed circular area); (c) fractured surfaces of MWCNTs-COOH/gelatin-chitosan composites (pulled out tips of the MWCNTs is visible inside the dashed circular area) and (d) pure gelatin film.
Composites bearing small amounts of chitosan possess micro pores which may be beneficial for the diffusion of metabolites and nutrients inside the three-dimensional structures. The fractured surfaces of the pure gelatin film (Fig. 9d) exhibited condensed structure with small pores and thick walls, whereas open structure (foliaceous morphology) in gelatin-chitosan composite (Fig. 9c). The fractured cross section also showed that nanotubes pulled out from the matrix and cracking due to stress. Open leafy structure might provide a higher ionic gradient due to easy penetration of the ions through its open structure which improved the bending in an electro-mechano-chemical experiment.
3.7. Drug release test (dissolution test)
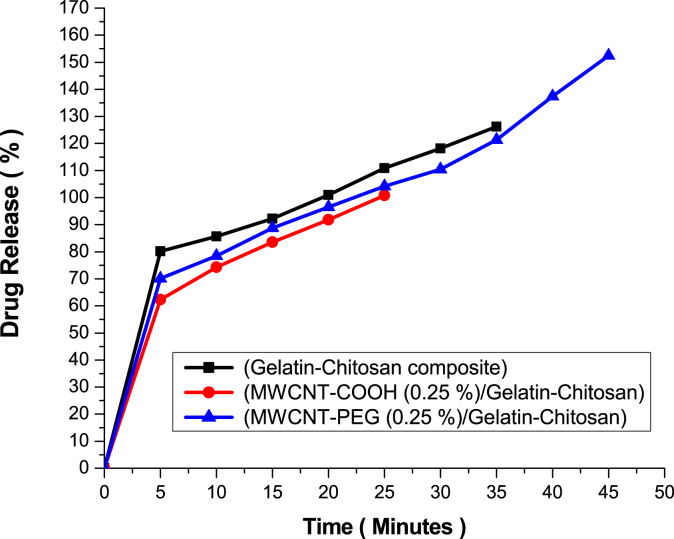
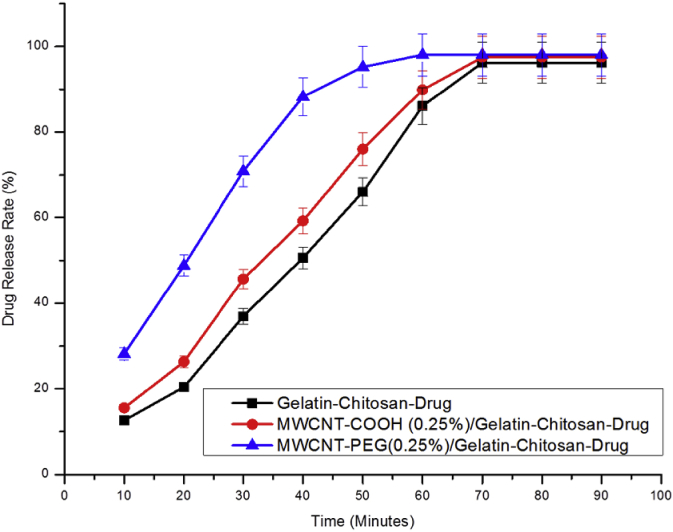
The time required to release drug completely from selected drug loaded films for eliciting its antimicrobial action was obtained by in vitro diffusion studies through the percentage drug release. The loaded drugs in the nanocomposite films were gradually diffused into the buffer solution and reached to the equilibrium at the end during swelling. As gelatin-chitosan film is water-soluble, ciprofloxacin loaded composite films could be easily dissolved in buffer solutions, causing a large initial burst at short time. Fig. 10 showed that release rate of ciprofloxacin was much higher in case of MWCNTs-PEG/gelatin-chitosan drug loaded films than MWCNTs-COOH/gelatin-chitosan nanocomposite films and drug loaded gelatin-chitosan films. However, ANOVA analysis did not show significance difference among the different composites in terms of their drug release rate as shown in Table S3 and S4 (Supporting Information). After 70 min of release, it reached a plateau. The feasible reason of the lower drug release rate with gelatin-chitosan might be due to the presence of existing ionic forces between gelatin-chitosan and presence of —COOH group in drug ciprofloxacin. In case of MWCNTs-COOH nanocomposite, MWCNTs offered diffusion of the drug because CNTs have -COOH groups attached in their outer tube shell as a product of acid treatment (primary functionalization), strengthened the interaction between MWCNTs-COOH and amino acid groups of films and thus drug ciprofloxacin got a much lesser opportunity to react with gelatin-chitosan matrix.
