Abstract
Background
Post-traumatic stress disorder (PTSD) is a chronic anxiety disorder that is often difficult to treat. Patients suffering from PTSD often fail to respond to antidepressants and may have a high incidence of positive symptoms of psychosis, though antipsychotic medications have been minimally studied in this population. The aim of this study was to assess the impact of the atypical antipsychotic ziprasidone (Geodon) on PTSD symptom clusters, as well as comorbid major depressive disorder. To our knowledge, this is the first completed randomized controlled trial investigating the potential efficacy and tolerability of ziprasidone in patients with chronic PTSD.
Methods
We conducted a 9-week prospective, randomized, double-blind, placebo-controlled trial of ziprasidone in 30 patients diagnosed with PTSD and comorbid depression. After screening and randomization, patients completed nine weekly study visits at which treatment safety and efficacy were evaluated. Primary measures of efficacy included total and subscale scores from the Clinician-Administered PTSD Scale (CAPS), while the Hamilton Rating Scale for Depression (HAM-D), Hamilton Anxiety Scale (HAM-A), Clinical Global Impression (CGI), and Treatment Outcome PTSD Scale (TOP-8) were implemented as secondary efficacy measures.
Results
We observed no significant effect of treatment on reduction of PTSD or depression symptoms from pre- to post-treatment.
Conclusions
Our findings suggest that ziprasidone treatment may not significantly improve symptoms of PTSD or comorbid depression, though further study is needed.
Keywords: Geodon, Ziprasidone, PTSD, Post-traumatic stress disorder
1. Introduction
Post-traumatic stress disorder (PTSD) is a chronic anxiety disorder with a high lifetime prevalence of 7.8% (10.4% for women and 5% for men) [1] that is often difficult to treat. PTSD is characterized by symptoms in three clusters: intrusive, avoidant, and hyperarousal. The intrusive symptom cluster, which includes flashbacks, nightmares, intrusive thoughts, and physiological and psychological arousal upon reminders of the trauma, is considered unique to PTSD and not seen in any other psychiatric condition. Additionally, the intrusive symptom cluster has proved difficult to treat successfully with conventional psychotherapeutic and pharmacotherapeutic approaches.
Presently, two medications, sertraline (Zoloft) [2], [3] and paroxetine (Paxil) [4], [5] have U.S. Food and Drug Administration (FDA) approval for the indication of treating PTSD. Unfortunately, many patients with PTSD are unresponsive, have only moderate or marginal responses, or have troubling side effects to first-line selective serotonin reuptake inhibitor (SSRI) treatment. In addition, current SSRI trials have found that more than 50% of patients still have significant residual symptoms, which can be highly incapacitating.
Recent studies suggest that patients suffering from combat-associated PTSD may have a high incidence of positive symptoms of psychosis [6], [7], [8], [9], and these patients especially frequently fail to respond to antidepressants. Antipsychotic medications have been minimally studied; however, some research suggests their usefulness in PTSD patients with marked paranoia, anger, and/or flashbacks [10], [11], [12], [13]. That hypothesis is also supported by biological study findings of abnormal dopamine function [14] and reports of elevated peripheral (urinary and plasma) dopamine levels in PTSD patients [15].
Ziprasidone (Geodon) is a new-generation (atypical) antipsychotic with a benign side effect profile (e.g., extrapyramidal side effects comparable to placebo). To date, there is little evidence concerning the efficacy and tolerability of ziprasidone in PTSD, though one report [16] suggests that ziprasidone can be effective and well tolerated in this population. There is one reported randomized placebo-controlled trial of ziprasidone as add-on to SSRI treatment for PTSD [17], though the trial was terminated early, making it difficult to draw conclusions regarding the efficacy of ziprasidone.
We conducted a 9-week, prospective double-blind trial of ziprasidone specifically designed to assess the impact of ziprasidone on PTSD symptom clusters. Also, as PTSD has an extensive comorbidity with major depressive disorder [18], the clinical trial of ziprasidone in PTSD was intended to help delineate its potential antidepressant spectrum of efficacy and anxiolytic profile. To our knowledge, this is the first completed randomized controlled trial investigating the potential efficacy and tolerability of ziprasidone in patients with chronic PTSD.
