Abstract
Objectives
Emerging evidence indicates that tendon disease is an active process with inflammation that is critical to disease onset and progression. However, the key cytokines responsible for driving and sustaining inflammation have not been identified.
Methods
We performed a systematic review of the literature using MEDLINE (U.S. National Library of Medicine, Bethesda, Maryland) in March 2017. Studies reporting the expression of interleukins (ILs), tumour necrosis factor alpha (TNF-α) and interferon gamma in diseased human tendon tissues, and animal models of tendon injury or exercise in comparison with healthy control tissues were included.
Results
IL-1β, IL-6, IL-10, and TNF-α are the cytokines that have been most frequently investigated. In clinical samples of tendinopathy and tendon tears, the expression of TNF-α tended not to change but IL-6 increased in tears. Healthy human tendons showed increased IL-6 expression after exercise; however, IL-10 remained unchanged. Animal tendon injury models showed that IL-1β, IL-6, and TNF-α tend to increase from the early phase of tendon healing. In animal exercise studies, IL-1β expression showed a tendency to increase at the early stage after exercise, but IL-10 expression remained unchanged with exercise.
Conclusions
This review highlights the roles of IL-1β, IL-6, IL-10, and TNF-α in the development of tendon disease, during tendon healing, and in response to exercise. However, there is evidence accumulating that suggests that other cytokines are also contributing to tendon inflammatory processes. Further work with hypothesis-free methods is warranted in order to identify the key cytokines, with subsequent mechanistic and interaction studies to elucidate their roles in tendon disease development.
Cite this article: W. Morita, S. G. Dakin, S. J. B. Snelling, A. J. Carr. Cytokines in tendon disease: A Systematic Review. Bone Joint Res 2017;6:656–664. DOI: 10.1302/2046-3758.612.BJR-2017-0112.R1.
Keywords: Tendon, Tendinopathy, Cytokine
Article focus
To investigate gene and protein expression of cytokines in the development and progression of tendon disease and during tendon healing compared with healthy tendon tissues in both humans and animals.
To investigate how exercise affects the gene and protein expression of cytokines in tendon tissues.
To determine how cytokines affect the expression of tendon extracellular matrix (ECM) genes and proteins.
Key messages
The most frequently investigated cytokines in the development and progression of tendon disease, during tendon healing or in response to exercise were interleukin (IL)-1β, IL-6, IL-10, and tumour necrosis factor alpha (TNF-α), with a paucity of research on others that may also contribute.
IL-6 was the only cytokine involved in human tendon disease and was increased in tendon tears, whereas IL-1β, IL-6, and TNF-α tended to be increased in animal models of tendon injury.
The effects of cytokines on the expression of tendon ECM genes and proteins could not be determined due to the lack of studies which, in turn, warrants further investigation.
Strengths and limitations
This review encompasses current literature by a systematic review and organises the evidence in human and animal studies separately.
The key cytokines and their dominant role in the development of tendon disease could not be determined due to the small number and heterogeneity of samples and models.
Introduction
Tendon disease is increasingly common and comprises a third of all musculoskeletal complaints.1 The tendon tissue of early-stage disease, commonly referred to as tendinopathy, is characterised by the development of fibrosis: disoriented collagen fibres; altered composition of extracellular matrix (ECM) proteins; formation of new vessels; and rounding of tendon cells.2 The accumulation of fibrotic tissue predisposes to injury and tendon tear.3,4 The aetiology of tendon disease is acknowledged to be multifactorial, with overuse, trauma, ageing, and genetic predisposition as notable factors;5 however, the involvement of inflammation has long been debated.6 Today, emerging evidence indicates a strong inflammatory component to the pathogenesis of tendon disease, with inflammatory cells and cytokines as important regulators of the tendon ECM.7,8 Cytokines such as interleukins (IL), tumour necrosis factor alpha (TNF-α) and interferon gamma (IFN-γ), alongside growth factors such as transforming growth factor beta (TGF-β) and platelet-derived growth factor, are released from tendon stromal and immunoregulatory cells in response to tissue injury, mechanical stress, and malfunction.3,9,10 They alter the cellular phenotype of the local cells and the persistence of the cellular change by chronic inflammation results in the production of excessive and inappropriate matrix proteins and fibrosis.10 Similar responses have been widely studied in fibrotic diseases in organs such as the liver, kidney, and lung.11
The aim of this study was to systematically review the key cytokines that are involved in the development of tendon disease with a focus on fibrosis. The first objective was to investigate the gene and protein expression of cytokines in diseased tendons along the development of tendinopathy to tear, in comparison with that of healthy tendon. The second objective was to investigate how exercise affects the expression of these cytokines in tendon tissues. We also reviewed how tendon cells respond to these cytokines in the expression of ECM genes and proteins. We hypothesised that the cytokines expressed would vary during the process of tendon disease development or injury healing, and that the tendon cells from normal tissue, early disease, late disease, and healing would have differential responses to these cytokines.
Materials and Methods
This systematic review was designed, undertaken, and reported based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. Scientific literature was obtained using the MEDLINE (U.S. National Library of Medicine, Bethesda, Maryland) electronic database using the term “tendon AND cytokine” in March 2017.
The studies were included if they reported the expression of ILs, TNF-α, and IFN-γ as these were found to be the cytokines that were consistently considered through the preliminary screening. The cytokines of the TGF-β superfamily were excluded as we have reviewed these previously.12 Studies that compared the expression of the cytokines in diseased human tendon tissues and animal models of tendon injury or exercise with that of healthy control tissue were included. The tendon tissues included in the search criteria were from the mid-substance of tendon or tendon-to-bone enthesis. Studies on muscle-tendon junctions and ligament reconstruction using tendon grafts and other soft tissues (muscles, ligaments, cartilage, fat, bursa, and synovial tissues), as well as fetal, knockout animal models, animal studies of endocrine disorders (hyperglycaemia, menopause), and ex vivo experimental studies were excluded. The in vitro studies that investigated the effects of the cytokines on the expression of tendon ECM genes and proteins (collagens, elastin, proteoglycans, metalloproteinases (MMPs), tissue inhibitor of metalloproteinases (TIMPs), and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)) by healthy, diseased, or injured tendon cells were also included. Review articles, protocols, commentaries, case reports, and studies that were not reported in English were excluded. There was no limitation as to the year of publication.
