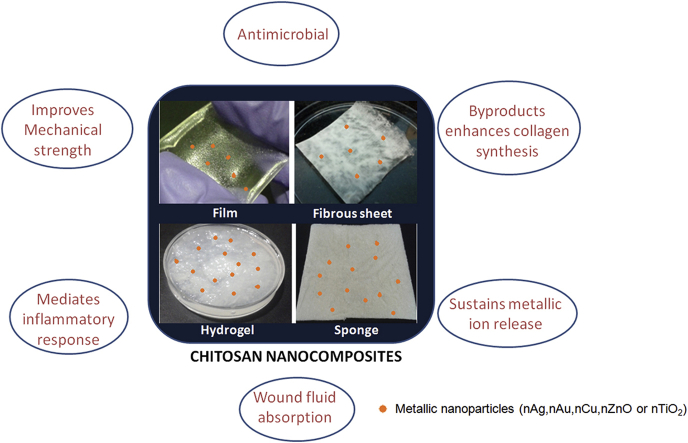
Abstract
Chitosan based nanocomposite scaffolds have attracted wider applications in medicine, in the area of drug delivery, tissue engineering and wound healing. Chitosan matrix incorporated with nanometallic components has immense potential in the area of wound dressings due to its antimicrobial properties. This review focuses on the different combinations of Chitosan metal nanocomposites such as Chitosan/nAg, Chitosan/nAu, Chitosan/nCu, Chitosan/nZnO and Chitosan/nTiO2 towards enhancement of healing or infection control with special reference to the antimicrobial mechanism of action and toxicity.
Keywords: Antimicrobial Chitosan nanocomposities, Silver, Zinc oxide, Gold, Copper, Wound healing
Graphical abstract
Highlights
-
•
Antimicrobial activity of chitosan/metallic nanocomposite scaffold is discussed.
-
•
Toxicity profile of chitosan/metallic nanocomposite scaffold is covered.
-
•
The mechanism of antimicrobial activity and toxicity is discussed in this review.
1. Introduction
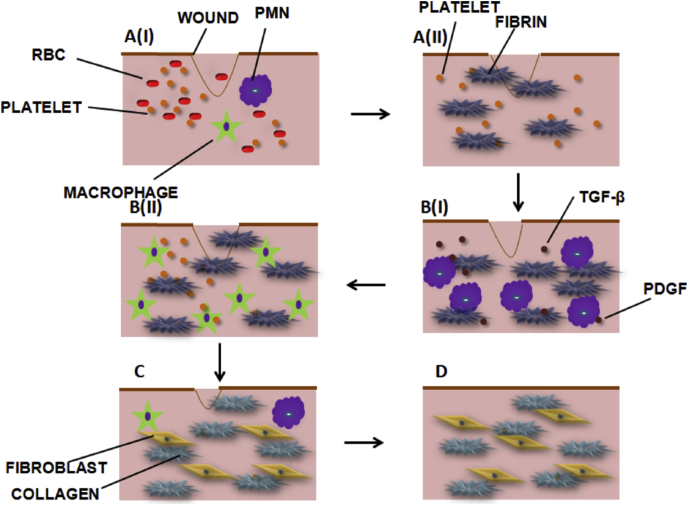
The wound is any type of injury occurring on the skin either due to cut or damages. Wound healing is a process by which any loss in the tissue integrity is being repaired by a series of phases [1]. Healing is dependent on several factors, including medical condition of the person. The phases are yet complex and are regulated by cellular and molecular mediators. The different stages include haemostasis, inflammation, proliferation and remodeling (Fig. 1). In an acute wound, haemostasis, which is the initial phase, is a mechanism that helps in the prevention of excess blood loss in the wound site. There is initiation of coagulation cascade, via extrinsic or intrinsic pathways leading to platelet accumulation and formation of a fibrin clot. The platelets would release several growth factors that would recruit and enable migration of cells, such as polymorphonuclear leucocytes (PMN), endothelial cells etc. Haemostasis occurs within minutes and is accompanied by the formation of a blood clot, which prevents further bleeding. Inflammation is marked by the activation of immune cells to defend the foreign substances at the wound site. Inflammatory phase is marked by the infiltration of neutrophils and macrophages for subsequent destruction of microbial flora. The stage is followed by proliferation, where fibroblast cells undergo rapid division, secrete collagen and a new extracellular matrix is built up. The inflammatory cells secrete many mediators required for the granulation tissue formation. Transforming growth factor-β (TGF-β) is an inflammatory cytokine that regulates cellular proliferation and apoptosis. Various other growth factors such as platelet derived growth factor (PDGF), vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), etc. are secreted to the site. All these pro-angiogenic factors initiate cell proliferation and differentiation. In remodeling, the collagen fibers mature and the wound heals with scar formation. When the healing phase is halted at any of the above mentioned stages, wounds become chronic. As a result, the wounds become more prone to secondary infections. Infected wounds are characterized by an increase in microbial burden, large volume of exudates, and an increase in the number of pus cells causing necrosis of tissues [2], [3]. Hence, the necrotized areas are debrided to allow the growth of new granulation tissue. The chance that diabetic patients get limb amputation is nearly 15% [4]. Therefore, special care has to be taken in dressing the wound to decrease the microbial burden and control infection. Hence, wound dressing materials are important in maintaining different phases of healing [5]. To assure a complete clinical solution, special emphasis is being laid to identify the types of wound and the design of dressing material. Ideally a dressing material, should cover the wound from foreign infection, allow gaseous exchange and maintain a moisture balance, which ultimately destines the fate of the wound [5].
Fig. 1.
Wound healing process (A) Haemostasis (I) Platelet activation (II) Fibrin clot (B) Inflammatory phase(I) Early Inflammation (24 h) (II) Late Inflammation (48 h) (C) Proliferation (72 h) and (D) Remodeling (Weeks to months).
Traditionally, people used cotton gauze as dressings which served only as a structural framework to cover the wounds. Such dressings are mentioned as passive dressings. However, modern dressings are either interactive or bioactive. Interactive dressings include films, sponges, hydrogels etc., which provide an antibacterial efficacy in addition to the structural framework [5]. Bioactive dressings play a role beyond the interactive dressings in the process of healing.
Currently, dressings are developed using different types of polymers whose origin is either synthetic or natural. There are synthetic dressings made from poly (Methacrylates), polyvinyl pyrrolidine, etc. These dressings render a low mechanical strength, making it difficult for patients to handle [5]. Though synthetic dressings impart good anti infectious property, their degradation products should not cause any harm to the skin [5]. This condition could not be met by synthetic polymers. Infected wounds are characterized by the presence of excess exudate formation with improper healing [6]. The infection spreads rapidly to other parts of tissue leading to surgical removal of affected parts. Management of such wounds is a major difficulty in the field of wound care. Therefore, much research has been focused to bring forward dressings developed from natural polymers. Need of the hour is an effective bandage that not only eliminates the microbial burden, but also assists in other healing activities such as control of scar formation, ECM remodeling, mediating inflammatory activities, etc. Often these are met by more than one active agent or a multitude of changes that happens within the dressing material.
Metal based active agents have received much interest in the clinical scenario. Extensive studies have been done on silver (Ag) nanoparticles or zinc oxide (ZnO) nanoparticles incorporated into Chitosan scaffolds/bandages. Certain redox-active transition metals, especially copper (Cu) can participate in the Fenton reaction resulting in the production of reactive oxygen species. Soft and borderline acids such as Ag+, Au+, Cu+, Cu++ and Zn++ respectively form covalent bonds with thiols or protein containing sulphur group [6]. This causes oxidation and subsequent depletion of microbial antioxidant reservoirs. Amino acids such as histidine, arginine, lysine and proline are susceptible to metal based oxidations leading to carbonyl products. Ag, gold (Au) and Cu attacks Iron-Sulphur- (Fe-S-) containing dehydratases. Ag has a high affinity with the highly electronegative polymeric membranes of bacteria. In addition to factors such as size, shape, surface charge, etc., the toxicity initiated by metallic nanoparticles is dependent on the mode of release of metallic ions from the material [6].
However, concerns regarding the possible environmental and health impacts prevail in the use of metallic nanoparticles, which calls for a detailed evaluation of cytotoxicity [7]. Toxicity of nanoparticles increases with lowering of the size of nanoparticles solely due to the enhancement in surface area to volume ratio, which amplifies the interaction of surface atoms or molecules with the outside environment. The type of interaction further depends on the surface moieties whether it is hydrophobic, hydrophilic, lipophilic, lipophobic, active or passive [8], [9]. Shape dependent toxicity adjoins aggregation of particles, surface coating or solubility resulting in the changes in transport properties whose accumulation may cause dose dependant toxicity in tissues. The major attribute to cytotoxicity of metallic nanoparticles include the reactive oxygen species (ROS) generated by the disruption of the electronic and ionic flux, permeability of transition pores and reduction of the intracellular glutathione level [9]. The overall cytotoxicity of metal based nanocomposites can be reduced by incorporating into suitable biodegradable matrices. Moreover, healing of wounds with larger tissue defects requires a biomaterial support for the regeneration of cells and matrix. Therefore, a bioactive dressing is largely encouraged and is extensively studied.
Dressings made out of marine based polymer, i.e. Chitosan, is the scope of this review. Chitosan is obtained from the deacetylation of amine groups in chitin, which is a component of exoskeleton of marine crustaceans and certain fungi. Degradation products of both chitin and chitosan can help in the regeneration of wounded area through fibroblast proliferation. It is reported that the oral administration of glucosamine enhances earlier synthesis of hyaluronic acid which helps in faster healing with minimal scarring [10]. Application of glucosamine gel helps in revascularization [11]. Glucosamine is transported to cells via glucose receptors and has a role in protecting against atherosclerosis. The protective effect was mediated by increased expression of perlecan in endothelial cells, which is an extracellular matrix heparin sulphate proteoglycan [12]. Glucosamine also reduced proliferation of smooth muscle cells in vitro, further confirming the anti-atherosclerotic property [11]. Therefore, chitosan based products tuned to degrade faster can also be used for application to prevent thrombotic lesions in peripheral arterial diseases. Chitin and its derivative, i.e. chitosan, are also known to control inflammatory mediators and help in faster healing [13]. This would be advantageous for the types of wound that has been halted at the inflammatory stage [14], [15]. Chitosan can be easily formed into films, hydrogel and scaffolds without use of toxic chemicals [13], [16], [17], [18], [19], [20]. The dressings that can be applied to wounds depends on the type of wound, amount of exudates formation, nature of damaged skin, pressure to be applied, etc.
