Abstract
Introduction
Statins may have pleiotropic effects in COPD, but mechanisms remain unclear.
Objectives
To assess the pleiotropic effect of statins in patients with stable COPD on (1): lung function (2); pulmonary and systemic inflammation (3); endothelial function (vascular stiffness) and circulating vascular growth factors; and (4), serum uric acid levels.
Method
Pilot, double-blind, randomized, placebo-controlled clinical trial in 24 patients with stable COPD, all statin-naïve, who were randomized (1:1) to receive simvastatin 40 mg/24 h during 12 weeks (n = 12; 69.0 ± 7.3 years; post-bd FEV1 53.4 ± 10.0% pred.) or placebo (n = 12; 66.4 ± 4.6 years; post-bd FEV1 48.2 ± 12.6% pred.). Nine patients per group (total n = 18) completed the study.
Results
Lung function, pulmonary and systemic inflammatory markers and the degree of vascular stiffness did not change significantly in any group. However, treatment with simvastatin increased the plasma levels of erythropoietin (Epo) (4.2 ± 2.2 mIU/mL to 6.8 ± 3.2 mlU/mL, p < 0.05) and reduced those of serum uric acid (7.1 ± 1.3 mg/dL to 6.5 ± 1.4 mg/dL, p < 0.01).
Conclusions
Short-term treatment with simvastatin in stable COPD patients did not modify lung function, pulmonary and systemic inflammation, or vascular stiffness, but it changed Epo and uric acid levels.
Keywords: Statins, COPD, Inflammation, Uric acid, Erythropoietin
1. Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by poorly reversible airflow limitation associated with pulmonary and systemic inflammation [1], [2], [3], [4]. The latter is clinically relevant since it relates to many of the comorbidities associated with the disease [3], [5], [6], [7], and is associated with greater exacerbation rates and mortality [8].
Statins inhibit HMC-CoA reductase, lower plasma cholesterol levels and reduce cardiovascular mortality. Besides, statins can have anti-inflammatory effects [9], [10]. These pleiotropic effects have been demonstrated in animal models [11] but studies in humans are controversial. On the one hand, several observational trials suggested that treatment with statins in COPD reduce the frequency and mortality rate from exacerbations and pneumonia, lung function decline and lung cancer risk [12], [13], [14], [15], [16], [17]. On the other, very recently, Criner et al. published the results of a randomized controlled trial showing that treatment with simvastatin (40 mg/day) did not affect exacerbation rates or time to a first exacerbation in COPD patients [18]. Of note, however, COPD patients were included in this study only if they had not had previous cardiovascular events, did not have cardiovascular risk nor were selected by the level of systemic inflammation [18].
The precise biological effects of statins in COPD are also unclear [19], [20]. Whereas some authors suggest that they may be related to their anti-inflammatory effects [20], [21], [22], particularly when COPD coexists with cardiac comorbidity [23], others [19] could not find any effect of statins on several markers of systemic inflammation, including fibrinogen, C-reactive protein [CRP], tumor necrosis factor-α [TNF-α] and interleukin [IL]-6).
To further explore the pleiotropic effects of statins in COPD, we designed a randomized pilot clinical trial to investigate comprehensively their potential effect on (1): lung function (2); systemic and pulmonary inflammation (3); endothelial function and growth factors involved in vascular homeostasis (erythropoietin [Epo] and vascular endothelial growth factor [VEGF]); and (4) serum uric acid (UA), a biomarker recently associated with the prognosis of COPD exacerbations [24].
2. Methods
2.1. Study design and ethics
Pilot, double-blind, randomized, placebo-controlled clinical trial (ClinicalTrial.gov number NCT02070133) performed according to the Declaration of Helsinki and Good Clinical Practice standards. The study was approved by the Ethics Committee of the Autonomous Community of the Balearic Islands, and all participants signed the informed consent forms after being fully informed of the nature, goals and design of the study.
Eligible patients (see below) were randomized (1:1) to one of the following groups: simvastatin 40 mg/24 h or placebo. Study variables were measured before and after 12 weeks of treatment. During the study period we monitored possible liver and muscle disorders (muscle weakness, rhabdomyolisis) caused by treatment with simvastatin through clinical data per medical history, physical examination and biomarkers for liver cytolysis and rhabdomyolisis (AST, ALT, CK).