Our search yielded 784 results. One article was identified by searching through references of listed articles. Following title screening, 594 abstracts were screened to determine eligibility and 57 papers met the inclusion criteria (Fig. 1). The papers that met the criteria are summarised in the additional files (supplementary tables i to iii).
Fig. 1.
Flowchart of the systematic review protocol.
Study characteristics
The studies that compared the expression of cytokines in diseased human tendons obtained tissue samples from healthy, tendinopathic, torn, and healing tendons after surgical repair of the rotator cuff (RC), Achilles, patella, posterior tibialis, digital flexors, and extensor carpal radialis brevis. The effect of exercise was studied in the Achilles and patellar tendons. Five out of 17 studies on human tendon tissues used a gene micro or cytometric bead array to determine the cytokines of interest in diseased human tendon tissues (supplementary table i).
The animal tendon injury models were wide-ranging, using the Achilles, RC, or flexor digitorum (FD) tendons in rats, dogs, and rabbits, with injuries created by collagenase, crush injury, partial transection, and full transection. Depending on the study, the transected tendons were repaired surgically or left to heal spontaneously. The animal tendon exercise models used the patellar, Achilles, RC, or FD tendons of rats or rabbits. Studies on horse tendon tissues obtained clinical samples of FD tendinopathy and compared the expression of cytokines with that of healthy tendon tissues. There were three out of 30 animal studies that used an array to determine the cytokines involved (supplementary table ii).
The in vitro studies investigated the effects of cytokines on the expression of tendon ECM genes and proteins in healthy, disease, or injured human and animal tendons. Human tendon cells were obtained from normal or tendinopathic tendons. The anatomy and species differed in all of the studies using animal tendon cells, which included healthy horse FD, injured mouse Achilles, and both healthy and injured rat patellar tendons (supplementary table iii).
Study methodology and assessing the risk of bias
The quality of study methodology was assessed in all papers by referring to the modified scoring system by Dean et al8 and Morita et al12 in order to highlight the studies with a high risk of potential bias. The median score was 8 (interquartile range, 7 to 9) out of 10 (supplementary table iv). Eight human tissue studies, six animal model tissue studies, and five in vitro experiment studies did not fully describe the age and gender of the included subjects. All of the included studies clearly described the control group. One study obtained the control tissue from the unaffected region of the tendinopathic tendon under confirmation by ultrasonography. There were three studies that sampled diseased tissues based on gross inspection, which may be a risk of bias. All except four studies clearly described the experimental procedures of tissue sampling and analysis, and 32 documented the validity or reliability of the methods used. Seven studies did not use quantitative measures or statistical analysis for comparison. Of the 51 studies that used quantitative analysis with statistical comparison, 45 stated the statistical level of significance, but only eight checked the data for normal distribution. Study limitations were not addressed in 21 studies. A meta-analysis was not carried out due to the heterogeneity of the data from clinical samples and animal models.
Results
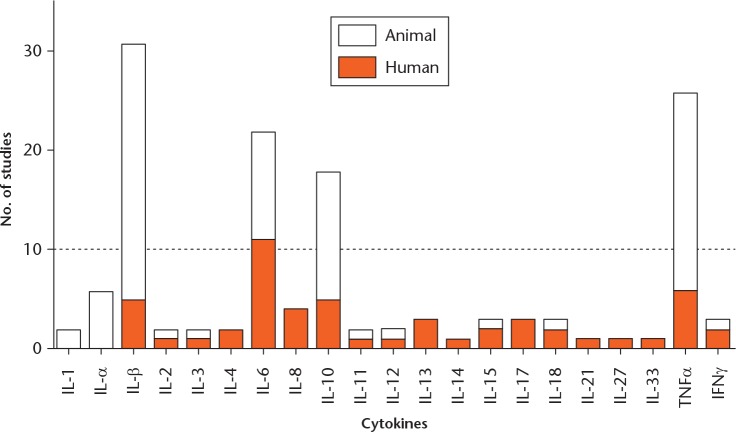
A total of 20 cytokines were implicated in the development of tendon disease, healing or in response to exercise: IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-6, IL-8, IL-10, IL-11, IL-12, IL-13, IL-14, IL-15, IL-17, IL-18, IL-27, TNF-α, and IFN-γ were identified by array-based studies (seven studies); and IL-21 and IL-33 were guided by literature. IL-1β, IL-6, IL-10, and TNF-α had been investigated in numerous (more than ten) hypothesis-free and literature-guided studies (Fig. 2). Hence, the results on these four cytokines were summarised (Table I). Results of other cytokines are summarised in the additional files (supplementary table v). Effects of cytokines on the expression of tendon ECM genes and proteins in tendon cells have been presented for IL-1β, IL-6, IL-10, and TNF-α, similarly (Table II). Results of other cytokines are shown in the additional files (supplementary table vi).
Fig. 2.
Number of studies of human tendon tissues and animal tendon injury or exercise models for each cytokine.
Table I.