Chitosan and various modifications of Chitosan [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21] have shown innate antimicrobial activity due to the presence of specific functional groups which can be further enhanced with the addition of metallic nanoparticles. In this review, we highlight the importance and antimicrobial effects of Chitosan with metallic nanoparticles. The review is structured into sections that elaborate on the development of Chitosan based nanocomposite scaffolds containing silver (nAg), gold (nAu), copper (nCu), zinc oxide (nZnO) or titania (nTiO2) in the nano form and its effect on microbial activity and wound healing.
2. Application of antimicrobial chitosan metallic nanocomposites in wound healing
2.1. Chitosan/nAg
In ancient times, silver has been used topically on burns and wounds in the form of silver nitrate [22], [23]. Metallic silver in the form of nAg is widely used as preservatives in cosmetics [24], in the fabrication of gels [25], sponges [26], antimicrobial agents [27], [28] films [29], [30], nanofibrous mat [31], [32], etc. These nAgs are mainly used in the treatment of several pathogenic bacteria that have developed resistance against various antibiotics. So, nAg has emerged up with a variety of biomedical applications ranging from silver based wound dressing to silver coated medical devices [33]. Inflammatory, oxidative, genotoxic, and cytotoxic effects are associated with nAg exposure and the target organ is liver [34], [35], [36], [37], [38]. Various in vitro and in vivo studies point out the toxicity effects and accumulation of Ag in the liver. A study reported that the nAg exposure to dermal sites significantly increased the Ag level in serum post surgery in human trials, but, it was observed that the normal liver functioning and coloration of skin was restored after the cessation of treatment without causing further deleterious effects [35]. nAg penetrates readily through damaged skin than intact skin [36], [39]. The toxic effects of nAg can be reduced by using suitable carrier systems or surface coatings [21], [40], [41]. Further incorporation of nAg in chitosan bandages would help in controlling the release of nAg thereby reducing the toxicity to normal cells. Travan et al., reported selective antimicrobial effect by encapsulating nAg in lactose substituted chitosan which strongly holds the nAg by coordination bonding and protects it by steric hindrance preventing eukaryotic cell uptake of nAgs [40]. Antimicrobial activity was observed, indicating the direct interaction of the nAg with the proteins localized on the bacterial surface [40]. Concentration dependant toxicity does prevail albeit its incorporation in bandages as observed in certain studies [26].
The activity of silver ions is more towards gram negative bacteria than a gram positive, due to the absence of the thick peptidoglycan layer as in gram positive bacteria. Silver ions at micro molar concentrations are studied for properties such as uncoupling of electron transport in respiration and membrane permeability [42]. nAg at a concentration as low as 0.4 nM inhibited E. coli proliferation in comparison with higher amount (6 μm) for AgNO3. Proteomic analysis revealed that silver treated E. coli expressed around eight proteins, namely outer membrane proteins A, C, and F (OmpA, OmpC and OmpF), periplasmic oligopeptide binding protein A (OppA), D-methionine binding lipoprotein (MetQ), inclusion body binding proteins A and B (IbpA and IbpB), and 30S ribosomal subunit S6. Also, the ATP level in E. coli was depleted after 5 min of incubation. The effect was observed for nano Ag as a result of follows membrane disruption by obstruction of potassium flux [43], [44].
The bactericidal action of Ag is well known and the capability of nAg to easily enter into the bacterial cell and form a less dense region in the center of the bacteria may be the mechanism of action. The antimicrobial activity of silver nanoparticles can be described in different ways. The silver ions are found to be reactive with thiol or sulphydryl groups. Therefore, once nAg enters a cell, it causes cell death by interacting with thiol containing important enzymes. The second proposed mechanism is that, Ag being a weak acid, has the tendency to react with the weak acid groups in the genetic material, such as phosphate, resulting in disruption of DNA/RNA and thereby preventing the translation of proteins [45], [46], [47]. nAg mainly get adsorbed on the bacterial membranes, thus forming pores in the membrane. The silver ions are known to enter inside bacterial cell membrane, causing reactive oxygen species and free radical synthesis which in turn causes structural and integral damage of the cell. In a thermal injury model, nAg showed better healing with 26.5 ± 0.93 days, whereas control and silver sulfadiazine exhibited slower wound healing rate by an average of around 35 to 37 days. The thermal injury model caused infection in 7 days, after which treatment with nAg showed no signs of further increase in the bacterial proliferation. The inflammatory cytokines such as IL-6, TGF-β1, IL-10, VEGF, and IFN-γ were found to be modulated in the wound after treatment of nAg [48].
Proteases in chronic wounds hinder healing by proteolytic cleavage of extracellular matrix and fibroblasts. It is already reported that the activity of MMP-2 and MMP-9 is down regulated by silver ions. Therefore, application of silver in optimum concentration would find application in infectious and chronic wounds [49], [50]. Silver ions, released from the nanoparticles inside the bacterial cells, enhance their bactericidal activity [27], [28]. nAgs were incorporated into the derivative of chitosan, 4-(ethoxycarbonyl) phenyl-1-amino-oxobutanoic acid-chitosan to form a nanocomposite film [29].4-(ethoxycarbonyl) phenyl-1-amino-oxobutanoic acid-chitosan, is a derivative of chitosan with reported cytotoxic effects. An initial concentration of 1 × 10−3 M silver nitrate was used in nanoparticle preparation. The activity of the components of nanofilms, Chitosan, acid (ETHA) and acid with Chitosan, was also compared. A combination of Chitosan, ETHA and Ag showed about 1.5 fold increase in the antimicrobial zone towards P. aeurugenosa, S. aureus and E. coli when compared to Chitosan control. However, the activity is improved when compared with commercially available silver dressings, Acticoat (Smith & Nephew) and Aquacel-Ag (Convatec). It is already reported that the toxicity of silver ions towards skin dermal cells are reduced by the presence of antioxidants such as thiols or cysteine [51]. The conjugation of chitosan with 4-(ethoxycarbonyl) phenyl-1-amino-oxobutanoic acid (ETHA) masks the free amine groups in chitosan. Therefore, the higher antimicrobial activity of Chitosan control could not be due to protonation of amine groups [52], rather it may be due to some unexplored possible mechanisms of microbial cellular antioxidant depletion by Chitosan. In a study conducted by Arockianathan et al., the properties of Chitosan/sago starch film impregnated with nAg and an antibiotic, i.e. gentamicin was evaluated by open excision wound model in vivo. Films are generally used in cases where the wound exudate is low. They protect damaged skin from compression and also help in debridement. Therefore, higher mechanical properties are often a ruling factor for such types of dressings. It would be interesting if such dressings can prevent further microbial infections [53]. Sago film, which had poor mechanical properties, was made flexible by the addition of ethylene glycol. Of the different prepared compositions, the film containing 10 mL of nAg containing 1 × 10−4 M of initial Ag nitrate solution showed better tensile strength of 13.98 ± 1.06 MPa and was used throughout the experiments. A recent study performed on evaluation of tensile strength of commercially available film dressing Medifoam® showed strength of 0.33 MPa which is much lesser than the reported value for chitosan-sago film [53]. In an open excision wound healing model, the wound closure by day 4 was found to be 21% and 30% for nAg containing chitosan/sago starch film and chitosan/sago starch film with gentamycin respectively. The faster rate of healing was evaluated through the measurement of wound biochemical markers such as protein content, collagen, DNA, hexosamine and uronic acid. The protein, collagen and DNA content were found to be higher for the nanocomposite films up to 12th day, when compared to the cotton gauze treated wounds. This corresponds to the higher fibroblast proliferation and tensile strength. There was a decrease in hexosamine and uronic acid levels, which is an indication of the higher extracellular matrix. The incorporation of nAg was favored for the enhancement of antimicrobial activity. However, the antimicrobial nature was estimated neither in vitro nor in vivo [30]. Chitosan blended with poly (vinyl alcohol) (PVA) was woven into a fibrous sheet by electrospinning technique, silver nanoparticles were incorporated at a concentration of 0.3142 g/100 mL of chitosan solution (i.e. 3142 ppm) [31]. Fibrous sheets can be applied on wounds where the active component is released for prolonged antimicrobial effects. The developed non-woven mat showed ability. However, 5 and 10 mmol L−1 containing nanofibers exhibited almost 99% inhibition halos after 12th hour, which was maintained throughout whereas, control Ag showed its peak activity at 5th hour, this corresponds to the release pattern of Ag from the fibrous scaffold. Viable cell counting showed that the fibre mats electrospun from different weight ratios of 90/10, 80/20, and 60/40 PVA/CS were effective in reducing E. coli growth up to 7 × 107 CFU/mL. Chitosan being polycationic, would attack the anionic bacterial membrane, causing damage which was further improved by the addition of nAg, which causes damage to cell membrane permeability and respiration. The interaction of silver ions with that of highly electron rich oxygen atoms and glucosidic groups in chitosan via ion-dipole interaction is an added advantage [31]. The activity of non-woven mat increased with increase in chitosan ratio. Further, the PVA/chitosan mat showed improved activity upon nAg addition. This was attained by increased chain flexibility and binding of carboxymethyl chitosan with Ag. Therefore, it acted as a good catalyst for reduction of Ag to nAg [54]. The PVA/CS-Ag-NPs with a chitosan concentration up to 20% was bacteriostatic, whereas chitosan concentration higher than 20% exhibited bactericidal activity [32]. PVA/N-carboxymethyl chitosan (PVA/CM) electrospun fibers containing in situ formed nAg showed improved bacterial growth inhibition against E coli at a concentration of 10 mmol L−1 Ag which was in accordance with the in vitro nAg release data [32]. nAg loaded into chitosan gels were evaluated for the antimicrobial activity towards biofilms of clinical strains and compared with silver sulphadiazene. The antimicrobial effect of the prepared gels was highly significant towards methicillin-resistant S. aureus (MRSA). Chitosan gel loaded with 300 ppm showed better activity against in vitro biofilm, which showed 75% viability towards fibroblast cells. The silver sulphadiazene on the other hand prevented the biofilm formation and at the same time, was highly toxic towards human fibroblast cells [25].
