Abstract
In this study, the SiO2—CaO—P2O5 ternary component of bioactive glass particles were successfully synthesized by sol-gel method, then the bioactive glass particles were pressed into tablets with dry pressing molding technology. The physicochemical structure, in-vitro bioactivity and biocompatibility of BG tablets were characterized by various methods, such as XRD、SEM、FTIR, etc. The results showed that the sol-gel bioactive glass particle was distinguished with its amorphous structure and micron-size. After being soaked in Tris-Hcl solution for 15 d, the bioactive glass tablets didn't collapse. Also, the mineralization assay in vitro showed that the bioactive glass tablets had good capability of inducing the formation of hydroxycarbonate apatite (HCA) after being immersed in simulated body fluid (SBF). In addition, the cytotoxicity assay indicated that the osteoblast (MC3T3) grew well on the surface of bioactive glass tablets. According to the above results, the bioactive glass tablets presented good mechanical strength, excellent apatite-forming activity and high biocompatibility, which demonstrated their potential applications in the field of bone defect repairing.
Keywords: Sol-gel bioactive glass, Dry pressing molding, BG tablets, Bioactivity, Cytotoxicity
Graphical abstract
Highlights
-
•
The constituent of tablets are pure sol-gel bioactive glass without any additives, so they exhibit high bioactivity.
-
•
The BG tablets are prepared by simple dry pressing molding technology that is convenient and economical.
-
•
The BG tablets are ideal bone repair material that have good mechanical property, bioactivity and biocompatibility.
1. Introduction
Accidental trauma and bone related diseases, including femoral head necrosis, bone tumors, osteoporosis, etc. could result in bone defects and impairment of bone tissue, which leads to an urgent clinical need for reconstructing and repairing of damaged bone tissue. As we all know, bone is one of the most commonly transplanted tissues, only inferior to blood. The autogenous bone graft has been regarded as golden standard in surgery for many years because of its optimal osteoconductive and osteogenic properties [1]. However, the autogenous bone graft still has many disadvantages. For example, the source of autograft is very limited and it adds the overall surgical procedure and causes secondary trauma and residual pain [2], [3]. In addition, autograft harvesting may bring about some complications, such as haematoma formation, blood loss, nerve injury, hernia formation, infection, arterial injury, ureteral injury, fracture, pelvic instability, cosmetic defects, etc [4], [5], [6]. So researchers have been exploring a variety of synthetic biomaterials which can be grafted in body to replace autogenous bone in the past decades, such as hydroxyapatite (HA), β-TCP, bioactive glass (BG), biopolymer, etc [7], [8]. Among them, bioactive glass has been widely studied since the first discovery of 45S5 BG in the 1970s by Hench [9]. It can chemically bond with host tissue by forming a bone-like apatite layer between materials and bone tissue [10]. Markedly, recent researches show that the ionic dissolution product of BG could promote proliferation and differentiation of osteoblasts by activating a series of genes that regulating cellular behaviours [11]. So numerous researchers around the world pay great attention to this superior material. The first generation of BG is prepared by melting-quenching method. Although traditional melting-derived BGs have excellent bioactivity, it was fired at a very high temperature (above 1300 °C), so it had dense structure and small specific surface area [12], which limits its application. Compared to melting-quenching method, sol-gel method is a chemistry-based synthesis route, of which a solution containing the compositional precursors undergoes polymer type reactions at room temperature to form a gel [13]. The second generation of sol-gel BGs possesses uniform composition, composed of numerous nanoparticles with microporous and mesoporous structure, and thus it has high specific surface area [14], [15]. These advantages grant sol-gel BGs excellent bioactivity. However, up to now, there is no commercial product made of pure sol-gel BG in clinical application. So it's meaningful to prepare a kind of biomedical material that is made of sol-gel BGs, which would be a promising application in the field of bone defect repairing.
Due to the development of industrial technology, material forming method become various. Additive manufacturing techniques are the most popular preparation methods of bioactive glass scaffolds. It includes freeze extrusion fabrication (FEF), selective laser sintering (SLS), solid freeform fabrication, etc. They can mimic the porous structure of cancellous bone and the material made by these methods have similar mechanical strengths comparing to cortical bone [16], [17], [18]. However, they all have the disadvantages of complicated procedure and high cost, even need additive to serve as binder or template, which may decrease the bioactivity of scaffolds. But the dry pressing molding doesn't need expensive experimental instrument and complicated experimental procedure. So it has been widely used in the fields of bio-pharmaceutical, inorganic materials, polymer, material analysis, etc [19]. Dry pressing molding technology was approximately developed in the 1960s in Europe and America [20]. It's a method to mix up the sifted powder and additive, then directly preform the mixture powder into tablet form. Importantly, it doesn't destroy BG particles structure. Turning BG particles into tablets is only physical reaction, so the high bioactivity and osteogenic property of BG are retained, which indirectly demonstrates that the bioactive glass tablets will possess excellent bioactivity and potential clinical application as bone healing materials.