Expression of interleukins IL-1β, IL-6, IL-10, and tumour necrosis factor alpha (TNF-α) in tissues of diseased human tendon, animal models of tendon injury or exercise versus healthy control tendon. Arrows indicate increased (↑), unchanged (→), or decreased (↓) expression of cytokines in tissues of diseased human tendon, animal models of tendon injury or exercise versus healthy control tendon. If two arrows are given, this indicates that more than one change in expression has been reported (for example, →/↑ indicates that both unchanged and increased expression have been reported)
| Cytokine | Animal | Disease model | Increased, unchanged, decreased in diseased vs control | |
|---|---|---|---|---|
| Gene | Protein | |||
| IL-1β | Human | Rotator cuff, tear | ↓13 | ↑ (descriptive)15 |
| Achilles, tear, post-operative (2 wks) | →*16 | |||
| Achilles, tendinopathy | →*17 | |||
| Achilles, tendinopathy + exercise (1 hr run) | →†14 | |||
| Rat | Rotator cuff, tendinopathy model, transection + repair | ↑ (1 wk)60 | ||
| Achilles, partial transection | ↑ (1 day) → (4 days)20 | |||
| Achilles, transection + repair | ↑ (3 days; 1, 2, 4 wks)18 | |||
| Achilles, crush | ↑ (1 day)21,22 → (3 days)22 ↑ (5 days)51 ↑ (1 wk)22 | |||
| Achilles, collagenase | ↑ (1, 2 wks)19 | |||
| Achilles, exercise | → (7 wks)27 | |||
| Achilles, transection + exercise (post-operative day 5) | ↑ (1, 3 hrs) → (12 hrs)28 | |||
| ↑ (1, 3 hrs) → (12 hrs)(IL-1RA)28 | ||||
| Achilles/patella, stress deprivation | ↑ (2, 6 wks)31 | |||
| Patella, cyclic exercise by surgery | ↑ (high strain) ↓ (low strain)29 | ↑ (high strain) ↓ (low strain)29 | ||
| Flexor digitorum, exercise | ↑ (training)26 →/↑ (3, 6, 8 wks)32-36 → (9 wks)36 →/↑ (12 wks)33,36-38 → (18, 24 wks)26 | |||
| Rabbit | Rotator cuff, partial transection (defect) | ↑ (1, 3 days; 1 wk) → (3 wks)(descriptive)23 | ↑ (1, 3 days; 1 wk) → (3 wks)23 | |
| Flexor digitorum superficialis, transection + repair | ↑ (3, 6 days) → (12, 24 days)24 | |||
| Flexor, electrical stimulation | → (14 wks)30 | |||
| Dog | Flexor, transection + repair | ↑ (1, 3, 9 days)25 | ||
| Horse | Flexor digitorum superficialis, tendinopathy | ↑ (descriptive)61 | ||
| IL-6 | Human | Rotator cuff, tear | ↑40 →41 | ↑40 |
| Rotator cuff/Achilles/patella/biceps/ECRB/flexor, tear | ↑ (OSM)62 ↓ (IL-6R)62 | |||
| Rotator cuff/posterior tibialis, tendinopathy | → 41,43 → (IL-6, OSM, LIF, IL-6R)42 | |||
| Achilles, tear | ↑ (IL-6, OSM, LIF)42 ↓ (IL-6R)42 | |||
| Achilles, tear, post-operative (2 wks) | ↑*16 | |||
| Achilles, tendinopathy | ↑ 42 → 43 ↓ (IL-6R)42 → (OSM, LIF)42 | →*17 | ||
| Achilles, exercise (1 hr/36 km run) | ↑* (post-exercise 2-6 hrs; 1, 2 days)44,45 | |||
| Patella, exercise (knee strenuous extension) | → (post-exercise 1, 3 days)*63 | |||
| Achilles, tendinopathy + exercise (1 hr run) | →†14 | |||
| Rat | Rotator cuff, exercise | ↑ (4 wks)40 | ||
| Achilles, transection + repair | ↑ (3 days; 1, 2, 4 wks)18 | |||
| Achilles, crush | ↑(1 day)21 | |||
| Achilles, collagenase | ↑ (2 hrs)46 ↑ (1, 2 wks)19 | |||
| Achilles, exercise | → (7 wks)27 | |||
| Flexor, exercise | ↑ (training)26 → (3, 6 wks)36 ↑ (9 wks)36 →/↑(12 wks)36,38 → (18 wks)26 ↑ (24 wks)26 | |||
| IL-10 | Human | Rotator cuff, tear | ↑13 → 41 | |
| Rotator cuff/Achilles, tendinopathy | ↓41 | ↑ (descriptive)*17 | ||
| Achilles, tear, post-operative (2 wks) | ↑*16 | |||
| Achilles, tendinopathy + exercise (1 hr run) | →†14 | |||
| Rat | Achilles, partial transection | → (1, 4 days)20 | ||
| Achilles, transection + repair | → (3 days; 1, 2 wks) ↑ (4 wks)18 | |||
| Achilles, crush | → (1 day)21 | |||
| Achilles, collagenase | ↑ (2 hrs)46 → (1, 2 wks)48,49 | |||
| Flexor digitorum, exercise | → (training, 3, 6, 8, 9, 12, 18, 24 wks)26,32,34,36,38 | |||
| TNFα | Human | Rotator cuff, tear | →40 ↑41 ↓ (TNFR1)13 | |
| Rotator cuff/Achilles tendinopathy | →41 | →(TNFα, TNFR2) ↑ (TNFR1)50 | ||
| Achilles, tear, post-operative (2 wks) | →*16 | |||
| Achilles, tendinopathy + exercise (1 hr run) | ↓†14 | |||
| Rat | Rotator cuff, tendinopathy model, transection + repair | → (1 wk)60 | ||
| Rotator cuff, exercise | ↑ (4 wks)40 | |||
| Achilles, partial transection | → (1 day) ↑ (4 days)20 | |||
| Achilles, crush | → (1 day)21 ↑ (5 days)51 | |||
| Achilles, collagenase | ↑ (2 hrs)46,64 ↑ (1 wk) → (2 wks)19 | |||
| Patella, stress deprivation | ↑ (2, 6 wks)31 | |||
| Flexor, exercise | ↑ (training)26,34 → (3, 6 wks)32,34,36 ↑ (8 wks)34,35 → (9 wks)36 ↓/→/↑ (12 wks)34,36-38 → (18 wks)26 ↑ (24 wks)26,34 | |||
| Dog | Flexor, transection + repair | ↑ (1, 3, 9 days)25 | ||
| Horse | Superficial flexor digitorum, tendinopathy | ↑ (acute) ↓ (chronic) (descriptive)65 | ↑ (acute) ↓ (chronic) (descriptive) (TNFα, R1, TRAF2)65 ↑ (descriptive)61 | |
studies that obtained clinical samples of tendon by microdialysis
studies that sampled control tissues from the healthy region of the same tendon
IL-1RA, interleukin-1 receptor antagonist; OSM, oncostatin M; IL-6R, interleukin 6 receptor; LIF, leukemia inhibitory factor; TNFR, tumour necrosis factor receptor; TRAF, TNF receptor-associated factor; ECRB, extensor carpi radialis brevis
Table II.