2.2. Patients
We consecutively evaluated former smokers with stable COPD and moderate to severe airflow limitation [1] from the outpatient clinic of the Pneumology Department of the Hospital Universitario Son Dureta/Son Espases. None of them had required hospitalization or treatment changes within the previous 12 weeks, and had no other concomitant chronic inflammatory disease. Exclusion criteria also included history of active coronary artery disease, cerebrovascular or peripheral vascular disease had a fasting level of total cholesterol ≥220 mg/dL and had received statin therapy before.
2.3. Measurements
2.3.1. Lung function
Forced spirometry (GS, Warren E. Collins, Braintree, MA, US) was performed according to international standards. Spirometric reference values were those of a Mediterranean population [25]. Arterial blood gases were measured in a blood sample obtained by radial artery puncture after local anesthesia (IL BG3, Izasa, Spain). The carbon monoxide diffusion capacity of the lungs (DLCO) (HypAir Compact +, Medisoft, Belgium) and the 6 min walking test were also determined according to international guidelines.
2.3.2. Circulating blood
Between 8 am and 10 am, fasting venous blood samples were obtained in tubes with EDTA (10 mL) or without EDTA (10 mL) for biochemical determinations. Immediately after, plasma was separated by centrifugation at 200 rpm for 10 min and stored at −80 °C. Total and differential leukocyte count was determined automatically in the whole blood sample (Sysmex K-4500, Toa Medical Electronics Co Ltd, Kobe, Japan). The serum levels of interleukin (IL) 6 (IL-6), IL-8 concentration and VEGF were determined by Luminex xMAP (MILLIPLEX MAP High Sensitivity Human Cytokine Magnetic Bead Panel Merck Millipore, Darmstadt, German) according to the manufacturer's instructions. The analytical sensitivity was 0.13–2.000 pg/mL for IL-6 and IL-8, and 3.2–10.000 pg/mL for VEGF. Circulating levels of Epo were determined by ELISA (eBioscioence, San Diego, USA) with a sensitivity of 100–1.6 mlU/mL. Ultrasensitive C-reactive protein (CRP) levels were determined by nephelometry.
2.3.3. Sputum
Two sputum specimens from each patient were obtained using the technique of induced sputum. Patients were pretreated with 400 mcg of salbutamol by inhalation. Pre-induction spirometry 10 min before and 10 min after salbutamol was done. After placing a nasal clip, induction was started with physiological hypertonic saline first and 3% hypertonic saline then, with an ultrasonic nebulizer (NE-U17, Omron Healthcare Co., Ltd., Japan). Patients were asked to expectorate whenever they feel or at every 5 min. FEV1 was also checked at every 5 min. Sputum samples were kept in cold place (temp. 4 °C) and processed within 2 h. The supernatant was frozen at −80 °C. The concentration of IL-6 and IL-8 in the sputum supernatant was determined by Luminex xMAP (MILLIPLEX MAP High Sensitivity Human Cytokine Magnetic Bead Panel Merck Millipore, Darmstadt, German) according to manufacturer's instructions. The analytical sensitivity of this method was 0.13–2000 pg/mL.
2.3.4. Vascular stiffness
Measurements of vascular stiffness were carried out according to international recommendations [26]. Briefly, in the morning, in a room with controlled temperature (20-25 °C) and the patient lying supine, we used applanation tonometry (SphygmoCor, AtCor Medical Pty Ltd, Sydney, Australia) to quantify the speed of the pulse wave (PWV) and the augmentation index (AI), as previously reported [27].
2.4. Statistical analysis
Quantitative results are presented as mean ± standard deviation and categorical variables as absolute number and percentage. All variables followed a normal distribution (Kolmogorov–Smirnov test). The statistical significance of baseline differences between the simvastatin and placebo groups was analyzed using the Student's t-test or Chi2 test for unpaired samples. Within each group, differences observed before and after the intervention were analyzed with the paired t-test or the McNemar test for paired samples. A p value of <0.05 was considered statistically significant.
3. Results
3.1. Patient characteristics
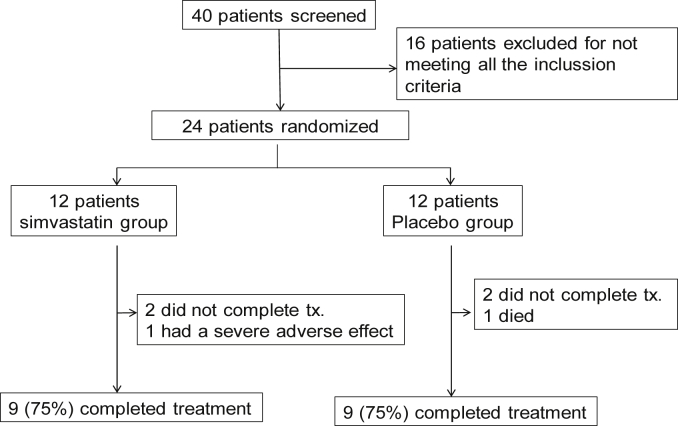
Fig. 1 presents the CONSORT flowchart diagram of the study. We screened 40 patients, of whom 16 did not meet all the inclusion criteria and had to be excluded. We finally randomized 24 patients (12 in each arm). No significant differences in age, gender or any major clinical variables between participants (n = 24) and non-participants (n = 16) were observed (data not shown).
