Abstract
Osteoarthritis (OA) is the most common degenerative joint disease and a growing health problem affecting more than half of the population over the age of 65. It is characterized by inflammation in the cartilage and synovium, resulting in the loss of joint structure and progressive damage to the cartilage. Many pro-inflammatory mediators are elevated in OA, including reactive oxygen species (ROS) such as nitric oxide (NO) and hydrogen peroxide (H2O2). Damaged articular cartilage remains a challenge to treat due to the limited self-healing capacity of the tissue and unsuccessful biological interventions. This highlights the need for better therapeutic strategies to heal damaged articular cartilage. Ozone (O3) therapy has been shown to have positive results in the treatment of OA; however the use of O3 therapy as a therapeutic agent is controversial. There is a perception that O3 is always toxic, whereas evidence indicates that when it is applied following a specified method, O3 can be effective in the treatment of degenerative diseases. The mechanism of action of O3 therapy in OA is not fully understood and this review summarizes the use of O3 therapy in the treatment of damaged articular cartilage in OA.
Keywords: Osteoarthritis (OA), Articular cartilage, Ozone (O3) therapy, Reactive oxygen species (ROS)
1. Introduction
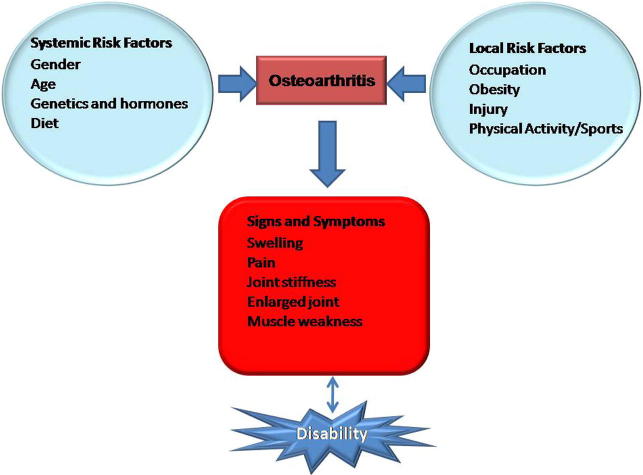
Osteoarthritis (OA) is the most common type of arthritis affecting more than half of people over the age of 65 and is more prevalent in women (18%) than in men (9.6%) after menopause (Musumeci et al., 2015). It is a chronic, degenerative joint disorder affecting millions of people worldwide (Ashkavand et al., 2013). OA is considered as one of the most common causes of disability affecting the joints of the knee, hips and hands. It can be classified into two different forms namely: primary and secondary OA. Primary or idiopathic OA is gene dependent while secondary or post traumatic OA occurs mainly after a traumatic event (Musumeci et al., 2015). Although primary and secondary OA are caused by different factors, they both result in the same abnormalities, a degenerative phenomenon complicated by inflammatory reactions (Reynard and Loughlin, 2012). OA is a complex disease causing a change in the tissue homeostasis of articular cartilage and the subchondral bone. The common features of OA include loss of cartilage, narrowing of joint spaces, hypertrophic bone changes and the formation of osteophyte (Ashkavand et al., 2013). The latter refers to the overgrowth of the bone and cartilage occurring at the joint margins. The stiffening of the subchondral bone causes the bone to be less able to absorb impact loads and thereby leading to increased stress in the cartilage (Li and Aspden, 1997). The primary cause of OA remains largely unknown and various factors can play a role in the development of OA (Fig. 1). The development of knee arthritis has been strongly associated with excess weight, obesity, gender and previous knee injury. Diabetes is also associated with the progression of knee OA (Musumeci et al., 2015). The signs and symptoms of OA usually include: pain, stiffness of the joints, muscle weakness and swelling of the knee.
Figure 1.
An illustration of the risk factors that are involved in osteoarthritis (adapted from Ashkavand et al., 2013).
Articular cartilage is a specialized connective tissue covering the joint surfaces that has no nerve supply and is therefore susceptible to early injuries (Rambani and Venkatesh, 2014). It is a hyaline cartilage characterized by a compact collagen network and extracellular matrix made up of proteoglycan, and is highly resistant to mechanical loads (Sato et al., 2014). Articular cartilage is avascular, aneural and alymphatic, and it is constituted by one cell type, the chondrocytes. Articular cartilage has poor repair properties due to a relatively low number of cells in the tissue, decreased metabolic rate, and matrix fibers that restrict the division of chondrocytes and their migration in the articular cartilage. The treatment of injured articular cartilage poses a major challenge to orthopedic surgeons because of its limited self-healing capacity. To date, there is no universally accepted successful treatment for injured or damaged articular cartilage (Rambani and Venkatesh, 2014).
