Abstract
Objective
Clinical trials have long been considered the ‘gold standard’ of research generated evidence in health care. Patient recruitment is an important determinant in the success of the trials, yet little focus is placed on the decision making process of patients towards recruitment. Our objective was to identify the key factors pertaining to patient participation in clinical trials, to better understand the identified low participation rate of patients in one clinical research facility within Ireland.
Design
Narrative literature review of studies focussing on factors which may act to facilitate or deter patient participation in clinical trials. Studies were identified from Medline, PubMed, Cochrane Library and CINAHL.
Results
Sixty-one studies were included in the narrative review: Forty-eight of these papers focused specifically on the patient's perspective of participating in clinical trials. The remaining thirteen related to carers, family and health care professional perspectives of participation. The primary factor influencing participation in clinical trials amongst patients was related to personal factors and these were collectively associated with obtaining a form of personal gain through participation. Cancer was identified as the leading disease entity included in clinical trials followed by HIV and cardiovascular disease.
Conclusion
The vast majority of literature relating to participation in clinical trials emanates predominantly from high income countries, with 63% originating from the USA. No studies for inclusion in this review were identified from low income or developing countries and therefore limits the generalizability of the influencing factors.
Keywords: Clinical trials, Participation, Recruitment, Patient factors, Physician factors
Abbreviations: ICH GCP, International Conference on Harmonisation Guidelines for Good Clinical Practice; GRACE, Gender Race And Clinical Experience; RCT, Randomized Control Trials; USA, United States of America
1. Introduction
Clinical trials, in which humans are prospectively assigned to one or more health-related interventions to evaluate the effects on health outcomes have long been considered the ‘gold standard’ of research generated evidence in health care. Clinical trials in key disease groups including the effectiveness of prevention strategies, early detection and treatment interventions for cancer [1], and the diagnosis, treatment, and prevention of chronic diseases [2] are among the most common areas where clinical trials are undertaken. During 2013–2014, one clinical research facility within Ireland had a total of fifty-six such trials in progress with a patient uptake of 291. The geographical region in which the clinical research facility is located has a catchment of 1,000,000 people with approximately 250,000 patients being treated in the associated hospitals per year. Thus the number of participants involved in clinical trials at this facility represented less than half of one percent of the patient population being treated. The extremely small numbers of clinical trial participants at this centre raised questions as to the potential factors which may be acting as deterrents to patient participation in clinical trials, and how such factors may be addressed to improve participation rates.
To address this issue and aid the sites in developing more effective recruitment procedures that encourage participation in clinical trials [3], a limited narrative literature review was undertaken to identify factors influencing the participation of patients in clinical trials.
2. Methods
2.1. Search strategy
A pragmatic approach to conducting the literature search was adopted influenced by an absence of research funding and limited time in which to undertake the study. A search on the three most appropriate key search terms; i) patient participation ii) clinical trials and iii) factors was undertaken. Search limitations were i) English language, ii) humans and iii) publication period – with publications between 2003 and 2014 being included in the review and iv) restriction to free full text availability - this restriction related to an absence of funding for the review. Databases searched included Medline, PubMed, Cochrane Library and CINAHL. These four databases were selected as they were the databases considered most appropriate to this study.
This phase of the search identified a total of 429 potential publications. Following initial review of all titles and abstracts of identified publications by researcher one (EW), 100 were identified as relevant to this study based on the following inclusion criteria; i) study identifiable as a clinical trial; participants were human adults over 18 years of age; papers identified factors which had potential to influence participation of patients in clinical trials.
2.2. Data extraction and management
Following initial review, a total of 100 publications were identified as potentially relevant for inclusion based on the criteria outlined above. The 100 articles were read in full and their relevance assessed, resulting in a total of sixty-one papers being identified as relevant for inclusion in this review. A total of thirty nine papers were excluded; twelve were identified as duplicates, eighteen examined participants who did not meet eligibility criteria relating to age; seven were not related to clinical trials; one examined a pattern of participation but was of no relevance and one was a published pilot study. The sixty-one articles selected for inclusion represented clinical trials in a variety of clinical settings utilising methods to increase patient recruitment onto healthcare studies and hypothetical trials. Forty-eight of these papers focused specifically on the patient's perspective of participating in clinical trials, including twelve randomized control trials (RCT) or hypothetical trials. The remaining thirteen related to carers, family and health care professional perspectives of participation.
3. Results
The vast majority of papers identified, 63% (n = 39), emanated from the United States of America (USA). Other countries represented included Asia, Australia, Canada, Europe and South America. A total of eight papers, representing 9% of the total identified, originated from Europe and of these, the majority (n = 6) were undertaken in the United Kingdom (UK). While no studies relating to participation in clinical trials conducted within an Irish context were identified, the past decade in Ireland has witnessed increasing emphasis in funding health research with the Irish Health Research Board, the principal funding body for health research, investing €100 million in building clinical research infrastructure with the majority of this infrastructure focused on undertaking clinical trials [4]. Thus the timing of this paper is relevant in contributing to a better understanding of the factors which contribute to patient participation in clinical trials.
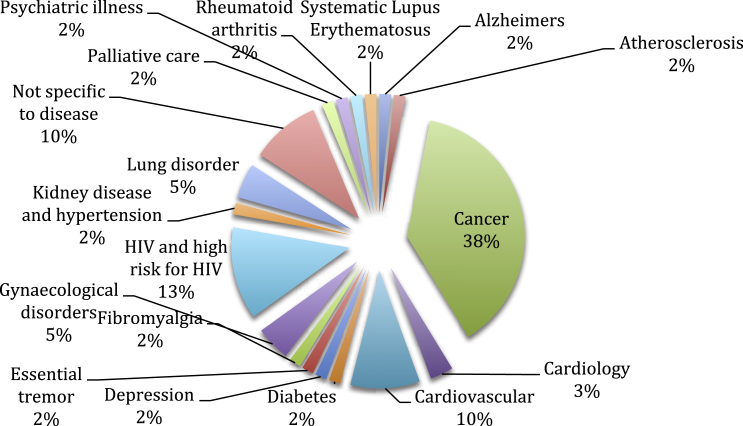
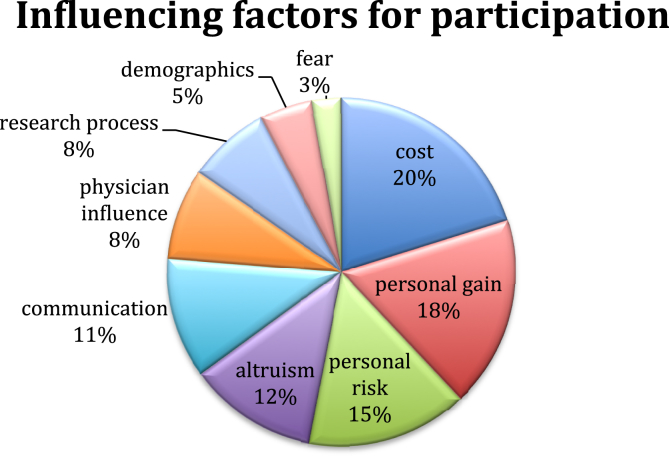
Cancer was identified as the leading disease entity included in clinical trials followed by HIV and cardiovascular disease. The distribution of disease range for the identified studies is demonstrated in Fig. 1. The review identified the key factors pertaining to patient participation in clinical trials and the primary factor associated with a decision to participate in a clinical trial related to personal factors. Secondary factors identified were cost, altruism, communication, physician influence, research process and demographics – see Fig. 2. These key factors identified form the basis of the remainder of this paper.
