Abstract
Background
Coffee consumption has been consistently associated with a lower risk of type 2 diabetes mellitus in cohort studies. In addition, coffee components increased insulin sensitivity in animal models. However, data from intervention studies on the effect of coffee consumption on glucose metabolism have been limited by small sample sizes, lack of blinding, short follow-up duration and the use of surrogate indices of insulin sensitivity. We designed the Coffee for Metabolic Health (COMETH) study to evaluate the effect of coffee consumption on insulin sensitivity.
Methodology
The COMETH study is a double-blind randomized placebo-controlled 24-week trial. Participants were overweight, male and female habitual coffee consumers who were of Chinese, Malay and Asian-Indian ethnicity. We excluded smokers, persons with diabetes, and persons with low insulin resistance (HOMA-IR < 1.30). Participants were randomly assigned to receive daily 4 cups of instant regular coffee or 4 cups of a coffee-like placebo beverage. The hyperinsulinemic euglycemic clamp was performed at baseline and at the end of 24 weeks to determine changes in the bodyweight standardized M-value. Secondary outcomes included changes in fasting glucose and insulin sensitivity mediators such as adiponectin, markers of inflammation, liver function, and oxidative stress.
We enrolled 128 participants, 126 (57.1% males; aged 35–67 years) of whom completed baseline assessments.
Discussion
If improvement in insulin sensitivity in the coffee group is significantly greater than that of the placebo group, this would support the hypothesis that coffee consumption reduced risk of type 2 diabetes through biological pathways involving insulin sensitivity.
Trial registration
ClinicalTrials.gov identifier: NCT01738399. Registered on 28 November 2012. Trial Sponsor: Nestlé Research Center, Lausanne, Switzerland. Trial Site: National University of Singapore.
Keywords: Coffee, Insulin resistance, Insulin sensitivity, Type 2 diabetes, Non-insulin dependent diabetes mellitus, Hyperinsulinemic euglycemic clamp
Abbreviations
- T2DM
Type 2 diabetes mellitus
- COMETH
Coffee for metabolic health
- BMI
Body mass index
- HOMA-IR
Homeostasis model assessment of insulin resistance
- DSRB
Domain specific review board
- NRC
Nestlé Research Center
- PTC
Product Technology Centre
- GERD
Gastroesophageal reflux disease
- SCCS
Singapore Consortium of Cohort Studies
- SINDI-2
Singapore Indian Eye Study-2
- SP2
Singapore Prospective Study Program
- IL-6
Interleukin-6
- IL-18
Interleukin-18
- FFQ
Food frequency questionnaire
- IPAQ
International physical activity questionnaire
- CES-D
Center for epidemiological studies depression scale
- PROMIS
Patient reported outcomes measurement information system
- NHANES
National health and nutrition survey
- IBS-SSS
Irritable bowel syndrome-symptom severity scale
- GDR
Glucose disposal rate
- M-value
Metabolizable glucose-value
- M/I ratio
Metabolizable glucose-value standardized to average steady-state plasma insulin levels
- BW
Bodyweight
- FFM
Fat free mass
- Mbw
Metabolizable glucose-value adjusted to bodyweight
- Mffm
Metabolizable glucose-value adjusted to fat free mass
- M/Ibw
Metabolizable glucose-value adjusted to bodyweight and standardized to average steady-state plasma insulin levels
- M/Iffm
Metabolizable glucose-value adjusted to fat free mass and standardized to average steady-state plasma insulin levels
- MSC
Space corrected M-value
- M
Uncorrected M-value
- SC
Space correction factor
- UC
Urinary correction factor
- DXA
Dual energy X-ray absorptiometry
- QC
Quality control
- CONSORT
Consolidated standards of reporting trials
- ANCOVA
Analysis of covariance
- ITT
Intention to treat
- PP
Per-protocol
- WC
Waist circumference
- WHTR
Waist-to-height ratio
- LDL
Low-density lipoprotein
- HDL
High-density lipoprotein
- CISI
Composite insulin sensitivity index
1. Introduction
The global burden of type 2 diabetes mellitus (T2DM) [1], calls for concerted efforts targeted at diabetes prevention. Insulin resistance is a major pathway to the development of T2DM [2] and it is influenced by both environmental and genetic factors [1]. The rapid rise in T2DM burden in recent decades has been attributed to a deterioration in diet quality [3], increase in obesity prevalence [2] and reduction of physical activity [2]. Dietary prevention of T2DM has largely focused on nutrients and food groups [3], but evidence is emerging that the choice of beverages, such as coffee, can also affect the development of T2DM.
Coffee is one of the most widely consumed beverages in the world [4]. Clues to the potential beneficial effect of coffee against T2DM were first published in 2002 [5]. Since 2002, more than 20 prospective cohort studies conducted in the U.S., Asia, and Europe showed an inverse association between coffee intake and T2DM [6]. In a recent meta-analysis, coffee intake was inversely associated with T2DM risk in a dose-dependent manner with 4 cups/day being associated with a 25% lower risk [6]. Although observational studies statistically adjusted for confounders, residual confounding by unmeasured or imperfectly measured confounders may persist, affecting the accuracy of results. Therefore, randomized trials are needed to provide more conclusive evidence about the effect of coffee on glucose homeostasis. In addition, trials can include detailed measurements that provide insights into the biological mechanisms that may underlie the putative effect of coffee on risk of T2DM.
Data on the modulation of glucose homeostasis by coffee from trials are limited. Limitations include small sample sizes, lack of a suitable placebo beverage to facilitate blinding, short follow-up periods, and the use of surrogate insulin sensitivity measures. In the trials conducted by Kempf et al. [7] and Wedick et al. [8] that evaluated 12 and 8 weeks of coffee consumption respectively, no change in insulin sensitivity or glucose tolerance was observed, but adiponectin levels significantly increased after coffee consumption. Ohnaka et al. [9], in a 16-week trial reported a decrease in 2-h glucose levels after coffee consumption although no change in insulin sensitivity was observed. In a crossover trial, van Dam et al. [10] reported an increase in fasting insulin levels after very high coffee consumption, which could reflect either an increase in insulin resistance or reduced insulin clearance. Furthermore, by assessing fasting hepatic glucose production in a 2-week randomized crossover trial, Lecoultre et al. [11] concluded that coffee attenuated fructose-induced hepatic insulin resistance.
Thus, results from conducted coffee trials have been inconclusive. We therefore designed the Coffee for Metabolic Health (COMETH) study, a double-blind randomized trial, with an aim to investigate the longer term (24 weeks) effects of coffee on insulin sensitivity in overweight participants using the gold standard technique of hyperinsulinemic euglycemic clamp. In addition, with a larger sample size and a coffee-like placebo beverage to facilitate masking, we sought to overcome the limitations of previous trials. In this article, we describe the rationale, methodology and baseline characteristics of participants in the COMETH study.
2. Methodology
2.1. Aims and hypothesis
The primary aim of the COMETH study was to examine the effects of 24 weeks of regular coffee consumption on insulin sensitivity in adults who were at higher risk of developing T2DM. We hypothesized that participants randomized to receive coffee (intervention group) would show a greater improvement in insulin sensitivity as measured by the hyperinsulinemic euglycemic clamp, and an improvement in mediators of insulin sensitivity after 24 weeks of coffee consumption, as compared to those receiving placebo (control group).
The secondary aims of the COMETH study were to examine the effects of regular coffee on glycemia (i.e. fasting plasma glucose); biological risk factors that may underlie a possible effect on insulin sensitivity (i.e. adiponectin, non-esterified fatty acids, and markers of inflammation, oxidative stress, liver function and renal function); cardiovascular risk factors (blood lipids, blood pressure); gut health; and psychological health (fatigue and depression).
2.2. Study design overview and ethics
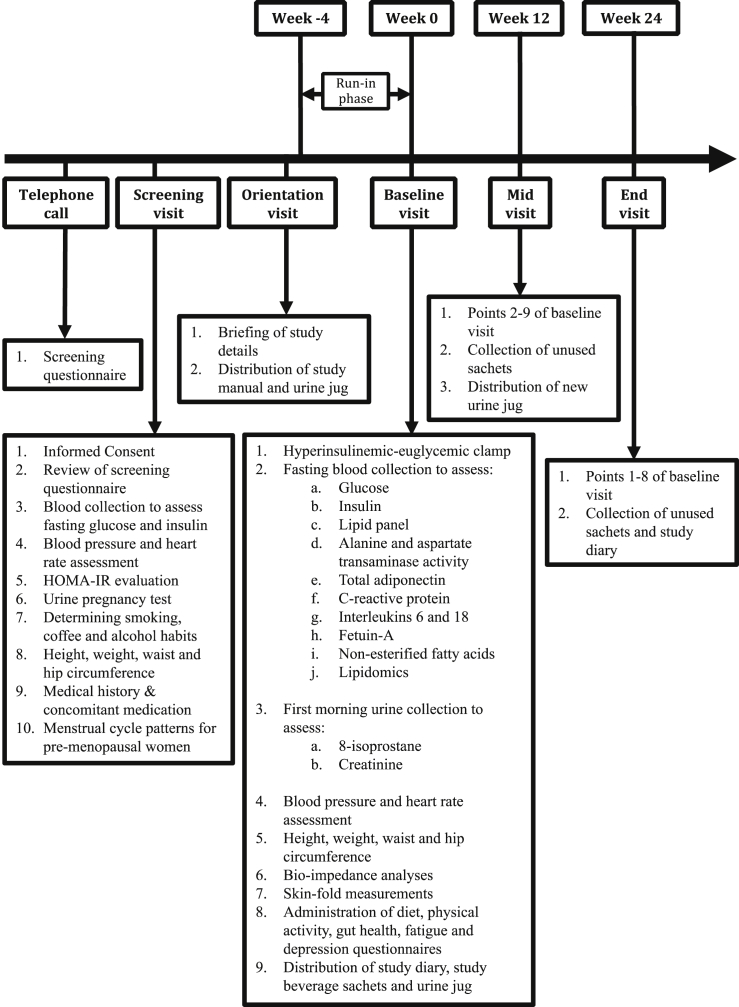
COMETH was a single-center, randomized, double-blind, placebo-controlled intervention trial comparing regular consumption of coffee and a coffee-like beverage in parallel (Fig. 1).
