Abstract
The study is focussing towards Metal Injection Moulding (MIM) of Mg-alloys for biomedical implant applications. Especially the influence of the sintering processing necessary for the consolidation of the finished part is in focus of this study. In doing so, the chosen high strength EZK400 Mg-alloy powder material was sintered using different sintering support bottom plate materials to evaluate the possibility of iron impurity pick up during sintering. It can be shown that iron pick up took place from the steel bottom plate into the specimen. Despite the fact that a separating boron nitrite (BN) barrier layer was used and the Mg-Fe phase diagram is not predicting any significant solubility to each other. As a result of this study a new bottom plate material not harming the sintering and the biodegradation performance of the as sintered material, namely a carbon plate material, was found.
Keywords: Magnesium, Mg alloy, Sintering, MIM, Powder metallurgy, Biodegradable implant, EZK400
Graphical abstract
1. Introduction
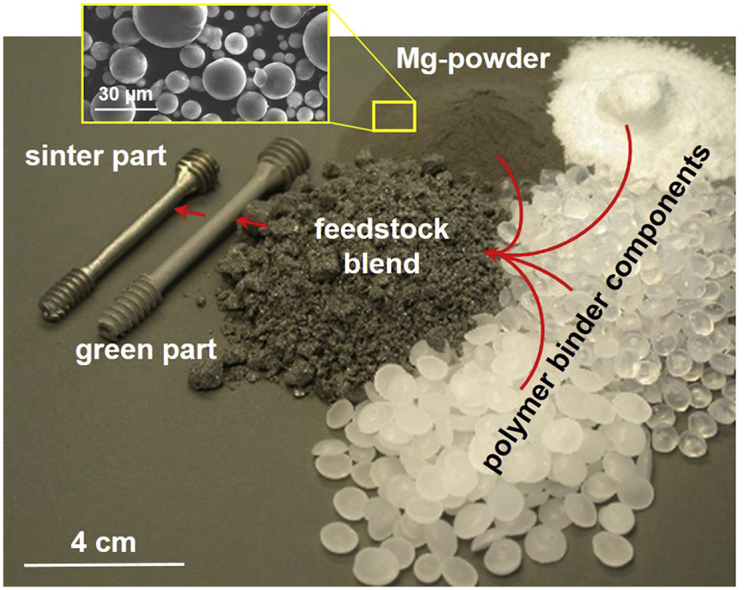
Biodegradable Mg-alloys are showing increasing interest regarding biomedical and orthopaedic implant applications. The biodegradable and biocompatible new material hold mechanical properties comparable to those of human cortical bone [[1], [2], [3], [4]]. Currently, first commercial implant applications are launched into the market [5]. These implants are made by extrusion of high strength Mg-alloy powders. However, the production process involves high effort and many individual machining steps to generate the finished part. Metal Injection Moulding (MIM) and Additive Manufacturing (AM) are alternative powder metallurgical production routes for both, mass production and manufacturing of individual single parts. MIM is an economic near net shape prototyping technique for small sized and complex shaped parts in high numbers. For the production of green parts via MIM, a feedstock blend made of spherical Mg-alloy powder and different polymer binder components as shown in Fig. 1 is needed. The shaping of the parts takes place via injection moulding of the semifluid or plasticised feedstock into a mould.
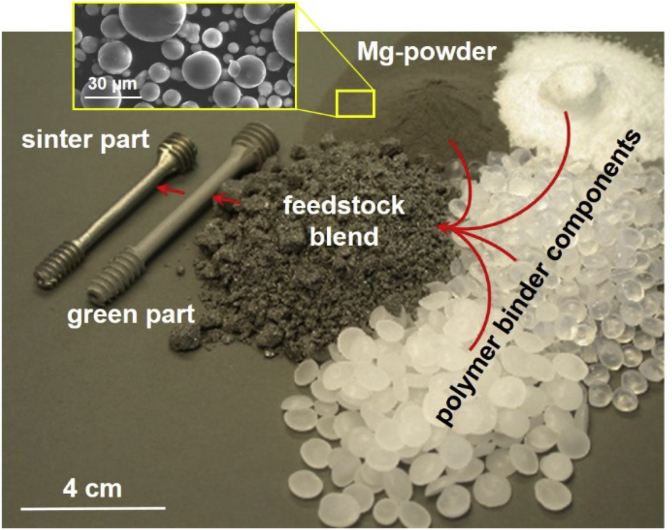
Fig. 1.