Fig. 1.
Distribution of disease range for all studies.
Fig. 2.
Key factors pertaining to patient participation.
3.1. Personal factors
The primary reasons that people committed to participating in a clinical trial were personal and critical to this was an expectation of some form of personal gain. However, as White and Hardy [5] identify, perceived personal gain is one among a number of motivational factors which together influence participation. Perceived personal gain, rather than being the singular influencing factor is likely mediated by other factors including the perception of personal risk and fear. As identified above, no studies conducted in Ireland were identified in this search. While the Irish health system differs significantly to that of the USA, other European countries and to a lesser extent that of the UK, and that these structural and funding differences are likely to impact participation of patients in clinical trials, it is also likely that significant similarities in terms of the factors which both encourage and deter clinical trial participation exist, particularly in areas of personal and altruistic motivation.
3.2. Personal gain
A total of 12 studies identified that clinical trial participants perceived they received better care and extra attention when partaking in clinical trials [6], [7], [8], [9], [10], [11] (see Table 1). The perception that participating in a clinical trial results in better care and extra attention is likely to be related to the nature and frequency of interaction with trial procedures which require stringent surveillance and monitoring to ensure adherence to trial protocols [12] as well as access to new treatments which, from the participants perspective, may be potentially more beneficial than existing treatments. Within oncology settings, while there is recognition that the chances of obtaining a personal cure are minimal, the belief that taking part in the clinical trial may result in eventual benefits to others, provides participants with a sense of purpose and hope [6], [8], [13]. Furthermore, exercising and maintaining personal autonomy [14] by having a say in care, an opportunity to improve the quality of their life with the concomitant impact on lifestyle [15] have also been identified as influential.
Table 1.
Influencing and deterring factors for clinical trial participation.
| Participation | |
|---|---|
| Factors | Studies |
| Perceived better care | Carroll et al., 2012; Meneguin and Cesar, 2012; Tallon et al., 2011; Truong et al., 2011; Wang et al., 2011; Voytek, Jones and Metzger, 2011; Biedrzycki, 2010; White and Hardy, 2010; Kasner et al., 2009; Costenbader et al., 2007; Sharp et al., 2006, Zullino et al., 2003 |
| Improve personal health | Brubaker et al., 2013; Squires et al., 2013; Biedrzycki, 2010; Houlihan et al., 2010; Udrea et al., 2009; Gadegbeku et al., 2008; Davison et al., 2007; Townsley et al., 2006; Borrayo, Lawsin and Coit, 2005 |
| Personal benefits | Meneguin and Cesar, 2012; Voytek, Jones and Metzger, 2011; Biedrzycki, 2010; White and Hardy, 2010; Costenbader et al., 2007 |
| Physician influence | Brubaker et al., 2013; Sprague et al., 2013; Lee et al., 2012; Meneguin and Cesar, 2012, McMahon et al., 2011; Tallon et al., 2011; Houlihan et al., 2010; Kohara and Inoue, 2010; Sabesan et al., 2010; Kinder et al., 2010; Costenbader et al., 2007; Townsley et al., 2006; Borrayo, Lawsin and Coit, 2005; Sahay et al., 2004; Brown and Topcu, 2003; Zullino et al., 2003 |
| Altruism | Brubaker et al., 2013; Squires et al., 2013; Chakrapani et al., 2012; Meneguin and Cesar, 2012; Rooney et al., 2011; Tallon et al., 2011; Troung et al., 2011; Sabesan et al., 2010; Kasner et al., 2009; Udrea et al., 2009; Gadegbeku et al., 2008; Moutsiakis and Chin, 2007; Townsley et al., 2006; Sharp et al., 2006 |
| Fight Stigma | Chakrapani et al., 2012 |
| Physician recommendation | Houlihan et al., 2010; Kinder et al., 2010; Costenbader et al., 2007 Sahay et al., 2004; Brown and Topcu, 2003 |
| Relevant adequate information | Biedrzycki, 2010 |
| Insurance cover | Sahay et al., 2004; Brown and Topcu, 2003 |
| Financial reward/incentive/cost | Brubaker et al., 2013; Carroll et al., 2012; Lee et al., 2012; Darnell, McGuire and Danner, 2011; Menezes et al., 2011; Rooney et al., 2011; Voytek, Jones and Metzger, 2011; Holman et al., 2010; Kasner et al., 2009; Udrea et al., 2009, Costenbader et al., 2007; Baquet et al., 2006; Sahay et al., 2005; Tu et al., 2005; Brown and Topcu, 2003; Zullino et al., 2003 |
| Medical cost | White and Hardy, 2010; Udrea et al., 2009; Zullino et al., 2003 |
| Flexibility | Rooney et al., 2011; Tallon et al., 2011, Sharp et al., 2006 |
| Transport provided | Baquet et al., 2006 |
| Information on newsletter | Udrea et al., 2009; Townsley et al., 2006; Sahay et al., 2005 |
| Relevant language | Rooney et al., 2011; Tu et al., 2005 |
| Non participation | |
|---|---|
| Factors | Studies |
| Perceived personal risk | Brubaker et al., 2013; Martin et al., 2013; Chakrapani et al., 2012; McMahon et al., 2011, Rooney et al., 2011; Tallon et al., 2011; Voytek, Jones and Metzger, 2011; Biedrzycki, 2010; Kinder et al., 2010; White and Hardy, 2010; Udrea et al., 2009; Gadegbeku et al., 2008; Moutsiakis and Chin, 2007; Costenbader et al., 2007; Hussain-Gambles, Atkin and Leese, 2006; Costenbader et al., 2005; Zullino et al., 2003 |
| Adverse effects of treatment | Brubaker et al., 2013, Wang et al., 2011; Sahay et al., 2005 |
| Guinea pig/Lab Rat | McMahon et al., 2011; Wang et al., 2011; Sabesan et al., 2010; Gadegbeku et al., 2008; Costenbader et al., 2005; Tu et al., 2005; Zullino et al., 2003 |
| Cost | McMahon et al., 2011; Kinder et al., 2010; Gadegbeku et al., 2008; Hussain-Gambles, Atkin and Leese, 2006; Sharp et al., 2006 |
| Time commitment/cost | Brubaker et al., 2013; Carroll et al., 2012; Lee et al., 2012; Houlihan et al., 2011; McMahon et al., 2011; Rooney et al., 2011; Kinder et al., 2010; Kasner et al., 2009; Gadegbeku et al., 2008; Baquet et al., 2006; Sharp et al., 2006; Zullino et al., 2003 |
