Abstract
Background
The PROtocolized Care to Reduce HYpotension after Spinal Anaesthesia (ProCRHYSA trial) is an unblinded, randomized, monocentric, prospective, three-arm, parallel-group trial aimed at assessing the role of a controlled volemic repletion in reducing both clinically significant hypotension rate and total amount of fluid administered in patients undergoing spinal anaesthesia.
Methods/Design
Aim of the study is assessing the effectiveness of a non-invasive tests to guide a titrated volemic repletion before spinal anesthesia in order to reduce post-spinal hypotension rate. After local ethical committee approval of the study (Comitato Etico Cantonale Ref. N. CE2796), we will randomize patients undergoing elective surgery under spinal anesthesia into two parallel groups: in the first vena cava ultrasound will be used in order to assess adequacy of patients’ volemic status and consequently guide the administration of crystalloids boluses; in the second passive legs raising test will be used instead of ultrasound for the same purpose.
Discussion
The hypothesis we want to test is that the using of these two experimental methods before spinal anaesthesia, compared to the standard method (empirical fluid administration) can reduce the impact of systemic hypotension through an adequate titrated volemic repletion, avoiding both hypotension and fluid overload. The final purpose is to ensure that spinal anaesthesia is performed in the safest way possible.
Conclusions
The study will offer a new insight on the possible role of vena cava ultrasound and passive legs raising test as screening tools to prevent hypotension after spinal anesthesia. These tests were already validated in a critical environment, but to the best of our knowledge this is the first time they are applied to an elective surgical population.
Trial registration
The trial was registered on May 2014 on www.clinicalstrial.gov with the number NCT02070276.
Keywords: Anaesthesia and Intensive Care; Statistic, epidemiology and research design; Anaesthesia – regional; Anaesthesia – safety; Haemodynamic monitoring
1. Background
Spinal anaesthesia is an ubiquitous clinical practice, its most feared and common side effect consisting of an abrupt reduction of systemic vascular resistances and a consequent risk of systemic hypotension [1], [2], [3]. To prevent this complication, an adequate correction of patients’ volume status is often obtained through a preventive fluids administration [4], [5], [6], [7].
Volume repletion is usually accomplished empirically, without having a real evaluation of patient’s hemodynamic status; this practice carries the risk of possible volume overload. The using of non-invasive methods has been proved to be accurate in identifying patients’ fluid-responsiveness [8], [9]; however it has not been investigated yet in this specific setting [10].
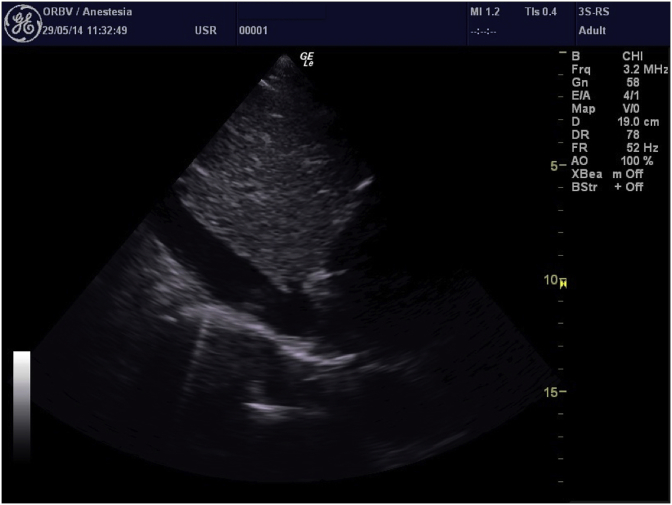
Ultrasound of inferior vena cava (IVC) studied by Trans-Thoracic Echocardiography (TTE) is considered an effective method to determine fluid responsiveness in mechanically ventilated patients [11], [12], however it has been proven to be inaccurate in spontaneous ventilated critically ill patients [13], but there are little data in non-critical patients [14]. IVC ultrasound study is based on measuring the size of the vessel in its own intra-abdominal portion (approximately 2 cm from the right atrium) through a subcostal view, and on its diameter’s changes with breathing, both in spontaneously breathing and in mechanical ventilated patients (Fig. 1).
Fig. 1.
IVC ultrasound analysis (subcostal view).