Mg-powder, polymer components, feedstock and implant screw demonstrator part in green and sintered condition made by MIM of Mg-alloy at Helmholtz-Zentrum Geesthacht.
Applying AM techniques, especially the Fused Filament Fabrication (FFF) or Fused Deposition Modelling (FDM), the same feedstock blend shown in Fig. 1 can be used for the shaping of individual single parts without use of a mould. The 3D printing techniques require feedstock granules (see Fig. 1) or feedstock filament. The consolidation of the final metal parts take place via sintering. The detailed processing about sintering of Mg and MIM of Mg is described in detail in Refs. [[6], [7], [8], [9], [10], [11]]. EZK400 tensile test specimen according to ISO 2740-B and different implant screw demonstrator parts could be successfully produced by MIM as shown in Fig. 1, Fig. 2b. The assessment of mechanical properties of the as sintered EZK400 implant prototypes and test specimen is described in detail in Ref. [12]. The tensile test of the dogbone shape MIM EZK400 specimen illustrated an ultimate tensile strength (UTS) of 164 MPa, tensile yield strength (YS) of 123 MPa and elongation at fracture of 3.4% [12]. Moreover, the binder-free press and sinter (P + S) parts, also used in the framework of this study for the biodegradation testing, obtain an UTS of 188 MPa, 122 MPa yield strength and 5% elongation at fracture [12]. However, this study is more focused on assessment of the biodegradation performance of the EZK 400 material. Biodegradable magnesium alloy parts for biomedical applications only tolerate limited amounts of impurities in the final part. Next to the existing impurity level of the used raw material the impurity uptake during processing is of high importance. Hence, the study focusses on the effect of impurity uptake during sintering of the Mg-Nd-Gd-Zr-Zn-alloy powder (EZK400) and the resulting effect on its biodegradation performance. Especially the influence of different sintering crucible bottom plate materials, necessary for the positioning of the green parts in the sintering furnace, on the degradation rate will be investigated within this study. This study demonstrated that both, good mechanical properties and biodegradation performance of the sintered Mg-material could be achieved.
Fig. 2.
a dogbone shape tensile test specimen [12] in: green condition (after injection moulding of the plasticised feedstock granules)- sintered condition (right side). b: suture anchor implant screw demonstrator parts made by MIM of EZK400 powder material in the green and sintered condition. Design: ConMed, USA.
2. Materials and methods
2.1. Powder, feedstock
The test specimen and implant screw demonstrator part production took place using a commercially available spherical gas atomised Mg-2.6Nd-1.3Gd-0.5Zr-0.3Zn-alloy powder, following up referred to as EZK400 (product name: MAP+21, Magnesium Elektron, UK). The particle size distribution was 20 μm–45 μm.
The feedstock blend as shown in Fig. 1, necessary for the MIM-process, was prepared using following polymer binder components: 60 wt% paraffin wax, 35 wt% polypropylene copolymer and 5 wt% stearic acid. The powder loading of the feedstock was 64 vol%. The blending of the feedstock components was performed in a planetary mixer (Thinky ARE-250 planetary mixer, Japan), applying 160 °C and 500 G acceleration. To avoid the uptake of additional oxygen during processing the powder handling took place under argon atmosphere in a glovebox system (Unilab, MBraun, Germany). The feedstock blend was granulated using a cutting mill (Wanner B08.10F, Germany).
2.2. MIM-specimen preparation
The manufacturing of dogbone shape tensile test specimen according ISO 2740-B and implant screw demonstrator parts as shown in Fig. 1, Fig. 2 took place using an injection moulding machine (Arburg Allrounder 320S) at up to 1500 bar injection pressure, 65 °C mould temperature and 135 °C feedstock temperature.
2.3. Debinding
To get rid of the waxy binder components and the stearic acid solvent debinding in a hexane bath (45 °C, 10–15 h) took place after the injection moulding process step (Lömi EBA50/2006, Germany). The thermal debinding of the polypropylene copolymer took place in a combined debinding and sintering hot wall furnace (RRO 350–900, MUT, Jena, Germany) using argon 6.0 as a protective gas.