Ozone (O3) is a colorless gas with an acrid odor and was first discovered in 1848 (Daif, 2012). The basic function of O3 is to protect living organisms from the harmful effects of UV radiation. It occurs at a concentration of less than 20 μg/m3 at the earth’s surface which is a concentration that is compatible with life (Elvis and Ekta, 2011). O3 was used for treating gaseous post-traumatic gangrene, burns, infected wounds and fistulas in German soldiers during World War I (Gupta and Abhishek, 2012). Since its discovery, O3 has been used as a therapeutic agent to treat different diseases. It exerts its effects, by preparing the host to undergo physiopathological events mediated by ROS (Inal et al., 2011). O3 possess both virucidal and bactericidal properties, and can be used for disinfection, sterilization and destruction of malignant cells (Eliakim et al., 2001). Hydrogen peroxide (H2O2) is produced during O3 therapy resulting from oxidative stress and lipid oxidation which mediates the biological effects of O3 therapy by acting as second messenger (Inal et al., 2011). Repeated exposure to O3 therapy may cause resistance against oxidative stress via inducing the antioxidative system.
O3 therapy activates the enzymes that are responsible for protecting the body against processes linked to the overproduction of superoxides. During O3 therapy, there is stimulation of transmembranous flow of oxygen and the induction of enzymes such as superoxide dismutase, peroxidases or catalases and the oxygen utilization in the mitochondrial respiratory chain becomes more effective (Madej et al., 2007). Superoxide is the most crucial radical, playing a key role in biological regulation (Inal et al., 2011). In the last decades a number of orthopedics in Europe have used ozone to treat acute and chronic knee arthritis and the results show rapid pain relief, disappearance of edema, decongestion and increased mobility. Unfortunately there are no experimental studies on the mechanism of action of ozone in the knee arthritis, and there is a large variability in terms of side of injection, volume and concentration of ozone (Borrelli et al., 2015).
2. History of ozone (O3) therapy
Van Mauren, first discovered the distinctive odor of O3 in 1785. The actual gas was later discovered by a German Chemist Christian Friedrich Schonbein at the University of Basel in Switzerland in 1840, when working with a voltaic pile in the presence of oxygen (Altman, 2007). Friedrich noticed the emergence of a gas with an electric and pungent smell, and named it ozone, which is derived from the Greek word for smell, because of its pungent smell (Bocci, 2011). In 1860, Jacques-Louis Soret, a Swiss chemist then demonstrated that the ozone molecule was made up of three atoms of oxygen (Altman, 2007). The disinfecting and oxidating properties of O3 were first demonstrated in the laboratory of an Irish chemist, Thomas Andrews (Bocci, 2011). O3 was used for the first time to disinfect operating rooms in a hospital in 1856, and in 1860, the first ozone water treatment plant was built in Monaco to disinfect water (Altman, 2007). Werner von Siemens practically applied the concept that O3 derives from oxygen when an electric discharge is generated by a voltaic arc. Siemens is the inventor of the super induction tube (Siemens’s tube), consisting of two interposed electrode plates set at high voltage, which could generate O3 in the presence of oxygen (Bocci, 2011). An outbreak of cholera in Hamburg which killed thirty thousand people led to the construction of the first waterworks to use O3 in Germany in 1901 (Altman, 2007). To date, the disinfecting properties of O3 are well known and there are more than three thousand municipal O3 water treatment facilities in the world (Bocci, 2011).
The first medical application of O3 was in the treatment of post traumatic gangrene in German soldiers during the first world war from 1914 to 1918 (Bocci, 2011). O3 was also used to prevent infection in local medical procedures and to control wound infections because of its prophylactic properties during World War I (Merin et al., 2007). The invention of a reliable O3 generator by a physicist, Joachim Hansler, was a major breakthrough in the use of O3 for medical applications. In the late 1980 s, there were reports that Germans were successfully treating HIV patients with ozonated autohemotherapy (O3-AHT). At that time, there was no pharmacological treatment of HIV, thus the Canadian government approved the testing of O3-AHT for its safety and efficacy in treating AIDS patients (Elvis and Ekta, 2011). O3 was effective at disinfecting extracorporeal blood samples of HIV, but was ineffective when applied in vivo (Elvis and Ekta, 2011).
3. Treatment of injured articular cartilage
Injured or damaged articular cartilage remains one of the most difficult tissues to treat. There are several techniques that have been developed in the past to treat damaged articular cartilage. These include: arthroscopic lavage and debridement, bone marrow stimulation technique, osteochondral autografting (OCG), autologous chondrocyte implantation (ACI) and now recently mesenchymal stem cells (MSCs) are used in treating injured articular cartilage.
3.1. Arthroscopic lavage and debridement
Arthroscopic lavage washes out loose cartilage, inflammatory mediators and collagen debris that can be present in the synovium causing synovitis and effusion (Falah et al., 2010). The debridement of cartilage (chondroplasty) aims to remove loose chondral flaps and fibrillated articular cartilage to a smoother surface while avoiding any damage to the healthy surrounding cartilage (Rambani and Venkatesh, 2014). Debridement chondroplasty can be done using several techniques including curettage, and mechanical debridement with a shaver; however this technique does not leave smooth cartilage and may cause further cartilage breakdown (Falah et al., 2010). Furthermore, thermal debridement uses radiofrequency energy and this technique causes chondrocyte death and matrix degeneration (Falah et al., 2010).