Considering the clinical requirement that bone defect healing and reconstruction, materials need good bone forming ability and certain mechnical property and combining the above advantages of sol-gel BG and dry pressing molding technology. So in this study, we aimed to synthesize high bioactivity BG particles by sol-gel method first, then directly preform sifted BG particles into tablet form with dry pressing molding technology. Also, we would characterize BG particles' and BG tablets' physicochemical properties, and systematically study the formation process of HCA in mineralization experiment and finally study the cytotoxicity of BG tablets in vitro.
2. Materials and methods
2.1. Preparation of 58S bioactive glass particles
The components (mol %) of bioactive glass were 60% SiO2, 36% CaO, 4% P2O5. The raw materials used were ethyl tetraethyl orthosilicate (TEOS), Ttriethylphosphate (TEP), calcium nitrate tetrahydrate (Ca(NO3)2.4H2O), deionized water and hydrochloric acid. All chemical reagents were purchased from China National Medicine Shanghai Chemical Reagent Corporation.
The preparation method was referred to the previous study [21]. It approximately included four steps. Firstly, according to the ratio of bioactive glass, 10.24 g TEOS, 1.16 g TEP, 7.02 g Ca (NO3) 2.4H2O were added into the mixture of 7.82 g water and 1.30 g hydrochloric acid (2 M) in turn, and undergo continuous stirring until it became uniform sol. Secondly, the sol was aged for 72 h at room temperature to make the hydrolysis of polycondensation fully react, then the sol was solidified into transparent gel. Thirdly, the gel was transferred to 60 °C and 120 °C oven to remove excess water and ethanol for 24 h respectively. Finally, the dried gel was put in the 650 °C furnace to remove unreacted organic matter.
2.2. Preparation of BG tablets
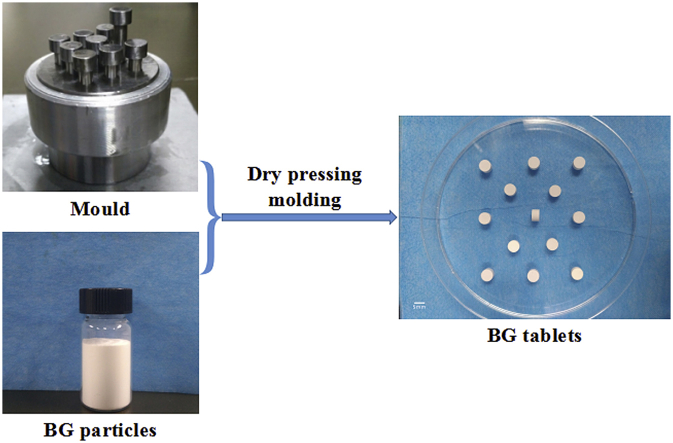
In order to prepare good mechnical strength tablets, it's necessary to levigate the big BG particles to decrease the stress concentration effect of BG tablets. Then the grinded 58S bioactive glass powder was put into multiple channels pelleting mould designed by Mike (Zhongshan, China), the load pressure and dwell time of tablet machine was set as 5 MPa, 60 s respectively, finally the diameter of 5 mm and thick of 3.5 mm bioactive glass tablets were successfully prepared by dry pressing molding technology.
2.3. In vitro bioactivity study
It's a common method to evaluate the bioactivity of material by observing the formation of apatite on material surface in SBF solution [22]. SBF was prepared as Kokubo explained [23]. The ions concentration of SBF is equal to plasma's ions concentration. Mineralization experiment adopts international standards (ISO/FDIS 23317), the volume of the SBF solution was determined according to the following formula [24]:
-the volume of SBF solution (mL)
-the surface of tablets (mm2)
2.4. Characterization studies
2.4.1. Morphology, structure and size distribution of BG particles
To observe the micro morphology and crystal structure of BG particles, the prepared BG particles were characterized by field emission scanning electron microscopy (MERLIN, Zeiss, Germany) and X-Ray Powder Diffraction (Empyrean, Panaco, Netherlands). Grain size distribution is the key factor that influences the mechanical strength of BG tablet. So the size distribution of BG particles was tested by Nanometer particle potential analyzer (MPT-2, Huayu, China).
2.4.2. Mechanical property of BG tablets
Ideal bone defect filling materials require moderate mechanical strength. It's essential to avoid collapse after implantation into body. So the mechanical property of BG tablets was tested by universal material testing machine (Instron 5967, Instron, America) with a 50 N load cell at a crosshead speed of 5 mm/min. The BG tablets were put in between two parallel plates, then the cross-sectional area of tablet was calculated and the stress strain curves were recorded. In addition, the degradation property of BG tablets were characterized by measuring the weight loss of BG tablets after immersing them into Tris-Hcl solution for specific time, finally the weight loss curve was recorded. In these two experiments, five same tablets named as S1, S2, S3, S4, S5 were tested in order to decrease random error.
2.4.3. Bioactivity of BG tablets in vitro
Simulated mineralization experiment was conducted in the thermostat shaking table of 37 °C. The BG tablets were hung in SBF with thin string to make it fully contact with SBF. After being soaked for certain time, the acetone was used to terminate the mineralization process of BG tablets, then they were washed with ethanol and deionized water two more times respectively, finally the BG tablets were put into 60 °C oven to dry out water. Then, the morphology of newly formed HCA on tablet's surface was observed with SEM. And the mineral composition and crystal structure of HCA was characterized by XRD and Fourier transform infrared spectroscopy (Vector 33-MIR, Brukev, Germany).