Fig. 1.
Timeline and activities of the Coffee for Metabolic Health (COMETH) study. Anthropometric measurements were performed at t = 0 min in all visits.
For 24 weeks, overweight and insulin resistant individuals aged 35–69 years, who satisfied our inclusion/exclusion criteria (see section 2.4), were randomly assigned to receive either 4 sachets (cups) of an instant regular coffee beverage (intervention) or 4 sachets (cups) of a coffee-like beverage (placebo) daily. The placebo beverage mimicked the taste and color of coffee, thereby allowing blinding to be possible. Being double-blind, neither the participants nor the researchers knew the identity of the beverage each subject consumed.
We used a variety of methods to recruit participants. Interested participants were screened using a telephonic survey. Participants who successfully completed the initial screening were invited for an in-person screening visit during which informed consent was obtained, height, weight and blood pressure was measured, and blood was drawn to measure glucose and insulin for homeostasis model assessment of insulin resistance (HOMA-IR) [12] calculations. Individuals who were insulin sensitive (HOMA-IR < 1.30) were excluded. Eligible participants who had a HOMA-IR ≥ 1.30 were invited for an orientation session, after which the participants were asked to refrain from coffee for a period of 4 weeks (run-in phase).
Following randomization to the respective intervention arms, hyperinsulinemic euglycemic clamps were performed at baseline, and at 24 weeks to measure insulin sensitivity. Questionnaire information, fasting blood samples, first morning urine samples and clinical measures were obtained at baseline, 12 weeks and 24 weeks. With an expected dropout rate of 20%, 104 out of a planned number of 130 enrolled participants were anticipated to complete the study.
The COMETH study was conducted in Singapore at a single site, the Investigational Medical Unit located at the Yong Loo Lin School of Medicine, National University of Singapore.
Our study was conducted according to the principles and rules laid down in the Declaration of Helsinki and its subsequent amendments. The study was approved by the Domain Specific Review Board (DSRB) of the National Healthcare Group, Singapore. Written informed consent was obtained from all participants prior to commencing study related activities.
An independent study monitor was appointed by the sponsor to: 1) review all aspects of the protocol and documentation before study commencement; 2) to evaluate clarity and consistency of information entered into database during the trial; and 3) to ensure completeness of documentation at the end of the trial. Safety of participants was monitored throughout the study by investigators. All adverse events were documented, evaluated as to whether they were expected to occur, determined as serious (death, life threatening, involves hospitalization, results in incapacitation, congenital anomaly/birth defect, or any event that is medically significant) or non-serious (all other adverse events not corresponding to the definition of serious events), and assessed for relationship with the study beverage by a physician. Serious adverse events (regardless of relationship with study beverage) were promptly reported to DSRB and the sponsor, and followed up until the outcome was known.
The study is registered at the US National Institute of Health clinical trial registry accessible at https://clinicaltrials.gov/ (NCT01738399).
2.3. Study beverage
2.3.1. Formulation and production
The Nestlé Research Center (NRC) in Lausanne, Switzerland, and the Nestlé Product Technology Centre (PTC), Orbe, Switzerland, formulated the placebo and coffee beverages. The placebo beverage was formulated with the aim to look, taste, and smell like coffee. Each sachet of the coffee beverage, contained 26.3% of regular Nescafé mixed with 73.7% of a non-dairy creamer (Table 1). Nestlé Nescafé was produced in the Shah Alam factory (Malaysia). The Nescafé recipe consists of 100% Robusta coffee (Table 1). Each sachet of the placebo beverage, contained 32.5% of a colored non-dairy creamer mixed with 67.5% of the same non-dairy creamer used for the coffee beverage (Table 1). Both the coffee and the coffee-like beverage contained 30 kcal per sachet.
Table 1.
Composition of study beverages in the Coffee for Metabolic Health (COMETH) study.
| Study beverage | Formulation | % Amount per sachet | Caloric content |
|---|---|---|---|
| Treatment | Regular Nestlé Nescafé | 26.3% | 30 kcal/sachet |
| Non-dairy creamer (Coffee mate light) | 73.7% | ||
| Placebo | Colored non-dairy creamer | 32.5% | 30 kcal/sachet |
| Non-dairy creamer (Coffee mate light) | 67.5% |
Sachets were labeled with expiration date and randomization code for each subject. All boxes were kept under lock and key at room temperature in a storeroom at the study site.
2.3.2. Instructions for consumption of the study beverage
Study beverages were distributed to participants at baseline and on their second clinic visit in the 12th week. Participants were instructed on the preparation of the beverage and to consume 4 cups of coffee (or placebo) per day for 24 weeks [at breakfast, one mid-morning, one with lunch, and one after lunch, with the last cup being consumed no later than 8:00 p.m.]. If participants missed a drink, they were advised to combine the missed dose with the next scheduled drink. Participants were allowed to adjust the amount of added water according to their preferred coffee strength. Participants were also allowed to add sweeteners or milk to the study beverage if they wished to do so but were instructed to add the same amount each time and report the amount.
2.4. Study population
Study staff ensured that potential participants complied with all inclusion criteria and possessed none of the exclusion criteria before enrolling individuals as study participants.
2.4.1. Inclusion criteria
All individuals had to comply with all the following inclusion criteria before they were considered for recruitment:
Individuals of Chinese, Malay or Asian Indian ethnicity
Aged ≥35 to ≤69 years
Individuals should have a non-diabetic fasting plasma glucose concentration (<7.0 mM).
Body mass index ≥22.5 to ≤35.4 kg/m2
Users of at least 1 cup of caffeinated coffee per day and who are willing to be randomized to any of the two intervention groups.
Individuals should be willing to stop consuming caffeinated soft drinks or supplements during the study and to drink coffee with non-dairy creamer.
Non-smokers (<1 cigarette per week)
Participants must have been weight stable for at least 8 weeks preceding the screening visit (±2.5 kgs).
2.4.2. Exclusion criteria
Individuals representing one or more of the following criteria were excluded from participation in the study:
Individuals deemed as being insulin-sensitive during the screening visit with a HOMA-IR of <1.30.
Any condition or illness that may affect study outcomes or would make participation potentially harmful such as pregnancy or breastfeeding, diabetes mellitus, heart disease, stroke, hypertension, malabsorption syndromes, gastroesophageal reflux disease (GERD), a history of ulcer, clotting or bleeding disorders, allergy to the test beverage and allergy to insulin, according to a detailed medical history.
Participants who had food allergies were excluded based on the investigator's discretion.
Participants who consumed more than 2 alcoholic servings/day on a regular basis and >8 caffeinated beverages (i.e. tea and coffee) per day.
Individuals who had a history of substance abuse or use of medications that could interfere with the intervention including bronchodilators, quinolone antibiotics, monoamine oxidase inhibitors, anxiolytics, ranitidine, corticosteroids, growth hormone and anti-hypertensives. These conditions were screened based on subject reporting. Potential participants were asked to bring in their current medications at the time of screening, and these were checked by study-staff.
Individuals who were consuming traditional medications, herbal or dietary supplements that could have affected study outcomes.
Individuals who could not be expected to comply with the study procedures.
Individuals who were participating in another trial or participated in another clinical trial during the 12 weeks prior to the beginning of our study.
Premenopausal women with self-reported irregular menstrual cycles or peri-menopausal women.
2.4.3. Recruitment
Rolling recruitment was employed in our study. Recruitment efforts were first directed towards overweight, coffee drinking men and women in ongoing cohort studies such as the Singapore Consortium of Cohort Studies (SCCS) [13] and the Singapore Indian Eye Study-2 (SINDI-2) [14], who gave prior consent to be re-contacted.
Participants were also recruited from the Singaporean Chinese, Malay and Indian communities through newspaper advertisements, and word-of-mouth methods. Further recruitment efforts included distributing study leaflets into mailboxes at various residential districts and door-to-door enquiry about interest in the study. Distribution of leaflets and enquiries about participant interest was also done at places of worship, community centers, taxi stands, malls and food centers, and various medical and health screening centres. Additionally, we engaged people through healthy lifestyle or active ageing programmes conducted by community centers and articles in community center newsletters. Finally, we encouraged enrolled individuals to refer other potential participants to the study. Most of the participants of our study were identified through responses to newspaper advertisements.
Our study staff administered a brief screening questionnaire over the telephone to persons who had responded positively to our recruitment efforts to conduct an initial eligibility assessment. In addition, a brief description of the study procedures was conveyed to the individuals.
2.4.4. Screening
Participants were provided information sheets describing the study and its possible risks and benefits. The screening process involved consent taking, reviewing medical history and lifestyle habits, measurements of blood pressure, heart rate, height and weight, a spot urine sample to rule out pregnancy (for women), and a fasting blood sample for measurement of glucose and insulin concentrations. We calculated the HOMA-IR score as [(fasting glucose × fasting insulin) ÷ 22.5] [12] (Fig. 1). Participants were excluded if the HOMA-IR score was <1.30. This cut-off corresponded to the 50th percentile of the HOMA-IR values in a Singaporean population-based cohort [Singapore Prospective Study Program (SP2)] [15]. At the commencement of trial, we had used a HOMA-IR threshold of 2.20 (corresponding to the 75th percentile of the SP2 cohort) but this was altered to 1.30 under the direction of our sponsor in view of difficulties encountered in participant recruitment. The HOMA-IR is an indicator of insulin resistance that is reasonably well correlated with values obtained by glucose clamps [16]. Individuals were enrolled after having fulfilled all inclusion criteria, presenting none of the exclusion criteria as assessed during the screening visit.