| Stigma | Chakrapani et al., 2012; Rooney et al., 2011 |
| Questionnaires | Houlihan et al., 2010; Tallon et al., 2011 |
| Research process | Martin et al., 2013; McMahon et al., 2011; Houlihan et al., 2010; Kinder et al., 2010; White and Hardy, 2010; Kasner et al., 2009; Costenbader et al., 2007 |
| Allocation of placebo/randomisation | Martin et al., 2013; Brubaker et al., 2013; Kinder et al., 2010; Kasner et al., 2009, udrea |
| Fear | Chakrapani et al., 2012; Kasner et al., 2009; Sahay et al., 2004; Zullino et al., 2003 |
| Side effects | Brubaker et al., 2013; Martin et al., 2013; Chakrapani et al., 2012; McMahon et al., 2011, Rooney et al., 2011; Tallon et al., 2011; Voytek, Jones and Metzger, 2011; Biedrzycki, 2010; Kinder et al., 2010; White and Hardy, 2010; Udrea et al., 2009; Gadegbeku et al., 2008; Moutsiakis and Chin, 2007; Costenbader et al., 2007; Hussain-Gambles, Atkin and Leese, 2006; Costenbader et al., 2005; Zullino et al., 2003 |
| Signing life away | Darnell, McGuire and Danner, 2011 |
| Decreased quality of life | McMahon et al., 2011; Sabesan et al., 2010 |
| Language barriers | Rooney et al., 2011; Cooke et al., 2010; Hussain-Gambles, Atkin and Leese, 2006; Borrayo, Lawsin and Coit, 2005; Tu et al., 2005 |
| Lack of understanding | Townsley et al., 2006; Smith et al. (2007) |
| Misconceptions | Chakrapani et al., 2012; Dhalla and Poole, 2011; Darnell, McGuire and Danner, 2011; Sabesan et al., 2010; Borrayo, Lawsin and Coit, 2005; Tu et al., 2005 |
| Work absence | Kinder et al. 2010; Sharp et al., 2006 |
| Severe treatment/unconfirmed treatment | Brubaker et al., 2013; Chakrapani et al., 2012; Lee et al., 2012; Menezes et al., 2011; Sabesan et al., 2010; Moutsiakis and Chin, 2007; Sahay et al., 2005 |
| Arrange transport | Martin et al., 2013; Kinder et al., 2010 |
| Unconfirmed treatment/severity of treatment | Brubaker et al., 2013; Chakrapani et al., 2012; Lee et al., 2012; Sabesan et al., 2010; Moutsiakis and Chin, 2007; Borrayo, Lawsin and Coit, 2005; Sahay et al., 2005 |
| Taking medication | Carroll et al., 2012; Borrayo, Lawsin and Coit, 2005 |
| Unknown physician | Brubaker et al., 2013 |
| Visual/audial impairment | Goode et al., 2008 |
| Inability to consent | Cooke et al., 2010 |
| Threat to health | Borrayo, Lawsin and Coit, 2005 |
| Last option | Tu et al., 2005 |
| Childcare cost | Baquet et al., 2006; Sahay et al., 2004 |
| Insurance cover cost | Biedrzycki, 2010 |
| Media cover | Lee et al., 2012 |
| Physician influence | Brubaker et al., 2013; Sprague et al., 2013; Lee et al., 2012; Meneguin and Cesar, 2012, McMahon et al., 2011; Tallon et al., 2011; Houlihan et al., 2010; Kohara and Inoue, 2010; Sabesan et al., 2010; Kinder et al., 2010; Costenbader et al., 2007; Townsley et al., 2006; Borrayo, Lawsin and Coit, 2005; Sahay et al., 2004; Brown and Topcu, 2003; Zullino et al., 2003 |
| Physician recommendation | McMahon et al., 2011; Sabesan et al., 2010; Zullino et al., 2003 |
The desire to improve personal health as a motivating factor influencing clinical trial participation was reported in nine studies. Gaining access to health care was among the key factors associated with the desire to improve health and this was of particular relevance in studies undertaken in the USA where agreement to participate in clinical trials could provide access to health care which would not otherwise be available to the person due to lack of health insurance. A GRACE (Gender, Race And Clinical Experience) trial identified participation in clinical trials was motivated by participants gaining access to treatment (33%) and altruism or the belief that participation enabled others to be helped (36%) [16].
3.3. Personal risk
3.3.1. Personal risk and fear
The perception of personal risk and fear were two interrelated but distinguishable constructs identified in this review as being important in decision to participate in clinical trials. Perceiving a risk to self was identified as the principal reason why people made a decision not to participate in clinical trials. Linked to this perception of personal risk was the feeling of fear, a feeling caused by the actual or perceived threat of danger or harm [17] resulting from participation in a clinical trial. Personal risk and feeling fearful were related to side effects of trial intervention or treatments along with the possibility of trial treatments interacting with existing treatments. Feeling fearful ranged from fear brought about by the procedures required within the clinical trial [18], fear about the nature of the research process [9] and fear about being cut off from family if confidentiality about trial participation was broken [19]. In the studies identifying personal risk of unconfirmed treatment and perception of its severity leading to participants declining, four focused on HIV and high risk of HIV, and reported that the stigma associated with the disease was a contributory factor to non-participation [18], [19], [20]. Fear of the treatment and the possibility of it being ineffective [11] was also evident among participants and physicians. Further causes for non-participation due to personal risk included patients having to take medications [6], [21], the impression that participation required signing ‘their life away’ [22], and being treated by an unknown physician when taking part in a trial [23].
However, increased interaction with trial staff and the improved level of information and understanding of the trial was identified as working to reduce fear and improve likelihood of participation. Thus while fear was identified as a critical factor deterring participation, there is evidence that this can be mitigated by research staff providing potential participants with adequate reassurance and detailed explanations of the procedural elements of the trial.