2.5. Assessment of biocompatibility in vitro
The cytotoxicity of BG tablet was assayed by coculturing with the osteoblast (MC3T3) cell line which was purchased from cell bank of Shanghai. The cells were cultured in complete medium that contains 90% Dulcecco's modified eagle medium (DMEM) and 10% Fetal Bovine Serum (FBS) in a humidified atmosphere at 5% CO2 and 37 °C. The BG tablets were sterilized by high temperature and high pressure before culturing with cells. MC3T3 cells were seeded in 48 well plate with density of 104 cells and volume of 200 μl complete medium per well. After material and cells coculured for 24 h, the culture medium was took out from plate and the BG tablets were washed with PBS three times, finally the Die dyeing kit (Invitrogen) was used according to the instruction. After cocultivation of the kit and cells for another 30 minutes, the growth condition of cells was observed by Inversed Fluorescent Microscope (Eclipsc Ti—U, NIKON, Japan). And in order to observe the shape of cells on BG tablet surface, 2.5% glutaraldehyde was used to fix the form of cells, then different concentration gradient of ethanol was used to dehydrate cells, finally the shape of cells was observed with SEM. Also, the quantitative analysis of cell viability was tested by CCK-8 method, the specific experiment procedures were referred to the cell counting kit (Invitrogen) instruction.
3. Results and discussion
3.1. Characterization of 58S bioactive glass particles
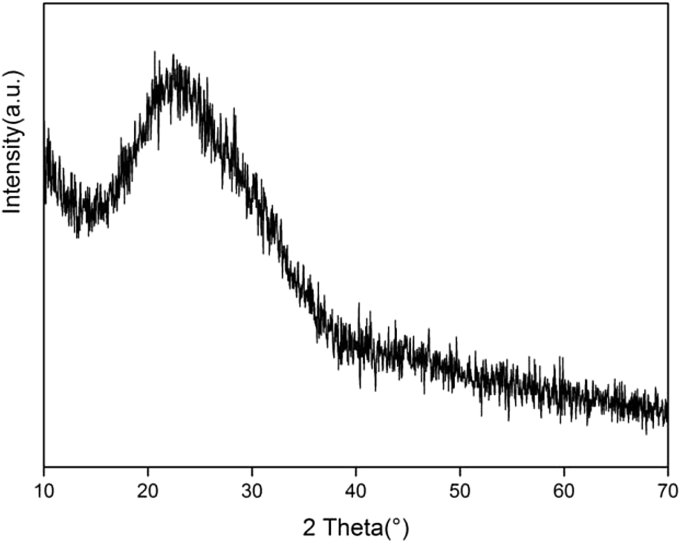
As shown in Fig. 1, the XRD peak of 58S BG particle were dispersive and there was no sharp diffraction peak, which demonstrated that the BG particles were typically with amorphous structure. And at 2 theta of around 23°, there appeared a broad peak that was a typical amorphous structure characteristic peak of silicate glass [25].
Fig. 1.
XRD pattern of 58S bioactive glass particles.
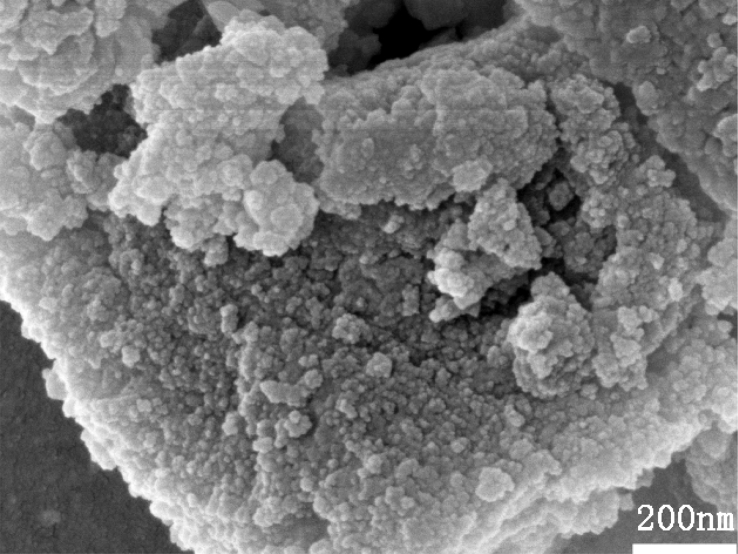
Fig. 2 is the SEM micrograph of 58S bioactive glass particle. The figure showed that the BG particle had irregular morphology and it consisted of a large number of nanoparticles with heterogeneous size, which turned the BG particles to multilevel pore structure. In the drying process, there were vapor-liquid interfaces between particles. Due to the effect of interfacial tension, there appeared meniscus between the particles and particle, which brought about strong pulling force that led to the gel skeleton collapse, finally the particles contacted closely, which caused the occurrence of soft and hard particles getting together. So the prepared BG particles need to be levigated before dry pressing molding.
Fig. 2.
SEM micrograph of 58S bioactive glass particles.
