Abstract
Background
Participation in cancer clinical trials has been shown to increase overall survival with minimal increase in cost, but enrollment in adult cancer clinical trials remains low. One factor limiting enrollment is lack of insurance coverage, but this barrier should be reduced under the 2010 Patient Protection and Affordable Care Act (ACA), which includes a provision requiring coverage for clinical trial participation as of 2014.
Methods
To assess the number of Kansas adults aged 19–64, newly covered with health insurance for participation in oncology clinical trials as a result of the ACA, a cross sectional design using extracted data from the 2012 American Community Survey, Public Use Microdata Sample to estimate the number of individuals covered by insurance and data from the 2014 Department of Health and Human Services Health Insurance Marketplace enrollment to estimate those newly enrolled through ACA.
Results
In 2014, there was an estimated increase of 3% (54,397; 95% CI: 44,149–64,244) for a total of 72% (1,171,041) of Kansans aged 19 to 64 with health insurance coverage for clinical trial participation.
Conclusion
Three main factors limit the effectiveness of the ACA provisions in expanding clinical trial coverage: 1) ‘grandfathered’ self-funded employer plans not subject to state Employee Retirement Income Security Act (ERISA) regulations, 2) Medicaid coverage limits not addressed under the ACA, 3) populations that remain uninsured. Kansas saw a negligible increase in insurance coverage as a result of the ACA thus lack of insurance coverage is likely to remain a concern for cancer patients.
Keywords: Cancer clinical trial, Insurance denial, Affordable Care Act, Barrier, Grandfathered policy
1. Introduction
An estimated 37 million individuals will be newly insured with health insurance coverage due to the ACA over the next 10 years; many of whom may have been without access to high-quality cancer prevention, early detection and treatment services [1]. Cancer is the second leading cause of death in the United States and in 2014, it was estimated that 1,665,540 new cancer cases would be diagnosed and about 585,720 Americans would die from cancer [2]. The development of investigational compounds is crucial in the quest for advancing treatment options and discovering the cure for cancer. Participants in clinical trials have access to cutting edge approaches to treatment and technology and trial participation has been shown to be associated with a higher survival rate [3], [4]. However, less than 5% of cancer patients participate in clinical trials despite nearly one-third of Americans indicating a willingness to participate if asked [5], [6].
The reasons affecting low accrual are varied and complex, but include cost-related hurdles, specifically lack of insurance coverage, especially those with private insurance compared to government-funded insurance [3], [7], [8]. Potential denial of coverage was reported as the reason for declining participation for 8–20% and as high as 85% of eligible patients [9], [10].
Removing the insurance coverage barrier is addressed in section 2709 of the 2010 Patient Protection and Affordable Care Act (ACA) as new policies are required to cover routine costs for participation in all phases of qualified clinical trials as of January 1, 2014. This study examines how effective the ACA provisions have been for increasing the number of Kansans with insurance coverage for cancer clinical trials.
2. PRE-ACA attempts to address coverage
Third party payers of health care costs have been covering routine care procedures for patients participating in clinical trials for decades, but this coverage has been steadily declining despite evidence suggesting the incremental costs of receiving treatment through a clinical trial is not significantly different than receiving treatment outside of the trial [11], [12], [13], [14]. Prior to the ACA, the federal government and many states attempted to address the issue of clinical trial coverage by enacting legislation or adopting cooperative agreements with insurance companies to ensure coverage for cancer patients' routine procedures while participating in a clinical trial [15]. Several factors limited the comprehensive nature of these efforts including inconsistent state legislation and regulations that do not apply to self-insured plans often offered by large employers, and varying Medicaid coverage rules set by each state. Research suggests these laws had varying effects on overall clinical trial accrual rates [9], [16], [17], [18] Nearly one in five individuals aged 19–64 were not covered for trial participation based solely on not having health insurance coverage in 2012 [19].
3. ACA addresses coverage for trial participation
As of January 1, 2014, the ACA requires insurance providers offering new policies as qualified health plans, to cover routine patient costs defined as “all items and services consistent with the coverage provided in the plan (or coverage) that is typically covered for a qualified individual who is not enrolled in a clinical trial, including hospital visits, imaging, laboratory tests and medications” and excludes any procedures or tests specifically related to the research project and data collection [20]. However, absence of regulations to enforce this mandate and exemptions for grandfathered plans, group plans and health insurance coverage with enrollees prior to March 23, 2010, may significantly impact the outcome of this mandate on clinical trial coverage [21], [22], [23].