Associated with perceptions of personal risk and fear was the perception that engaging in a trial and taking medications or other interventions as yet untested or unproven to result in a positive outcome, was the perception of being experimented upon and this was a further important factor in declining participation. Seven of the studies within this review identified that unconfirmed treatment and the perception of its severity was influential in participants declining to enter clinical trials [23], [24], [25]. Other participants refused to enter because they viewed clinical trials as being invasive [5]. While evidence from clinical trials may result in confirmation of treatment efficacy, cases where the disease is resistant to the particular treatment type or where individual participants are severely impacted by adverse effects of treatment can result in non-participation or discontinuation. Thus the in the absence of a guarantee that the trial intervention will be effective in an individual case was for some potential participants a deterrent.
It is anticipated that fear and personal risks would have similar effects on patient participation within clinical trials in Ireland as they would worldwide, however it is possible that these perceptions may be heightened due to clinical trials not being as widely recognised within Ireland as they are in other countries such as USA.
3.3.2. Research process
This review identified variables relating to the clinical trials process as influential in patient's decision to participate. These were categorised under the umbrella term research process, which refers to any activities that are undertaken specifically for the clinical trial. The research process was discussed twenty eight times throughout this reviewed literature, accounting for an overall reported influence of 8% (Fig. 2). The main influencing factor of the research process is the trial design with 99% of the studies reviewed referring to it as a factor discouraging participation [5], [9], [14], [26], [27], [28], [29].
Patients are more inclined towards non-participation due to the stringent nature of the clinical trials protocols [27], [28]. From the patient perspective, whether based in USA, Europe or Ireland, it is likely that such protocols appear overly strict. Since clinical trials are experimental processes utilising new and as yet unproven drugs or procedures in human beings, stringent practices are required to be in place to avoid and/or mitigate any potential harm to study participants. However patients participating in clinical trials may not always fully appreciate that such protocols are required to protect patient safety. Protocols that require regular and stringent monitoring of participants necessitating frequent hospital visits are cited as a reason to decline participation [24] as are the frequency of medical consultations [30], the time spent within hospital [9] and the duration of the trial [6], [9]. Other trial requirements including the randomisation process, including the risk of being randomised to the control arm of the study and receiving placebo [5], [9], [14], [26], [29], having to complete questionnaires [7], [28] and keeping a diary log [28] are also noted as research process barriers to participation.
3.3.3. Cost
Cost was the second leading factor influencing the decision to participate in clinical trials and was identified by sixteen studies. Three constituents of cost identified in this review included i) financial cost, ii) medical cost and iii) time cost.
3.3.4. Financial cost
Financial cost was identified in this review as the most influential element of cost consisting of receiving an incentive or financial reward, the greatest influencing factor within the literature is incentive or financial reward. Eight of the studies included in this review provided an incentive to participants to engage in the clinical trial and these consisted of a 12.5-megabyte jump drive; entry to a free gift raffle; $20 cash; two public transportation tokens, and a $20 gift certificate. While the use of incentives, financial and benefit in kind, are identified as a factor influencing participation in clinical trials, evidence also exists to suggest that providing financial incentives alone do not guarantee entry to or completion by participants of clinical trials. A study undertaken by Stock [31], examined if providing a monetary incentive increased attendance. No difference between the group receiving a $25 shopping voucher and those receiving no incentive was identified. In a separate study, Holman [32] examined retention rates in patients participating in an RCT using a placebo. They identified that while incentives such as a payment did constitute an influencing factor in participation, it was the factor considered least important by participants. It should also be noted that approximately half of the studies utilising financial incentives were undertaken in the USA where such provision is legal. In Ireland, the use of incentives for patient participation in research and specifically clinical trials is governed by the International Conference on Harmonisation Guidelines for Good Clinical Practice (ICH GCP, [33]), and use of such incentives are rare and are generally associated with defraying costs incurred by participation in the trial. A number of studies have identified that a significant cost related barrier to participation in clinical trials is the financial burden associated with trial participation [14], [18], [34], [35]. In particular, the loss of income resulting from work absence, and the costs of child care are of particular concern to potential participants. This review identified that proximity of the study and income level influenced clinical trial participation. Potential participants were more likely to participate if the location of the research unit was in close proximity to where they lived [26] and were less likely to participate if they lived in rural locations distant from the trial centre [27], [34]. Likewise income level was viewed as a predictor for patient participation in clinical trials [35] and having a greater income status was more likely to lead to a higher chance of participation [36], [37]. However, employment was seen as a more reliable predictor than income [38]. It is possible that those not employed were more likely to participate, as the need to take time off work and losing income to participate was not a factor.
3.3.5. Medical cost
Medical Insurance cover overlapped financial cost and medical cost. While most frequently identified as a cost barrier [39], two studies, Sahay [18] and Brown and Topcu [36] identify medical insurance cover as a motivational and incentivising factor. Reference to medical cost as a barrier was primarily identified in studies undertaken in the USA and this is likely to be related to the requirement in the USA necessitating patients to be in possession of health insurance to enable them to receive healthcare and to participate in clinical trials. In contrast the current organisation of the Irish health care system is broadly based on a welfare model whereby citizens are not required to have private health insurance to enable access to health care. While patients are responsible to meet some costs associated with receipt of health care, this is based on the citizens ability to pay, thus those on low income, in receipt of unemployment benefit, social welfare or other state related benefits such as disability benefit will receive health care free of charge. Thus, within the USA context participation in clinical trials is likely to be significantly influenced by the involvement of those who would otherwise not have access to health care. When participants agree to take part in clinical trials, they are according to Menezes [40] likely to attain improved health status gaining access to free investigative procedure such as blood testing and other investigations [41] without incurring financial costs. By contrast, the availability of free health care to Irish citizens who are unable to pay is likely to negate the impact of this factor within an Irish context. A further factor requiring consideration is treatment availability. Taking part in clinical trials is likely to advantage trial participants whereby they gain access to treatments that are new, innovative, expensive and not available elsewhere especially within the oncology setting [24].
3.3.6. Time
A number of studies included in this review identified time as a significant influencing factor. The time commitment required to participate in a clinical trial is identified as an important barrier to participation. The time costs included the inconvenience of trial related requirements and visits; travel; following specific regimens and completing a daily diary log [6], [9], [42], [43]. The time to arrange transport was the greatest barrier for participation [14], [26] and this is likely to be due to the lack of clinical trials available in rural areas [27]. A separate but related element of time was also identified and this related to the time taken by staff recruiting study participants. If recruitment practices involve clearly outline eligibility criteria and allow for recruitment staff to spend sufficient time and effort on eligible candidates, potential health benefits and associated risks, participation rates can be increased effectively [38].