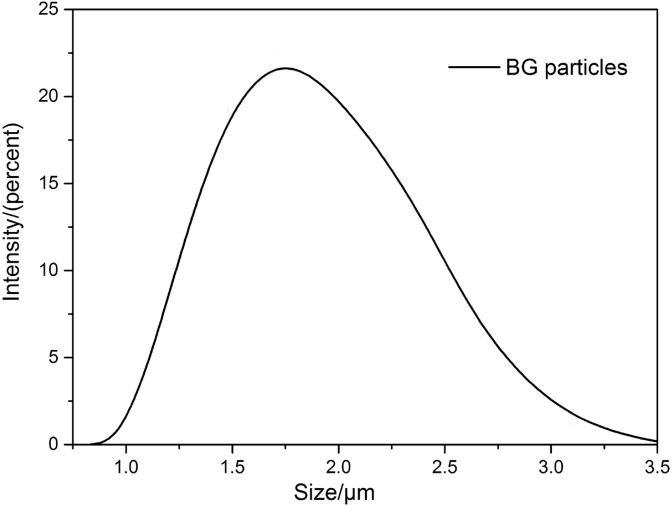
Fig. 3 is the particles size distribution diagram of bioactive glass. From the diagram, it showed that the milled 58S BG particles were micron-sized, the particles size ranged from around 1 μm–3 μm, more than 60% of BG particles were between 1.5 μm and 2 μm, the average particle diameter calculation was 1.8 μm according to scientific calculation.
Fig. 3.
Particle size distribution diagram of 58S bioactive glass particles.
3.2. Mechanical property study of BG tablets
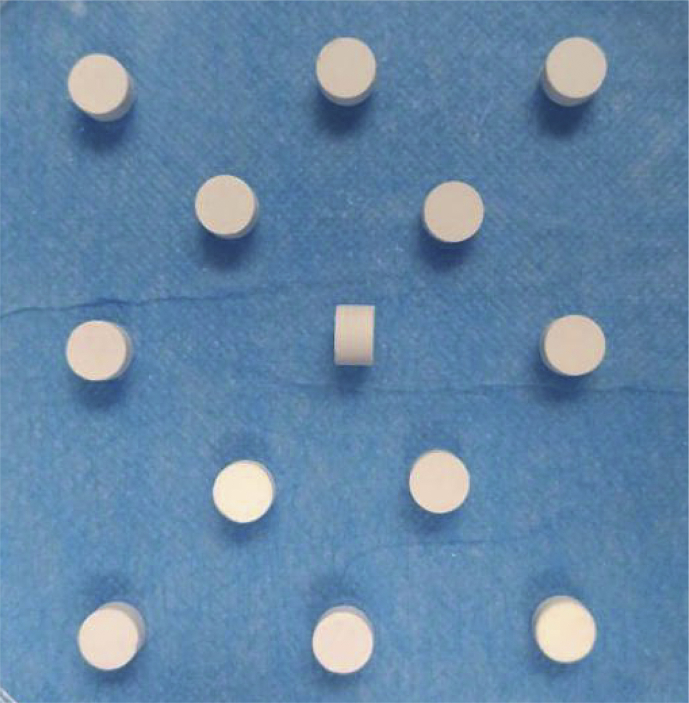
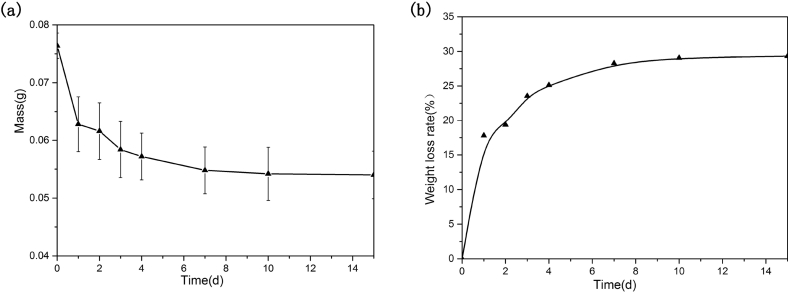
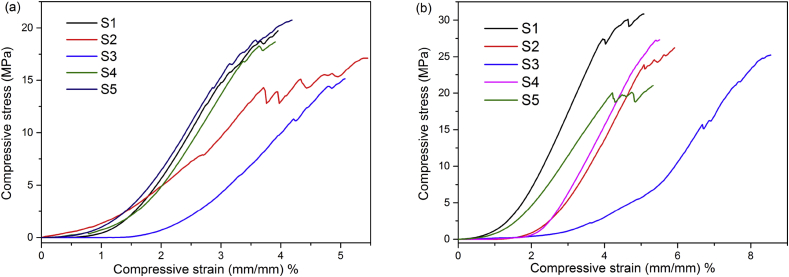
The photograph of the fabricated BG tablets are shown in Fig. 4. It could be seen that the BG tablets made by dry pressing molding technology were wafer-like and uniformly-structured, and the surface was a little rough. The thickness and diameter of the BG tablet depended on the dosage of BG particles and the mould respectively. Also, the weight loss curve (a) and weight loss rate of BG tablets (b) after soaking in Tris-Hcl solution are shown in Fig. 5. It could be seen that the weight of BG tablets decreased fastest after soaking for 1 d, the weightlessness rate was 17.8%. When soaking for more than 1 d, the rate of weight loss of BG tablets decreased. From 2 d to 7 d, the weightlessness rate of BG tablets was only 11%. After 7 d, the weight of BG tablets remained stable, and there was no sign of collapsion. At that time, the degradation and deposition of material reached to a dynamic balance. Fig. 6 is the stress-strain curve of BG tablets (a) before and (b) after soaking in Tris-Hcl for 1 d. It demonstrated that the compressive stress of five identical samples were greater than 15 MPa. By calculation, the average compressive stress of BG tablets was 18.31 MPa, and the average modulus of elasticity was 799.18 MPa. The strength of BG tablets was stronger than the spongy bone (2–12 MPa) [26], [27], [28]. In addition, the mechanical property of BG tablets were studied after they were soaking in Tris-Hcl for 1 d. It could be seen from Fig. 6 (b) that the compressive stress of all BG tablets were more than 20 MPa. According to scientific calculation, the average compressive stress and elasticity modulus became 26.12 MPa and 893.28 MPa respectively. The reason why the BG tablets became stronger after soaking in Tris-Hcl solution was probably that there were bridging oxygen bonds formed between bioactive glass particles, which made particles attach closer. On the other hand, when the BG tablets were soaking in Tris-Hcl, the liquid filled fully in the pores of tablets, then after evaporation of liquid, the newly formed bridging oxygen bonds made the pores become smaller, which decreased the porosity of BG tablets. Hence, the above results showed that the BG tablets made by dry pressing molding technology were capable of serving as bone filling materials because of their excellent mechanical strength. And the BG tablets were expected to be applied in the field of bone defect repairing.
Fig. 4.
Photograph of the BG tablets.
Fig. 5.
Weight loss curve (a) and weight loss rate of BG tablets (b) after soaking in Tris-Hcl for different time.
