Summary
A growing literature has highlighted important differences in transplant-related outcomes between men and women. In the United States there are fewer women than men on the liver transplant waitlist and women are two times less likely to receive a deceased or living-related liver transplant. Sex-based differences exist not only in waitlist but also in post-transplant outcomes, particularly in some specific liver diseases, such as hepatitis C. In the era of individualized medicine, recognition of these differences in the approach to pre and post-liver transplant care may impact short and long-term outcomes.
Keywords: Liver transplantation, Sex, Women’s health, Hepatitis C virus, Waitlist outcome, Quality of life, Liver allocation, MELD score
Introduction
A growing literature has highlighted important differences in transplant-related outcomes between men and women. In the United States, there are fewer women than men on the liver transplant (LT) waitlist (38% vs. 62%) [1], and women are two times less likely to receive a deceased or living-related LT [2–4] (Fig. 1). While the MELD-based allocation system has decreased waitlist mortality by prioritizing the sickest patients awaiting LT [5], sex-based disparities in waitlist outcomes (Fig. 2) have not been overcome. Indications for transplant (Figs. 3 and 4) and their respective disease course post-LT also vary by sex. This review focuses on the current knowledge of transplant-related outcomes in women with the goal of facilitating a more gender-specific management of transplant patients.
Fig. 1. Post-MELD era waitlist and transplant numbers by sex.
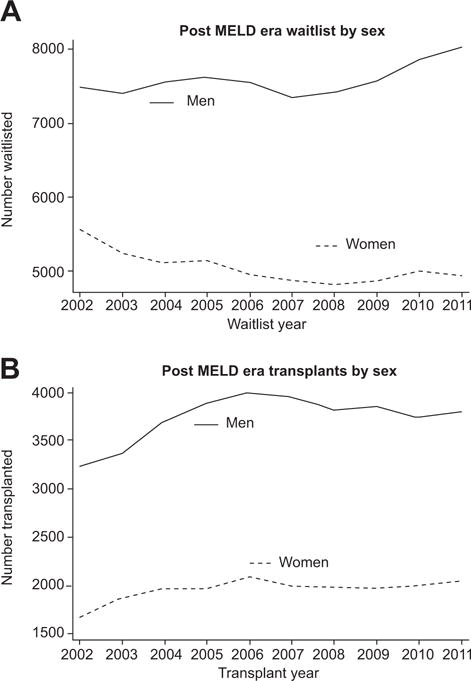
(A) Post-MELD era waitlist by sex. Number of women and men in the U.S. listed for liver transplant based on data from the Scientific Registry of Transplant Recipients (SRTR) in the post-MELD era. (B) Post-MELD era transplant numbers by sex. Number of women and men receiving live and deceased donor liver transplants in the U.S. based on SRTR data in the post-MELD era.
Fig. 2. Post-MELD era waitlist mortality by sex.
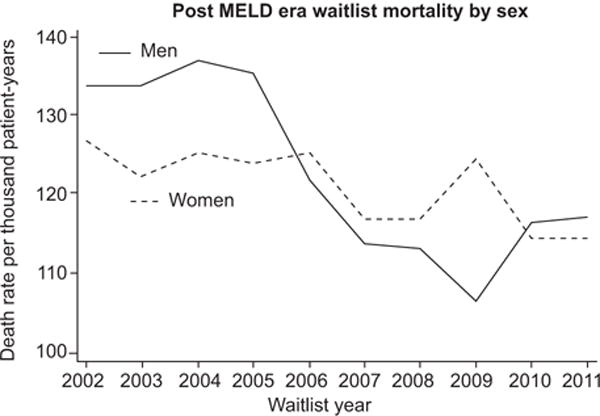
Number of deaths among women and men in the U.S. based on SRTR data in the post-MELD era. Those delisted as too sick for transplant are not included.
Fig. 3. Indications for liver transplantation in 2013 in the US by sex.

(A) Indications for liver transplants in 2013 among U.S. women based on UNOS data. (B) Indications for liver transplants in 2013 among U.S. men based on UNOS data. ALD, alcohol liver disease; PBC, primary biliary cirrhosis; HCV, hepatitis C virus; HCC, hepatocellular carcinoma; PSC, primary sclerosing cholangitis; AIH, autoimmune hepatitis; HBV, hepatitis B virus; NAFLD, non-alcoholic fatty liver disease.
Fig. 4. Indications for liver transplantation in 2012 in Europe by sex.
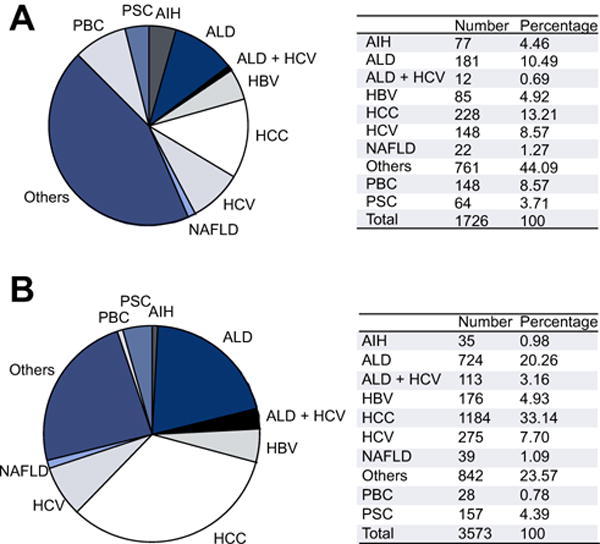
(A) Indications for liver transplants in 2012 among European women based on ELTR data. (B) Indications for liver transplants in 2012 among European men based on ELTR data (data kindly provided by V. Karam).
Age at transplant
Both men and women are most commonly transplanted between 50 and 64 years of age, though a somewhat higher percentage of women are transplanted in the older and younger age ranges. Among U.S. LT recipients in 2012, approximately 15% of women were transplanted at >65 years of age compared to 13% of men, and approximately 7% of women compared to 4% of men were transplanted between ages 18 and 34 years [1].
Transplant rates and waitlist mortality
Women remain disadvantaged in the post-MELD era with worse waitlist outcomes (Table 1) [6–9], with women 30% less likely than men to receive a transplant within 3 years of listing (OR 0.7; 95% CI 0.6–0.8; p <0.001) [9]. A recent US investigation of trends in LT rates found that in the pre-MELD era, women had a 9% lower adjusted transplant rate compared to men, which increased to a 14% difference (p <0.004) after implementation of MELD. The lower transplant rates among women compared to men appeared to predominate at higher MELD scores with 20% lower rates at MELD scores 20–29 and a 12% lower rate at MELD scores of 30–40 (p values <0.05). However, similar transplant rates were noted at MELD scores <15 [9]. Disparities in transplantation rates may translate into higher healthcare expenditures for waitlisted women, given the longer wait times and lower risk of non-liver-related removal from the waitlist [9,10].
Table 1.
Key sex differences in waitlist outcomes in the post-MELD era.
| Waitlist times and transplant rates | • Women spend longer on waitlist [2-4,7-9] • Transplant rates are higher in men [7-9] |
| Waitlist mortality | • Many studies note higher mortality [7,12,13] in women, but not all [9] |
| Size | • Patient height/liver volume contribute to sex disparity in transplant rates and waitlist mortality [6,13] |
| Renal function | • Waitlisted women have lower creatinine for similar degree of renal failure [8,12,14-16] • Creatinine and MELD scores inadequately reflect renal dysfunction in women |
| Sarcopenia | • Sarcopenia is strongly associated with waitlist mortality • Women may have lower risk of sarcopenia [35] |
Recent data investigating the rate of waitlisting relative to those potentially eligible for transplant, have found that women may have greater access to the transplant waitlist, despite lower rates of transplantation than men [9,11]. These data also highlight an ongoing racial/ethnic disparity for Hispanics, who have lower transplant rates compared to other racial/ethnic groups in the post-MELD era, although sex-based disparities in transplant rates by race/ethnicity have not specifically been identified [11].
Women have also been shown to be at higher risk of death or becoming too sick for LT (OR 1.3; 95% CI 1.1–1.5; p = 0.003) [7] (Fig. 2). This higher risk of waitlist mortality in women has been observed in most [12,13], but not all studies [9]. There are several hypotheses to explain sex differences in waitlist outcomes. A major focus has been on renal function measures. It has been proposed that less muscle mass in women results in lower creatinine levels for similar degrees of renal impairment than men, resulting in overall lower MELD scores [14,15]. Though creatinine levels do contribute to sex differences in waitlist outcomes, studies adjusting for estimated glomerular filtration rate (eGFR), and thereby accounting for gender differences in renal function, reveal persistent disparities in liver allocation and waitlist mortality [8,12,14]. In a study using iothalamate as a direct measure of GFR in wait-listed patients, women were more likely than men to have a pre-transplant GFR <60 ml/min (29% vs. 21%) and <30 ml/min (10% vs. 6%, respectively) [16]. A U.S.-based study in the post-MELD era (n = 42,322) noted similar overall waitlist mortality, but among patients with non-dialysis dependent End Stage Renal Disease (ESRD), women were more likely to die on the waitlist than men (26% vs. 20%; p = 0.001) [17]. Moreover, despite lower GFR levels, women were less likely to receive dialysis [18], and lower rates of dual liver-kidney transplant were reported in women compared to men with non-dialysis dependent ESRD (OR 0.5; p <0.001) [19].