3.3.7. Altruism
Altruism or selflessness is described as the principle or practice of concern for the welfare of others. It is frequently considered as a core virtue in many cultures and is a central principle in many religious traditions and secular worldviews. Altruism is also considered to be the undertaking of good acts without the thought of personal reward. Altruism was identified as a key factor which positively influences participation in clinical trials. In the clinical trial setting altruism is seen as an act for the betterment of mankind [43]. The most common explanations of altruism within this review was the act of unselfishness for contribution to medicine and science [7], [8], [10], [19], [25], [30], and to help others, be it the community or nursing care, family or future patients [8], [9], [16], [19], [23], [38], [42], [44]. This review identified that the act of altruism is most likely to be observed within the haematology and oncology settings with it occurring less prominently in settings such as cardiology, gastrointestinal diseases and diabetes. The prominence of altruism in a specific setting may be linked to the nature and progression of disease. Those entering haematology or oncology trials are likely to be influenced significantly by the life limiting or life threatening nature of their condition, may view the trial as a means to contribute to treatment advances as well as obtaining some sense of personal meaning and purpose within their illness. There is no reason to assume that altruism as a positive indicator for participation in clinical trials would differ in impact upon patients worldwide by it’s very definition.
3.3.8. Communication
Communication was an important variable when looking at increasing participation into clinical trials. There are a variety of positive and negative influences identified when considering communication as a variable [43] and language, knowledge, understanding and misconception are among the collective terms used to identify communication factors. Knowledge and understanding are dependent upon the information provided to participants. Having a lack of information, along with a lack of understanding of clinical trials and concerns about the effectiveness of clinical trials [44] leads to negative influences giving further cause for non-participation. If the information provided is adequate and user friendly [39], and is provided in the relevant language [43], [45], it is likely to result in positively influencing participants to participate in clinical trials. Furthermore, factors relating to language [21], [34], [43], [45], [46], as well as those associated with visual and auditory impairment combine to negatively impact the consent process [46], thus reducing the likelihood of individuals participating in clinical trials.
Communication is also impacted by how information about clinical trials is presented and advertised. Information which is provided in a newsletter format or publicised by the media resulted in patients being more willing to participate in the clinical trial as well as being likely to give potential participants more confidence in the research being undertaken [18], [41], [44].
This review identified a number of misconceptions related to understanding and knowledge about clinical trials. Clinical trials were perceived as a potential threat to participants health [21] and partaking in the trial was viewed as the last option [45]; doctors and clinicians were considered to have more interest in the trial than the person per se and the individual perceived they had less say in their care when participating in trial [25]. Issues pertaining to under-representation of ethnic minorities were also identified with some potential participants considering that clinical trials are designed for a pre-dominantly Caucasian population with findings positively biased towards the white population [22]. Providing sufficient time to address these perceptions further may result in potential participant becoming more informed and thus result in a greater uptake in participation in clinical trials. Awareness of the nature and types of misconceptions among potential trial participants is essential as understanding these will assist researchers to accurately inform and re-educate potential participants [19], [47] and by doing so increase recruitment. Within Ireland clinical trials are identified though internet base platforms and through word of mouth by consultants. If Ireland was to utilise resources like other countries worldwide for example they used advertisement or increased media via news letters, this may have a more positive influence on patients for participation within Ireland as greater knowledge would be accessible about the different trials and it is anticipated that communication would no longer be a deterrent.
3.3.9. Physician influence
Sixteen of the papers reviewed discussed Physician influence and identified it to be one of the most provocative variables in influencing patient participation in clinical trials [7], [11], [18], [21], [23], [24], [25], [27], [28], [29], [30], [36], [44], [48]. Physician recommendation was identified as a variable influencing patient participation in seven of the studies reviewed, with a further five identifying physicians as having a positive impact on participation while two identified physicians acting as a discouragement to patient participation. Patients appear to be more influenced to take part if they have a good relationship with and trusts the physician [18], [28], [36]; the physician is experienced, involved in the clinical trial and is reputable [14], [28], [29], [49]. Patients are more likely to decline participation if the physician discouraged due to either their dissatisfaction with trial design, or the physician's feelings of responsibility if a patient suffered as a result of partaking in the trial [27], appears to have more interest in the trial than patient care [25], and patients have a lack of confidence in the physician [11]. The nature of the Irish healthcare system means that patients will have a single primary physician, as opposed to multiple physicians in other countries health care settings such as United Kingdom. We would anticipate this having a positive impact upon patients' willingness to participate in Clinical Trials in Ireland as they will build trusted relationships with their physician.
3.3.10. Demographics
This part played by patient demographics, as a predicting variable for participating in clinical trials, continues to be debated. Demographic variables considered influential included the social and economic characteristics of a specific population as well as gender, race and ethnicity, sexual orientation, age, religion and income level [2], [9], [13], [15], [20], [26], [31], [35], [36], [37], [38], [40], [46], [49], [50].
In clinical trials and any research, it is important to have true representation of the overall population. This includes minority groups, which incorporate women and ethnic communities as observed throughout this literature [46]. Despite the perceived importance of these groups, only two of the articles within this review focus on these [2], [36].
One demographic characteristic, that of age, and whether or not it was a predictor for patient participation was identified as a contentious issue within the literature reviewed. This review identified that older groups were considered as less willing to participate [49], [50]. However it may well that be older people are less likely to be invited to participate due to a perception of potential cognitive impairment or they may be seen as less likely to comply with rigorous protocols thus potentially jeopardising the study outcomes. Nevertheless, this review also identified that older people felt that they should not be judged by their age, and that when considering a clinical trial, they should be looked at as an individual in their entirety [40]. While age was identified as potentially controversial, the issue of religion was only addressed within one study and in this case it was viewed as exerting a negative influence on participation, as the participants believed their fate was in the hands of God [37].
However, while demographics and the likelihood of the different characteristics to predict participation in clinical trials are identified as important, some disagreement continues to exist as to the true part demographics play in patients' willingness to participate in clinical trials. According to Kasner [9] demographics were not identified as being influential in the decision to participate in clinical trials and Biedrzycki [13] found that socio-demographic factors including age, race and gender, and had no significant impact on the decision to participate in clinical trials.