Fig. 6.
Stress-strain curve of BG tablets (a) before and (b) after soaking in Tris-Hcl for 1 d.
3.3. In vitro bioactivity study
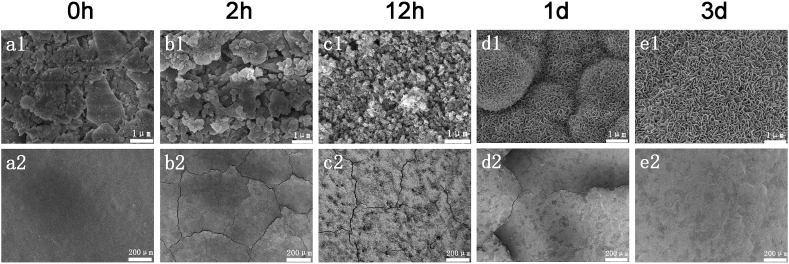
The surface morphology and microstructure of BG tablets before and after immersing in SBF were characterized by SEM, XRD, FTIR. As shown in Fig. 7, BG tablets were accumulated by numerous inhomogenous nanoparticles and the surface was flat and had no cracks. But after be immersed in SBF solution for 2 h, the big BG particles began to break up and more macropores appeared within BG tablets, which was clearly showed in Fig. 7 (b2). The reason why the surface of BG tablets appeared many microcracks was that the particles composing of BG tablets were inhomogenous, so it produced stress concentration easily. And the tablets would swell after being soaked in SBF. Under the effect of water erosion and ion exchange between BG tablets and SBF solution, cracks formed as result of the dual function of physical and chemical reactions. As the mineralization process went on, it could be seen from Fig. 7 (c1) that the surface of BG tablets were covered with a layer of loose strip of floccule after soaking in SBF solution for 12 h, which indicated that the crystal nucleus of HCA came into being in epitaxy on the material surface. At the same time, the number of microcracks became smaller, and chemical attack caused some pits appear on the BG tablet surface, as shown in Fig. 7 (c2). Research results also found that HCA crystal nucleus were produced at the corner of the crack initially. Ion exchange reaction between the Ca2+ ions from BG network and the H+ ions from the SBF solution took place at the corner of the cracks easily. The H2O destroyed Si—O bonds in the BG network, so the porous silica gel layer was formed on the surface of material, which contained a large number of ≡ Si—OH groups. The groups of OH— had strong attraction to the Ca2+, H2PO4-, HPO42− and PO43− of SBF solution, thus crack intersections provided a favorable HCA nucleation site. As shown in Fig. 7 (d1), when the mineralization time reached 1 d, the morphology of HCA has developed from the original bar flocculent shape to fragment, then converged into ball shape spontaneously in order to reduce the surface energy. The density of mineralization layer becomes higher, gradually the microcracks were covered, which was owing to the growth of HCA crystal along the crystal direction. The PO43− and the Ca2+of BG tablets diffused through porous silica gel layer into the SBF solution, leading to the ionic supersaturation in solution, thus the water rich CaO—P2O5 sediments were formed, then covered the silica gel layer. When mineralization time reached 3 d, as shown in Fig. 7 (e2), the microcracks on the surface of the BG tablets were completely covered with dense mineral compound. Meanwhile, the HCA crystals became layer form and it grew upright randomly on the BG tablets surface. It was due to the silica gel layer went through dehydration, polymerization and crystallization. Eventually, the mineralized crystalline bone-like HCA layer was built.