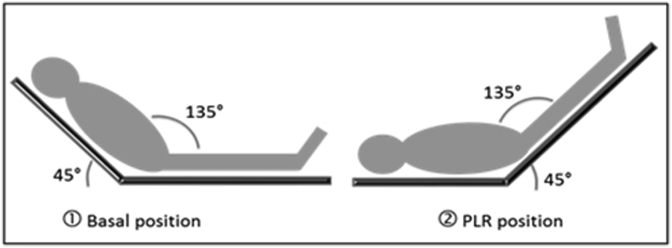
Passive leg raising test (PLRT) has been shown to be a highly accurate method in predicting fluid responsiveness in spontaneously breathing critically ill patients [15]. The method is based on the following assumption: active leg elevation, in addition to its effect of venous pool shifting from the lower limbs to the chest, exerts a simultaneous stimulation effect on the iliac-femoral peri-arterial sympathetic system, causing an orthosympathetic reflex which can increase cardiac output (Fig. 2). Alternatively, passive lower limb test has the advantage of mobilizing lower limb venous blood (estimated 300–500 ml) without activating the orthosympathetic reflex. Thus, the test makes it possible to quantify the clinical response after a bolus of fluids [15].
Fig. 2.
Passive leg Raising Test.
We report our statistical analysis plan before performing the outcomes assessment. We have conceived the ProCRHYSA trial with the aim of determining whether these two non-invasive methods are effective in guiding titrated fluid repletion in non-critical patients, both to decrease post-procedural significant hypotension rate (primary end-point), and to avoid unnecessary fluid overload in patients undergoing spinal anaesthesia in elective surgical procedures [16] and to compare the time needed to complete the entire anaesthetic procedure (secondary end-point).
2. Methods
2.1. Design of the study
This trial is conducted in our Anesthesiology Unit beginning on May 2014; Ethical Committee approved the protocol (Comitato Etico Cantonale Ref. N. CE2796) [16], [17], [18] and written informed consent is obtained from all subjects during pre-operative anaesthesiologist visit.
Here we report our statistical analysis plan before the trial conclusion. This is an unblinded, randomized, single-center, prospective, three-arm, parallel-group trial of a cohort of consecutive patients undergoing spinal anaesthesia for any elective surgical procedure scheduled under spinal anesthesia. Patients are screened for exclusion criteria. If one of these is met patients are excluded from the study.
Inclusion criteria:
-
-
adult patients
-
-
elective intervention requiring spinal anaesthesia
-
-
classified according to the American Society of Anesthesiology risk (ASA) as level I, II or III.
Exclusion criteria:
-
-
patients requiring invasive blood pressure monitoring (arterial/pulmonary catheter, thermo-dilution catheter);
-
-
pre-existing hypotension defined as two measurements of systolic arterial pressure less than 80 mmHg and/or mean arterial pressure less than 60 mmHg;
-
-
patients unable to give informed consent due to language barriers, mental retard or any reduction in own ability to understand or give own informed consent;
-
-
patients in whom it was not possible to perform spinal anaesthesia due to patient’s refusal or technical difficulties in sampling;
-
-
patients with International Normalized Ratio (INR) greater than 1.5 and/or aPTT in therapeutic range (more than 1.5–2 times the patient’s normal values) and/or anti-factor-Xa activity in therapeutic range;
-
-
patients with thrombocytopenia less than 50 G/l;
-
-
parturients and pregnant women;
2.1.1. Randomization and allocation
Eligible patients are allocated 1:1:1 to one of the three treatment groups by randomization blocks via an electronic software [19]. An investigator not involved in the enrolment handles the randomization-list, in order to guarantee allocation concealment; doctors enrolling patients do not have access to it. Patients are randomised using the method of minimisation incorporating a random element; the minimisation factors are: age (cut off 65 years), ASA status (level III or level I/II intended as “not III”), any assumption of anti-hypertensive therapy (intended as B-blockers or ACE-inhibitors), any psychoactive therapy (SSRI, tricyclic antidepressant, MAO-inhibitors). Patients can only be included and randomised by an authorised member of staff as detailed on the delegation form.
Each participant is followed-up till the end of surgical operation; participation in the study begins when patient enter the operating block (time 0) and ends 30 min after spinal anaesthesia; different study frames are classified as:
-
•
Pre-anaesthetic phase: from time 0 to the execution of spinal anaesthesia
-
•
Anaesthetic phase: from the beginning to the end of the spinal anaesthesia
-
•
Post-anaesthetic phase: from the end of spinal anaesthesia to 30 min after the conclusion of the procedure.
All Serious Adverse Events (SAE) defined as all possible complications related to hypotension after spinal anaesthesia (cerebral ischemia, neurological deficit related to hypotension, chest pain, myocardial ischemia/infarction, renal failure, cardiac arrest and all clinical complication due to iatrogenic hypotension) are checked till the end of the surgical procedure.