Cellular responses to treatment by cytokines in tendon cells. Arrows indicate increased (↑), unchanged (→), or decreased (↓) expression of cytokines in tissues of diseased human tendon, animal models of tendon injury or exercise versus healthy control tendon
| Treatment | Cells | Increased, unchanged, decreased in response to treatment versus control | |
|---|---|---|---|
| Gene | Protein | ||
| IL-1β | Human, various, normal | Collagen I ↓ MMP1 ↑ MMP2 → MMP3 ↑ MMP13 ↑ TIMP1 → TIMP2→ ADAMTS-4 →66,67 | MMP1 ↑ MMP3 ↑67 |
| Human, not described, tendinopathy | MMP1 ↑ MMP2 → MMP3 ↑68 | MMP1 ↑ MMP2 ↑ MMP3 ↑68 | |
| Rat, patella, normal | MMP13 ↑*39 | ||
| Rat, patella, injured | MMP13 ↑*39 | ||
| Mouse, Achilles, injured | Collagen I ↓ Collagen III ↓ Biglycan ↓ Decorin ↑ Fibromodulin ↓ Lumican → Aggrecan ↓ MMP13 ↑69 | ||
| IL-6, IL-10 | Human, various, normal | Elastin → MMP1 →47 | Collagen I →47 |
| TNFα | Human, various, normal | Elastin ↑ MMP1 ↑47 | Collagen I ↓47 |
| Horse, FDS, normal | Collagen I ↑ MMP9 → MMP13 ↓52 | ||
significant difference between tendon cells from normal and injured patellar tendons (p < 0.05)
IL, interleukin; MMP, matrix metalloproteinase; TIMP, tissue inhibitor of metalloproteinase; ADAMTS, a disintegrin and metalloproteinase with thrombospondin motifs; TNF, tumour necrosis factor; FDS, flexor digitorum superficialis
IL-1β
In clinical samples, gene expression was decreased and protein expression increased in torn RC, but it remained unchanged in torn Achilles tendon tissues and after repair, exercise, or tendinopathy.13-17 The role of IL-1β in human tendon disease or after exercise could not be concluded. In animal injury models, gene and protein expression of IL-1β tended to increase at the early stages of tendon injury or healing until two weeks after the intervention.18-25 Similarly, exercise tended to increase the gene and protein expression of IL-1β in the early stages.26-38 Treatment of tendon cells with IL-1β showed catabolic effects such as increased expression of matrix metalloproteinase (MMPs), with a significant difference between cells from healthy and injured patellar tendons.39
IL-6
Gene and protein expression of IL-6 tended to be increased in RC and Achilles tendon tear patient samples,40-42 which continued until two weeks post-surgical repair,16 but not in patients with Achilles, RC, or posterior tibialis tendinopathy.17,41-43 Increased IL-6 protein expression was noted after prolonged running in healthy tendons,44,45 but not in tendinopathic Achilles tendons.14 In animal injury models, gene, and protein expression of IL-6 was increased from two hours to four weeks after the intervention.18,19,21,26,46 The effect of exercise was inconsistent in animal models.26,27,36,38,40 IL-6 treatment on tendon cells did not have any effect on the expression of tendon ECM genes and proteins.47
IL-10
The expression of IL-10 was inconsistent in clinical samples13,14,16,17,41 and animal injury models.18,20,21,46,48,49 No effects of exercise on the expression of IL-10 were noted in either humans or animals.26,32,34,36,38 IL-10 treatment on tendon cells did not have any effect on the expression of tendon ECM genes and proteins.47
TNF-α
Gene and protein expression of TNF-α tended not to change in clinical samples of RC and Achilles tendinopathy and tear patients.16,40,41,50 The effect of exercise on the expression of TNF-α in healthy human tendons has not been reported. In animal injury models, gene expression of TNF-α was elevated from two hours to nine days and declined at two weeks after the intervention,19,20,21,25,51 whereas the protein expression increased after four days. The effect of exercise on the gene and protein expression of TNF-α was inconclusive in animal models.26,31,32,34-38 The effects of TNF-α on tendon cells could not be concluded as genes of interest differed between studies.47,52
Discussion
This systematic review shows that IL-1β, IL-6, IL-10, and TNF-α are the most frequently investigated cytokines in the development and progression of tendon disease, during tendon healing and in response to exercise. Most studies focused on inflammatory cytokines based on previous literature, and only a few studies used hypothesis-free approaches to define the implicated cytokines.