Sponge dressings are used in the case of high to moderate exudate wounds. Chitosan-Hyaluronic acid sponges incorporating nAg showed excellent antimicrobial activity towards S. aureus, MRSA, P. aeruginosa and K. pneumonia as evaluated by disc diffusion and membrane potential analysis. However, the cytocompatibility was dependent on the concentration of nAg. The chitosan-HA sponge containing 0.001% nAg showed slight decrease in viability towards human dermal fibroblasts (HDF) to nearly 46%. However, nAg incorporated into the chitosan-HA with a concentration of 0.001% and 0.005% showed 60–70% viability. This indicates that with increasing concentrations of nAg, there is increase in the toxicity towards human dermal fibroblasts [25]. The membrane potential of bacteria as assessed by DiOC6 showed a decrease in fluorescence with an increase in nAg concentration which indicates changes in membrane permeability due to the collapse of bacterial membrane [26]. nAg get oxidized in the presence of moist wound fluid, thereby releasing Ag+ ions. The silver ions would bind to the sulphur containing proteins in the cell membrane disrupting the prokaryotic genetic material. The silver ions also cause inhibition of bacterial respiratory chain [27], [28].
2.2. Chitosan/nAu
nAu have found multitude applications owing to its easy synthesis routes, inert nature and easy and efficient surface functionalization. Toxicity pertaining to nAu accounts to the significantly low extent compared to other nanoparticles like Ag, ZnO, TiO2 etc [55], [57]. Interestingly, size and shape dependant toxicity was observed for nAu, sizes lower than 2 nm showed higher toxicity compared to larger particles. Spherical particles are easily uptaken by cells compared to rods or triangles with higher aspect ratio [55], [56], [57]. The various chemical synthesis routes for nAu development create varying toxicity levels. The presence of toxic capping agents also contributes to toxicity [57]. Nanoparticle uptake of different size and shape are mediated by receptor-ligand binding, receptor recycling rates, and exocytosis [55], [56]. Skin penetration of nAu has shown to be higher in damaged skin [35], [58]. nAu toxicity also accounts to the ROS mediated cell apoptosis [59]. The careful determination of surface capping agents or modifiers like cysteine, citrate and glucose can greatly control the toxicity pertaining to nAu [59].
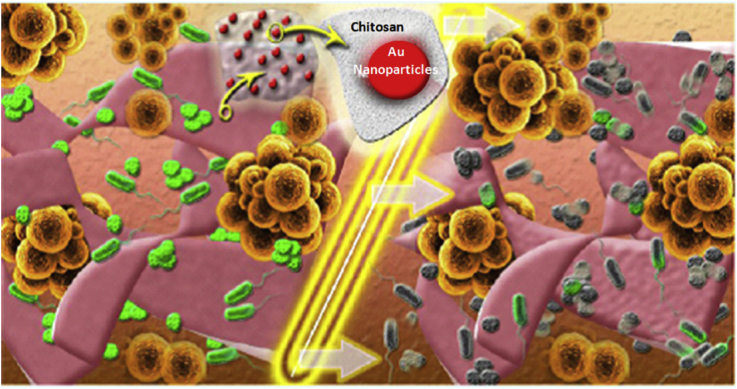
nAu of average diameter 10 nm were prepared by green synthesis using chitosan. The chitosan with medium molecular weight acted as a better stabilizing agent. Chitosan based films containing nAu of high antibacterial activity were prepared [60]. Cytotoxicity tests showed no toxicity up to a concentration of 700 μM for A549 cells and 1 mM for HaCaT cells, respectively. The cytotoxicity was mainly reduced by the surface coating with biodegradable and non toxic polymer, i.e. chitosan. The prepared films were characterized for cytotoxicity. The in vitro cytotoxicity tested on A549 showed higher toxicity within the range from 143 μM up to 714 μM. The morphology of S. aureus and P. aeruginosa was assessed by SEM. The nAu at 5 and 10 mM of initial precursor showed significant changes in bacterial cell morphology with pores and structural changes on bacterial membrane as confirmed by transmission electron microscopy. The nAu released from the film on swelling in a fluid medium, showed almost 10 fold decreases in microbial colonies in vitro, a concentration dependent manner [60]. The higher antimicrobial activity against gram negative biofilm than gram positive one was shown by SEM analysis of membrane destruction of S. aureus upon nAu treatment. The activity of chitosan is favored by the higher amino groups contributed by the degree of deacteylation. The positive charge of chitosan enables cell wall interactions and further destabilization. The toxicity of nAu was size dependent which was due to the interaction with the chitosan films. Figure (Fig. 2) shows that upon contact with wound dressing materials, i.e. chitosan/nAu film, the microbes are deactivated [60].
Fig. 2.
Mechanism of antibacterial activity of gold nanoparticles released from Chitosan scaffold. [Copyright 2015. Reproduced with permission from ACS publications Ltd].
Spherical nAu of size 25–50 nm exhibits anti-inflammatory properties by inhibiting IL-6, IL-12 and TNF-α level [61]. Topical application of mixture of nAu, epigallocatechin gallate (EGCG), and α-lipoic acid promoted greater cutaneous healing. This was probably induced by greater absorption of the formulation by opening tight junctions in the stratum corneum. The wound tissue at day 7 showed higher expression of VEGF and Ang-1expression [62]. Similarly the same formulation was applied on to full thickness wounds in mice induced with diabetes. The nAu + EGCG + α-lipoic acid treated wounds showed a faster healing rate. The expression of receptors for advanced glycated end products was found to be lower in the experimental group. The expression of pro-angiogenic markers such as Ang-1, Ang-2 and VEGF was modulated and there was no neutrophil infiltration preventing inflammatory response [63].
In another study, colloidal gold was self assembled onto chitosan film [64]. The nanoparticles of size 25 nm were used for preparing film with the molar ratio of precursors, trichlorogold tetrahydrate hydrochloride to trisodium citrate being 0.50. The chitosan control showed better attachment of keratinocytes in the 6th hour, however, at later time points, the three dimensional film of chitosan gold showed improved keratinocyte attachment, in vitro. The 3-D structure of the scaffold could also prevent the fibroblast growth, thereby reducing the mixing of the two cells. The in vitro keratinocyte culture studies were marked by the presence of heterochromatin, chondriosomes and desmosomes which finds importance in skin regeneration [64].
2.3. Chitosan/nCu
Copper is an essential trace element that facilitates the activity of various enzymes and is an essential element in many wound-healing-related processes. In the context of toxicity studies on nCu, the main factors relates to the production of oxyradicals which initiates the formation of ROS resulting in oxidative stress [65], [66]. Dose dependant toxicity occurs due to the dissolution of Cu ions form the system which accumulates in tissues resulting in ROS generation [67], [68]. The systemic toxicity of Cu can occur if the nCu reaches the dermis layer where the nCu can enter the extensive blood vasculature. The incorporation of nCu in chitosan based bandages has shown less toxicity which is indicative of the interaction of nCu with chitosan [69]. nCu is also reported to be a potent antibacterial material [69], [70]. nCu mainly act by two known mechanisms; one is depletion of potassium ions leading to disturbances in the membrane redox potential [71]. Other mechanism is by the hydrolysis of peptidoglycan layer causing changes in osmotic pressure [72]. nCu (100 μg/mL) biosynthesised from P. aeruginosa had better activity than native copper towards B. subtilis, P. aeruginosa,S. aureus and E. coli. Higher activity of copper towards gram positive bacteria is due to the higher affinity with proteins and peptidoglycans. nCu diffuses readily than native copper and interlinks the cellular proteins to cause cellular destruction [73]. Particle size is inversely proportional to the toxicity. According to toxicological parameters such as maximum tolerated dose (MTD), LD50 and LD100 values, nCu are lesser toxic than copper salts. An ointment incorporated with oil suspension of nCu in an open wound caused complete healing by two days [74]. Chitosan based copper nanocomposite (0.3%) was shown to enhance healing in an excision wound model in a rat [69]. When compared to the chitosan control, there was a decrease in the pro-inflammatory cytokines for chitosan based copper nanocomposite treated group. The increase in the pro-inflammatory cytokines was prolonged for chitosan control, whereas it was attenuated in the presence of nCu. The expression of VEGF, which is an enhancer of angiogenesis, was found to increase by day 3 and 7. Also the expression of inflammatory cytokines such as TGF-β1 and IL-10 was found to increase before day 3, and thereby a decrease then after. This is indicative of requirement of inflammatory markers in the initial stages for the activation of monocytes and macrophages and later decrease, for the collagen synthesis. TNF- α and IL-10 are anti-inflammatory cytokines which are not required at the later stages of healing. The inflammatory activity of chitosan is masked and further inhibited by the presence of copper. nCu also stimulated proliferation and migration of fibroblasts as evidenced by the histology studies. Copper dependent enzymes, prolyl 4- hydroxylases and lysyl oxidase helps in the synthesis of collagen, further chitosan can also contribute to the higher collagen deposition in chitosan based copper nanocomposite group. Decreased TGF-β1 would inhibit the formation of keloids and hypertrophic scars. Therefore, the wound healing property nCu was enhanced with chitosan. Fig. 3 shows the activity of nCu at different stages of healing towards wound re-epithelialisation and wound contraction. The fibroblast proliferation and angiogenesis is favored by modulating immune cytokines. Copper is a trace element and plays a role in skin regeneration and angiogenesis [75].
Fig. 3.
Mechanism of action of Chitosan-based copper nanocomposite. [Copyright 2010 Reproduced with permission from Elsevier Ltd].