Lack of health insurance coverage for clinical trial participation does not necessarily preclude a patient from participating in the trial, but likely means the patient must assume responsibility for all cancer treatment-related care while on the trial and risk denial of coverage for other health services if the insurance company determines that the cost is related to trial participation. As individuals consider trial participation, they weigh their perceived benefits, including broader societal benefits from cancer research, with monetary and non-monetary costs of participation [24]. Although research suggests patients are more likely to decline being a research participant due to preferring the standard therapy or not wanting to be on a trial, financial and insurance issues were frequently cited as reasons for non-enrollment when patients desired treatment through a clinical trial [8].
4. Methods
We used current population survey data from the 2012 American Community Survey, Public Use Microdata Sample (PUMS) and adjusted insurance coverage using estimates from the literature and ACA marketplace enrollment numbers to estimate post-ACA cancer clinical trial coverage [25]. ACA health insurance marketplace data was obtained from the United States (US) Census Bureau and the United States Department of Health and Human Services (HHS) report dated May 1, 2014 [26]. PUMS includes individual and household-level data for a one percent sample of the US population and is meant to be nationally representative of the US civilian, non-institutionalized population. PUMS data are gathered through interview and mail questionnaires on an ongoing basis and made public yearly. The HHS report derives its numbers from the ACA health insurance open enrollment period from October 1, 2013 to March 31, 2014, and includes the special enrollment period activity through April 19, 2014.
PUMS data are used to estimate the number of Kansans covered by type of health insurance in 2012. Estimates are adjusted for sampling and response bias using the available weights. The HHS report is used to estimate post-ACA insurance status. Note that we use 2012 insurance status as the baseline to isolate the effects of ACA coverage. Specifically, we apply percent changes in the uninsured to calculate 2014 estimated coverage assuming that the underlying distribution in insurance status remains steady. This allows us to capture ACA-related changes separate from trends in insurance coverage between 2012 and 2014. State laws and regulations and results from the literature are used to estimate the proportion of individuals with each insurance coverage type (i.e. employer, government, uninsured) who are covered for clinical trial participation. Individual and household-level data for Kansas adults aged 19 to 64 was chosen because older Americans were generally covered prior to the ACA through Medicare, which began covering trial participation in 2000 with the enactment of the clinical trial policy national coverage determination (NCD) by the Centers for Medicare and Medicaid Services [27]. Children up to the age of 19 were generally covered through Medicaid or the Children's Health Insurance Program and were not expected to have significant changes in coverage rates due to the ACA.
The seven insurance types collected in PUMS were grouped into three categories: commercial payer (employer or union sponsored, purchased directly from insurance company); government payer (Medicare, Medicaid, TriCare, Veterans Health Administration (VA))1; and no coverage2. When an individual was covered by more than one insurance plan, they were assigned to the category with the highest cancer trial coverage rates (e.g. Medicare first, employer coverage second, Medicaid third). Once these categories were developed, the proportion of individuals with insurance coverage for clinical trial participation was estimated.
Point estimates from national survey data and the literature were used to calculate the approximation of the newly insured. Ninety-five percent confidence intervals are used to calculate lower and upper bound estimates of the newly insured. The results are based on a study of Kansas and provide a framework for identifying the policy areas fundamental to determining the ACA's impact nationally. In Kansas, it was estimated that 14,630 new cancer cases would be diagnosed and 5460 Kansans would die from cancer in 2014 [2].
Using the PUMS data as a baseline, changes in coverage type were estimated using: ACA enrollment through the Marketplaces; changes in employer coverage; changes in government coverage; and changes in uninsured rates. PUMS data estimation and confidence intervals were calculated using Stata version 12.0. Estimates of coverage changes were developed using Microsoft Office Excel.