Expression of IL-1β, IL-6, IL-10, and TNF-α differed depending on the stage of the tendon disease development, injury healing, and in response to exercise: IL-1β tended to increase in the early stage of tendon injury or exercise in animal models; IL-6 was suggested to increase at tendon tear, after prolonged exercise in healthy human tendons, and in the early stage of tendon injury in animal models; IL-10 remained unchanged in response to exercise in both humans and animals; and TNF-α tended to increase in the early stage of animal tendon injury models. Their functional mechanism in tendon disease development or healing could not be determined due to the small number of studies. The cellular response to IL-1β treatment significantly differed between injured and healthy tendon cells, but the involvement of other cytokines in tendon disease, healing, or after exercise could not be determined due to the paucity of studies or the inconsistency of the results thus far.
We were not able to identify the key cytokines through array studies alone due to the small number of studies, and due to the heterogeneity of clinical samples and animal models. An arbitrary number of more than ten studies was set to focus on the cytokines that had been investigated frequently in the literature. However, there was a wide variety of the anatomical locations of the diseased and control tendons, diagnostic criteria of tendinopathy, intervention, and the methods of gene or protein expression analysis in both human and animal studies. Tendons respond differently despite similar intervention based on anatomical location, function,53 and the content of exercises.37 Variances of the study limited the performing of a meta-analysis.
Schulze-Tanzil et al3 and Millar, Murrell, and McInnes54 have indicated through narrative reviews that multiple cytokines such as IL-1β, IL-4, IL-6, IL-13, IL-15, IL-17, IL-18, IL-21, IL-33, and TNF family members alongside TGF-β contribute to the development of tendon disease. By carrying out a systematic review, we captured all current literature and organised the evidence in human and animal studies separately. This largely supported the data presented in previous narrative reviews. Animal studies have been helpful in understanding tendon healing and the effect of exercise, but only represent limited features of the pathophysiology and clinical diseases.55 Our data on the four most investigated cytokines suggest that the expression of cytokines in diseased human tendons and animal models does not act in the same manner.
Numerous cytokines have been proposed to contribute to the development of tendon pathology, but there is a clear shortage of analyses on human-derived tendon tissue and cells. There is a risk of noting IL-1β, IL-6, IL-10, and TNFα as the key cytokines in tendon disease just for their frequent investigation. More work is warranted through individual interrogation to specify the cytokines that actually play prominent roles. Dakin et al41 indicated that advanced-stage disease tendon tissues from large to massive tears have a tissue inflammation signature characterised by the activation of signal transducer and activator of transcription 6, which is predominantly activated by cytokines such as IL-4. Millar et al,56,57 through mechanistic studies, have proposed IL-33 as an alarmin that triggers inflammation and IL-17A as an inflammatory modulator regulating cytokines, which contribute to the development and progress of tendon disease. There is clearly a strong inflammatory component in the development of tendon disease, with cytokines, which thus far have not been investigated frequently, potentially playing a substantial role. In the case of rheumatoid arthritis, TNF-α unexpectedly turned out to be the master regulator of the pro-inflammatory cytokines contributing to the disease.58 Moreover, it may also be important to consider interplay of numerous inflammatory processes with regard to different cell types, receptors, and biological and physical environments, and not just to focus on a limited number of molecules.
The cellular response of tendon cells to cytokines in the expression of tendon ECM genes differed depending on cell phenotype. A study by Tohyama et al39 suggested that infiltrating fibroblasts show significantly decreased expression of an ECM gene (MMP13) in response to IL-1β treatment when compared with the response of healthy tendon cells, and this comes in line with the studies by Dakin et al41,59 reporting that diseased tendon stromal cells may be primed for inflammation. Although the directions of the stimulatory or inhibitory effect of IL-1β in the gene and protein expression of collagens and MMPs were similar, it should be noted that the magnitude of the change may differ in cells isolated from diseased or healing tissues in comparison with healthy cells.59 Currently, only a few studies have focused on the mechanisms of action of the cytokines in cells derived from tissues of different phenotypes.
Determining both the temporal expression of cytokines during tendon disease progression and the mechanism of interaction of tendon cells with focus on cell phenotype is essential in order to improve our understanding of tendon disease pathophysiology and tendon healing. A systematic approach using well-defined clinical specimens to identify the cytokines that play a prominent role in the development of tendon disease is warranted. Further mechanistic studies on cytokine biology based on context of expression and surrounding inflammatory milieu should conduce identification of fundamental therapies to improve disease management through enhancing the quality of tissue repair or slowing disease progression.
Footnotes
Author Contribution: W. Morita: Study concept and design, Data collection, Analysis, Interpretation of data, Drafting the manuscript.
S. G. Dakin: Analysis, Interpretation of data, Critical revision of manuscript.
S. J.B Snelling: Analysis, Interpretation of data, Critical revision of manuscript.
A. J. Carr: Supervision of study, Interpretation of data, Critical revision of manuscript.
Conflict of Interest Statement: None declared
Supplementary material
Tables and figures showing the main characteristics of the papers included, results of assessment of study methodology quality, and summarised data of the expression of other cytokines in tendon tissues are available alongside the online version of this article at www.bjr.boneandjoint.org
Funding Statement
The research was funded by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC). Dr Morita was funded by 28th term scholarship No. 274 of the INOAC International Education and Scholarship Foundation. Dr Dakin is a recipient of an Oxford UCB Prize Fellowship in Biomedical Research.
We would like to thank Miss J. Mimpen and Dr M. Baldwin for critical feedback of the manuscript.
