Abstract
Introduction
The benefit of surfactant prescription for respiratory distress syndrome (RDS) has been approved. Curosurf and Survanta are two commonly used natural surfactants in Iran. Previous studies did not report priority for one of these two drugs. The present study aimed to compare the effectiveness and safety of Curosurf and Survanta in treatment of RDS.
Methods
In this randomized clinical trial, neonates were born with RDS diagnosis in two governmental and referral hospitals of Tehran (the capital of Iran) in 2014 were randomly selected. Neonates were randomly assigned into two groups receiving 100 mg/kg Curosurf or Survanta as soon as possible after randomization. Complications, mortality and needing the second dose were compared between the two groups.
Results
A total 112 patients with the mean gestational age of 32.59 ± 3.39 weeks were evaluated (56 patients in each group). There were no significant differences regarding birth weight, gestational age, delivery method, and parity between the two groups (P > 0.05). The complications were occurred in 18 neonates (32.1%) of Curosurf group and 20 neonates (35.7%) of Survanta group (RR = 0.922, 95% CI = 0.617–1.379). There were no significant differences regarding complications, mortality, and needing nasal CPAP and endotracheal tube between the two groups. In the neonates with gestational age of 29–32 weeks the IVH and NEC incidence were significantly more in Curosurf group compared to Survanta group (27.8% vs 0% and 22.3% vs 0%, P < 0.05).
Conclusion
There was no significant difference in complications or mortality between those two groups; however Curosurf was associated with less need of ET tube (in >32 birth weeks subgroup) and NCPAP (in 29–32 birth weeks subgroup) (p = 0.008). Further evaluations with longer follow-up duration are needed for comparing these two surfactants.
Keywords: Respiratory distress syndrome, Beractant, Poractant alfa, Complications, Treatment outcome
1. Introduction
Involving approximately 60% of infants with gestational age of lower than 30 weeks and 42% of those with birthweight lower than 1500 gr, respiratory distress syndrome (RDS) or hyaline membrane disease (HMD) is the most common respiratory disease and most important cause of mortality in premature infants [1], [2].
RDS has a progressive trend and its severity increases during the first two days of life which may be resulted in death due to hypoxemia and respiratory failure [3]. Various studies were conducted to determine the physiopathology of the disease and eventually the effect of surfactant in lung maturity was discovered in 1929. Successful application of surfactant in RDS was first reported in 1980 [4], [5].
Respiratory protection, endotracheal mechanical ventilation (EMV) and nasal continuous positive air way pressure (N.CPAP) and surfactant prescription is the basis of management in RDS [6], [7]. Associated complications with mechanical ventilation have led to design of new strategies [7]. Early application of N.CPAP and Surfactant has been shown effective in reducing need to EMV and complications as well as improving RDS prognosis in infants [7], [8]. Previous studies have reported INSURE as an effective method for reducing side effects of RDS management as well as hospitalization duration and expenses [9], [10], [11].
Thus the benefit of surfactant prescription for RDS treatment has been approved. At the present time, surfactant is available in Iran with various trading names and there are some controversies about their effectiveness. Curosurf and Survanta are two commonly used natural surfactants in Iran. Previous studies did not show significant priority for one of these two drugs. So in the present study we aimed to compare the effectiveness and safety of Curosurf versus Survanta in treatment of RDS.
2. Methods
This randomized clinical trial was approved by ethics committee of Baqiyatallah University of Medical Sciences (IR.BMSU.REC.1394.107) and was registered at Iranian Registry of Clinical Trials (IRCT) with unique ID of IRCT2015082817413N12.