4. Discussion
There is an absence of Irish data about the factors which operate to influence patient participation in clinical trials. However, data originating in other international clinical trial facilities have sought to identify the factors that facilitate and or inhibit patient participation in clinical trials both from a patient and a health care perspective and with some exceptions, these are likely to be transferable to the Irish context. One clinical research facility located in Ireland serves a population of over one million, and the numbers of clinical trial participation was less than half of one percent of the 250,000 patients directly treated in the associated hospitals. While it is unknown whether these patients were eligible for ongoing studies within this facility, the review presented in this paper was undertaken to address the factors that contribute to participation of patients. This narrative literature review has identified that the vast majority of literature relating to participation in clinical trials emanates predominantly from high income countries, with 63% originating from the USA. It is a notable finding that no studies for inclusion in this review were identified from low income or developing countries. Although the trials carried out are global, and participants may be from low and middle income countries, it is difficult to identify if specific factors identified as influencing patient participation in clinical trials in higher income countries have the same relevance for participants in middle and low income countries.
The review identified the primary factor influencing participation in clinical trials amongst patients was related to personal factors and that these were collectively associated with obtaining a form of personal gain through participation. The type of personal gain identified was that related to receiving better or more treatment and care. While it appears that, obtaining access to care which would otherwise not be possible was particularly relevant in insurance based care systems such as the USA, it is also likely that factor influences trial participation within the Irish context. While obtaining access to care not otherwise available may be less influential due to the Irish health systems broadly welfare based model whereby citizens do not require private health insurance, getting access to novel treatments with their perceived greater efficacy may be more influential. Personal gain also included obtaining more positive outcomes. However, it is likely that personal gain is mediated by other factors including the perception of personal risk and fear as well as personal cost including time and financial costs; altruism, communication, physician influence, research process and demographics.
Clinical research regulations mandate that the decision making for clinical trial participation should be independent [13]. However, information giving is a significant part of the trials process and independent decision making may not be the preference for participants who accept or decline participation in clinical trials. This review has identified that patients are influenced to take part in clinical trials based on their relationship with the physician. A relationship perceived by a patients as good and trusting with the physician is likely to result in participation. However, if relationships were not perceived as good or if the physician was in any way discouraging, a decline in invitation to participate was more likely. Thus physician influence was identified as potentially the most challenging variable in influencing patient participation. Within the Irish context, regulation clearly states that the physician should not coerce or unduly influence the patient to participate, and that participants are the final decision makers whether or not to participate in trials [33], although this review clearly identifies that patients are influenced by the physician.
In the absence of Irish evidence, it is difficult to estimate if potential clinical trial participants experience these factors differently. With regard to experience of risk, it is possible that within the Irish context that this may be mediated by the influence of physicians and other health professionals. In Ireland cultural norms pertaining to the conduct of medical practice and professional – patient based relationships are still largely based within a public welfare model. The ideology underpinning this model of serving the poor, while becoming less dominant, in reality still retains elements of professional dominance and paternalism whereby significant proportions of patients, particularly those who are older, emanating from lower socio-economic groups and with lower educational levels abdicate responsibility for decision making to health care professionals. Resultantly, these cultural differences in the conduct of health care practice may affect the degree of influence of physicians and other health care professionals in that patients are more likely to be guided by and accept the advice of the health professionals in an unquestioning way when compared to patients in health systems where patients by virtue of being required to purchase health insurance operate more as consumers of health care as a product.
While striving to avoid any undue influence in decision making, it is also important to note that physician communication may also act as a deterrent to participation. Therefore it is critical to recognise that physician attitude relating to decision making along with their ability to communicate complex information in an appropriate manner will impact trial participation. Therefore, attention to recruitment, information giving and consent strategies do require further exploration to attempt to achieve a balanced approach to recruitment of trial participants. If roles of patients as potential participants as competent decision makers is underestimated by physicians, it is likely to result in less effective communication about clinical trials.
4.1. Limitations
This study has several limitations acknowledged by the authors. The literature review was limited to papers that were in the English language, participants that were human and over eighteen years of age. The search that was carried out was within a publication period of 2003–2014 and articles included were required to be free and in full text. Neither translators or paper articles costs were feasible, due to financial constraints and therefore limited the study. Only four databases were used to carry out this literature review, however these were seen as the most relevant databases appropriate to this study.
5. Conclusion
Despite its limitations, this review makes an important contribution for clinicians and researchers in understanding the factors which influence participation by patients or act to deter their participation and this is identified from the perspective of patients. Factors affecting patient participation in clinical trials has not as yet been examined in Ireland and this review provides initial insight into the decision making process of patients – having to make life altering decisions while facing a chronic illness. To address the absence of evidence within the Irish context, it is recommended that future research should continue to focus on innovation in trial methodologies and that these innovations incorporate exploration of factors that act to both support and or deter patient participation to enhance enrolment.
Acknowledgement
The authors would like to acknowledge the support of the following individuals; Dr. Sherly George for her help and guidance with the initial writing process and Dr. Veronica McInerney for her contributing thoughts and guidance. The authors would also like to thank The HRB Clinical Research Facility, Galway for giving the time to carry out the research.
References
- 1.Wallington S.F., Luta G., Noone A.M., Caicedo L., Lopez-Class M., Sheppard V., Spencer C., Mandelblatt J. Assessing the awareness of and willingness to participate in cancer clinical trials among immigrant Latinos. J. Community Health. 2012;37(2):335–343. doi: 10.1007/s10900-011-9450-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Markman M., Petersen J., Montgomery R. An examination of the influence of patient race and ethnicity on expressed interest in learning about cancer clinical trials. J. Cancer Res. Clin. Oncol. 2008;134(1):115–118. doi: 10.1007/s00432-007-0263-4. [DOI] [PubMed] [Google Scholar]
- 3.Voytek C.D., Jones K.T., Metzger D.S. Selectively willing and conditionally able: HIV vaccine trial participation among women at “high risk” of HIV infection. Vaccine. 2011;29(36):6130–6135. doi: 10.1016/j.vaccine.2011.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Health Research Board (HRB) [Internet] 2015 [cited 2015 December 15]. Available from: http://www.hrb.ie/about/in-the-news/?no_cache=1&tx_ttnews%5Btt_news%5D=655&tx_ttnews%5BbackPid%5D=19&cHash=251a1700b9a6090a5424da8aa8190ef8.