3.2. Bone marrow stimulation technique
This technique exposes the chondral defect to the bone marrow, thereby creating an environment which causes fibrocartilage healing. The bone marrow stimulation technique includes microfracture and subchondral drilling of cartilage (Rambani and Venkatesh, 2014). The drilling technique was developed by Pridie in 1959 in order to bring the pluripotent stem cells into a chondral defect. The subchondral bone is drilled with a high speed drill through the trabecular bone and blood is allowed to perfuse into the defect resulting in the formation of a blood clot, thereby initiating the repairing of the defect (Falah et al., 2010). The repaired cartilage contains a mixture of hyaline and fibrocartilage. Subchondral drilling causes thermal necrosis which is a major setback of this technique. To reduce thermal damage caused by drilling, Steadman and Rokey, developed the microfracture technique in the late 1980 s. This technique allows the accurate debridement of all unstable and damaged articular cartilage, down to the subchondral bone plate while maintaining a stable perpendicular edge of healthy cartilage (Smith et al., 2005). Multiple holes in the defect are made 3–4 mm apart using an arthroscopic awl. Following the making of a microfracture, the defect is filled with fibrin clot, which is an optimal environment for the pluripotential marrow cells to differentiate into stable cells (Steadman et al., 2001). Histological analysis after a microfracture repair shows that a hybrid of hyaline and fibrocartilage dominates the site of defect (Falah et al., 2010).
3.3. Osteochondral autografting
OCG is useful in small lesions with subchondral bone loss (Rambani and Venkatesh, 2014). OCG involves the harvesting of multiple individual osteochondral plugs from the donor site, usually from a non-weight bearing area of the knee. Using a press fit technique; the grafts from the donor site are inserted into the lesion in a mosaic like fashion (mosaicplasty) to replace damaged or missing articular cartilage and to supply the chondral lesion with islands of viable and immediately functional hyaline cartilage (Miniaci and Martineau, 2007). This results in the formation of a surface consisting of the transplanted hyaline cartilage and fibrocartilage from the abrasion arthroplasty. The fibrocartilage acts a grout between the individual grafts. The disadvantage of OCG is donor site morbidity, but there are advantages in this being a one-step procedure with consistent survival of the hyaline cartilage (Rambani and Venkatesh, 2014).
3.4. Autologous chondrocyte implantation (ACI)
ACI was first conducted by Peterson and colleagues in Gothenburg in 1987 and was the first application of cell engineering in orthopedic surgery (Smith et al., 2005). In ACI, a healthy cartilage is obtained from the affected knee and chondrocytes are cultured in a suitable environment (Rambani and Venkatesh, 2014). After a few weeks, the cells are then reimplanted into the defect underneath a patch of periosteum (Smith et al., 2005). Newer generations of ACI are available and this includes, characterized chondrocyte implantation (CCI) and matrix induced ACI (MACI) which involve the placing of cells underneath the scaffolds thereby reducing the complications of periosteal hypertrophy seen in ACI (Rambani and Venkatesh, 2014).
3.5. Mesenchymal stem cells (MSCs) therapy
Currently, the most innovative approach to investigate and treat joint disorders is the use of MSCs. MSCs are multipotent stem cells that have the ability to migrate and engraft onto multiple musculoskeletal tissues, especially sites of injury and undergo site specific differentiation (Chen and Tuan, 2008). The chondrogenic differentiation potential of MSCs has made them a hopeful candidate progenitor cell source for cartilage tissue engineering (Qi et al., 2011). MSCs can be isolated from many types of tissues, such as adipose tissue, bone marrow, umbilical cord blood, placenta, synovium, periosteum and muscle. MSCs can secrete a broad spectrum of bioactive molecules with regenerative and/or immunoregulatory activities (Caplan and Dennis, 2006). MSCs therapy allows the repairing of both the cartilage and bone, and results in improved remodeling and integration with the host surface zone (Fan et al., 2006). In general, MSCs combined with three dimensional scaffolds and growth factors, are implanted into the cartilage defect, and result in the repairing of the cartilage defect.
4. Reactive oxygen species (ROS) and osteoarthritis (OA)
ROS are the most abundant free radicals in the biological system and are continuously formed as normal by products of cellular metabolism (Rajendran et al., 2014). When the oxidant level does not exceed the reducing capabilities of cells, ROS are vital for physiological processes such as cell differentiation, protein phosphorylation, transcription factor activation, apoptosis, cell immunity, steroidogenesis and cell defense against microorganisms (Miller et al., 1993). Under normal conditions, a delicate balance exists between free radical production and their scavenging mechanism (Daif, 2012). In pathological conditions, there is an excessive generation of ROS which can harm cell functionality because it damages cellular lipids, proteins and DNA. ROS modify proteins by the oxidation of amino acids resulting in impaired biological activity, altered protein structure and the accumulation of damaged proteins in tissue (Henrotin et al., 2003). Oxidized molecules can react with non-oxidized molecules resulting in the formation of more oxidized molecules which may further damage the tissue (Henrotin et al., 2003). Oxidative stress may eventually result in cell death and the release of cellular contents into the surrounding environment.