Neonates were born with RDS diagnosis in Najmieh and Baqiyatallah hospitals, two governmental and referral hospitals from south and north areas of Tehran (the capital of Iran) in 2014 were randomly selected. The selection was done based on a randomization list among RDS neonates in 2014. Other inclusion criteria were birth weight more than 750 gr; gestational age less than 35 weeks; O2 saturation 85%–96%; signed informed consent by parents, and age ≤6 h at the time of randomization. RDS diagnosis was based on clinical picture of infant with the onset of progressive respiratory failure shortly after birth (manifested by an increase in the work of breathing and an increase in the oxygen requirement), in conjunction with a characteristic chest radiograph (low lung volume and the classic diffuse reticulogranular ground-glass appearance with air bronchograms).
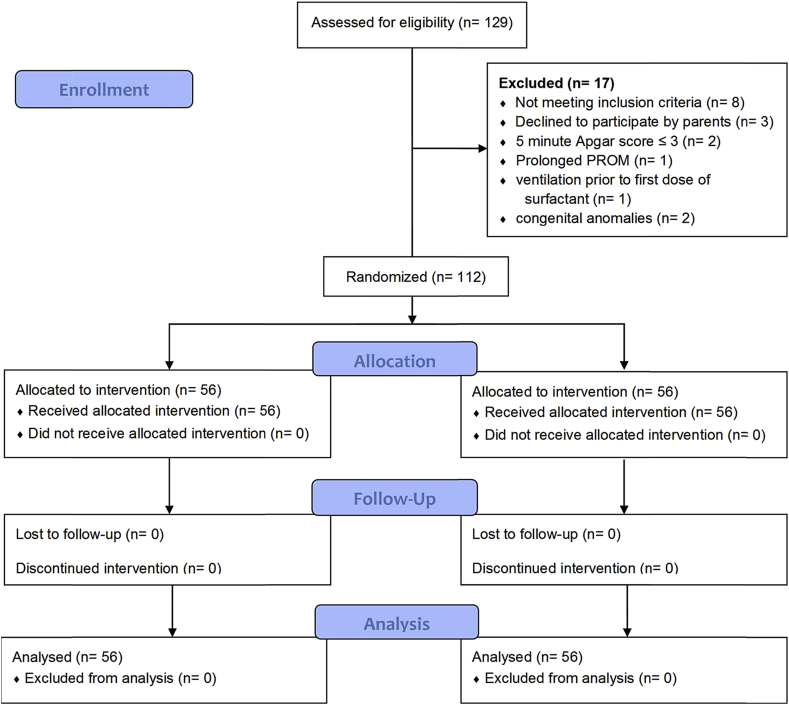
Neonates with congenital heart diseases and other life threatening congenital anomalies, respiratory failure due to other causes except RDS, 5 min Apgar score ≤3, proven fetal lung maturity profile from amniocentesis, prior treatment with exogenous surfactant, prolonged (≥3 weeks) rupture of membranes, untreated hypotension, or hypoglycemia, use of high-frequency ventilation prior to first dose of surfactant, and severe grades of intra-ventricular hemorrhage (grades III or IV) by cranial ultrasound prior to surfactant were excluded from study. The study flowchart is shown in Fig. 1.
Fig. 1.
Study flowchart.
Neonates were randomly assigned into two groups. Randomization was stratified by birth weight with two birth weight strata (below 1250 gr and more than 1250 gr). A randomization list was generated from 1 to 112 by SPSS software and neonates were randomly assigned into each intervention group by their numbers. The block randomization technique with 1:1 ratio was used to achieve balanced group sizes.
2.1. Intervention
After intubation the group one infants were received 100 mg/kg Curosurf and group two infants were received 100 mg/kg Survanta as soon as possible after randomization. Infants were extubated after surfactant injection and nasal continuous positive airway pressure (CPAP) set at 4 cm H2O pressure. The nasal CPAP were discontinued if the symptoms resolve and in case of FIO2 < 0–40, PEEP <5 cm H2O, and in the arterial blood gases PaCO2 < 60 mmHg, PaO2 > 50 mmHg, and PH > 7.25. In case of oxygen saturation by pulse oximetry less than 85%, PaCO2 > 60 mmHg, PaO2 < 50 mmHg, and PH < 7.2 the PEEP was increased to 6 cm H2O and in case of resistant oxygen saturation less than 85% the intubation was done and continued by mechanical ventilation. Additional dose of surfactant was given in 12–24 h if the infant continued to require mechanical ventilation and an FIO2 of 0.30 or greater to maintain an oxygen saturation by pulse oximetry greater than 85%.
