Abstract
Objectives
Recently, high failure rates of metal-on-metal (MOM) hip implants have raised concerns of cobalt toxicity. Adverse reactions occur to cobalt nanoparticles (CoNPs) and cobalt ions (Co2+) during wear of MOM hip implants, but the toxic mechanism is not clear.
Methods
To evaluate the protective effect of zinc ions (Zn2+), Balb/3T3 mouse fibroblast cells were pretreated with 50 μM Zn2+ for four hours. The cells were then exposed to different concentrations of CoNPs and Co2+ for four hours, 24 hours and 48 hours. The cell viabilities, reactive oxygen species (ROS) levels, and inflammatory cytokines were measured.
Results
CoNPs and Co2+ can induce the increase of ROS and inflammatory cytokines, such as tumour necrosis factor α (TNF-α), interleukin-1β (IL-1β) and interleukin-6 (IL-6). However, Zn pretreatment can significantly prevent cytotoxicity induced by CoNPs and Co2+, decrease ROS production, and decrease levels of inflammatory cytokines in Balb/3T3 mouse fibroblast cells.
Conclusion
These results suggest that Zn pretreatment can provide protection against inflammation and cytotoxicity induced by CoNPs and Co2+ in Balb/3T3 cells.
Cite this article: Y. Liu, H. Zhu, H. Hong, W. Wang, F. Liu. Can zinc protect cells from the cytotoxic effects of cobalt ions and nanoparticles derived from metal-on-metal joint arthroplasties? Bone Joint Res 2017;6:649–655. DOI: 10.1302/2046-3758.612.BJR-2016-0137.R2.
Keywords: Cobalt cytotoxicity, Protective effects, Zinc, Cobalt nanoparticles, Cobalt ions
Article focus
The use of cobalt-chromium metal-on-metal (MOM) hip arthroplasty causes a new source of cobalt exposure, induced wear particles in nanometric size and metal ions.
Cytotoxicity was observed in cobalt ions (Co2+) and nanoparticles (CoNPs).
The study aimed to investigate whether zinc protects cells from the cytotoxic effects of Co2+ and CoNPs derived from MOM joint arthroplasties.
Key messages
CoNPs and Co2+ can induce dose and time dependent cytotoxicity, increases in reactive oxygen species (ROS) and increased levels of inflammatory cytokines in Balb/3T3 cells
Zn pretreatment can provide protection against CoNPs and Co2+ induced cytotoxicity and inflammation in Balb/3T3 mouse fibroblast cells.
Strengths and limitations
Strengths: this study confirmed that 50 μM Zn could decrease CoNPs- and Co2+- induced cytotoxicity, ROS production and inflammatory cytokines.
Limitations: future research should focus on clinically relevant experimental research in vivo.
Introduction
With the introduction of the metal-on-metal (MOM) bearings to hip resurfacing, the use of cobalt-chromium (Co-Cr) MOM hip arthroplasty has caused a new source of Co exposure.1 Co-containing metal alloy releases a large number of metal debris particles and metal ions.2 Co and Cr ions have been detected in solution during corrosion of metal alloys.3 Furthermore, there is an increase in circulating metal ions in the blood of MOM hip arthroplasty patients,4 which may be exposed to metal for many years post-operatively.
Co nanoparticles (CoNPs) are of special toxicological importance because of the ability of the nanoparticles to directly enter cells and cause subsequent tissue damage.5 Due to the possible risks for human health, the toxic effects of CoNPs and Co ions (Co2+) have attracted widespread attention. However, the precise mechanism of Co-induced toxicity is still unknown. Among the proposed potential mechanisms, reactive oxygen species (ROS) and oxidative stress were thought to induce cytotoxicity and genotoxicity.6-8 ROS are not only a key factor in the apoptosis process but also play an important role in DNA damage and other cellular processes.9 The toxicity of metal oxides may also be dependent on the extent of oxidative stress.10,11 In other words, the cellular redox status and the imbalance between oxygen free radical generation and detoxification can influence the apoptosis process. In addition, CoNPs and Co2+ not only cause cytotoxicity and genotoxicity but also induce an inflammatory response.12,13 Pro-inflammatory cytokines, such as tumour necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and interleukin-6 (IL-6), have been found in the tissue surrounding failed implants.14
Zinc plays an essential role in cell membrane integrity, and it is an important element in more than 70 different enzymes functions, such as cellular metabolism, cell proliferation, apoptosis and defence against free radicals.15 It functions as an antioxidant, as reported in in vitro studies,16-19 through increasing the stability of biomembranes20 and protecting thiol groups from oxidation via complexation.21 Additionally, previous research demonstrated that Zn deficiency caused cellular oxidative stress,22 and that Zn deficiency potentiates the inflammatory response mediated by certain lipids and cytokines in endothelial cells, possibly via mechanisms associated with increased cellular oxidative stress.23 Zn supplementation protects vascular system from oxidative damage.24
Considering the aforementioned properties of Zn, we hypothesised that Zn may hold potential for inhibiting the inflammatory response and cytotoxicity induced by CoNPs and Co2+ in Balb/3T3 mouse fibroblast cells. The present study was carried out to investigate the underlying toxic effects induced by CoNPs and Co2+ and the possible protective effects of Zn pretreatment in Balb/3T3 cells.