References
- 1. Forde MS, Punnett L, Wegman DH. Prevalence of musculoskeletal disorders in union ironworkers. J Occup Environ Hyg 2005;2:203-212. [DOI] [PubMed] [Google Scholar]
- 2. Dean BJ, Franklin SL, Carr AJ. A systematic review of the histological and molecular changes in rotator cuff disease. Bone Joint Res 2012;1:158-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schulze-Tanzil G, Al-Sadi O, Wiegand E, et al. The role of pro-inflammatory and immunoregulatory cytokines in tendon healing and rupture: new insights. Scand J Med Sci Sports 2011;21:337-351. [DOI] [PubMed] [Google Scholar]
- 4. Neer CS, Welsh RP. Shoulder in Sports. Orthop Clin North Am 1977;8:583-591. [PubMed] [Google Scholar]
- 5. Wang JH, Iosifidis MI, Fu FH. Biomechanical basis for tendinopathy. Clin Orthop Relat Res 2006;443:320-332. [DOI] [PubMed] [Google Scholar]
- 6. Rees JD, Stride M, Scott A. Tendons–time to revisit inflammation. Br J Sports Med 2014;48:1553-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Millar NL, Dean BJ, Dakin SG. Inflammation and the continuum model: time to acknowledge the molecular era of tendinopathy. Br J Sports Med 2016. (Epub ahead of print) PMID: 27259752. [DOI] [PubMed]
- 8. Dean BJ, Gettings P, Dakin SG, Carr AJ. Are inflammatory cells increased in painful human tendinopathy? A systematic review. Br J Sports Med 2016;50:216-220. [DOI] [PubMed] [Google Scholar]
- 9. Sziksz E, Pap D, Lippai R, et al. Fibrosis related inflammatory mediators: role of the IL-10 cytokine family. Mediators Inflamm 2015;2015:764641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Medzhitov R. Origin and physiological roles of inflammation. Nature 2008;454:428-435. [DOI] [PubMed] [Google Scholar]
- 11. Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol 2008;214:199-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Morita W, Snelling SJ, Dakin SG, Carr AJ. Profibrotic mediators in tendon disease: a systematic review. Arthritis Res Ther 2016;18:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chaudhury S, Xia Z, Thakkar D, Hakimi O, Carr AJ. Gene expression profiles of changes underlying different-sized human rotator cuff tendon tears. J Shoulder Elbow Surg 2016;25:1561-1570. [DOI] [PubMed] [Google Scholar]
- 14. Pingel J, Fredberg U, Mikkelsen LR, et al. No inflammatory gene-expression response to acute exercise in human Achilles tendinopathy. Eur J Appl Physiol 2013;113:2101-2109. [DOI] [PubMed] [Google Scholar]
- 15. Gotoh M, Hamada K, Yamakawa H, et al. Significance of granulation tissue in torn supraspinatus insertions: an immunohistochemical study with antibodies against interleukin-1 beta, cathepsin D, and matrix metalloprotease-1. J Orthop Res 1997;15:33-39. [DOI] [PubMed] [Google Scholar]
- 16. Ackermann PW, Domeij-Arverud E, Leclerc P, Amoudrouz P, Nader GA. Anti-inflammatory cytokine profile in early human tendon repair. Knee Surg Sports Traumatol Arthrosc 2013;21:1801-1806. [DOI] [PubMed] [Google Scholar]
- 17. Waugh CM, Morrissey D, Jones E, et al. In vivo biological response to extracorporeal shockwave therapy in human tendinopathy. Eur Cell Mater 2015;29:268-280. [DOI] [PubMed] [Google Scholar]
- 18. Sugg KB, Lubardic J, Gumucio JP, Mendias CL. Changes in macrophage phenotype and induction of epithelial-to-mesenchymal transition genes following acute Achilles tenotomy and repair. J Orthop Res 2014;32:944-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pires D, Xavier M, Araujo T, et al. Low-level laser therapy (LLLT; 780 nm) acts differently on mRNA expression of anti- and pro-inflammatory mediators in an experimental model of collagenase-induced tendinitis in rat. Lasers Med Sci 2011;26:85-94. [DOI] [PubMed] [Google Scholar]
- 20. Da Ré Guerra F, Vieira CP, Oliveira LP, et al. Low-level laser therapy modulates pro-inflammatory cytokines after partial tenotomy. Lasers Med Sci 2016;31:759-766. [DOI] [PubMed] [Google Scholar]
- 21. Haslerud S, Lopes-Martins RA, Frigo L, et al. Low-level laser therapy and cryotherapy as mono- and adjunctive therapies for Achilles tendinopathy in rats. Photomed Laser Surg 2017;35:32-42. [DOI] [PubMed] [Google Scholar]
- 22. de Jesus JF, Spadacci-Morena DD, dos Anjos, Rabelo ND, et al. Low-level laser therapy in IL-1β, COX-2, and PGE2 modulation in partially injured Achilles tendon. Lasers Med Sci 2015;30:153-158. [DOI] [PubMed] [Google Scholar]
- 23. Koshima H, Kondo S, Mishima S, et al. Expression of interleukin-1beta, cyclooxygenase-2, and prostaglandin E2 in a rotator cuff tear in rabbits. J Orthop Res 2007;25:92-97. [DOI] [PubMed] [Google Scholar]
- 24. Berglund M, Hart DA, Wiig M. The inflammatory response and hyaluronan synthases in the rabbit flexor tendon and tendon sheath following injury. J Hand Surg Eur Vol 2007;32:581-587. [DOI] [PubMed] [Google Scholar]
- 25. Manning CN, Havlioglu N, Knutsen E, et al. The early inflammatory response after flexor tendon healing: a gene expression and histological analysis. J Orthop Res 2014;32:645-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gao HG, Fisher PW, Lambi AG, et al. Increased serum and musculotendinous fibrogenic proteins following persistent low-grade inflammation in a rat model of long-term upper extremity overuse. PLoS One 2013;8:e71875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pingel J, Wienecke J, Kongsgaard M, et al. Increased mast cell numbers in a calcaneal tendon overuse model. Scand J Med Sci Sports 2013;23:e353-e360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eliasson P, Andersson T, Aspenberg P. Influence of a single loading episode on gene expression in healing rat Achilles tendons. J Appl Physiol (1985) 2012;112:279-288. [DOI] [PubMed] [Google Scholar]