2. Materials and methods
2.1. Participants
Participants were recruited from the outpatient mental health clinic at Creighton University and community referrals. The diagnosis of PTSD was made during a comprehensive screening evaluation for PTSD program entry. The study protocol was approved by the Creighton University Institutional Review Board. Informed consent of all participants was obtained after the nature of the procedures had been fully explained and prior to study participation.
Patients had to meet the following inclusion criteria: (1) male or female patients aged 19–64 years meeting DSM-IV criteria for PTSD; (2) competent to provide informed consent; (3) able to attend weekly clinic appointments; (4) if female, using an approved contraceptive if of childbearing potential. Patients were excluded from the study if they had any of the following: (1) history of prior treatment with ziprasidone; (2) medical condition that may prevent safe administration of ziprasidone, such as clinically significant/severe hepatic, cardiac, kidney, or pulmonary disease and seizure disorders, with the exception of childhood seizure disorders; (3) primary major psychotic disorder (i.e., schizophrenia, schizoaffective disorder, or bipolar disorder); (4) suicidal or homicidal ideation or other clinically significant dangerousness; (5) change in psychotropic medication within 90 days of study entry.
2.2. Study procedures
During the screening evaluation, patients received a comprehensive psychiatric evaluation, as well as a physical examination and urine drug screen. Laboratory tests (clinical chemistry and hematology) were also performed if indicated by the patient's medical history. Patients who met eligibility criteria were randomized either to ziprasidone group or placebo group. Patients completed a total of nine weekly study visits and were provided with a pager number to contact the study coordinator 24 h per day for adverse event reporting. The beginning dose of ziprasidone was 20 mg administered twice daily. The dose was increased in 20 mg increments twice daily, up to 80 mg twice daily. Concomitant antidepressant and other psychotropic (including antipsychotic) medications were permitted if they were maintained at a constant dose for at least 3 months before baseline visit.
2.3. Assessment of effectiveness
The primary outcome measure was the Clinician-Administered PTSD Scale (CAPS), which was administered at study visits 1, 7, and 9. The CAPS is a clinician-administered scale used to assess the core PTSD symptoms of the DSM-IV. Higher scores on the CAPS indicate greater severity of PTSD symptoms. The primary efficacy variable was the change from visit 1 (baseline) to visit 9 (endpoint) in the global scores on the CAPS. Clinically significant improvement on the CAPS score was defined a priori as at least a 50% decrease from baseline to endpoint. We also examined the number of patients showing at least a 30% decrease on the CAPS over the course of the study.
The Hamilton Anxiety Scale (HAM-A), Hamilton Rating Scale for Depression (HAM-D), Clinical Global Impression (CGI), and Treatment Outcome PTSD Scale (TOP-8) were administered as secondary outcome measures at each study visit. Higher scores on the HAM-A reflect greater severity of anxiety symptoms, while higher scores on the HAM-D reflect greater severity of depression symptoms. The CGI is a clinician-administered scale with two items used to rate illness severity (CGI-S) and global improvement from baseline (CGI-I). Higher scores on the CGI-S reflect greater illness severity, while lower scores on the CGI-I indicate greater improvement. Higher scores on the TOP-8 reflect greater severity of PTSD symptoms. Utilizing the secondary measures, the data also helped to determine whether the improvement in PTSD symptoms was entirely a function of treating depression and/or anxiety symptoms, or whether there were additional effects on PTSD. The change from baseline to endpoint was measured. At the end of the acute phase, patients who elected to continue in treatment in the research clinic were administered open-label ziprasidone at individual doses for an additional 12 weeks.
2.4. Assessment of safety and tolerability
Adverse events and vital signs were evaluated at each visit. Each patient had a pager number to contact the study coordinator 24 h per day for adverse event reporting.
2.5. Statistical analyses
Categorical data, including demographics, were assessed with descriptive statistics. Treatment group was compared over time with repeated measures, mixed-effects models. The outcome measures of interest were total CAPS score, and CAPS B (Intrusion), C (Avoidance), and D (Hyperarousal) subscale scores. The repeated measures model included treatment, visit (as a categorical variable), and treatment*visit interaction fixed effects. An unstructured covariance matrix was used to fit the within-patient repeated measures. Pairwise comparison p-values from the mixed models were adjusted with Tukey's method. Fisher's exact test was also used to compare treatment response between groups.