2.4.5. Subject withdrawal during the run-in period
As assessed by study investigators, individuals were withdrawn during the run-in period of the study in the event of: 1) illness, after examination by the medical staff; 2) lack of compliance; 3) observation of exclusion criteria, and; 4) subject's decision. Individuals who withdrew before baseline were replaced in order to achieve enrolment of 130 participants commencing intervention at baseline.
2.5. Timeline of study visits
After screening, eligible and consented individuals were asked to avoid caffeine or coffee containing products and medications, or medications that may interact with caffeine for one month prior to the start of the study as part of the run-in phase and for the entire duration of the study. The purpose of the run-in phase was to gradually wean participants from coffee to facilitate adequate washout, assist with adherence to study protocol which required participants to not consume non-study caffeinated foods and beverages, and to minimize differential dropout rates between the placebo and the intervention arms. In addition, to reduce symptoms related to caffeine withdrawal, we advised participants to gradually reduce their coffee consumption at the start of the run-in period over a duration of 1 week.
Participants were invited for an orientation session prior to the run-in-phase during which the conduct of the study was described in detail. During the 30 min orientation visit, participants were given a study manual and a urine jug for the collection of first morning urine on the day of baseline clinic visit. Study staff reminded participants of the commencement of the run-in phase over the telephone. In addition, study staff contacted participants one day prior to the baseline visit to deliver further instructions with regards to fasting and collection of urine for baseline visit (Fig. 1).
Following the orientation visit, there were three clinic visits (baseline, week-12, week-24). Week-12 and week-24 visits will henceforth be referred to as “Mid” and “End” visits respectively. Pre-menopausal women were scheduled at the same phase of their menstrual cycle for all three clinic visits. Participants were instructed to come in after an overnight fast and stop drinking caffeinated beverages including coffee after 8:00 p.m. on the day before clinic visits. In addition, participants were instructed to collect first-morning urine samples in the jugs provided before each clinic visit (Fig. 1). When distributing study beverage sachets, participants received instructions about the consumption of study beverage (coffee/placebo).
Data on anthropometrics, body fatness, blood pressure, and heart rate, were obtained, and fasting blood samples were collected at each clinic visit. A hyperinsulinemic euglycemic clamp was performed at the baseline and end clinic visits. Participants were instructed to complete questionnaires related to their diet, physical activity, gut health and mental well-being at each clinic visit (Fig. 1). Information on adverse events and concomitant medication were obtained during each clinic visit. After the baseline and mid clinic visits, participants were given a study diary and sufficient study beverage sachets to last them for the next 3 months. In addition, participants were instructed to return unused study beverage sachets and the completed study diary at the mid and end clinic visits. Participants were regularly contacted over the telephone to discuss concerns and provide motivation to encourage their continued participation in the study.
2.6. Randomization and intervention allocation
Two lists of 70 individual non-speaking randomization codes (e.g. 3K5, 7W4), were created for coffee and placebo products by a biostatistician at NRC. Both lists were given to a product manager at the Nestlé Product Technology Centre (PTC) who assigned one list as the coffee product codes and the other list as the placebo product codes. The product manager at Nestlé PTC was therefore unblind. On the day of baseline visit, participants were randomly assigned to the coffee or placebo group by means of minimization method of randomization [17] based on gender and ethnicity (Malay, Chinese or Indian) as stratification factors. Using this strategy, the first participant was truly randomly assigned to either coffee or placebo. Intervention allocation for subsequent participants were made dynamically to minimize imbalance between the two intervention arms with regards to each level of the above-mentioned stratification factors. Randomization was performed at the study site using a secure online “TRIALSYS” system (developed internally at NRC). In order to randomize a subject, study staff responsible for distributing the product box (containing sachets of coffee or placebo) entered the subject's gender and race (Chinese, Malay or Indian) in the TRIALSYS system to generate a randomization code. The product box with the assigned randomization code was then located before the study beverage sachets in it were distributed to participants. The biostatistician responsible for creating the randomization codes and configuring “TRIALSYS” at NRC was semi-blind [possessing knowledge of which participants belonging to one intervention group or the other but not the identity (coffee or placebo) of the groups]. However, these individuals (biostatistician at NRC, product manager at PTC and product distributor at study site) were not involved in any other study tasks besides their primary tasks. The rest of the research staff (including investigators, project manager, other biostatisticians, data managers, clinical project manager, monitor, and laboratory analysts) involved in the conduct of the study were truly blind.
2.7. Physical examination
Physical examinations including anthropometric measurements, blood pressure measurements, and blood and urine collection were performed according to a standardized protocol at baseline, mid and end clinic visits. Participants were measured in thin, light clothes in the morning typically from 8:00 a.m. to 9:00 a.m. Study subjects were instructed to visit the washroom before commencement of measurements. Without footwear, body weight was measured to the nearest 0.1 kg using a SECA 708 digital scale (Hamburg, Germany). Height was measured to the nearest centimeter without footwear using a SECA 708 height gauge (Hamburg, Germany). Waist and hip circumferences were measured under the subjects' clothes using a Butterfly brand measuring tape (China). Waist circumference was measured to the nearest centimeter midway between the lowest rib margin and the iliac crest. Hip circumference was measured to the nearest centimeter over the greater trochanters, perpendicular to the length axis of the body. We measured triceps, biceps, subscapular and supra-iliac skin-folds over the right side of the body in triplicates to the nearest millimeter using the Holtain Tanner/Whitehouse Skinfold Caliper (Crymych, Pembrokeshire, Wales, United Kingdom). Bio-impedance measurements such as fat free mass and fat mass were carried out to the nearest 0.1 kg according to manufacturer's recommendations using the Tanita Body Composition Analyzer TBF-300 (Tokyo, Japan).
With participants seated upright, systolic and diastolic blood pressure measurements were performed to the nearest millimeter of mercury (mmHg) in duplicates while heart rate was measured in duplicates to the nearest beat per minute (bpm) using the Omron blood pressure and heart rate monitor HEM-7203 (Kyoto, Japan).
Participants were instructed to fast overnight for at least 10 h prior to blood draw. At baseline, mid and end clinic visits, 35 ml of fasting blood was obtained by venipuncture for the analyses of fasting glucose, insulin, triglycerides, HDL, LDL and total cholesterol, alanine and aspartate aminotransferase, interleukin-6 (IL-6), IL-18, fetuin-A, total adiponectin, C-reactive protein, and non-esterified fatty acid levels. Fasting glucose levels were measured by an enzymatic method (GLU Reagent OSR6221), triglycerides were assessed by a colorimetric method (TRIG Reagent OSR61118), HDL-cholesterol was measured by a colorimetric method (HDL-C Reagent OSR6287), total cholesterol was determined in a colorimetric assay (CHOL Reagent OSR6116), alanine (ALT IFCC Reagent OSR6107) and aspartate (AST Reagent OSR6209) aminotransferase levels were determined by enzymatic assays, and C-reactive protein was measured in an immunoturbidimetric assay (CRP Latex Reagent OSR6199) in a AU5800 analyzer (Beckman Coulter, Brea, CA, United States). Insulin levels were determined by a sandwich immunoassay using ADVIA Centaur XP 02230141 (Siemens Medical Solutions Diagnostics, Tarrytown, NY, United States). LDL-cholesterol was estimated using the Friedewald's formula: ; where LDL is LDL-cholesterol; TC is total cholesterol; HDL is HDL-cholesterol and TG is triglycerides [18]. IL-6 (HS600B, R&D Systems, Inc, Minneapolis, MN, United States), IL-18 (7620, MBL International Corporation, Woburn, MA, United States), fetuin-A (DFTA00, R&D Systems, Inc, Minneapolis, MN, United States), and total adiponectin (RD195023100, BioVendor, Inc, Czech Republic) were measured using enzyme-linked immunosorbent assays on the EVOLIS™ System (Bio-Rad Laboratories, Inc, Hercules, CA, United States). Non-esterified fatty acids (434-91795 & 436-91995, Wako Chemicals GmbH, Neuss, Germany) were measured by an enzymatic colorimetric method in the Cobas C111 analyzer (Roche, Basel, Switzerland). During hyperinsulinemic euglycemic clamp at baseline and end visits, a further 5 ml of blood was drawn at 0, 90 and 120 min time points to determine fasting and steady state insulin levels. On the night before each clinic visit, participants were instructed to empty their bladder before going to bed. In the morning, participants were instructed to collect their first-morning urine in the bottles provided to them as soon as they woke up. Participants were also advised to collect urine passed in the middle of the night into the same bottle. About 18 ml of the first-morning urine was collected primarily for the analyses of creatinine and 8-isoprostane.
2.8. Questionnaires
We collected information on lifestyle (diet and physical activity), mental health (depression and fatigue), and gut health at baseline, mid-study, and the final study visit using standardized questionnaires. A validated [19] food frequency questionnaire (FFQ), adapted from the Singapore National Nutrition Survey 2010 [20], was used to assess dietary intakes over the past month. Dietary information obtained included intakes of a wide variety of foods and, alcoholic and non-alcoholic beverages. To assess physical activity in the last seven days, the validated [21] International Physical Activity Questionnaire (IPAQ) (short version comprising 4 generic items) [22] was administered. The interviewer-administered IPAQ consisted of queries regarding light, moderate and vigorous physical activities during leisure, transportation, at work, and in the household.