Fig. 1.
CONSORT flowchart diagram of the trial. For further explanations, see text.
Of the 24 patients originally randomized, 18 patients (75%) completed the study, nine in each arm. Two patients (one per arm) decided to quit the study. One patient in the simvastatin group required admission to the intensive care unit due to a COPD exacerbation and was later excluded from the study. In the placebo group, one patient died after hospital admission for severe community acquired pneumonia. Table 1 shows the main demographic, clinical and functional characteristics at recruitment of the 18 patients who finalized the study.
Table 1.
Characteristics of participants at recruitment.
| Simvastatin group | Placebo group | |
|---|---|---|
| N | 9 | 9 |
| Age, years | 69.3 ± 7.2 | 66.4 ± 4.6 |
| Male, n (%) | 8 (88.9) | 7 (77.8) |
| BMI, kg/m2 | 30.0 ± 4.7 | 28.5 ± 6.5 |
| Smoking exposure, pack-years | 50.0 ± 33.5 | 50.4 ± 14.0 |
| GOLD A, n (%) | 4 (44.4) | 3 (33.3) |
| GOLD B, n (%) | 1 (11.1) | 1 (11.1) |
| GOLD C, n (%) | 3 (33.3) | 3 (33.3) |
| GOLD D, n (%) | 1 (11.1) | 2 (22.2) |
| Total cholesterol, mg/dL | 183.6 ± 29.2 | 197.6 ± 27.9 |
| Glycemia, mg/dL | 106.3 ± 17.9 | 107.6 ± 18.7 |
| Hemoglobin, g/dL | 14.9 ± 1.1 | 14.2 ± 1.6 |
| Comorbidities Arterial hypertension, n (%) Diabetes mellitus, n (%) |
6 (66.7) 2 (22.2) |
6 (66.7) 2 (22.2) |
| LA-β2, n (%) | 8 (88.9) | 7 (77.8) |
| LA Anticholinergic, n (%) | 7 (77.8) | 6 (66.7) |
| Inhaled corticosteroids, n (%) | 6 (66.7) | 7 (77.8) |
| SA-β2, n (%) | 4 (44.4) | 2 (22.2) |
| SA Anticholinergic, n (%) | 1 (11.1) | 2 (22.2) |
| ASA, n (%) ACEI, n (%) ARBs, n (%) Diuretics, n (%) |
2 (22.2) 2 (22.2) 2 (22.2) 2 (22.2) |
2 (22.2) 3 (33.3) 2 (22.2) 2 (22.2) |
N: number of patients; BMI: body mass index, LA: Long-acting, SA: short-acting, ASA: acetylsalicylic acid, ACEI: angiotensin converting enzyme inhibitor; ARBs angiotensin II receptor blocker.
3.2. Effects of treatment
After 12 weeks of treatment with simvastatin, a reduction in total cholesterol (183.6 ± 29.2 mg/dL to 149.2 ± 23.9 mg/dL, p<0.01) and LDL cholesterol (114.4 ± 25.6 mg/dL to 78.7 ± 18.2 mg/dL, p<0.01) was observed in the interventional study arm but not in the control group (total cholesterol from 197.6 ± 27.9 mg/dL to 200.4 ± 27.5 mg/dL and LDL cholesterol from 121.1 ± 18.9 mg/dL to 124.4 ± 20.5 mg/dL). No participants reported adverse effects from simvastatin treatment.