5. Results
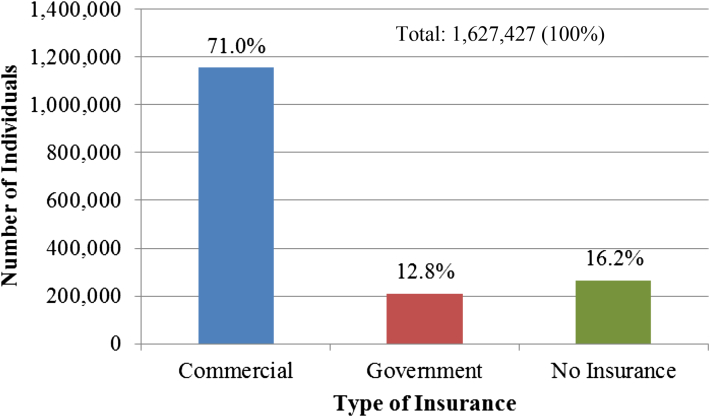
The 2012 population of Kansans aged 19–64 was 1,627,427 (95% CI: 1,602,848–1,652,007). Insurance coverage by category for these individuals is represented in Fig. 1. Pre-ACA, eighty-four percent of these individuals had at least one type of health insurance. Most Kansans (71%. 95% CI: 69.8%–72.2%) were covered by commercial health insurance policies through an employer, union or as a direct purchase from an insurance carrier. Government sponsored policies including Medicare, Medicaid or other government assistance, TriCare, and the Veteran's Administration, covered nearly 13% of Kansans. An estimated 16% of Kansans were uninsured.
Fig. 1.
Health Insurance Coverage by provider pre-ACA, Adults aged 19–64.
Source: Authors' calculations of PUMS data
According to a study by Klamerus et al., 13.6% of individuals were denied coverage for participation in a clinical trial by their commercial insurance carrier [28]. Based on these data, a 13.6% denial rate was used to calculate the number of Kansans in the commercial category without insurance coverage for clinical trial participation pre- and post-ACA. Although not reported in the Klamerus et al. article, the 95% confidence interval estimates provided by the study authors (S.S. Bruinooge, personal communication, January 23, 2015) of 12.6%–14.6% were used to calculate lower and upper bound estimates.
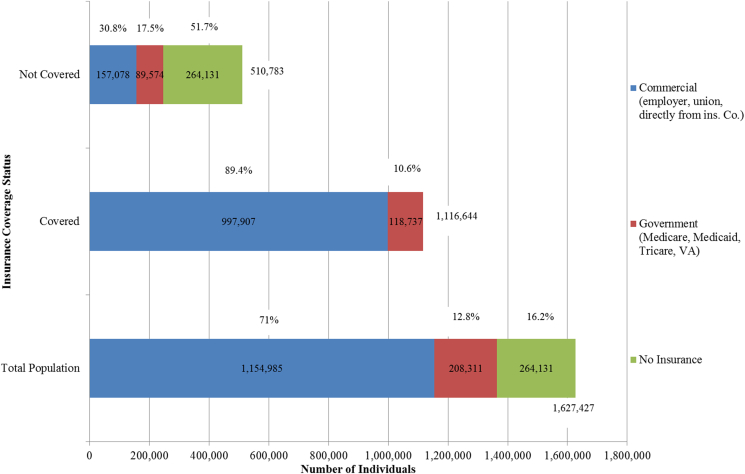
The government category assumes 100% coverage for clinical trial participation for Medicare, (based on the 2000 NCD),3 as well as TriCare4 and the VA5. Clinical trial participation is not covered by Medicaid in Kansas, therefore those with Medicaid (43% of the government category) were not counted as having coverage either pre- or post-ACA. Uninsured individuals were coded as having no insurance coverage for clinical trial participation. A summary of pre-ACA clinical trial coverage is presented in Fig. 2. An estimated total of 69% (1,116,644: 95% CI: 1,091,912–1,141,445) of Kansans had insurance coverage for clinical trial participation pre-ACA. Of those without clinical trial coverage, 51% (95% CI: 48.53%–54.76%) had no insurance coverage, 18% (95% CI: 16.42%–18.69%) were covered by Medicaid, and 31% (95% CI: 30.27%–31.33%) were covered by commercial insurance plans. Therefore, to achieve universal coverage, the ACA would have needed to address trial participation coverage for each of these three groups.
Fig. 2.
Estimated Coverage for Clinical Trial Participation pre-ACA.