2.1.2. Safety
To reduce the risk of exposing patient to hypotension or to a fluid overload, we exclude patients with ASA score equal or greater than IV. The use of both TTE than PLRT were assumed to be risk-free. All serious adverse events and side effects are recorded in the electronic case report form (eCRF) throughout the study regardless of their relation to study participation. The principal investigator submits a weekly report of all SAEs (expected and unexpected) to the Ethics Committee; the study may be prematurely discontinued on the basis of eventual new safety concerns by the research Ethics Committee or sponsor concerned.
2.1.3. Setting
All patients are scheduled for elective surgical procedures under spinal anesthesia in the operating suits of Bellinzona Regional Hospital, Bellinzona (Switzerland).
During the induction phase, patients are monitored with non-invasive blood pressure, three-lead ECG, pulse-oxymetry. These parameters are recorded in the data collecting sheet. Total amount of fluid is recorded before and after spinal anaesthesia. Spinal anaesthesia technique is standardized for every patient: patients are positioned in lateral decubitus, L3-L4 space is identified and spinal anaesthesia administered via a 27G pencil point beveled spinal needle (BBraun, Melsingen, Germany). A standard dose of bupivacaine 0.5% 10 mg is slowly injected with needle oriented cranially and no barbotage. After injection, patients are immediately positioned supine for 30 min before the beginning of surgery, than NIBP is measured every minute and recorded in a data collecting form. If the patient develops any sign or symptom of hypotension, he/she is treated according to standard internal protocol with a crystalloids bolus and eventually with neosynephrine, ephedrine or atropine according to the clinical setting. Total quantity and type of amine used is also recorded.
2.1.4. Interventions
In the IVC ultrasound group, a TTE is performed before spinal anaesthesia, with the aim of assessing patients’ volume status; in particular, the exam is performed to assess size and collapse of the IVC during breathing. According to a set of pre-established parameters [13], patients are defined as fluid-responsive or unresponsive; we use the echocardiographic evaluation of IVC with M-mode during spontaneous breathing, adopting a cut-off value of collapsibility of 36% from baseline in order to define a patient as fluid-responsive [13]. If the patient is not responsive, investigators proceed to spinal anaesthesia; otherwise they administer a crystalloid bolus (500 ml of NaCl 0.9% or Hartmann’s solution). Patients are than re-assessed and they may receive further boluses until reaching a non-responsive echocardiographic pattern. After spinal anaesthesia, patients are immediately positioned supine for 30 min before the beginning of surgery.
In the passive legs raising arm of the study (PLRT), a measurement of etCO2 with patients in semi-recumbent position (45°) was performed [15]. Measurements were performed before and after the test (after 60–90 s): an etCO2 increase of 5% from baseline is interpreted as fluid-responsive. If the patient is not responsive investigators proceed to spinal anaesthesia, otherwise they administer a fluid bolus (500 ml of crystalloid) and reassess him/her, until no response to fluids is found. After spinal anaesthesia patients are immediately positioned supine for 30 min before the beginning of surgery.
2.2. Data collection
All investigator fill the CRF in during the anaesthetic procedure reporting all demographic data (age, sex, weight and height), date and hour of the procedure, all vitals parameters (NIBP, heart rate and peripheral arterial saturation), some pre-procedural clinical data (ASA score, anti-hypertensive therapy, procedure timing), post-procedural clinical data (lowest NIBP value, heart rate and oxygen saturation, eventual symptoms of hypotension), need for of amines and total amount of fluid administered (Table 1). These data are subsequently collected in the electronic data base (EDC).
Table 1.
Summary of study assessment.
| Summary of study assessment | Baseline | Pre-assessment | Pre-anesthesia | Anesthesia | Post-anesthesia | End of trial | Withdrawl |
|---|---|---|---|---|---|---|---|
| Registration/Demographics | x | ||||||
| Informed consent | x | x | |||||
| Eligibility form | x | ||||||
| Inclusion criteria | x | ||||||
| Exclusion criteria | x | ||||||
| Randomization | x | ||||||
| Medical history | x | x | |||||
| Concomitant medications | x | x | |||||
| Systolic blood pressure | x | x | x | x | |||
| Diastolic blood pressure | x | x | x | x | |||
| Mean blood pressure | x | x | x | x | |||
| Heart rate | x | x | x | x | |||
| Peripheral saturation | x | x | x | x | |||
| Type of fluid administrated | x | x | x | x | |||
| Quantity of fluid administrated | x | x | x | x | |||
| Time arrival-anesthesia | x | x | x | ||||
| Time anesthesia / post-anesthesia | x | x | x | ||||
| Vasoactive drugs administered | x | x | x | x | |||
| Withdrawl form | x | x | x | x | x | ||
| SAE | x | x | x | x | x | ||
| Adherence status | x |
Data management procedures for the trial are developed and overseen by the Ciesse-Sistemi; all data are registered in the EDC system; this system is compliant to the Good Clinical Practice standards. An eCRF using the EDC is programmed by Ciesse-Sistemi in collaboration with the principal investigator and the statistician and hosted on a dedicated secure server within the hospital. The eCRF system has full audit trail, data discrepancy functionality, database lock functionality and log reporting. The Ciesse-Sistemi provides training, the essential documentation, and user support to the study centres and remote monitoring; a detailed standard operating procedure regulates data recording, online entry, checking, central backup and storage. A regularly updated coding manual is developed with regard to the study database. Each investigator has a personal username and password for the eCRF.
