Abstract
Background
Recent introduction of computer-aided design/computer-aided manufacturing (CAD/CAM) monolithic zirconia dental prostheses raises the issue of material low thermal degradation (LTD), a well-known problem with zirconia hip prostheses. This phenomenon could be accentuated by masticatory mechanical stress. Until now zirconia LTD process has only been studied in vitro. This work introduces an original protocol to evaluate LTD process of monolithic zirconia prostheses in the oral environment and to study their general clinical behavior, notably in terms of wear.
Methods/design
101 posterior monolithic zirconia tooth elements (molars and premolars) are included in a 5-year prospective clinical trial. On each element, several areas between 1 and 2 mm2 (6 on molars, 4 on premolars) are determined on restoration surface: areas submitted or non-submitted to mastication mechanical stress, glazed or non-glazed. Before prosthesis placement, ex vivo analyses regarding LTD and wear are performed using Raman spectroscopy, SEM imagery and 3D laser profilometry. After placement, restorations are clinically evaluated following criteria of the World Dental Federation (FDI), complemented by the analysis of fracture clinical risk factors. Two independent examiners perform the evaluations. Clinical evaluation and ex vivo analyses are carried out after 6 months and then each year for up to 5 years.
Discussion
For clinicians and patients, the results of this trial will justify the use of monolithic zirconia restorations in dental practice. For researchers, the originality of a clinical study including ex vivo analyses of material aging will provide important data regarding zirconia properties.
Trial registration: ClinicalTrials.gov Identifier: NCT02150226.
Keywords: Dental prosthesis, Zirconia, Low thermal degradation, Computer-aided design/computer-aided manufacturing, Wear, Raman spectroscopy
1. Background
Dental caries and periodontal diseases affect nearly 100% of the adults worldwide [1], [2]. Crowns are intended to restore a tooth with extensive decay, while bridges are intended to replace at least one missing tooth. Crowns and bridges can also be used on dental implants. Thanks to the emergence of computer-aided design/computer-aided manufacturing (CAD/CAM) processes, zirconia (yttria-tetragonal zirconia-polycrystal, Y-TZP), a polycrystalline ceramic material, was introduced to replace metal in dental prostheses because of its good mechanical, better optical properties and good biocompatibility. These prostheses are typically bilayered structures, with a framework that gives mechanical resistance and a porcelain layer that provides aesthetics to the restoration. Unfortunately, clinical reports on zirconia-based restorations have indicated a high rate of short-term failures related to cohesive fracture of the porcelain layer [3], which constitutes a weak link from a mechanical point of view. Therefore, manufacturers have recently introduced monolithic prostheses, which are fully composed of zirconia, without any porcelain layer, except for a thin layer of glaze.
Currently, few clinical studies have been published on zirconia monolithic restorations [4], [5], [6], [7], [8], [9], [10], [11], [12]. Yet a critical issue with those restorations is the material low thermal degradation (LTD), which generates zirconia surface degradation, loss of mechanical properties and risk of fracture [13], [14], [15], [16], [17]. Indeed, zirconia LTD is an aging phenomenon occurring when the material is in contact with water, which induces a change in zirconia metastable crystalline structure. LTD was intensely investigated in the orthopaedic field following numerous zirconia hip prosthesis fractures encountered in the 2000’s [18]. Consequently, several in vitro studies were performed concerning LTD of dental prostheses [13], [14], [19], [20], [21], [22], [23], [24]. Most particularly, LTD was shown to be responsible for a decrease in material flexural strength when 50% of sample surface crystals are transformed [21], [25], [26]. For zirconia dental implants, International Standard Rules [27], [28] state that the crystalline transformation must not exceed a maximum of 25% after aging in an autoclave at 134 °C, 2 bar for 5 h, while no guidelines are available for zirconia prostheses. Nonetheless, extrapolation of in vitro results to clinical behavior is debatable with respect to the differences between oral environment and autoclave aging. Moreover, in vitro studies did not take into account the effect of mastication mechanical stress on restorations [26], [29], [30], [31]. Consequently, the prediction of LTD kinetics and its impact on the lifespan of dental prostheses remains an unsolved problem. To author’s knowledge, no clinical studies about in vivo LTD of dental zirconia prostheses has been published up to now. This issue is particularly critical for monolithic zirconia restorations that have no porcelain layer to act as a barrier against water penetration [31], [32] and which can be submitted to glaze wear. Additionally, some high translucency Y-TZP developed for monolithic restorations are reputed to be more metastable and, thus, more sensitive to LTD [33].
2. Aims and objectives
The main objective of this 5-year prospective study is to evaluate the in vivo LTD of monolithic zirconia restorations on implants and natural teeth using an original protocol, which includes ex vivo analyses of zirconia crystalline microstructure. Secondary objectives include the investigation of the overall quality of monolithic restorations and of the wear process effect on both restorations and antagonistic teeth. The glaze LTD protective effect is investigated through a comparison of glazed and unglazed areas, submitted or not to mastication mechanical stress.
3. Design and methods
3.1. Study design
A 5-year prospective trial was designed. It received approval from the Ethics Committee of the University of Liège (Comité d’Ethique Hospitalo-Facultaire Universitaire de Liège, number B7107201317778, reference 2013/138).