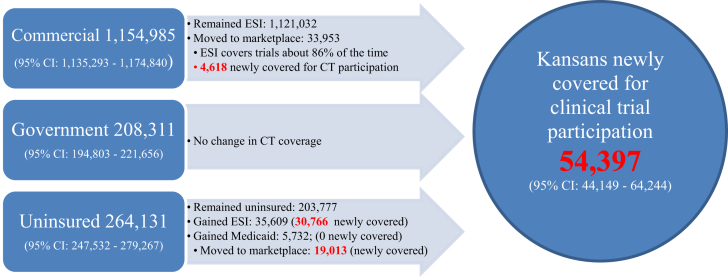
Kansas did not develop its own state-based marketplace and therefore uses the federally facilitated marketplace. As of May 1, 2014, 52,966 Kansans aged 18 to 64 had enrolled through the marketplace6. Although it is unclear from the reported government statistics how many individuals were newly insured and how many were previously insured through another source, researchers have estimated that 35.9% (95% CI: 17.95%–53.85%) of marketplace enrollees were newly insured [29]. Estimates of insurance transitions post-ACA are presented in Fig. 3. As noted earlier, it is estimated that 13.6% of commercial plans did not cover trial participation in 2012 therefore individuals who switched from employer sponsored insurance (ESI) to a marketplace plan would be newly covered. This yields 4618 individuals who were previously employer insured without clinical trial participation coverage that are now newly covered for clinical trial participation.
Fig. 3.
Estimated Coverage for Clinical Trial Participation post-ACA.
Another group potentially affected by ACA provisions includes those uninsured pre-ACA. Based on Marketplace enrollment statistics, most of the uninsured pre-ACA remained uninsured post-ACA, largely attributable to no Medicaid expansion in Kansas. As noted earlier, Kansas Medicaid does not cover clinical trial participation and Kansas opted not to expand Medicaid coverage. Thus, individuals who were previously uninsured but eligible for Medicaid, and newly enrolled in Medicaid because of ACA provisions, gained health insurance coverage not clinical trial coverage.
The largest gains in insurance among the uninsured came from the employer category. It is estimated that 30,766 of the 35,609 individuals with new employer-provided insurance are newly covered for cancer clinical trial participation, due to approximately 14% of commercial plans not covering trial participation7. Finally, it is estimated that a total of 19,013 individuals transitioned from uninsured in 2012 to a Marketplace plan in 2014. All of these individuals were newly covered for cancer clinical trial participation. In total, 54,397 (95% CI: 44,149–64,244) Kansans, or about 3% of adults aged 19 to 64 were newly covered for cancer trial participation following the 2014 ACA provisions.
6. Discussion
Advancements in cancer treatment hinge critically on the ability of researchers to conduct timely clinical trials. The United States Food and Drug Administration requires all new treatments to undergo rigorous testing through the clinical trial process before granting approval for the treatment to be available on the market [30]. Lack of adequate enrollment to clinical trials limits the generalizability of results, can result in trial cancellation and increases the timeline for regulatory approval of new therapies, thus increasing financial and health-related costs [4], [9].
Most cancer clinical trials are designed to follow the current guidelines of delivering cancer care with the investigational agent being added to this treatment schema. The procedures and tests a patient would receive for their disease are referred to as “routine or standard of care” and are billed to the patient's insurance, provided the definition of a qualified trial is met. The investigational procedures are paid for by the sponsor of the clinical trial, such as a pharmaceutical company.
The ACA provision requiring coverage of routine care procedures as part of a clinical trial seemed like a promising policy for eliminating lack of insurance as a barrier to trial participation. However, the effectiveness of the provision in expanding coverage is limited by three key factors that differ across states: 1) the provision does not apply to grandfathered self-insured plans, 2) the provision does not address coverage limitations in Medicaid, 3) and the provision does not address individuals who remain uninsured.
Prior to the ACA, trends in employer-sponsored insurance coverage from 1999 to 2011 showed an average decline of 11.75% for adults aged 19-64 [31]. Using the state of Kansas for illustration, this analysis shows an increase in insurance coverage for trial participation for adults aged 19–64 from 69% to 72%. While over fifty thousand Kansans gained clinical trial coverage under the ACA mandate and other provisions, nearly half a million individuals in the state of Kansas remain at risk for not being able to access treatment through a clinical trial. Although community organizations have historically had high enrollment rates [8], recent experience suggests that insurance is a limiting factor for as many as four patients a week at one of the high enrolling National Cancer Institute's Community Oncology Research Program sites (K. Humphries, personal communication, January 5, 2015).