- 5.White C., Hardy J. What do palliative care patients and their relatives think about research in palliative care?-a systematic review. Support. Care Cancer. 2010;18(8):905–911. doi: 10.1007/s00520-009-0724-1. [DOI] [PubMed] [Google Scholar]
- 6.Carroll R., Antigua J., Taichman D., Palevsky H., Forfia P., Kawut S., Halpern S.D. Motivations of patients with pulmonary arterial hypertension to participate in randomized clinical trials. Clin. Trials. 2012;9(3):348–357. doi: 10.1177/1740774512438981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tallon D., Mulligan J., Wiles N., Thomas L., Peters T.J., Elgie R., Sharp D., Lewis G. Involving patients with depression in research: survey of patients' attitudes to participation. Br. J. General Pract. J. R. Coll. General Pract. 2011;61(585):134–141. doi: 10.3399/bjgp11X567036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Truong T.H., Weeks J.C., Cook E.F., Joffe S. Altruism among participants in cancer clinical trials. Clin. Trials. 2011;8(5):616–623. doi: 10.1177/1740774511414444. [DOI] [PubMed] [Google Scholar]
- 9.Kasner S.E., Del Giudice A., Rosenberg S., Sheen M., Luciano J.M., Cucchiara B.L., Messe S.R., Sansing L.H., Baren J.M. Who will participate in acute stroke trials? Neurology. 2009;72(19):1682–1688. doi: 10.1212/WNL.0b013e3181a55fbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharp L., Cotton S.C., Alexander L., Williams E., Gray N.M., Reid J.M. Reasons for participation and non participation in a RCT: postal questionnaire surveys of women eligible for TOMBOLA (Trial of Management of Borderline and Other Low grade Abnormal smears) Soc. Clin. Trials. 2006;3:431–442. doi: 10.1177/1740774506070812. [DOI] [PubMed] [Google Scholar]
- 11.Zullino D., Conus P., Borgeat F., Bonsack C. Readiness to participate in psychiatric research. Can. J. Psychiatry. 2003;48(7):480–484. doi: 10.1177/070674370304800709. [DOI] [PubMed] [Google Scholar]
- 12.Wang L.H., Tsai Y.F., Chen J.S., Tsay P.K. Intention, needs, and expectations of cancer patients participating in clinical trials. Cancer Nurs. 2011;34(2):117–123. doi: 10.1097/NCC.0b013e3181efe1c0. [DOI] [PubMed] [Google Scholar]
- 13.Biedrzycki B.A. Factors and outcomes of decision making for cancer clinical trial participation. Oncol. Nurs. Forum. 2011;38(5):542–552. doi: 10.1188/11.ONF.542-552. [DOI] [PubMed] [Google Scholar]
- 14.Kinder B.W., Sherman A.C., Young L.R., Hagaman J.T., Oprescu N., Byrnes S., McCormack F.X. Predictors for clinical trial participation in the rare lung disease lymphangioleiomyomatosis. Respir. Med. 2010;104(4):578–583. doi: 10.1016/j.rmed.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davison B.J., So A., Goldenberg S.L., Berkowitz J., Gleave M.E. Measurement of factors influencing the participation of patients with prostate cancer in clinical trials: a Canadian perspective. BJU Int. 2007;101(8):982–987. doi: 10.1111/j.1464-410X.2007.07349.x. [DOI] [PubMed] [Google Scholar]
- 16.Squires K., Feinberg J., Bridge D.A., Currier J., Ryan R., Seyedkazemi S., Dayaram Y.K., Mrus J. Insights on GRACE (Gender, Race, And Clinical Experience) from the patient's perspective: GRACE participant survey. AIDS Patient Care STDs. 2013;27(6):352–362. doi: 10.1089/apc.2013.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oxford dictionary. Definition of fear in English. [Internet] 2014 [cited 2014 March 1] Available from http://www.oxforddictionaries.com/definition/english/fear.
- 18.Sahay S., Mehendale S., Sane S., Brahme R., Brown A., Charron K., Beyrer C., Bollinger R., Paranjape R. Correlates of HIV vaccine trial participation: an Indian perspective. Vaccine. 2004;23(11):1351–1358. doi: 10.1016/j.vaccine.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 19.Chakrapani V., Newman P.A., Singhal N., Jerajani J., Shunmugam M. Willingness to participate in HIV vaccine trials among men who have sex with men in Chennai and Mumbai, India: a social ecological approach. PLOS One. 2012;7(12) doi: 10.1371/journal.pone.0051080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moutsiakis D.L., Chin P.N. Why blacks do not take part in HIV vaccine trials. J. Natl. Med. Assoc. 2007;99(3):254–257. [PMC free article] [PubMed] [Google Scholar]
- 21.Borrayo E.A., Lawsin C., Coit C. Latinas' appraisal of participation in breast cancer prevention clinical trials. Cancer control. 2005:107–110. doi: 10.1177/1073274805012004S18. [DOI] [PubMed] [Google Scholar]
- 22.Darnell K.R., McGuire C., Danner D.D. African American participation in Alzheimer's disease research that includes brain donation. Am. J. Alzheimer’s Dis. Other Dementias. 2011;26(6):469–476. doi: 10.1177/1533317511423020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brubaker L., Richter H.E., Barber M.D., Hsu Y., Rahn D.D., Menefee S., Visco A., Spino C., Martin S., Meikle S.F. Pelvic floor disorders clinical trials: participant recruitment and retention. Int. Urogynecol. J. 2013;24(1):73–79. doi: 10.1007/s00192-012-1824-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee S.J., Park L.C., Lee J., Kim S., Choi M.K., Hong J.Y., Park S., Maeng C.H., Chang W., Kim Y.S., Park S.H., Park J.O., Lim H.Y., Kang W.K., Park Y.S. Unique perception of clinical trials by Korean cancer patients. BMC Cancer. 2012;12(594) doi: 10.1186/1471-2407-12-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sabesan S., Burgher B., Buettner P., Piliouras P., Otty Z., Varma S., Thaker D. Attitudes, knowledge and barriers to participation in cancer clinical trials among rural and remote patients. Asia-Pacific J. Clin. Oncol. 2011;7(1):27–33. doi: 10.1111/j.1743-7563.2010.01342.x. [DOI] [PubMed] [Google Scholar]
- 26.Martin S.S., Ou F.S., Newby L.K., Sutton V., Adams P., Felker G.M., Wang T.Y. Patient- and trial-specific barriers to participation in cardiovascular randomized clinical trials. J. Am. Coll. Cardiol. 2013;61(7):762–769. doi: 10.1016/j.jacc.2012.10.046. [DOI] [PubMed] [Google Scholar]
- 27.McMahon V.A., Matthews S., Capper H., Chudleigh J.B., McLachlan C.S. Understanding decision and enabling factors influencing clinical trial participation in Australia: a view point. Asian Pac. J. Cancer Prev. APJCP. 2011;12(11):3153–3156. [PubMed] [Google Scholar]
- 28.Houlihan R.H., Kennedy M.H., Kulesher R.R., Lemon S.C., Wickerham D.L., Hsieh C., Altieri D.C. Identification of accrual barriers onto breast cancer prevention clinical trials: a case-control study. Cancer. 2011;116(15):3569–3576. doi: 10.1002/cncr.25230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costenbader K.H., Brome D., Blanch D., Gall V., Karlson E., Liang M.H. Factors determining participation in prevention trials among systemic lupus erythematosus patients: a qualitative study. Arthritis Rheum. 2007;57(1):49–55. doi: 10.1002/art.22480. [DOI] [PubMed] [Google Scholar]
- 30.Meneguin S., Cesar L.A. Motivation and frustration in cardiology trial participation: the patient perspective. Clin. (Sao Paulo, Braz. 2012;67(6):603–608. doi: 10.6061/clinics/2012(06)10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stocks N., Allan J., Frank O., Williams S., Ryan P. Improving attendance for cardiovascular risk assessment in Australian general practice: an RCT of a monetary incentive for patients. BMC Fam. Pract. 2012;13(54) doi: 10.1186/1471-2296-13-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holman A.J., Neradilek M.B., Dryland D.D., Neiman R.A., Brown P.B., Ettlinger R.E. Patient-derived determinants for participation in placebo-controlled clinical trials for fibromyalgia. Curr. Pain & Headache Rep. 2010;14(6):470–476. doi: 10.1007/s11916-010-0152-4. [DOI] [PubMed] [Google Scholar]
- 33.International Conference on Harmonization guidelines for good clinical practice. (ICH GCP)) [Internet] 1996 [cited 2014 April 1]. Available from: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6_R1/Step4/E6_R1__Guideline.pdf.