2.2. Evaluations and measurements
Gestational age was measured by mean of two first trimester Crown Rump Length measurements at the first antenatal visit.
The Apgar score was calculated using heart rate, respiratory effort, muscle tone, reflex irritability, and color given values of 0, 1, or 2 [12]. The diagnosis of sepsis was based on positive blood culture and pneumonia was approved by chest radiography, seeing bilateral alveolar densities with air bronvhograms or irregular patchy infiltrates. Cranial ultrasonography was also used for diagnosis of intraventricular hemorrhage (IVH).
2.3. Statistical analysis
Data were analyzed by statistical package for social sciences (SPSS) version 21 (SPSS Inc. Chicago, IL) for windows. Study infants described using mean descriptive statistics, standard deviation, and frequency then two groups compared by independent sample t-test or its nonparametric equivalent (Mann-Whitney, if indicated) for quantitative variables, then Chi-square or Fisher exact (if indicated) tests used for qualitative variables. The P-values that were less than 0.05 were considered statistically significant.
3. Results
A total 112 patients with the mean gestational age of 32.59 ± 3.39 weeks and birth weight of 1911.3 ± 786.5 gr were evaluated (56 patients in each group). There were no significant differences regarding birth weight, gestational age, delivery method, and parity between the two groups (P > 0.05, Table 1). Also there were no significant differences in mother's diabetes, steroid injection, IUGR, placental abruption, PROM, first and 5 min Apgar, and needing CPR between the two groups (P > 0.05, Table 1). The mother's hypertension was significantly more in Curosurf group in comparison of Survanta group (16.1% vs. 3.6%, P = 0.026). Mothers/neonates-related risk factors are shown in Table 1.
Table 1.
Baseline characteristics and risk factors of neonates.a,b
| Variables | Curosurf (N = 56) | Survanta (N = 56) | P Value |
|---|---|---|---|
| Gender | 0.338 | ||
| Male | 30 (53.6) | 35 (62.5) | |
| Female | 26 (46.4) | 21 (37.5) | |
| Birth weight, gr | 1945.61 ± 723.9 | 1856.98 ± 848.5 | 0.544 |
| Gestational age, weeks | 33.21 ± 3.01 | 31.96 ± 3.66 | 0.051 |
| ≤ 32 weeks | 24 (42.9) | 34 (60.7) | 0.059 |
| > 32 weeks | 32 (57.1) | 22 (39.3) | |
| Delivery method | 0.801 | ||
| C/S | 46 (82.1) | 47 (83.9) | |
| NVD | 10 (17.9) | 9 (16.1) | |
| Parity | 0.534 | ||
| Nulliparous | 41 (73.2) | 38 (67.9) | |
| Multiparous | 15 (26.8) | 18 (32.1) | |
| Mother's underlying diseases | |||
| Diabetes | 2 (3.6) | 1 (1.8) | 0.558 |
| Hypertension | 9 (16.1) | 2 (3.6) | 0.026 |
| Steroid injection | 37 (66.1) | 44 (78.6) | 0.102 |
| IUGR | 3 (5.4) | 1 (1.8) | 0.309 |
| Placental abruption | 1 (1.8) | 2 (3.6) | 0.558 |
| PROM | 5 (8.9) | 11 (19.6) | 0.088 |
| First minute Apgar | 8.20 ± 1.09 | 7.80 ± 1.49 | 0.114 |
| Five minute Apgar | 9.29 ± 1.07 | 9.02 ± 1.10 | 0.196 |
| CPR | 2 (3.6) | 5 (8.9) | 0.242 |
a Abbreviations: cesarean section (C/S), normal vaginal delivery (NVD), intrauterine growth retardation (IUGR), premature rupture of membrane (PROM), and cardiopulmonary resynchronization (CPR).
b Data are presented as number (%) or mean ± standard deviation.