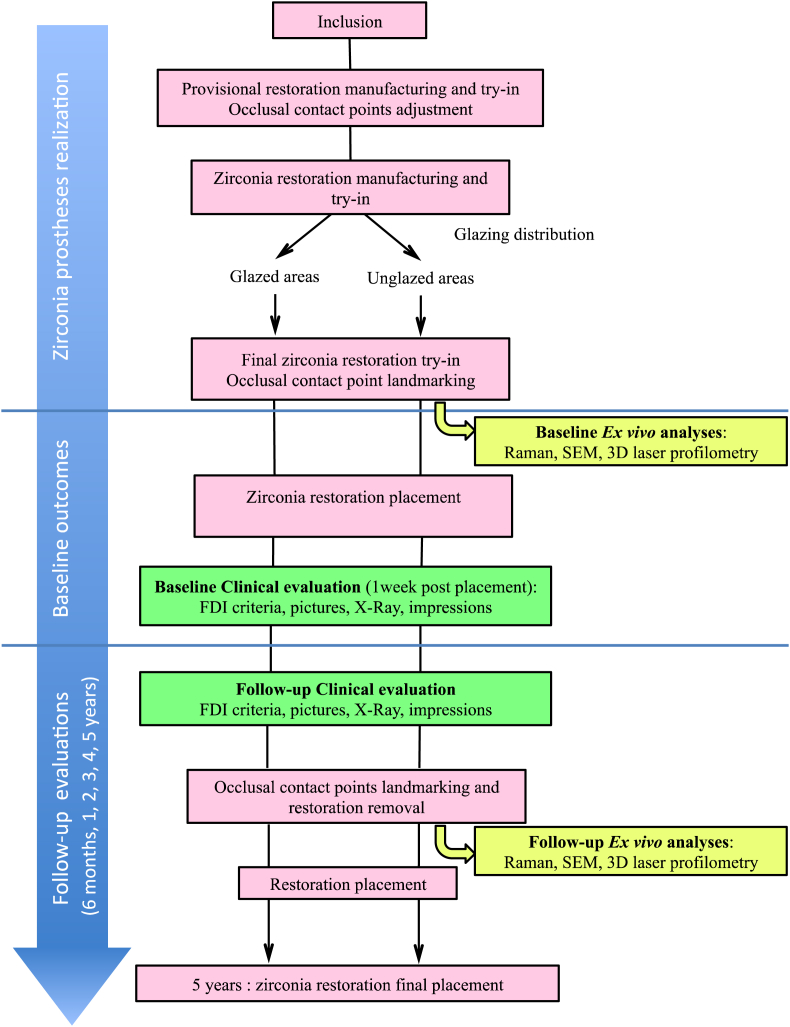
Table 1 gives an overview of the study, which is composed of three stages: zirconia prostheses realisation, baseline data gathering and follow-up evaluations (after 6 months and every year for up to 5 years). Evaluations include clinical evaluation and ex vivo analyses.
Table 1.
Study design.
3.2. Participants and settings
3.2.1. Settings
Patients are included and treated in the Department of Fixed Prosthodontics, Institute of Dentistry, University Hospital, Liège, Belgium. Any patient with the eligible criteria visiting the Institute of Dentistry is asked to participate in the study.
3.2.2. Inclusion/exclusion criteria
Patients are eligible to participate in the trial if they need restoration(s) in the posterior region (molar or premolar). The restorations can be carried out either on implants or teeth. Multi-unit restorations on implants are included if limited to 3 elements (maximum 2 bridges per patient). Several teeth per patient are eligible (maximum 6 elements per patient).
Patients presenting parafunctions such as bruxism, masticatory muscle discomfort, articular disorders or severe wear facets were also included. Exclusion criteria are severe and acute periodontal, carious disease or poor oral hygiene. Patients with removable prosthesis as an antagonist are excluded. Once eligibility is established, the protocol is presented and explained to patients. Inclusion is validated after consent signature.
3.2.3. Operators and evaluators standardization
Operators carry out prosthetic treatment. Evaluators assign scores according to FDI criteria. Both operators and evaluators are experienced dentists in the field of fixed prosthodontics. They are trained in the FDI criteria by means of the e-calib web based software (http://zep01793.dent.med.uni-muenchen.de/moodle/website) and group training sessions. Operators cannot evaluate their own treatments. Trained researchers and technicians perform ex vivo analyses.
3.2.4. Participant incentives
Participants receive no financial compensation. However, their treatment and prostheses are provided free of charge. If the patient wishes to withdraw from the study, a conventional crown will be made at his expense. If an experimental crown fails during the study, a conventional crown will be provided as a replacement.
3.3. Procedure
3.3.1. Tooth preparation and impression for tooth or implant-supported prostheses
All clinical and technical procedures are performed in strict agreement with the clinical and technical instruction protocol validated by the ethics committee and following manufacturer’s recommendations. Teeth are prepared following standardized criteria (1.0–1.5 mm occlusal depth cut to achieve appropriate occlusal anatomy, 1.0–1.5 mm functional cusp tip reduction, 0.5 mm gingival chamfer reduction, and a 6–8° taper to the axial walls). A double-mix impression is performed with a high- and a low-viscous A-silicone impression material (Aquasil Heavy/XLV, Dentsply De Trey, Konstanz, Germany) and the same impression procedure is used for implant restorations. Shade is registered using Vita Classic System (Vita Zahnfabrik, Bad Säckingen, Germany) and if needed, restorations on antagonistic teeth are replaced.