- 34.Hussain-Gambles M., Atkin K., Leese B. South Asian participation in clinical trials: the views of lay people and health professionals. Health Policy (Amsterdam, Neth. 2006;77(2):149–165. doi: 10.1016/j.healthpol.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 35.Baquet C.R., Commiskey P., Mullins D., Shiraz I.M. Recruitment and participation in clinical trials: socio-demographic, rural/urban, and health care access predictors. Cancer Detect. Prev. 2006;30(1):24–33. doi: 10.1016/j.cdp.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown D.R., Topcu M. Willingness to participate in clinical treatment research among older African Americans and Whites. Gerontologist. 2003;43(1):62–72. doi: 10.1093/geront/43.1.62. [DOI] [PubMed] [Google Scholar]
- 37.Advani A.S., Atkeson B., Brown C.L., Peterson B.L., Fish L., Johnson J.L., Gockerman J.P., Gautier M. Barriers to the participation of African-American patients with cancer in clinical trials: a pilot study. Cancer. 2003;97(6):1499–1506. doi: 10.1002/cncr.11213. [DOI] [PubMed] [Google Scholar]
- 38.Gadegbeku C.A., Stillman P.K., Huffman M.D., Jackson J.S., Kusek J.W., Jamerson K.A. Factors associated with enrollment of African Americans into a clinical trial: results from the African American study of kidney disease and hypertension. Contemp. Clin. Trials. 2008;29(6):837–842. doi: 10.1016/j.cct.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Biedrzycki B.A. Decision making for cancer clinical trial participation: a systematic review. Oncol. Nurs. Forum. 2010;37(6):387–399. doi: 10.1188/10.ONF.E387-E399. [DOI] [PubMed] [Google Scholar]
- 40.Menezes P., Eron J.J., Leonea P.A., Adimoraa A.A., Wohla D.A., Millera W.C. Recruitment in HIV/AIDS treatment naïve clinical trials in the HAART era – influence of gender, sexual orientation and race. HIV Med. 2011;2(3):183–191. doi: 10.1111/j.1468-1293.2010.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Udrea G., Dumitrescu B., Purcarea M., Balan I., Rezus E., Deculescu D. Patients' perspectives and motivators to participate in clinical trials with novel therapies for rheumatoid arthritis. J. Med. Life. 2009;2(2):227–231. [PMC free article] [PubMed] [Google Scholar]
- 42.Goode P.S., Fitzgerald M.P., Richter H.E., Whitehead W.E., Nygaard I., Wren P.A., Zyczynski M.H., Cundiff G., Menefee S., Senka J.M., Gao X., Weber A.M. Enhancing participation of older women in surgical trials. J. Am. Coll. Surg. 2008;207(3):303–311. doi: 10.1016/j.jamcollsurg.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rooney L.K., Bhopal R., Halani L., Levy M.L., Partridge M.R., Netuveli G., Car J., Griffiths C., Atkinson J., Lindsay G., Sheikh A. Promoting recruitment of minority ethnic groups into research: qualitative study exploring the views of South Asian people with asthma. J. Public Health. 2011;33(4):604–615. doi: 10.1093/pubmed/fdq100. [DOI] [PubMed] [Google Scholar]
- 44.Townsley C.A., Chan K.K., Pond G.R., Marquez C., Siu L.L., Straus S.E. Understanding the attitudes of the elderly towards enrolment into cancer clinical trials. BMC Cancer. 2006;6(34) doi: 10.1186/1471-2407-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tu S.P., Chen H., Chen A., Lim J., May S., Drescher C. Clinical trials: understanding and perceptions of female Chinese-American cancer patients. Cancer. 2005;104(12):2999–3005. doi: 10.1002/cncr.21524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cooke C.R., Erickson S.E., Watkins T.R., Matthay M.A., Hudson L.D., Rubenfeld G.D. The national heart, lung, and blood institute acute respiratory distress syndrome network. Age-, sex-, and race-based differences among patients enrolled versus not enrolled in acute lung injury clinical trials. Crit. Care Med. 2010;38(6):1450–1457. doi: 10.1097/CCM.0b013e3181de451b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dhalla S., Poole G. Barriers of enrolment in HIV vaccine trials: a review of HIV vaccine preparedness studies. [Review] Vaccine. 2011;29(35):5850–5859. doi: 10.1016/j.vaccine.2011.06.055. [DOI] [PubMed] [Google Scholar]
- 48.Kohara I., Inoue T. Searching for a way to live to the end: decision-making process in patients considering participation in cancer phase I clinical trials. Oncol. Nurs. Forum. 2010;37(2):124–132. doi: 10.1188/10.ONF.E124-E132. [DOI] [PubMed] [Google Scholar]
- 49.Sprague D., Russo J., LaVallie D.J., Buchwald D. Barriers to cancer clinical trial participation among American Indian and Alaska native tribal college students. J. Rural Health Assoc. 2013;29:55–60. doi: 10.1111/j.1748-0361.2012.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nene B., Jayant K., Arrossi S., Shastri S., Budukh A., Hingmire S., Muwonge R., Malvi S., Dinshaw K., Shankaranarayanan R. Determinants of women's participation in cervical cancer screening trial, Maharashtra, India. Bull. World Health Organ. 2006;85(4):264–272. doi: 10.2471/BLT.06.031195. [DOI] [PMC free article] [PubMed] [Google Scholar]