Paired t-tests were used to compare the change on secondary efficacy measures between the treatment groups from baseline to visit 9. For the primary outcome variable a p-value less than 0.05 is considered to be statistically significant, while other statistical comparisons are exploratory. SAS software version 9.1 (SAS Institute, Cary, NC) was used for the analyses.
3. Results
3.1. Patient characteristics
Fifty two patients were recruited for the study, including 15 who screen failed and seven who were lost to follow up, resulting in a total of 30 randomized in the study. Patients randomized to the ziprasidone group (n = 15) included 3 male and 12 female patients (Mean age = 39.5, SD = 14.8), while the placebo group (n = 15) included 1 male and 14 female patients (Mean age = 38.3, SD = 8.4). The two groups did not differ significantly with regard to age (t (28) = −0.26, P > 0.7) or sex distribution (Fisher's exact test, P > 0.5).
3.2. Efficacy of ziprasidone
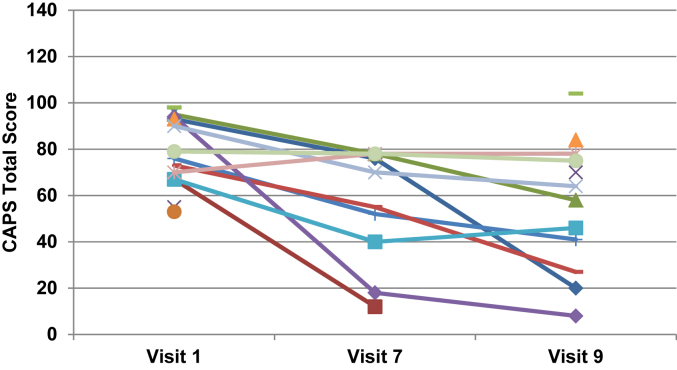
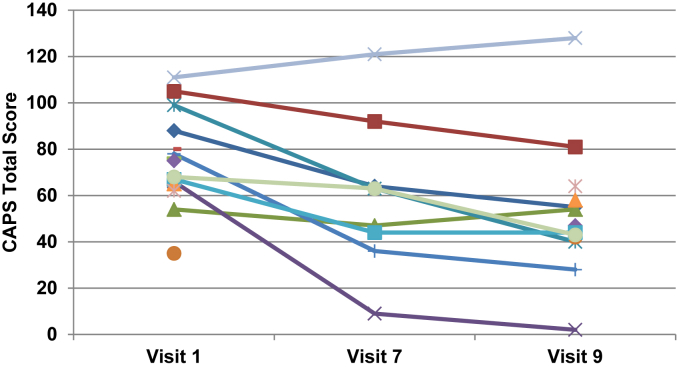
Results for CAPS total score, and CAPS B (Intrusion), C (Avoidance), and D (Hyperarousal) subscale scores at visit 1 (baseline), visit 7, and visit 9 (endpoint) are summarized in Table 1. The placebo group had patient numbers of n = 15 at visit 1, n = 9 at visit 7, and n = 13 at visit 9. The ziprasidone group had patient numbers of n = 15 at visit 1, n = 10 at visit 7, and n = 12 at visit 9. The two groups did not differ significantly on any of the CAPS scores at baseline. When analyzing the CAPS total score as the outcome variable, the treatment*visit interaction was not significant (F (2,28) = 0.49, P = 0.62), indicating that the effect of treatment did not differ by visit, and therefore, the interaction was excluded from the model. The main effects model showed a significant visit effect (F (2,28) = 10.36, P = 0.0004), but not a treatment effect (F (1,28) = 0.18, P = 0.67). On average, CAPS total score decreased by 18.2 (SE = 4.3) from visit 1 to visit 7 (t (28) = 4.01, adjusted P = 0.0012) and by 25.2 (SE = 5.6) from visit 1 to visit 9 (t (28) = 4.49, adjusted P = 0.0003). After adjusting for multiple comparisons the difference between visit 7 and 9 was not statistically significant (t (28) = 2.03, adjusted P = 0.12). CAPS total scores across visits for each group are illustrated in Figs A1 and A2.
Table 1.