To assess mental health over the past week, participants self-administered a validated [23] 20-item Center for Epidemiological Studies Depression Scale (CES-D) [24] questionnaire. The CES-D evaluates the most noticeable signs of depression in the general population such as feelings of hopelessness, guilt and worthlessness. Each item on the CES-D is scored on a four-point scale and the sum of all scores ranges from 0 to 60. High scores suggest a high level of distress while low scores suggest a low level of distress. Fatigue over the past week was assessed using the self-administered 7-item Patient Reported Outcomes Measurement Information System (PROMIS) fatigue scale short form [25], [26] questionnaire which has been validated in women with premenstrual symptoms [27] and in osteoarthritis patients [28].
Gut health over the past week was evaluated using a self-administered questionnaire adapted from bowel health component of the National Health and Nutrition Survey (NHANES) [29], [30] and the Irritable Bowel Syndrome-Symptom Severity Scale (IBS-SSS) [31]. The gut health questionnaire entailed queries on frequency of bowel movements, type of stool, history of constipation and diarrhea, and abdominal discomfort. All questionnaires were administered at baseline, mid and end visits.
2.9. The hyperinsulinemic-euglycemic clamp
The hyperinsulinemic-euglycemic clamp [32] is regarded as the gold standard method of evaluating insulin sensitivity in humans [33] that offers the most direct measurement of insulin-mediated glucose disposal under steady state conditions in vivo [12]. A 2-h hyperinsulinemic euglycemic clamp procedure was carried out after an overnight fast. During the clamp procedure, participants were intravenously saturated with exogenous insulin at a high infusion rate (40 mU of insulin per m2 of body surface area per min). This results in increased glucose disposal [uptake into peripheral tissues; mainly skeletal muscles (∼85%)] [34] in vivo while hepatic glucose production is suppressed. Concurrently, exogenous glucose was infused to replenish the increased glucose disposal mediated by insulin infusion. Using a bedside glucose analyzer (YSI Incorporated, USA), the rate of glucose infusion was adjusted at 5-min intervals to maintain euglycemic levels (5.0 mmol/L). Blood samples were obtained at 0, 90 and 120 min to determine plasma insulin levels for HOMA-IR and metabolized glucose (M-value) computations. By maintaining a euglycemic steady-state, the rate of glucose infusion would reflect the rate of systemic glucose disposal into peripheral tissues such as skeletal muscles. Therefore, individuals with slower rates of glucose infusion (i.e. lower M values) are acknowledged to be more resistant to insulin than individuals with faster rates of infusion.
The GIR over the final 30 min of the clamp procedure reflects the steady-state glucose disposal rate (GDR) or metabolizable glucose (M-value). The M-value is the measure of insulin sensitivity of the clamp procedure and it is typically standardized to body weight (Kg) or fat free mass (Kg); less frequently to body surface area (m2) or metabolic size (KgFFM + 17.7) [35]. In addition to the M-value, the M/I ratio, known as the insulin sensitivity index of euglycemic clamp, is frequently reported. The M/I ratio (amount of glucose metabolized per unit of plasma insulin) [36] is obtained by normalizing the M-value with the average plasma insulin levels during the final 30 min of the clamp procedure (also known as steady-state plasma insulin levels) [37]. Average steady-state insulin levels were calculated by averaging the 90 and 120-min plasma insulin values.
In this study, the primary outcome refers to the amount of glucose metabolized (M-value) per kilogram of body weight per minute (mg kg−1 min−1) (Mbw). The decision to adjust the M-value to bodyweight instead of fat free mass (FFM) stems from the controversy about the extent of glucose disposal in adipose tissue [38]; studies have shown glucose uptake to be possible in adipocytes under insulin mediated conditions in vivo [34]. Furthermore, FFM, as determined by bio-impedance analyses has been shown to overestimate and underestimate FFM in lean and obese individuals respectively as compared to the gold standard Dual Energy X-Ray Absorptiometry (DXA) [39]. The M/I ratio, in this study, refers to the percentage of the amount of glucose metabolized per kilogram of bodyweight per minute standardized to per unit of steady state plasma insulin levels (100 × mg kg−1 min−1 mU−1 L−1) [36]. However, due to the higher degree of variability in the M/I ratio as compared to M-value, normalizing the insulin sensitivity measure to steady state plasma insulin levels during glucose clamps may not be advantageous [40]. Therefore, the M-value adjusted to bodyweight (Mbw) will be the primary outcome of our study while the M/I ratio (M/Ibw) will be reported in secondary analyses. In addition, the FFM-adjusted M-value (Mffm) and M/I ratio (M/Iffm) will be evaluated in secondary analyses.
Typically, a correction factor, termed ‘space correction’ by DeFronzo et al. [32], is applied to the M-value to correct for non-insulin mediated glucose uptake and changes in glucose infusion rate that are not due to real changes in glucose disposal. Space correction for a 30-min steady-state is calculated as (G2 − G1) x 0.06333; where G2 and G1 are plasma glucose concentrations (mg/dl) at the end and beginning of the 30-min time period, respectively [34]. The space corrected M-value is then obtained using MSC = M − SC − UC; where MSC is the space corrected M-value; M is the uncorrected M-value; SC is the space correction factor; and UC is the correction factor for urinary glucose loss. Urinary correction is negligible as urinary glucose loss does not generally occur during clamp procedures performed at euglycemia [34].
For valid insulin sensitivity assessment, the quality of clamp data was taken into account in the analyses of the M-value and M/I ratio. Individuals who had not fasted before the glucose clamp (with fasting glucose ≥ 7.0 mmol/L and fasting insulin >50 mU/L) or possessed steady state plasma insulin levels that were not adequate to suppress hepatic glucose production (<50 mU/L) were excluded from the analyses of the M-value and M/I ratio [34].
During the study, there was a change in the assay (to the UniCel DxI Immunoassay System, Beckman Coulter, Brea, CA, United States) used to measure insulin levels. The agreement between the two assays used for these insulin measurements were high (r2 = 0.97) in a calibration study using 30 individuals and we calculated the expected older assay values for samples measured with the newer assay using the following formula: ; where old’ is formula-derived old assay value; and new is the insulin value measured under the new assay.
2.10. Compliance assessments
A variety of strategies were employed to identify non-compliance in our study. The 4-week run-in phase of coffee intake abstention allowed precipitation of non-compliance among potential participants who were unable to refrain from coffee consumption. Individuals who dropped out of the study at this stage did not affect our dropout rate because they were yet to be enrolled and randomized.
During the trial phase, counting of returned unused sachets at mid and end clinic visits was used to assess compliance. Adherence to study protocol was encouraged by study staff by regular phone calls. During the orientation visit, participants were provided with a wallet insert card containing information about coffee/caffeine containing foods to avoid. The wallet insert contained contact information of study staff should the participant need clarification.
A study diary was provided to the participants at baseline clinic visit. In the course of the 24 weeks of intervention, participants were instructed to record the number of sachets consumed on a daily basis in their study diary. Participants were also instructed to record any coffee/caffeine containing food/medicine consumed. The study diaries were checked on the week 12 and week 24 clinic visits. Any discrepancies were clarified with the participant during the clinic visit.
Questions regarding consumption of coffee/caffeine containing foods or beverages in the past one month were also included in the FFQ. Study staff evaluated compliance from the answers provided. Together with the physical activity questionnaire, the FFQ also serves to monitor changes in lifestyle during the intervention phase.
We will also measure chlorogenic acid metabolites in plasma using reversed-phase ultra-performance liquid chromatography (Acquity, UPLC BEH C18 column, Waters AG, Baden-Dättwil, Switzerland) electrospray ionization-tandem mass spectrometry (Triple QUAD 5500 System, AB Sciex, Zug, Switzerland). Coffee is the major source of chlorogenic acid in the diet, but various fruits and vegetables also contain chlorogenic acid [41]. As a result, plasma chlorogenic acid metabolites will not be a good measure of compliance on an individual level, but differences in concentrations between the coffee and the placebo group will be used to assess compliance at a group level.
2.11. Quality control of laboratory analysis
To ensure that laboratory measurements did not vary substantially within and across batches, we collected fasting blood and overnight urine samples in a convenience sample of 15 people to serve as quality control (QC) samples. Written informed consent was obtained before a brief screener was used to assess the eligibility of participants. Participants were between 21 and 69 years old with Malay, Chinese or Indian ethnicity. Persons who regularly smoked (1 cigarette/week or more) or had heavy alcohol consumption (>2 drinks/day) or antibiotics use in the past 3 months and those with pre-existing diabetes mellitus, heart disease, stroke, hypertension, clotting or bleeding disorders, and women who reported to be pregnant or breastfeeding were excluded.
Participants were instructed to fast for at least 10 h prior to their blood draw. Participants who were also willing to provide an overnight urine sample were provided with a urine jug and instructions on how to collect overnight urine.
2.12. Data analysis and statistics
2.12.1. Sample size calculations
The sample size calculations were based on the anticipated mean difference in final M/Iffm between the intervention and control arms. Given the design of our study, the sample size formula for a statistical superiority trial design [42] was used: ; where N is the sample size per group; z1-α/2 is the z score for a 2-tailed test at 5% significance level; z1-β is the z score at a statistical power of 80%; δ is the anticipated mean difference in the absolute M/Iffm between the coffee and placebo groups at 24-week and s is the standard deviation which is assumed to be common for both groups.
Given that there have been no previous trials of the effect of coffee on clamp measured insulin sensitivity, the anticipated mean difference in M/Iffm was obtained from an article by McAuley et al. [38] on the influence of a lifestyle intervention on insulin sensitivity in normoglycemic insulin resistant individuals. After 4 months of an intense diet and exercise intervention, McAuley et al. [38] observed a 1.2 mg kg−1 min−1 mU−1 L−1 difference in final M/Iffm between the intervention and control groups. Based on their data we estimated a corresponding SD of 2.19 mg kg−1 min−1 mU−1 L−1. Therefore, at 80% power and 5% significance level, we obtained . We thus required 52 individuals per intervention group with a total of 104 individuals. This number will be achieved if 17% or fewer of the 126 participants who completed our baseline assessment drop out.
