Abstract
Background
Randomized controlled trials (RCTs) are considered the most robust research design to determine the effectiveness of interventions. RCTs comparing surgery to non-surgical alternatives are particularly difficult to perform, partly due to difficulties with recruitment. Low recruitment rates can limit the internal and external validity of a trial thus understanding their causes may be important for avoiding protracted recruitment periods. This study aimed to report patient factors that influenced participation in a trial comparing surgery to a non-surgical treatment approach.
Methods
This study was a cross-sectional study nested within CROSSBAT (Combined Randomized and Observational Study of Surgery For Type B Ankle Fracture Treatment). Eligible participants willing to be randomized were randomized while those who declined randomization were offered participation in an observational cohort. Participants from both groups (randomized and observational) were asked to indicate their level of agreement on a 100 mm line with statements concerning reasons for acceptance or rejection of randomization. A subset were asked to state the primary reason for agreeing to participate or not in the trial.
Results
The nested study included 312 participants; 113 who accepted and 199 who declined randomization. Participants unwilling to be randomized (those in the observational arm of the study) predominantly received a non-surgical intervention. They were significantly more worried about receiving treatment by chance (55 mm vs. 33 mm; p < 0.0001) and had a significantly higher preference for one particular treatment (less equipoise) (82 mm vs 43 mm; p < 0.0001) compared to participants willing to be randomized. Influence from clinicians and risk avoidance were primary influences of participation. Participants’ responses regarding protocol burden, study follow-up requirements and altruism did not significantly differ between groups.
Conclusion
Patient non-participation in an RCT comparing surgery to no surgery is related to concern about receiving a treatment through chance and the presence of a strong preference for a particular treatment, particularly a non-surgical one. To avoid protracted recruitment periods, investigators can increase the number of study sites and ensure personnel involved have equipoise and are trained to provide a balanced view of both treatment arms.
Keywords: Orthopedic, Randomized controlled trials, Patient participation
Abbreviations: RCT, Randomized controlled trial, CROSSBAT, Combined randomized and observational study of surgery for type B ankle fracture treatment, OTA, Orthopedic trauma association
1. Introduction
Randomized controlled trials (RCTs) are considered the most robust research design yet they are often arduous to perform [1]. Patient recruitment to RCTs in general is difficult, often because people have a preference for one treatment over another [2]. Surgical RCTs are reported to be more problematic than drug trials especially as the results of surgery are more permanent and may be associated with increased risks and costs. This issue is particularly highlighted in surgical vs. non-surgical RCTs [3], [4], [5], [6]. Low patient recruitment can lead to a decrease in the scientific quality of an RCT in a number of ways, including loss of statistical power and poorer generalizability, and may lead to early termination [4].
There are many factors affecting patient participation in RCTs. These can be related to the patient or to other factors such as clinician or systematic factors [6]. Patient factors including altruism, self-benefit, fear of chance depicting treatment and patient preference have been identified previously as they influence a patient’s decision to participate in an RCT [7], [8], [9]. Clinician factors that can affect recruitment include time constraints, lack of staff and training, concern for patients, loss of professional autonomy, difficulty with the consent procedure, lack of rewards and recognition, or insufficiently interesting research question [6]. Institutional factors such as procedural, structural and infrastructural obstacles can also affect patient recruitment. Identifying and addressing these factors can improve patient recruitment and, thus, improve the quality of surgical RCTs. Although these factors have been studied in the medical RCTs, there is limited evidence on how these factors influence patient participation in surgical vs. non-surgical RCTs [4], [6], [8], [9], [10]. This study aimed to report patient factors that influenced participation in a trial comparing surgery to a non-surgical treatment approach.
2. Methods
CROSSBAT (Combined randomized and observational study of surgery for type B ankle fracture treatment) involved 22 hospitals across Australia and New Zealand that were a mix of rural, regional and metropolitan hospitals. It included a randomized group, and participants declining randomization were invited to participate in the observational cohort. CROSSBAT was designed to assess if surgical management was superior to non-surgical management for the treatment of type B ankle fractures with minimal talar shift. Consecutive adult patients presenting to a recruiting hospital during the study period with an isolated, closed OTA (Orthopedic trauma association) type 44-B1 distal fibula fracture without significant talar shift were screened for eligibility. Written, informed consent was obtained from all participants willing to participate.
In addition to the above, inclusion criteria included patients with no other concomitant fractures/dislocations; aged between 18 and 65 years inclusive; mobilizing unaided/independently pre-injury and willing to be followed up for 12 months. Exclusion criteria included participants that were medically unfit for anesthesia/surgery; skeletally immature; previous trauma or surgery to the fractured ankle; inability to consent; pregnancy; the presence of other injuries or co-morbidities that impede mobilization; and non-English speaking. Eligible participants willing to be randomized were randomly allocated in a 1:1 ratio to either the surgical or non-surgical intervention. Those not willing to be randomized were invited to participate in the study as part of the observational cohort. Members of the orthopedic team recruited participants either in the emergency department or fracture clinics of the participating hospitals.