Another potential reason for the higher waitlist mortality among women relates to physical stature. Deceased donors are more often male, thus with size matching, more likely to be allocated to men. In addition, preferential allocation of small or split livers to children may also limit the pool of available organs for women, contributing to longer waitlist times and higher risk waitlist dropout [6]. A UNOS-based study found that women had a 20% higher risk of death than men, after adjusting for age, region, blood type, disease etiology, race, and MELD but this difference largely disappeared with the addition of height to the model (HR 1.04; 95% CI 0.98–1.1; p = 0.2) [13]. Another UNOS-based study found that although women were 25% less likely to undergo LT in a given month compared to men, this decreased to 17% with adjustment for renal function, and to 13% with further adjustment for liver volume [6]. These data suggest that like renal function, physical stature contributes to, but does not fully explain sex differences in waitlist outcomes.
Interestingly, while 35% of deceased-donor LT recipients are women, 44% of live donor transplants are in female recipients. The higher percentage of women receiving a live as compared to deceased donor transplant may be reflective of their smaller stature, and therefore better suitability for smaller, live donor grafts [1].
The implications of these findings are several-fold. First, continued efforts to find markers of renal function that are gender independent are essential. Second, nephrologists need to consider gender differences in their recommendations for renal replacement therapy and kidney transplant. Third, women should be encouraged to pursue living donation, and programs may need to consider splitting more organs to offset the longer wait times for women. Finally, continued evaluation of gender disparities as the MELD system evolves is critical for affecting outcomes in waitlisted women and informing future allocation policy.
Post-transplant outcomes
Patient and graft survival
Whether there are sex differences in post-transplant survival, remains controversial. A recent study from Germany (n = 266) found that female sex in the post-MELD era was a strong and independent risk factor for 90-day post LT mortality (OR 3.2; 95% CI 1.3–7.6; p = 0.009). Women had higher MELD scores at transplant than men in this study, and higher post LT mortality was only identified among individuals with pre-transplant MELD scores >20 (33% vs. 14%; p <0.05), and not among those with MELD scores <20 (10 vs. 4%; p >0.05) [20]. Recent data from the U.S. Scientific Registry of Transplant Recipients (SRTR) (n = 19,249) found higher donor risk indices in women than men (1.46 vs. 1.4; p <0.001), with a 24% higher odds among women receiving a low quality graft (OR 1.24; p <0.001). This is possibly driven by use of smaller grafts allocated to women, but with no difference in graft survival between women and men after adjustment for differences in graft quality [21].
Donor gender has also been investigated as a factor influencing graft survival. Older studies report worse post-LT survival in gender mismatched transplants, with particularly high risk of graft loss noted in male recipients of female donors [22,23]. Recent data from Germany noting higher post-transplant mortality in women did not identify donor gender mismatch as a contributing factor [20]. Other studies indicate that donor quality, rather than donor gender or sex mismatch are more important in predicting graft survival. In composite, it remains unclear to what extent sex mismatch or receipt of a female donor, contribute to potential differential post-transplant outcomes.
Rejection
The immune profile of men and women are distinct, with women having been described as more “immunogenic” than men, with greater antibody production and higher rates of autoimmune conditions [24]. Sex differences in immune activity may also translate into different post-transplant immunosuppression needs. A recent multicenter trial investigating immunosuppression withdrawal after LT identified male sex as an independent predictor of successful weaning and subsequent development of immune tolerance (OR 4.7; p = 0.016) [25]. Interestingly, the opposite may hold true in the pediatric population, and maternal grafts in female recipients may also protect against rejection in children with biliary atresia [26,27]. In the hepatitis C population, female gender has been shown to predict early acute rejection (HR 1.8; 95% CI 1.2–2.7; p = 0.004) [28]. Although older studies suggest that female recipients of male livers may be predisposed to chronic rejection, in studies that include all liver conditions no definitive sex differences in risk of acute or chronic rejection have been demonstrated [29–31].
Renal dysfunction
Female sex has been shown to be an independent risk factor for post-transplant renal impairment [32–34] for similar reasons as described above. While earlier studies have been limited by use of indirect measures of renal function, a recent study using iothalamate as a direct measure of GFR also identified female sex as an independent predictor of Pstage 3 CKD at 1 (OR 3.0; 95% CI 1.7–5.1; p <0.001) and 5 years post-transplant (OR 2.5; 95% CI 1.3–4.7; p = 0.004). This finding appeared to be related to worse renal function in women compared to men in the pre-transplant setting [16].
Sarcopenia or physical condition
Recent data indicate that sarcopenia, or severe muscle depletion, is strongly associated with waitlist mortality, but with men at greater risk than women (OR 5.9; 2.4–14.6; p <0.001) [35]. Pre-transplant muscle mass is also emerging as an important predictor of post-transplant outcomes. A study of 338 transplant candidates (223 men, 115 women) found that low muscle mass as determined by CT scan was strongly associated with post-transplant length of ICU stay, total length of hospital stay, and number of days requiring intubation, although this effect was modest in women and quite strong in men. In men, but not women, low muscle mass was also associated with worse post LT survival and hospital disposition [36,37]. The differential effect may be related to greater baseline muscle mass in men for whom a greater degree of cachexia and catabolism is reflected in the presence of sarcopenia, though further investigation is required.
Quality of life
Data on sex differences in overall quality of life (QOL) after LT have been conflicting [38]. Older studies found no differences [39,40] whereas more recent data report lower QOL scores at 1 and 2 years in women compared to men [41], as well as worse psychosocial adjustment in women [42]. In contrast, a small study (n = 52) of patients transplanted for hepatitis C virus (HCV) found that women had significantly better mental health, emotional role functioning, as well as lower pain scores than men, whereas men felt they had better physical functionality after LT [43]. A larger post LT cohort (n = 386) including all liver diseases noted worse measures of physical distress and personal function in women, without apparent sex differences in psychological distress or general health perception [44]. Despite different methodologies for measuring specific QOL indictors, the overall findings indicate that sex differences in post LT QOL are apparent.
Cirrhosis is known to impair sexual function, an important QOL measure. Most men and women experience improved sexual function after LT [45], though one large study of 233 transplanted women found no improvement in multiple measures of sexual satisfaction following transplant [46]. De novo sexual dysfunction following LT has been identified in 33% of men and 26% of women (p value not reported) [47]. Persistent sexual dysfunction following transplant may relate to depression, and psychosocial interventions in the post LT setting may be underutilized [48]. Interestingly, a recent study found that marital happiness was not affected by LT in men, while women in the post LT setting experienced marked improvement in conjugal satisfaction, which correlated with sexual function in women (r = 0.4; p = 0.02), but not in men (r = 0.1; p = 0.3) [45]. Chronic anovulation and symptoms of premature menopause are common problems in women with end-stage liver disease and most pre-menopausal women do have restoration of ovarian function and fertility after transplant [49]. Pregnancy outcomes in the post-LT setting have been well studied, though beyond the scope of the current review [50–53].
Non-hepatic complications following liver transplantation
With increased life expectancy, de novo tumors and cardiovascular disease are now leading causes of non-graft related death in long-term liver transplant survivors [54–56]. This high incidence of non-hepatic events is theoretically explained by the presence of pre-existing risk factors, as well as the introduction of additional risk factors associated with the organ transplant process, such as chronic exposure to immunosuppressive agents, life-style habits (weight gain, tobacco use), and/or the development of de novo metabolic disorders including post-transplant arterial hypertension, diabetes and/or dyslipidemia. With respect to cardiovascular risk factors and disease, no gender association has been found in most studies to date [54,57]. Interestingly, the fact that male gender is a known risk factor for malignancy in the general population but not in post-transplant studies suggests that women have closed the gap, and hence are at higher risk than women in the general population.
Overall, the risk of malignancy is 2 to 4 times higher in transplant recipients than in an age- and sex-matched population [58–62]. With the exception of a few studies where men appear to be more affected than women [62–65], there does not appear to be a clear gender-based difference in the incidence of de novo malignancy. Importantly, since the incidence rates of breast cancer is not increased in organ transplant recipients, there is no evidence to suggest the need for breast cancer screening that would differ from the general population [54].
Post-transplant outcomes in specific liver diseases (Table 2)
Table 2.