3.3.2. Provisional restoration
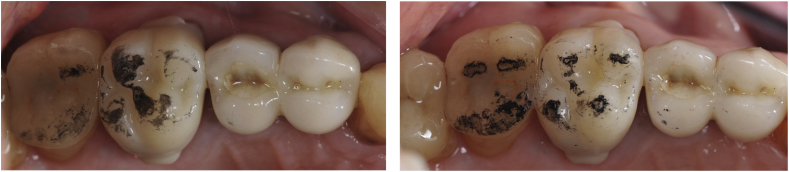
Before the manufacture of zirconia restorations, CAD-CAM composite provisional crowns (Lava Ultimate, 3M ESPE, Seefeld, Germany) or PMMA provisional bridges are made. After die scanning, the restoration design is carried out with CAD/CAM software, either Exocad (Darmstadt, Germany) or Dental Wings (Montreal, Canada) (DPI Lava milling center, Anderlecht, Belgium). Specific buccal and palatal grips are added to the crown design to facilitate cemented crown removal. The file is then transferred to the milling machine for manufacturing (Lava CNC 500, Serial Number: 07019 (2009), 3M ESPE). The provisional restorations are adapted in-mouth and used as a template for the design of the zirconia restoration. Particular attention is paid to occlusal contact points adjustment, in order to obtain at least one flat contact surface of approximately 1 mm2 per cusp, by either grinding or by adding composite (Fig. 1).
Fig. 1.
Occlusal contact points before and after adjustment on a Lava Ultimate crown (tooth #16).
3.3.3. Zirconia prostheses
Provisional restorations are scanned for zirconia restorations fabrication (Lava Plus, 3M ESPE, Seefeld, Germany) with the same milling system. Sintering is performed according to manufacturer’s instructions, i.e. at 1450 °C for 2 h. Implant-supported restorations are bonded on to a specific titanium abutment (1000er-Serie, Medentika, Hugelsheim, Germany) with a resin composite cement: either RelyX Ultimate (3M ESPE, Seefeld, Germany) for the first 16 restorations of the study, or Multilink abutment (Ivoclar Vivadent, Schaan, Liechtenstein) for the 40 next, according to manufacturer’s recommendations, after sandblasting of the abutment and of the zirconia restoration with 50 μm alumina particles, 2 bar. Zirconia restorations are tried-in and occlusal contact points are adjusted and polished with a specific bur kit if needed (Diasynt Plus/Diacera Zirconium, Eve Ernst Vetter, Pforzheim, Germany). Adjusted areas are encoded.
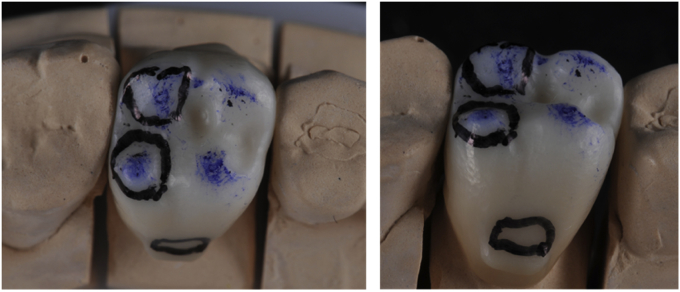
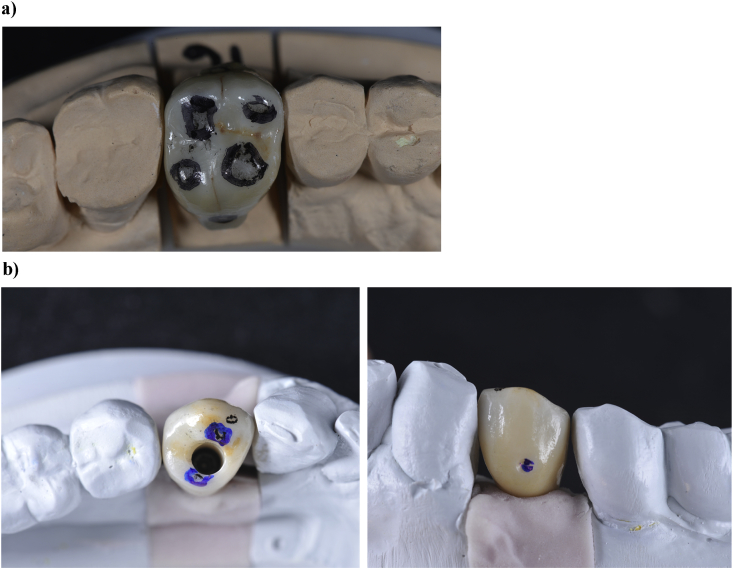
Occlusal surface contact areas, which will not be glazed, are randomly determined (Fig. 2, Fig. 3). Four occlusal contact points (one contact per cusp) are determined on molars and two on premolars. For molars, two cusps are randomly selected to remain unglazed: one centric cusp (unglazed centric cusp (UCC)) and one non-centric (unglazed non-centric cusp (UNCC)). The two other cusps are called “glazed centric cusp” (GCC) and “glazed non-centric cusp” (GNCC). For premolars, one cusp is randomly selected to remain unglazed. Control areas are the buccal face (glazed) and the lingual/palatal face (unglazed) of the restoration. The glaze (IPS empress stains and eMax Ceram glaze, Ivoclar Vivadent, Schaan, Liechtenstein) is sintered at 780 °C for 1 min. Definitive bonding (bond is eliminated during the glaze firing) on the specific titanium abutment is performed following the procedure described previously. The glazed restorations are tried-in and occlusal contact points, as well as lingual/palatal and buccal areas, are marked for ex vivo analyses and registered with a picture (Fig. 4).
Fig. 2.
Landmarking with permanent ink of areas, which will not be glazed (tooth #16).
Fig. 3.
Glazed Lava Plus crown (tooth #16).
Fig. 4.