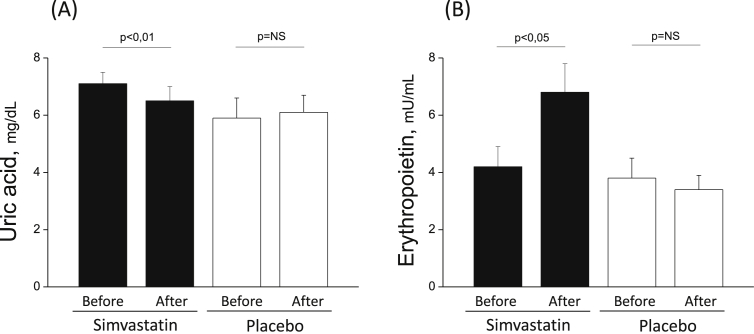
Table 2 presents the study effects on pulmonary and systemic inflammation, lung function and vascular biology. After 12 weeks of simvastatin or placebo treatment, we did not observe any significant intragroup differences in markers for pulmonary inflammation (sputum), systemic inflammation, or lung function (Table 2), except for a reduction in the serum levels of UA (from 7.1 ± 1.3 mg/dL to 6.5 ± 1.4 mg/dL; p<0.01) in the simvastatin-treated group but not in the placebo group (5.9 ± 2.0 mg/dL to 6.1 ± 1.8 mg/dL) (Table 2 and Fig. 2, panel A).
Table 2.
Effects of treatment on pulmonary and systemic inflammatory markers, lung function, vascular physiology and uric acid.
| Simvastatin group | Placebo group | |||||
|---|---|---|---|---|---|---|
| Pre | Post | p | Pre | Post | p | |
| Sputum inflammation | ||||||
| IL-6, pg/mL | 77.6 ± 92.1 | 63.4 ± 91.9 | 0.77 | 93.8 ± 53.9 | 154.1 ± 70.5 | 0.15 |
| IL-8, pg/mL | 1069.2 ± 722.8 | 1304.0 ± 526.8 | 0.49 | 1322.2 ± 808.7 | 1445.5 ± 652.2 | 0.74 |
| Blood inflammation | ||||||
| Leukocytes, 103/μL | 7.0 ± 1.9 | 7.6 ± 1.8 | 0.15 | 9.0 ± 2.7 | 8.3 ± 2.3 | 0.11 |
| Neutrophils, 103/μL | 4.5 ± 1.6 | 4.9 ± 1.5 | 0.20 | 6.0 ± 2.3 | 5.3 ± 1.9 | 0.13 |
| IL-6, pg/mL | 4.4 ± 2.2 | 5.6 ± 3.8 | 0.46 | 8.3 ± 9.5 | 7.4 ± 8.5 | 0.79 |
| IL-8, pg/mL | 9.3 ± 3.6 | 7.4 ± 2.6 | 0.40 | 8.4 ± 3.5 | 8.4 ± 8.2 | 0.98 |
| CRP, mg/L | 3.8 ± 2.0 | 5.1 ± 5.3 | 0.51 | 3.1 ± 2.5 | 3.8 ± 2.9 | 0.11 |
| Lung Function | ||||||
| FEV1/FVC, % pred. post-bd. | 47.0 ± 9.0 | 46.3 ± 11.5 | 0.63 | 42.0 ± 7.8 | 41.9 ± 8.2 | 0.93 |
| FEV1, L post-bd. | 1.5 ± 0.4 | 1.5 ± 0.4 | 0.55 | 1.3 ± 0.5 | 1.3 ± 0.4 | 0.82 |
| FEV1, % pred. post-bd. | 53.4 ± 10.0 | 53.1 ± 12.9 | 0.85 | 48.2 ± 12.6 | 48.1 ± 10.7 | 0.97 |
| DLCO, % pred. | 60.0 ± 21.1 | 61.1 ± 22.1 | 0.77 | 59.1 ± 12.1 | 58.6 ± 19.3 | 0.90 |
| PaO2, mmHg | 73.8 ± 5.5 | 73.5 ± 7.6 | 0.92 | 68.7 ± 6.8 | 70.3 ± 7.6 | 0.65 |
| 6 MWD, m | 453.9 ± 58.3 | 455.6 ± 69.6 | 0.87 | 446.3 ± 99.7 | 444.4 ± 106.0 | 0.33 |
| Vascular effects | ||||||
| C-AP, mmHg | 15.4 ± 3.3 | 14.3 ± 5.2 | 0.55 | 13.1 ± 4.7 | 16.1 ± 5.3 | 0.16 |
| AI, % | 33.8 ± 6.9 | 30.5 ± 5.7 | 0.23 | 27.5 ± 4.4 | 30.5 ± 7.5 | 0.32 |
| PWV, m/s | 12.7 ± 4.5 | 11.8 ± 4.6 | 0.32 | 12.2 ± 1.8 | 13.5 ± 4.3 | 0.74 |
| Epo, mlU/mL | 4.2 ± 2.2 | 6.8 ± 3.1 | 0.04 | 3.8 ± 2.8 | 3.4 ± 1.6 | 0.76 |
| VEGF, pg/mL | 162.4 ± 59.4 | 135.5 ± 27.6 | 0.13 | 153.8 ± 72.6 | 174.7 ± 68.6 | 0.50 |
| Other markers | ||||||
| Uric acid, mg/dL | 7.1 ± 1.3 | 6.5 ± 1.4 | 0.01 | 5.9 ± 2.0 | 6.1 ± 1.8 | 0.56 |
FEV1/FVC: forced expiratory volume in the first second/forced vital capacity, FEV1: forced expiratory volume in the first second; PaO2: partial pressure of arterial oxygen. 6 MWD: 6 min walking distance; C-AP: central pressure, AI: augmentation index, PWV: pulse wave velocity.