Sex differences in overall post LT outcomes.
| Outcome | Sex difference | Comment |
|---|---|---|
| Patient/graft survival | Controversial | German study identified higher 90-day mortality in women [20]. U.S. study did not identify sex difference [21] |
| Rejection risk | No | No overall sex difference in acute or chronic rejection [29-31] though higher risk of early acute rejection in women with HCV [28]. Women less likely to wean from IMS and develop immune tolerance [25] |
| Quality of life | Yes | Considerable variability in the definition of specific QOL indicators though differences in sexual function, emotional and physical well-being are apparent |
| Renal function | Yes | Women at higher risk of CKD post LT [16,32-34] |
| Post LT recovery and sarcopenia | Yes | In men, but less so women, sarcopenia is associated with worse post LT survival and post operative recovery [36-37] |
Chronic hepatitis C
Chronic hepatitis C is an important cause of cirrhosis and hepatocellular carcinoma (HCC) globally. In the United States, Europe and Japan, HCV is the most common indication for LT. In recent years, the proportion of patients with HCC as the primary indication for LT has increased, likely reflecting prioritization of small HCC for LT as well as the increased prevalence of cirrhosis among HCV-infected persons [66,67]. Cirrhosis and its complications are less frequent in women than men [67] and this difference is likely due to the higher rates of spontaneous clearance among women [68], the protective effects of estrogens on fibrosis in premenopausal women [69], as well as lower frequency of cofactors associated with fibrosis in women, such as heavy alcohol use.
Recurrent disease is essentially universal among viremic patients after LT and the estimated median time to recurrent cirrhosis is 8–10 years [70] with rapid progressors advancing to cirrhosis within 3–5 years [71]. Approximately 10% develop severe early recurrence with cholestatic features within the first year post LT, which can rapidly progress to graft loss in the absence of antiviral therapy. Higher rates of severe HCV disease and reduced graft survival are associated with several recipient and donor factors, including African-American race, HIV co-infection, older donor age and IL28B polymorphisms. Women have more severe recurrent disease [72–74] with a 23% higher risk of advanced fibrosis than men after a median of 3 years after liver transplant [72]. Viral eradication prior to or after LT can prevent complications of HCV recurrence. Few studies have focused on sex differences in response to therapy, but the lower response rates to interferon-based therapy in women [75] are likely due to lower adherence to therapy and a higher rate of therapy discontinuation related to ribavirin-induced anemia. Sex differences in treatment response with direct acting antiviral therapies have not been studied, but the greater risk of ribavirin-associated toxicity may continue to limit therapy tolerability and efficacy in women (Table 3).
Table 3.
Sex differences in liver transplant by disease.
Non alcoholic fatty liver disease (NAFLD)
Non-alcoholic steatohepatitis (NASH) is the third most common indication for LT in the United States and is predicted to surpass HCV as the most common indication for LT in 10 years’ time [76]. Most population-based studies note a higher prevalence of NAFLD in men, though clinically diagnosed and biopsy proven NASH appears to be higher in women [77–79]. Hormonal factors may contribute to this difference, as a recent cross sectional study noted increased liver fibrosis in men with NASH compared to pre-menopausal women, but similar fibrosis scores as postmenopausal women [80]. Sex differences in NAFLD prevalence may also equalize after women reach menopause [81,82]. Patients receiving LT for NASH are equally distributed by sex [78], with no apparent sex differences in post LT patient or graft survival [83,84], or risk of recurrent NASH [85,86].
Alcoholic liver disease (ALD)
There are clear sex differences in the hepatotoxic effects of alcohol, with higher risk of hepatic damage at lower doses of alcohol exposure in women (>10 g daily) compared to men (>20 g daily) [87]. This is in part related to lower levels of gastric alcohol dehydrogenase in women, which is involved in first pass alcohol metabolism [88]. Once diagnosed with ALD, women have more rapid acceleration of liver fibrosis than men, which may persist even after alcohol cessation [89]. Consistent with this finding, men on the waiting list for ALD tend to have longer median durations of alcohol abuse than women [90,91]. However, the overall prevalence of ALD remains higher in men, and a greater proportion of men undergo LT for ALD [90,92,93]. UNOS-based data from 2002 to 2012 indicate that men account for 75% of patients transplanted for a primary diagnosis of ALD [78].
Most studies have not identified sex differences in post LT graft or patient survival for ALD [91,93], though one older study noted a higher percentage of women than men surviving at 5 years (78% vs. 58%, respectively, no p-value provided) [94]. A French study identified de novo malignancies as an independent risk factor for lower post LT survival in ALD and though sex was not predictive on multivariate analysis, male sex was strongly associated with the risk of de novo malignancy [93].
Data on sex differences in recidivism rates are conflicting. A Scandinavian study (n = 103) found no association between sex and recidivism [95], while a Canadian study (n = 80) noted higher recidivism in women, accounting for 5/8 patients that resumed problem drinking. Interestingly, 4/5 of these women had a pre-transplant diagnosis of depression, which may contribute to the higher observed recidivism in women [96]. A U.S.-based study has since reported depression to be a strong predictor of post LT recidivism, though sex was not specifically investigated in this model [90].
Autoimmune hepatitis (AIH)
Like most autoimmune conditions, AIH is more prevalent in women than men, with a sex ratio of 3.6:1 [97]. Women comprise the majority of patients that receive LT for AIH, though a recent study (n = 1318) identified male sex as an independent predictor of mortality or need for LT (HR 1.5 compared to women, 95% CI 1.2–2.2; no p-value reported). In this study, cirrhotic women with AIH also had a lower HCC incidence rate per 1000 person-years compared to men (0.6 vs. 5.5) [98]. A smaller study (n = 138) noted a higher unadjusted risk of cirrhosis at the time of AIH diagnosis in men than women (OR 2.8; 95% CI 1.2–6.2; p = 0.01) though risk of mortality or need for LT was not different [99]. Interestingly, there are no apparent recipient sex differences in risk of de novo autoimmune hepatitis (HR 0.8; 95% CI 0.3–2.3; p = 0.7), although the risk of de novo AIH appears to be higher in recipients of female donors regardless of recipient sex (HR 3.0; 95% CI 1.1–8.3; p = 0.03) [100]. To date, sex differences in risk of post LT survival, recurrent AIH, or risk of rejection have not been identified [101–103].
Primary biliary cirrhosis (PBC)
Like AIH, PBC is more common in women accounting for ~90% of PBC cases [104]. Women with PBC tend to be younger at the time of diagnosis, with worse fatigue and pruritus than men, and higher risk for concomitant autoimmune conditions. Interestingly, recent data note similar fatigue and cognitive symptoms in women after LT compared to sex matched non-transplant controls (p values >0.05), whereas transplanted men compared to non-transplant controls had worse fatigue (p <0.05) and cognitive symptoms (p <0.005) [105]. Pre-LT serologic profiles are similar in men and women, though men have more progressive disease and overall worse outcomes [101,106,107]. A recent large study (n = 2,353) found that men were less responsive to ursodeoxycholic acid, based on ALT, total bilirubin and alkaline phosphatase levels (OR 0.9; 95% CI 0.83–0.97; p = 0.007) [108]. Similar to most chronic liver diseases, the incidence of HCC in PBC patients with cirrhosis is lower in women than men, with a recent study noting a 10-year HCC incidence of 2.0% in women vs. 6.5% in men (p <0.001) [109–112]. Though HCC in women is predominantly seen in cirrhosis, men with PBC have been diagnosed with HCC at all stages of fibrosis [112]. Data are limited on post LT sex differences in patient or graft survival, though recurrent PBC or risk of rejection appears to be similar between sexes [101,113].
Primary sclerosing cholangitis (PSC)
Unlike PBC and AIH, PSC is less common in women, with more than 60% of cases diagnosed in men, and no major differences in clinical presentation [114]. While most studies have not identified a difference in overall survival [115,116] a regional study from Sweden (142 men and 57 women) identified female sex as a strong and independent risk factor for death or need for LT (RR 2.0; 95% CI 1.1–3.7; p = 0.02) [117]. The reasons for the discrepancy in survival outcomes between this and other studies are not clear, but may be related to delayed diagnosis in women as this condition is more typically associated with men. Sex differences in genetic factors contributing to PSC-related outcomes have been identified. The rs738409 variant (I148M) of the PNPLA3 gene was recently shown to predict survival in patients with PSC with concurrent dominant strictures, although this effect was restricted to men (mean survival 11.9 years in I148M carriers vs. 18.8 years in wildtype; p <0.001), and did not predict survival in women (p = 0.65) [118].
Data reporting sex differences in post LT outcomes in PSC are limited. A single center study (n = 83) noted a smaller proportion of women than men with post LT biliary complications, revisions of the transplanted liver, and/or death (32% vs. 65%, p = 0.02) [119]. To date, no studies have demonstrated differences in risk for recurrent PSC on adjusted analyses [120–122] or differences in risk of rejection [101]. A study of 61 women and 119 men following LT for PSC identified a higher incidence of de novo colorectal cancer in women (SIR 17.6; 95% CI 3.6–51.4) [123], though other studies have not identified sex differences in risk of post LT malignancy for patients with PSC [124,125].