CAPS scores by treatment group and visit.
| Outcome measure | Visit | Ziprasidone Mean (SE) |
Placebo Mean (SE) |
Adjusted P-value |
|---|---|---|---|---|
| CAPS Total score | 1 7 9 |
79.9 (4.6) 62.1 (7.8) 51.7 (8.4) |
75.4 (4.6) 56.7 (7.9) 53.1 (8.3) |
0.98 0.99 1.0 |
| CAPS B (Intrusion) | 1 7 9 |
21.0 (2.2) 16.7 (2.4) 14.0 (2.9) |
21.0 (2.2) 16.8 (2.4) 13.7 (2.9) |
1.0 1.0 1.0 |
| CAPS C (Avoidance) | 1 7 9 |
33.9 (1.7) 26.8 (3.5) 22.5 (3.8) |
31.0 (1.7) 23.3 (3.6) 22.8 (3.8) |
0.85 0.98 1.0 |
| CAPS D (Hyperarousal) | 1 7 9 |
25.1 (1.7) 18.8 (2.7) 15.6 (2.4) |
23.4 (1.7) 17.9 (2.7) 16.8 (2.4) |
0.98 1.0 1.0 |
CAPS: Clinician Administered PTSD Scale.
Note: CAPS scores at visit 7 were unavailable for 11 patients (5 in ziprasidone group and 6 in placebo group). CAPS scores at visit 9 were unavailable for five patients (3 in ziprasidone group and 2 in placebo group).
For CAPS B, C, and D subscale scores, the treatment*visit interaction was not significant (Ps > 0.5), indicating that the effect of treatment did not differ by visit, and therefore, the interaction was excluded from the model. For CAPS B, the main effects model showed a significant visit effect (F (2,28) = 6.12, P = 0.0062), with scores decreasing by 4.3 (SE = 1.5) from visit 1 to visit 7 (t (28) = 2.83, adjusted P = 0.022) and by 7.2 (SE = 2.1) from visit 1 to visit 9 (t (28) = 3.5, adjusted P = 0.0044). After adjusting for multiple comparisons the difference between visit 7 and 9 was not statistically significant (t (28) = 2.45, adjusted P = 0.053). For CAPS C, the main effects model showed a significant visit effect (F (2,28) = 8.22, P = 0.0016), with scores decreasing by 7.4 (SE = 2.1) from visit 1 to visit 7 (t (28) = 3.52, adjusted P = 0.0041) and by 9.7 (SE = 2.4) from visit 1 to visit 9 (t (28) = 3.97, adjusted P = 0.0013). After adjusting for multiple comparisons the difference between visit 7 and 9 was not statistically significant (t (28) = 1.43, adjusted P = 0.34). For CAPS D, the main effects model showed a significant visit effect (F (2,28) = 11.36, P = 0.0002), with scores decreasing by 5.9 (SE = 1.7) from visit 1 to visit 7 (t (28) = 3.49, adjusted P = 0.0045) and by 8.0 (SE = 1.7) from visit 1 to visit 9 (t (28) = 4.76, adjusted P = 0.0002). After adjusting for multiple comparisons the difference between visit 7 and 9 was not statistically significant (t (28) = 1.65, adjusted P = 0.24). No significant main effects of treatment were observed (Ps > 0.3).
3.3. Response rate
The response rate was defined as a greater than 50% decrease in total CAPS score from visit 1 (baseline) to visit 9 (endpoint). We also looked at the rate of response defined as a 30% decrease. Based on the 50% decrease criterion, response rate was 25% in the active treatment group and 23% in the placebo group. Using the 30% decrease criterion, response rate was 50% in the active treatment group and 54% in the placebo group. Response rates were not significantly different between the groups for total CAPS score or any of the subscales (as shown in Table 2).
Table 2.