Fig. 10.
The release rate (%) of ciprofloxacin from different composite films at pH 7.4.
The nanoparticles which require long circulation times in blood need particular types of ligand. PEG is exceptionally compatible as a ligand in high concentrations of salts and in extremes of pH; it decreases the degree of opsonisation and thus provides extra-ordinary long-term stability. Biofunctionalization and selective attachment to nanotube surfaces can be achieved by altering and coupling the PEG group derivatives. As MWCNTs-PEG contains -COO-PEG groups attached in their outer tube shell as a product of PEG treatment (secondary functionalization), it showed faster diffusion of the drug. This procedure was carried out concerning the body fluid environment, thus it would also be effective for the simulated condition of animal study. It was found that 98% of ciprofloxacin drug was liberated within 90 min, indicating the films can be used as a carrier for sustained release as they do not interfere with drug release on wound.
3.8. Brine shrimp lethality (cytotoxicity)
Dispersed aqueous solution (100mg/100 ml) of raw MWCNTs and MWCNTs-PEG/gelatin-chitosan nanocomposite was used to determine the cytotoxic property. For each sample concentration, the mortality (in percent) of the brine shrimp nauplii was calculated. A plot of log concentration of the sample versus percent of mortality displayed a linear correlation between them. The result of the brine shrimp lethality test revealed that raw MWCNTs exhibited no toxicity towards the brine shrimp. The mortality rate of the brine shrimp was found to be very small extent of the highest concentration of MWCNTs (400 μm/ml) for both raw MWCNTs and MWCNTs-PEG/gelatin-chitosan nanocomposite. From the first dilution, the mortality affected by both raw MWCNTs and MWCNTs-PEG/gelatin-chitosan nanocomposite was nil. The results of cytotoxicity are shown in Tables S5 and S6 (Supporting Information).
3.9. Antimicrobial susceptibility testing
The result of the antimicrobial activities of the individual drug loaded gelatin-chitosan, drug loaded MWCNTs-COOH/gelatin-chitosan and drug loaded MWCNTs-PEG/gelatin-chitosan nanocomposite films has been represented in Table 1. The investigation was conveyed by Mueller-Hinton [ [47]] agar disk diffusion susceptibility testing method according to NCCLS (National Committee for Clinical Laboratory Standards). The loading of the drug was fixed (10μg/0.28 cm2) for gelatin-chitosan blend, MWCNTs-COOH/gelatin-chitosan composite, MWCNTs-PEG/gelatin-chitosan nanocomposite film and standard disks.
Table 1.
Antibacterial activity of drug loaded Gelatin-chitosan, MWCNTs-COOH/gelatin-chitosan, MWCNTs-PEG/gelatin-chitosan nanocomposites and standard Ciprofloxacin.