- 29. Sun HB, Li Y, Fung DT, et al. Coordinate regulation of IL-1beta and MMP-13 in rat tendons following subrupture fatigue damage. Clin Orthop Relat Res 2008;466:1555-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Asundi KR, King KB, Rempel DM. Evaluation of gene expression through qRT-PCR in cyclically loaded tendons: an in vivo model. Eur J Appl Physiol 2008;102:265-270. [DOI] [PubMed] [Google Scholar]
- 31. Uchida H, Tohyama H, Nagashima K, et al. Stress deprivation simultaneously induces over-expression of interleukin-1beta, tumor necrosis factor-alpha, and transforming growth factor-beta in fibroblasts and mechanical deterioration of the tissue in the patellar tendon. J Biomech 2005;38:791-798. [DOI] [PubMed] [Google Scholar]
- 32. Frara N, Abdelmagid SM, Tytell M, et al. Growth and repair factors, osteoactivin, matrix metalloproteinase and heat shock protein 72, increase with resolution of inflammation in musculotendinous tissues in a rat model of repetitive grasping. BMC Musculoskelet Disord 2016;17:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fedorczyk JM, Barr AE, Rani S, et al. Exposure-dependent increases in IL-1beta, substance P, CTGF, and tendinosis in flexor digitorum tendons with upper extremity repetitive strain injury. J Orthop Res 2010;28:298-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barbe MF, Elliott MB, Abdelmagid SM, et al. Serum and tissue cytokines and chemokines increase with repetitive upper extremity tasks. J Orthop Res 2008;26:1320-1326. [DOI] [PubMed] [Google Scholar]
- 35. Coq JO, Barr AE, Strata F, et al. Peripheral and central changes combine to induce motor behavioral deficits in a moderate repetition task. Exp Neurol 2009;220:234-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kietrys DM, Barr-Gillespie AE, Amin M, et al. Aging contributes to inflammation in upper extremity tendons and declines in forelimb agility in a rat model of upper extremity overuse. PLoS One 2012;7:e46954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Barbe MF, Gallagher S, Massicotte VS, et al. The interaction of force and repetition on musculoskeletal and neural tissue responses and sensorimotor behavior in a rat model of work-related musculoskeletal disorders. BMC Musculoskelet Disord 2013;14:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Elliott MB, Barr AE, Clark BD, et al. High force reaching task induces widespread inflammation, increased spinal cord neurochemicals and neuropathic pain. Neuroscience 2009;158:922-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tohyama H, Yasuda K, Uchida H, Nishihira J. The responses of extrinsic fibroblasts infiltrating the devitalised patellar tendon to IL-1beta are different from those of normal tendon fibroblasts. J Bone Joint Surg [Br] 2007;89-B:1261-1267. [DOI] [PubMed] [Google Scholar]
- 40. Millar NL, Wei AQ, Molloy TJ, Bonar F, Murrell GA. Cytokines and apoptosis in supraspinatus tendinopathy. J Bone Joint Surg [Br] 2009;91-B:417-424. [DOI] [PubMed] [Google Scholar]
- 41. Dakin SG, Martinez FO, Yapp C, et al. Inflammation activation and resolution in human tendon disease. Sci Transl Med 2015;7:311ra173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Legerlotz K, Jones ER, Screen HR, Riley GP. Increased expression of IL-6 family members in tendon pathology. Rheumatology (Oxford) 2012;51:1161-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Courneya JP, Luzina IG, Zeller CB, et al. Interleukins 4 and 13 modulate gene expression and promote proliferation of primary human tenocytes. Fibrogenesis Tissue Repair 2010;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gump BS, McMullan DR, Cauthon DJ, et al. Short-term acetaminophen consumption enhances the exercise-induced increase in Achilles peritendinous IL-6 in humans. J Appl Physiol (1985) 2013;115:929-936. [DOI] [PubMed] [Google Scholar]
- 45. Langberg H, Olesen JL, Gemmer C, Kjaer M. Substantial elevation of interleukin-6 concentration in peritendinous tissue, in contrast to muscle, following prolonged exercise in humans. J Physiol 2002;542:985-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Torres-Silva R, Lopes-Martins RA, Bjordal JM, et al. The low level laser therapy (LLLT) operating in 660 nm reduce gene expression of inflammatory mediators in the experimental model of collagenase-induced rat tendinitis. Lasers Med Sci 2015;30:1985-1990. [DOI] [PubMed] [Google Scholar]
- 47. John T, Lodka D, Kohl B, et al. Effect of pro-inflammatory and immunoregulatory cytokines on human tenocytes. J Orthop Res 2010;28:1071-1077. [DOI] [PubMed] [Google Scholar]
- 48. Xavier M, de Souza RA, Pires VA, et al. Low-level light-emitting diode therapy increases mRNA expressions of IL-10 and type I and III collagens on Achilles tendinitis in rats. Lasers Med Sci 2014;29:85-90. [DOI] [PubMed] [Google Scholar]
- 49. Casalechi HL, Leal-Junior EC, Xavier M, et al. Low-level laser therapy in experimental model of collagenase-induced tendinitis in rats: effects in acute and chronic inflammatory phases. Lasers Med Sci 2013;28:989-995. [DOI] [PubMed] [Google Scholar]