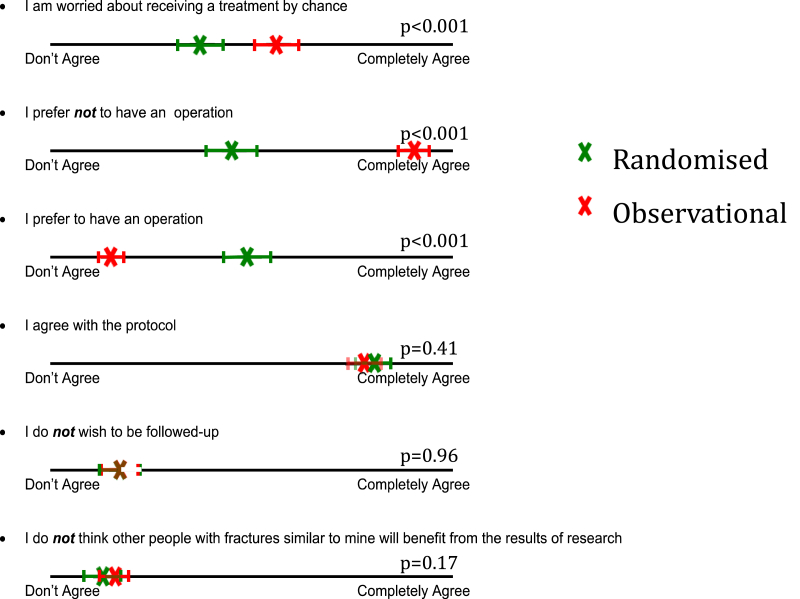
At baseline, along with demographics questions, all participants were asked to complete a survey indicating their level of agreement with a series of statements by placing an “X” through a 100 mm line (Fig. 1). The survey questions were based on common patient concerns regarding participation in RCTs that have been raised in other studies, such as uncertainty of treatment; lack of participant equipoise; protocol rationale, follow-up burden and altruism [6], [7], [8]. As the study progressed, it was evident that the majority of people who participated in CROSSBAT (almost 2:1), opted to become involved in the observational arm (and thus avoid the possibility of surgery). In order to understand further what was the primary reason for participation or not in the randomized arm, participants in the latter stage of recruitment were probed further at six weeks post-surgery via a telephone interview.
Fig. 1.
Participant level of agreement with questions. X denotes the mean response on the 10 mm line. Horizontal bars indicate 95% confidence interval.
The primary outcome was participant level of agreement with each statement expressed in millimetres. As this study was nested within the CROSSBAT study, the sample size was dictated by the CROSSBAT sample. Baseline characteristics of the two cohorts were compared using Student’s t-test or chi-squared test. The survey data were compared using Students’ t-test. Missing data were not imputed. The probed responses were categorized by two investigators (RM, JN) at the conclusion of the study. The number of participants in each category was calculated and expressed as a percent. The percentages in each study group were compared using chi-squared test.
The CROSSBAT protocol was approved by the relevant ethics committees associated with each site. Approval for the nested study was granted by the Hunter New England HREC covering the New South Wales participants.
3. Results
A total of 312 participants were recruited in the sub-study from August 2010 to October 2013; 113 in the randomized cohort and 199 in the observational cohort. The majority of people in the observational cohort received a non-surgical intervention (185 vs. 14) whilst, as expected, treatment distribution was balanced in the randomized cohort.
There were significantly more females in the randomized cohort. The observational cohort had a significantly higher number of participants with a tertiary level education. Other baseline characteristics were similar between the two cohorts and are shown in Table 1.
Table 1.
Baseline demographics.
| Variable | Observational cohort n = 199 | Randomized cohort n = 113 | p Value |
|---|---|---|---|
| Age, mean (SD), years | 38.4 (13.5) | 39.4 (13.5) | 0.53 |
| Female, no. (%) | 80 (40) | 62 (55) | 0.012 |
| BMI, mean (SD), kg/m2 | 27.1 (4.5) | 28.1 (6.2) | 0.14 |
| Education, no. (%) | 0.015 | ||
| High school or lower | 75 (39) | 55 (49) | |
| TAFE/diploma | 60 (31) | 40 (35) | |
| University or above | 60 (31) | 18 (16) | |
| Working, no. (%) | 159 (80) | 91 (81) | 0.89 |
| Insurance status, no. (%) | 0.57 | ||
| Public | 116 (59) | 73 (65) | |
| Private | 65 (33) | 31 (27) | |
| Compensation | 15 (8) | 9 (8) |
Participants in the observational cohort (i.e. those declining randomization) were significantly more worried about receiving treatment by chance (mean 55 mm, 95%CI: 50.0 to 59.7 vs. 36 mm, 95%CI: 30.3 to 40.8; p < 0.0001) and had a significantly higher level of preference for non-surgical management, indicating they had less equipoise (mean 82 mm, 95%CI: 78.6 to 85.0 vs. 43 mm, 95%CI: 37.2 to 48.9; p < 0.0001) when compared with the randomized cohort, respectively. Participants in both cohorts (observational and randomized) had similar levels of agreement with the protocol rationale (mean 77 mm vs. 79 mm; p = 0.41), follow-up burden (mean 17 mm vs. 17 mm; p = 0.96) and altruism (mean 16 mm vs. 13 mm; p = 0.17). The results are shown in Table 2 and Fig. 1.