Fig. 2.
Mean values (±SD) of circulating levels of uric acid and erythropoietin in COPD patients treated with simvastatin (black bars) and in the placebo group (white bars). Simvastatin reduces (p < 0.01) serum uric acid levels (A), while raising (p < 0.05) the erythropoietin plasma levels (B). No changes were observed in placebo group (A and B).
Finally, there were no significant differences in endothelial function (vascular stiffness) or VEGF levels (Table 2). Yet, plasma Epo levels increased significantly (4.2 ± 2.2 mlU/ml to 6.8 ± 3.2 mlU/ml, p< 0.05) in the simvastatin-treated but not in the placebo group (Table 2 and Fig. 2, panel B).
4. Discussion
This pilot randomized placebo-controlled trial failed to demonstrate a significant effect of 12-weeks treatment with simvastatin on most of the variables studied, including lung function, pulmonary and systemic inflammation and vascular stiffness. Yet, to our knowledge, it is the first to show that treatment with simvastatin in patients with stable COPD who were simvastatin-naïve reduces serum UA levels and increases the plasma Epo levels. The implications of these findings deserve further research.
4.1. Previous studies
Several previous observational studies have explored the effects of treatment with statins in COPD [28], [29], [30], [31]. Lahousse L et al. studied a population of nearly 2700 middle-aged COPD patients who were followed up to 17 years, and reported that statin treatment was associated with a 39% decrease in all-cause mortality, and that this effect was particularly evident (78%) in those individuals with more systemic inflammation (hsCRP ≥ 3 mg/L) [31]. Yet, the precise mechanisms underlying these effects are unclear, since two previous randomized clinical trials reported opposite results. Hence, whereas Lee et al [20] observed a significant reduction in some markers of systemic inflammation (IL-6 and CRP), following 6 months of treatment, which appeared to be associated with an increase in exercise tolerance and a decrease in respiratory symptoms, Kaczmarek et al [19] were unable to observe an anti-inflammatory effect of statins (simvastatin) or to show any effect on lung function (FEV1). Our results are in keeping with this latter trial [19], since we did not find either a significant effect of statins on systemic inflammation or lung function. We concur, however, that the small sample size and short duration of our study may have limited our ability to detect a significant effect of simvastatin on these variables. More recently, Criner et al reported negative results of simvastatin treatment on exacerbation rate and time to first exacerbation in a large, prospective, randomized clinical trial [18]. The short duration of our study did not allowed us to study these outcomes. Of note however, patients included in Criner's study represent a very selected subpopulation of COPD patients (i.e., those without any previous cardiovascular event and/or need of statin therapy to reduce their cardiovascular risk) whereas ours included all comers fulfilling the established inclusion criteria of our study (see above).
4.2. Novel findings
Our study provides three novel observations. First, we explored if treatment with simvastatin could have an anti-inflammatory pulmonary effect, an aspect that, to our knowledge, had not been investigated before. Unfortunately, our results (IL-6 and IL-8 sputum concentrations) were negative, and similar to the lack of effects observed on systemic inflammatory markers (Table 2).
Second, since cardiovascular morbidity and mortality is highly prevalent in COPD [32], [33], and some studies link systemic inflammation and airflow limitation with arterial stiffness, carotid intima thickness and endothelial dysfunction [34], we also evaluated the potential effects of simvastatin on vascular stiffness and some vascular growth factors involved in vascular homeostasis. Results of baseline vascular stiffness in our population were similar to those reported by other authors [27] and did not change after treatment with simvastatin (Table 2). However, treatment with simvastatin increased Epo plasma levels by 24.8%. Besides stimulating erythroid precursor cell proliferation and differentiation, Epo also stimulates the recruitment of circulating endothelial progenitor cells (EPC) and induces angiogenesis [35], [36], [37]. Since low circulating EPC levels have been associated with the risk of dying in patients with cardiovascular disease [38] it is conceivable that increased serum Epo levels in COPD patients treated with simvastatin may be one of the mechanisms underlying the beneficial effects of statins in this disease.