Fig. 7.
SEM micrograph of BG tablets after soaking in SBF solution for different times.
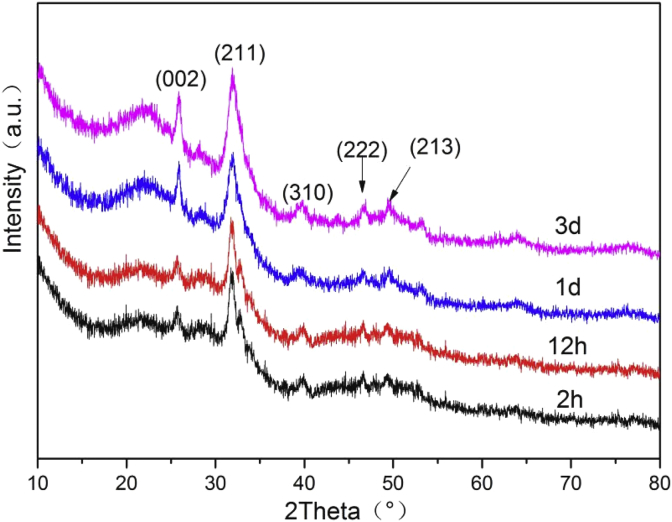
Fig. 8 showed the XRD patterns of the BG tablets after soaking in SBF solution for different times. After mineralizing for 2 h, the diffraction peak at 2θ = 26°, 32°, 39° were observed, which corresponded to the (002), (211), (310) reflections of HCA crystals. After mineralizing for 12 h, low crystallinity of HCA had formed, the diffraction peak became intense. As the mineralization time increased, the diffraction peak at 2θ = 26°, 32° became more and more intense. After immersing for 3 d, the two diffraction peaks at 2θ = 46°, 49° that corresponded with the (222), (213) reflections of HCA became strongest and most obvious than other experiment groups. The above newly formed diffraction peaks were equal to the standard card of HCA (JCPD 24-0033). Hence, the XRD results demonstrated that the BG tablets had good bioactivity in vitro and their mineralization product was HCA. And the crystallinity of HCA became higher as immersion time increased.
Fig. 8.
XRD patterns of BG tablets after soaking in SBF solution for different times.
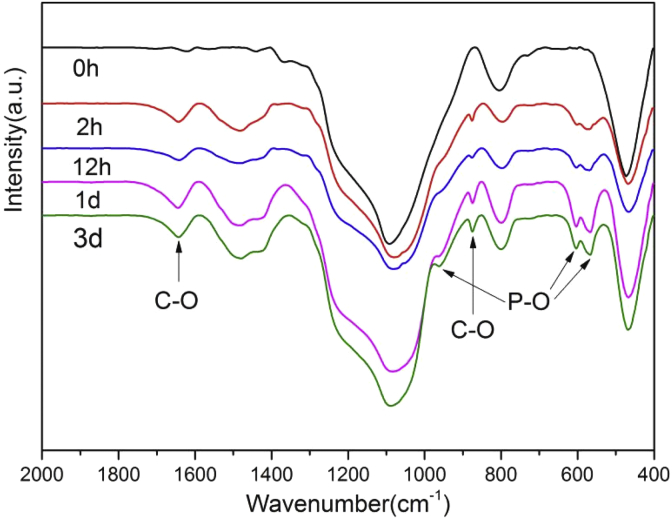
Fig. 9 summarized the FTIR spectra of BG tablets before and after immersing in SBF, it could reflect the surface composition and structure of HCA. After soaking for 2 h, there were two split peaks appearing at 603 cm−1 and 568 cm−1, they were assigned to the P—O bending vibration. The reason why there appeared two split peaks was that the molecules in the crystal lattice arranged orderly due to the strengthened intermolecular interaction, finally led to the band split. And when mineralization time increased, the process of HCA crystallization accelerated, so the intensity of split peak became stronger as the mineralization time increased. When mineralization time reached 1 d, the new peak appeared at 961 cm−1 that corresponded to the P—O stretching vibration. But the intensity of 961 cm−1 peak was very weak and not obvious if mineralization time was less than 1 d. In addition, then new peak appearing at 1644−1 m, 875 cm−1 were assigned to C—O stretching vibration, which indirectly indicated that the mineralization product contained carbon element. As the conclusion, the mineralization product of BG tablets was HCA after soaking in SBF solution.
Fig. 9.
FTIR spectra of BG tablets after soaking in SBF solution for different times.