Time of surfactant injection was 8.04 ± 10.6 h in Curosurf group and 8.35 ± 9.76 h in Survanta group (P = 0.874). There were also no significant difference in needing the second surfactant dose between the two groups (OR = 2.179, 95% CI = 0.90–5.28). The mean hospital stay was 16.57 ± 11.43 days in Curosurf group and 15.36 ± 14.39 days in Survanta group (P = 0.622, Table 2).
Table 2.
Comparison of the time of first surfactant injection, needing second dose, and mean hospital stays between the two groups.a
| Variables | Curosurf (N = 56) | Survanta (N = 56) | P Value |
|---|---|---|---|
| Time of first surfactant | 8.04 ± 10.6 | 8.35 ± 9.76 | 0.874 |
| < 2 h | 23 (41.1) | 21 (37.5) | 0.789 |
| 2–12 h | 22 (39.3) | 21 (37.5) | |
| > 12 h | 11 (19.6) | 14 (25) | |
| Needing second dose | 10 (17.9) | 18 (32.1) | 0.081 |
| Hospital stay, days | 16.57 ± 11.43 | 15.36 ± 14.39 | 0.622 |
a Data are presented as number (%) or mean ± standard deviation.
3.1. Complications
Thirty eight neonates were complicated, the complications were occurred in 18 neonates (32.1%) of Curosurf group and 20 neonates (35.7%) of Survanta group (RR = 0.922, 95% CI = 0.617–1.379).
The sepsis was occurred in 7 neonates, pneumonia 16 neonates, IVH in 11 neonates, pulmonary hemorrhage in 9 neonates, NEC in 8 neonates, pneumothorax in 7 neonates, and ROP in 4 neonates. There were no significant differences regarding complications between the two groups (Table 3).
Table 3.
Comparing the occurrence of complications between the two groups.a,b
| Complications | Curosurf (N = 56) | Survanta (N = 56) | RR (95% CI) |
|---|---|---|---|
| Sepsis | 2 (3.6) | 5 (8.9) | 1.80 (0.550–5.89) |
| Pneumonia | 5 (8.9) | 11 (19.6) | 1.70 (0.802–3.60) |
| IVH | 7 (12.5) | 4 (7.1) | 0.762 (0.476–1.24) |
| Pulmonary hemorrhage | 3 (5.4) | 6 (10.7) | 1.54 (0.601–3.96) |
| NEC | 5 (8.9) | 3 (5.4) | 0.785 (0.443–1.39) |
| Pneumothorax | 2 (3.6) | 5 (8.9) | 1.80 (0.550–5.89) |
| ROP | 3 (5.4) | 1 (1.8) | 0.654 (0.360–1.19) |
| Total complications | 0.922 (0.617–1.379) | ||
| With complication | 18 (32.1) | 20 (35.7) | |
| Without complication | 38 (67.9) | 36 (64.3) | |
| Mortality | 2 (3.6) | 6 (10.7) | 2.08 (0.617–6.99) |
| Nasal CPAP | 11 (19.6) | 14 (25) | 0.851 (0.523–1.38) |
| ET Tube | 17 (30.4) | 27 (48.2) | 0.674 (0.440–1.03) |
a Abbreviations: intra-ventricular hemorrhage (IVH), necrotizing enteric colitis (NEC), retinopathy of prematurity (ROP), continuous positive airway pressure (CPAP), endotracheal (ET). b Data are presented as number (%).