2.4. Chitosan/nZnO
nZnO induced a time and concentration dependant toxicity to human cells since nZnO readily dissolves in aqueous medium [75], [76]. A study on human epidermal cell line showed toxicity from a concentration of 8 μg/ml as determined by MTT assay [77], [78]. Genotoxicity and loss of mitochondrial activity were the baseline effects of nZnO in human cells which results from the ROS species dependant oxidative stress [79]. The readily dissolving capability of nZnO would lead to enhanced Zn penetration and accumulation in tissues, the penetration rate enhanced in acidic environment [80]. Careful optimization in the concentration of nZnO incorporated into chitosan based bandages would help in balancing the antimicrobial activity and cytotoxicity [79]. Several factors affect the antimicrobial properties of metallic nanoparticles. The factors include particle morphology, larger number of polar facets, surface modification, size, concentration etc. The shape or morphology of nanoparticles is largely dependent on the active facets of the nanoparticle [81]. Rods have greater number of facets compared to spherical particles thereby having greater antimicrobial activity. Again internalization of particles becomes easier for nanorods, nanowire and flower shaped, the least being for spherical ones [81].
Larger number of polar facets confers higher number of oxygen vacancies, which in turn would help in greater amount of ROS generation. Surface modification by oxygen also improves the antibacterial activity. The particle size influences the toxicity of nZnO. Lesser the particle size, greater would be the surface area and higher the interaction of particle to bacterial cell membrane. The activity is largely dependent on the fluid environment, which helps in generation of H2O2. Therefore, nZnO finds applications in wounds where there are more infection and fluid accumulation [80], [81], [82], [83], [84], [85].
nZnO possess excellent antibacterial and antifungal activity. Thus, in order to enhance the antimicrobial property, nZnO was incorporated into wound dressing and cosmetics materials [82]. Reports have suggested that a size scale of <100 nm nZnO showed efficient antibacterial activity with less toxicity to normal cells under in vitro (MG-63, HDF) and in vivo (cells in proximity to applied bandage) conditions [80]. In addition, the leaching of Zn2+ ions from nZnO would help in keratinocyte migration towards the wound site and promote epithelialisation [82].
Sudheesh Kumar et al., developed flexible and microporous chitosan hydrogel/nZnO composite bandages (CZBs) using nZnO and chitosan hydrogel [79]. Chitosan hydrogel incorporating nZnO was further lyophilised to obtain Chitosan/nZnO sponges which showed higher activity towards E. coli as compared to S. aureus which was probably due to the thicker peptidoglycan layer of S. aureus. Eventhough higher antimicrobial activity for chitosan was expected. The in vitro results show lesser activity. This could be due to the neutral pH of the sponges where there are lesser amine groups. The human dermal fibroblast cell attachment and infiltration studies proved the viability of nanocomposite bandages with respect to the concentrations of nZnO present in the chitosan sponges. Chitosan sponges with nZnO of 0.0025% and 0.001% showed a viability of 60–80%. Whereas, on increasing the nZnO concentration to 0.01% or 0.005%, the nanocomposite sponges exhibited toxicity range from 30-60% in vitro. This confirms the toxicity of nZnO against human dermal fibroblast cells with increasing concentrations. The viability towards human dermal fibroblast cells was found to improve by 90% after 72 h. The highly porous nature of the chitosan sponges, facilitated cell penetration and the exchange of nutrients and gases. In vivo evaluations revealed that these nanocomposite bandages showed an improved collagen deposition and re-epithelialisation with rete-peg formation. The in vivo antibacterial study also confirmed that nZnO incorporated composite bandages showed 1000 fold high antibacterial activity when compared with controls. The number of viable bacteria's P. aeruginosa, Staphylococcus intermedius, and Staphylococcus hyicus was found to decrease at second week when compared to chitosan control and kaltostat bandages (Table 1).
Table 1.
Data showing the in vivo antimicrobial activity (Colony Forming Units) of Chitosan/nZnO bandage in comparison to commercially available kaltostat, at two different concentrations.
| Sample |
Pseudomonas aeruginosa |
Staphylococcus intermedius |
Staphylococcus hyicus |
|||
|---|---|---|---|---|---|---|
| Week 1 | Week 2 | Week 1 | Week 2 | Week 1 | Week 2 | |
| Bare wound | 2 × 109 | 2 × 108 | 3 × 1010 | 2 × 108 | 4 × 1010 | 2 × 109 |
| Kaltostat | 4 × 109 | 2 × 108 | 5 × 1010 | 2 × 109 | 6 × 1010 | 8 × 109 |
| Chitosan control | 7 × 109 | 4 × 108 | 9 × 1010 | 4 × 109 | 6 × 1010 | 3 × 109 |
| Chitosan + 0.01% nZnO | 5 × 106 | 2 × 103 | 8 × 106 | 2 × 103 | 4 × 106 | 3 × 104 |
| Chitosan + 0.005% nZnO | 8 × 107 | 2 × 104 | 4 × 107 | 3 × 104 | 8 × 108 | 2 × 105 |
Table 1 reports the in vivo data showing the wound microbial flora after treatment with chitosan/nZnO bandages. P. aeruginosa, S. intermedius, and S. hyicus were found to be reduced by week 2 when compared to the commercial kaltostat dressing. Similarly, a combination of Alginate/Chitosan sponges incorporating nZnO showed higher activity towards E. coli, S. aureus and C. albicans [16]. The sponges with nZnO at concentrations of 5% and 7.5% showed activity towards MRSA in vitro. The resistance of bacterial cell against lower concentration of nZnO was due to the secretion of staphyloxanthin by MRSA [85]. Similar activity of nZnO released from β-chitin scaffold was studied [86].
The antibacterial activity showed a direct relationship with nZnO concentration. The antibacterial activity of nZnO was mainly contributed by the ROS formation by the zinc ions released from the nanocomposite [79], [84]. The ROS together with Zn ions, destructs the negatively charged bacterial cell wall causing cell wall leakage and thereby death of bacteria [86], [87], [88], [89], [90], [91]. Since nZnO addition in chitosan composite exhibits excellent antibacterial activity, this composite could find practical applications in infectious wound healing.
2.5. Chitosan/nTiO2
nTiO2 results in the formation of superoxide radical by electron capture, which produces ROS through Fenton chemistry, occurs after a chronic inflammation or mitochondrial dysfunction [8]. nTiO2 extracts electrons from neighbouring atoms upon exposure to light and moisture giving rise to hydroxyl and superoxide anion radicals [92]. This could lead to damage of lipids and peptidoglycan layers in microbes. Also, nTiO2 could break the chains of DNA [93]. ROS induced toxicity includes DNA damage, p53 mediated DNA damage check signal activation, G2M cell cycle arrest, mutagenesis, generation of reactive nitrogen species etc which leads to oxidative stress resulting in cellular apoptosis [8], [94]. The prepared nTiO2 showed lower toxicity compared to microparticles due to the highly crystalline nature of titania [95]. Titanium is known to cause skin irritation. The dermal application of nTiO2 has been reported to not penetrate beyond the stratum corneum layer in intact skin [96], but in damaged skin the penetration significantly enhances the systemic toxicity thus it would be preferable to provide coatings for the nanoparticles or encapsulate in scaffolds that can hold the burst release of nTiO2.
nTiO2 due to its favorable biological properties have found reasonable role in wound healing applications. nTiO2 is subjected to photocatalysis. The slow release of titanium ions have shown to inhibit microbial proliferation and hence accelerate wound healing [97], [98]. Origanum vulgare engineered nTiO2 showed improved wound closure in an excision wound model. Wound contraction was observed from day 4 onwards with almost 94% by day 12. Histopathological studies of wound tissue examined larger number of macrophages, fibroblasts and collagen deposition on TiO2 treatment [99]. An in vitro antimicrobial activity of nTiO2 containing electrospun graphene exhibited higher activity towards gram negative E . Coli. This was achieved probably by ROS production in microbes [100]. In a study, commercially available Duoderm was compared with composite n-TiO2-chitosan artificial skin (NTCAS) [97]. The matrix had higher water absorption and oxygen permeability. This might be due to higher porosity of the composite matrix than chitosan or gelatin controls. The biodegradation properties were improved upon nTiO2 incorporation. The in vivo antibacterial efficacy determined better healing in dermis injury model. The pro-inflammatory TNF-α and anti-inflammatory cytokine IL-6 was observed to be reduced in NTCAS group when compared to controls and a commercial product, e.g. Duoderm. The bactericidal activity was dependent on the TiO2 concentrations, where 0.1% to 0.40% of matrix showed an increasing trend of antimicrobial activity [98]. nTiO2 (nanorods) were incorporated into chitosan-poly (N-vinylpyrrolidone) scaffold to evaluate the antimicrobial property against E. coli, S. aureus, P. aeruginosa and B. subtilis [100]. For a good tensile strength (34.6 ± 1.0 Mpa) the chitosan PVP ratio was maintained to be 1:1. Whereas upon nanoparticle addition the tensile strength was increased by 1 fold. The average particle size of nTiO2 was 25–35 nm. An initial amount of 10 mg was loaded into the dressing. The titanium ions released from the scaffolds containing nTiO2 were responsible for the activity. The wounds were healed with minimal scarring. The in vivo open excision study reveals that the wound closure rate of the chitosan-PVP-TiO2 treated group for 3, 7, 11 and 16 days are 31.48, 62.33, 91.49 and 99.09%, respectively which was higher than chitosan/nZnO incorporated bandages as reported earlier [79]. A study conducted with chitosan-pectin ternary dressing containing nTiO2 (0.001%) was evaluated for the in vitro antimicrobial activity towards, E. coli, S. aureus, P. aeruginosa, B. subtilis and A. niger [101]. A polyelectrolyte complex formation occurred between the amine group of chitosan and negatively charged carboxyl group of pectin. The dressing possesses high tensile strength with improved swelling ratio. Open excision wounds treated with chitosan-pectin ternary dressing containing nTiO2 showed improved healing when compared to the controls. Histological observation revealed that the skin were intact with epidermis and matured dermis. The wound closure in chitosan control was 95% by day 16, where the wound exhibited lesser scarring than control wounds. This further supports the anti-scarring property of chitosan. Also, the presence of chitosan pectin matrix provided easy removal without hurting the skin [101]. The chitosan based nanocomposites is known to activate inflammatory cells and reduce scar formation. The additional role of pectin in wound dressing is not addressed in the literature. The antimicrobial activity of titanium is due to respiratory chain inhibition, defective signaling, loss of membrane integrity, changes in transport mechanism of ions and assimilation of the heme center in co-factors [102]. Further, the toxicity issues related to nTiO2 was reduced by incorporation into chitosan matrix. The applications of different chitosan based metallic nanoparticle composites discussed in the review are summarized in Table 2.
Table 2.