3.4. Biocompatibility assessment of BG tablets
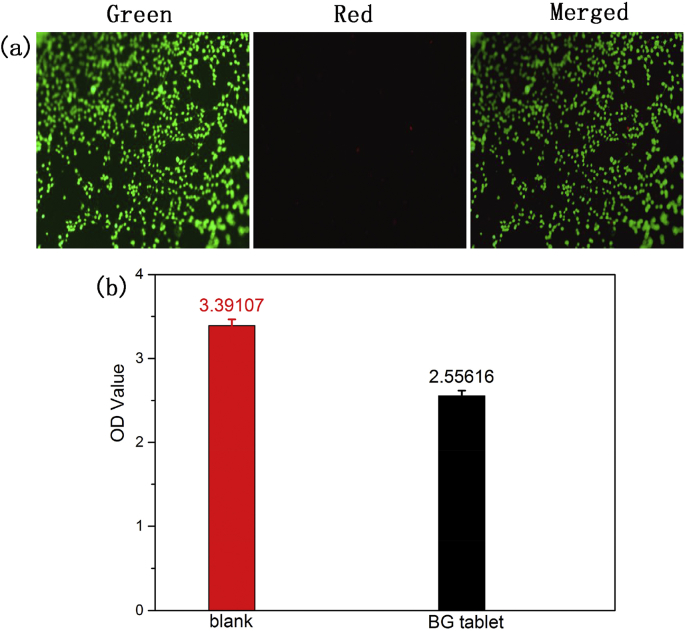
Previous studies had demonstrated that the BG particles have good biocompatibility, it showed low cytotoxicity and could even promote the osteoblastic proliferation and differentiation. However, the effects of tablet made up of 58S BG powder on cell viability have not been studied yet. In this study, the cytotoxicity of the BG tablet was assayed by cultivating the osteoblast (MC3T3) with material, then the growth of MC3T3 cells was observed by SEM and fluorescence microscope. Fig. 10 was the result of adhesion condition of MC3T3 cells on BG tablet. It showed that the cells attached to the surface of BG tablet tightly (as shown in red arrows) and spread their body to polygonal shapes, which indicated that the slightly rough surface of BG tablets were beneficial for osteoblasts to migrate and grow. In addition, as shown in Fig. 11 (a), the live-dead staining also showed that the cells could grow well after culturing with BG tablets. The cells exhibited vigorous activities and there were affluent cellular pseudopods and few dead cells, which could be seen from the fluorescence images. Fig. 11 (b) was the result of cell viability after it cocultured with BG tablets for 1 d. It could be seen that the OD value of BG tablets was lower than blank group. The cell viability was only 75.38%. Because when the BG tablets were soaked in 96-well plates containing culture medium, the dissolution product of Ca2+ released from BG tablets, then the pH of medium was increased inevitably, which was slightly harmful for cell surviving. However, this weakness of BG tablets was evident only in micro-environment, when the BG tablets were applied in dynamic environment, such as animal or human body, this disadvantage may be negligible because of the internal environment homeostasis. Hence, the above results demonstrated that the BG tablets presented excellent cellular biocompatibility, which was good for biomedical applications in the field of bone defect repairing.
Fig. 10.
SEM images of cells attaching on the surface of BG tablets with different magnification (a) 300x, (b) 3000x.
Fig. 11.
Fluorescence images (a) and cell viability (b) of cells after coculturing with BG tablets.
4. Conclusions
In this study, BG particles were successfully synthetized by sol-gel method. It was distinguished with its amorphous structure and micron-size. And the BG tablets were prepared by dry pressing molding technology. The physicochemical properties and bioactivity of BG tablets in vitro were investigated scientifically. The results showed that a dense layer of biological apatite formed on the surface of BG tablets after being soaked in SBF solution for 1 d, which indicated that the BG tablets had excellent bioactivity. The mechanical testing and degradation experiment proved that the BG tablets possessed good mechanical property. In addition, the cytological study demonstrated that BG tablets could offer ideal physical environment for osteoblast surviving, the MC3T3 cells could grow very well on its surface. Therefore, the BG tablets could serve as a kind of ideal bone filling materials and were expected to be applied in the field of bone defect repairing.
Acknowledgments
This work was supported by the Joint Funds of the National Natural Science Foundation of China (Grant No. U1501245), the Fundamental Research Funds for the Central Universities (2015ZP020), the National Natural Science Foundation of China (Grant No. 51672088), the science and technology innovation team project of Foshan (No. 2015IT100062).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Jianhui Chen, Email: 705218927@qq.com.
Xiaofeng Chen, Email: chenxf@scut.edu.cn.