Baseline data are collected and entered by researchers before the randomisation; to each enrolled patient is assigned a unique trial identification number at the beginning of the assessment process. This number is written on all clinical assessment forms, datasheets and databases used to record participant data. The datasheets are checked for completeness and accuracy at the end of the entire procedure. A hard copy of a record sheet linking patient identity, contact details and trial ID number for all participants is stored in accordance to clinical trial GCP regulations.
Each investigator transcribes data into the eCRF; the Clinical Trial Unit reviews the data and eventual inquiries are addressed to the investigator concerned. At the end of the trial, the principal investigator reviews all the data for each participant and provides electronic sign-off to certify that all data are complete and correct, in order to be approved to undergo statistical analysis.
2.3. Objectives of study
Primary objective:
-
•
To compare rates of arterial hypotension [16] after spinal anaesthesia in patients undergoing inferior vena cava US guided versus PLRT guided volemic optimization. Significant hypotension is defined, according to international guidelines, as a fall in systolic arterial blood pressure of either more than 50 mmHg or more than 25% from the baseline value, an absolute value of systolic pressure less than 80 mmHg, an absolute value of mean pressure of less than 60 mmHg, a reduction in mean arterial pressure of more than 30% from the baseline value and/or clinical signs/symptoms of inadequate perfusion [20].
Secondary objectives:
-
•
To compare rates of arterial hypotension after spinal anaesthesia in patients undergoing volemic optimization according to PLRT with patients treated according to the current clinical standards.
-
•
To compare rates of arterial hypotension after spinal anaesthesia in patients undergoing volemic optimization according to vena cava ultrasound with patients treated according to the current clinical standards.
-
•
To carry out subgroup analyses in order to determine the effects of the vena cava ultrasound and PLRT methods on arterial hypotension after spinal anaesthesia according to: age, ASA score, anti-hypertensive and anti-psychotic therapy.
-
•
To assess an eventual difference between the three treatments in the quantity of fluids administered in the pre-anaesthetic phase (from time 0 to spinal anaesthesia).
-
•
To assess if there is a difference between the three treatments in the total quantity and the quantity of vasoactive drugs required.
-
•
To compare the time needed to complete the entire anaesthetic procedure into the three groups.
2.4. Ethical issues
The conduction of this study is in accordance with the recommendations for physicians involved in research on human subjects adopted by the 18th World Medical Assembly, Helsinki 1964 (and later revisions). This protocol and related documents have been approved by the National Research Ethics Service Committee London - Dulwich. Local approval is required before recruitment. The Study Coordination Centre requires a written copy of all the documentation before starting to recruit participants into the study. TTE and PLRT are non-invasive tests known risks. During the pre-clinical assessment, investigators explain all study details [18]. When it is not possible to obtain consent for whatsoever reasons, the patient cannot be involved.
2.5. Statistical analyses
2.5.1. Sample size calculation
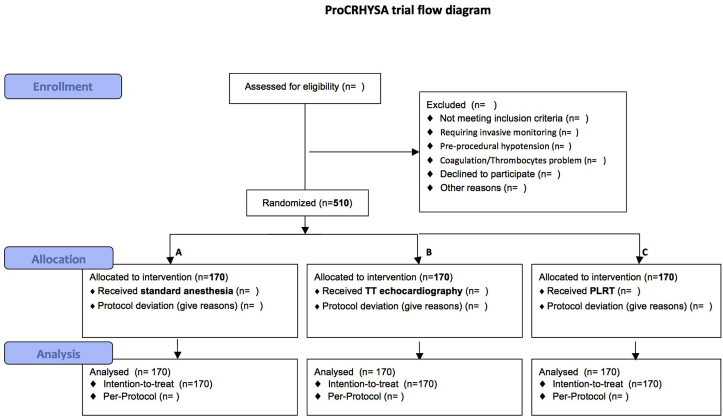
From previous studies21 we estimate the incidence of arterial hypotension after spinal anesthesia to be of about 50% in the standard treatment group [22], [23], [24], [25], [26], [27], [28]. In order to have a significant difference between each treatment group, with a power of 80% and a significance level of 0.05 (two-tailed z test) we calculated a numerosity of 170 patients per group (G-Power 3.1.5). Based on patients routinely treated in last two previously year, we estimate an accrual rate around 30–35 patients each month (low value 15 patients/month), with the necessity to enroll patient for 2 years. The study flow-chart is provided [21], including total patients screened, number of patients who met inclusion and exclusion criteria and number of patients who were included. To assess randomization success, we tabulate the distribution of baseline variables across the study’s sections, summarizing categorical variables by frequencies and percentage and numerical variables either by mean and standard deviations (±SDs), or by medians and interquartile ranges (IQR).