And third, some prospective observational studies suggest that statin therapy is associated with a reduction in hospitalizations due to COPD exacerbations [28], [29]. Recently, Bartziokas K et al. [24] suggested that increased serum UA levels were associated with increased mortality and risk of future exacerbations in COPD. Besides, high serum UA levels have been associated with increased cardiovascular risk and, in patients with cardiovascular disease, statins have been shown to decrease serum UA levels [39]. Our results show that treatment with simvastatin in patients with COPD also reduces serum UA levels (up to 10.1%). It can be speculated that this may contribute to reduce cardiovascular risk in COPD and, perhaps, to reduce the severity and/or frequency of exacerbations.
4.3. Strengths and limitations
The fact that this is a randomized, double-blind, placebo-controlled prospective study is a clear strength of our study. We acknowledge, however, that due to its pilot nature, the reduced number of patients completing the study and its short duration are clear limitations. In fact, the reduced number of patients can be responsible for a type I error whereas the lack of a treatment effect on some of the variables studied might be due to a lack of statistical power. However, our results provide novel interesting observations that we think deserve further exploration in larger and longer cohorts of COPD patients that somehow can be done as a response to the study of Criner GJ et al. widening the profile of COPD patients included in it [18].
5. Conclusions
The results of this pilot randomized clinical trial do not support any significant effect of simvastatin treatment in patients with stable COPD on pulmonary and systemic inflammation, lung function and/or vascular biology. Yet, we found that it significantly increases Epo plasma levels and reduces serum UA levels. The potential clinical implications of these findings deserve further research.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
CB: patient selection and follow up, acquisition, analysis and interpretation of data, and drafted the manuscript.
AP: patient recruitment and acquisition of data, and drafted the manuscript.
AR: patient recruitment and follow up, and acquisition of data.
AI: carried out all ELISAS.
JV: performed lung function tests.
AN: carried out and validated all laboratory analysis.
JS: participated in the design of the study and has been involved in revising the manuscript critically.
AA: conceived of the study, participated in the design of the study and has been involved in revising the manuscript critically.
ES: acquisition of funding, conceived of the study, participated in its design and coordination, performed the statistical analysis, drafted the manuscript and has given final approval of the version to be published.
All authors read and approved the final manuscript.
Acknowledgments
Authors thank study participants for their willingness to contribute to medical research. This study was funded by SEPAR 2006. All funds were used to carry out all laboratory analysis and ELISAS.
Contributor Information
Catalina Balaguer, Email: catalina.balaguer@hcin.es.
Alejandro Peralta, Email: dperalta@ufm.edu.
Ángel Ríos, Email: angel.rios@ssib.es.
Amanda Iglesias, Email: amanda.iglesias@ssib.es.
Josep Lluís Valera, Email: josel.valera@ssib.es.
Aina Noguera, Email: aina.noguera@ssib.es.
Joan B. Soriano, Email: jbsoriano2@gmail.com.
Àlvar Agustí, Email: aagusti@clinic.ub.es.
Ernest Sala-Llinas, Email: ernest.sala@ssib.es.