Chitosan based metallic nanoparticle composites with reference to their mode of action.
| Metallic Nanoparticles | Matrix | Tested microorganisms | In vivo | Properties |
|---|---|---|---|---|
| n Ag | 4-(ethoxycarbonyl) phenyl-1-amino oxobutanoic acid-chitosan (Nanocomposite film) | P.aeruginosa, S. aureus & E. coli | – | Antimicrobial [29] |
| n Ag | Chitosan/sago starch (Nanocomposite film) | – | Open excision wound (Male wistar rats) | Improved Mechanical properties, Antimicrobial, Higher granulation tissue & tensile strength [30] |
| n Ag | Chitosan blended with poly (vinyl alcohol) (Fibrous sheet) | E. coli | – | Antimicrobial, damage to microbial cell membrane permeability and respiration [31] |
| n Ag | PVA/N-carboxymethyl chitosan (Fibrous sheet) | E. coli | – | Antimicrobial [32] |
| n Ag | Chitosan gel | Biofilm of Methicillin-resistant Staphylococcus aureus (MRSA) & P.aeruginosa | – | Bactericidal[25] |
| n Ag | Chitosan-Hyaluronic acid (Sponges) | S. aureus, MRSA, P. aeruginosa and Klebsiella pneumonia | Antimicrobial (silver ions inhibits mitochondrial membrane potential and respiratory chain) [26] | |
| n Au | Chitosan (Film) | S.aureus, P.aeurigensoa | Stabilizing agent, Antimicrobial Cell wall interaction and destabilization(Dependent on positive amine groups in chitosan) [60] | |
| n Au | Colloid/Chitosan (Film) | - | – | Higher keratinocyte proliferation [64] |
| n Cu | Chitosan (Nanocomposite) | Open excision wound model (Male wistar rats) | Higher VEGF & TGF-β1- Increased angiogenesis, fibroblast proliferation & collagen deposition. Lesser TNF-α & IL-10- Decreased inflammatory response [69], [70] | |
| n ZnO | Chitosan (Nanocomposite) | E. coli & S. aureus | – | Antimicrobial [84] |
| n ZnO | Chitosan hydrogel (Composite bandages) | E. coli & S. aureus | Open excision wound model (Sprague Dawley) | Antimicrobial, Improved re-epithelialisation & collagen deposition [79] |
| nZnO | Alginate/Chitosan (Sponges) | E. coli, MRSA, S. aureus & C.albicans | – | Antimicrobial [16] |
| nTiO2–chitosan | Collagen (Artificial skin substitute) | - | – | Bactericidal, Immune enhancing (TNF-α, IL-6) [97] |
| Nanorods of TiO2 | Chitosan, poly(N-vinylpyrrolidone) (PVP) | E. coli,S. aureus, P. aeruginosa, B. subtilis | open excision type wounds (Albino rat) | Antibacterial, N-acetylglucosamine, from chitosan degradation contributes fibroblast Proliferation [5], [98] |
| nTiO2 | Chitosan-pectin (Ternary dressing) | E. coli, Staphylococcus aureus, Pseudomonas aeruginosa, B. subtilis & A. niger | open excision type wounds (Albino rat) | Antibacterial, Completely regenerated wound with epidermis & dermis [100] |
3. Chemical interactions
The structural interaction of chitosan is determined by the amine group. The electron doublet that is free on the nitrogen atom of amine group is responsible for interaction with metallic ions [103]. This in turn is dependent on the degree of deacetylation of chitosan. The more the deacetylation, more is the number of free amine groups for interactions. Crosslinking of chitosan has got influence in the metal interactions. The greater the degree of cross linking, lesser will be the free amine group available for interaction of metal ions with chitosan [104]. There can be two types of interactions. One is chelation and other being electrostatic interaction. If chitosan is in a protonated state, then an electrostatic interaction exists between metallic anion and amine of chitosan. The interaction is dependent on several properties such as pH, concentration of metallic ions, the size, solvents, nature of chitosan (whether protonated or deprotonated) etc. For example, the structural morphology of chitosan hydrogel varied with different concentrations of Cu ions. Stronger interactions lead to multi layers from oriented fibres [105]. This is because Cu ions caused the shrinking of gel solution due to ionic cross linking leading to structural variation. Further modifications in interactions can be modulated by chemical conjugation of chitosan with other groups. This would favor enhanced bonding with metallic ions.
4. Conclusion and future perspectives
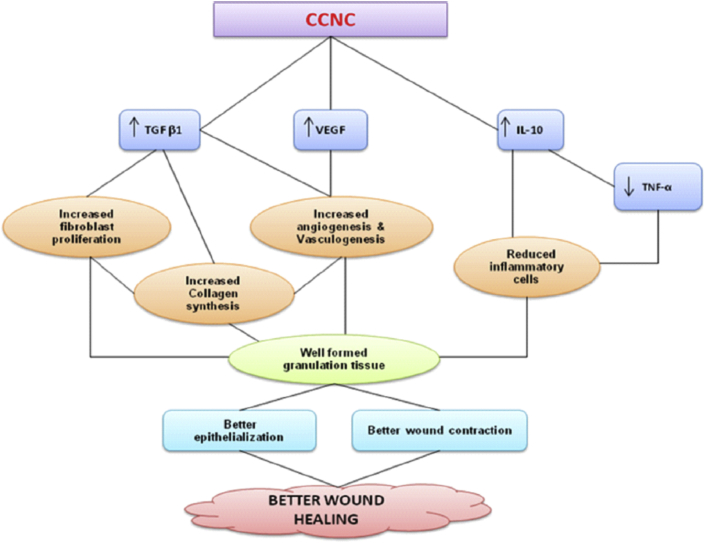
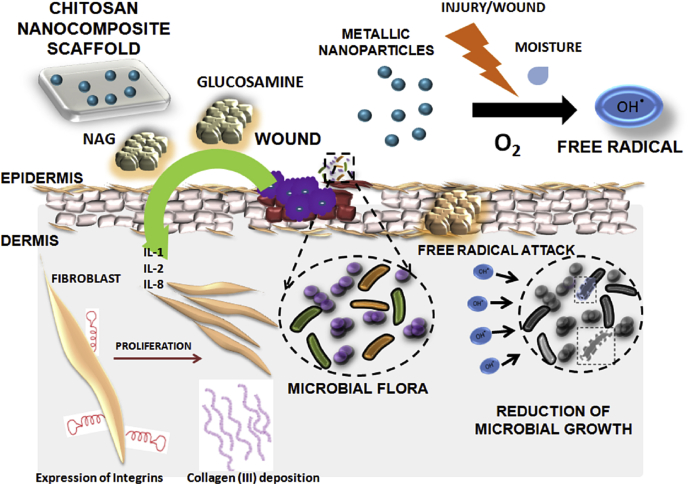
Addition of metallic nanoparticles to chitosan favors properties that drives wound healing processes (Fig. 4). Chitosan based nanocomposite films, hydrogels or sponges have gained significant attraction in the area of wound management. We have discussed about few studies done using chitosan based materials on the antimicrobial activity. This review is focused on different activities of chitosan and its application towards wound healing; metallic ions or nanoparticles activity being the same, leads to the controlled release of metallic ions, improved antimicrobial activity and modified cytotoxicity upon its incorporation into chitosan matrices. In addition, healing is being enhanced by these metallic ions through the regulation of re-epithelialisation and inflammation. The incorporation of metallic nanoparticles into chitosan based matrix favors sustained antimicrobial activity in proportion to matrix degradation without affecting the viability of normal cells. Most of the studies, in general prove the antimicrobial activity by in vitro assays. The mechanism of action of metallic nanoparticles is concentration dependent and is mostly through free radical formation. Although few works report the antimicrobial properties of other metallic nanocomposites, the efficacy of nanocomposites could only be confirmed by evaluation in an infectious model in vitro and in vivo. However, there has been no effort taken to develop an in vivo infectious model either in small or large animals to prove the effectiveness of the chitosan matrix based metallic nanocomposites. The in vivo toxicity of these particles further remains to be addressed. The nanocomposite scaffolds need to be subjected to further preclinical and clinical trials. Moreover definite mechanism of action pertaining to the antimicrobial behavior of these metallic nanoparticles still remains a future perspective. A general trend towards enhancement of antimicrobial effect is seen on combination of chitosan with metallic nanoparticles. Also the loading efficiencies and release of metallic ions from scaffolds can be modified based on different fabrication techniques. For, example if there is a need for bandage as to promote healing in a moist wound, then metallic nanocomposite scaffolds made from lyophilisation technique would be encouraged. This would favor moisture absorption and gradual release of ions from scaffold removing any secondary infections if present. But if a wound requires immediate infection control, then lyophilised scaffols or fibrous films may not be sufficient, instead a topical metallic nanocomposite topical hydrogel would be entertained. Therefore, the goal of fabrication of scaffolds should be entirely dependent on the type of end point applications. The possible reasons for the augmented effect by the addition of metallic nanoparticles such as silver, zinc, copper, gold, titanium etc., is a much unexplored area. Antimicrobial nature is preserved, despite the absence of free amine groups or protonation in the chitosan matrices. These could be dependent on the type of metallic ions, the molecular weight and degree of deacetylation of chitosan, presence of other antibiotics and interaction with them etc. There could be some synergistic activities between metallic ions and chitosan. The possible interactions need to be understood and studied carefully. Genes or proteins that are getting over expressed only in the combination systems need to be evaluated thoroughly.
Fig. 4.
Mechanism of action of chitosan metallic nanocomposite in wound healing. Chitosan matrix degrades and releases N-acetyl-glucosamine and D-glucosamine which helps in secretion of pro-inflammatory cytokines that regulates fibroblast proliferation and collagen deposition. The metallic ions released from the matrix in turns provide antimicrobial effect by formation of free radicals.
Different chitosan based metallic nanocomposites have been appreciated for the antimicrobial mechanism of action. The activity of a nanocomposite material is largely based on the method of fabrication, pH and mechanical strength of the matrix. The release of metallic nanoparticles from matrix is dependent on the material property. However, a proper design of metal chitosan nanocomposite and dosing with thorough pre-clinical evaluations is required prior to clinical applications.