| Test Microorganisms | Diameter of zone of inhibition (mm), (Mean ± S.D.) |
|||
|---|---|---|---|---|
| Gelatin-chitosan | MWCNTs-COOH/gelatin-chitosan | MWCNTs-PEG/gelatin-chitosan | Ciprofloxacin | |
| Gram-positive bacteria | ||||
| Bacillus subtilis | 25.73 (±0.03) | 26.03 (±0.0911) | 26.93 (±0.0818) | 21.98 (±0.0361) |
| Staphylococcus aureus | 25.11 (±0.1559) | 30.23 (±0.0819) | 30.8 (±0.1414) | 25.85 (±0.0424) |
| Listeria monocytogenes | 24.88 (±0.1473) | 37.04 (±0.0748) | 37.14 (±0.0173) | 24.53 (±0.0224) |
| Gram-negative bacteria | ||||
| Escherichia coli 0157 | 28.22 (±0.1414) | 29.13 (±0.1367) | 31.85 (±0.0707) | 23.98 (±0.1393) |
| Salmonella enteritidis | 24.20 (±0.1414) | 28.05 (±0.1308) | 28.75 (±0.0374) | 24.23 (±0.0141) |
| Salmonella typhi | 21.42 (±0.1204) | 31.93 (±0.0819) | 30.62 (±0.0141) | 21.07 (±0.1797) |
| Klebsiella pneumoniae | 24.93 (±0.0866) | 25.27 (±0.1634) | 29.5 (±0.1044) | 23.99 (±0.0332) |
Present investigation confirmed that the zone of inhibition for drug loaded MWCNTs-PEG/gelatin-chitosan nanocomposite was the highest against all the bacterial species compared to drug loaded gelatin-chitosan blend, MWCNTs-COOH/gelatin-chitosan composite and even standard disc as well. It might be possibly due to the fact that MWCNTs and PEG have some additional antimicrobial activity. In order to confirm the fact we have also studied the antibacterial activities of the blank films of gelatin, chitosan, gelatin-chitosan blend, MWCNTs-COOH/gelatin-chitosan composite and MWCNTs-PEG/gelatin-chitosan composite. The test results are presented in Table S7 (Supporting Information) and it was found that pure gelatin films did not show any antibacterial activity whereas pure chitosan and all the composite films showed moderate sensitivity against all the bacterial species. The reason for no antibacterial activity of pure gelatin is that gelatin is a protein and might not inhibit the growth or may promote the growth of bacteria. To find out whether there is any significant difference among the three drug loaded composites on the zone of inhibition, statistical significance was determined by means of ANOVA test and the results thus obtained are summarized in Tables S8 and S9 (Supporting Information). It is evident from ANOVA table that the zones of inhibition for nanocomposites MWCNTs-COOH/gelatin-chitosan and MWCNTs-PEG/gelatin-chitosan are significantly different from composite gelatin-chitosan (P < 0.05), but nanocomposite MWCNTs-PEG/gelatin-chitosan is not significantly different from nanocomposite MWCNTs-COOH/gelatin-chitosan (P > 0.05). However, we conclude that the MWCNTs-PEG/gelatin-chitosan nanocomposite has the strongest antibacterial activity among the tested samples (Table 1) and the results completely support the drug release assessment in dissolution tests for the samples (Fig. 10). Even though some bacterial plates were found to contain colonies, but the zones of inhibition were very clear and the zones of inhibition obtained for each sample against seven different bacterial species are shown in Fig. S1–S7 (Supporting Information).
4. Conclusions
The effect of addition of MWCNTs on mechanical, thermal, swelling properties of gelatin-chitosan composites have been successfully explored in this paper. By accomplishing successful covalent functionalization of MWCNTs facilitated the interfacial interaction between the natural polymer blend and the nanotubes, which further enhanced the dispersion within the matrix and thus ultimately enhanced the mechanical properties of the blends. Surface and interface structures of the composites were studied by SEM and the intimate relationship between the structure and overall performances of the composite was revealed. The thermal, swelling, drug releasing properties were also found to superior compared with gelatin-chitosan blend due to addition of nanotubes. Besides the effectiveness in drug release rate, prepared MWCNTs/gelatin-chitosan nanocomposites did not show any cytotoxicity and we believe that such nanocomposites can be employed as targeted drug delivery agent in nanomedicine, targeted thermal tumor ablation and magnetic field targeting of tumors.
This research provides an alternative novel strong and light-weight material for versatile biomedical applications. Although these MWCNTs conjugates displayed no cytotoxicity in vitro, for further development, it is important to assess them by metabolism in vivo tests, biodistribution and clearance from the body.
Conflicts of interest
The authors declare no competing financial interests.
5. Contributions
A. F. M. M. Rahman designed the project, while Sadia Sharmeen conducted the experiments. A. F. M. M. Rahman and Sadia Sharmeen wrote the main manuscript text and all authors reviewed the paper.