Twenty one patients had gestational age of below 28 weeks (6 neonates in Curosurf and 15 neonates in Survanta groups). There were no significant differences regarding complications between the two groups in below 28 weeks neonates; sepsis (16.7% vs 26.7%, P = 0.672), pneumonia (16.7% vs 13.3%, P = 0.844), IVH (16.7% vs 26.7%, P = 0.627), pulmonary hemorrhage (11.1% vs 5.3%, P = 0.442), NEC (16.7% vs 20%, P = 0.648), pneumothorax (16.7%, vs 20%, P = 0.648), and ROP (16.7% vs 6.7%, P = 0.481).
There was also no significant difference for occurrence of total complications in the neonates with gestational age of below 28 weeks (RR = 1.0, 95% CI = 0.238–4.198).
In the neonates with below 28 weeks gestational age the needing nasal CPAP was significantly more in Curosurf group (RR = 4.25, 95% CI = 1.32–13.73) but there was no significant difference in needing ET tube between the two groups (RR = 0.80, 95% CI = 0.196–3.27).
Thirty seven neonates had gestational age of 29–32 weeks (18 neonates in Curosurf and 19 neonates in Survanta groups). The IVH and NEC incidence were significantly more in Curosurf group compared to Survanta group (27.8% vs 0% and 22.3% vs 0%, P < 0.05). There were no significant difference in other complications between the two groups in 29–32 weeks neonates (sepsis: 5.6% vs 5.3%, pneumonia: 0% vs 10.5%, pulmonary hemorrhage: 11.1% vs 5.3%, pneumothorax: 5.6% vs 5.3%, and ROP: 5.6% vs 0%, P > 0.05).
There was also no significant difference in occurrence of total complications in the neonates with gestational age of 29–32 weeks (RR = 1.0, 95% CI = 0.238–4.198).
In the neonates with 29–32 weeks gestational age, the needing nasal CPAP was significantly more in Survanta group (RR = 2.50, 95% CI = 1.61–3.88) but there was no significant difference in needing ET tube between the two groups (RR = 1.69, 95% CI = 0.351–1.34).
In the neonates with below 32 weeks gestational age, there were no significant differences in occurrence of each complication, total complications, mortality, and needing nasal CPAP and ET tube between the two groups (P > 0.05, Table 4).
Table 4.
Comparing the occurrence of complications between the two groups separated by gestational age less and more than 32 weeks.
| Complications | Curosurf | Survanta | P Value |
|---|---|---|---|
| Gestational age ≤32 weeks (N = 58) | |||
| Number | 24 | 34 | |
| Sepsis | 2 (8.3) | 5 (14.7) | 0.381 |
| Pneumonia | 1 (4.2) | 4 (11.8) | 0.304 |
| IVH | 6 (25) | 4 (11.8) | 0.189 |
| Pulmonary hemorrhage | 3 (12.5) | 6 (17.6) | 0.594 |
| NEC | 5 (20.8) | 3 (8.8) | 0.179 |
| Pneumothorax | 2 (8.3) | 4 (11.8) | 0.514 |
| ROP | 2 (8.3) | 1 (2.9) | 0.370 |
| Total complications | |||
| With complication | 12 (50) | 13 (38.2) | |
| Without complication | 12 (50) | 21 (61.8) | |
| Mortality | 1 (4.2) | 6 (17.6) | 0.221 |
| Nasal CPAP | 3 (12.5) | 8 (23.5) | 0.291 |
| ET Tube | 14 (58.3) | 18 (52.9) | 0.446 |
| Gestational age > 32 weeks (N = 54) | |||
| Number | 32 | 22 | |
| Sepsis | 0 (0) | 0 (0) | – |
| Pneumonia | 4 (12.5) | 7 (31.8) | 0.083 |
| IVH | 1 (3.1) | 0 (0) | 0.593 |
| Pulmonary hemorrhage | 0 (0) | 0 (0) | – |
| NEC | 0 (0) | 0 (0) | – |
| Pneumothorax | 0 (0) | 1 (4.5) | 0.407 |
| ROP | 1 (3.1) | 0 (0) | 0.593 |
| Total complications | |||
| With complication | 6 (18.8) | 7 (31.8) | |
| Without complication | 26 (81.2) | 15 (68.2) | |
| Mortality | 1 (3.1) | 0 (0) | 0.403 |
| Nasal CPAP | 8 (25) | 6 (27.3) | 0.547 |
| ET Tube | 3 (9.4) | 9 (40.9) | 0.008 |
In the neonates with more than 32 weeks gestational age, there were no significant differences in occurrence of each complication, total complications, mortality, and needing nasal CPAP between the two groups (P > 0.05, Table 4), but needing ET tube was significantly more in Survanta Group (P = 0.008).