Acknowledgements
Authors are grateful to the Department of Biotechnology (DBT), India, for providing funding (BT/PR6758/NNT/28/620/2012 dated 23-08-2013). Annapoorna Mohandas acknowledges the University Grants Commission (UGC, India) for Five year Fellowship (EU-IV dtd 31/07/2010 SRNo. 2120930570). One of the author S. Deepthi is thankful to the Council of Scientific and Industrial Research for supporting financially under the CSIR-SRF award no: 9/963(0034)2K13-EMR-I.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Velnar T., Bailey T., Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009;37:1528–1542. doi: 10.1177/147323000903700531. [DOI] [PubMed] [Google Scholar]
- 2.Hunt T.K., Hopf H., Hussain Z. Physiology of wound healing. Adv. Skin. Wound. Care. 2000;13:6–11. [PubMed] [Google Scholar]
- 3.Sen C.K., Gordillo G.M., Roy S., Kirsner R., Lambert L., Hunt T.K., Gottrup F., Gurtner G.C., Longaker M.T. Human skin wounds: a major and snowballing threat to public health and the economy. Wound. Repair. Regen. 2009;17:763–771. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sood A., Granick M.S., Tomaselli N.L. Wound dressings and comparative effectiveness data. Adv. Wound. Care. 2014;3:511–529. doi: 10.1089/wound.2012.0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jayakumar R., Prabaharan M., Sudheesh Kumar P.T., Nair S.V., Tamura H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011;29:322–337. doi: 10.1016/j.biotechadv.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Lemire J.A., Harrison J.J., Turner R.J. Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013;11:371–384. doi: 10.1038/nrmicro3028. [DOI] [PubMed] [Google Scholar]
- 7.Schrand A.M., Rahman M.F., Hussain S.M., Schlager J.J., Smith D.A., Syed A.F. Metal-based nanoparticles and their toxicity assessment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010;2:544–568. doi: 10.1002/wnan.103. [DOI] [PubMed] [Google Scholar]
- 8.Nel A., Xia T., Madler L., Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 9.Auffan M., Rose J., Wiesner M.R., Bottero J.Y. Chemical stability of metallic nanoparticles: a parameter controlling their potential cellular toxicity in vitro. Environ. Pollut. 2009;157:1127–1133. doi: 10.1016/j.envpol.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Mc Carty M.F. Glucosamine for wound healing. Med. Hypotheses. 1996;47:273–275. doi: 10.1016/s0306-9877(96)90066-3. [DOI] [PubMed] [Google Scholar]
- 11.Ashkani-Esfahani S., Emami Y., Esmaeilzadeh E., Bagheri F., Namazi M.R. Glucosamine enhances tissue regeneration in the process of wound healing in rats as animal model: a stereological study. J. Cytol. Histol. 2012;3:1000150. [Google Scholar]
- 12.Duan W., Paka L., Pillarisetti S. Distinct effects of glucose and glucosamine on vascular endothelial and smooth muscle cells: evidence for a protective role for glucosamine in atherosclerosis. Cardiovasc. Diabetol. 2005;4:1–16. doi: 10.1186/1475-2840-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madhumathi K., Sudheesh Kumar P.T., Abhilash S., Sreeja V., Tamura H., Manzoor K., Nair S.V., Jayakumar R. Development of novel chitin/nanosilver composite scaffolds for wound dressing applications. J. Mater. Sci. Mater. Med. 2009;21:807–813. doi: 10.1007/s10856-009-3877-z. [DOI] [PubMed] [Google Scholar]
- 14.Paul W., Sharma C.P. Chitin and alginates wound dressings: a short review. Trends. Biomater. Artif. Organs. 2004;18:18–23. [Google Scholar]
- 15.Egger S., Lehmann R.P., Height M.J., Loessner M.J., Schuppler M. Antimicrobial properties of a novel silver-silica nanocomposite material. Appl. Environ. Microbiol. 2009;75:2973–2976. doi: 10.1128/AEM.01658-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Annapoorna M., Sudheesh Kumar P.T., Lakshman L.R., Lakshmanan V.K., Nair S.V., Jayakumar R. Biochemical properties of Hemigraphis alternata incorporated chitosan hydrogel scaffold. Carbohydr. Polym. 2013;92:1561–1565. doi: 10.1016/j.carbpol.2012.10.041. [DOI] [PubMed] [Google Scholar]
- 17.Shalumon K.T., Anulekha K.H., Nair S.V., Nair S.V., Chennazhi K.P., Jayakumar R. Sodium alginate/poly (vinyl alcohol)/nano ZnO composite nanofibers for antibacterial wound dressings. Int. J. Biol. Macromol. 2011;49:247–254. doi: 10.1016/j.ijbiomac.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Annapoorna M., Anisha B.S., Chennazhi K.P., Jayakumar R. Chitosan-hyaluronic acid/VEGF loaded fibrin nanoparticles composite sponges for enhancing angiogenesis in wounds. Colloids Surf. B. 2015;127:105–113. doi: 10.1016/j.colsurfb.2015.01.024. [DOI] [PubMed] [Google Scholar]
- 19.Jayakumar R., Deepthy M., Manzoor K., Nair S.V., Tamura H. Biomedical applications of chitin and chitosan based nanomaterials-A short review. Carbohydr. Polym. 2010;82:227–232. [Google Scholar]
- 20.Anitha A., Sowmya S., Sudheesh Kumar P.T., Deepthi S., Chennazhi K.P., Ehrlich H., Tsurkan M., Jayakumar R. Chitin and chitosan in selected biomedical applications. Prog. Polym. Sci. 2014;39:1644–1667. [Google Scholar]
- 21.Pinto R.J., Fernandes S.C., Freire C.S., Sadocco P., Causio J., Neto C.P., Trindade T. Carbohydr. Res. 2012;348:77–83. doi: 10.1016/j.carres.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Klasen H.J. Historical review of the use of silver in the treatment of burns. I. Early uses. Burns. 2000;26:117–130. doi: 10.1016/s0305-4179(99)00108-4. [DOI] [PubMed] [Google Scholar]
- 23.Fong J., Wood F. Nanocrystalline silver dressings in wound management: a review. Int. J. Nanomed. 2006;1:441–449. doi: 10.2147/nano.2006.1.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kokura S., Handa O., Takagi T., Ishikawa T., Naito Y., Yoshikawa T. Silver nanoparticles as a safe preservative for use in cosmetics. Nanomed. Nanotechnol. 2010;6:570–574. doi: 10.1016/j.nano.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Diaz M.P., Gomez E.A., Aquino M.M., Sanchez R.S., Velasquillo C., Gonzalez C., Rondero A.G., Castanon G.M., Alonso N.Z., Gutierrez F.M. Anti biofilm activity of chitosan gels formulated with silver nanoparticles and their cytotoxic effect on human fibroblasts. Mater. Sci. Eng. C. 2016;60:317–323. doi: 10.1016/j.msec.2015.11.036. [DOI] [PubMed] [Google Scholar]
- 26.Anisha B.S., Biswas R., Chennazhi K.P., Jayakumar R. Chitosan-hyaluronic acid/nano silver composite sponges for drug resistant bacteria infected diabetic wounds. Int. J. Biol. Macromol. 2013;62:310–320. doi: 10.1016/j.ijbiomac.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Rai M., Yadav A., Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009;27:2776–2783. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Kim J.S., Kuk E., Yu K.N., Kim J.H., Park S.J., Lee H.J., Kim S.H., Park Y.K., Park Y.H., Hwang C.Y., Kim Y.K., Lee Y.S., Jeong D.H., Cho M.H. Antimicrobial effects of silver nanoparticles. Nanomedicine. 2007;3:95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Srivastava R., Tiwari D.K., Dutta P.K. 4-(Ethoxycarbonyl) phenyl-1-amino-oxobutanoic acid- chitosan complex as a new matrix for silver nanocomposite film: preparation, characterization and antibacterial activity. Int. J. Biol. Macromol. 2011;49:863–870. doi: 10.1016/j.ijbiomac.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 30.Arockianathan P.M., Sekar S., Kumaran B., Sastry T.P. Preparation, characterization and evaluation of biocomposite films containing chitosan and sago starch impregnated with silver nanoparticles. Int. J. Biol. Macromol. 2012;50:939–946. doi: 10.1016/j.ijbiomac.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 31.Abdelgawad A.M., Hudson S.M., Rojas O.J. Antimicrobial wound dressing nanofiber mats from multicomponent (chitosan/silver-NPs/poly vinyl alcohol) systems. Carbohydr. Polym. 2014;100:166–178. doi: 10.1016/j.carbpol.2012.12.043. [DOI] [PubMed] [Google Scholar]
- 32.Zhao Y., Zhou Y., Wu X., Wang L., Xu L., Wei S. A facile method for electrospinning of Ag nanoparticles/poly (vinyl alcohol)/carboxymethyl chitosan nanofibers. Appl. Surf. Sci. 2012;258:8867–8873. [Google Scholar]
- 33.Pishbin F., Mourino V., Gilchrist J.B., Mc Comb D.W., Kreppel S., Salih V., Ryan M.P., Boccaccini A.R. Single-step electrochemical deposition of antimicrobial orthopaedic coatings based on a bioactive glass/chitosan/nano-silver composite system. Acta Biomater. 2013;9:469–479. doi: 10.1016/j.actbio.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 34.Reidy B., Haase A., Luch A., Dawson K.A., Lynch I. Mechanisms of silver nanoparticle release, transformation and toxicity: a critical review of current knowledge and recommendations for future studies and applications. Materials. 2013;6:2295–2350. doi: 10.3390/ma6062295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnston H.J., Hutchison G., Christensen F.M., Peters S., Hankin S., Stone V. A review of the in vivo and in vitro toxicity of silver and gold particulates: particle attributes and biological mechanisms responsible for the observed toxicity. Crit. Rev. Toxicol. 2010;40:328–346. doi: 10.3109/10408440903453074. [DOI] [PubMed] [Google Scholar]