Acknowledgements
The authors sincerely thank Prof. Dr. Siegmar Roth, Sineurop Nanotech GmbH, Stuttgart, Germany for providing purified multi-walled carbon nanotubes.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.bioactmat.2018.03.001.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Sun Y.-P., Fu K., Lin Y., Huang W. Functionalized carbon nanotubes: properties and applications. Accounts Chem. Res. 2002;35:1096–1104. doi: 10.1021/ar010160v. [DOI] [PubMed] [Google Scholar]
- 2.Ajayan P. Nanotubes from carbon. Chem. Rev. 1999;99:1787–1800. doi: 10.1021/cr970102g. [DOI] [PubMed] [Google Scholar]
- 3.Esawi A.M., Farag M.M. Carbon nanotube reinforced composites: potential and current challenges. Mater. Des. 2007;28:2394–2401. [Google Scholar]
- 4.Baughman R.H., Zakhidov A.A., De Heer W.A. Carbon nanotubes-the route toward applications. Science. 2002;297:787–792. doi: 10.1126/science.1060928. [DOI] [PubMed] [Google Scholar]
- 5.Zhang L., Webster T.J. Nanotechnology and nanomaterials: promises for improved tissue regeneration. Nano Today. 2009;4:66–80. [Google Scholar]
- 6.Iijima S., Brabec C., Maiti A., Bernholc J. Structural flexibility of carbon nanotubes. J. Chem. Phys. 1996;104:2089–2092. [Google Scholar]
- 7.Edwards S.L., Church J.S., Werkmeister J.A., Ramshaw J.A. Tubular micro-scale multiwalled carbon nanotube-based scaffolds for tissue engineering. Biomaterials. 2009;30:1725–1731. doi: 10.1016/j.biomaterials.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 8.Firkowska I., Olek M., Pazos-Peréz N., Rojas-Chapana J., Giersig M. Highly ordered MWNT-based matrixes: topography at the nanoscale conceived for tissue engineering. Langmuir. 2006;22:5427–5434. doi: 10.1021/la053067e. [DOI] [PubMed] [Google Scholar]
- 9.Polizu S., Savadogo O., Poulin P., Yahia L.H. Applications of carbon nanotubes-based biomaterials in biomedical nanotechnology. J. Nanosci. Nanotechnol. 2006;6:1883–1904. doi: 10.1166/jnn.2006.197. [DOI] [PubMed] [Google Scholar]
- 10.Galvan-Garcia P., Keefer E.W., Yang F., Zhang M., Fang S., Zakhidov A.A., Baughman R.H., Romero M.I. Robust cell migration and neuronal growth on pristine carbon nanotube sheets and yarns. J. Biomat. Sci., Polymer Edition. 2007;18:1245–1261. doi: 10.1163/156856207782177891. [DOI] [PubMed] [Google Scholar]
- 11.Liopo A.V., Stewart M.P., Hudson J., Tour J.M., Pappas T.C. Biocompatibility of native and functionalized single-walled carbon nanotubes for neuronal interface. J. Nanosci. Nanotechnol. 2006;6:1365–1374. doi: 10.1166/jnn.2006.155. [DOI] [PubMed] [Google Scholar]
- 12.Abarrategi A., Gutiérrez M.C., Moreno-Vicente C., Hortigüela M.J., Ramos V., López-Lacomba J.L., Ferrer M.L., del Monte F. Multiwall carbon nanotube scaffolds for tissue engineering purposes. Biomaterials. 2008;29:94–102. doi: 10.1016/j.biomaterials.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 13.Sorkin R., Gabay T., Blinder P., Baranes D., Ben-Jacob E., Hanein Y. Compact self-wiring in cultured neural networks. J. Neural. Eng. 2006;3:95–101. doi: 10.1088/1741-2560/3/2/003. [DOI] [PubMed] [Google Scholar]
- 14.Tjong S.C. Structural and mechanical properties of polymer nanocomposites. Mater. Sci. Eng.: R. Rep. 2006;53:73–197. [Google Scholar]
- 15.Bokobza L. Multiwall carbon nanotube elastomeric composites: a review. Polymer. 2007;48:4907–4920. [Google Scholar]
- 16.Zhang X., Liu T., Sreekumar T., Kumar S., Moore V.C., Hauge R.H., Smalley R.E. Poly (vinyl alcohol)/SWNT composite film. Nano Lett. 2003;3:1285–1288. [Google Scholar]
- 17.Murphy A.R., Fréchet J.M. Organic semiconducting oligomers for use in thin film transistors. Chem. Rev. 2007;107:1066–1096. doi: 10.1021/cr0501386. [DOI] [PubMed] [Google Scholar]