The mean gestational age was 31.32 ± 3.85 weeks in complicated neonates and 33.24 ± 2.96 weeks in neonates without complications (P = 0.004). The mean birth weight was 1694.87 ± 800 gr in complicated neonates and 2022.43 ± 761 gr in non-complicated neonates (P = 0.036).
The complicated neonates were 71.1% boy and 28.9% girl while the non-complicated neonates were 51.4% boy and 48.6% girl (OR = 2.325, 95% CI = 1.01–5.37).
4. Discussion
Despite proper randomization in our study, there were slight differences in birth weight and gestational age showing superiority of Curosurf group. Thus the subgroup analyses were done for removing confounders' effect. We found that among complications, intraventricular hemorrhage (IVH) and necrotizing enterocollitis (NEC) are more frequent in Curosurf group in comparison with Survanta in 29–32 week neonates, while there was no significant difference between two groups for occurrence of other complications. We also realized that lower birthweight and lower gestational age were associated with more complications in both Curosurf and Survanta groups. Prescription of Curosurf was associated with lower CPAP or endotracheal tube requirement.
Ramanathan et al., comparing the effectiveness of Curosurf and Survanta, concluded that Curosurf group had significantly lower oxygen demand [13]. Confirming the results of the present study, Dizdar et al. have reported equal mortality rate and complications for both Curosurf and Survanta in a clinical trial. They also concluded lower oxygen demand in Curosurf group in first three days of treatment which is in agreement with the present study [14]. In another similar study on 150 premature infants by Gharehbaghi et al., they mentioned a significantly lower intubation days in Curosurf group in comparison with Survanta group, while there was no significant difference between two groups for occurrence of other complications such as pneumothorax, patent ductus arteriosus (PDA), IVH, bronchopulmonary dysplasia and mortality rate [15].
Evaluating 52 premature infants with RDS, allocated to two Curosurf and Survanta groups, Fujii et al. reported a lower oxygen therapy demand in first 72 h of treatment for Curosurf group. Curosurf treatment was also associated with lower PDA and air leak complication in comparison with Survanta, while two groups had same mortality rates [16].
In another clinical trial by Bozdag et al. they concluded that both Curosurf and Survanta improved oxygenation in VLBW infants with pulmonary hemorrhage but there was no difference for these two drugs for effectiveness on BPD as well as mortality rate [17]. Terek et al., comparing effectiveness of Curosurf and Survanta in preterm infants with RDS, mentioned that improvement of perfusion index (PI) was achieved earlier in Survanta group in comparison with Curosurf group. They also reported that inflammatory or oxidative stress was normalized earlier with Curosurf [18]. Eras et al. concluded that Survanta and Curosurf have similar effects in neurodevelopmental outcomes when used for treatment of RDS in preterm infants. There was no significant difference between two groups for incidence of cerebral palsy in their study [19]. In a retrospective cohort study, Paul et al. reported no superiority or preferential use of neither Survanta nor Curosurf in treatment of RDS in preterm infants [20].