Materials and Methods
Chemicals and reagents
Co chloride hexahydrate (CoCl2-6H2O, Puratronic, 99.998% on metal basis, Chemical Abstracts Service No. 7791-13-1) and CoNPs (median size 30 nm) were supplied by Sigma-Aldrich (St Louis, Missouri). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS) and penicillin/streptomycin were purchased from Gibco (Life Technologies, Paisley, United Kingdom). Dichlorofluorescin diacetate (DCFH-DA) was obtained from Sigma-Aldrich. Mouse TNF-α, IL-6 and IL-1β enzyme-linked immunosorbent assay (ELISA) kits were purchased from R&D Systems (Minneapolis, Minnesota). A Cell-Counting Kit 8 (CCK8) was purchased from Dojindo (Kumamoto, Japan).
Preparation of Co nanoparticle and Co2+ solutions
CoCl2-6H2O was dissolved in ultrapure water at 10 mM concentration, and diluted in culture medium to achieve the required working concentrations (1 μM, 5 μM, 10 μM, 50 μM, 100 μM, 500 μM). The CoNPs samples were suspended in ultrapure water at the initial concentration of 1000 μM, sonicated in a sonicator bath at room temperature for 30 minutes at 40 W in order to minimise particle aggregates and finally diluted with complete culture medium to the final working concentrations (1 μM, 5 μM, 10 μM, 50 μM, 100 μM, 500 μM). The freshly prepared stock solution was processed three times by sonication immediately before exposure to cells.
Cell culture and sample treatment
Balb/3T3 mouse fibroblast cells were procured from the China Center for Type Culture Collection (Shanghai, China). Cells were cultured in DMEM medium supplemented with 10% FBS and 100 U/ml penicillin–streptomycin at 5% CO2 and 37°C in a humidified atmosphere. At 85% confluence, cells were harvested by using 0.25% trypsin and were plated into 60 mm dishes or 96-well plates according to the type of experiment. Cells were allowed to attach to the surface for 24 hours prior to treatment. For the dose-related experiments, the cells were exposed to CoNPs and Co2+ for four, 24 and 48 hours. To examine the protective effects of Zn ions (Zn2+) on the viability of cells, the cells were pretreated with 50 μM Zn2+ for four hours and subsequently exposed to varying concentrations of CoNPs and Co2+ for 24 hours. The cells were randomly divided into six groups: control group (Balb/3T3 cells treated with normal medium), CoNPs group, CoNPs + 50μM Zn2+ group, Co2+ group, Co2+ + 50 μM Zn2+ group and 50 μM Zn2+ group.
Cell viability assay
The cytotoxicity of Balb/3T3 cells was analysed by the CCK8 assay according to the manufacturer's protocol. Balb/3T3 cells grown in 96-well culture plates were treated with the agents for various time periods and washed twice with medium. The CCK-8 solution (10 μl) was immediately added to 100 μl of a culture medium per well and the cells were then incubated for an additional 2 hours at 37°C in a humidified atmosphere of 5% CO2 and 95% air. The absorbance of each sample was measured at 450 nm by a microplate reader (Japan Intermed, Tokyo, Japan). The cell viability ratio was expressed as a percentage of the control. Each experimental condition was repeated in triplicate.
ROS measurement
Spectrofluorometry analysis was used to assess ROS generation in cells exposed to target concentrations of CoNPs and Co2+ and the protective effects of Zn2+ pretreatment. For the spectrofluorometry analysis, cells (1 × 105/well) were seeded in 96-well plates. Before the cells were exposed to CoNPs and Co2+ at target concentrations for 24 hours, the cells were pretreated with 50 μM Zn2+ for four hours. After exposure, cells were incubated with DCFH-DA for 30 minutes at 37°C in a 5% CO2 incubator. Following completion of the reaction, the reaction mixture was washed three times by 200 μl of phosphate-buffered saline in each well. The fluorescence intensity of the cell was measured on a multi-well microplate reader (Japan Intermed) using excitation and emission of 485 nm and 525 nm, respectively. Values were expressed as the percentage of fluorescence intensity relative to the control wells.
Detection of cytokines
Cytokines levels were measured by collecting the supernatants from cell cultures at 24 hours after the cells were exposed to Co2+ and CoNPs groups. The concentrations of TNF-α, IL-1β and IL-6 in the culture media were determined using ELISA kits according to the manufacturer’s instructions. Each sample was analysed in triplicate.
In order to evaluate the protective effect of Zn2+ pretreatment, cells were pretreated with 50 μM Zn2+ for four hours. Then, the cells were exposed to different concentrations of Co2+ and CoNPs for 24 hours. Values were collected as described above.
Statistical analysis
This was performed using SPSS 19.0 (IBM Corp, Armonk, New York). Data were analysed by one-way analysis of variance followed by Tukey’s post hoc test. Results shown summarise data from three independent experiments and were represented as the mean ± standard deviation (sd). Differences between groups were considered significant if p-values < 0.05.