2.5.2. Adherence effects
For every patient, we check the adherence to the protocol during the study; for each patient who didn’t completely meet the protocol, we wrote on the CRF the reasons. It could be linked to any pre-anaesthetic fluid administration different from crystalloid infusion, administration of any sedation different than standard therapy (Fentanyl more than 1 μg/Kg IV or Midazolam more than 0.05 mg/kg IV) both in quantity than in quality (Ketamine, Morphine, etc …), post-anaesthetic position different than the supine one or difference in spinal drug administration, both in quantity (different than hyperbaric Bupivacaine 0.5% 10 mg) and in quality (co-administration of any intra-thecal substance). We assessed and report total adherence rate to our protocol in order to analyse data on the intention-to-treat (ITT) and on the per-protocol (PP) population. Primary analysis was carried out on the ITT population, which consisted of all patients enrolled in the study taken in their randomized groups and irrespective of potential non-adherence to the study protocol. Secondary analyses included analysis on the PP population, which consisted of patients who received fluid replacement and all treatments as specified by the protocol; our results of the analyses on the two populations were compared. All hypothesis tests were two-sided and considered significant if p-value ≤ 0.05. Analyses were carried out with the intention to treat if not otherwise specified.
Primary analysis:
-
•
We tested the hypothesis of difference in the proportion of arterial hypotension in subjects allocated to the TTE group and in subjects of the standard treatment group, with the ITT. We carried out a z-test for comparison of the two proportions. The null hypothesis of no difference between the two proportions wasn’t accepted if the p-value was ≤0.05.
Secondary analyses:
-
•
We tested the hypothesis of difference in the proportion of arterial hypotension in subjects allocated to the PLRT group and subjects of the standard treatment group, with the ITT analysis and also on the per-protocol population. We carried out z-tests for comparison of proportions.
-
•
A z-test for comparison of two proportions was also applied to test the hypothesis of difference in the proportion of arterial hypotension in subjects allocated to the TTE group and subjects of the standard treatment group, on the per-protocol population.
-
•
We assessed the protective effect of the TTE and of the PLRT methods compared to the standard method on the occurrence of arterial hypotension by means of a univariable logistic regression model. The outcome “arterial hypotension” was binary coded (yes/no). We calculated crude odds ratio and their relative 95% confidence intervals (CI). A multivariable logistic regression model was applied to account for potential confounders which consist of independent variables that were deemed to be unbalanced after randomization process or that are of clinical importance. This multivariable logistic regression model allowed calculating adjusted odds ratio with their relative 95% CI.
-
•
Explorative subgroup analyses was carried out to investigate the effects of the TTE and the PLRT methods on arterial hypotension after spinal anaesthesia according to different subgroups. The subgroups of interest included the following variables: age (cut off 65 years), ASA score (I–II vs III), assumption of B-blockers or ACE-inhibitors (yes vs no), patient positioning after spinal anaesthesia (supine vs lateral decubitus). We carried out multivariable logistic regression models, to calculating the odds ratio adjusted for the same baseline variables as previous models, and their relative 95% CI.
-
•
We tested the hypothesis that there was a difference in the quantity of fluids (ml) administered to the subjects in each groups in the pre-anaesthetic phase. We first checked data distribution; if this was shown to be normal we summarized the total amount of fluids by means and standard deviations (±SDs) for each group; additionally, the comparison between the three groups was carried out by one-way ANOVA (Analysis of variance) and post-doc tests. If data were not normally distributed we summarized the amounts of fluids by median and IQR carrying out the comparison by a Kruskal-Wallis test to assess, with a non-parametric method, the null hypothesis of no difference in the amount of fluids between each groups. The null hypothesis wasn’t be accepted if p-value was ≤0.05. If the distribution of data allowed it, we applied multivariable linear regression model to account for potential confounders which consisted of independent variables that were deemed to be unbalanced after randomization process or that were of clinical importance.
-
•
We tested the hypothesis that there was a difference in the total amount of fluids (ml) and in the quantity of administered drugs (μg) as well as in the time needed to complete the entire anaesthetic procedure in the three groups the same way as for the amount of fluids in the pre-anaesthetic phase (see above).