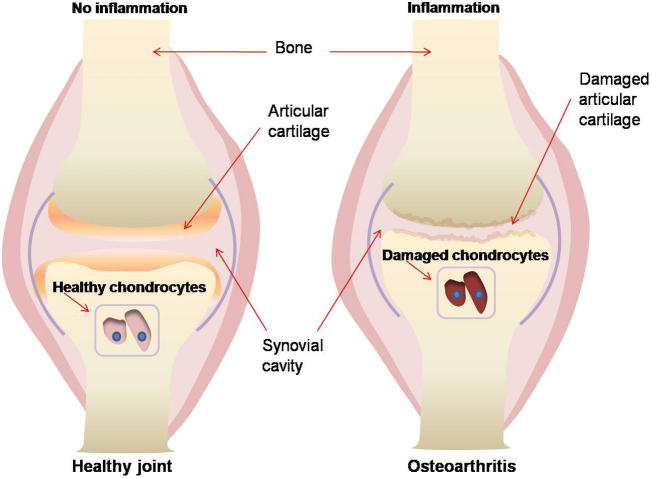
The development and progression of OA, as with other degenerative diseases is associated with the continuous exposure to oxidants (Frei, 1994). In normal physiological conditions, chondrocytes live in conditions with low oxygen supply and some of their metabolic functions require oxygen which is supplied by the synovial fluid (Henrotin et al., 2003). Chondrocytes thus have a metabolism adapted to anaerobic conditions, and in response to changes in partial oxygen pressure, mechanical stress, inflammatory and immunomodulatory mediators, chondrocytes produce increased levels of ROS (Henrotin et al., 2003). The main ROS produced by chondrocytes are nitric oxide (NO) and superoxide anion (O2−) that can result in the formation of derivative radicals such as H2O2 and peroxynitrite (ONOO−) (Hiran et al., 1997). The ROS produced by chondrocytes can damage the cartilage and synovial fluid by reducing its viscosity (McCord, 1974) (Fig. 2). The production of NO is stimulated by tumor necrosis factor (TNF)-β, interleukin (IL)-β, interferon (IFN)-γ and lipopolysaccharides and inhibited by IL-4, IL-10, IL-13 and transforming growth factors (TGF)-β (Henrotin et al., 2000, Borderie et al., 2002). Excessive production of NO contributes to the pathogenesis of chronic arthritis (Vailant et al., 2013). NO can also interfere with chondrocyte function resulting in the loss of cartilage matrix by apoptosis induction, type 2 collagen synthesis inhibition and matrix metalloproteinase activation (Otero et al., 2005, Musumeci et al., 2015). Besides ROS itself, lipid peroxidation products may also result in the formation of reactive aldehydes and lead to inflammations (Yin et al., 2015). These lipid peroxidation products have longer biological half-lives than free radicals and can diffuse from the site of formation to reach a distant target and induce cellular injury (Dwivedi et al., 2007).
Figure 2.
An illustration of a healthy joint cushioned by articular cartilage and an osteoarthritic joint where there articular cartilage is completely damaged and the bone ends are exposed to each other.
5. Ozone (O3) therapy in osteoarthritis (OA)
O3 therapy has been widely used in the treatment of OA (Guo and Zhang, 2010). The intra articular injection of O3 has been suggested for the treatment of osteoarthritis and has yielded positive results (Riva, 1989). Daif, 2012 showed that direct injection of ozone into the superior joint space of patients with internal derangement of the temporomandibular joint yielded a good clinical outcome. The application of O3 seems to be empirical and there are limited studies showing the histological and biochemical evidence for the effects of its use (Iliakis et al., 2008). O3 acts as a bioregulator by releasing factors from endothelial cells and normalizing the cellular redox balance when it comes into contact with a biological fluid (Iliakis et al., 2008). O3 can change the levels of cytokines such as interleukin 8, TNF-α, transforming factor beta1 (TGF beta1) and platelet derived growth factor (PDGF) (Paulesu et al., 1991, Bocci et al., 1998). When O3 is administered intravenously, it dissolves in biological fluids such as plasma, urine and lymph, where it reacts with polyunsaturated fatty acids, antioxidants, reduced glutathione and albumin (Guven et al., 2008). All of these compounds can act as an electron donor and undergo oxidation. O3 causes oxidation of these compounds resulting in the formation of H2O2 and lipid oxidation products (LOPs). The following reaction shows the simultaneous formation of one mole of H2O2 included among ROS and two moles of lipid oxidation products (LOPs) (Bocci, 2006).
H2O2 is able to act as an ozone messenger for initiating therapeutic and biological effects (Guven et al., 2008). Excessive amounts of H2O2 can be detrimental to cells while at physiological amounts H2O2 acts as a regulator of signal transduction and is an important mediator of the host defense and immune responses (Reth, 2002). H2O2 acts immediately and disappears (early and short acting messengers) while LOPs remains in the circulation and distributes throughout the tissues and thus becoming late and long lasting messengers (Bocci, 2006). This process leads to the stimulation of the innate immune system and helps the cells to survive injury. O3 therapy stimulates the production of interferon and interleukins in the body and initiates the production of antioxidant enzymes (Bocci, 2006). The immune system can also be stimulated by activating neutrophils and stimulating the release of cytokines. O3 increases the levels of TGFβ which is important in tissue remodeling.