Glazed crowns after try-in and landmarking of areas to be ex vivo analysed. a) Final crown on tooth #16. Landmarking of areas to be analysed: occlusal contact points and control areas on buccal and palatal faces, which are located up to the undercut created to remove the crown. b) Screw-retained crown on implant (tooth #34). Landmarking of areas to be analysed: occlusal contact points and control areas on buccal and lingual faces, which are located up to a small groove performed in the restoration surface.
3.3.4. Zirconia prostheses placement and removal
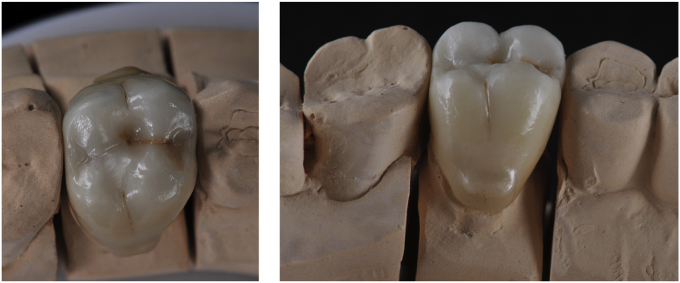
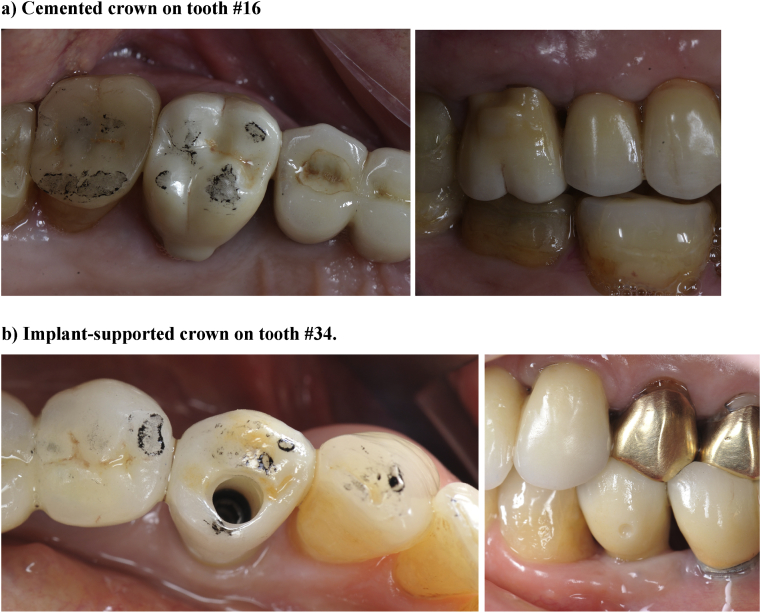
Baseline ex vivo analyses of zirconia restorations are performed before placement. Screw-retained restorations are torqued with 35 N cm−1 (Fig. 5). Cemented restorations are sealed with eugenol-free cement (RelyX Temp NE, 3M ESPE) and prior to cementation, restorations are cleaned with alcohol in an ultrasonic bath and teeth are disinfected with 2% chlorhexidine. Clinical evaluation is performed one week after placement. After 6 months, restorations are clinically evaluated and then removed for ex vivo analyses. Provisional restorations replace zirconia restorations during ex vivo analyses. After these analyses, zirconia restorations are placed in the mouth of the patient, following the same procedure as the first time. Evaluations will be repeated after a one-year in-mouth stay, and then each year for up to 5 years.
Fig. 5.
Crowns after placement. a) Cemented crown on tooth #16. b) Implant-supported crown on tooth #34.
3.4. Data collection
3.4.1. Primary outcome: LTD evaluation
LTD is evaluated directly on zirconia restorations through zirconia crystalline microstructure analysis with Raman spectroscopy. Indeed, LTD is characterized by a shift from the tetragonal crystalline form (t) to the monoclinic form (m). The presence of monoclinic, tetragonal or a combination of both forms is distinguishable and quantifiable on Raman spectra, allowing the measurement of the transformation volume ratio (Vfm).
Raman spectra are recorded with a Labram Raman spectrometer (Horiba-Jobin Yvon, Kyoto, Japan). The excitation laser is provided by a HeNe laser (632 nm) with 1 mW power focused at the surface of the specimen and the Raman spectra are acquired by a charge-coupled device detector (Horiba-Jobin Yvon, Kyoto, Japan) with 1 cm−1 spectral resolution (1800 grooves/mm grating). The Raman spectrometer is combined with an optical microscope (Olympus LX71; Olympus Corporation, Tokyo, Japan). A confocal pinhole with adjustable diameter is used for a confocal detection and an objective 80× (numerical apertures 0.75) is used to reach 1 μm3 resolution (lateral × axial).
Analysis of collected spectra enables Vfm calculation in the confocal probed volume, estimated using the Eq. (1) [34]:
| (1) |
where Im and It are the intensities of the peaks (wave numbers in superscript) of the monoclinic and tetragonal phases. The Raman peak positions and intensities are obtained by fitting the Raman spectra with Lorentzian curves (Origin 8 software, OriginLab, Northampton, MA). 5 points per area are investigated and the outcome is the highest (worst) Vfm (%) for each area and tooth.