2.4. Binder-free specimen preparation
To avoid any additional influences of binder components or binder residuals on the biodegradation test, binder free specimens produced by P + S were used for the biodegradation test procedure. In doing so, the EZK400 powder was directly filled into a die plate with cylindrical cavity. Cylindrical specimen with 11 mm in diameter and approximately 12–16 mm in high were produced using die plate, stamper, punching tool and a manual mode press (Enerpac RC 55, USA) as shown in Fig. 3a. The applied surface pressure was 100 MPa. The cylindrical specimens were placed in the sintering furnace using a BN coated sintering support bottom plate made of unalloyed steel as shown in Fig. 3b.
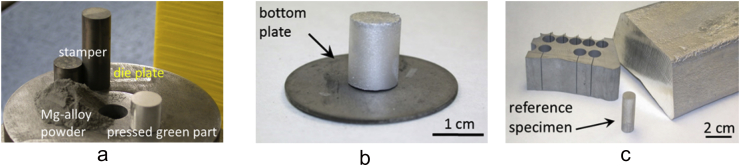
Fig. 3.
a: preparation of binder free biodegradation test specimen by pressing of Mg-alloy powder. b: sintered cylindrical test specimen for further finishing regarding the biodegradation test. c: exemplary cylindrical reference specimen made by wire spark erosion of EZK400 as cast bulk material.
For the biodegradation test, additional EZK 400 material in the condition as cast was needed to serve as a reference material (product name: Elektron21, Magnesium Elektron, UK). Platelets of 10 mm in diameter and 1.5 mm in thickness were produced using electrical discharge machining of cylinders from the bulk material (see Fig. 3c) followed by cutting into discs (Isomet 4000, Bühler, Germany). Equally discs from the as sintered material were prepared using the cutting machine. The single discs were numbered according their position in the sintered/heat treated cylinder as shown schematically in Fig. 4a.
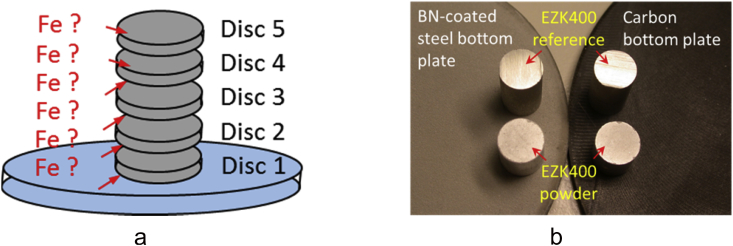
Fig. 4.
a: sintered/heat treated and fitted cylinder into single biodegradation test specimens, considering their correct ascending order. b: using different sintering support bottom plates for both: EZK400 powder material and EZK400 reference as cast material.
In the next step, a trace metal analysis of corrosion relevant elements like Fe, Ni and Cu was done performing Spark Emission Spectroscopy (SES) on each side of the single discs as shown schematically in Fig. 4a (Spectrolab, Spectro, Germany). After performing the SES analysis, the biodegradation specimen were finished using additional grinding (SiC grinding paper grid 1000) and cleaning treatments in ethanol (ATM, Saphir 360, Germany), followed by drying in a vacuum chamber (Epovac, Struers, Germany).
2.5. Sintering
Based on former sintering studies about Mg-Ca alloys [[6], [7], [8]] the sintering time was set onto 3–64 h. A labyrinth-like crucible setup and Mg-getter material as described in Ref. [6] was used. BN coated steel plates were used as standard sintering support plates for the parts. To avoid any additional influences of binder components or binder residuals on the biodegradation test, the binder free specimens, produced by P + S were used for the test procedure, as described in the former chapter.
A second sintering support plate setup was used performing an additional set of biodegradation specimen. In this second setup, both, pressed EZK400 powder green part cylinders and EZK400 as cast reference material cylinders were placed on different sintering support plates. (BN coated steel plate and carbon plate) as shown in Fig. 4b. This sintering plate setup aims on understanding if impurity uptake from the sintering support plate onto the specimen took place. The final test geometry of 10 mm in diameter and 1–2 mm in height was produced after the sintering heat treatment using the cutting machine. Single discs were numbered according to their position in the sintered/heat treated cylinder as shown schematically in Fig. 4a.
2.6. Trace metal analysis
Next to the trace metal analysis supported by a customers data sheet, additional analysis were performed using spark emission spectroscopy (Spectrolab, Spectro, Germany).