- 50. Gaida JE, Bagge J, Purdam C, et al. Evidence of the TNF-alpha system in the human Achilles tendon: expression of TNF-alpha and TNF receptor at both protein and mRNA levels in the tenocytes. Cells Tissues Organs 2012;196:339-352. [DOI] [PubMed] [Google Scholar]
- 51. Dohnert MB, Venâncio M, Possato JC, et al. Gold nanoparticles and diclofenac diethylammonium administered by iontophoresis reduce inflammatory cytokines expression in Achilles tendinitis. Int J Nanomedicine 2012;7:1651-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hosaka YZ, Uratsuji T, Ueda H, Uehara M, Takehana K. Comparative study of the properties of tendinocytes derived from three different sites in the equine superficial digital flexor tendon. Biomed Res 2010;31:35-44. [DOI] [PubMed] [Google Scholar]
- 53. Davies MR, Ravishankar B, Laron D, et al. Rat rotator cuff muscle responds differently from hindlimb muscle to a combined tendon-nerve injury. J Orthop Res 2015;33:1046-1053. [DOI] [PubMed] [Google Scholar]
- 54. Millar NL, Murrell GA, McInnes IB. Inflammatory mechanisms in tendinopathy - towards translation. Nat Rev Rheumatol 2017;13:110-122. [DOI] [PubMed] [Google Scholar]
- 55. Dirks RC, Warden SJ. Models for the study of tendinopathy. J Musculoskelet Neuronal Interact 2011;11:141-149. [PubMed] [Google Scholar]
- 56. Millar NL, Akbar M, Campbell AL, et al. IL-17A mediates inflammatory and tissue remodelling events in early human tendinopathy. Sci Rep 2016;6:27149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Millar NL, Gilchrist DS, Akbar M, et al. MicroRNA29a regulates IL-33-mediated tissue remodelling in tendon disease. Nat Commun 2015;6:6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Feldmann M, Maini RN. Lasker Clinical Medical Research Award. TNF defined as a therapeutic target for rheumatoid arthritis and other autoimmune diseases. Nat Med 2003;9:1245-1250. [DOI] [PubMed] [Google Scholar]
- 59. Dakin SG, Buckley CD, Al-Mossawi MH, et al. Persistent stromal fibroblast activation is present in chronic tendinopathy. Arthritis Res Ther 2017;19:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tucker JJ, Riggin CN, Connizzo BK, et al. Effect of overuse-induced tendinopathy on tendon healing in a rat supraspinatus repair model. J Orthop Res 2016;34:161-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hosaka Y, Kirisawa R, Yamamoto E, et al. Localization of cytokines in tendinocytes of the superficial digital flexor tendon in the horse. J Vet Med Sci 2002;64:945-947. [DOI] [PubMed] [Google Scholar]
- 62. Jelinsky SA, Rodeo SA, Li J, et al. Regulation of gene expression in human tendinopathy. BMC Musculoskelet Disord 2011;12:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pingel J, Langberg H, Skovgard D, et al. Effects of transdermal estrogen on collagen turnover at rest and in response to exercise in postmenopausal women. J Appl Physiol (1985) 2012;113:1040-1047. [DOI] [PubMed] [Google Scholar]
- 64. Marcos RL, Leal-Junior EC, Arnold G, et al. Low-level laser therapy in collagenase-induced Achilles tendinitis in rats: analyses of biochemical and biomechanical aspects. J Orthop Res 2012;30:1945-1951. [DOI] [PubMed] [Google Scholar]
- 65. Hosaka Y, Kirisawa R, Ueda H, Yamaguchi M, Takehana K. Differences in tumor necrosis factor (TNF)alpha and TNF receptor-1-mediated intracellular signaling factors in normal, inflamed and scar-formed horse tendons. J Vet Med Sci 2005;67:985-991. [DOI] [PubMed] [Google Scholar]
- 66. Thampatty BP, Li H, Im HJ, Wang JH. EP4 receptor regulates collagen type-I, MMP-1, and MMP-3 gene expression in human tendon fibroblasts in response to IL-1 beta treatment. Gene 2007;386:154-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tsuzaki M, Guyton G, Garrett W, et al. IL-1 beta induces COX2, MMP-1, -3 and -13, ADAMTS-4, IL-1 beta and IL-6 in human tendon cells. J Orthop Res 2003;21:256-264. [DOI] [PubMed] [Google Scholar]
- 68. Corps AN, Harrall RL, Curry VA, et al. Ciprofloxacin enhances the stimulation of matrix metalloproteinase 3 expression by interleukin-1beta in human tendon-derived cells. A potential mechanism of fluoroquinolone-induced tendinopathy. Arthritis Rheum 2002;46:3034-3040. [DOI] [PubMed] [Google Scholar]
- 69. Zhang K, Asai S, Yu B, Enomoto-Iwamoto M. IL-1β irreversibly inhibits tenogenic differentiation and alters metabolism in injured tendon-derived progenitor cells in vitro. Biochem Biophys Res Commun 2015;463:667-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Campbell AL, Smith NC, Reilly JH, et al. IL-21 receptor expression in human tendinopathy. Mediators Inflamm 2014;2014:481206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Attia M, Huet E, Gossard C, et al. Early events of overused supraspinatus tendons involve matrix metalloproteinases and EMMPRIN/CD147 in the absence of inflammation. Am J Sports Med 2013;41:908-917. [DOI] [PubMed] [Google Scholar]
- 72. Leumann A, Longino D, Fortuna R, et al. Altered cell metabolism in tissues of the knee joint in a rabbit model of Botulinum toxin A-induced quadriceps muscle weakness. Scand J Med Sci Sports 2012;22:776-782. [DOI] [PubMed] [Google Scholar]
- 73. Wang W, Tang X, Zhang J, Yan X, Ma Y. Complete stress shielding of the Achilles tendon: ultrastructure and level of interleukin-1 and TGF-beta. Orthopedics 2010;33:810. [DOI] [PubMed] [Google Scholar]