Results
Effect of Zn2+ on cell viability in Balb/3T3 cells
As shown in Figure 1, CoNPs and Co2+ induced a concentration- and time-dependent reduction in the viability of Balb/3T3 cells. Whilst 10 μM Co2+ did not significantly affect cell viability at four hours, > 50 μM Co2+ significantly decreased cell viability. Whereas 5 μM CoNPs had no influence on cell viability, > 10 μM induced reduction of cell viability (Fig. 1a). At 48 hours, 50 μM Co2+ had a significant reduction of cell viability and 5 μM CoNPs induced reduction of cell viability (Fig. 1c). The inhibitory concentration 50 values at 24 hours were approximately 50 μM CoNPs and 100 μM Co2+, respectively (Fig.1b). Therefore, those concentration values were used for the subsequent tests. Our results showed that pretreatment with 50 μM Zn exhibited significant protective effects on the cell viability (p < 0.05), which showed 51.36% (sd 5.09) and 67.91% (sd 6.97) increased survival respectively, compared with that in Co2+ and CoNPs treated cells. In addition, pretreatment with 50 μM Zn2+ did not show cytotoxicity and in fact an increase in cell viability was observed (Fig. 2).
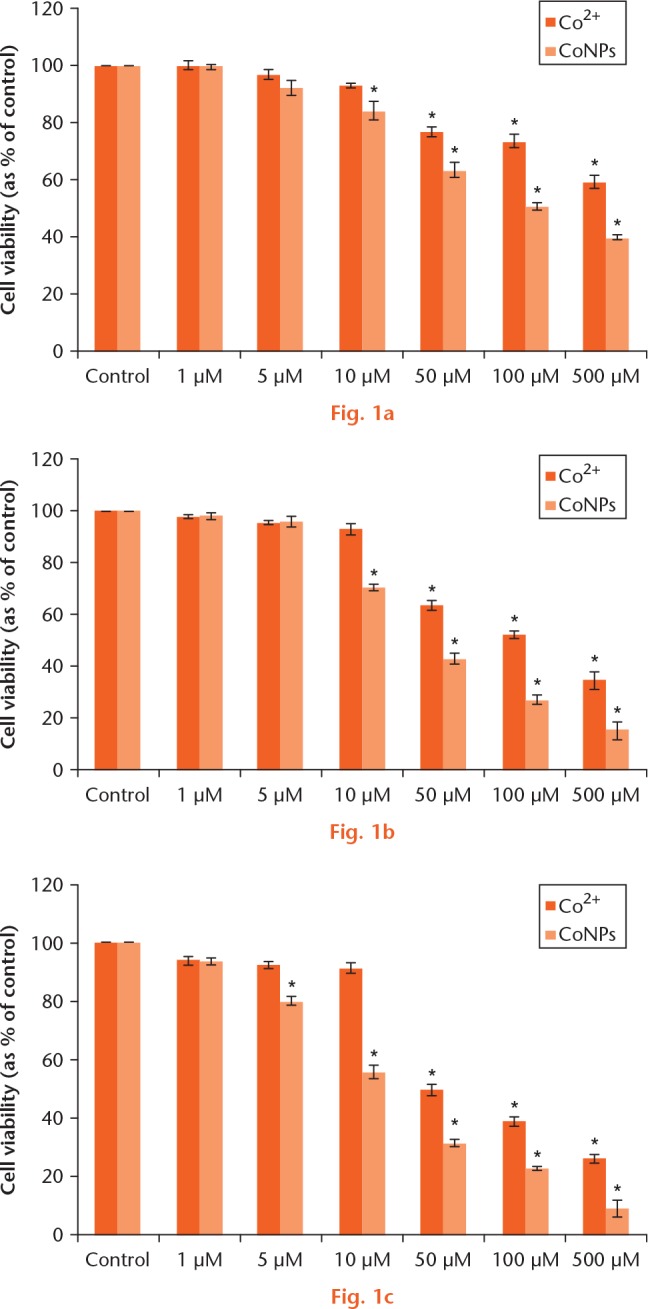
Viability of Balb/3T3 cells exposed to cobalt nanoparticles (CoNPs) and cobalt ions (Co2+) for a) four, b) 24, c) and 48 hours, as determined by Cell-Counting Kit 8 assay. All data were expressed as means ± standard deviation of three independent experiments performed in triplicate. *p < 0.05 compared with the control group.
Fig. 2.