References
- 1.Vestbo J., Hurd S.S., Agusti A.G. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 2013;187(4):347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 2.Miravitlles M., Soler-Cataluna J.J., Calle M. Spanish COPD Guidelines (GesEPOC): pharmacological treatment of stable COPD. Spanish Society of Pulmonology and Thoracic Surgery. Arch. Bronconeumol. 2012;48(7):247–257. doi: 10.1016/j.arbres.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Agusti A.G., Noguera A., Sauleda J., Sala E., Pons J., Busquets X. Systemic effects of chronic obstructive pulmonary disease. Eur. Respir. J. 2003;21(2):347–360. doi: 10.1183/09031936.03.00405703. [DOI] [PubMed] [Google Scholar]
- 4.Gan W.Q., Man S.F., Senthilselvan A., Sin D.D. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;59(7):574–580. doi: 10.1136/thx.2003.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lange L.A., Carlson C.S., Hindorff L.A. Association of polymorphisms in the CRP gene with circulating C-reactive protein levels and cardiovascular events. JAMA. 2006;296(22):2703–2711. doi: 10.1001/jama.296.22.2703. [DOI] [PubMed] [Google Scholar]
- 6.Man S.F., Connett J.E., Anthonisen N.R., Wise R.A., Tashkin D.P., Sin D.D. C-reactive protein and mortality in mild to moderate chronic obstructive pulmonary disease. Thorax. 2006;61(10):849–853. doi: 10.1136/thx.2006.059808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ridker P.M., Hennekens C.H., Buring J.E., Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N. Engl. J. Med. 2000;342(12):836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 8.Agusti A., Edwards L.D., Rennard S.I. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS One. 2012;7(5):e37483. doi: 10.1371/journal.pone.0037483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ridker P.M., Rifai N., Pfeffer M.A., Sacks F., Braunwald E. Long-term effects of pravastatin on plasma concentration of C-reactive protein. The Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1999;100(3):230–235. doi: 10.1161/01.cir.100.3.230. [DOI] [PubMed] [Google Scholar]
- 10.Arnaud C., Burger F., Steffens S. Statins reduce interleukin-6-induced C-reactive protein in human hepatocytes: new evidence for direct antiinflammatory effects of statins. Arterioscler. Thromb. Vasc. Biol. 2005;25(6):1231–1236. doi: 10.1161/01.ATV.0000163840.63685.0c. [DOI] [PubMed] [Google Scholar]
- 11.Lee J.H., Lee D.S., Kim E.K. Simvastatin inhibits cigarette smoking-induced emphysema and pulmonary hypertension in rat lungs. Am. J. Respir. Crit. Care Med. 2005;172(8):987–993. doi: 10.1164/rccm.200501-041OC. [DOI] [PubMed] [Google Scholar]
- 12.Mancini G.B., Etminan M., Zhang B., Levesque L.E., FitzGerald J.M., Brophy J.M. Reduction of morbidity and mortality by statins, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers in patients with chronic obstructive pulmonary disease. J. Am. Coll. Cardiol. 2006;47(12):2554–2560. doi: 10.1016/j.jacc.2006.04.039. [DOI] [PubMed] [Google Scholar]
- 13.Soyseth V., Brekke P.H., Smith P., Omland T. Statin use is associated with reduced mortality in COPD. Eur. Respir. J. 2007;29(2):279–283. doi: 10.1183/09031936.00106406. [DOI] [PubMed] [Google Scholar]
- 14.Young R.P., Hopkins R., Eaton T.E. Forced expiratory volume in one second: not just a lung function test but a marker of premature death from all causes. Eur. Respir. J. 2007;30(4):616–622. doi: 10.1183/09031936.00021707. [DOI] [PubMed] [Google Scholar]
- 15.Young R.P., Hopkins R., Eaton T.E. Pharmacological actions of statins: potential utility in COPD. Eur. Respir. Rev. 2009;18(114):222–232. doi: 10.1183/09059180.00005309. [DOI] [PubMed] [Google Scholar]
- 16.Janda S., Park K., FitzGerald J.M., Etminan M., Swiston J. Statins in COPD: a systematic review. Chest. 2009;136(3):734–743. doi: 10.1378/chest.09-0194. [DOI] [PubMed] [Google Scholar]
- 17.Wang M.T., Lo Y.W., Tsai C.L. Statin use and risk of COPD exacerbation requiring hospitalization. Am. J. Med. 2013;126(7):598–606. doi: 10.1016/j.amjmed.2013.01.036. [DOI] [PubMed] [Google Scholar]
- 18.Criner G.J., Connett J.E., Aaron S.D. Simvastatin for the prevention of exacerbations in moderate-to-severe COPD. N. Engl. J. Med. 2014;370(23):2201–2210. doi: 10.1056/NEJMoa1403086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaczmarek P., Sladek K., Skucha W. The influence of simvastatin on selected inflammatory markers in patients with chronic obstructive pulmonary disease. Pol. Arch. Med. Wewn. 2010;120(1–2):11–17. [PubMed] [Google Scholar]
- 20.Lee T.M., Lin M.S., Chang N.C. Usefulness of C-reactive protein and interleukin-6 as predictors of outcomes in patients with chronic obstructive pulmonary disease receiving pravastatin. Am. J. Cardiol. 2008;101(4):530–535. doi: 10.1016/j.amjcard.2007.09.102. [DOI] [PubMed] [Google Scholar]