Hepatocellular carcinoma
The risk ratio of HCC in cirrhotic women vs. men ranges from 1:2 to 1:4 [126]. Sex differences in HCC risk also extend to chronic non-cirrhotic HBV infection for which the AASLD recommends initiation of HCC screening in non-cirrhotic Asian women at age 50 years compared to 40 years in Asian men [127]. A large Italian study (482 women and 1352 men) investigating all etiologies of liver disease found that women were older at HCC diagnosis, had higher alpha-fetoprotein levels, and were more likely to have smaller, unifocal and well-differentiated HCCs, with lower likelihood of presenting with metastases. Though overall survival was better in women than men, there were no differences in likelihood of undergoing curative treatment such as transplant or resection. In this study survival differences disappeared when subgroup analyses were performed among individuals diagnosed with HCC by surveillance imaging opposed to symptomatic presentation, suggesting that sex differences in presentation and outcomes may have been related to differential receipt of HCC surveillance, rather than sex differences in tumor biology [128]. In a recent study from the U.S. Surveillance, Epidemiology, and End Results database, women with HCC were found to have a significantly greater median overall survival compared with men, independent of age, race, disease stage, or treatment (11 vs. 10 months; HR 0.93; 95% CI, 0.91–0.96, p <0.001). Interestingly, greater survival in women was noted among those who received surgical resection (HR 0.87; 95% CI 0.78–0.96; p = 0.01) but not among those receiving liver-directed therapy or LT [129]. In another U.S.-based study women were more likely to receive curative resection than men. Though adjusted analyses revealed lower risk of decompensation in women than men (OR 0.79; p <0.001), these authors found that among those with compensated cirrhosis and HCC, women were still more likely to be offered curative therapy. No differences in rates of LT were noted for patients with HCC, similar to previously reported UNOS data [7,130]. In the latter study, tumor characteristics were not available, therefore higher rates of resection in women may be related to lower tumor burden, as demonstrated in the Italian study [128]. Recipient sex does not appear to predict risk of recurrent HCC post LT [131–133].
Acute liver failure
A high female predominance is observed in acute liver failure of most etiologies, not only those associated with autoimmunity, though the reason for this association is not clear. In particular, acute liver failure due to Wilson disease occurs predominantly in young females with a female to male ratio of 4:1. Furthermore, approximately 10% of patients with hepatotoxicity due to medical/recreational drugs or herbal products may progress to fulminant hepatic failure, potentially requiring LT. While sex does not seem to increase the overall risk of drug-induced liver injury, the severity may differ by sex, with a predominance of severe cases observed in women [134–136].
Conclusions
LT remains the optimal treatment for patients with end-stage liver disease, though sex differences in access to transplant persist. In this review, we highlight sex-based disparities in transplant outcomes, as well as sex differences in transplant indications, some of which are quite marked and others more subtle. Despite clear differences in waitlist outcomes, the reasons for this particular disparity remain only partially understood. Further data are clearly needed to narrow the gender gap in transplant-related events, and to facilitate interventions that may optimize the management of women in both the pre- and post-transplant period.
Key Points.
Transplant indications, waitlist outcomes, and post-transplant course vary by sex
Waitlist outcomes (liver allocation and waitlist mortality) remain worse in women in the post model for end-stage liver disease (MELD) era, particularly at high MELD scores. Inadequate renal function measures that underestimate renal impairment in women, as well as differences in physical stature contribute to, but do not fully explain sex differences in waitlist outcomes
Despite different methodologies for measuring specific quality of life (QOL) indicators, the overall findings indicate that women have lower QOL scores post-liver transplantation (LT) compared to men
While overall post-transplant graft and patient survival do not seem to differ by sex, in some specific liver diseases, particularly in hepatitis C, sex differences are evident (more severe recurrent disease and lower response rates to interferon-ribavirin based therapies in women)
Data on sex differences in alcohol recidivism rates are conflicting
Recipient sex does not appear to predict risk of recurrent hepatocellular carcinoma (HCC) post-LT
Acknowledgments
Financial support
Ciberehd is partially funded by the Instituto de Salud Carlos III.
Abbreviations
- MELD
model for end-stage liver disease
- QOL
quality of life
- LT
liver transplantation
- IFN
interferon
- RBV
ribavirin
- HCC
hepatocellular carcinoma
- eGFR
estimated glomerular filtration rate
- SRTR
Scientific Registry of Transplant Recipients
- HCV
Hepatitis C virus
- NAFLD
non-alcoholic Fatty Liver Disease
- NASH
non-alcoholic steatohepatitis
- ALD
alcoholic liver disease
- AIH
autoimmune hepatitis
- PBC
primary biliary cirrhosis
- PSC
primary sclerosing cholangitis
Footnotes
Conflict of interest
The authors declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
Author contribution
All authors contributed to the literature review, analysis and interpretation of the data. Monika Sakar compiled the first draft of the manuscript which was then critically reviewed by the remaining authors.
References
- 1.In: Scientific registry of transplant recipients.
- 2.Bryce CL, Chang CC, Angus DC, Arnold RM, Farrell M, Roberts MS. The effect of race, sex, and insurance status on time-to-listing decisions for liver transplantation. J Transplant. 2010;2010:467976. doi: 10.1155/2010/467976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thuluvath PJ, Guidinger MK, Fung JJ, Johnson LB, Rayhill SC, Pelletier SJ. Liver transplantation in the United States, 1999–2008. Am J Transplant. 2010;10:1003–1019. doi: 10.1111/j.1600-6143.2010.03037.x. [DOI] [PubMed] [Google Scholar]
- 4.Hermann HC, Klapp BF, Danzer G, Papachristou C. Gender-specific differences associated with living donor liver transplantation: a review study. Liver Transpl. 2010;16:375–386. doi: 10.1002/lt.22002. [DOI] [PubMed] [Google Scholar]
- 5.Freeman RB, Jr, Wiesner RH, Harper A, McDiarmid SV, Lake J, Edwards E, et al. The new liver allocation system: moving toward evidence-based transplantation policy. Liver Transpl. 2002;8:851–858. doi: 10.1053/jlts.2002.35927. [DOI] [PubMed] [Google Scholar]
- 6.Mindikoglu AL, Emre SH, Magder LS. Impact of estimated liver volume and liver weight on gender disparity in liver transplantation. Liver Transpl. 2013;19:89–95. doi: 10.1002/lt.23553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moylan CA, Brady CW, Johnson JL, Smith AD, Tuttle-Newhall JE, Muir AJ. Disparities in liver transplantation before and after introduction of the MELD score. JAMA. 2008;300:2371–2378. doi: 10.1001/jama.2008.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma P, Schaubel DE, Messersmith EE, Guidinger MK, Merion RM. Factors that affect deceased donor liver transplantation rates in the United States in addition to the model for end-stage liver disease score. Liver Transpl. 2012;18:1456–1463. doi: 10.1002/lt.23548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathur AK, Schaubel DE, Gong Q, Guidinger MK, Merion RM. Sex-based disparities in liver transplant rates in the United States. Am J Transplant. 2011;11:1435–1443. doi: 10.1111/j.1600-6143.2011.03498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Axelrod DA, Dzebisashvili N, Lentine K, Segev DL, Dickson R, Tuttle-Newhall E, et al. Assessing variation in the costs of care among patients awaiting liver transplantation. Am J Transplant. 2014;14:70–78. doi: 10.1111/ajt.12494. [DOI] [PubMed] [Google Scholar]