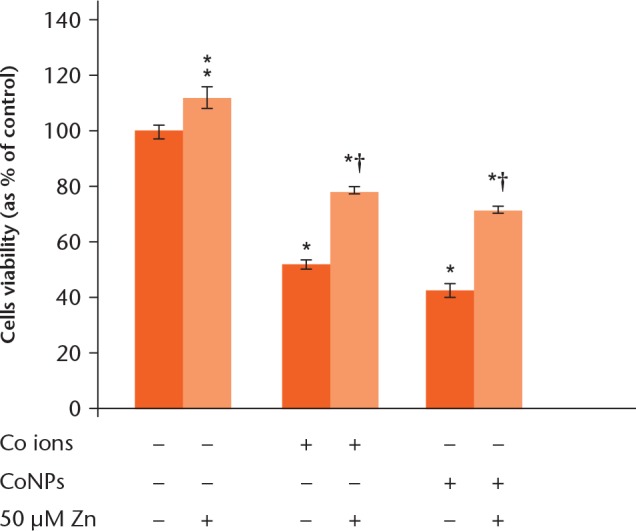
Protective effect of zinc ions (Zn2+) pretreatment against cytotoxicity induced by cobalt nanoparticles (CoNPs) and cobalt ions (Co2+) was measured in Balb/3T3cells. The cells were pretreated with 50 μM Zn2+ for four hours and subsequently exposed to 100 μM CoNPs and 500 μM Co2+for 24 hours. Cells cultured with serum-free medium served as control group. Cell viability was measured by Cell-Counting Kit 8 assay. The values were presented as means ± standard deviation from three independent experiments. *p < 0.05 compared with the control group. †p < 0.05 compared with the relevant treatment group.
ROS formation
ROS production, plotted as a function of intracellular Co content, is shown in Figure 3. An increase of ROS formation dependent on concentration was observed in cells exposed to Co2+ and CoNPs. The formation of ROS induced by CoNPs and Co2+ was prevented by pretreatment with 50 μM Zn in Balb/3T3 cells.
Fig. 3.
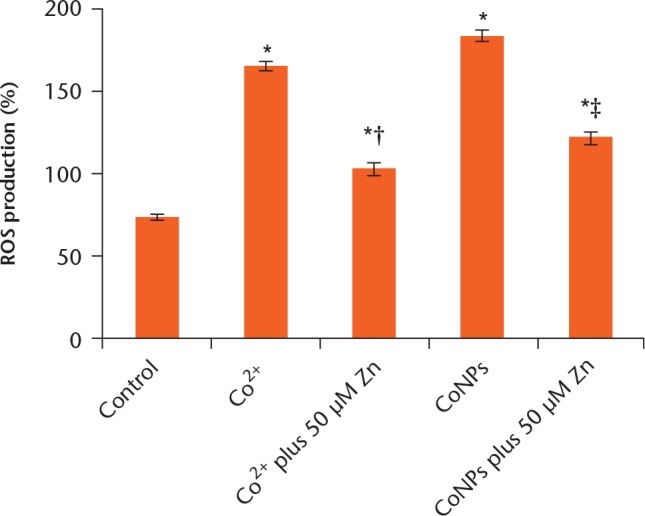
Relative percentage change in the production of reactive oxygen species (ROS) was measured on cobalt ions (Co2+), cobalt nanoparticles (CoNPs) and zinc ions (Zn2+) pretreated groups. Each value represents the mean ± standard deviation of three independent experiments, performed in triple. *p < 0.05 compared with control group. †p < 0.05 compared with Co2+ treated group. ‡p < 0.05 compared with the CoNPs treated group.
Zn inhibited inflammatory cytokines
The results as illustrated in Figure 4 showed that the levels of TNF-α, IL-1β and IL-6 exposed to CoNPs and Co2+ groups were significantly raised as compared with the control group. Pretreatment of cells with 50 μM Zn provided significant protection as compared with the exposed to CoNPs and Co2+ groups. Specifically, Zn pretreatment reduced the level of TNF-α induced by Co2+ and CoNPs by 62.55% (sd 2.71) and 58.22% (sd 5.49), respectively. Zn pretreatment inhibited IL-6 level by 48.41% (sd 3.76) and 55.28% (sd 4.33) and IL-1β levels by 46.97% (sd 3.18) and 54.52% (sd 1.84), compared with that in Co2+ and CoNPs treated cells, respectively.