References
- 1.Cypher T.J., Grossman J.P. Biological principles of bone graft healing. J. Foot Ankle Surg. 1996;35:413–417. doi: 10.1016/s1067-2516(96)80061-5. [DOI] [PubMed] [Google Scholar]
- 2.Arrington E.D., Smith W.J., Chambers H.G. Complications of iliac crest bone graft harvesting. Clin. Orthop. 1996;329:300–309. doi: 10.1097/00003086-199608000-00037. [DOI] [PubMed] [Google Scholar]
- 3.Younger E.M., Chapman M.W. Morbidity at bone graft donor sites. J. Orthop. Trauma. 1989;3:192–195. doi: 10.1097/00005131-198909000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Banwart J.C., Asher M.A., Hassanein R.S. Iliac crest bone graft harvest donor site morbidity: a statistical evaluation. Spine. 1995;20:1055–1060. doi: 10.1097/00007632-199505000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Seiler J.G., Johnson J. Iliac crest autogenous bone grafting:donor site complications. J. South Orthop. Assoc. 2000;9:91–97. [PubMed] [Google Scholar]
- 6.Summers B.N., Eisenstein S.M. Donor site pain from the ilium: a complication of lumbar spine fusion. J. Bone Jt. Surg. Br. 1989;71:677–680. doi: 10.1302/0301-620X.71B4.2768321. [DOI] [PubMed] [Google Scholar]
- 7.Giannoudis P.V., Dinopoulos H., Tsiridis E., Tsiridis E. Bone substitutes: an update. Injury-international J. Care Inj. 2005;36(3):S20–S27. doi: 10.1016/j.injury.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 8.LeGeros R.Z. Properties of osteoconductive biomaterials: calcium phosphates. Clin. Orthop. Relat. Res. 2002;395:81–98. doi: 10.1097/00003086-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Hench L.L. The story of Bioglass. J. Mater Sci. – Mater Med. 2006;17:967–978. doi: 10.1007/s10856-006-0432-z. [DOI] [PubMed] [Google Scholar]
- 10.Hench L.L., Splinter R.J., Allen W.C. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. Part A. 1971;5(6):117–141. [Google Scholar]
- 11.Hench L.L., Polak J.M. Third-generation biomedical materials. Science. 2002;295:1014–1017. doi: 10.1126/science.1067404. [DOI] [PubMed] [Google Scholar]
- 12.Jones J.R. Reprint of: review of bioactive glass: from Hench to hybrids. Acta Biomater. 2015;23(S53) doi: 10.1016/j.actbio.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 13.Hench L L, West J K. The sol-gel process-Chemical Reviews (ACS Publications)[J]. American Chemical Society.
- 14.Sepulveda P., Jones J.R., Hench L.L. Characterization of melt-derived 45S5 and sol-gel-derived 58S bioactive glasses. J. Biomed. Mater Res. 2001;58:734–740. doi: 10.1002/jbm.10026. [DOI] [PubMed] [Google Scholar]
- 15.Owens Gareth J. Sol–gel based materials for biomedical applications. Prog. Mater. Sci. 2016;77:1–79. [Google Scholar]
- 16.Huang T.S. Porous and strong bioactive glass (13–93) scaffolds fabricated by freeze extrusion technique. Mater. Sci. Eng. C. 2011;31(7):1482–1489. [Google Scholar]
- 17.Kolan K.C.R., Leu M.C., Hilmas G.E., Brown R.F., Velez M. Fabrication of 13–93 bioactive glass scaffolds for bone tissue engineering using indirect selective laser sintering. Biofabrication. 2011;3:025004. doi: 10.1088/1758-5082/3/2/025004. [DOI] [PubMed] [Google Scholar]
- 18.Wu C., Luo Y., Cuniberti G., Xiao Y., Gelinsky M. Three-dimensional printing of hierarchical and tough mesoporous bioactive glass scaffolds with a controllable pore architecture, excellent mechanical strength and mineralization ability. Acta Biomater. 2011;7:2644–2650. doi: 10.1016/j.actbio.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Degli A. A survey of current industrial practices in the formulation and manufacture of tablets and capsules. Pharm. Technol. 1993;17 32–32. [Google Scholar]
- 20.Meeus Liesbeth. Direct compression versus granulation. Dev. Med. Child Neurology. 2011;58(3):539–540. [Google Scholar]
- 21.Lei Bo. Versatile fabrication of nanoscale sol–gel bioactive glass particles for efficient bone tissue regeneration. J. Mater. Chem. 2012;22(33):16906–16913. [Google Scholar]
- 22.Kokubo T., Takadama H. How useful is SBF in predicting in vivo bone bioactivity. Biomaterials. 2006;27:2907–2915. doi: 10.1016/j.biomaterials.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 23.Oyane A., Kim H.M., Furuya T. Preparation and assessment of revised simulated body fluids. J. Biomed. Mater. Res. Part A. 2003;65:188–195. doi: 10.1002/jbm.a.10482. [DOI] [PubMed] [Google Scholar]
- 24.Implants for Surgery - In Vitro Evaluation for Apatite-forming Ability of Implant Materials. 2007. Aware Wtare, and T. Documentation. [Google Scholar]
- 25.Hench L.L. Bioceramics Proceedings of International Symposium on Ceramics in Medicine. 1994. Bioactive ceramics: theory and clinical applications - bioceramics: proceedings of the 7th international symposium on ceramics in medicine; pp. 3–14. [Google Scholar]
- 26.Baino F., Fiorilli S., Vitalebrovarone C. Bioactive glass-based materials with hierarchical porosity for medical applications: review of recent advances. Acta Biomater. 2016;42:18–32. doi: 10.1016/j.actbio.2016.06.033. [DOI] [PubMed] [Google Scholar]
- 27.Giesen E.B.W., Ding M., Dalstra M., Van Eijden T.M.G.J. Mechanical properties of cancellous bone in the human mandibular condyle are anisotropic. J. Biomech. 2001;34:799–803. doi: 10.1016/s0021-9290(01)00030-6. [DOI] [PubMed] [Google Scholar]
- 28.Yeni Y.N., Fyhrie D.P. Finite element calculated uniaxial apparent stiffness is a consistent predictor of uniaxial apparent strength in human vertebral cancellous bone tested with different boundary conditions. J. Biomech. 2001;34:1649–1654. doi: 10.1016/s0021-9290(01)00155-5. [DOI] [PubMed] [Google Scholar]