3.4.2. Secondary outcomes
3.4.2.1. Clinical evaluation
Clinical evaluation follows World Dental Federation recommendations and uses World Dental Federation instruments for assessing dental restorations, described in 2007 [35] and updated in 2010 [36]. This instrument contains three dimensions (18 items): biological (six items), functional (seven items) and aesthetic (five items). Each item is assessed by clinical examination on a 5-point Likert scale (1 corresponding to a perfect restoration and 5 corresponding to a restoration that needs to be replaced) and collected. The dentist assesses all items except one; the remaining item is the patient-reported satisfaction. The outcome is the worst score of all items (ranging from 1 to 5) at follow-up. These evaluations are performed at baseline, at 6 months and then each year for up to 5 years by two independent evaluators. Moreover, occlusal risk factors are registered [3]: occlusal relationships characterized as favourable or unfavourable based on the clinical examination (class III or class II.2 malocclusion, anterior or posterior crossbite, edge to edge or open bite, were considered as unfavourable occlusal relationships), the presence of parafunctional habits, the use of an occlusal nightguard, the type of support (tooth or implant) and the nature of the antagonistic tooth. Impressions of restorations and antagonistic teeth are performed in order to cast polyrurethane replicas (Alphadie, Schütz Dental GmbH, Rosbach, Germany). Beside radiographs, pictures of restorations and antagonistic teeth, with occlusal contact point registering, are performed. To prepare ex vivo analyses, occlusal contact points, as well as lingual/palatal and buccal areas are marked with permanent ink.
3.4.2.2. Wear
Wear is studied with ex vivo analyses of zirconia restorations, which include scanning electron microscopy (SEM) and 3D laser profilometry. Polyurethane replicas of teeth will be used to study wear of antagonistic teeth in the same manner, while replicas of zirconia restorations are stored as a control.
3.4.2.3. SEM observations
After Raman spectroscopy, restorations are gold-coated and observed with a JSM-6400 Scanning Electron Microscope (JEOL Limited, Tokyo, Japan). Interpretation of fracture patterns, if occurs, is based on the descriptions by Scherrer et al. [37], particularly to determine the origin and direction of the crack propagation.
3.4.2.4. 3D laser profilometry
Samples are placed in the scanner on a die replica embedded in resin, for repeatable positioning at each evaluation. Occlusal, buccal and lingual surfaces are scanned with a custom-made device including a XY motorized board stage and a 100 nm-resolution laser sensor (Keyence LK G30 with LK GD500 controller, Keyence Corporation, Osaka, Japan). Raw data acquisition and processing are performed using a custom-developed software using C# language (Microsoft Visual Studio 2013, Microsoft Corporation, Redmond, WA, USA/Measurement Studio 2014, National Instrument Corporation, Austin, TX, USA) coupled to a digital data acquisition PCI board (NI PCI-6534, National Instruments Corporation, Austin, TX, USA). Resulting matrices of Z values are then transferred to a surface matching software Geomagic Control 2014 (Geomagic Inc, Morrisville, C.C., USA).
3.4.3. Data management
Data are collected, stored and processed in the Department of Fixed Prosthodontics, Institute of Dentistry, University Hospital, Liège, Belgium. Patients are identified by their inclusion number in order to preserve their privacy. Data are entered twice by operators and checked by a data manager. Only the data manager and statisticians have unrestricted access. Adverse events are also assessed at each study visit.
4. Statistical analysis
4.1. Sample size
The determination of the sample size (N) was based on the following considerations. The statistical unit was the tooth characterized by its maximum LTD value recorded at each time point (baseline, 6 months, 1, 2, 3, 4 and 5 years). An LTD value above 50% was considered as treatment failure for the tooth. The overall proportion (π) of such treatment failures was defined as the primary outcome measure of the study. The study rationale was to reject the proposed treatment if π > 0.20, i.e. more than 20% treatment failures over time. Assuming a significance level α of 1% (Bonferroni correction for multiple time testing), a power 1-β of 90%, a proportion π of at most 0.08 (margin 0.12) and a one-sided Z test for a Binomial proportion of 0.20, a sample of 91 teeth would be needed to detect a percentage > 20% of treatment failures at each data point collection. To account for correlations between teeth within subjects and for study withdrawals, the sample size was increased to a minimum of N = 100 teeth.
4.2. Statistical methods
Quantitative variables characterizing patients and teeth are summarized by mean and standard deviation (SD) or by median and interquartile range (IQR) for skewed data; frequency tables are used for categorical variables. The association between two quantitative variables is assessed by the correlation coefficient. Cohen kappa coefficient is used to assess the degree of agreement between clinical evaluations made by different evaluators. The observed percentage of treatment failures at each time point (interim analysis) is tested at the 1% critical level by a one-sided Z test for a Binomial proportion of 0.20 as described in the sample size section. In case of rejection, the study will be terminated unless prostheses are not fractured and still functional in which case it will go on to analyze the LTD kinetic process. To assess the effect of fixed experimental factors (e.g. time, glaze, mechanical stress) and random effects (subjects and teeth) on LTD, wear measures and other clinical parameters, a generalized linear mixed model approach is used. Unless otherwise stated, results are considered significant at the 5% critical level. All calculations will be performed with the SAS (version 9.4) statistical package.