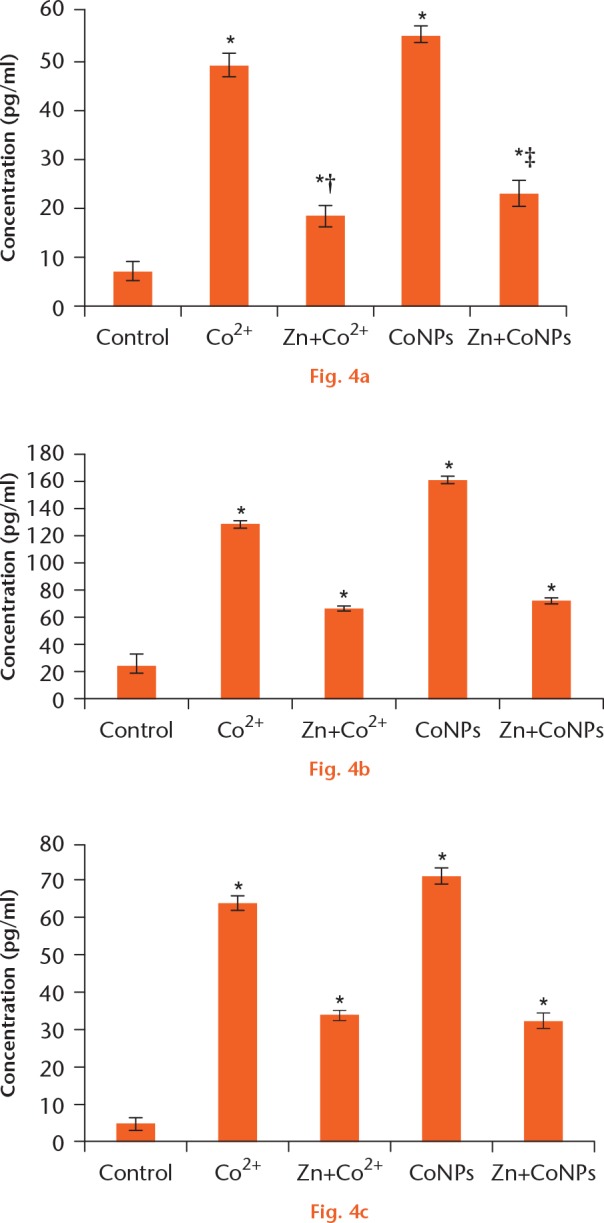
Effect of zinc ions (Zn2+) pretreatment on a) tumour necrosis factor-α (TNF-α), b) interleukin-6 (IL-6) and c) interleukin-1β (IL-1β) levels in Balb/3T3 cells treated with cobalt nanoparticles (CoNPs) and cobalt ions (Co2+). Balb/3T3 cells were pretreated with 50 μM Zn2+ for four hours, followed by treatment with 100 μM Co2+ and 50 μM CoNPs for 24 hours. Data were expressed as the mean ± standard deviation. *p < 0.05 compared with the control group. †p < 0.05 compared with the Co2+ treated group. ‡p < 0.05 compared with the CoNPs treated group.
Discussion
Wear debris from orthopaedic patients with MOM bearing surfaces is produced by mechanical wear, surface corrosion, or both. It contains insoluble CoNPs and Co2+, which may lead to prosthesis failure and adverse effects. Daniel et al25 demonstrated that in resurfacing arthroplasty patients, mean whole blood Co and Cr concentrations were 1.26 μ/L and 2.41 μ/L at one year and 1.17 μ/L and 1.11 μ/L at six years, respectively. However, the molecular mechanisms involved in cytotoxicity induced by CoNPs and Co2+ remains to be elucidated. In the present study, we have focused on the mechanisms of the adverse effects and evaluated the protective effect of Zn pretreatment on CoNPs and Co2+ cytotoxicity on Balb/3T3 cells.
In this study, the CCK8 assay was used to assess Balb/3T3 cells viability, exposed to CoNPs and Co2+. The results showed concentration and time-dependent decreases in absorbance in the CoNPs and Co2+ groups when compared with control cells. These results correspond well with those of previous studies,1,19,26,27 and CoNPs were more cytotoxic than Co2+. It is suggested that Co2+ firstly saturates the binding sites of molecules in the extracellular milieu and on the cell surface, becoming unavailable to enter cells; after saturation, Co2+ are actively transported inside cells.28 The cytotoxicity of nanoparticles is likely to be mediated by the nanoparticles themselves rather than by their corrosion into ions in the culture medium.28
CoNPs and Co2+ induced a concentration-dependent increase in ROS, as previously shown with Co oxide nanoparticles.6 ROS can cause DNA damage, oxidative stress and activate other cellular processes.9 Oxidative stress has been proposed to play a key role in the mechanism of toxicity for a number of nanoparticles, through either the excessive generation of ROS or depletion of cellular antioxidant capacity.6 On the one hand, ROS function as homeostatic signaling molecules that regulate cell growth, and on the other hand, as adaptation responses at physiological concentrations. However, ROS production can result in cellular injury and death at higher concentrations. In the present study, Zn2+ pretreatment significantly decreased ROS production compared with exposure to CoNPs and Co2+ alone. The cytotoxic effect of 100 μM of Co2+ and 50 μM CoNPs were inhibited by pretreatment of cells with 50 μM Zn2+. Gürbay19 reported the preventive effect of Zn2+ against cytotoxicity of Co2+ and Zn deficiency increased chromium(IV)-induced cytotoxicity.29 It has been known that intracellular zinc levels influence cell survival.30 Furthermore, Zn is an essential component of numerous proteins involved in the defense against oxidative stress.17 Present results, along with our previous study, verify that Zn pretreatment is important for protection against Co-induced cytotoxicity.31
Previous studies have shown that periprosthetic Co-Cr wear particles induced diverse pro-inflammatory cytokines release, such as IL-6, IL-1β and TNF-α.32-34 The increased pro-inflammatory responses stimulated the cell death process. This study confirms the pro-inflammatory character of Co2+ and CoNPs by an increase in TNF-α, IL-1β and IL-6. IL-1β is a component of the inflammasome and is processed by active caspase-1 cleaving the precursor IL-1β.35 The effects of chronic inflammation include induction of oxidative stress,36 while oxidative stress can induce inflammatory cytokines.37 Thus, there is a vicious cycle among inflammation, oxidative stress, and induction of toxic inflammatory cytokines. Zn pretreatment led to a significant decrease in the number of inflammatory cytokines induced by Co2+and CoNPs that were released after 24 hours of exposure. Zn deficiency in diabetic patients increased cardiovascular events,38 which may be related to the increased inflammatory response in the renal system and vascular systems.39,40 Moreover, the generation of inflammatory cytokines is increased as a result of zinc deficiency.41 In the current study, we determined whether Zn pretreatment can reduce increasing inflammatory cytokines levels induced by CoNPs and Co2+. Zn uniquely functions not only as an antioxidant but also as an anti-inflammatory agent. In this study, elevated levels of IL-6, IL-1β and TNF-α in the Balb/3T3 cells exposed to 50 μM CoNPs and 100 μM Co2+ were significantly decreased by pretreatment with 50 μM Zn for four hours, which suggested that Zn can protect against inflammatory cytokines of Balb/3T3 cells induced by 50 μM CoNPs and 100 μM Co2+.