Both Curosurf and Survanta vials are available in Iran and there is no tendency for more prescription of one of these drugs among Iranian physicians. These vials have the same price as 370$ in Iran and both of them are under appropriate cover of insurance. Our findings show that prescription of Survanta is more associated with need to injection of additional dose of surfactant; so prescribing Survanta may impose higher expenses to the healthcare system, Further studies, comparing more surfactant types, with more follow-up duration are suggested. One of our study limitations was non-matched patients in the two groups, thus future studies could evaluate a narrower age group of neonates.
5. Conclusion
Among complications, intraventricular hemorrhage and necrotizing enterocollitis were more frequent in Curosurf group in comparison with Survanta but there were no significant differences in occurrence of other complications between the two groups. Our results showed a slight superiority in prescription of Survanta compared to Curosurf due to lower complications; however Curosurf was observed to have less need to second doses injection, ET tube and NCPAP. Our study did not show a prominent difference between the two groups. Further evaluations with more follow-up duration are needed for comparing these two surfactants.
Authors' contribution
Data collection: Bita Najafian, Majid Shohrati, Sobhan Amin; data analysis: Hamidreza Karimi-Sari; drafting the manuscript: Hamidreza Karimi-Sari, Mohamad Hossein Khosravi, Niloofar Nikjoo; critical revision of paper: Bita Najafian, Majid Shohrati, Niloofar Nikjoo; study supervision: Bita Najafian.
Acknowledgment
Authors would like to thank Students' Research Committee of Baqiyatallah University of Medical Sciences.
References
- 1.Hintz S., Poole W., Wright L., Fanaroff A., Kendrick D., Laptook A. Changes in mortality and morbidities among infants born at less than 25 weeks during the post-surfactant era. Archives Dis. Childhood-Fetal Neonatal Ed. 2005;90(2):F128–F133. doi: 10.1136/adc.2003.046268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Helve O., Pitkänen O., Janér C., Andersson S. Pulmonary fluid balance in the human newborn infant. Neonatology. 2009;95(4):347–352. doi: 10.1159/000209300. [DOI] [PubMed] [Google Scholar]
- 3.Somaschini M., Nogee L.M., Sassi I., Danhaive O., Presi S., Boldrini R. Unexplained neonatal respiratory distress due to congenital surfactant deficiency. J. Pediatr. 2007;150(6):649–653. doi: 10.1016/j.jpeds.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Whitsett J.A. The molecular era of surfactant biology. Neonatology. 2014;105(4):337–343. doi: 10.1159/000360649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suresh G.K., Soll R.F. Overview of surfactant replacement trials. J. Perinatology. 2005;25:S40–S44. doi: 10.1038/sj.jp.7211320. [DOI] [PubMed] [Google Scholar]
- 6.Henderson-Smart D.J., Wilkinson A., Raynes-Greenow C.H. Mechanical ventilation for newborn infants with respiratory failure due to pulmonary disease. Cochrane Database Syst. Rev. 2002;4 doi: 10.1002/14651858.CD002770. (CD002770) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevens T.P., Harrington E., Blennow M., Soll R. Early surfactant administration with brief ventilation vs. selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome. Cochrane Database Syst. Rev. 2007;4(4) doi: 10.1002/14651858.CD003063.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verder H., Robertson B., Greisen G., Ebbesen F., Albertsen P., Lundstrom K. Surfactant therapy and nasal continuous positive airway pressure for newborns with respiratory distress syndrome. N. Engl. J. Med. 1994;331(16):1051–1055. doi: 10.1056/NEJM199410203311603. [DOI] [PubMed] [Google Scholar]