- 36.George S., Lin S., Ji Z., Thomas C., Li L.J., Mecklenburg M., Meng H., Wang X., Zhang H., Xia T., Hohman J.N., Lin S., Zink J.I., Weiss P.S., Nel A.E. Surface defects on plate-shaped silver nanoparticles contribute to its hazard potential in a fish gill cell line and zebrafish embryos. ACS Nano. 2012;6:3745–3759. doi: 10.1021/nn204671v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asha Rani P.V.G., Mun L.K., Hande M.P., Valiyaveettil S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 2009;2:279–290. doi: 10.1021/nn800596w. [DOI] [PubMed] [Google Scholar]
- 38.Gliga A.R., Skoglund S., Wallinder I.O., Fadeel B., Karlsson H.L. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: the role of cellular uptake, agglomeration and Ag release. Part. Fibre Toxicol. 2014;11:1–11. doi: 10.1186/1743-8977-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samberg M.E., Oldenburg S.J., Monteiro-Riviere N. Evaluation of silver nanoparticle toxicity in skin in vivo and keratinocytes in vitro. Environ. Health Perspect. 2010;118:407–413. doi: 10.1289/ehp.0901398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Travan A., Pelillo C., Donati I., Marsich E., Benincasa M., Scarpa T., Semeraro S., Turco G., Gennaro R., Paoletti S. Non-cytotoxic silver nanoparticle-polysaccharide nanocomposites with antimicrobial activity. Biomacromolecules. 2009;10:1429–1435. doi: 10.1021/bm900039x. [DOI] [PubMed] [Google Scholar]
- 41.Yang X., Gondikas A.P., Marinakos S.M., Auffan M., Liu J., Hsu-Kim H., Meyer J.N. Mechanism of silver nanoparticle toxicity is dependent on dissolved silver and surface coating in Caenorhabditis elegans. Environ. Sci. Technol. 2012;46:1119–1127. doi: 10.1021/es202417t. [DOI] [PubMed] [Google Scholar]
- 42.Morones J.R., Elechiguerra J.L., Camacho A., Holt K., Kouri J.B., Yacaman M.J. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346–2353. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 43.Lok C.N., Ho C.M., Chen R., He Q.Y., Yu W.Y., Sun H., Tam P.K., Chiu J.F., Che C.M. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J. Proteome Res. 2006;5:916–924. doi: 10.1021/pr0504079. [DOI] [PubMed] [Google Scholar]
- 44.Epstein W. The roles and regulation of potassium in bacteria. Prog. Nucleic Acid. Res. Mol. Biol. 2003;75:293–320. doi: 10.1016/s0079-6603(03)75008-9. [DOI] [PubMed] [Google Scholar]
- 45.Wright J.B., Lam K., Burrell R.E. Wound management in an era of increasing bacterial antibiotic resistance: a role for topical silver treatment. Am. J. Infect. Control. 1998;26:572–577. doi: 10.1053/ic.1998.v26.a93527. [DOI] [PubMed] [Google Scholar]
- 46.Prabhu S., Poulose E.K. Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012;29:2–32. [Google Scholar]
- 47.Mamonova I.A., Babushkina I.V., Norkin I.A., Gladkova E.V., Matasov M.D., Puchinyan D.M. Biological activity of metal nanoparticles and their oxides and their effect on bacterial cells. Nanotechnol. Russ. 2015;10:128–134. [Google Scholar]
- 48.Tian J., Wong K.K., Ho C.M., Lok C.N., Yu W.Y., Che C.M., Chiu J.F., Tam P.K. Topical delivery of silver nanoparticles promotes wound healing. Chem. Med. Chem. 2007;2:129–136. doi: 10.1002/cmdc.200600171. [DOI] [PubMed] [Google Scholar]
- 49.Walker M., Bowler P.G., Cochrane C.A. Ostomy, In vitro studies to show sequestration of matrix metalloproteinase by silver-containing wound care products. Wound Manage. 2007;53:18–25. [PubMed] [Google Scholar]
- 50.McCarty S.M., Percival S.L. Proteases and delayed wound healing. Adv. Wound Care New Rochelle. 2013;2:438–447. doi: 10.1089/wound.2012.0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mulley G., Tobias A., Jenkins A., Waterfield N.R. Inactivation of the antibacterial and cytotoxic properties of silver ions by biologically relevant compounds. Plos One. 2014;9 doi: 10.1371/journal.pone.0094409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Srivastava R., Tiwari D.K., Dutta P.K. 4-(Ethoxycarbonyl) phenyl-1-amino-oxobutanoic acid- chitosan complex as a new matrix for silver nanocomposite film: preparation, characterization and antibacterial activity. Int. J. Biol. Macromol. 2011;49:863–870. doi: 10.1016/j.ijbiomac.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 53.Lee S.M., Park K., Kim Y.S., Kim H.J., Moon H., Mueller S., Jeong Y.I. Erratum to: physical, morphological, and wound healing properties of a polyurethane foam-film dressing. J. Biomed. Mater. Res. 2016;20:37. doi: 10.1186/s40824-016-0084-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Laudenslager M.J., Schiffman J.D., Schauer C.L. Carboxymethyl chitosan as a matrix material for platinum, gold, and silver nanoparticles. Biomacromolecules. 2008;9:2682–2685. doi: 10.1021/bm800835e. [DOI] [PubMed] [Google Scholar]
- 55.Chithrani B.D., Ghazani A.A., Chan W.C.W. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6:662–668. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 56.Pan Y., Neuss S., Leifert A., Fischler M., Wen F., Simon U., Schmid G., Brandau W., Jahnen-Dechent W. Size-dependent cytotoxicity of gold nanoparticles. Small. 2007;3:1941–1949. doi: 10.1002/smll.200700378. [DOI] [PubMed] [Google Scholar]
- 57.Albanese A., Chan W.C.W. Effect of gold nanoparticle aggregation on cell uptake and toxicity. ACS Nano. 2011;5:5478–5489. doi: 10.1021/nn2007496. [DOI] [PubMed] [Google Scholar]
- 58.Filon F.L., Crosera M., Adami G., Bovenzi M., Rossi F., Maina G. Human skin penetration of gold nanoparticles through intact and damaged skin. Nanotoxicology. 2011;5:493–501. doi: 10.3109/17435390.2010.551428. [DOI] [PubMed] [Google Scholar]
- 59.Connor E.E., Mwamuka J., Gole A., Murphy C.J., Wyatt M.D. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005;1:325–327. doi: 10.1002/smll.200400093. [DOI] [PubMed] [Google Scholar]
- 60.Futyra A.R., Liskiewicz M.K., Sebastian V., Irusta S., Arruebo M., Stochel G., Kyziol A. Development of non cytotoxic chitosan-gold nanocomposites as efficient antibacterial materials. ACS Appl. Mater. Interfaces. 2015;7:1087–1099. doi: 10.1021/am508094e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pivodova V., Frankova J., Galandakova A., Ulrichova J. In vitro AuNPs' cytotoxicity and their effect on wound healing. Nanomed. Nanotechnol. Biol. Med. 2015;2 doi: 10.5772/61132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leu J.G., Chen S.A., Chen H.M., Wu W.M., Hung C.F., Yao Y.D., Tu C.S., Liang Y.J. The effects of gold nanoparticles in wound healing with antioxidant epigallocatechin gallate and α-lipoic acid. Nanomedicine. 2012;8 doi: 10.1016/j.nano.2011.08.013. 775–767. [DOI] [PubMed] [Google Scholar]
- 63.Chen S.A., Chen H.M., Yao Y.D., Hung C.F., Tu C.S., Liang Y.J. Topical treatment with anti-oxidants Au nanoparticles promote healing of diabetic wound through receptor for advance glycation end-products. Eur. J. Pharm. Sci. 2012;47:875–883. doi: 10.1016/j.ejps.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Y., He H., Gao W.J., Lu S.Y., Liu Y., Gu H.Y. Rapid adhesion and proliferation of keratinocytes on the gold colloid/chitosan film scaffold. Mater. Sci. Eng. C. 2009;29:908–912. [Google Scholar]
- 65.Wang T., Long X., Cheng Y., Liu Z., Yan S. Copper nanoparticles and copper sulphate induced cytotoxicity in hepatocyte primary cultures of epinepheluscoioides. Int. J. Genom. 2015;2015 doi: 10.1371/journal.pone.0149484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li F., Lei C., Shen Q., Li L., Wang M., Guo M., Huang Y., Nie Z., Yao S. Analysis of copper nanoparticles toxicity based on a stress-responsive bacterial biosensor array. Nanoscale. 2013;5:653–662. doi: 10.1039/c2nr32156d. [DOI] [PubMed] [Google Scholar]
- 67.Song L., Vijver M.G., Peijnenburg W.J., Galloway T.S., Tyler C.R. A comparative analysis on the in vivo toxicity of copper nanoparticles in three species of freshwater fish. Chemosphere. 2015;139:181–189. doi: 10.1016/j.chemosphere.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 68.Studer A.M., Limbach L.K., Van Duc L., Krumeich F., Athanassiou E.K., Gerber L.C., Moch H., Stark W.J. Nanoparticle cytotoxicity depends on intracellular solubility: comparison of stabilized copper metal and degradable copper oxide nanoparticles. Toxicol. Lett. 2010;197:169–174. doi: 10.1016/j.toxlet.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 69.Gopal A., Kant V., Gopalakrishnan A., Tandan S.K., Kumar D. Chitosan-based copper nanocomposite accelerates healing in excision wound model in rats. Eur. J. Pharm. Sci. 2014;731:8–19. doi: 10.1016/j.ejphar.2014.02.033. [DOI] [PubMed] [Google Scholar]
- 70.Borkow G., Gabbay J., Dardik R., Eidelman A.I., Lavie Y., Grunfeld Y., Ikher S., Huszar M., Zatcoff R.C., Marikovsky M. Molecular mechanisms of enhanced wound healing by copper oxide-impregnated dressings. Wound Repair Regen. 2010;18:266–275. doi: 10.1111/j.1524-475X.2010.00573.x. [DOI] [PubMed] [Google Scholar]