2.12.2. Analysis of baseline characteristics
Given that two enrolled individuals did not complete baseline assessment, 126 out of the 128 enrolled participants were included in baseline analyses. Nominal scale variables were described as percentages and compared using chi-square tests. Normally distributed interval and ratio scale variables were described as mean and standard deviation, and compared using student's T-test with equal variances. Interval ratio scale variables with skewed distributions were described as median and interquartile ranges, and compared using Mann-Whitney U tests. For baseline analyses of clamp-based insulin sensitivity measures (M and M/I ratio), an additional 4 participants with invalid clamp data (subjects with steady state insulin levels there were not sufficiently high to adequately suppress hepatic glucose production) out of the 126 enrolled subjects with baseline data were excluded.
2.12.3. Analysis of the effect of coffee on primary outcomes
The primary outcome was the difference between coffee and placebo groups in Mbw at the end of the study corrected for the baseline Mbw values. Our null hypothesis is that consumption of 4 cups of coffee per day for 24 weeks will not influence changes in insulin sensitivity. Under this hypothesis, we expect to find no significant difference between Mbw values of the coffee and placebo groups at the end of intervention after adjusting for baseline Mbw values. The alternative hypothesis is that individuals receiving the coffee beverage will experience an increase in insulin sensitivity. Under this hypothesis and with sufficient statistical power, we expect to find a significant improvement in insulin sensitivity, as indicated by the significantly higher Mbw values among individuals in the coffee group compared to the placebo group at week 24.
As indicated in the Consolidated Standards of Reporting Trials (CONSORT) [43] statement, results from an unadjusted analysis (t-test comparing the difference in mean Mbw between the placebo and coffee groups at week 24) will be first reported. The difference in the mean Mbw at week 24 will further be adjusted for the baseline Mbw using Analysis of Covariance (ANCOVA) with treatment as the main effect and baseline Mbw as a covariate. Log transformation of the Mbw values will be performed as needed to achieve normality and equal variances; t-tests with unequal variances will be performed if log-transformation is insufficient to ensure equivariance.
Intention to treat (ITT) analysis (regarded as the gold standard endpoint analysis of randomized trials) on the primary outcome will be performed according to original assignments at randomization without taking into account overt dropouts and unplanned crossovers resulting from silent dropouts and drop-ins (contamination). Only if necessary (depending on compliance of participants), per protocol (PP) analysis may be done according to whether coffee was actually consumed (determined from compliance assessments described earlier) during the intervention period.
2.12.4. Analysis of the effect of coffee on secondary outcomes
Secondary outcomes on insulin sensitivity included differences between coffee and placebo groups in the week 24 values for M/Ibw, Mffm and M/Iffm with and without adjustment for their corresponding baseline values. Other secondary outcomes included differences between coffee and placebo arms at week 12 or week 24 for the following outcomes: fasting glucose metabolism (fasting plasma glucose, HOMA-IR); potential mediators of effects of coffee on insulin sensitivity including non-esterified fatty acids, adipokines and markers of inflammation (total adiponectin, C-reactive protein, interleukins 6 and 18), markers of liver function (alanine and aspartate aminotransferases, fetuin-A), markers of renal function and oxidative stress (serum creatinine, urinary 8-isoprostane); cardiovascular risk factors including heart rate, blood pressure (systolic and diastolic), and blood lipids (fasting triglycerides, LDL-cholesterol, HDL-cholesterol); reported gut health (frequency of bowel movements, type of stool, abdominal pain); and psychological measures (depression, fatigue).
With the exception of secondary insulin sensitivity outcomes, the secondary endpoints listed above were assessed at baseline, week-12 and week-24 visits. The efficacy of coffee consumption on the above-mentioned secondary outcomes will be assessed using mixed models that account for within participant correlated errors. As an additional analysis, differences in secondary endpoints between the coffee and placebo groups at week 12 and at week 24 will also be analyzed using ANCOVA with adjustment for baseline values.
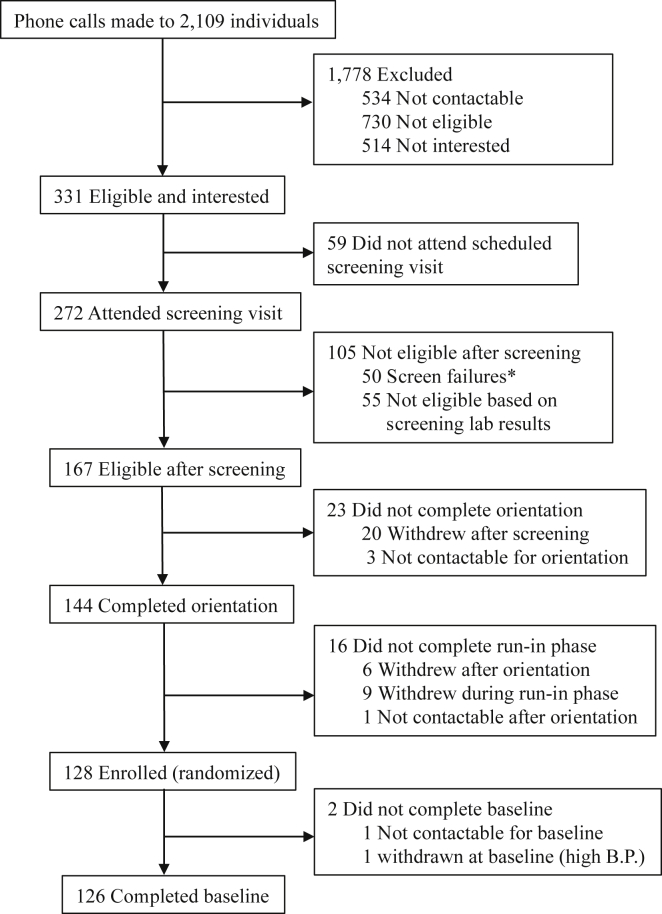
2.13. Trial profile
Of at least 2109 phone calls made to individuals who were identified through various recruitment activities, 331 individuals were potentially eligible and interested to participate in the study (Fig. 2). Of the calls made, 534 individuals were not contactable and 730 were not eligible based on a screening questionnaire administered over the phone (predominant reasons include medical conditions such as hypertension or cardiac problems). Upon learning more about the study, 514 individuals declined participation because they were either too busy or not interested. Although screening visits were scheduled for all 331 potentially eligible individuals, 59 of them either cancelled or did not show up at our study site. Of the 272 individuals who attended screening, 50 were deemed as screen failures while 55 did not qualify based on their screening laboratory results. Screen failures were individuals who were found to have issues such high blood pressure or ineligible BMI and were known to be ineligible without a need to draw blood for laboratory analyses. The most common reason for screen failure was the presence of high blood pressure (76.0%). Exclusions based on screening lab results were mostly (90.9%) due to HOMA-IR values of less than 1.30.
Fig. 2.
Trial profile of the Coffee for Metabolic Health (COMETH) study. *Screen failures were individuals who were found to be ineligible based on criteria such as high blood pressure and BMI, etc. These individuals were excluded without the need for a blood sample assessment.
Of the 167 eligible individuals after screening, 20 withdrew after the screening visit and 3 were not contactable. The remaining 144 individuals successfully completed their orientation visit (Fig. 2). After completing orientation, 6 individuals withdrew from the study while 1 individual was not contactable. Since coffee drinkers were recruited, a 4-week run in period ensued after orientation in which participants were asked to refrain from consuming coffee or other caffeinated products. During the run-in phase, 9 individuals withdrew from the study. A total of 128 individuals successfully completed the run-in phase and were enrolled into the study before the enrolment deadline (Fig. 2). These 128 individuals were randomized to either the coffee or placebo arm. Although enrolled, 2 subjects did not complete the baseline visit. This was because early in the study, randomization was done before the baseline clinic visit. As such, one subject was not contactable although he had successfully completed the run-in phase and had been randomized. The other subject was found to have high blood pressure at the baseline visit. After multiple readings, the elevated blood pressure persisted and an on-site study physician decided to withdraw the subject from the study. Therefore, a total of 126 participants successfully completed the baseline visit.
2.14. Baseline characteristics
Males formed the majority (57.1%) of our study population with a median age of 48 years while females had a median age of 47 years. Table 2 shows the baseline characteristics of the participants according to sex. Most of our participants were Chinese, reflecting the ethnic composition of the general Singapore population. However, unlike the general population, we had a higher proportion of Asian Indians compared to Malays in our study population. One reason could be that initially we restricted recruitment to Chinese and Indian participants but later expanded recruitment to Malay individuals. Furthermore, in view of the fasting month of Ramadan, which may interfere with adherence to the study protocol, we stopped recruiting Malay participants before enrolment deadline. Majority (65.1%) of our study participants had a history of consuming 2 or more but less than 4 cups of coffee daily.
Table 2.