State-level policies interacting with ACA provisions would lead to differences in coverage if the analysis were repeated for other states. Prior to the 2014 mandate, although not documented in previous literature [9], [16], [17], Kansas had a regulation in place requiring insurance providers issuing policies in Kansas to cover routine procedures as part of a cancer clinical trial.8 The regulation does not apply to self-insured plans and these plans have been granted grandfathered status for the purposes of ACA, meaning if the plan was in existence prior to March 23, 2010 and no significant changes to the policy or cost have been made, these plans are not subject to the mandates of the ACA. According to the Kansas Insurance Commissioner's office, approximately 40% of the plans sold in Kansas are self-insured. Due to the process of how plans are filed with the State, it is not possible to determine how many of these plans exclude clinical trial participation. To further delay access to care through a clinical trial, the Kansas Insurance Department notes it remains permissible for “companies selling health insurance in the state to continue to renew policies that don't meet the requirements of the ACA … for policy years beginning on or before October 1, 2016” [32]. Over time, it is likely that companies will stop issuing grandfathered plans as the cost of retaining the grandfathered status will be outweighed by the need to revise policies due to the current economic climate. In the context of the analysis presented above, elimination of grandfather status could lead to significant gains in cancer clinical trial coverage greater than the current effects of the ACA implementation.
Kansas did not opt to expand Medicaid eligibility to 138% of the federal poverty level (FPL) under the ACA, so adults without children cannot qualify for Medicaid benefits at any income level and parents qualify for benefits only with an income of 38% FPL or below. Ultimately, this policy decision had little effect on the number of individuals newly covered for clinical trials as Kansas Medicaid does not cover trial participation. However, Medicaid expansion would have reduced the number of individuals with no health insurance coverage, providing for access to cancer screening and prevention services.
The complexities of state-level policy differences have been noted by others in the literature [9], [16], [17], [18]. A pre-ACA announcement issued by the HHS and the Department of Labor in the form of “Frequently Asked Questions” stated there was not a plan to issue a regulation on the implementation of the clinical trial coverage mandate therefore leaving “the implementation details of this provision up to the individual states, which is likely to produce a patchwork of uneven and unpredictable coverage that will confuse patients and their health care providers and impact timely access to potentially life-extending research.” [23], [33] This led to more than 50 organizations including patient advocacy and leading oncology professional organizations to sign a letter dated June 18, 2013 urging the Administration to issue regulations or guidance before January 2014 for fear that, “implementation of this provision will be very uneven across the country and many consumers may be denied a new protection they should be guaranteed under the law” [23]. This guidance was never issued. This study highlights some of the areas where state policies continue to have uneven effects.
Limitations of this analysis include the cross-use of the databases which include different samples. The ACS database is maintained by the US Census Bureau and the availability of geographic data is limited [25]. Marketplace enrollment reported by HHS included enrollments reported to CMS and does not include state based exchanges. CMS reports a limitation of this data includes having not captured insurance status prior to enrollment on through the marketplace [26].
This study does not try to quantify the number of newly diagnosed cancer cases affected by the policy but rather attempts to highlight challenges encountered once other identified barriers that affect clinical trial participation are overcome.
7. Conclusions
Three main factors limit the effectiveness of the ACA provisions in expanding clinical trial coverage: 1) ‘grandfathered’ self-funded employer plans not subject to state Employee Retirement Income Security Act (ERISA) regulations, 2) Medicaid coverage limits not addressed under the ACA, 3) populations that remain uninsured. Kansas saw a negligible increase in insurance coverage as a result of the ACA and lack of insurance coverage is likely to remain a concern for cancer clinical trial participants.
Author contributions
Christine Mackay and Tami Gurley-Calvez had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Mackay, Gurley-Calvez.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Mackay, Gurley-Calvez.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Mackay, Gurley-Calvez.
Administrative, technical, or material support: Gurley-Calvez.
Study supervision: Mackay.
Conflict of interest disclosures
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Acknowledgments
This study was not supported by outside funding.
Footnotes
Medicare is federal health insurance coverage for individuals 65 years and older and for those younger than 65 deemed disabled. Medicaid is a government health insurance program for low-income households operated by states with federal matching funds. TriCare is a health insurance program for active duty military members, reserve members, military retirees and their families. The VA is an integrated health insurance and health care system for Veterans.