Zn has been shown to have antioxidant, anti-inflammatory and membrane-stabilising properties. Zinc’s function as an antioxidant involves two different mechanisms: 1) the protection of sulphydryl groups of proteins against free radical attack and 2) the reduction of free radical formation through the antagonism of redox-active transition metals, such as iron and copper.42 Evidence shows that cellular oxidative stress is markedly induced by exposure to linoleic acid and that this oxidative stress can be partially blocked by Zn.43 Research indicates that Zn is a potent inhibitor of apoptosis44 and that Zn deficiency can induce apoptosis.45 Recently, it was reported that the protective effects of Zn may occur through inhibition of caspases, such as caspase-3,46 and methollthionein (MT) expression.47 MT is a cysteine-rich metal-binding protein that has several biological roles including antioxidant properties. It is shown that Co can displace intracellular Zn2+ in cells exposed to Co2+ and CoNPs, which decreases the intracellular Zn2+ level.28 Thus, the replacement of essential divalent cations might contribute to the cytotoxicity induced by Co particles.48
In the present study, we showed that Co2+ and CoNPs had concentration- and time-dependent cytotoxicity in Balb/3T3 cells, and that Zn pretreatment could reduce the cytotoxicity, ROS production, and levels of TNF-α, IL-1β, and IL-6, which suggested that Zn2+ pretreatment has a protective effect. Our study provided a new insight into the roles of Zn pretreatment in ameliorating cytotoxicity and aseptic inflammation induced by CoNPs and Co2+. Further research into the functional roles of Zn and metal ions in tissue damage due to MOM arthroplasty corrosion should focus on mitigating the toxic effects of these proceses.
Acknowledgments
This work is supported by Jiangsu Province Natural Science Foundation of China (No. BK20150399) and Technology Innovation Programme of Nantong University (No. YKC16060).
Footnotes
Author Contribution: Y. Liu: Designing the study, Collecting the data, Analysing and interpreting the data, Drafting the manuscript.
H. Zhu: Collecting the data, Analysing and interpreting the data, Drafting the manuscript.
H. Hong: Analysing the data, Preparing the data.
W. Wang: Inception of the study, Preparing the paper.
F. Liu: Designing the study, Analysing and interpreting the data, Critically revising the paper.
*Y. Liu and H. Zhu contributed to this paper equally.
Conflicts of Interest Statement: The authors declare that there are no conflicts of interest.
Funding Statement
This work is supported by Jiangsu Province Natural Science Foundation of China (No. BK20150399) and Technology Innovation Programme of Nantong University (No. YKC16060).
References
- 1. Kwon YM, Xia Z, Glyn-Jones S, et al. Dose-dependent cytotoxicity of clinically relevant cobalt nanoparticles and ions on macrophages in vitro. Biomed Mater 2009;4:025018. [DOI] [PubMed] [Google Scholar]
- 2. Jacobs JJ, Hallab NJ, Skipor AK, Urban RM. Metal degradation products: a cause for concern in metal-metal bearings? Clin Orthop Relat Res 2003;417:139-147. [DOI] [PubMed] [Google Scholar]
- 3. Germain MA, Hatton A, Williams S, et al. Comparison of the cytotoxicity of clinically relevant cobalt-chromium and alumina ceramic wear particles in vitro. Biomaterials 2003;24:469-479. [DOI] [PubMed] [Google Scholar]
- 4. Firkins PJ, Tipper JL, Ingham E, et al. A novel low wearing differential hardness, ceramic-on-metal hip joint prosthesis. J Biomech 2001;34:1291-1298. [DOI] [PubMed] [Google Scholar]
- 5. Gill HS, Grammatopoulos G, Adshead S, Tsialogiannis E, Tsiridis E. Molecular and immune toxicity of CoCr nanoparticles in MoM hip arthroplasty. Trends Mol Med 2012;18:145-155. [DOI] [PubMed] [Google Scholar]
- 6. Alarifi S, Ali D, Y AO, et al. Oxidative stress contributes to cobalt oxide nanoparticles-induced cytotoxicity and DNA damage in human hepatocarcinoma cells. Int J Nanomedicine 2013;8:189-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nyga A, Hart A, Tetley TD. Importance of the HIF pathway in cobalt nanoparticle-induced cytotoxicity and inflammation in human macrophages. Nanotoxicology 2015;9:905-917. [DOI] [PubMed] [Google Scholar]