Response rate comparison by group.
| Outcome measure | Response rate | Ziprasidone N (%) |
Placebo N (%) |
P-value |
|---|---|---|---|---|
| CAPS total score | <50% decrease >=50% decrease |
9 (75%) 3 (25%) |
10 (77%) 3 (23%) |
1.0 |
| <30% decrease >=30% decrease |
6 (50%) 6 (50%) |
6 (46%) 7 (54%) |
1.0 | |
| CAPS B (Intrusion) | <50% decrease >=50% decrease |
8 (67%) 4 (33%) |
8 (62%) 5 (38%) |
1.0 |
| <30% decrease >=30% decrease |
6 (50%) 6 (50%) |
6 (46%) 7 (54%) |
1.0 | |
| CAPS C (Avoidance) | <50% decrease >=50% decrease |
8 (67%) 4 (33%) |
10 (77%) 3 (23%) |
0.67 |
| <30% decrease >=30% decrease |
6 (50%) 6 (50%) |
7 (54%) 6 (46%) |
1.0 | |
| CAPS D (Hyperarousal) | <50% decrease >=50% decrease |
9 (75%) 3 (25%) |
10 (77%) 3 (23%) |
1.0 |
| <30% decrease >=30% decrease |
6 (50%) 6 (50%) |
8 (62%) 5 (38%) |
0.70 |
CAPS: Clinician Administered PTSD Scale.
3.4. Secondary efficacy
Results for secondary efficacy measures are summarized in Table 3. Scores on secondary efficacy measures were unavailable at visit 9 for four patients (2 in ziprasidone group and 2 in placebo group). Baseline CGI-S was unavailable for one patient in the ziprasidone group, and visit 2 CGI-I was unavailable for one patient in the placebo group. There was no significant difference between treatment groups at baseline on any of the measures. Change from baseline to visit 9 was also not significantly different between groups on the HAM-A (t (24) = −1.12, P > 0.2), HAM-D (t (24) = −1.28, P > 0.2) CGI-S (t (23) = −0.64, P > 0.5), CGI-I (t (23) = −0.86, P > 0.4), or TOP-8 (t (24) = −0.85, P > 0.4).
Table 3.
Change from baseline to endpoint on secondary efficacy measures.
| Outcome measure | Baseline |
Change from baseline to endpoint |
||||
|---|---|---|---|---|---|---|
| Ziprasidone Mean (SD) |
Placebo Mean (SD) |
P-value | Ziprasidone Mean (SD) |
Placebo Mean (SD) |
P-value | |
| HAM-A | 22.5 (7.0) | 22.5 (8.5) | 1.0 | −11.8 (12.2) | −6.7 (10.8) | 0.27 |
| HAM-D | 19.0 (4.4) | 17.8 (5.4) | 0.51 | −9.8 (8.3) | −5.8 (7.6) | 0.21 |
| TOP-8 | 20.0 (5.8) | 18.7 (4.4) | 0.53 | −10.1 (8.2) | −7.4 (8.0) | 0.41 |
| CGI-S | 4.9 (0.9) | 4.5 (1.0) | 0.38 | −1.9 (1.9) | −1.5 (1.7) | 0.53 |
| CGI-I | 3.5 (0.6) | 3.4 (1.2) | 0.92 | −1.2 (1.3) | −0.7 (1.5) | 0.40 |
HAM-A: Hamilton Anxiety Scale; HAM-D: Hamilton Rating Scale for Depression; TOP-8: Treatment Outcome PTSD Scale; CGI-S: Clinical Global Impression-Severity; CGI-I: Clinical Global Impression-Improvement.
Note: As the CGI-I is not obtained at baseline (Visit 1), Visit 2 scores are reported for Baseline.
Endpoint scores on secondary efficacy measures were unavailable for four patients (2 in ziprasidone group and 2 in placebo group). Baseline CGI-S was unavailable for one patient in the ziprasidone group, and visit 2 CGI-I was unavailable for one patient in the placebo group.
3.5. Safety and tolerability
Seven of the patients randomized in the study discontinued due to an adverse event, including four (26.7%) in the placebo group and three (20%) in the ziprasidone group. The most commonly reported adverse events in our sample were irritability, drowsiness, insomnia, dizziness, and nausea. There were two serious adverse events reported during the study, both in patients randomized to the placebo group. One patient reported chest pain and hypertension, and a second patient experienced a seizure. Both patients were discontinued from the trial.
4. Discussion
The primary aim of this study was to assess the efficacy and tolerability of ziprasidone in patients with chronic PTSD. Contrary to expectations, we observed no significant difference in outcomes between ziprasidone treatment compared with placebo. These findings are in contrast to two previous studies that reported improvement in PTSD symptoms with ziprasidone treatment [19], [16]. However, this discrepancy may be attributed to differences in study design. In particular, both previous studies involved inpatients treated with ziprasidone for approximately a week on average, while our study focused on outpatients treated with ziprasidone over a 9-week period. In addition, one of these studies [19] reported on two patients with chronic combat-related PTSD, while many of the participants in the current study were recruited through area women’s trauma groups and treatment centers.