2.5.3. Missing data
Missing data due to incomplete CRF or withdrawal from the study were left empty and were not be completed later. Subjects were not be replaced.
2.6. Figures and tables
Planned figures include:
-
•
a standard CONSORT flow diagram [29] illustrating patients’ flow through the trial (Fig. 3);
-
•
a graph showing the mean cumulative fluids received in all three groups;
-
•
a graph showing hypotension rate in all groups and subgroups;
Fig. 3.
ProCRHYSA trial flow diagram.
Planned tables include:
-
•
baseline characteristics by treatment group;
-
•
clinical management by treatment group;
-
•
non-compliance with allocated treatment by all three treatment groups;
-
•
primary and secondary outcomes by treatment group;
-
•
serious adverse events until the end of surgical operation;
-
•
results of subgroups and secondary analyses;
3. Discussion
To the best of our knowledge this is the first study to investigate the potential role of non-invasive methods (like TTE and PLRT) to accomplish a titrated and targeted volemic optimization before spinal anesthesia in elective surgical patients in order to prevent hypotension.
Post-spinal transient hypotensive episodes can be generally well tolerated by healthy patients, however they may lead to major complications in patients with increased cardiovascular risk. In the common clinical practice, it is usual to administer fluids empirically. An empirical preventive fluid repletion however can represent per se a risk in patients with impaired cardiac and renal functions, since a volume overload in case of reduced ventricular compliance, can cause pulmonary oedema or congestive heart failure.
Since about ten years both non-invasive and invasive techniques have been developed, with the specific purpose of optimizing fluid status on a more rational basis. This methods are based on the correlation between the levels of mean arterial pressure (MAP), systemic vascular resistance (SVR) and cardiac output (CO), according to the equation: MAP = CO * SVR. The latter explains why, in case of vasodilation, unless CO is increased, a lowering of SVR will inevitably cause a decrease of MAP. Determinants of blood pressure are: volemic status, vascular resistances (determined by the sympathetic tone) and cardiac output. In case of spinal anaesthesia the reduction in SVR causes relative hypovolemia. For this reason after spinal anaesthesia is often necessary an optimization of patients’ volemic status through fluids administration.
The use of different methods to assess fluid responsiveness in order to guide volume repletion avoiding blind fluid challenges has been extensively studied in critical care populations, however these techniques have never been studied in non-critical patients. The hypothesis that investigators want to test is that the use of TTE and PLRT before spinal anaesthesia, compared to an empirical fluid administration can reduce the rate of systemic hypotension through a titrated volemic repletion, avoiding both hypotension and fluid overload. The final purpose is to administer spinal anaesthesia in the safest possible way.
Trial status
The ProCRHYSA trial is still ongoing at the time of submission, since patients recruitment has not been completed yet.
Competing interests
The content of this article is the sole responsibility of the Authors and does not necessarily reflect the official views of the Sponsor (Service of Anesthesiology of Ospedale Regionale di Bellinzona e Valli, Tessin, Switzerland). All Authors declare they do not have conflict of interest.
Authors’ contributions
SC conceived the study, designed and coordinated the protocol and recruited patients, furthermore he drafted the manuscript and performed the statistical analysis. AS participated in the design of the study, coordinated the protocol recruited patients, furthermore he drafted the manuscript and performed the statistical analysis. BM, MP, SDV, LA participated in the design of the study and protocol, recruited patients and helped to draft the manuscript. All authors read and approved the final manuscript.
Authors’ information
SC is Intensive Care Specialist at the Department of Intensive Care Medicine, Hôpitaux Universitaires de Genève. AS is Specialist Anesthesiologist of the Department of Anesthesiology at Ospedale Regionale di Bellinzona e Valli. LA is Head of the Department of Anesthesiology at Ospedale Regionale di Bellinzona e Valli. BM and SDV are resident physicians of the Department of Anesthesiology at Ospedale Regionale di Bellinzona e Valli.
Acknowledgements
All Authors declare they do not have any personal financial gain, consultancy or board activity, nor any commercial interest related to this study. We thank Alessandro Spila (Ciesse Sistemi) who provided informatic support and designed the program to collect the data. We would like to thank all the nursing and medical staff for the quality surveys. We also thank Daniele Franceschini and all the research team who conducted this study, for their support.