- 8. Raghunathan VK, Devey M, Hawkins S, et al. Influence of particle size and reactive oxygen species on cobalt chrome nanoparticle-mediated genotoxicity. Biomaterials 2013;34:3559-3570. [DOI] [PubMed] [Google Scholar]
- 9. Patlolla A, Patlolla B, Tchounwou P. Evaluation of cell viability, DNA damage, and cell death in normal human dermal fibroblast cells induced by functionalized multiwalled carbon nanotube. Mol Cell Biochem 2010;338:225-232. [DOI] [PubMed] [Google Scholar]
- 10. Kim S, Choi JE, Choi J, et al. Oxidative stress-dependent toxicity of silver nanoparticles in human hepatoma cells. Toxicol In Vitro 2009;23:1076-1084. [DOI] [PubMed] [Google Scholar]
- 11. Park EJ, Yi J, Chung KH, et al. Oxidative stress and apoptosis induced by titanium dioxide nanoparticles in cultured BEAS-2B cells. Toxicol Lett 2008;180:222-229. [DOI] [PubMed] [Google Scholar]
- 12. Alinovi R, Goldoni M, Pinelli S, et al. Oxidative and pro-inflammatory effects of cobalt and titanium oxide nanoparticles on aortic and venous endothelial cells. Toxicol In Vitro 2015;29:426-437. [DOI] [PubMed] [Google Scholar]
- 13. Caicedo MS, Pennekamp PH, McAllister K, Jacobs JJ, Hallab NJ. Soluble ions more than particulate cobalt-alloy implant debris induce monocyte costimulatory molecule expression and release of proinflammatory cytokines critical to metal-induced lymphocyte reactivity. J Biomed Mater Res A 2010;93:1312-1321. [DOI] [PubMed] [Google Scholar]
- 14. Goodman SB, Huie P, Song Y, et al. Cellular profile and cytokine production at prosthetic interfaces. Study of tissues retrieved from revised hip and knee replacements. J Bone Joint Surg [Br] 1998;80-B:531-539. [DOI] [PubMed] [Google Scholar]
- 15. Valko M, Morris H, Cronin MTD. Metals, toxicity and oxidative stress. Curr Med Chem 2005;12:1161-1208. [DOI] [PubMed] [Google Scholar]
- 16. Iqbal M, Noor R, Mizuno R, Okada S. Protective role of zinc-metallothionein (Zn-MT) in iron nitrilotriacetate (Fe-NTA)-induced renal oxidative damage. Redox report: communications in free radical research. Redox Rep 2003;8:163-167. [DOI] [PubMed] [Google Scholar]
- 17. Prasad AS. Zinc: an antioxidant and anti-inflammatory agent: role of zinc in degenerative disorders of aging. J Trace Elem Med Biol 2014;28:364-371. [DOI] [PubMed] [Google Scholar]
- 18. Akita K, Okamura H, Yoshida K, et al. Cobalt chloride induces apoptosis and zinc chloride suppresses cobalt-induced apoptosis by Bcl-2 expression in human submandibular gland HSG cells. Int J Oncol 2007;31:923-929. [PubMed] [Google Scholar]
- 19. Gürbay A. Protective effect of zinc chloride against cobalt chloride-induced cytotoxicity on vero cells: preliminary results. Biol Trace Elem Res 2012;148:110-116. [DOI] [PubMed] [Google Scholar]
- 20. Verstraeten SV, Zago MP, MacKenzie GG, Keen CL, Oteiza PI. Influence of zinc deficiency on cell-membrane fluidity in Jurkat, 3T3 and IMR-32 cells. Biochem J 2004;378:579-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Powell SR. The antioxidant properties of zinc. J Nutr 2000;130(5S Suppl):1447S-54S. [DOI] [PubMed] [Google Scholar]
- 22. Oteiza PI, Clegg MS, Zago MP, Keen CL. Zinc deficiency induces oxidative stress and AP-1 activation in 3T3 cells. Free Radic Biol Med 2000;28:1091-1099. [DOI] [PubMed] [Google Scholar]
- 23. Meerarani P, Ramadass P, Toborek M, et al. Zinc protects against apoptosis of endothelial cells induced by linoleic acid and tumor necrosis factor alpha. Am J Clin Nutr 2000;71:81-87. [DOI] [PubMed] [Google Scholar]
- 24. Jenner A, Ren M, Rajendran R, et al. Zinc supplementation inhibits lipid peroxidation and the development of atherosclerosis in rabbits fed a high cholesterol diet. Free Radic Biol Med 2007;42:559-566. [DOI] [PubMed] [Google Scholar]
- 25. Daniel J, Ziaee H, Pradhan C, McMinn DJ. Six-year results of a prospective study of metal ion levels in young patients with metal-on-metal hip resurfacings. J Bone Joint Surg [Br] 2009;91-B:176-179. [DOI] [PubMed] [Google Scholar]
- 26. Ponti J, Sabbioni E, Munaro B, et al. Genotoxicity and morphological transformation induced by cobalt nanoparticles and cobalt chloride: an in vitro study in Balb/3T3 mouse fibroblasts. Mutagenesis 2009;24:439-445. [DOI] [PubMed] [Google Scholar]