5. Discussion
CAD-CAM processes have revolutionized the world of dental prostheses and the replacement of artisanal work by industrial processes has enhanced the reproducibility and the productivity of manufacturing. But one of the main advantages of CAD-CAM processes is the opportunity to use high performance materials, such as zirconia, particularly yttria-tetragonal zirconia-polycrystal (Y-TZP), a popular material, which was introduced in the early 2000’s as an alternative to metal for crowns and bridges. Zirconia has good optical and biocompatibility properties in comparison with metal alloys and it is also the most resistant material among dental ceramics, combining high strength and toughness due to its unique phase transformation toughening property. Indeed, Y-TZP is a polycrystalline ceramic material in a metastable state: yttrium oxide acts as a dopant to stabilize the crystalline tetragonal form at room temperature, this tetragonal form being able to further transform to the monoclinic form under the effect of stress. This transformation is characterized by a crystal volume increase, which is able to counteract the propagation of cracks [38]. Unfortunately, this phase transformation can also occur with time, when the material is in contact with water, which is able to penetrate the crystalline structure. This aging phenomenon, called the low temperature degradation (LTD), generates zirconia surface degradation, loss of mechanical properties and risk of fracture [13], [14], [15], [16], [17]. LTD was at the origin of catastrophic failures encountered with zirconia hip prostheses in the early 2000’s. This problem was extensively studied in vitro, particularly by Chevalier et al. [18], but surprisingly, this issue was not raised by the dental community before the introduction of zirconia prostheses to the dental market. Yet temperature, moisture and mastication mechanical stress characterizing the oral environment are ideal conditions for LTD to develop and to impact the prognosis of dental prostheses. This is particularly true for monolithic zirconia restorations that are not covered by a porcelain layer preventing water penetration [31], [32] and that are, for aesthetic reasons, composed of specific high translucency varieties of zirconia, which can be particularly LTD-sensitive. Indeed, to increase translucency, some manufacturers increase grain size or reduce dopant content, which give more metastable zirconia [33].
Consequently, the primary outcome of this study protocol is to evaluate the in-mouth LTD of monolithic zirconia restorations on natural teeth and implants. Indeed, if, as suspected, LTD occurs in the oral environment, the question is the kinetic of this process and its impact compared to the lifespan of dental prostheses (around 15 years). To the author’s knowledge, no clinical study about LTD of dental zirconia prostheses has been published up to now and the clinical background with monolithic restorations is too short to highlight potential failures. However, several in vitro studies were dedicated to this issue using artificial aging with an autoclave [13], [14], [19], [20], [21], [22], [23], [24]. A recent systematic review [26] concluded that aging in an autoclave promotes Y-TZP LTD, decreases its flexural strength, while the monoclinic content increases. When increasing time (more than 20 h), pressure (more than 2 bars) and temperature (134 °C), the flexural strength significantly decreases, which was observed when the monoclinic content was superior to 50% in the sample surface. It must be noticed that none in vitro studies took into account the additional effect of mechanical stress on LTD [26], [29], [30], [31]. Some authors showed a lower resistance to LTD for some high translucency zirconia than for standard zirconia, with the presence of around 75% of monoclinic content after 200 h of autoclave aging [39] and a decrease of 30% in crown resistance to cyclic mechanical loading after 100 h aging [40]. It must also be noted that only 1 h of exposure in a steam vapor autoclave at 134 °C and 2 bar is considered to correspond to 3 or 4 years of clinical use [41]. Yet extrapolation of in vitro aging to clinical behavior is doubtful, notably in regards to the important differences between oral environment and autoclave conditions, such as the absence of mechanical stress. If International Standard Rules [27], [28] established for zirconia dental implants (not prostheses) state that the crystalline transformation must not exceed a maximum of 25% after aging in an autoclave at 134 °C, 2 bar for 5 h, there are no guidelines regarding Y-TZP dental prostheses. Consequently, the present protocol, which combines clinical evaluation and ex vivo analyses, was designed to allow the monitoring of LTD in the oral environment through quantification of zirconia t-m phase transformation with Raman spectroscopy. Raman spectroscopy is a powerful and reliable method, which is an alternative to X-ray diffraction [42], [43]. Its advantage lies in its 1 μm2-resolution, which is particularly appropriate for the evaluation of occlusal contact points.
Regarding secondary outcomes of this study protocol, they include the investigation of the overall quality of monolithic restorations and of wear of both restorations and antagonistic teeth. Few clinical studies have been published in the literature concerning monolithic zirconia restorations and the clinical background is short [4], [5], [6], [7], [8], [9], [10], [11], [12]. Three studies focused on the evaluation of zirconia crowns and antagonistic teeth wear. They all used impressions and casting of replicas for an indirect quantification of the wear by 3D surface laser analysis, which can generate some bias related to the accuracy of replicas. The ex vivo analyses performed in the present protocol are intended to avoid this bias. Moreover, a supplementary advantage of ex vivo analyses is the direct observation of restoration with SEM, which allows the visual detection of glaze wear. As glaze wear could promote LTD, glaze protective effect is investigated through a comparison of glazed and unglazed areas, submitted or not to mastication mechanical stress, to evaluate the effect of this stress on LTD. Additionally, the general clinical behavior of monolithic zirconia tooth- and implant-supported restorations is seriously evaluated taking into account international standard criteria complemented by the analysis of a variety of risk factors, particularly occlusal, that can significantly influence the performance of the restorations, notably in terms of wear or fracture [3].
In conclusion, this new clinical protocol including in-depth ex vivo evaluation of Y-TZP microstructure will provide important data regarding its phase transformation process, which is still not fully understood, particularly in regards to the effect of the combination of mechanical stress to moisture and temperature [32]. The novel approach of restoration removal at the different evaluation times allows for the use of Raman spectroscopy, SEM imagery and 3D laser profilometry to provide quantitative and qualitative information about Y-TZP aging and degradation of monolithic restorations. For future research, this trial should be able to provide reliable data to compute in silico models of dental zirconia in-mouth aging kinetic [41], [44]. Indeed, there is an urgent and crucial need to establish standards regarding LTD of zirconia materials for dental prostheses on an international level in order to avoid potential failures in these restorations, used daily in dental offices.
Trial status
The trial was submitted for registration at ClinicalTrials.gov on May 26, 2014. Patient recruitment started on February 2014. This protocol was submitted for publication on March 7, 2016.