2.7. Biodegradation test
The biodegradation immersion test was performed in vitro for the measurement of the biodegradation performance of the cylindrical platelets, described in the former chapter. Before starting the immersion test, weight and density of the single discs were determined using the Archimedes method (Satorius LA230S, Germany). Cleaning of the specimens was performed using n-hexane for 20 min, followed by acetone for 20 min and additionally ethanol (100 vol%) for 3 min. Finally, sterilization was done using ethanol (70 vol%) for 20 min. All steps were performed in ultrasonic bathing with subsequent drying in vacuum and storage in argon.
The immersion test was done under semi-static conditions for 7 days in an incubator using 2 mL DMEM (Dulbecco's Modified Eagle Medium) + GlutaMAX™ + 10% FBS (Fetal Bovine Serum) solution under cell culture conditions (37 °C, 5% CO2, 19.7% O2, 95% rH). The immersion test was performed in 24 multi-well plates (Thermo Fisher Scientific, Denmark) incubated in the clean bench under sterile environment. The immersion medium was changed every 2–3 days.
After immersion testing, the corrosion products onto the specimen surfaces were removed via immersing the corroded specimens in chromic acid solution (180 g/L in distilled water) for 20 min. Afterwards the platelets were cleaned with distilled water and ethanol. The average degradation rate (DR) was calculated by weight loss method in mm/year using following equation:
where at Δg is the weight change in gram, A is the surface area of the sample in cm2, t is the immersion time in hours, and ρ is the density in g/cm3.
3. Results and discussion
3.1. Biodegradation test results – 1. sinter-experiment
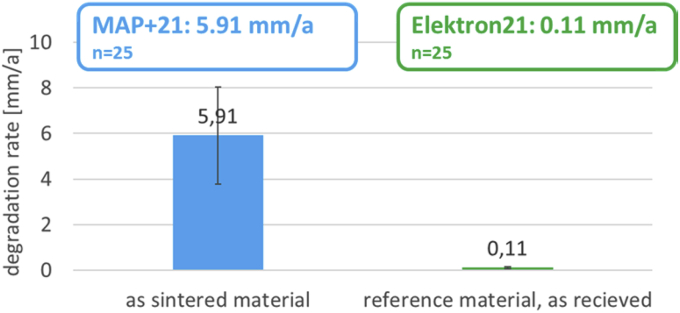
Biodegradation test results of the as sintered EZK400 in comparison to as cast EZK400 reference material are shown in the following diagram in Fig. 5. The column stained in blue is representing the average degradation rate result of the as sintered material. The column stained in green is representing the as cast reference material. All over all, 25 as sintered and 25 as cast reference specimens were tested.
Fig. 5.
Biodegradation test results of the as sintered EZK400 material (blue) in comparison to the as cast EZK400 material in the as received condition (no heat treatment).
Fig. 5 pointed out that the as sintered EZK400 material obtains a significantly higher average degradation rate of 5.91 mm/a in comparison to the as cast EZK400 reference material, with 0.11 mm/a. This result is surprising due to the fact that both, the as sintered EZK400 material and the as cast EZK400 material obtain nearly the same amount of alloying elements as proven by the customers analysis data sheet and additional SES analysis data, shown in Table 1a, Table 1b. The very promising result of the low average degradation rate of the EZK400 reference material in the as cast condition shown in Fig. 5 pointed out the general suitability of the chosen alloy composition for biodegradable applications.
Table 1a.
Analysis data about alloying elemental concentration and trace metal impurity levels of both EZK400 materials; powder and as cast. Both in the condition as received. Provided by customers data sheet (Table 1a) and SES-analysis (Table 1b).
| EZK400 | powder material | as cast reference material |
|---|---|---|
| Nd | 2.58 wt% | 2.8 wt% |
| Gd | 1.3 wt% | 1.3 wt% |
| Zr | 0.48 wt% | 0.44 wt% |
| Zn | 0.28 wt% | 0.29 wt% |
| Fe | <10 ppm | 30 ppm |
| Ni | <10 ppm | <5 ppm |
| Cu | <20 ppm | 10 ppm |
Table 1b.