- 11.Mathur AK, Ashby VB, Fuller DS, Zhang M, Merion RM, Leichtman A, et al. Variation in access to the liver transplant waiting list in the United States. Transplantation. 2014;98:94–99. doi: 10.1097/01.TP.0000443223.89831.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Myers RP, Shaheen AA, Aspinall AI, Quinn RR, Burak KW. Gender, renal function, and outcomes on the liver transplant waiting list: assessment of revised MELD including estimated glomerular filtration rate. J Hepatol. 2011;54:462–470. doi: 10.1016/j.jhep.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Lai JC, Terrault NA, Vittinghoff E, Biggins SW. Height contributes to the gender difference in wait-list mortality under the MELD-based liver allocation system. Am J Transplant. 2010;10:2658–2664. doi: 10.1111/j.1600-6143.2010.03326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huo SC, Huo TI, Lin HC, Chi CW, Lee PC, Tseng FW, et al. Is the corrected-creatinine model for end-stage liver disease a feasible strategy to adjust gender difference in organ allocation for liver transplantation? Transplantation. 2007;84:1406–1412. doi: 10.1097/01.tp.0000282867.92367.d0. [DOI] [PubMed] [Google Scholar]
- 15.Cholongitas E, Marelli L, Kerry A, Senzolo M, Goodier DW, Nair D, et al. Different methods of creatinine measurement significantly affect MELD scores. Liver Transpl. 2007;13:523–529. doi: 10.1002/lt.20994. [DOI] [PubMed] [Google Scholar]
- 16.Fussner LA, Charlton MR, Heimbach JK, Fan C, Dierkhising R, Coss E, et al. The impact of gender and NASH on chronic kidney disease before and after liver transplantation. Liver Int. 2014;34:1259–1266. doi: 10.1111/liv.12381. http://dx.doi.org/10.1111/liv.12381. [DOI] [PubMed] [Google Scholar]
- 17.Mindikoglu AL, Regev A, Seliger SL, Magder LS. Gender disparity in liver transplant waiting-list mortality: the importance of kidney function. Liver Transpl. 2010;16:1147–1157. doi: 10.1002/lt.22121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mindikoglu AL, Raufman JP, Seliger SL, Howell CD, Magder LS. Simultaneous liver-kidney versus liver transplantation alone in patients with end-stage liver disease and kidney dysfunction not on dialysis. Transplant Proc. 2011;43:2669–2677. doi: 10.1016/j.transproceed.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mindikoglu AL, Madger LS, Seliger SL, Raufman JP, Howell CD. Outcomes and predictors of simultaneous liver-kidney transplantation among patients with kidney dysfunction who are not on dialysis on the liver transplant waiting list. Gastroenterology. 2010;138:S-774. [Google Scholar]
- 20.Bruns H, Lozanovski VJ, Schultze D, Hillebrand N, Hinz U, Buchler MW, et al. Prediction of postoperative mortality in liver transplantation in the era of MELD-based liver allocation: a multivariate analysis. PLoS One. 2014;9:e98782. doi: 10.1371/journal.pone.0098782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathur AK, Schaubel DE, Zhang H, Guidinger MK, Merion RM. Disparities in liver transplantation: the association between donor quality and recipient race/ethnicity and sex. Transplantation. 2014;97:862–869. doi: 10.1097/01.tp.0000438634.44461.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rustgi VK, Marino G, Halpern MT, Johnson LB, Umana WO, Tolleris C. Role of gender and race mismatch and graft failure in patients undergoing liver transplantation. Liver Transpl. 2002;8:514–518. doi: 10.1053/jlts.2002.33457. [DOI] [PubMed] [Google Scholar]
- 23.Velidedeoglu E, Mange KC, Frank A, Abt P, Desai NM, Markmann JW, et al. Factors differentially correlated with the outcome of liver transplantation in hcv+ and HCV recipients. Transplantation. 2004;77:1834–1842. doi: 10.1097/01.tp.0000130468.36131.0d. [DOI] [PubMed] [Google Scholar]
- 24.Fairweather D, Frisancho-Kiss S, Rose NR. Sex differences in autoimmune disease from a pathological perspective. Am J Pathol. 2008;173:600–609. doi: 10.2353/ajpath.2008.071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benitez C, Londono MC, Miquel R, Manzia TM, Abraldes JG, Lozano JJ, et al. Prospective multicenter clinical trial of immunosuppressive drug withdrawal in stable adult liver transplant recipients. Hepatology. 2013;58:1824–1835. doi: 10.1002/hep.26426. [DOI] [PubMed] [Google Scholar]
- 26.Sanada Y, Kawano Y, Miki A, Aida J, Nakamura K, Shimomura N, et al. Maternal grafts protect daughter recipients from acute cellular rejection after pediatric living donor liver transplantation for biliary atresia. Transpl Int. 2014;27:383–390. doi: 10.1111/tri.12273. [DOI] [PubMed] [Google Scholar]
- 27.Waki K, Sugawara Y, Mizuta K, Fujita H, Kadowaki T, Kokudo N. Living-donor liver transplantation at the University of Tokyo, 1996-2011 the impact of HLA matching and a positive crossmatch on long-term survival and tolerance. Clinical Transplants. 2011:223–235. [PubMed] [Google Scholar]
- 28.McTaggart RA, Terrault NA, Vardanian AJ, Bostrom A, Feng S. Hepatitis C etiology of liver disease is strongly associated with early acute rejection following liver transplantation. Liver Transpl. 2004;10:975–985. doi: 10.1002/lt.20213. [DOI] [PubMed] [Google Scholar]
- 29.Thurairajah PH, Carbone M, Bridgestock H, Thomas P, Hebbar S, Gunson BK, et al. Late acute liver allograft rejection; a study of its natural history and graft survival in the current era. Transplantation. 2013;95:955–959. doi: 10.1097/TP.0b013e3182845f6c. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez-Peralvarez M, Germani G, Papastergiou V, Tsochatzis E, Thalassinos E, Luong TV, et al. Early tacrolimus exposure after liver transplantation: relationship with moderate/severe acute rejection and long-term outcome. J Hepatol. 2013;58:262–270. doi: 10.1016/j.jhep.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 31.Bathgate AJ, Hynd P, Sommerville D, Hayes PC. The prediction of acute cellular rejection in orthotopic liver transplantation. Liver Transpl Surg. 1999;5:475–479. doi: 10.1002/lt.500050608. [DOI] [PubMed] [Google Scholar]
- 32.Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931–940. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 33.Burra P, Senzolo M, Masier A, Prestele H, Jones R, Samuel D, et al. Factors influencing renal function after liver transplantation. Results from the MOST, an international observational study. Dig Liver Dis. 2009;41:350–356. doi: 10.1016/j.dld.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 34.Leithead JA, Ferguson JW, Hayes PC. Modifiable patient factors are associated with the late decline in renal function following liver transplantation. Clin Transplant. 2012;26:E316–E323. doi: 10.1111/j.1399-0012.2012.01650.x. [DOI] [PubMed] [Google Scholar]
- 35.Tandon P, Ney M, Irwin I, Ma MM, Gramlich L, Bain VG, et al. Severe muscle depletion in patients on the liver transplant wait list: its prevalence and independent prognostic value. Liver Transpl. 2012;18:1209–1216. doi: 10.1002/lt.23495. [DOI] [PubMed] [Google Scholar]
- 36.DiMartini A, Cruz RJ, Jr, Dew MA, Myaskovsky L, Goodpaster B, Fox K, et al. Muscle mass predicts outcomes following liver transplantation. Liver Transpl. 2013;19:1172–1180. doi: 10.1002/lt.23724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montano-Loza AJ, Meza-Junco J, Baracos VE, Prado CM, Ma M, Meeberg G, et al. Severe muscle depletion predicts postoperative length of stay but is not associated with survival after liver transplantation. Liver Transpl. 2014;20:640–648. doi: 10.1002/lt.23863. [DOI] [PubMed] [Google Scholar]
- 38.Tome S, Wells JT, Said A, Lucey MR. Quality of life after liver transplantation. A systematic review. J Hepatol. 2008;48:567–577. doi: 10.1016/j.jhep.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 39.Bravata DM, Olkin I, Barnato AE, Keeffe EB, Owens DK. Health-related quality of life after liver transplantation: a meta-analysis. Liver Transpl Surg. 1999;5:318–331. doi: 10.1002/lt.500050404. [DOI] [PubMed] [Google Scholar]
- 40.Moore KA, Mc LJR, Burrows GD. Quality of life and cognitive function of liver transplant patients: a prospective study. Liver Transpl. 2000;6:633–642. doi: 10.1053/jlts.2000.9743. [DOI] [PubMed] [Google Scholar]