- 71.Lebedev V.S., Veselovskii A.V., Deinega E.Y., Fedorov Y.I. The role of reactive oxygen species in copper-induced permeability of plasma membranes in Escherichia coli. Biofizika. 2002;2:295–309. [PubMed] [Google Scholar]
- 72.Raffi M., Mehrwan S., Bhatti T.M., Akhter J.I., Hameed A., Yawar W., ul Hasan M.M. Investigations into the antibacterial behavior of copper nanoparticles against Escherichia coli. Ann. Microbiol. 2010;60:75–80. [Google Scholar]
- 73.Tiwari M., Narayanan K., Thakar M.B., Jagani H.V., Rao J.V. Biosynthesis and wound healing activity of copper nanoparticles. IET Nanobiotechnol. 2014;8:230–237. doi: 10.1049/iet-nbt.2013.0052. [DOI] [PubMed] [Google Scholar]
- 74.Rakhmetova A.A., Alekseeva T.P., Bogoslovskaya O.A., Leipunskii I.O., Olkhovskaya I.P., Zhigach A.N., Glushchenko N.N. Wound-healing properties of copper nanoparticles as a function of physicochemical parameters. Nanotechnol. Russ. 2010;5:271–276. [Google Scholar]
- 75.Miao A.J., Zhang X.Y., Luo Z., Chen C.S., Chin W.C., Santschi P.H., Quigg A. Zinc oxide-engineered nanoparticles: dissolution and toxicity to marine phytoplankton. Environ. Toxicol. Chem. 2010;29:2814–2822. doi: 10.1002/etc.340. [DOI] [PubMed] [Google Scholar]
- 76.Xia T., Kovochich M., Liong M., Mädler L., Gilbert B., Shi H., Yeh J.I., Zink J.I., Nel A.E. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano. 2008;2:2121–2134. doi: 10.1021/nn800511k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sharma V., Singh S.K., Anderson D., Tobin D.J., Dhawan A. Zinc oxide nanoparticle induced genotoxicity in primary human epidermal keratinocytes. J. Nanosci. Nanotechnol. 2011;11:3782–3788. doi: 10.1166/jnn.2011.4250. [DOI] [PubMed] [Google Scholar]
- 78.Sharma V., Anderson D., Dhawan A. Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria mediated apoptosis in human liver cells (HepG2) Apoptosis. 2012;17:852–870. doi: 10.1007/s10495-012-0705-6. [DOI] [PubMed] [Google Scholar]
- 79.Sudheesh Kumar P.T., Lakshmanan V.K., Anilkumar T.V., Ramya C., Reshmi P., Unnikrishnan A.G., Nair S.V., Jayakumar R. Flexible and microporous chitosan hydrogel/nano ZnO composite bandages for wound dressing: in vitro and in vivo evaluation. ACS Appl. Mater. Interfaces. 2012;4:2618–2629. doi: 10.1021/am300292v. [DOI] [PubMed] [Google Scholar]
- 80.Sirelkhatim A.S.S., Mahmud A., Seeni N.H.M., Kaus, Ann L.C., Bakhori S.K.M., Hasan H., Mohamad D. Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano Micro Lett. 2015;7:219–242. doi: 10.1007/s40820-015-0040-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Talebian N., Amininezhad S.M., Doudi M. Controllable synthesis of ZnO nanoparticles and their morphology-dependent antibacterial and optical properties. J. Photochem. Photobiol. B. 2013;120:66–73. doi: 10.1016/j.jphotobiol.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 82.Kuo C.L., Wang C.L., Ko H.H., Hwang W.S., Chang K., Li W.L., Huang H.H., Chang Y.H., Wang M.C. Synthesis of zinc oxide nanocrystalline powders for cosmetic applications. Ceram. Int. 2010;36:693–698. [Google Scholar]
- 83.Nair S., Sasidharan A., Divya Rani V.V., Menon D., Nair S., Manzoor K., Raina S. Role of size scale of ZnO nanoparticles and microparticles on toxicity toward bacteria and osteoblast cancer cells. J. Mater. Sci. Mater. Med. 2009;20:S235–S241. doi: 10.1007/s10856-008-3548-5. [DOI] [PubMed] [Google Scholar]
- 84.Wahab R., Mishra A., Yun S.I., Hwang I., Mussarat J., Al-Khedhairy A.A., Kim Y.S., Shin H.S. Fabrication, growth mechanism and antibacterial activity of ZnO micro-spheres prepared via solution process. Biomass. Bioenergy. 2012;39:227–236. [Google Scholar]
- 85.Sakai K., Koyama N., Fukuda T., Mori Y., Onaka H., Tomoda H. Search method for inhibitors of Staphyloxanthin production by methicillin-resistant Staphylococcus aureus. Biol. Pharm. Bull. 2012;35:48–53. doi: 10.1248/bpb.35.48. [DOI] [PubMed] [Google Scholar]
- 86.Sudheesh Kumar P.T., Lakshmanan V.K., Raj M., Biswas R., Hiroshi T., Nair S.V., Jayakumar R. Evaluation of wound healing potential of β-chitin hydrogel/nano zinc oxide composite bandage. Pharm. Res. 2013;30:523–537. doi: 10.1007/s11095-012-0898-y. [DOI] [PubMed] [Google Scholar]
- 87.Rahman P.M., Muraleedaran K., Mujeeb V.M.A. Applications of chitosan powder with in situ synthesized nano ZnO particles as an antimicrobial agent. Int. J. Biol. Macromol. 2015;77:266–272. doi: 10.1016/j.ijbiomac.2015.03.058. [DOI] [PubMed] [Google Scholar]
- 88.Sasidharan A., Chandran P., Menon D., Raman S., Nair S., Koyakutty M. Rapid dissolution of ZnO nanocrystals in acidic cancer microenvironment leading to preferential apoptosis. Nanoscale. 2011;3:3657–3669. doi: 10.1039/c1nr10272a. [DOI] [PubMed] [Google Scholar]
- 89.Stoimenov P.K., Klinger R.L., Marchin G.L., Klabunde K.J. Metal oxide nanoparticles as bactericidal agents. Langmuir. 2002;18:6679–6686. [Google Scholar]
- 90.Liu Y., He L., Mustapha A., Li H., Hu Z., Lin M. Antibacterial activities of zinc oxide nanoparticles against Escherichia coli O157:H7. J. Appl. Microbiol. 2009;107:1193–1201. doi: 10.1111/j.1365-2672.2009.04303.x. [DOI] [PubMed] [Google Scholar]
- 91.Yamamoto O., Komatsu M., Sawai J., Nakagawa Z. Effect of lattice constant of zinc oxide on antibacterial characteristics. J. Mater. Sci. Mater. Med. 2004;15:847–851. doi: 10.1023/B:JMSM.0000036271.35440.36. [DOI] [PubMed] [Google Scholar]
- 92.Nadtochenko V.A., Denisov N., Sarkisov O., Gumy D., Pulgarin C., Kiwi J. Laser kinetic spectroscopy of the interfacial charge transfer between membrane cell walls of E. coli and TiO2. J. Photochem. Photobiol. A Chem. 2006;18:401–407. [Google Scholar]
- 93.Serpone N., Salinaro A., Emeline A. Deleterious effects of sunscreen titanium dioxide nanoparticles on DNA: efforts to limit DNA damage by particle surface modification. Proc. SPIE. 2001;86:4258. [Google Scholar]
- 94.Jeng H.A., Swanson J. Toxicity of metal oxide nanoparticles in mammalian cells. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2006;41:2699–2711. doi: 10.1080/10934520600966177. [DOI] [PubMed] [Google Scholar]
- 95.Karlsson H.L., Gustafsson J., Cronholm P., Möller L. Size-dependent toxicity of metal oxide particles-A comparison between nano- and micrometer size. Toxicol. Lett. 2009;188:112–118. doi: 10.1016/j.toxlet.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 96.Shi H., Magaye R., Castranova V., Zhao J. Titanium dioxide nanoparticles: a review of current toxicological data. Part. Fibre Toxicol. 2013;10:1–15. doi: 10.1186/1743-8977-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Peng C.C., Yang M.H., Chiu W.T., Chiu C.H., Yang C.S., Chen Y.W., Chen K.C., Peng R.Y. Composite nano-titanium oxide-chitosan artificial skin exhibits strong wound-healing effect-An approach with anti-inflammatory and bactericidal kinetics. Macromol. Biosci. 2008;8:316–327. doi: 10.1002/mabi.200700188. [DOI] [PubMed] [Google Scholar]
- 98.Archana D., Dutta J., Dutta P.K. Evaluation of chitosan nano dressing for wound healing: characterization, in vitro and in vivo studies. Int. J. Biol. Macromol. 2013;57:193–203. doi: 10.1016/j.ijbiomac.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 99.Sankar R., Dhivya R., Shivashangari K.S., Ravikumar V. Wound healing activity of Origanum vulgare engineered titanium dioxide nanoparticles in wistar albino rats. J. Mater. Sci. Mater. Med. 2014;25:1701–1708. doi: 10.1007/s10856-014-5193-5. [DOI] [PubMed] [Google Scholar]
- 100.Madhavan A.A., Mohandas A., Licciulli A., Sanosh K.P., Praveen P., Jayakumar R., Nair S.V., Nair A.S., Balakrishnan A. Electrospun continuous nanofibers based on a TiO2–ZnO–graphene composite. RSC Adv. 2013;3:25312–25316. [Google Scholar]
- 101.Archana D., Singh K.B., Dutta J., Dutta P.K. In vivo evaluation of chitosan-PVP-titanium dioxide nanocomposite as wound dressing material. Carbohydr. Polym. 2013;95:530–539. doi: 10.1016/j.carbpol.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 102.Kubacka A., Diez M.S., Rojo D., Bargiela R., Ciordia S., Zapico I., Albar J.P., Barbas C., Santos V.A.P.M.D., Garcia M.F., Ferre M. Understanding the antimicrobial mechanism of TiO₂-based nanocomposite films in a pathogenic bacterium. Sci. Rep. 2014;4:4134. doi: 10.1038/srep04134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Guibal E. Interactions of metal ions with chitosan-based sorbents: a review. Sep. Sci. Technol. 2004;38:43–74. [Google Scholar]
- 104.Hsien T.Y., Rorrer G.L. Heterogeneous cross-linking of chitosan gel beads: kinetics, modeling, andi on cadmium ion adsorption capacity. Ind. Eng. Chem. Res. 1997;36:3631–3638. [Google Scholar]
- 105.Nie J., Wang Z., Hu Q. Chitosan hydrogel structure modulated by metal ions. Sci. Rep. 2016;6:36005. doi: 10.1038/srep36005. [DOI] [PMC free article] [PubMed] [Google Scholar]