COMETH baseline characteristics by gender for all subjects who completed baseline (n = 126).
| Characteristics | Males (n = 72) | Females (n = 54) |
|---|---|---|
| Age (years) | 48 (40–55) | 47 (41–53) |
| Ethnicity n (%) | ||
| Chinese | 50 (69.4) | 31 (57.4) |
| Malay | 9 (12.5) | 8 (14.8) |
| Indian | 13 (18.1) | 15 (27.8) |
| History of coffee consumption n (%) | ||
| < 2 cups/day | 17 (23.6) | 12 (22.2) |
| ≥ 2 to < 4 cups/day | 46 (63.9) | 36 (66.7) |
| ≥ 4 cups/day | 9 (12.5) | 6 (11.1) |
| Weight (Kg) | 78.0 (71.5–83.2) | 70.6 (63.6–75.9) |
| Height (cm) | 171.0 (167.5–173.5) | 158.0 (153.0–161.0) |
| Waist circumference (cm) | 92.5 (89.0–98.0) | 89.0 (81.0–93.0) |
| Hip circumference (cm) | 101.5 (98.5–106.0) | 106.0 (100.0–111.0) |
| Body mass index (Kg/m2) | 26.7 (24.9–28.1) | 28.4 (25.9–30.4) |
| Waist-to-hip ratio | 0.91 (0.054) | 0.83 (0.064) |
| Waist-to-height ratio | 0.54 (0.52–0.57) | 0.57 (0.52–0.59) |
| Skinfold thickness (mm) | ||
| Triceps | 24.5 (16.1–30.6) | 31.0 (27.5–36.3) |
| Biceps | 11.4 (9.3–16.0) | 20.2 (15.7–23.6) |
| Subcapsular | 24.2 (19.3–30.1) | 28.9 (24.4–32.9) |
| Supra-iliac | 26.8 (21.4–38.4) | 30.9 (25.8–35.5) |
| Fat free mass (Kg) | 55.8 (51.6–60.9) | 40.7 (38.5–44.4) |
| Fat mass (Kg) | 20.9 (17.3–24.9) | 28.7 (23.8–36.0) |
| Body fat percentage (%) | 26.2 (23.5–31.0) | 40.3 (35.7–45.5) |
| Systolic blood pressure (mmHg) | 129.0 (122.8–135.0) | 122.0 (113.5–129.0) |
| Diastolic blood pressure (mmHg) | 79.0 (72.8–85.3) | 75.0 (70.0–82.0) |
| Heart rate (bpm) | 69 (64–76) | 74 (70–80) |
| Total cholesterol (mmol/L) | 4.97 (4.57–5.80) | 5.26 (4.72–5.94) |
| Triglycerides (mmol/L) | 1.44 (1.04–1.84) | 1.05 (0.91–1.40) |
| HDL-cholesterol (mmol/L) | 1.10 (0.98–1.22) | 1.36 (1.12–1.59) |
| LDL-cholesterol (mmol/L) | 3.31 (0.80)a | 3.30 (0.66) |
| Aspartate aminotransferase (U/L) | 25 (21–28) | 21 (18–26) |
| Alanine aminotransferase (U/L) | 29 (24–37) | 20 (16–27) |
| Plasma insulin at 0 min (mU/L) | 9.6 (6.7–15.3) | 10.4 (5.7–15.8) |
| Plasma insulin at 90 min (mU/L) | 115.6 (80.7–138.5) | 108.7 (74.6–130.8) |
| Plasma insulin at 120 min (mU/L) | 111.3 (79.9–136.3) | 109.1 (82.5–139.4) |
| Average steady state plasma insulin (mU/L) | 113.2 (79.6–134.4) | 107.6 (80.9–130.9) |
| Fasting plasma glucose (mmol/L) | 4.7 (4.6–5.1) | 4.7 (4.5–5.1) |
| Glucose infusion rate (ml/hr) | 92.4 (71.6–130.6) | 96.4 (74.9–123.7) |
| HOMA-IR | 2.08 (1.44–3.23) | 2.19 (1.24–3.24) |
| M-value (BWc) (mg/Kg/min) | 4.10 (3.04–5.56)ˆ | 4.28 (3.44–6.18)b |
| Space corrected M-value (BW) (mg/Kg/min) | 4.11 (3.10–5.53)ˆ | 4.32 (3.64–6.25)b |
| M-value (FFMd) (mg/Kg/min) | 5.83 (4.36–7.92)ˆ | 7.51 (5.95–10.51)b |
| Space corrected M-value (FFM) (mg/Kg/min) | 5.89 (4.35–7.97)ˆ | 7.45 (6.04–10.49)b |
| M/I ratio (BW) (100 × mg kg−1 min−1 mU−1 L−1) | 3.80 (2.65–6.35)ˆ | 4.40 (2.99–5.78)b |
| Space Corrected M/I ratio (BW) (100 × mg kg−1 min−1 mU−1 L−1) | 3.90 (2.73–6.35)ˆ | 4.50 (3.01–5.81)b |
| M/I ratio (FFM) (100 × mg kg−1 min−1 mU−1 L−1) | 5.18 (3.63–8.65)ˆ | 7.00 (5.20–10.73)b |
| Space Corrected M/I ratio (FFM) (100 × mg kg−1 min−1 mU−1 L−1) | 5.28 (3.64–8.58)ˆ | 7.03 (5.23–10.71)b |
Nominal variables described as percentages; normally distributed interval ratio scale variables described as mean and SD; interval ratio scale variables with skewed distributions described as median and IQR.
Invalid data from 2 subjects; ˆ1 subject excluded from analysis due to invalid baseline clamp data.
3 Subjects excluded from analysis due to invalid baseline clamp data.
Body Weight.
Fat Free Mass.
Based on BMI recommended for Asian populations [44], participants in our study were at least overweight as expected and stated in the inclusion criteria, with 77.0% of subjects being obese (≥25 kg/m2). Median waist circumferences (WC) [44], [45] and waist-to-height ratios (WHTR) [46] suggest abdominal obesity among men (WCmen ≥ 90 cm; WHTRmen ≥ 0.52) and women (WCwomen ≥ 80 cm; WHTRwomen ≥ 0.53). Among commonly measured anthropometric indices, males had a significantly greater waist circumference and waist-to-hip ratio, whereas females had a larger hip circumference and BMI (Table 2). Both men and women had median body fat percentages that were higher than values typical for the threshold of obesity (BMI of 30 kg/m2) in persons of European origin (25% for men and 35% for women) [47].
As expected from our exclusion criteria, the median systolic and diastolic blood pressure values were below cut-offs for high blood pressure, and all participants had normal heart rates of below 100 beats per minute.
The median levels of total cholesterol, triglycerides, HDL-cholesterol, aspartate [48] and alanine [49] aminotransferase activity levels, and mean levels of LDL-cholesterol were within the normal range [50]. As expected from our inclusion criteria, median and interquartile ranges of HOMA-IR suggested that our study population consisted of individuals who were more likely to be insulin resistant. Median fasting glucose levels were in the normoglycemic range with a 3.2% baseline prevalence of impaired fasting glucose among all subjects [51]. Clamp based insulin sensitivity measures (M and M/I ratio), when adjusted for bodyweight, indicated that males and females had similar levels of insulin sensitivity. (Table 2).
3. Discussion
In a large number of cohort studies, coffee consumption was associated with a lower risk for type 2 diabetes [6]. Although most observational studies statistically address confounding, residual confounding may persist. Randomized trials are therefore needed to provide more conclusive evidence. However, to date, few trials have been conducted to investigate the effect of coffee on glucose metabolism [7], [9], [8], [10], [11]. Furthermore, trials conducted thus far had limitations such as small sample sizes, lack of randomization or blinding, short duration of follow-up and use of surrogate measures of insulin sensitivity. Therefore, we designed the COMETH study, a randomized placebo-controlled 6-month trial that examined the effect of consuming 4 cups of coffee per day for 24 weeks on insulin sensitivity assessed with a glucose clamp.
3.1. Recruitment
According to our sample size calculations, we had planned to recruit and enroll 130 participants after accounting for an expected dropout rate of 20% to obtain a minimum sample size of 104. Recruitment for coffee trial was challenging, because it required the selection of participants who were willing to either consume 4 cups of coffee for 6 months or consume only the placebo beverage for six months. Long-term adherence to coffee consumption requires selection of persons who regularly consume coffee. However, given the strict inclusion and exclusion parameters, recruitment of subjects proved to be challenging from the commencement of study activities. As such, we expanded the scope and increased the intensity of recruitment activities and achieved a total enrolment of 128 participants by the enrolment deadline.
3.2. Strengths and limitations
This study had several strengths. Firstly, this study was double-blinded. Central to masking was the production of a well-designed placebo beverage that tastes, smells and looks like coffee unlike in previous trials [9], [8], where water was used. This allowed the aversion of subconscious or conscious preconceptions in investigators, thereby minimizing diagnostic suspicion bias in the assessment of insulin sensitivity. In addition, the blinded placebo group allowed us to account for a possible Hawthorne effect because lifestyle modifications, such as those culminating in weight changes, could influence insulin sensitivity. Importantly, the placebo beverage allows for a standardized replacement of coffee, otherwise, people who typically consume coffee may be tempted to drink some other beverage (e.g. tea or malt beverages) instead of water.
This study provided a larger sample size and a longer duration of intervention and follow up. Previous trials [7], [9], [8], [10] were relatively short ranging from 2 to 16 weeks with sample sizes ranging from 26 to 49 subjects. The longer intervention period with greater statistical power increases the likelihood that we will be able detect longer term effects of coffee on insulin sensitivity.
An important advantage of this study over previous trials was the employment of the gold standard measure of insulin sensitivity (hyperinsulinemic euglycemic clamp) to assess the potential influence of coffee on the risk of diabetes. Previous trials [10], [9], [8], [7] had relied on surrogate measures such as the HOMA-IR or the Composite Insulin Sensitivity Index (CISI) [52] derived from fasting glucose and insulin data or data from an oral glucose tolerance test.
The 4-week run-in period not only conferred a washout period but also enabled us to evaluate long-term tolerance to caffeine depletion among participants who are habitual coffee drinkers. Given the relatively longer intervention period compared to earlier trials, the run-in period was critical in identifying overt non-compliers who had problems abstaining from coffee consumption and were likely to drop out from the study should they be enrolled.