O3 therapy reduces TNF-α concentrations, which is a proinflammatory cytokine, activating the nuclear factor-κB (NFκB) pathway, leading to a downstream cascade of other proinflammatory cytokines giving rise to a vicious cycle which perpetuates the chronic inflammatory process (Karouzakis et al., 2006, Vailant et al., 2013). TNF-α is strongly associated with joint and bone injury (Vailant et al., 2013). This cytokine is also known to increase the mitochondrial ROS production and inhibition of TNF-α may decrease ROS production in OA, thereby causing the improvement and prevention of joint erosion. The inhibition of TNF-α may break the noxious NFκB pathway causing a reduction of the inflammation. ROS can function as a second messenger to activate NFκB, which co-ordinates the expression of a wide range of genes involved in the inflammatory response. The efficacy of O3 therapy is not only through the actions of cytokines, O3 also has the ability to re-establish the cellular redox homeostasis (Vailant et al., 2013). O3 can protect against overproduction of NO and this is likely because of ozone’s action on NFκB, since inducible nitric oxide synthase (iNOS) is regulated at the transcriptional level by NFκB (Karouzakis et al., 2006).
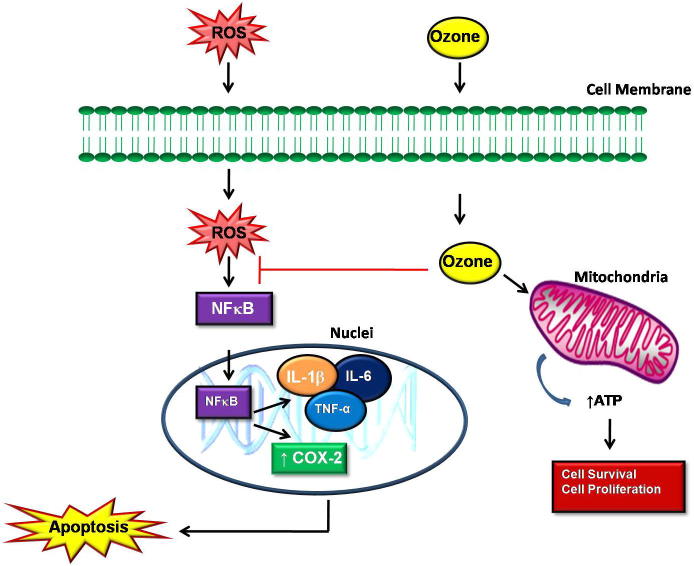
It has been shown that the controlled administration of O3 may promote an oxidative preconditioning or adaptation to oxidative stress that in turn stimulates the antioxidant endogenous systems resulting in the protection against tissue damage (Ajamieh et al., 2004). However, the exact mechanisms underlying preconditioning are not fully understood. NFκB is a major regulator of inflammations in mammalian cells (Yin et al., 2015). NFκB includes subunits such as NFκB1 (p50/p105), NFκB2 (p52/p100), RelA (p65), RelB, c-Rel and regulates inflammatory and immune responses, cell proliferation or cell death (Mitsiades et al., 2002, Hwang et al., 2012). Activation of NFκB seems to be a positive regulator of cell growth and an inhibitor of apoptosis in fibroblasts like synoviocytes (Yin et al., 2015). On the contrary, some studies have shown that NFκB activation may promote apoptosis of neural cells (Grilli et al., 1996). Inhibition of NFκB activation prevents cell death by apoptosis in human cultured thymocytes (Bessho et al., 1994). The functions of NFκB in whether a cell should undergo cell proliferation or apoptosis seem to be cell type dependent. O3 inhibition of NFκB activation may cause a reduction in inflammation and apoptotic cell death (Fig. 3). ROS can activate NFκB directly or through other stimuli such as TNF-α (Chapple et al., 2013). In OA, ROS activates NFκB pathways by increasing its translocation into the nuclei and this causes the release of proinflammatory cytokines such as IL-1β, IL-6, TNF-α and COX-2. These cytokines inhibit the synthesis of aggrecan and collagen type II, which are the major components of the cartilage matrix (Saklatvala, 1986, Goldring et al., 1994). In addition, these cytokines increase the release of matrix metalloproteinases and aggrecanase, enzymes that degrade the matrices, causing cartilage damage, disturbance of the metabolic balance of the cartilage matrix and eventually apoptosis (Kondo et al., 2014). IL-1β and TNF-α can induce chondrocytes apoptosis by promoting the production of other proinflammatory factors such as prostaglandin E2 (PGE2) and NO (Lotz et al., 1999). O3 therapy can block the activation of NFκB and therefore inhibiting the release of proinflammatory cytokines. This inhibits the degradation of the cartilage matrix and initiation of the apoptotic pathway, thereby causing cell survival and proliferation. O3 can stimulate the production of adenosine triphosphate (ATP) through the glycolysis enzymatic pathway and increase oxygenation to injured cells and thus prevent cell death (Guven et al., 2008).
Figure 3.
Proposed mechanisms of action of ozone (O3) therapy in osteoarthritis (OA). ROS formed during OA activates NFκB pathways by increasing its translocation into the nuclei and this causes the activation of intracellular inflammation pathways such as IL-1β, IL-6, TNF-α and COX-2 which then open the apoptotic cascade. O3 can inhibit apoptosis and degradation of the cartilage matrix by inhibiting the activation NFκB resulting in cell survival.