We cannot explain the lack of a treatment effect by insufficient response to ziprasidone. Total CAPS score changes from baseline were −28.2 in the ziprasidone treatment group and −22.3 in placebo-treated patients, and there was no statistically significant difference in response rates between the two groups (25% and 23% for ziprasidone and placebo, respectively). In addition, no relevant differences in symptom severity were observed between the groups at baseline (CAPS mean baseline scores of 79.9 (4.6) and 75.4 (4.6) in the active treatment and placebo-controlled groups, respectively), making it unlikely that differences in symptomology contributed to the lack of treatment effect. Placebo response in clinical trials has been well documented in a variety of psychiatric patient populations [20], [21], [22]. While the double-blind design and use of standardized measures reduced the potential for bias in our study, there are other factors (e.g., patient expectancy of improvement, benefit of contact with health care providers, recruitment strategy) that may have contributed to the high placebo response rate and reduced our ability to detect a difference between drug and placebo.
There are a number of limitations of the current study that should be acknowledged. First, due to the small sample and limited duration of our study, additional studies in larger samples and over longer periods will be needed to further evaluate the efficacy of antipsychotic medications like ziprasidone in treating PTSD. Second, our sample was predominantly comprised of women with PTSD mostly related to sexual or physical assault, and the findings from this study may not generalize to all individuals with PTSD (e.g., military personnel with combat-related PTSD). Finally, according to a study by Hamner [23], severity of specific PTSD symptoms, as measured by the CAPS and respective symptom cluster scores, did not predict psychotic features. That is, the psychotic PTSD patients did not have more severe current illness as measured by these subscales. For example, the “re-experiencing symptoms” (CAPS cluster B) was not higher in patients with psychotic features. While we did not specfically measure psychotic symptoms in our study, we believe that future work should include assessment of the frequency and intensity of psychotic features in patients using a clinical rating for psychosis, such as the Brief Psychiatric Rating Scale or the Positive and Negative Symptom Scale (PANSS).
5. Conclusion
In conclusion, we did not find any significant effect of ziprasidone treatment compared to placebo in the reduction of PTSD or depression symptoms. The high placebo response rate in our study highlights the importance of better understanding the nature of placebo response and taking appropriate measures to minimize its impact in clinical trials.
Acknowledgments
The study was funded by an investigator initiated grant from Pfizer Inc.
Appendices.
Fig. A.1CAPS total scores by visit for the ziprasidone group. Note: Data were unavailable for five patients at visit 7 and three patients at visit 9.1
Fig. A.2CAPS total scores by visit for the placebo group. Note: Data were unavailable for six patients at visit 7 and two patients at visit 9.