- 21.Melbye H., Halvorsen D.S., Hartz I. Bronchial airflow limitation, smoking, body mass index, and statin use are strongly associated with the C-reactive protein level in the elderly. The Tromso Study 2001. Respir. Med. 2007;101(12):2541–2549. doi: 10.1016/j.rmed.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 22.Boschetto P., Beghe B., Fabbri L.M., Ceconi C. Link between chronic obstructive pulmonary disease and coronary artery disease: implication for clinical practice. Respirology. 2012;17(3):422–431. doi: 10.1111/j.1440-1843.2011.02118.x. [DOI] [PubMed] [Google Scholar]
- 23.Fabbri L.M., Beghe B., Agusti A. Cardiovascular mechanisms of death in severe COPD exacerbation: time to think and act beyond guidelines. Thorax. 2011;66(9):745–747. doi: 10.1136/thoraxjnl-2011-200406. [DOI] [PubMed] [Google Scholar]
- 24.Bartziokas K., Papaioannou A.I., Loukides S. Serum uric acid as a predictor of mortality and future exacerbations of COPD. Eur. Respir. J. 2014;43(1):43–53. doi: 10.1183/09031936.00209212. [DOI] [PubMed] [Google Scholar]
- 25.Roca J., Sanchis J., gusti-Vidal A. Spirometric reference values from a Mediterranean population. Bull. Eur. Physiopathol. Respir. 1986;22(3):217–224. [PubMed] [Google Scholar]
- 26.Laurent S., Cockcroft J., Van B.L. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur. Heart J. 2006;27(21):2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 27.Maclay J.D., McAllister D.A., Mills N.L. Vascular dysfunction in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2009;180(6):513–520. doi: 10.1164/rccm.200903-0414OC. [DOI] [PubMed] [Google Scholar]
- 28.Huang C.C., Chan W.L., Chen Y.C. Statin use and hospitalization in patients with chronic obstructive pulmonary disease: a nationwide population-based cohort study in Taiwan. Clin. Ther. 2011;33(10):1365–1370. doi: 10.1016/j.clinthera.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Bartziokas K., Papaioannou A.I., Minas M. Statins and outcome after hospitalization for COPD exacerbation: a prospective study. Pulm. Pharmacol. Ther. 2011;24(5):625–631. doi: 10.1016/j.pupt.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Lawes C.M., Thornley S., Young R. Statin use in COPD patients is associated with a reduction in mortality: a national cohort study. Prim. Care Respir. J. 2012;21(1):35–40. doi: 10.4104/pcrj.2011.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lahousse L., Loth D.W., Joos G.F. Statins, systemic inflammation and risk of death in COPD: the Rotterdam study. Pulm. Pharmacol. Ther. 2013;26(2):212–217. doi: 10.1016/j.pupt.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 32.Ludman A., Venugopal V., Yellon D.M., Hausenloy D.J. Statins and cardioprotection–more than just lipid lowering? Pharmacol. Ther. 2009;122(1):30–43. doi: 10.1016/j.pharmthera.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Young R.P., Hopkins R.J. Possible role of statins in COPD-related pulmonary hypertension. Chest. 2010;137(5):1250–1251. doi: 10.1378/chest.09-2778. [DOI] [PubMed] [Google Scholar]
- 34.Barr R.G. The epidemiology of vascular dysfunction relating to chronic obstructive pulmonary disease and emphysema. Proc. Am. Thorac. Soc. 2011;8(6):522–527. doi: 10.1513/pats.201101-008MW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der M.P., Voors A.A., Lipsic E., van Gilst W.H., van Veldhuisen D.J. Erythropoietin in cardiovascular diseases. Eur. Heart J. 2004;25(4):285–291. doi: 10.1016/j.ehj.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 36.Sahinarslan A., Yalcin R., Kocaman S.A. The relationship of serum erythropoietin level with coronary collateral grade. Can. J. Cardiol. 2011;27(5):589–595. doi: 10.1016/j.cjca.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Xu W., Guo Z., Mi L., Wang G. Serum erythropoietin: a useful biomarker for coronary collateral development and potential target for therapeutic angiogenesis among the patients with coronary chronic total occlusion. Biomarkers. 2013;18(4):343–348. doi: 10.3109/1354750X.2013.787459. [DOI] [PubMed] [Google Scholar]
- 38.Werner N., Kosiol S., Schiegl T. Circulating endothelial progenitor cells and cardiovascular outcomes. N. Engl. J. Med. 2005;353(10):999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 39.Moutzouri E., Liberopoulos E.N., Florentin M., Liamis G., Elisaf M.S. Effects of statin monotherapy versus statin plus ezetimibe combination on serum uric acid levels. J. Cardiovasc Pharmacol. Ther. 2013;18(1):13–18. doi: 10.1177/1074248412444463. [DOI] [PubMed] [Google Scholar]