6. Conclusion
Once articular cartilage is damaged, it has limited potential to repair itself. Many treatment modalities that are used to repair damaged articular cartilage show limited efficiency. O3 therapy is an efficient therapy in the treatment of damaged articular cartilage in OA. O3 inhibits the inflammatory milieu which damages the cartilage matrix and induces apoptosis of chondrocytes in OA. It produces its beneficial effects by normalizing the cellular redox balance and through the actions of cytokines. Further in vitro studies are required in order to understand the exact mechanisms involved in the stimulation of cells by O3.
Author contributions
Sello Lebohang Manoto conducted the literature review and wrote the manuscript. Makwese Johaness Maepa assisted in writing the manuscript and proofreading. Shirley Keolebogile Motaung revised the manuscript and approved the paper.
Acknowledgment
The authors thank the Tshwane University of Technology for providing support.
The material in this review paper submitted to Saudi Journal of Biological Sciences has neither been published, nor is being considered elsewhere for publication.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Sello Lebohang Manoto, Email: manotosello@gmail.com.
Makwese Johaness Maepa, Email: MaepaMJ@tut.ac.za.
Shirley Keolebogile Motaung, Email: motaungsckm@tut.ac.za.
References
- Ajamieh H.H., Menendez S., Martinez-Sanchez G., Candelario-Jalil E., Re L., Giuliani A., Fernandez O.S.L. Effects of ozone oxidative preconditioning on nitric oxide generation and cellular redox balance in a rat model of hepatic ischaemia–reperfusion. Liver Int. 2004;24:55–62. doi: 10.1111/j.1478-3231.2004.00885.x. [DOI] [PubMed] [Google Scholar]
- Altman N. Healing Arts Press; Rochester, Vermont: 2007. The Oxygen Prescription: The Miracle of Oxidative Therapies. pp. 30–31. [Google Scholar]
- Ashkavand Z., Malekinejad H., Vishwanath B.S. The pathophysiology of osteoarthritis. J. Pharm. Res. 2013;7:132–138. [Google Scholar]
- Bessho R., Matsubara K., Kubota M. Pyrrolidine dithiocarbonate, a potent inhibitor of nuclear factor κB (NFκB) activation, prevents apoptosis in human promyelocytic leukemia HL-60 cells and thymocytes. Biochem. Pharmacol. 1994;48:1883–1889. doi: 10.1016/0006-2952(94)90586-x. [DOI] [PubMed] [Google Scholar]
- Bocci V.A. Scientific and medical aspects of ozone therapy. State of the art. Arch. Med. Res. 2006;37:425–435. doi: 10.1016/j.arcmed.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Bocci V. Springer; Dordrecht, Netherlands: 2011. Ozone: A New Medical Drug. pp. 1–307. [Google Scholar]
- Bocci V., Valacci G., Corradeschi, Fanetti G. Studies on the biological effects of ozone. Effects of the total antioxidant status and on interleukin 8 production. Mediators Inflamm. 1998;7:313–317. doi: 10.1080/09629359890820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borderie D., Hilliquin P., Hernvann A., Lamarechal H., Kahan A., Menkes C.J., Ekindjian O.G. Inhibition of inducible NO synthase by TH2 cytokines and TGF beta in rheumatoid arthritic synoviocytes: effects on nitrosothiol production. Nitric Oxide. 2002;6:271–282. doi: 10.1006/niox.2001.0418. [DOI] [PubMed] [Google Scholar]
- Borrelli E., Alexandre A., Illiakis E., Alexandre A., Bocci V. Disc herniation and knee arthritis as chronic oxidative stress diseases: the therapeutic role of oxygen ozone therapy. J. Arthritis. 2015;4:1–7. [Google Scholar]
- Caplan A.I., Dennis J.E. Mesenchymal stem cells in immunoregulation. Immunol. Cell Biol. 2006;98:1076–1084. doi: 10.1111/j.1440-1711.2006.01458.x. [DOI] [PubMed] [Google Scholar]
- Chapple S.J., Cheng X., Mann G.E. Effects of 4-hydroxy-nonenal on vascular endothelial and smooth muscle cell redox signaling and function in health and disease. Redox Biol. 2013;1:319–331. doi: 10.1016/j.redox.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F.H., Tuan R.S. Mesenchymal stem cells in arthritic diseases. Arthritis Res. Ther. 2008;10:223. doi: 10.1186/ar2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daif E.T. Role of intra-articular ozone gas injection in the management of internal derangement of the temporomandibular joint. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012;113:10–14. doi: 10.1016/j.tripleo.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Dwivedi S., Sharma A., Patrick B., Sharma R., Awasthi Y.C. Role of 4-hydroxynonenal and its metabolites in signaling. Redox Rep. 2007;1:4–10. doi: 10.1179/135100007X162211. [DOI] [PubMed] [Google Scholar]