- 18.Lebrón-Colón M., Meador M.A., Gaier J.R., Solá F., Scheiman D.A., McCorkle L.S. Reinforced thermoplastic polyimide with dispersed functionalized single wall carbon nanotubes. ACS Appl. Mater. Interfaces. 2010;2:669–676. doi: 10.1021/am900682s. [DOI] [PubMed] [Google Scholar]
- 19.Cherukuri P., Bachilo S.M., Litovsky S.H., Weisman R.B. Near-infrared fluorescence microscopy of single-walled carbon nanotubes in phagocytic cells. J. Am. Chem. Soc. 2004;126:15638–15639. doi: 10.1021/ja0466311. [DOI] [PubMed] [Google Scholar]
- 20.O'connell M.J., Bachilo S.M., Huffman C.B., Moore V.C., Strano M.S., Haroz E.H., Rialon K.L., Boul P.J., Noon W.H., Kittrell C. Band gap fluorescence from individual single-walled carbon nanotubes. Science. 2002;297:593–596. doi: 10.1126/science.1072631. [DOI] [PubMed] [Google Scholar]
- 21.Kong J., Franklin N.R., Zhou C., Chapline M.G., Peng S., Cho K., Dai H. Nanotube molecular wires as chemical sensors. Science. 2000;287:622–625. doi: 10.1126/science.287.5453.622. [DOI] [PubMed] [Google Scholar]
- 22.Collins P.G., Bradley K., Ishigami M., Zettl A. Extreme oxygen sensitivity of electronic properties of carbon nanotubes. Science. 2000;287:1801–1804. doi: 10.1126/science.287.5459.1801. [DOI] [PubMed] [Google Scholar]
- 23.Chen J., Liu H., Weimer W.A., Halls M.D., Waldeck D.H., Walker G.C. Noncovalent engineering of carbon nanotube surfaces by rigid, functional conjugated polymers. J. Am. Chem. Soc. 2002;124:9034–9035. doi: 10.1021/ja026104m. [DOI] [PubMed] [Google Scholar]
- 24.Shim M., Javey A., Shi Kam N.W., Dai H. Polymer functionalization for air-stable n-type carbon nanotube field-effect transistors. J. Am. Chem. Soc. 2001;123:11512–11513. doi: 10.1021/ja0169670. [DOI] [PubMed] [Google Scholar]
- 25.Kim H.S., Yoon S.H., Kwon S.-M., Jin H.-J. pH-sensitive multiwalled carbon nanotube dispersion with silk fibroins. Biomacromolecules. 2008;10:82–86. doi: 10.1021/bm800896e. [DOI] [PubMed] [Google Scholar]
- 26.Zheng M., Jagota A., Semke E.D., Diner B.A., Mclean R.S., Lustig S.R., Richardson R.E., Tassi N.G. DNA-assisted dispersion and separation of carbon nanotubes. Nat. Mater. 2003;2:338–342. doi: 10.1038/nmat877. [DOI] [PubMed] [Google Scholar]
- 27.Tsyboulski D.A., Bakota E.L., Witus L.S., Rocha J.-D.R., Hartgerink J.D., Weisman R.B. Self-assembling peptide coatings designed for highly luminescent suspension of single-walled carbon nanotubes. J. Am. Chem. Soc. 2008;130:17134–17140. doi: 10.1021/ja807224x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng M., Jagota A., Strano M.S., Santos A.P., Barone P., Chou S.G., Diner B.A., Dresselhaus M.S., Mclean R.S., Onoa G.B. Structure-based carbon nanotube sorting by sequence-dependent DNA assembly. Science. 2003;302:1545–1548. doi: 10.1126/science.1091911. [DOI] [PubMed] [Google Scholar]
- 29.Wu P., Chen X., Hu N., Tam U.C., Blixt O., Zettl A., Bertozzi C.R. Biocompatible carbon nanotubes generated by functionalization with glycodendrimers. Angew. Chem. 2008;120:5100–5103. doi: 10.1002/anie.200705363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Longmire M., Choyke P.L., Kobayashi H. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine. 2008;3:703–717. doi: 10.2217/17435889.3.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Z., Sun X., Nakayama-Ratchford N., Dai H. Supramolecular chemistry on water-soluble carbon nanotubes for drug loading and delivery. ACS Nano. 2007;1:50–56. doi: 10.1021/nn700040t. [DOI] [PubMed] [Google Scholar]