Contributor Information
S. Ceruti, Email: samuele.ceruti@hcuge.ch.
B. Minotti, Email: bruno.minotti@eoc.ch.
S. De Vivo, Email: sergio.devivo@eoc.ch.
P. De Christophoris, Email: paola.decristophoris@gmail.com.
L. Anselmi, Email: luciano.anselmi@eoc.ch.
A. Saporito, Email: andrea.saporito@eoc.ch.
List of abbreviations used:
- ANOVA
Analysis of variance
- aPTT
Activated Partial Thrombin Time
- ASA
American Society of Anesthesiology
- CI
Confidence Intervals
- CRF
Case Report Form
- eCRF
Electronic Case Report Form
- EDC
Electronic Data Capture
- INR
International Normalized Ratio
- IQR
Interquartile Range
- ITT
Intention-To-Treat
- IVC
Inferior Vena Cava
- MAP
Mean arterial pressure
- NIBP
Non Invasive Blood Pressure
- P
Principal Investigator
- PLRT
Passive Leg Raising Test
- PP
Per-Protocol
- SAE
Serious Adverse Events
- SDs
Standard Deviations
- SVR
Systemic vascular resistance
- TTE
Trans-Thoracic Echocardiography
References
- 1.Carpenter R.L., Caplan R.A., Brown D.L., Stephenson C., Wu R. Incidence and risk factors for side effects of spinal anesthesia. Anesthesiology. 1992 Jun;76(6):906–916. doi: 10.1097/00000542-199206000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Kim H.J., Kim J.S. A cardiovascular collapse following vigorous cough during spinal anesthesia. Korean J. Anesthesiol. 2013 Dec;65(6 Suppl):S49–S50. doi: 10.4097/kjae.2013.65.6S.S49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nogueira C.S., Lima L.C., Paris V.C., Neiva P.M., Otani E.T., Couceiro Rde O., Burim F., Ferreira J.A., Jr., Cadecaro P.A. Comparative study between bupivacaine (S75-R25) and ropivacaine in spinal anesthesia for labor analgesia. Rev. Bras. Anestesiol. 2010 Sep-Oct;60(5):484–494. doi: 10.1016/S0034-7094(10)70060-X. [DOI] [PubMed] [Google Scholar]
- 4.Cherpanath T.G., Geerts B.F., Lagrand W.K., Schultz M.J., Groeneveld A.B. Basic concepts of fluid responsiveness. Neth Heart J. 2013 Dec;21(12):530–536. doi: 10.1007/s12471-013-0487-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jabalameli M., Soltani H.A., Hashemi J., Behdad S., Soleimani B. Prevention of post-spinal hypotension using crystalloid, colloid and ephedrine with three different combinations: a double blind randomized study. Adv. Biomed. Res. 2012;1:36. doi: 10.4103/2277-9175.100129. Epub 2012 Aug 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu S., Wu H., Zhao Q., Shen X., Guo X., Wang F. The median effective volume of crystalloid in preventing hypotension in patients undergoing cesarean delivery with spinal anesthesia. Rev. Bras. Anestesiol. 2012 May-Jun;62(3):312–324. doi: 10.1016/S0034-7094(12)70132-0. [DOI] [PubMed] [Google Scholar]
- 7.Buggy D.J., Power C.K., Meeke R., O’Callaghan S., Moran C., O’Brien G.T. Prevention of spinal anaesthesia-induced hypotension in the elderly: i.m. methoxamine or combined hetastarch and crystalloid. Br. J. Anaesth. 1998 Feb;80(2):199–203. doi: 10.1093/bja/80.2.199. [DOI] [PubMed] [Google Scholar]
- 8.Pinsky M.R., Payen D. Functional hemodynamic monitoring. Crit. Care. 2005;9:566–572. doi: 10.1186/cc3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vieillard-Baron A., Chergui K., Rabiller A., Peyrouset O., Page B., Beauchet A., Jardin F. Superior vena caval collapsibility as a gauge of volume status in ventilated septic patients. Intensive Care Med. 2004;30:1734–1739. doi: 10.1007/s00134-004-2361-y. [DOI] [PubMed] [Google Scholar]
- 10.Zöllei E., Bertalan V., Németh A., Csábi P., László I., Kaszaki J., Rudas L. Non-invasive detection of hypovolemia or fluid responsiveness in spontaneously breathing subjects. BMC Anesthesiol. 2013 Nov 5;13(1):40. doi: 10.1186/1471-2253-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbier C., Loubières Y., Schmit C., Hayon J., Ricôme J.L., Jardin F., Vieillard-Baron A. Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients. Int. Care Med. 2004 Sep;30(9):1740–1746. doi: 10.1007/s00134-004-2259-8. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z., Xu X., Ye S., Xu L. Ultrasonographic measurement of the respiratory variation in the inferior vena cava diameter is predictive of fluid responsiveness in critically ill patients: systematic review and meta-analysis. Ultrasound Med. Biol. 2014 Feb 1 doi: 10.1016/j.ultrasmedbio.2013.12.010. pii: S0301–S5629(13)01237-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Lamia B., Ochagavia A., Monnet X., Chemla D., Richard C., Teboul J.L. Echocardiographic prediction of volume responsiveness in critically ill patients with spontaneously breathing activity. Int. Care Med. 2007;33:1125–1132. doi: 10.1007/s00134-007-0646-7. [DOI] [PubMed] [Google Scholar]