- Eliakim R., Karmeli F., Rachmilewitz D., Cohen P., Zimran A. Ozone enema: a model of microscopic colitis in rats. Dig. Dis. Sci. 2001;46:2515–2520. doi: 10.1023/a:1012348525208. [DOI] [PubMed] [Google Scholar]
- Elvis A.M., Ekta J.S. Ozone therapy: a clinical review. J. Nat. Sci. Biol. Med. 2011;2:66–70. doi: 10.4103/0976-9668.82319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falah M., Nierenberg G., Soundry M., Hayden M., Volpin G. Treatment of articular cartilage lesions of the knee. Int. Orthop. 2010;34:621–630. doi: 10.1007/s00264-010-0959-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H., Hu Y., Zhang C., Li X., Lv R., Qin L., Zhu R. Cartilage regeneration using mesenchymal stem cells and a PLGA-gelatin/chondroitin/hyaluronate hybrid scaffold. Biomaterials. 2006;27:4573–4580. doi: 10.1016/j.biomaterials.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Frei B. Reactive oxygen species and antioxidant vitamins: mechanisms of action. Am. J. Med. 1994;97:S5–S13. doi: 10.1016/0002-9343(94)90292-5. [DOI] [PubMed] [Google Scholar]
- Goldring M.B., Fukuo K., Birkhead J.R., Dudek E., Sandell L.J. Transcriptional suppression by interleukin-1 and interferon-gamma of type II collagen gene expression in human chondrocytes. J. Cell. Biochem. 1994;54:85–99. doi: 10.1002/jcb.240540110. [DOI] [PubMed] [Google Scholar]
- Grilli M., Pizzi M., Memo M., Spano P. Neuroprotection by aspirin and sodium salicylate through blockade of NFκB activation. Science. 1996;5291:1383–1385. doi: 10.1126/science.274.5291.1383. [DOI] [PubMed] [Google Scholar]
- Guo D., Zhang X. Study on treatment for knee osteoarthritis by medical ozone. Gansu Med. J. 2010;1:10–11. [Google Scholar]
- Gupta M., Abhishek An emerging prospect in dentistry. Indian J. Dent. Sci. 2012;4:47–50. [Google Scholar]
- Guven A., Gundogdu G., Sadir S., Topal T., Erdogan E., Korkmaz A., Surer I., Ozturk H. The efficacy of ozone therapy in experimental caustic esophageal burn. J. Pediatr. Surg. 2008;43:1679–1684. doi: 10.1016/j.jpedsurg.2008.01.064. [DOI] [PubMed] [Google Scholar]
- Henrotin Y., Zheng S.X., Labasse A.H., Deby G.P., Crielaard J.M., Reginster J.Y. Modulation of human chondrocyte metabolism by recombinant interferon. Osteoarthritis Cartilage. 2000;8:474–482. doi: 10.1053/joca.1999.0323. [DOI] [PubMed] [Google Scholar]
- Henrotin Y.E., Bruckner P., Pujol J.P.L. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthritis Cartilage. 2003;11:747–755. doi: 10.1016/s1063-4584(03)00150-x. [DOI] [PubMed] [Google Scholar]
- Hiran T.S., Moulton P.J., Hancock J.T. Detection of superoxide and NADPH oxidase in porcine articular chondrocytes. Free Radic. Biol. Med. 1997;23:736–743. doi: 10.1016/s0891-5849(97)00054-3. [DOI] [PubMed] [Google Scholar]
- Hwang J.R., Jo K., Lee Y., Sung B.J., Park Y.W., Lee J.H. Upregulation of CD9 in ovarian cancer is related to the induction of TNF-α gene expression and constitutive NFκB activation. Carcinogenesis. 2012;33:77–83. doi: 10.1093/carcin/bgr257. [DOI] [PubMed] [Google Scholar]
- Iliakis E., Petropoulos I., Iliaki A., Agapitos E., Agrogiannis G. Is medical ozone safe when injected intra articularly. Int. J. Ozone Ther. 2008;7:7–15. [Google Scholar]
- Inal M., Dokumacioglu A., Ozcelik E. The effects of ozone therapy and coenzyme Q10 combination on oxidative stress markers in healthy subjects. Ir. J. Med. Sci. 2011;180:703–707. doi: 10.1007/s11845-011-0675-7. [DOI] [PubMed] [Google Scholar]
- Karouzakis E., Neidhart M., Gay R.E., Gay S. Molecular and cellular basis of rheumatoid joint destruction. Immunol. Lett. 2006;106:8–13. doi: 10.1016/j.imlet.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Kondo M., Yamaoka K., Tanaka Y. Acquiring chondrocyte phenotype from human mesenchymal stem cells under inflammatory conditions. Int. J. Mol. Sci. 2014;15:21270–21285. doi: 10.3390/ijms151121270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Aspden R.M. Composition and mechanical properties of cancellous bone from the femoral head of patients with osteoporosis or osteoarthritis. J. Bone Miner. Res. 1997;12:641–651. doi: 10.1359/jbmr.1997.12.4.641. [DOI] [PubMed] [Google Scholar]
- Lotz M., Hashimoto S., Kuhn K. Mechanisms of chondrocyte apoptosis. Osteoarthritis Cartilage. 1999;74:389–391. doi: 10.1053/joca.1998.0220. [DOI] [PubMed] [Google Scholar]
- Madej P., Plewka A., Madej J.A., Nowak M., Plewka D., Franik G., Golka D. Ozonotherapy in an induced septic shock I: effect of ozonotherapy on rat organs in evaluation of free radical reactions and selected enzymatic systems. Inflammation. 2007;30:52–58. doi: 10.1007/s10753-007-9021-7. [DOI] [PubMed] [Google Scholar]