Indian Health Service (IHS) access was included in the no insurance tabulation as IHS is not an insurance provider.
Statistics for federally run marketplaces include 18-year olds in their reporting.
We use national estimates from Carman and Eibner (2014) to assess the share in the reduction of the uninsured attributable to employer provided insurance. Specifically, we calculate the percent with new employer insurance by dividing the number newly insured in ESI (7.2 million) by the sum of those newly insured (EST + Medicaid + Marketplace = 7.2 + 3.6 + 1.4 = 12.2 million) to arrive at 59 percent of newly insured from these categories in employer plans.
K.A.R. 40-4-43.
Contributor Information
Christine B. Mackay, Email: cmackay@kumc.edu.
Tami Gurley-Calvez, Email: tgurley-calvez@kumc.edu.
Kirsten D. Erickson, Email: kerickson2@kumc.edu.
Roy A. Jensen, Email: rjensen@kumc.edu.
References
- 1.Hutchins V.A., Samuels M.B., Lively A.M. Analyzing the affordable care act: Essential health benefits and implications for oncology. J. Oncol. Pract. 2013;9:73–77. doi: 10.1200/JOP.2012.000881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Cancer Society Cancer: Cancer Facts and Figures. http://www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf.
- 3.Lara P.N., Jr., Higdon R., Lim N. Prospective evaluation of cancer clinical trial accrual patterns: Identifying potential barriers to enrollment. J. Clin. Oncol. 2001;19:1728–1733. doi: 10.1200/JCO.2001.19.6.1728. [DOI] [PubMed] [Google Scholar]
- 4.Unger J.M., Barlow W.E., Martin D.P. Comparison of survival outcomes among ancer patients treated in and out of clinical trials. J. Natl. Cancer Inst. 2014;106:1–13. doi: 10.1093/jnci/dju002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weckstein D.J., Thomas C.A., Emery I.F. Assessment of perceived cost to the patient and other barriers to clinical trial participation. J. Oncol. Pract. 2011;7:330–333. doi: 10.1200/JOP.2011.000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Comis R.L., Miller J.D., Aldigé C.R. Public attitudes toward participation in cancer clinical trials. J. Clin. Oncol. 2003;21:830–835. doi: 10.1200/JCO.2003.02.105. [DOI] [PubMed] [Google Scholar]
- 7.Ward E., Halpern M., Schrag N. Association of insurance with cancer care utilization and outcomes. CA Cancer J. Clin. 2008;58:9–31. doi: 10.3322/CA.2007.0011. [DOI] [PubMed] [Google Scholar]
- 8.St Germain D., Denicoff A.M., Dimond E.P. Use of the national cancer institute community cancer centers program screening and accrual log to address cancer clinical trial accrual. J. Oncol. Pract. 2014;10:e73–e80. doi: 10.1200/JOP.2013.001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellis S.D., Carpenter W.R., Minasian L.M. Effect of state-mandated insurance coverage on accrual to community cancer clinical trials. Contemp. Clin. Trials. 2012;33:933–941. doi: 10.1016/j.cct.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byrne M.M., Tannenbaum S.L., Glück S. Participation in cancer clinical trials: why are patients not participating? Med. Decis. Mak. 2014;34:116–126. doi: 10.1177/0272989X13497264. [DOI] [PubMed] [Google Scholar]
- 11.Berlyn D. Routine patient care in clinical trials: whose cost is it anyway? J. Law Health. 2001;16:77–102. [PubMed] [Google Scholar]
- 12.Goldman D.P., Berry S.H., McCabe M.S. Incremental treatment costs in national cancer institute-sponsored clinical trials. J. Am. Med. Assoc. 2003;289:2970–2977. doi: 10.1001/jama.289.22.2970. [DOI] [PubMed] [Google Scholar]
- 13.Bennett C.L., Adams J.R., Knox K.S. Clinical trials: are they a good buy? J. Clin. Oncol. 2001;19:4330–4339. doi: 10.1200/JCO.2001.19.23.4330. [DOI] [PubMed] [Google Scholar]
- 14.Chirikos T.N., Ruckdeschel J.C., Krischer J.P. Impact of clinical trials on the cost of cancer care. Med. Care. 2001;39:373–383. doi: 10.1097/00005650-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 15.American Society of Clinical Oncology: State Laws and Cooperative Agreements. http://www.asco.org/insurance-coverage-clinical-trial-participants.