Competing interests
The authors declare that they have no competing interests.
Author’s contributions
V. Koenig, C. P. Wulfman, M.A. Derbanne and A.K. Mainjot contributed to conception and design, data analysis and interpretation, drafted the manuscript. N.M. Dupont, S.O. Le Goff, M-L. Tang, L.Seidel, T.Y. Dewael, A.J. Vanheusden contributed to data analysis and interpretation, critically revised the manuscript.
Acknowledgments
The authors thank 3M ESPE for providing the restorations used in this study. This company did not have any authority in the study design and will not have any on the decision to submit the report for publication. The authors also thank the University of Liège Hospital (CHU) for funding profilometry equipment.
Contributor Information
Vinciane Koenig, Email: vinciane.koenig@chu.ulg.ac.be.
Amélie K. Mainjot, Email: a.mainjot@chu.ulg.ac.be.
References
- 1.Petersen P.E. The world oral health report 2003: continuous improvement of oral health in the 21st century–the approach of the WHO global oral health programme. Community Dent. Oral Epidemiol. 2003;31(Suppl. 1):3–23. doi: 10.1046/j..2003.com122.x. [DOI] [PubMed] [Google Scholar]
- 2.Bagramian R.A., Garcia-Godoy F., Volpe A.R. The global increase in dental caries. A pending public health crisis. Am. J. Dent. 2009;22(1):3–8. [PubMed] [Google Scholar]
- 3.Koenig V. Clinical risk factors related to failures with zirconia-based restorations: an up to 9-year retrospective study. J. Dent. 2013;41(12):1164–1174. doi: 10.1016/j.jdent.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Batson E.R. Clinical outcomes of three different crown systems with CAD/CAM technology. J. Prosthet. Dent. 2014;112(4):770–777. doi: 10.1016/j.prosdent.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Carames J. Clinical advantages and limitations of monolithic zirconia restorations full arch implant supported reconstruction: case series. Int. J. Dent. 2015;2015:392496. doi: 10.1155/2015/392496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cardelli P. Full-Arch, implant-supported monolithic zirconia rehabilitations: pilot clinical evaluation of wear against natural or composite teeth. J. Prosthodont. 2015;00:1–5. doi: 10.1111/jopr.12374. [DOI] [PubMed] [Google Scholar]
- 7.Cheng C.W. Complete-mouth implant rehabilitation with modified monolithic zirconia implant-supported fixed dental prostheses and an immediate-loading protocol: a clinical report. J. Prosthet. Dent. 2013;109(6):347–352. doi: 10.1016/S0022-3913(13)00109-1. [DOI] [PubMed] [Google Scholar]
- 8.Dhima M. Practice-based clinical evaluation of ceramic single crowns after at least five years. J. Prosthet. Dent. 2014;111(2):124–130. doi: 10.1016/j.prosdent.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 9.Moscovitch M. Consecutive case series of monolithic and minimally veneered zirconia restorations on teeth and implants: up to 68 months. Int. J. Periodontics Restor. Dent. 2015;35(3):315–323. doi: 10.11607/prd.2270. [DOI] [PubMed] [Google Scholar]
- 10.Mundhe K. Clinical study to evaluate the wear of natural enamel antagonist to zirconia and metal ceramic crowns. J. Prosthet. Dent. 2015;114(3):358–363. doi: 10.1016/j.prosdent.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Stober T. Enamel wear caused by monolithic zirconia crowns after 6 months of clinical use. J. Oral Rehabil. 2014;41(4):314–322. doi: 10.1111/joor.12139. [DOI] [PubMed] [Google Scholar]
- 12.Venezia P. Retrospective analysis of 26 complete-arch implant-supported monolithic zirconia prostheses with feldspathic porcelain veneering limited to the facial surface. J. Prosthet. Dent. 2015;114(4):506–512. doi: 10.1016/j.prosdent.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Deville S., Guenin G., Chevalier K. Martensitic transformation in zirconia - Part I. Nanometer scale prediction and measurement of transformation induced relief. Acta Mater. 2004;52(19):5697–5707. [Google Scholar]
- 14.Cattani-Lorente M. Low temperature degradation of a Y-TZP dental ceramic. Acta Biomater. 2011;7(2):858–865. doi: 10.1016/j.actbio.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 15.Chevalier J. Low-temperature degradation in zirconia with a porous surface. Acta Biomater. 2011;7(7):2986–2993. doi: 10.1016/j.actbio.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Hallmann L. The influence of grain size on low-temperature degradation of dental zirconia. J. Biomed. Mater Res. B Appl. Biomater. 2011;100(2):447–456. doi: 10.1002/jbm.b.31969. [DOI] [PubMed] [Google Scholar]
- 17.Kim J.W. Concerns of hydrothermal degradation in CAD/CAM zirconia. J. Dent. Res. 2010;89(1):91–95. doi: 10.1177/0022034509354193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chevalier J. What future for zirconia as a biomaterial? Biomaterials. 2006;27(4):535–543. doi: 10.1016/j.biomaterials.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 19.Samodurova A. The combined effect of alumina and silica co-doping on the ageing resistance of 3Y-TZP bioceramics. Acta Biomater. 2015;11:477–487. doi: 10.1016/j.actbio.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Egilmez F. Factors affecting the mechanical behavior of Y-TZP. J. Mech. Behav. Biomed. Mater. 2014;37:78–87. doi: 10.1016/j.jmbbm.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Ban S. Biaxial flexure strength and low temperature degradation of Ce-TZP/Al2O3 nanocomposite and Y-TZP as dental restoratives. J. Biomed. Mater Res. B Appl. Biomater. 2008;87(2):492–498. doi: 10.1002/jbm.b.31131. [DOI] [PubMed] [Google Scholar]