- 14.Muller L., Bobbia X., Toumi M., Louart G., Molinari N., Ragonnet B., Quintard H., Leone M., Zoric L., Lefrant J.Y., the AzuRea group Respiratory variations of inferior vena cava diameter to predict fluid responsiveness in spontaneously breathing patients with acute circulatory failure: need for a cautious use. Crit. Care. 2012 Oct 8;16(5):R188. doi: 10.1186/cc11672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monge García M.I., Gil Cano A., Gracia Romero M., Monterroso Pintado R., Pérez Madueño V., Díaz Monrové J.C. Non-invasive assessment of fluid responsiveness by changes in partial end-tidal CO2 pressure during a passive leg-raising maneuver. Ann. Int. Care. 2012 Mar 26;2:9. doi: 10.1186/2110-5820-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ceruti S., Peruzzo M., De Vivo S., De Bianchi D., Anselmi L., Saporito A. Can non invasive methods for fluid responsive assessment optimize preventive volemic repletion in order to prevent significant hypotension after spinal anesthesia? A randomized trial. www.clinicaltrial.gov NCT02070276.
- 17.SwissEthic Commissions suisses d’éthique pour la recherche sur l’être humain. www.swissethics.ch/index_f.html
- 18.Koordinationsstelle Forschung am Menschen, Kofam Number SNCTP000000500 www.kofam.ch.
- 19.Random Allocation Software, http://mahmoodsaghaei.tripod.com/Softwares/randalloc.html.
- 20.Antonelli M., Levy M., Andrews P.J., Chastre J., Hudson L.D., Manthous C. Hemodynamic monitoring in shock and implications for management. International Consensus Conference, Paris, France, 27–28 April 2006. Int. Care Med. 2007;33(4):575–590. doi: 10.1007/s00134-007-0531-4. [DOI] [PubMed] [Google Scholar]
- 21.Chinachoti T., Tritrakarn T. Prospective study of hypotension and Bradycardia during spinal anesthesia with Bupivacaine: incidence and risk factors, part two. J. Med. Assoc. Thai. 2007;90(3):492–501. [PubMed] [Google Scholar]
- 22.Carpenter R.L., Caplan R.A., Brown D.L., Stephenson C., Wu R. Incidence and risk factors for side effects of spinal anesthesia. Anesthesiology. 1992;76:906–916. doi: 10.1097/00000542-199206000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Tarkkila P., Isola J. A regression model for identifying patients at high risk of hypotension, bradycardia and nausea during spinal anesthesia. Acta Anaesthesiol. Scand. 1992;36:554–558. doi: 10.1111/j.1399-6576.1992.tb03517.x. [DOI] [PubMed] [Google Scholar]
- 24.Hanss R., Bein B., Weseloh H., Bauer M., CAvus E., Steinfath M. Heart rate variability predicts severe hypotension after spinal anesthesia. Anesthesiology. 2006;104:537–545. doi: 10.1097/00000542-200603000-00022. [DOI] [PubMed] [Google Scholar]
- 25.Park S. Prediction of hypotension in spinal anesthesia. Korean J. Anesthesiol. 2013;65(4):291. doi: 10.4097/kjae.2013.65.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith I., Jackson I. Beta-blockers, calcium channel blockers, angiotensin converting enzyme inhibitors and angiotensin receptor blockers: should they be stopped or not before ambulatory anaesthesia? Curr. Opin. Anesthesiol. 2010;23:687–690. doi: 10.1097/ACO.0b013e32833eeb19. [DOI] [PubMed] [Google Scholar]
- 27.Alecu C., Cuignet-Royer E., Mertes P.M., Salvi P., Vespignani H., Lambert M. Pre-existing arterial stiffness can predict hypotension during induction of anesthesia in the elderly. Br. J. Anaesth. 2010;105:583–588. doi: 10.1093/bja/aeq231. [DOI] [PubMed] [Google Scholar]
- 28.Nakasuji M., Suh S.H., Nomura M., Nakamura M., Imanaka N., Tanaka M., Nakasuji K. Hypotension from spinal anesthesia in patients aged greater than 80 years is due to a decrease in systemic vascular resistance. J. Clin. Anesth. 2012 May;24(3):201–206. doi: 10.1016/j.jclinane.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 29.http://www.consort-statement.org