Several limitations of our study should also be noted. Because the extent of glucose disposal in adipose tissues remains unclear, previous studies based on glucose clamps have typically reported insulin sensitivity measures standardized to both body weight and fat free mass [35], [38]. In our study, because of logistical reasons, we did not include ‘gold standard’ assessments of FFM such as DXA scans and we will therefore only report body weight standardized M-values as the primary outcome while the M/I ratios and FFM standardized insulin sensitivity measures will be reported as secondary outcomes.
Due to methodological reasons (see section 2.9), we decided to slightly change our primary outcome from M/Iffm to Mbw. It is unclear how this change will affect our statistical power. On the one hand not dividing by insulin values may increase our power as Mbw is not affected by additional measurement error in the assessment of insulin concentrations [53]. On the other hand, it may reduce our power if fluctuations in insulin concentrations during the final phase of the clamp introduce extraneous variation. It is less likely that the use of body weight instead of fat free mass in the calculation will have a substantial impact as we do not expect the coffee intervention to affect body composition.
Although this study required abstention from coffee consumption, covert non-compliance (silent drop outs or drop-ins) is a possibility given the ubiquitous availability of coffee. Inadvertent consumption of coffee among placebo group subjects or abstention of study product use among coffee group subjects could potentially diminish the effect of coffee in our study. We employed several strategies to assess compliance bias such as counting of unused study beverage sachets and the use of a study diary. Still, these methods rely on cooperation of subjects and complementary objective biomarkers of compliance would have been preferable. Unfortunately, identifying biomarkers that specific to coffee proved to be difficult. Chlorogenic acid metabolites, although not specific to coffee, will be measured and used as a marker for compliance at a group level.
Incomplete blinding is a possibility because our recruited participants are habitual coffee drinkers who could recognize the effects of caffeine. At the end of the study, we will ask subjects to guess which arm they had been randomized to and this will inform us whether blinding was successful.
Given the randomized controlled and double-blind nature of our trial, the measurement error in insulin sensitivity assessment in participants is unlikely to differ between the coffee and the placebo group. As a result, this measurement error is likely to underestimate the treatment effect.
Finally, we investigated the possible efficacy of coffee in improving insulin sensitivity in a population consisting of non-diabetic, non-smoking, Chinese, Malay and Asian Indians. As a result, our results may not be generalizable to persons with diabetes, smokers, or other ethnic groups.
3.3. Interpretation of outcomes
There are three possible outcomes in our study. Firstly, we may find no difference in insulin sensitivity over the 24-week period between the placebo and coffee groups. This would suggest that there is no evidence of coffee's beneficial influence on insulin sensitivity. One explanation could be that our study was not sufficiently powered or the follow duration was not long enough to observe the appreciable effects of coffee on insulin sensitivity. Another explanation could be that the association seen in observation studies could have been due to coffee modulating diabetes risk through pathways other than that involving insulin sensitivity. A null finding could also indicate that coffee may act in a later stage of diabetes development when individuals are more insulin-resistant or have pre-diabetes.
Secondly, we may find that the improvement of insulin sensitivity in the placebo group is significantly greater than the coffee group. Such a result would be in conflict with our hypothesis that coffee could reduce diabetes risk. A previous report had indicated that caffeine adversely affects glucose metabolism by reducing insulin sensitivity following acute administration [54]. So, an increase in insulin resistance among the coffee group could reflect a long term persistent adverse effect of caffeine on glucose metabolism.
Thirdly, we may find that the improvement of insulin sensitivity in the coffee group is significantly greater than the placebo group. This would corroborate the abundance of observational studies in which coffee consumption was inversely associated with risk of type 2 diabetes. Such a result would suggest that 24 weeks of daily coffee intake is sufficient to improve insulin sensitivity. Importantly, this evidence would suggest that coffee modulates type 2 diabetes risk through biological mechanisms that involve insulin sensitivity.
Trial status
As of October 2015, enrollment of participants and data collection are complete. Data analysis is ongoing.
Funding source
This trial was funded by Nestec SA, the research and development arm of Nestlé SA.
Competing interests
None declared.
Author contributions
D.J.A. was responsible for the recruitment of participants, managing logistics of the trial, coordinating with the ethics committee, data collection and management, analysis and interpretation of the data, contributing to discussions about the results, drafting the initial manuscript and critically revising and editing the final manuscript.
S.A.R. co-supervised the conduct of the trial, co-obtained ethics approval and coordinated with the ethics committee, co-supervised data management, contributed to discussions about the results, and critically revised and edited the final manuscript.
E.Y.H.K. co-supervised the conduct of the trial, co-obtained ethics committee approval, provided medical expertise, co-supervised data management, contributed to discussions about the results, and critically revised and edited the final manuscript.
Z.T. and S.S.Y.S. were responsible for the screening and recruitment of participants, managing logistics of the trial, data collection and management.
B.C.T. and S.E.A provided statistical expertise in the analysis and interpretation of the data, contributed to discussions about the results and critically revised and edited the final manuscript. S.E.A drew up the statistical plan for analysis.
C.D. and C.J.C contributed to the conception of the study, development of the placebo beverage, discussions about the results and critically revised and edited the final manuscript.
R.M.V.D. conceived and designed the study, co-obtained ethics committee approval, co-supervised the conduct of the trial, contributed to discussions about the results and critically reviewed and edited the final manuscript. R.M.V.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgements
Derrick Johnston Alperet was supported with funding by the National University of Singapore Graduate School for Integrative Sciences and Engineering PhD scholarship. We would also like to thank the staff of the Investigational Medical Unit, Yong Loo Lin School of Medicine, National University of Singapore, for their assistance and cooperation in the conduct of clinic visits, and for sharing their expertise on the hyperinsulinemic euglycemic clamp. We are also grateful to Nasheen Naidoo, Wu Yi, and Sun Ye for serving as product managers, Julie Chambard for her valuable help in coordinating study related activities between NRC and NUS, the Singapore Consortium of Cohort Studies team for their support with our recruitment efforts, Francoise Le Derff and Valérie Leloup (Nestlé PTC Orbe, Switzerland) for test products formulation and preparation.
References
- 1.IDF Diabetes Atlas. sixth ed. International Diabetes Federation; 2013. [Google Scholar]
- 2.Kahn S.E., Cooper M.E., Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet. 2014;383(9922):1068–1083. doi: 10.1016/S0140-6736(13)62154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ley S.H., Hamdy O., Mohan V., Hu F.B. Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet. 2014;383(9933):1999–2007. doi: 10.1016/S0140-6736(14)60613-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global Coffee Trends: Finding the Premiumisation Opportunity. Euromonitor International; 2011. [Google Scholar]
- 5.van Dam R.M., Feskens E.J. Coffee consumption and risk of type 2 diabetes mellitus. Lancet. 2002;360(9344):1477–1478. doi: 10.1016/S0140-6736(02)11436-X. [DOI] [PubMed] [Google Scholar]
- 6.Ding M., Bhupathiraju S.N., Chen M., van Dam R.M., Hu F.B. Caffeinated and decaffeinated coffee consumption and risk of type 2 diabetes: a systematic review and a dose-response meta-analysis. Diabetes care. 2014;37(2):569–586. doi: 10.2337/dc13-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kempf K., Herder C., Erlund I., Kolb H., Martin S., Carstensen M. Effects of coffee consumption on subclinical inflammation and other risk factors for type 2 diabetes: a clinical trial. Am. J. Clin. Nutr. 2010;91(4):950–957. doi: 10.3945/ajcn.2009.28548. [DOI] [PubMed] [Google Scholar]
- 8.Wedick N.M., Brennan A.M., Sun Q., Hu F.B., Mantzoros C.S., van Dam R.M. Effects of caffeinated and decaffeinated coffee on biological risk factors for type 2 diabetes: a randomized controlled trial. Nutr. J. 2011;10:93. doi: 10.1186/1475-2891-10-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohnaka K., Ikeda M., Maki T., Okada T., Shimazoe T., Adachi M. Effects of 16-week consumption of caffeinated and decaffeinated instant coffee on glucose metabolism in a randomized controlled trial. J. Nutr. Metab. 2012;2012:207426. doi: 10.1155/2012/207426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Dam R.M., Pasman W.J., Verhoef P. Effects of coffee consumption on fasting blood glucose and insulin concentrations: randomized controlled trials in healthy volunteers. Diabetes care. 2004;27(12):2990–2992. doi: 10.2337/diacare.27.12.2990. [DOI] [PubMed] [Google Scholar]
- 11.Lecoultre V., Carrel G., Egli L., Binnert C., Boss A., MacMillan E.L. Coffee consumption attenuates short-term fructose-induced liver insulin resistance in healthy men. Am. J. Clin. Nutr. 2014;99(2):268–275. doi: 10.3945/ajcn.113.069526. [DOI] [PubMed] [Google Scholar]
- 12.Singh B., Saxena A. Surrogate markers of insulin resistance: a review. World J. diabetes. 2010;1(2):36–47. doi: 10.4239/wjd.v1.i2.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Singapore Consortium of Cohort Studies (SCCS). Singapore. http://www.nus-cme.org.sg/home.html. Accessed 21 July 2015.
- 14.The Singapore Indian Eye Study 2 (SINDI–2) – Prospective cohort study of 6-year incidence, risk factors, and impact of retinal and other major eye diseases. Singapore Eye Research Institute. https://http://www.seri.com.sg/key-programmes/singapore-epidemiology-of-eye-diseases-seed/. Accessed 21 July 2015.