- 22.Roy M.E. Phase transformation, roughness, and microhardness of artificially aged yttria- and magnesia-stabilized zirconia femoral heads. J. Biomed. Mater Res. A. 2007;83(4):1096–1102. doi: 10.1002/jbm.a.31438. [DOI] [PubMed] [Google Scholar]
- 23.Chowdhury S. Accelerating aging of zirconia femoral head implants: change of surface structure and mechanical properties. J. Biomed. Mater Res. B Appl. Biomater. 2007;81(2):486–492. doi: 10.1002/jbm.b.30688. [DOI] [PubMed] [Google Scholar]
- 24.Kosmac T. The effect of surface grinding and sandblasting on flexural strength and reliability of Y-TZP zirconia ceramic. Dent. Mater. 1999;15(6):426–433. doi: 10.1016/s0109-5641(99)00070-6. [DOI] [PubMed] [Google Scholar]
- 25.Kim H.T. The effect of low temperature aging on the mechanical property & phase stability of Y-TZP ceramics. J. Adv. Prosthodont. 2009;1(3):113–117. doi: 10.4047/jap.2009.1.3.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pereira G.K. Low-temperature degradation of Y-TZP ceramics: a systematic review and meta-analysis. J. Mech. Behav. Biomed. Mater. 2015;55:151–163. doi: 10.1016/j.jmbbm.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 27.ISO 13356-2008 Implants for surgery – ceramic materials based on yttria-stabilizes tetragonal zirconia (Y-TZP) Int. Organ. Stand. 2008 [Google Scholar]
- 28.ISO 6872-2008 Dentistry – ceramic materials. Int. Organ. Stand. 2008 [Google Scholar]
- 29.Flinn B.D. Accelerated aging characteristics of three yttria-stabilized tetragonal zirconia polycrystalline dental materials. J. Prosthet. Dent. 2012;108(4):223–230. doi: 10.1016/S0022-3913(12)60166-8. [DOI] [PubMed] [Google Scholar]
- 30.Kohorst P. Low-temperature degradation of different zirconia ceramics for dental applications. Acta Biomater. 2012;8(3):1213–1220. doi: 10.1016/j.actbio.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 31.Pereira G. Effect of low-temperature aging on the mechanical behavior of ground Y-TZP. J. Mech. Behav. Biomed. Mater. 2015;45:183–192. doi: 10.1016/j.jmbbm.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 32.Denry I., Kelly J.R. Emerging ceramic-based materials for dentistry. J. Dent. Res. 2014;93(12):1235–1242. doi: 10.1177/0022034514553627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y. Making yttria-stabilized tetragonal zirconia translucent. Dent. Mater. 2014;30(10):1195–1203. doi: 10.1016/j.dental.2014.08.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim C.S., Finlayson T.R., Ninio F., Griffiths J.R. In-situ measurement of the stress-induced phase transformations in magnesia-partially-stabilized zirconia using Raman spectroscopy. J. Am. Ceram. Soc. 1992;75(6):1570–1573. [Google Scholar]
- 35.Hickel R. Recommendations for conducting controlled clinical studies of dental restorative materials. Science Committee Project 2/98–FDI World Dental Federation study design (Part I) and criteria for evaluation (Part II) of direct and indirect restorations including onlays and partial crowns. J. Adhes. Dent. 2007;9(Suppl. 1):121–147. [PubMed] [Google Scholar]
- 36.Hickel R. FDI World Dental Federation - clinical criteria for the evaluation of direct and indirect restorations. Update and clinical examples. J. Adhes. Dent. 2010;12(4):259–272. doi: 10.3290/j.jad.a19262. [DOI] [PubMed] [Google Scholar]
- 37.Scherrer S.S. Fractographic ceramic failure analysis using the replica technique. Dent. Mater. 2007;23(11):1397–1404. doi: 10.1016/j.dental.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chevalier J. The tetragonal-monoclinic transformation in zirconia: lessons learned and future trends. J. Am. Ceram. Soc. 2009;92(9):1901–1920. [Google Scholar]
- 39.Flinn B.D. Effect of hydrothermal degradation on three types of zirconias for dental application. J. Prosthet. Dent. 2014;112(6):1377–1384. doi: 10.1016/j.prosdent.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura K. The influence of low-temperature degradation and cyclic loading on the fracture resistance of monolithic zirconia molar crowns. J. Mech. Behav. Biomed. Mater. 2015;47:49–56. doi: 10.1016/j.jmbbm.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 41.Chevalier J., Cales B., Drouin J. Low-temperature aging of Y-TZP ceramics. J. Am. Ceram. Soc. 1999;82(8):2150–2154. [Google Scholar]
- 42.Pezzotti G., Porporati A.A. Raman spectroscopic analysis of phase-transformation and stress patterns in zirconia hip joints. J. Biomed. Opt. 2004;9(2):372–384. doi: 10.1117/1.1647547. [DOI] [PubMed] [Google Scholar]
- 43.Clarke D.R.A.F. Measurement of the cristallographically transformed zone produced by fracture in ceramics containing tetragonal zirconia. J. Am. Ceram. Soc. 1982;65(6):284–288. [Google Scholar]
- 44.Gremillard L. Modeling the aging kinetics of zirconia ceramics. J. Am. Ceram. Soc. 2004;24(13):3483–3489. [Google Scholar]