SES-analysis.
| Fe | 11 ppm | 2,5 ppm |
| Ni | 37 ppm | 8.2 ppm |
| Cu | 26 ppm | 21.3 ppm |
However, mainly iron (Fe), cupper (Cu) and nickel (Ni) impurities can be responsible for an unexpected increase of the corrosion behaviour of the as sintered EZK400 material [13,14]. Just little changes of Fe impurities in the lower ppm range of Mg-alloys can take major influence on their corrosion behaviour [15]. The iron, nickel and copper concentrations of the materials in the as received condition are shown in Table 1a, Table 1b. The Fe impurity level of 2.5 ppm for the as cast reference material and 11 ppm for the powder material is still in a low range. A huge difference in degradation behaviour cannot be assumed due to these impurity levels [13,15]. Hence, the reason for the observed significant difference in the biodegradation performance of the two material routes might be based on an impurity uptake of the as sintered material that can only occur during sintering.
4. Sinter-experiment
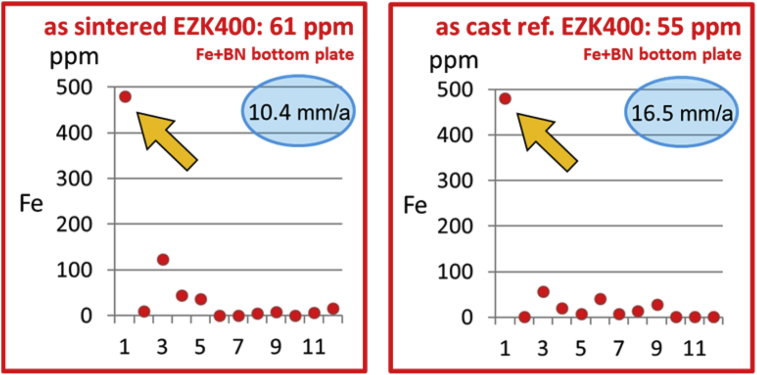
Therefore, a second sintering heat treatment using two different crucible plate materials was performed as described in the former chapter. This second sintering setup aims on understanding if impurity uptake from the sintering support plate to the specimen took place. The single red dots in the following two diagrams shown in Fig. 6 displays the SES analysis test results regarding the Fe content in ppm of each single disc of both, the EZK400 powder material and the as cast reference EZK400 material both in the as sintered/heat treated condition.
Fig. 6.
The diagram pointed out the impurity gradient of the cylindrical materials heat treated onto BN coated unalloyed steel bottom plates. The single red dots displays the SES analysis test results regarding the Fe content in ppm.
Both cylindrical specimens (powder material and as cast reference material) were placed onto the BN-coated bottom plate made of unalloyed steel (see Fig. 4b, left hand side) and heat treated together in the same sintering furnace run. The average Fe contents are shown in the headlines of the diagrams in Fig. 6. It can be seen that both, the Fe level of the as sintered material (61 ppm) and that of the as cast reference material (55 ppm) are quite high. Hence, the mean degradation depth shown in the blue sphere is consistently high, corresponding to their high level of Fe impurity.
The assumption that high Fe impurity levels can be traced back to Fe pick up from the bottom plate during sintering could be confirmed regarding the single SES results in the diagrams in Fig. 6, Fig. 7. It can be seen that the first disc (see orange arrows), which was in direct contact to the bottom plate during heat treatment, obtain a significant higher Fe value than the following discs. The Fe content of the first disc is above 480 ppm which is the maximum value, the used SES analysis system detected. The following disc 2–13 discloses in average, approximately an exponential decrease in the Fe contamination. The experimental results give a very strict indication that Fe pick up took place, despite of the fact that specimen and steel plate were disconnected through the used BN coating.
Fig. 7.
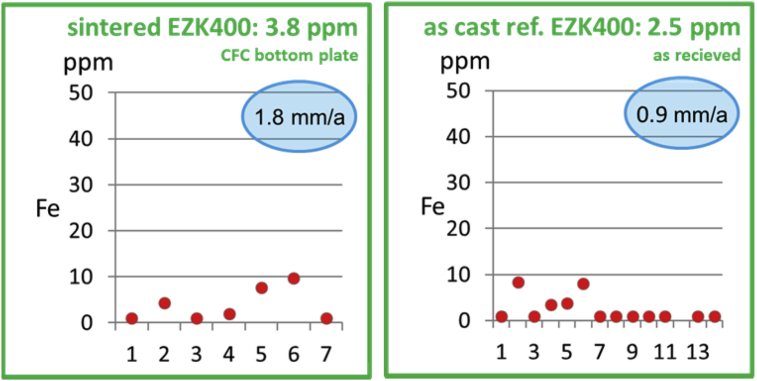
The diagram pointed out the impurity gradient of the cylindrical powder material heat treated onto carbon bottom plate material, as well as the gradient in the as cast reference material in the condition as received. The single red dots displays the SES analysis test results regarding the Fe content in ppm.