- 27. Behl B, Papageorgiou I, Brown C, et al. Biological effects of cobalt-chromium nanoparticles and ions on dural fibroblasts and dural epithelial cells. Biomaterials 2013;34:3547-3558. [DOI] [PubMed] [Google Scholar]
- 28. Sabbioni E, Fortaner S, Farina M, et al. Cytotoxicity and morphological transforming potential of cobalt nanoparticles, microparticles and ions in Balb/3T3 mouse fibroblasts: an in vitro model. Nanotoxicology 2014;8:455-464. [DOI] [PubMed] [Google Scholar]
- 29. Kimura T, Onodera A, Okumura F, Nakanishi T, Itoh N. Chromium (VI)-induced transformation is enhanced by Zn deficiency in BALB/c 3T3 cells. J Toxicol Sci 2015;40:383-387. [DOI] [PubMed] [Google Scholar]
- 30. Pulido MD, Parrish AR. Metal-induced apoptosis: mechanisms. Mutat Res 2003;533:227-241. [DOI] [PubMed] [Google Scholar]
- 31. Zhu H, Liu Y, Hong H, Wang W, Liu F. Protective effects of Zn(2+) against cobalt nanoparticles and cobalt chloride-induced cytotoxicity of RAW 264.7cells via ROS pathway. Biochem Biophys Res Commun 2017;486:357-363. [DOI] [PubMed] [Google Scholar]
- 32. Thomas V, Halloran BA, Ambalavanan N, Catledge SA, Vohra YK. In vitro studies on the effect of particle size on macrophage responses to nanodiamond wear debris. Acta Biomater 2012;8:1939-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hallab NJ, Jacobs JJ. Biologic effects of implant debris. Bull NYU Hosp Jt Dis 2009;67:182-188. [PubMed] [Google Scholar]
- 34. Kaufman AM, Alabre CI, Rubash HE, Shanbhag AS. Human macrophage response to UHMWPE, TiAlV, CoCr, and alumina particles: analysis of multiple cytokines using protein arrays. J Biomed Mater Res A 2008;84:464-474. [DOI] [PubMed] [Google Scholar]
- 35. Lamkanfi M, Kanneganti TD, Franchi L, Núñez G. Caspase-1 inflammasomes in infection and inflammation. J Leukoc Biol 2007;82:220-225. [DOI] [PubMed] [Google Scholar]
- 36. Simon DI. Inflammation and vascular injury: basic discovery to drug development. Circ J 2012;76:1811-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Prasad AS, Bao B, Beck FW, Sarkar FH. Zinc-suppressed inflammatory cytokines by induction of A20-mediated inhibition of nuclear factor-κB. Nutrition 2011;27:816-823. [DOI] [PubMed] [Google Scholar]
- 38. Soinio M, Marniemi J, Laakso M, et al. Serum zinc level and coronary heart disease events in patients with type 2 diabetes. Diabetes Care 2007;30:523-528. [DOI] [PubMed] [Google Scholar]
- 39. Tomat AL, Costa MdeL, Arranz CT. Zinc restriction during different periods of life: influence in renal and cardiovascular diseases. Nutrition 2011;27:392-398. [DOI] [PubMed] [Google Scholar]
- 40. de Oliveira Otto MC, Alonso A, Lee DH, et al. Dietary intakes of zinc and heme iron from red meat, but not from other sources, are associated with greater risk of metabolic syndrome and cardiovascular disease. J Nutr 2012;142:526-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Prasad AS, Beck FW, Bao B, et al. Zinc supplementation decreases incidence of infections in the elderly: effect of zinc on generation of cytokines and oxidative stress. Am J Clin Nutr 2007;85:837-844. [DOI] [PubMed] [Google Scholar]
- 42. Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011;283:65-87. [DOI] [PubMed] [Google Scholar]
- 43. Hennig B, Meerarani P, Toborek M, McClain CJ. Antioxidant-like properties of zinc in activated endothelial cells. J Am Coll Nutr 1999;18:152-158. [DOI] [PubMed] [Google Scholar]
- 44. Hennig B, Toborek M, Mcclain CJ. Antiatherogenic properties of zinc: implications in endothelial cell metabolism. Nutrition 1996;12:711-717. [DOI] [PubMed] [Google Scholar]
- 45. Shankar AH, Prasad AS. Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr 1998;68(suppl):447S-463S. [DOI] [PubMed] [Google Scholar]
- 46. Aiuchi T, Mihara S, Nakaya M, et al. Zinc ions prevent processing of caspase-3 during apoptosis induced by geranylgeraniol in HL-60 cells. J Biochem 1998;124:300-303. [DOI] [PubMed] [Google Scholar]
- 47. Miao X, Wang Y, Sun J, et al. Zinc protects against diabetes-induced pathogenic changes in the aorta: roles of metallothionein and nuclear factor (erythroid-derived 2)-like 2. Cardiovasc Diabetol 2013;12:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Moulis JM. Cellular mechanisms of cadmium toxicity related to the homeostasis of essential metals. Biometals 2010;23:877-896. [DOI] [PubMed] [Google Scholar]