- 41.Cowling T, Jennings LW, Goldstein RM, Sanchez EQ, Chinnakotla S, Klintmalm GB, et al. Liver transplantation and health-related quality of life: scoring differences between men and women. Liver Transpl. 2004;10:88–96. doi: 10.1002/lt.20013. [DOI] [PubMed] [Google Scholar]
- 42.Blanch J, Sureda B, Flavia M, Marcos V, de Pablo J, De Lazzari E, et al. Psychosocial adjustment to orthotopic liver transplantation in 266 recipients. Liver Transpl. 2004;10:228–234. doi: 10.1002/lt.20076. [DOI] [PubMed] [Google Scholar]
- 43.Bianco T, Cillo U, Amodio P, Zanus G, Salari A, Neri D, et al. Gender differences in the quality of life of patients with liver cirrhosis related to hepatitis C after liver transplantation. Blood Purif. 2013;36:231–236. doi: 10.1159/000356362. [DOI] [PubMed] [Google Scholar]
- 44.Ruppert K, Kuo S, DiMartini A, Balan V. In a 12-year study, sustainability of quality of life benefits after liver transplantation varies with pretransplantation diagnosis. Gastroenterology. 2010;139:1619–1629. 1629, e1611–e1614. doi: 10.1053/j.gastro.2010.06.043. [DOI] [PubMed] [Google Scholar]
- 45.Klein J, Tran SN, Mentha-Dugerdil A, Giostra E, Majno P, Morard I, et al. Assessment of sexual function and conjugal satisfaction prior to and after liver transplantation. Ann Transplant. 2013;18:136–145. doi: 10.12659/AOT.883860. [DOI] [PubMed] [Google Scholar]
- 46.Gomez-Lobo V, Burgansky A, Kim-Schluger L, Berkowitz R. Gynecologic symptoms and sexual function before and after liver transplantation. J Reprod Med. 2006;51:457–462. [PubMed] [Google Scholar]
- 47.Ho JK, Ko HH, Schaeffer DF, Erb SR, Wong C, Buczkowski AK, et al. Sexual health after orthotopic liver transplantation. Liver Transpl. 2006;12:1478–1484. doi: 10.1002/lt.20831. [DOI] [PubMed] [Google Scholar]
- 48.Burra P, Germani G, Masier A, De Martin E, Gambato M, Salonia A, et al. Sexual dysfunction in chronic liver disease: is liver transplantation an effective cure? Transplantation. 2010;89:1425–1429. doi: 10.1097/TP.0b013e3181e1f1f6. [DOI] [PubMed] [Google Scholar]
- 49.Burra P. Sexual dysfunction after liver transplantation. Liver Transpl. 2009;15:S50–S56. doi: 10.1002/lt.21899. [DOI] [PubMed] [Google Scholar]
- 50.Brosens I, Pijnenborg R, Benagiano G. Risk of obstetrical complications in organ transplant recipient pregnancies. Transplantation. 2013;96:227–233. doi: 10.1097/TP.0b013e318289216e. [DOI] [PubMed] [Google Scholar]
- 51.Rupley DM, Janda AM, Kapeles SR, Wilson TM, Berman D, Mathur AK. Preconception counseling, fertility, and pregnancy complications after abdominal organ transplantation: a survey and cohort study of 532 recipients. Clin Transplant. 2014;28:937–945. doi: 10.1111/ctr.12393. http://dx.doi.org/10.1111/ctr.12393. [DOI] [PubMed] [Google Scholar]
- 52.Coffin CS, Shaheen AA, Burak KW, Myers RP. Pregnancy outcomes among liver transplant recipients in the United States: a nationwide case-control analysis. Liver Transpl. 2010;16:56–63. doi: 10.1002/lt.21906. [DOI] [PubMed] [Google Scholar]
- 53.Deshpande NA, James NT, Kucirka LM, Boyarsky BJ, Garonzik-Wang JM, Cameron AM, et al. Pregnancy outcomes of liver transplant recipients: a systematic review and meta-analysis. Liver Transpl. 2012;18:621–629. doi: 10.1002/lt.23416. [DOI] [PubMed] [Google Scholar]
- 54.Watt KD, Pedersen RA, Kremers WK, Heimbach JK, Charlton MR. Evolution of causes and risk factors for mortality post-liver transplant: results of the NIDDK long-term follow-up study. Am J Transplant. 2010;10:1420–1427. doi: 10.1111/j.1600-6143.2010.03126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aberg F, Gissler M, Karlsen TH, Ericzon BG, Foss A, Rasmussen A, et al. Differences in long-term mortality among liver transplant recipients and the general population: a population-based Nordic study. Hepatology. 2015;61:668–677. doi: 10.1002/hep.27538. [DOI] [PubMed] [Google Scholar]
- 56.Rubin A, Sanchez-Montes C, Aguilera V, Juan FS, Ferrer I, Moya A, et al. Long-term outcome of ‘long-term liver transplant survivors’. Transpl Int. 2013;26:740–750. doi: 10.1111/tri.12118. [DOI] [PubMed] [Google Scholar]
- 57.Nicolau-Raducu R, Gitman M, Ganier D, Loss G, Cohen A, Patel H, et al. Adverse cardiac events after orthotropic liver transplantation: a cross-sectional study in 389 consecutive patients. Liver Transpl. 2015;21:13–21. doi: 10.1002/lt.23997. http://dx.doi.org/10.1002/lt.23997. [DOI] [PubMed] [Google Scholar]
- 58.Watt KD, Pedersen RA, Kremers WK, Heimbach JK, Sanchez W, Gores GJ. Long-term probability of and mortality from de novo malignancy after liver transplantation. Gastroenterology. 2009;137:2010–2017. doi: 10.1053/j.gastro.2009.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adami J, Gabel H, Lindelof B, Ekstrom K, Rydh B, Glimelius B, et al. Cancer risk following organ transplantation: a nationwide cohort study in Sweden. Br J Cancer. 2003;89:1221–1227. doi: 10.1038/sj.bjc.6601219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baccarani U, Piselli P, Serraino D, Adani GL, Lorenzin D, Gambato M, et al. Comparison of de novo tumours after liver transplantation with incidence rates from Italian cancer registries. Dig Liver Dis. 2010;42:55–60. doi: 10.1016/j.dld.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 61.Collett D, Mumford L, Banner NR, Neuberger J, Watson C. Comparison of the incidence of malignancy in recipients of different types of organ: a UK Registry audit. Am J Transplant. 2010;10:1889–1896. doi: 10.1111/j.1600-6143.2010.03181.x. [DOI] [PubMed] [Google Scholar]
- 62.Aberg F, Pukkala E, Hockerstedt K, Sankila R, Isoniemi H. Risk of malignant neoplasms after liver transplantation: a population-based study. Liver Transpl. 2008;14:1428–1436. doi: 10.1002/lt.21475. [DOI] [PubMed] [Google Scholar]
- 63.Aseni P, Vertemati M, De Carlis L, Sansalone CV, Bonacina E, Minola E, et al. De novo cancers and post-transplant lymphoproliferative disorder in adult liver transplantation. Pathol Int. 2006;56:712–715. doi: 10.1111/j.1440-1827.2006.02035.x. [DOI] [PubMed] [Google Scholar]
- 64.Maggi U, Consonni D, Manini MA, Gatti S, Cuccaro F, Donato F, et al. Early and late de novo tumors after liver transplantation in adults: the late onset of bladder tumors in men. PLoS One. 2013;8:e65238. doi: 10.1371/journal.pone.0065238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chak E, Saab S. Risk factors and incidence of de novo malignancy in liver transplant recipients: a systematic review. Liver Int. 2010;30:1247–1258. doi: 10.1111/j.1478-3231.2010.02303.x. [DOI] [PubMed] [Google Scholar]
- 66.Kim WR, Terrault NA, Pedersen RA, Therneau TM, Edwards E, Hindman AA, et al. Trends in waiting list registration for liver transplantation for viral hepatitis in the United States. Gastroenterology. 2009;137:1680–1686. doi: 10.1053/j.gastro.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Biggins SW, Bambha KM, Terrault NA, Inadomi J, Shiboski S, Dodge JL, et al. Projected future increase in aging hepatitis C virus-infected liver transplant candidates: a potential effect of hepatocellular carcinoma. Liver Transpl. 2012;18:1471–1478. doi: 10.1002/lt.23551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 69.Di Martino V, Lebray P, Myers RP, Pannier E, Paradis V, Charlotte F, et al. Progression of liver fibrosis in women infected with hepatitis C: long-term benefit of estrogen exposure. Hepatology. 2004;40:1426–1433. doi: 10.1002/hep.20463. [DOI] [PubMed] [Google Scholar]
- 70.Berenguer M, Prieto M, Rayon JM, Mora J, Pastor M, Ortiz V, et al. Natural history of clinically compensated hepatitis C virus-related graft cirrhosis after liver transplantation. Hepatology. 2000;32:852–858. doi: 10.1053/jhep.2000.17924. [DOI] [PubMed] [Google Scholar]
- 71.Carrion JA, Torres F, Crespo G, Miquel R, Garcia-Valdecasas JC, Navasa M, et al. Liver stiffness identifies two different patterns of fibrosis progression in patients with hepatitis C virus recurrence after liver transplantation. Hepatology. 2010;51:23–34. doi: 10.1002/hep.23240. [DOI] [PubMed] [Google Scholar]