- 15.Nang E.E., Khoo C.M., Tai E.S., Lim S.C., Tavintharan S., Wong T.Y. Is there a clear threshold for fasting plasma glucose that differentiates between those with and without neuropathy and chronic kidney disease?: the Singapore prospective study program. Am. J. Epidemiol. 2009;169(12):1454–1462. doi: 10.1093/aje/kwp076. [DOI] [PubMed] [Google Scholar]
- 16.Katsuki A., Sumida Y., Gabazza E.C., Murashima S., Furuta M., Araki-Sasaki R. Homeostasis model assessment is a reliable indicator of insulin resistance during follow-up of patients with type 2 diabetes. Diabetes care. 2001;24(2):362–365. doi: 10.2337/diacare.24.2.362. [DOI] [PubMed] [Google Scholar]
- 17.Pandis N. Randomization. Part 2: minimization. Am. J. Orthod. Dentofac. Orthop. Off. Publ. Am. Assoc. Orthod. its Const. Soc. Am. Board Orthod. 2011;140(6):902–904. doi: 10.1016/j.ajodo.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 18.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 19.Deurenberg-Yap M., Li T., Tan W.L., van Staveren W.A., Deurenberg P. Validation of a semiquantitative food frequency questionnaire for estimation of intakes of energy, fats and cholesterol among Singaporeans. Asia Pac. J. Clin. Nutr. 2000;9(4):282–288. doi: 10.1046/j.1440-6047.2000.00187.x. [DOI] [PubMed] [Google Scholar]
- 20.Report of the National Nutrition Survey. Health Promotion Board; Singapore: 2010. (Division RSP) [Google Scholar]
- 21.Craig C.L., Marshall A.L., Sjostrom M., Bauman A.E., Booth M.L., Ainsworth B.E. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. sports Exerc. 2003;35(8):1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 22.International Physical Activity Questionnaire: Short Last 7 Days Self-administered Format for Use with Young and Middle-aged Adults (15-69 Years) The IPAQ Group; 2002. [Google Scholar]
- 23.Schroevers M.J., Sanderman R., van Sonderen E., Ranchor A.V. The evaluation of the Center for Epidemiologic Studies Depression (CES-D) scale: depressed and positive affect in cancer patients and healthy reference subjects. Qual. life Res. Int. J. Qual. life aspects Treat. care Rehabil. 2000;9(9):1015–1029. doi: 10.1023/a:1016673003237. [DOI] [PubMed] [Google Scholar]
- 24.Radloff L.S. The CES-D scale: a self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977;1(3):385–401. [Google Scholar]
- 25.Lai J.S., Cella D., Choi S., Junghaenel D.U., Christodoulou C., Gershon R. How item banks and their application can influence measurement practice in rehabilitation medicine: a PROMIS fatigue item bank example. Arch. Phys. Med. Rehabil. 2011;92(10 Suppl):S20–S27. doi: 10.1016/j.apmr.2010.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia S.F., Cella D., Clauser S.B., Flynn K.E., Lad T., Lai J.S. Standardizing patient-reported outcomes assessment in cancer clinical trials: a patient-reported outcomes measurement information system initiative. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007;25(32):5106–5112. doi: 10.1200/JCO.2007.12.2341. [DOI] [PubMed] [Google Scholar]
- 27.Junghaenel D.U., Schneider S., Stone A.A., Christodoulou C., Broderick J.E. Ecological validity and clinical utility of Patient-reported outcomes measurement information system (PROMIS(R)) instruments for detecting premenstrual symptoms of depression, anger, and fatigue. J. Psychosom. Res. 2014;76(4):300–306. doi: 10.1016/j.jpsychores.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Broderick J.E., Schneider S., Junghaenel D.U., Schwartz J.E., Stone A.A. Validity and reliability of patient-reported outcomes measurement information system instruments in osteoarthritis. Arthritis care & Res. 2013;65(10):1625–1633. doi: 10.1002/acr.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Markland A.D., Palsson O., Goode P.S., Burgio K.L., Busby-Whitehead J., Whitehead W.E. Association of low dietary intake of fiber and liquids with constipation: evidence from the National Health and Nutrition Examination Survey. Am. J. Gastroenterol. 2013;108(5):796–803. doi: 10.1038/ajg.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Health and Nutrition Examination Survey Bowel Health Questionnaire (NHANES BHQ). http://wwwn.cdc.gov/nchs/nhanes/2009-2010/BHQ_F.htm. Accessed 15 May 2015.
- 31.Francis C.Y., Morris J., Whorwell P.J. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Alimentary Pharmacol. Ther. 1997;11(2):395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- 32.DeFronzo R.A., Tobin J.D., Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am. J. Physiol. 1979;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 33.Muniyappa R., Lee S., Chen H., Quon M.J. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am. J. Physiol. Endocrinol. Metab. 2008;294(1):E15–E26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- 34.Brehm A., Roden M. Glucose clamp techniques. In: Roden M., editor. Clinical Diabetes Research: Methods and Techniques. John Wiley & Sons, Inc.; 2007. pp. 43–76. [Google Scholar]
- 35.Tam C.S., Xie W., Johnson W.D., Cefalu W.T., Redman L.M., Ravussin E. Defining insulin resistance from hyperinsulinemic-euglycemic clamps. Diabetes care. 2012;35(7):1605–1610. doi: 10.2337/dc11-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nerpin E., Riserus U., Ingelsson E., Sundstrom J., Jobs M., Larsson A. Insulin sensitivity measured with euglycemic clamp is independently associated with glomerular filtration rate in a community-based cohort. Diabetes care. 2008;31(8):1550–1555. doi: 10.2337/dc08-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuan T., Zhao W.G., Sun Q., Fu Y., Dong Y.Y., Dong Y.X. Association between four adipokines and insulin sensitivity in patients with obesity, type 1 or type 2 diabetes mellitus, and in the general Chinese population. Chin. Med. J. 2010;123(15):2018–2022. [PubMed] [Google Scholar]
- 38.McAuley K.A., Williams S.M., Mann J.I., Goulding A., Chisholm A., Wilson N. Intensive lifestyle changes are necessary to improve insulin sensitivity: a randomized controlled trial. Diabetes care. 2002;25(3):445–452. doi: 10.2337/diacare.25.3.445. [DOI] [PubMed] [Google Scholar]
- 39.Sun G., French C.R., Martin G.R., Younghusband B., Green R.C., Xie Y.G. Comparison of multifrequency bioelectrical impedance analysis with dual-energy X-ray absorptiometry for assessment of percentage body fat in a large, healthy population. Am. J. Clin. Nutr. 2005;81(1):74–78. doi: 10.1093/ajcn/81.1.74. [DOI] [PubMed] [Google Scholar]
- 40.Bergman R.N., Finegood D.T., Ader M. Assessment of insulin sensitivity in vivo. Endocr. Rev. 1985;6(1):45–86. doi: 10.1210/edrv-6-1-45. [DOI] [PubMed] [Google Scholar]
- 41.Clifford M.N. Chlorogenic acids and other cinnamates – nature, occurrence and dietary burden. J. Sci. Food Agric. 1999;79(3):362–372. doi:10.1002/(SICI)1097-0010(19990301)79:3<362::AID-JSFA256>3.0.CO;2-D. [Google Scholar]
- 42.Zhong B. How to calculate sample size in randomized controlled trial? J. Thorac. Dis. 2009;1(1):51–54. [PMC free article] [PubMed] [Google Scholar]
- 43.Schulz K.F., Altman D.G., Moher D. Group C. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Trials. 2010;11:32. [Google Scholar]
- 44.The Asia Pacific Perspective: Redefining Obesity and its Treatment. World Health Organization Western Pacific Region, International Association for the Study of Obesity & International Obesity Task Force; 2000. [Google Scholar]
- 45.WHO . 2008. Waist Circumference and Waist-hip Ratio: Report of a WHO Expert Consultation. Geneva, Switzerland. 8–11 December 2008. [Google Scholar]
- 46.Browning L.M., Hsieh S.D., Ashwell M. A systematic review of waist-to-height ratio as a screening tool for the prediction of cardiovascular disease and diabetes: 0.5 could be a suitable global boundary value. Nutr. Res. Rev. 2010;23(2):247–269. doi: 10.1017/S0954422410000144. [DOI] [PubMed] [Google Scholar]
- 47.Deurenberg-Yap M., Chew S.K., Deurenberg P. Elevated body fat percentage and cardiovascular risks at low body mass index levels among Singaporean Chinese, Malays and Indians. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2002;3(3):209–215. doi: 10.1046/j.1467-789x.2002.00069.x. [DOI] [PubMed] [Google Scholar]
- 48.AST: MedlinePlus Medical Encyclopedia. U.S. National Library of Medicine, National Institutes of Health; United States of America: 2013. http://www.nlm.nih.gov/medlineplus/ency/article/003472.htm Accessed 31 July 2014. [Google Scholar]
- 49.ALT: MedlinePlus Medical Encyclopedia. U.S. National Library of Medicine, National Institutes of Health; United States of America: 2013. http://www.nlm.nih.gov/medlineplus/ency/article/003473.htm Accessed 31 July 2014. [Google Scholar]
- 50.Conditions & Treatments: Cholesterol Management. SingHealth Group; Singapore: 2006. http://www.singhealth.com.sg/PatientCare/ConditionsAndTreatments/Pages/Cholesterol-Management.aspx Accessed 31 July 2014. [Google Scholar]
- 51.Nolan C.J., Damm P., Prentki M. Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet. 2011;378(9786):169–181. doi: 10.1016/S0140-6736(11)60614-4. [DOI] [PubMed] [Google Scholar]
- 52.Matsuda M., DeFronzo R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes care. 1999;22(9):1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 53.Bokemark L., Froden A., Attvall S., Wikstrand J., Fagerberg B. The euglycemic hyperinsulinemic clamp examination: variability and reproducibility. Scand. J. Clin. Lab. Investig. 2000;60(1):27–36. doi: 10.1080/00365510050185010. [DOI] [PubMed] [Google Scholar]
- 54.Natella F., Scaccini C. Role of coffee in modulation of diabetes risk. Nutr. Rev. 2012;70(4):207–217. doi: 10.1111/j.1753-4887.2012.00470.x. [DOI] [PubMed] [Google Scholar]