- McCord J.M. Free radicals and inflammation: protection of synovial fluid by superoxide dismutase. Science. 1974;185:529–531. doi: 10.1126/science.185.4150.529. [DOI] [PubMed] [Google Scholar]
- Merin O., Attias E., Elstein D., Schwalb H., Bitran D., Zimran A., Silberman S. Ozone administration reduces reperfusion injury in an isolated rat heart model. J. Card. Surg. 2007;22:339–342. doi: 10.1111/j.1540-8191.2007.00419.x. [DOI] [PubMed] [Google Scholar]
- Miller J.K., Brzezinska-Slebodzinska E., Madsen F.C. Oxidative stress, antioxidants and animal function. J. Dairy Sci. 1993;76:2812–2823. doi: 10.3168/jds.S0022-0302(93)77620-1. [DOI] [PubMed] [Google Scholar]
- Miniaci A., Martineau P.A. Technical aspects of osteochondral autograft transplantation. Instr. Course Lect. 2007;56:447–455. [PubMed] [Google Scholar]
- Mitsiades C.S., Mitsiades N., Poulaki V., Schlossman R., Akiyama M., Chauhan D., Hideshima T., Treon S.P., Munshi N.C., Richardson P.G., Anderson K.C. Activation of NFκB and upregulation of intracellular anti-apoptotic proteins via the IGF-1/Akt signaling in human multiple myeloma cells: therapeutic implications. Oncogene. 2002;21:5673–5683. doi: 10.1038/sj.onc.1205664. [DOI] [PubMed] [Google Scholar]
- Musumeci G., Aillo F.C., Szychlinska M.A., Di Rosa M., Castrogiovanni P., Mobasheri A. Osteoarthritis in the XXIst century: risk factors and behaviours that influence disease onset and progression. Int. J. Mol. Sci. 2015;16:6093–6112. doi: 10.3390/ijms16036093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero M., Lago R., Lago F., Reino J.J., Gualilo O. Signalling pathway involved in nitric oxide synthase type II activation in chondrocytes: synergistic effect of leptin with interleukin-1. Arthritis Res. Ther. 2005;7:581–591. doi: 10.1186/ar1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulesu L., Luzzi E., Bocci V. Studies on the biological effects of ozone: 2. Induction of tumour necrosis factor (TNF-alpha) on human leucocytes. Lymphokine Cytokine Res. 1991;10:121–126. [PubMed] [Google Scholar]
- Qi Y., Feng G., Yan W. Mesenchymal stem cell based treatment for cartilage defects in osteoarthritis. Mol. Biol. Rep. 2011;39:5683–5689. doi: 10.1007/s11033-011-1376-z. [DOI] [PubMed] [Google Scholar]
- Rajendran P., Nandakumar N., Rengarajan T., Palaniswami R., Gnanadhas E.N., Lakshminarasaiah U., Gopa J., Nishigaki I. Antioxidants and human diseases. Clin. Chem. Acta. 2014;436:332–347. doi: 10.1016/j.cca.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Rambani R., Venkatesh R. Current concepts in articular cartilage repair. J. Arthrosc. Joint Surg. 2014;1:59–65. [Google Scholar]
- Reth M. Hydrogen peroxide as second messenger in lymphocyte activation. Nat. Immun. 2002;3:1129–1134. doi: 10.1038/ni1202-1129. [DOI] [PubMed] [Google Scholar]
- Reynard L.N., Loughlin J. Genetics and epigenetics of osteoarthritis. Maturitas. 2012;71:200–204. doi: 10.1016/j.maturitas.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Riva S.E. Knee joint disorders treated by oxygen ozone therapy. Eur. Med. Phys. 1989;25:163. [Google Scholar]
- Saklatvala J. Tumour necrosis factor alpha stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature. 1986;322:547–549. doi: 10.1038/322547a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M., Yamato M., Hamahashi K., Okano T., Mochida J. Articular cartilage regeneration using cell sheet technology. Anat. Rec. 2014;297:36–43. doi: 10.1002/ar.22829. [DOI] [PubMed] [Google Scholar]
- Smith G.D., Knutsen G., Richardson J.B. A clinical review of cartilage repair techniques. J. Bone Joint Surg. 2005;47:445–449. doi: 10.1302/0301-620X.87B4.15971. [DOI] [PubMed] [Google Scholar]
- Steadman J.R., Rodkey W.G., Rodrigo J.J. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin. Orthop. 2001;391:362–369. doi: 10.1097/00003086-200110001-00033. [DOI] [PubMed] [Google Scholar]
- Vailant J.D., Fraga A., Diaz M.T., Mallok A., Viebahn-Hansler R., Fahmy Z., Barbera A., Delgado L., Menendez S., Fernandez O.S.L. Ozone oxidative postconditioning ameliorates joint damage and decreases pro inflammatory cytokine levels and oxidative stress in PG/PS induced arthritis in rats. Eur. J. Pharmacol. 2013;714:318–324. doi: 10.1016/j.ejphar.2013.07.034. [DOI] [PubMed] [Google Scholar]
- Yin G., Wang Y., Cen X., Yang M., Liang Y., Xie Q. Lipid peroxidation mediated inflammation promotes cell apoptosis through activation of NFκB pathway in rheumatoid arthritis synovial cells. Mediators Inflamm. 2015:1–10. doi: 10.1155/2015/460310. [DOI] [PMC free article] [PubMed] [Google Scholar]