Hence, a closer look for an appropriate bottom plate material, not harming the Mg was done. The change of bottom plate material from BN coated unalloyed steel to carbon results in a significant decrease of the Fe impurity level and hence, in a significant better biodegradation performance (1.8 mm/a) of the as sintered EZK400 powder material as shown in the left hand side diagram in Fig. 7. The average Fe value is about 16 times lower using a carbon plate instead of BN coated steel plate. For comparison, the test result of the as cast reference material in the as received condition is given in the right hand side diagram, too. Hence, the Fe value in the as received condition increases of about 22 times using a sintering heat treatment onto BN coated steel bottom plate material. Therefore, future work will be done using the new carbon bottom plate crucible material for the sintering of MIM or P + S Mg-parts.
5. Conclusion
The study pointed out that EZK400 alloy obtains a high potential for biomedical application due to the low mean degradation rate of below 0.9 mm/a. However, the materials degradation performance is very sensitive to impurities as there are Fe, Ni and Cu. It was demonstrated that boron nitrite is not suitable for coating sintering bottom plates made of unalloyed steel. Iron pick up is not prevented consequently. The basically good biodegradation performance of the EZK400 material is deteriorated. The problem of Fe pick during sintering could be overcome using novel carbon sintering support bottom plates. Hence further work will be done using the new carbon bottom plate crucible material for the sintering of MIM or P + S Mg parts.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Poumarat G., Squire P. Biomaterials. 1993;14:337–349. doi: 10.1016/0142-9612(93)90051-3. [DOI] [PubMed] [Google Scholar]
- 2.Cunha A.R. Hindawi Publishing Co; 2012. International Journal of Hypertension. Art.-ID 754250. [Google Scholar]
- 3.Janning C., Willbold E., Vogt C., Nellesen J., Meyer-Lindenberg A., Windbergen H., Thorey F., Witte F. Magnesium hydroxide temporarily enhancing osteoblast activity and decreasing the osteoclast number in peri-implant bone remodelling. Acta Biomater. 2010;6:1861–1868. doi: 10.1016/j.actbio.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 4.Witte F., Fischer J., Nellesen J., Crostack H.A., Kraese V., Pisch A., Beckmann F., Windhagen H. Biomaterials. 2006;27:1013–1018. doi: 10.1016/j.biomaterials.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 5.http://www.syntellix.de/en/home.html.
- 6.Wolff M., Dahms M., Ebel T. Sintering of magnesium. Adv. Eng. Mater. 2010;12:829–836. [Google Scholar]
- 7.Wolff M., Guelck T., Ebel T. vol. 2. 2009. Sintering of Mg and Mg-Ca Alloys for Biomedical Applications; pp. 417–422. (Euro PM2009 Proceed). [Google Scholar]
- 8.Wolff M., Bischof C., Dahms M., Ebel T., Klassen T. 9th International Conference on Magnesium and Their Applications, Vancouver, Canada. 2012. p. 102. [Google Scholar]
- 9.Wolff M., Schaper J.G., Dahms M., Ebel T., Kainer K.U., Klassen T. Powder Metall. 2014;57(5):331–340. [Google Scholar]
- 10.Wolff M., Schaper J.G., Suckert M.R., Dahms M., Feyerabend F., Ebel T., Willumeit-Römer R., Klassen T. Metal injection molding - MIM of magnesium and its alloys. Metals. 2016;6(118) [Google Scholar]
- 11.Wolff M., Schaper J.G., Suckert M.R., Dahms M., Ebel T., Willumeit-Römer R., Klassen T. Magnesium powder injection molding (MIM) of orthopedic implants for biomedical application. JOM (J. Occup. Med.) 2016;68(4) [Google Scholar]
- 12.The Minerals, Metals & Materials Society, TMS . 147th Annual Meeting & Exhibition Supplemental Proceedings, the Minerals, Metals & Materials Series. 2018. ISBN: 978-3-319-72525-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.J.E. Hillis et al, Soc Auto Engineers Technical Paper Series #860288, Detroit.
- 14.Liu M. Corrosion Sci. 2009;51:602–619. [Google Scholar]
- 15.Atrens A. Mater. Sci. Eng. B. 2011;176:1609–1636. [Google Scholar]