- 32.Schipper M.L., Nakayama-Ratchford N., Davis C.R., Kam N.W.S., Chu P., Liu Z., Sun X., Dai H., Gambhir S.S. A pilot toxicology study of single-walled carbon nanotubes in a small sample of mice. Nat. Nanotechnol. 2008;3:216–221. doi: 10.1038/nnano.2008.68. [DOI] [PubMed] [Google Scholar]
- 33.Tagmatarchis N., Prato M. Functionalization of carbon nanotubes via 1,3-dipolar cycloadditions. J. Math. Chem. 2004;14:437–439. [Google Scholar]
- 34.Kim J., Kim H.S., Lee N., Kim T., Kim H., Yu T., Song I.C., Moon W.K., Hyeon T. Multifunctional uniform nanoparticles composed of a magnetite nanocrystal core and a mesoporous silica shell for magnetic resonance and fluorescence imaging and for drug delivery. Angew. Chem. Int. Ed. 2008;47:8438–8441. doi: 10.1002/anie.200802469. [DOI] [PubMed] [Google Scholar]
- 35.Sakura T., Takahashi T., Kataoka K., Nagasaki Y. One-pot preparation of mono-dispersed and physiologically stabilized gold colloid. Colloid Polym. Sci. 2005;284:97–101. [Google Scholar]
- 36.Dubertret B., Skourides P., Norris D.J., Noireaux V., Brivanlou A.H., Libchaber A. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science. 2002;298:1759–1762. doi: 10.1126/science.1077194. [DOI] [PubMed] [Google Scholar]
- 37.Shi C., Zhu Y., Ran X., Wang M., Su Y., Cheng T. Therapeutic potential of chitosan and its derivatives in regenerative medicine. J. Surg. Res. 2006;133:185–192. doi: 10.1016/j.jss.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 38.Kim Y., Minami N., Kazaoui S. Highly polarized absorption and photoluminescence of stretch-aligned single-wall carbon nanotubes dispersed in gelatin films. Appl. Phys. Lett. 2005;86:073103. [Google Scholar]
- 39.Kim I.-Y., Seo S.-J., Moon H.-S., Yoo M.-K., Park I.-Y., Kim B.-C., Cho C.-S. Chitosan and its derivatives for tissue engineering applications. Biotechnol. Adv. 2008;26:1–21. doi: 10.1016/j.biotechadv.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 40.Denkbas E.B., Ottenbrite R.M. Perspectives on: chitosan drug delivery systems based on their geometries. J. Bioact. Compat Polym. 2006;21:351–368. [Google Scholar]
- 41.Saraswathy G., Pal S., Rose C., Sastry T. A novel bio-inorganic bone implant containing deglued bone, chitosan and gelatin. Bull. Mater. Sci. 2001;24:415–420. [Google Scholar]
- 42.Meyer B.N., Ferringni N.R., Puam J.E., Lacobsen L.B., Nichols D.E., McLaughlin J.L. Brine shrimp: a convenient general bioassay for active constituents. Planta Med. 1982;45:31–32. [PubMed] [Google Scholar]
- 43.Bakota E.L., Aulisa L., Tsyboulski D.A., Weisman R.B., Hartgerink J.D. Multidomain peptides as single-walled carbon nanotube surfactants in cell culture. Biomacromolecules. 2009;10:2201–2206. doi: 10.1021/bm900382a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salem K.S., Lubna M.M., Rahman A.F.M., NurNabi M., Islam R., Khan M.A. The effect of multiwall carbon nanotube additions on the thermo-mechanical, electrical, and morphological properties of gelatin–polyvinyl alcohol blend nanocomposite. J. Compos. Mater. 2015;49:1379–1391. [Google Scholar]
- 45.Al Islam M.A., Rahman A.F.M.M., Iftekhar S., Salem K.S., Sultana N., Bari M.L. Morphology, thermal stability, electrical, and mechanical properties of graphene incorporated poly(vinyl alcohol)-gelatin nanocomposites. Int. J. Compos. Mater. 2016;6:172–182. [Google Scholar]
- 46.Li H., Wang D.Q., Chen H.L., Liu B.L., Gao L.Z. A novel gelatin–carbon nanotubes hybrid hydrogel. Macromol. Biosci. 2003;3:720–724. [Google Scholar]
- 47.Mueller J.H., Hinton J. A protein-free medium for primary isolation of the gonococcus and meningococcus. Proc. Soc. Exp. Biol. Med. 1941;48:330–333. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.