- 16.McBride G. More states mandate coverage of clinical trial costs, but does it make a difference? J. Natl. Cancer Inst. 2003;95:1268–1269. doi: 10.1093/jnci/95.17.1268. [DOI] [PubMed] [Google Scholar]
- 17.Gross C.P., Murthy V., Li Y. Cancer trial enrollment after state-mandated reimbursement. J. Natl. Cancer Inst. 2004;96:1063–1069. doi: 10.1093/jnci/djh193. [DOI] [PubMed] [Google Scholar]
- 18.Taylor P.L. State payer mandates to cover care in US oncology trials: do science and ethics matter? J. Natl. Cancer Inst. 2010;102:376–390. doi: 10.1093/jnci/djq028. [DOI] [PubMed] [Google Scholar]
- 19.Kaiser Family Foundation: State Health Facts: Health Insurance Coverage of Adults 19-64. http://kff.org/other/state-indicator/adults-19-64/.
- 20.Phillips C. National Cancer Institute; 2010. NCI Cancer Bulletin Special Issue: Clinical Trials Enrollment: Insurance Coverage Expanding for cancer Clinical Trials. [Google Scholar]
- 21.Edge S.B., Zwelling L.A., Hohn D.C. The anticipated and unintended consequences of the patient protection and affordable care act on cancer research. Cancer J. 2010;16:606–613. doi: 10.1097/PPO.0b013e318201fdac. [DOI] [PubMed] [Google Scholar]
- 22.Kircher S.M., Benson A.B., 3rd, Farber M. Effect of the accountable care act of 2010 on clinical trial insurance coverage. J. Clin. Oncol. 2012;30:548–553. doi: 10.1200/JCO.2011.37.8190. [DOI] [PubMed] [Google Scholar]
- 23.American Society of Clinical Oncology: ASCO Urges Administration to Provide Clear ACA Guidance on Protecting Patient Access to Clinical Trials http://www.asco.org/press-center/asco-urges-administration-provide-clear-aca-guidance-protecting-patient-access-clinical.
- 24.Biedrzycki B.A. Decision making for cancer clinical trial participation: a systematic review. Oncol. Nurs. Forum. 2010;37:E387–E399. doi: 10.1188/10.ONF.E387-E399. [DOI] [PubMed] [Google Scholar]
- 25.United States Census Bureau: American Community Survey: Public Use Microdata Sample. https://www.census.gov/acs/www/data_documentation/public_use_microdata_sample/.
- 26.The Affordable Care Act Research Briefs: Health Insurance Marketplace: Summary Enrollment Report. U.S. Department of Health & Human Services; May 2014. https://aspe.hhs.gov/sites/default/files/pdf/76876/ib_2014Apr_enrollment.pdf ASPE Issue Brief. [Google Scholar]
- 27.Center for Medicare and Medicaid Services: Medicare Clinical Trial Policies. http://www.cms.gov/Medicare/Coverage/ClinicalTrialPolicies/.
- 28.Klamerus J.F., Bruinooge S.S., Ye X. The impact of insurance on access to cancer clinical trials at a comprehensive cancer center. Clin. Cancer Res. 2010;16:5997–6003. doi: 10.1158/1078-0432.CCR-10-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.K.G. Carman, C. Eibner, Survey Estimates Net Gain of 9.3 Million American Adults with Health Insurance. http://www.rand.org/blog/2014/04/survey-estimates-net-gain-of-9-3-million-american-adults.html.
- 30.United States Food and Drug Administration: How Drugs Are Developed and Approved. http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/default.htm.
- 31.State Health Access Data Assistance Center . 2013. State level trends in employer sponsored health insurance.http://www.rwjf.org/en/library/research/2013/04/state-level-trends-in-employer-sponsored-health-insurance.html SHADAC Report. [Google Scholar]
- 32.Praeger S. The Kansas Insurance Department; State of Kansas: Winter 2014. Insurance Quarterly. [Google Scholar]
- 33.United States Department of Labor: FAQs about the Affordable Care Act Implementation Part XV. http://www.dol.gov/ebsa/faqs/faq-aca15.html.