- 9.Najafian B., Saburi A., Fakhraei S.H., Afjeh A., Eghbal F., Noroozian R. Predicting factors of INSURE failure in low birth-weight neonates with respiratory distress syndrome: a logistic regression model. Iran. J. Neonatol. 2014;5(4):31. [Google Scholar]
- 10.Cherif A., Hachani C., Khrouf N. Risk factors of the failure of surfactant treatment by transient intubation during nasal continuous positive airway pressure in preterm infants. Am. J. Perinatology. 2008;25(10):647–652. doi: 10.1055/s-0028-1090590. [DOI] [PubMed] [Google Scholar]
- 11.Dani C., Corsini I., Bertini G., Fontanelli G., Pratesi S., Rubaltelli F.F. The INSURE method in preterm infants of less than 30 weeks' gestation. J. Maternal-Fetal Neonatal Med. 2010;23(9):1024–1029. doi: 10.3109/14767050903572174. [DOI] [PubMed] [Google Scholar]
- 12.Casey B.M., McIntire D.D., Leveno K.J. The continuing value of the Apgar score for the assessment of newborn infants. N. Engl. J. Med. 2001;344(7):467–471. doi: 10.1056/NEJM200102153440701. [DOI] [PubMed] [Google Scholar]
- 13.Ramanathan R., Rasmussen M.R., Gerstmann D.R., Finer N., Sekar K., Group N.A.S. A randomized, multicenter masked comparison trial of poractant alfa (Curosurf) versus beractant (Survanta) in the treatment of respiratory distress syndrome in preterm infants. Am. J. Perinatology. 2004;21(3):109–119. doi: 10.1055/s-2004-823779. [DOI] [PubMed] [Google Scholar]
- 14.Dizdar E.A., Sari F.N., Aydemir C., Oguz S.S., Erdeve O., Uras N. A randomized, controlled trial of poractant alfa versus beractant in the treatment of preterm infants with respiratory distress syndrome. Am. J. Perinatology. 2012;29(2):95–100. doi: 10.1055/s-0031-1295648. [DOI] [PubMed] [Google Scholar]
- 15.Gharehbaghi M.M., Sakha S.H.P., Ghojazadeh M., Firoozi F. Complications among premature neonates treated with beractant and poractant alfa. Indian J. Pediatr. 2010;77(7):751–754. doi: 10.1007/s12098-010-0097-y. [DOI] [PubMed] [Google Scholar]
- 16.Fujii A., Patel S., Allen R., Doros G., Guo C., Testa S. Poractant alfa and beractant treatment of very premature infants with respiratory distress syndrome. J. Perinatology. 2010;30(10):665–670. doi: 10.1038/jp.2010.20. [DOI] [PubMed] [Google Scholar]
- 17.Bozdağ Ş Dilli D., Gökmen T., Dilmen U. Comparison of two natural surfactants for pulmonary hemorrhage in very low-birth-weight infants: a randomized controlled trial. Am. J. Perinatology. 2015;32(3):211–218. doi: 10.1055/s-0034-1389090. [DOI] [PubMed] [Google Scholar]
- 18.Terek D., Gonulal D., Koroglu O.A., Yalaz M., Akisu M., Kultursay N. Effects of two different exogenous surfactant preparations on serial peripheral perfusion index and tissue carbon monoxide measurements in preterm infants with severe respiratory distress syndrome. Pediatr. Neonatol. 2014;56(4):248–255. doi: 10.1016/j.pedneo.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Eras Z., Dizdar E.A., Kanmaz G., Guzoglu N., Aksoy H.T., Altunkaya G.B. Neurodevelopmental outcomes of very low birth weight preterm infants treated with poractant alfa versus beractant for respiratory distress syndrome. Am. J. Perinatology. 2014;31(6):463–468. doi: 10.1055/s-0033-1351659. [DOI] [PubMed] [Google Scholar]
- 20.Paul S., Rao S., Kohan R., McMichael J., French N., Zhang G. Poractant alfa versus beractant for respiratory distress syndrome in preterm infants: a retrospective cohort study. J. Paediatr. Child Health. 2013;49(10):839–844. doi: 10.1111/jpc.12300. [DOI] [PubMed] [Google Scholar]