- 72.Lai JC, Verna EC, Brown RS, Jr, O’Leary JG, Trotter JF, Forman LM, et al. Hepatitis C virus-infected women have a higher risk of advanced fibrosis and graft loss after liver transplantation than men. Hepatology. 2011;54:418–424. doi: 10.1002/hep.24390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Belli LS, Burroughs AK, Burra P, Alberti AB, Samonakis D, Camma C, et al. Liver transplantation for HCV cirrhosis: improved survival in recent years and increased severity of recurrent disease in female recipients: results of a long term retrospective study. Liver Transpl. 2007;13:733–740. doi: 10.1002/lt.21093. [DOI] [PubMed] [Google Scholar]
- 74.Walter T, Dumortier J, Guillaud O, Hervieu V, Scoazec JY, Boillot O. Factors influencing the progression of fibrosis in patients with recurrent hepatitis C after liver transplantation under antiviral therapy: a retrospective analysis of 939 liver biopsies in a single center. Liver Transpl. 2007;13:294–301. doi: 10.1002/lt.21000. [DOI] [PubMed] [Google Scholar]
- 75.Giannelli V, Giusto M, Farcomeni A, Ponziani FR, Pompili M, Vigano R, et al. Treatment of hepatitis C recurrence is less successful in female than in male liver transplant recipients. Transpl Int. 2012;25:448–454. doi: 10.1111/j.1432-2277.2012.01440.x. [DOI] [PubMed] [Google Scholar]
- 76.Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249–1253. doi: 10.1053/j.gastro.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 77.Neuschwander-Tetri BA, Clark JM, Bass NM, Van Natta ML, Unalp-Arida A, Tonascia J, et al. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology. 2010;52:913–924. doi: 10.1002/hep.23784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wong RJ, Chou C, Bonham CA, Concepcion W, Esquivel CO, Ahmed A. Improved survival outcomes in patients with non-alcoholic steatohepatitis and alcoholic liver disease following liver transplantation: an analysis of 2002–2012 United Network for Organ Sharing data. Clin Transplant. 2014;28:713–721. doi: 10.1111/ctr.12364. [DOI] [PubMed] [Google Scholar]
- 79.Bambha K, Belt P, Abraham M, Wilson LA, Pabst M, Ferrell L, et al. Ethnicity and nonalcoholic fatty liver disease. Hepatology. 2012;55:769–780. doi: 10.1002/hep.24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang JD, Abdelmalek MF, Pang H, Guy CD, Smith AD, Diehl AM, et al. Gender and menopause impact severity of fibrosis among patients with nonalcoholic steatohepatitis. Hepatology. 2014;59:1406–1414. doi: 10.1002/hep.26761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122:1649–1657. doi: 10.1053/gast.2002.33573. [DOI] [PubMed] [Google Scholar]
- 82.Kojima S, Watanabe N, Numata M, Ogawa T, Matsuzaki S. Increase in the prevalence of fatty liver in Japan over the past 12 years: analysis of clinical background. J Gastroenterol. 2003;38:954–961. doi: 10.1007/s00535-003-1178-8. [DOI] [PubMed] [Google Scholar]
- 83.Wang X, Li J, Riaz DR, Shi G, Liu C, Dai Y. Outcomes of liver transplantation for nonalcoholic steatohepatitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2014;12:e391. doi: 10.1016/j.cgh.2013.09.023. [DOI] [PubMed] [Google Scholar]
- 84.Heuer M, Kaiser GM, Kahraman A, Banysch M, Saner FH, Mathe Z, et al. Liver transplantation in nonalcoholic steatohepatitis is associated with high mortality and post-transplant complications: a single-center experience. Digestion. 2012;86:107–113. doi: 10.1159/000339344. [DOI] [PubMed] [Google Scholar]
- 85.Angulo P. Nonalcoholic fatty liver disease and liver transplantation. Liver Transpl. 2006;12:523–534. doi: 10.1002/lt.20738. [DOI] [PubMed] [Google Scholar]
- 86.Khullar V, Dolganiuc A, Firpi RJ. Pre-and-post transplant considerations in patients with nonalcoholic fatty liver disease. World J Transplant. 2014;4:81–92. doi: 10.5500/wjt.v4.i2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Becker U, Deis A, Sorensen TI, Gronbaek M, Borch-Johnsen K, Muller CF, et al. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology. 1996;23:1025–1029. doi: 10.1002/hep.510230513. [DOI] [PubMed] [Google Scholar]
- 88.Frezza M, di Padova C, Pozzato G, Terpin M, Baraona E, Lieber CS. High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. N Engl J Med. 1990;322:95–99. doi: 10.1056/NEJM199001113220205. [DOI] [PubMed] [Google Scholar]
- 89.Poynard T, Mathurin P, Lai CL, Guyader D, Poupon R, Tainturier MH, et al. A comparison of fibrosis progression in chronic liver diseases. J Hepatol. 2003;38:257–265. doi: 10.1016/s0168-8278(02)00413-0. [DOI] [PubMed] [Google Scholar]
- 90.DiMartini A, Day N, Dew MA, Javed L, Fitzgerald MG, Jain A, et al. Alcohol consumption patterns and predictors of use following liver transplantation for alcoholic liver disease. Liver Transpl. 2006;12:813–820. doi: 10.1002/lt.20688. [DOI] [PubMed] [Google Scholar]
- 91.Pfitzmann R, Schwenzer J, Rayes N, Seehofer D, Neuhaus R, Nussler NC. Long-term survival and predictors of relapse after orthotopic liver transplantation for alcoholic liver disease. Liver Transpl. 2007;13:197–205. doi: 10.1002/lt.20934. [DOI] [PubMed] [Google Scholar]
- 92.Pfitzmann R, Benscheidt B, Langrehr JM, Schumacher G, Neuhaus R, Neuhaus P. Trends and experiences in liver retransplantation over 15 years. Liver Transpl. 2007;13:248–257. doi: 10.1002/lt.20904. [DOI] [PubMed] [Google Scholar]
- 93.Dumortier J, Guillaud O, Adham M, Boucaud C, Delafosse B, Bouffard Y, et al. Negative impact of de novo malignancies rather than alcohol relapse on survival after liver transplantation for alcoholic cirrhosis: a retrospective analysis of 305 patients in a single center. Am J Gastroenterol. 2007;102:1032–1041. doi: 10.1111/j.1572-0241.2007.01079.x. [DOI] [PubMed] [Google Scholar]
- 94.Burra P, Mioni D, Cillo U, Fagiuoli S, Senzolo M, Naccarato R, et al. Long-term medical and psycho-social evaluation of patients undergoing orthotopic liver transplantation for alcoholic liver disease. Transpl Int. 2000;13:S174–S178. doi: 10.1007/s001470050320. [DOI] [PubMed] [Google Scholar]
- 95.Bjornsson E, Olsson J, Rydell A, Fredriksson K, Eriksson C, Sjoberg C, et al. Long-term follow-up of patients with alcoholic liver disease after liver transplantation in Sweden: impact of structured management on recidivism. Scand J Gastroenterol. 2005;40:206–216. doi: 10.1080/00365520410009591. [DOI] [PubMed] [Google Scholar]
- 96.Karim Z, Intaraprasong P, Scudamore CH, Erb SR, Soos JG, Cheung E, et al. Predictors of relapse to significant alcohol drinking after liver transplantation. Can J Gastroenterol. 2010;24:245–250. doi: 10.1155/2010/596246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Manns MP, Czaja AJ, Gorham JD, Krawitt EL, Mieli-Vergani G, Vergani D, et al. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51:2193–2213. doi: 10.1002/hep.23584. [DOI] [PubMed] [Google Scholar]
- 98.Gronbaek L, Vilstrup H, Jepsen P. Autoimmune hepatitis in Denmark: incidence, prevalence, prognosis, and causes of death. A nationwide registry-based cohort study. J Hepatol. 2014;60:612–617. doi: 10.1016/j.jhep.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 99.Ngu JH, Gearry RB, Frampton CM, Stedman CA. Predictors of poor outcome in patients with autoimmune hepatitis: a population-based study. Hepatology. 2013;57:2399–2406. doi: 10.1002/hep.26290. [DOI] [PubMed] [Google Scholar]
- 100.Montano-Loza AJ, Vargas-Vorackova F, Ma M, Bain VG, Burak K, Kumar T, et al. Incidence and risk factors associated with de novo autoimmune hepatitis after liver transplantation. Liver Int. 2012;32:1426–1433. doi: 10.1111/j.1478-3231.2012.02832.x. [DOI] [PubMed] [Google Scholar]
- 101.Carbone M, Neuberger JM. Autoimmune liver disease, autoimmunity and liver transplantation. J Hepatol. 2014;60:210–223. doi: 10.1016/j.jhep.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 102.Duclos-Vallee JC, Sebagh M. Recurrence of autoimmune disease, primary sclerosing cholangitis, primary biliary cirrhosis, and autoimmune hepatitis after liver transplantation. Liver Transpl. 2009;15:S25–S34. doi: 10.1002/lt.21916. [DOI] [PubMed] [Google Scholar]
- 103.Tripathi D, Neuberger J. Autoimmune hepatitis and liver transplantation: indications, results, and management of recurrent disease. Semin Liver Dis. 2009;29:286–296. doi: 10.1055/s-0029-1233531. [DOI] [PubMed] [Google Scholar]
- 104.Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353:1261–1273. doi: 10.1056/NEJMra043898. [DOI] [PubMed] [Google Scholar]