References
- 1.Kessler R.C. Posttraumatic stress disorder in the national comorbidity survey. Arch. Gen. Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 2.Brady K. Efficacy and safety of sertraline treatment of posttraumatic stress disorder: a randomized controlled trial. JAMA. 2000;283:1837–1844. doi: 10.1001/jama.283.14.1837. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz A.C., Rothbaum B.O. Review of sertraline in post-traumatic stress disorder. Expert Opin. Pharmacother. 2002;3:1489–1499. doi: 10.1517/14656566.3.10.1489. [DOI] [PubMed] [Google Scholar]
- 4.Marshall R.D. A controlled trial of paroxetine for chronic PTSD, dissociation, and interpersonal problems in mostly minority adults, depress. Anxiety. 2007;24:77–84. doi: 10.1002/da.20176. [DOI] [PubMed] [Google Scholar]
- 5.Tucker P. Paroxetine in the treatment of chronic posttraumatic stress disorder: results of a placebo-controlled, flexible-dosage trial. J. Clin. Psychiatry. 2001;62:860–868. doi: 10.4088/jcp.v62n1105. [DOI] [PubMed] [Google Scholar]
- 6.Mueser K.T., Butler R.W. Auditory hallucinations in combat-related chronic posttraumatic stress disorder. Am. J. Psychiatry. 1987;144:299–302. doi: 10.1176/ajp.144.3.299. [DOI] [PubMed] [Google Scholar]
- 7.Wilcox J.A., Briones D.F., Suess L. Substance abuse, post-traumatic stress, and ethnicity. J. Psychoact. Drugs. 1991;23:83–84. doi: 10.1080/02791072.1991.10472577. [DOI] [PubMed] [Google Scholar]
- 8.Hamner M.B. Psychotic features and combat-associated PTSD. Depress. Anxiety. 1997;5:34–38. doi: 10.1002/(sici)1520-6394(1997)5:1<34::aid-da6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 9.Hamner M.B. Psychotic features and illness severity in combat veterans with chronic posttraumatic stress disorder. Biol. Psychiatry. 1999;45:846–852. doi: 10.1016/s0006-3223(98)00301-1. [DOI] [PubMed] [Google Scholar]
- 10.Lindley S.E., Carlson E., Sheikh J. Psychotic symptoms in posttraumatic stress disorder. CNS Spectr. 2000;5:52–57. doi: 10.1017/s1092852900021659. [DOI] [PubMed] [Google Scholar]
- 11.Butterfield M.I. Olanzapine in the treatment of post-traumatic stress disorder: a pilot study. Int. Clin. Psychopharmacol. 2001;16:197–203. doi: 10.1097/00004850-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Petty F. Olanzapine treatment for post-traumatic stress disorder: an open-label study. Int. Clin. Psychopharmacol. 2001;16:331–337. doi: 10.1097/00004850-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Hamner M.B. Adjunctive risperidone treatment in post-traumatic stress disorder: a preliminary controlled trial of effects on comorbid psychotic symptoms. Int. Clin. Psychopharmacol. 2003;18:1–8. doi: 10.1097/00004850-200301000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Segman R.H. Association between the dopamine transporter gene and posttraumatic stress disorder. Mol. Psychiatry. 2002;7:903–907. doi: 10.1038/sj.mp.4001085. [DOI] [PubMed] [Google Scholar]
- 15.Yehuda R. Urinary catecholamine excretion and severity of PTSD symptoms in Vietnam combat veterans. J. Nerv. Ment. Dis. 1992;180:321–325. doi: 10.1097/00005053-199205000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Annitto W. Ziprasidone treatment for posttraumatic stress disorder: 128 cases. Psychiatry (Edgmont) 2005;2:19. [PMC free article] [PubMed] [Google Scholar]
- 17.Kellner M., Muhtz C., Wiedemann K. Primary add-on of ziprasidone in sertraline treatment of posttraumatic stress disorder: lessons from a stopped trial? J. Clin. Psychopharmacol. 2010;30:471–473. doi: 10.1097/JCP.0b013e3181e79600. [DOI] [PubMed] [Google Scholar]
- 18.Breslau N. A second look at comorbidity in victims of trauma: the posttraumatic stress disorder-major depression connection. Biol. Psychiatry. 2000;48:902–909. doi: 10.1016/s0006-3223(00)00933-1. [DOI] [PubMed] [Google Scholar]
- 19.Siddiqui Z. Ziprasidone therapy for post-traumatic stress disorder. J. Psychiatry Neurosci. 2005;30:430–431. [PMC free article] [PubMed] [Google Scholar]
- 20.Fava M. The problem of the placebo response in clinical trials for psychiatric disorders: culprits, possible remedies, and a novel study design approach. Psychother. Psychosom. 2003;72:115–127. doi: 10.1159/000069738. [DOI] [PubMed] [Google Scholar]
- 21.Rutherford B.R. Placebo response in antipsychotic clinical trials: a meta-analysis. JAMA Psychiatry. 2014;71:1409–1421. doi: 10.1001/jamapsychiatry.2014.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walsh B.T. Placebo response in studies of major depression: variable, substantial, and growing. JAMA. 2002;287:1840–1847. doi: 10.1001/jama.287.14.1840. [DOI] [PubMed] [Google Scholar]
- 23.Hamner M.B. Psychotic features and combat-associated PTSD. Depress. Anxiety. 1997;5:34–38. doi: 10.1002/(sici)1520-6394(1997)5:1<34::aid-da6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]