- 105.Pells G, Mells GF, Carbone M, Newton JL, Bathgate AJ, Burroughs AK, et al. The impact of liver transplantation on the phenotype of primary biliary cirrhosis patients in the UK-PBC cohort. J Hepatol. 2013;59:67–73. doi: 10.1016/j.jhep.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lasker JN, Sogolow ED, Short LM, Sass DA. The impact of biopsychosocial factors on quality of life: women with primary biliary cirrhosis on waiting list and post liver transplantation. Br J Health Psychol. 2011;16:502–527. doi: 10.1348/135910710X527964. [DOI] [PubMed] [Google Scholar]
- 107.Lammers WJ, Kowdley KV, van Buuren HR. Predicting outcome in primary biliary cirrhosis. Ann Hepatol. 2014;13:316–326. [PubMed] [Google Scholar]
- 108.Carbone M, Mells GF, Pells G, Dawwas MF, Newton JL, Heneghan MA, et al. Sex and age are determinants of the clinical phenotype of primary biliary cirrhosis and response to ursodeoxycholic acid. Gastroenterology. 2013;144:560–569, e567. doi: 10.1053/j.gastro.2012.12.005. quiz e513–e564. [DOI] [PubMed] [Google Scholar]
- 109.Shibuya A, Tanaka K, Miyakawa H, Shibata M, Takatori M, Sekiyama K, et al. Hepatocellular carcinoma and survival in patients with primary biliary cirrhosis. Hepatology. 2002;35:1172–1178. doi: 10.1053/jhep.2002.33157. [DOI] [PubMed] [Google Scholar]
- 110.Suzuki A, Lymp J, Donlinger J, Mendes F, Angulo P, Lindor K. Clinical predictors for hepatocellular carcinoma in patients with primary biliary cirrhosis. Clin Gastroenterol Hepatol. 2007;5:259–264. doi: 10.1016/j.cgh.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 111.Silveira MG, Suzuki A, Lindor KD. Surveillance for hepatocellular carcinoma in patients with primary biliary cirrhosis. Hepatology. 2008;48:1149–1156. doi: 10.1002/hep.22458. [DOI] [PubMed] [Google Scholar]
- 112.Harada K, Hirohara J, Ueno Y, Nakano T, Kakuda Y, Tsubouchi H, et al. Incidence of and risk factors for hepatocellular carcinoma in primary biliary cirrhosis: national data from Japan. Hepatology. 2013;57:1942–1949. doi: 10.1002/hep.26176. [DOI] [PubMed] [Google Scholar]
- 113.Manousou P, Arvaniti V, Tsochatzis E, Isgro G, Jones K, Shirling G, et al. Primary biliary cirrhosis after liver transplantation: influence of immunosuppression and human leukocyte antigen locus disparity. Liver Transpl. 2010;16:64–73. doi: 10.1002/lt.21960. [DOI] [PubMed] [Google Scholar]
- 114.Singh S, Talwalkar JA. Primary sclerosing cholangitis: diagnosis, prognosis, and management. Clin Gastroenterol Hepatol. 2013;11:898–907. doi: 10.1016/j.cgh.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Toy E, Balasubramanian S, Selmi C, Li CS, Bowlus CL. The prevalence, incidence and natural history of primary sclerosing cholangitis in an ethnically diverse population. BMC Gastroenterol. 2011;11:83. doi: 10.1186/1471-230X-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Boonstra K, Weersma RK, van Erpecum KJ, Rauws EA, Spanier BW, Poen AC, et al. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology. 2013;58:2045–2055. doi: 10.1002/hep.26565. [DOI] [PubMed] [Google Scholar]
- 117.de Valle MB, Bjornsson E, Lindkvist B. Mortality and cancer risk related to primary sclerosing cholangitis in a Swedish population-based cohort. Liver Int. 2012;32:441–448. doi: 10.1111/j.1478-3231.2011.02614.x. [DOI] [PubMed] [Google Scholar]
- 118.Friedrich K, Rupp C, Hov JR, Steinebrunner N, Weiss KH, Stiehl A, et al. A frequent PNPLA3 variant is a sex specific disease modifier in PSC patients with bile duct stenosis. PLoS One. 2013;8:e58734. doi: 10.1371/journal.pone.0058734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mogl MT, Albert K, Pascher A, Sauer I, Puhl G, Gul S, et al. Survival without biliary complications after liver transplant for primary sclerosing cholangitis. Exp Clin Transplant. 2013;11:510–521. doi: 10.6002/ect.2013.0051. [DOI] [PubMed] [Google Scholar]
- 120.Alabraba E, Nightingale P, Gunson B, Hubscher S, Olliff S, Mirza D, et al. A re-evaluation of the risk factors for the recurrence of primary sclerosing cholangitis in liver allografts. Liver Transpl. 2009;15:330–340. doi: 10.1002/lt.21679. [DOI] [PubMed] [Google Scholar]
- 121.Moncrief KJ, Savu A, Ma MM, Bain VG, Wong WW, Tandon P. The natural history of inflammatory bowel disease and primary sclerosing cholangitis after liver transplantation – A single-centre experience. Can J Gastroenterol. 2010;24:40–46. doi: 10.1155/2010/830291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vera A, Moledina S, Gunson B, Hubscher S, Mirza D, Olliff S, et al. Risk factors for recurrence of primary sclerosing cholangitis of liver allograft. Lancet. 2002;360:1943–1944. doi: 10.1016/S0140-6736(02)11861-7. [DOI] [PubMed] [Google Scholar]
- 123.Schrem H, Kurok M, Kaltenborn A, Vogel A, Walter U, Zachau L, et al. Incidence and long-term risk of de novo malignancies after liver transplantation with implications for prevention and detection. Liver Transpl. 2013;19:1252–1261. doi: 10.1002/lt.23722. [DOI] [PubMed] [Google Scholar]
- 124.Singh S, Edakkanambeth Varayil J, Loftus EV, Jr, Talwalkar JA. Incidence of colorectal cancer after liver transplantation for primary sclerosing cholangitis: a systematic review and meta-analysis. Liver Transpl. 2013;19:1361–1369. doi: 10.1002/lt.23741. [DOI] [PubMed] [Google Scholar]
- 125.Sint Nicolaas J, de Jonge V, Steyerberg EW, Kuipers EJ, van Leerdam ME, Veldhuyzenvan Zanten SJ. Risk of colorectal carcinoma in post-liver transplant patients: a systematic review and meta-analysis. Am J Transplant. 2010;10:868–876. doi: 10.1111/j.1600-6143.2010.03049.x. [DOI] [PubMed] [Google Scholar]
- 126.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 127.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2010;53:1–35. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Farinati F, Sergio A, Giacomin A, Di Nolfo MA, Del Poggio P, Benvegnu L, et al. Is female sex a significant favorable prognostic factor in hepatocellular carcinoma? Eur J Gastroenterol Hepatol. 2009;21:1212–1218. doi: 10.1097/MEG.0b013e32831a86f8. [DOI] [PubMed] [Google Scholar]
- 129.Yang D, Hanna DL, Usher J, LoCoco J, Chaudhari P, Lenz HJ, et al. Impact of sex on the survival of patients with hepatocellular carcinoma: a surveillance, epidemiology, and end results analysis. Cancer. 2014;120:3707–3716. doi: 10.1002/cncr.28912. http://dx.doi.org/10.1002/cncr.28912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cauble S, Abbas A, Balart L, Bazzano L, Medvedev S, Shores N. United States women receive more curative treatment for hepatocellular carcinoma than men. Dig Dis Sci. 2013;58:2817–2825. doi: 10.1007/s10620-013-2731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cucchetti A, Cescon M, Bertuzzo V, Bigonzi E, Ercolani G, Morelli MC, et al. Can the dropout risk of candidates with hepatocellular carcinoma predict survival after liver transplantation? Am J Transplant. 2011;11:1696–1704. doi: 10.1111/j.1600-6143.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 132.Senkerikova R, Frankova S, Sperl J, Oliverius M, Kieslichova E, Filipova H, et al. Incidental hepatocellular carcinoma: risk factors and long-term outcome after liver transplantation. Transplant Proc. 2014;46:1426–1429. doi: 10.1016/j.transproceed.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 133.Schlansky B, Chen Y, Scott DL, Austin D, Naugler WE. Waiting time predicts survival after liver transplantation for hepatocellular carcinoma: a cohort study in the UNOS registry. Liver Transpl. 2014;20:1045–1056. doi: 10.1002/lt.23917. http://dx.doi.org/10.1002/lt.23917. [DOI] [PubMed] [Google Scholar]
- 134.Reuben A, Koch DG, Lee WMAcute Liver Failure Study G Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–2076. doi: 10.1002/hep.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Andrade RJ, Lucena MI, Fernandez MC, Pelaez G, Pachkoria K, Garcia-Ruiz E, et al. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005;129:512–521. doi: 10.1016/j.gastro.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 136.Fontana RJ. Acute liver failure including acetaminophen overdose. Med Clin North Am. 2008;92:761–794, viii. doi: 10.1016/j.mcna.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