Table 2.
Participant level of agreement with questions.
| Question | Observational cohort | Randomized cohort | P value |
|---|---|---|---|
| I am worried about receiving treatment by chance | 55 (2.5) | 36 (2.7) | <0.001 |
| I prefer an operation | 14 (1.4) | 48 (2.9) | <0.001 |
| I prefer no operation | 82 (1.6) | 43 (3.0) | <0.001 |
| I agree with the protocol | 77 (1.7) | 79 (2.0) | 0.41 |
| I do not wish to be followed up | 17 (1.9) | 17 (2.5) | 0.96 |
| I do not think that other people with fractures similar to mine will benefit from this research | 16 (1.8) | 13 (1.9) | 0.17 |
Values are mean (standard deviation) in millimetres, based on a 100 mm line.
Of the subset (n = 84) who were probed about their primary reasons for participation in the randomized or observational cohort, 25 were from the randomized arm, and 59 were from the observational cohort. The demographics details of these 84 participants were similar to the whole cohort as shown in Table 3.
Table 3.
Participants that were probed vs. not probed regarding primary reason for participation.
| Variable | Not probed n = 228 | Probed n = 84 | p Value |
|---|---|---|---|
| Age, mean (SD), years | 38.5 (12.3) | 39.3 (14.1) | 0.65 |
| Female, no. (%) | 102 (45) | 40 (48) | 0.65 |
| BMI, mean (SD), years | 27.3 (5.3) | 27.7 (4.9) | 0.55 |
| Education, no. (%) | 0.13 | ||
| High school or lower | 103 (46) | 27 (33) | |
| TAFE/diploma | 68 (30) | 32 (39) | |
| University or above | 55 (23) | 23 (28) | |
| Working, no. (%) | 183 (80) | 67 (80) | 0.92 |
| Insurance status, no. (%) | 0.39 | ||
| Public | 140 (62) | 49 (60) | |
| Private | 67 (30) | 29 (35) | |
| Compensation | 20 (8) | 4 (5) |
The primary reasons for participation or not in the randomized arms were broadly categorized into two themes - health professional influence and risks of surgery. – A higher proportion of participants from the observational cohort preferred non-surgical management (n = 57 (97%)) and identified influence from a health professional regarding their choice of participation (observational vs. randomized) compared to those in the randomized arm as shown in Table 4.
Table 4.
Health professional influence on patient participation.
| Health professional influence, no. (%) | Observational cohort n = 59 | Randomized cohort n = 25 | p Value |
|---|---|---|---|
| No | 30 (51%) | 19 (76%) | p = 0.033 |
| Yes | 29 (49%) | 6 (24%) |
A higher proportion of participants from the observational cohort also identified risks of surgery as a factor that affected their choice of participation (observational vs. randomized) compared to those in the randomized arm as shown in Table 5.
Table 5.
Risks of surgery affecting patient participation.
| Risks of surgery, no. (%) | Observational cohort n = 59 | Randomized cohort n = 25 | p Value |
|---|---|---|---|
| No | 19 (32%) | 19 (76%) | p = 0.0002 |
| Yes | 40 (68%) | 6 (24%) |
4. Discussion
Recruiting for randomized controlled trials is challenging and recruiting for surgical randomized controlled trials is associated with particular barriers. These barriers have been broadly grouped into patient-, surgeon- and institutional-related factors. Within the context of an orthopedic trial, we provide some insights into patient factors influencing participation in a trial comparing surgery to a non-surgical intervention.
In our study, females were more likely to participate in the randomized cohort. There is equivocal evidence regarding whether females are more or less likely to participate in RCTs. Pearson et al. noted that females are less likely to participate while Jenkins et al. found that females were more likely to participate in RCTs [11], [12]. We also noted that participants with a higher level of education were more likely to decline randomization. As for gender, there is conflicting evidence about whether patients with a higher level of education are more or less likely to participate in RCTs [2], [4], [13], [14].
Studies have shown that patients participating in randomized trials have altruism as a motivation for participation and are less worried about uncertainty of treatment [6], [15], [16], [17], [18], [19], [20], [21], [22]. Our study showed that patients who were less worried about uncertainty of treatment were more likely to participate in the randomized cohort. We did not observe a difference in altruism between the groups, however, and this is possibly explained by the inclusion of the observational arm. It is possible that people in both cohorts (randomized and observational) had elements of altruistic behavior guiding their choice to participate in the study. As all patients who were eligible to participate agreed to be in either the randomized or observational arm, we are not able to confirm if people who would do neither had less altruistic tendencies.
Most of the patients who declined randomization preferred non-surgical management, stating risks associated with surgery as the main reason for declining randomization and preferring non-surgical management. Our findings were consistent with other studies as others have shown that patients with a strong preference for a treatment or those who are worried about receiving treatment by chance are less likely to participate in an RCT [2], [4], [6], [23]. Further, it is known that health professionals can influence a patient’s decision to participate in a trial [19], [24], [25]. Our study supports this as a number of patients stated that influence from a healthcare professional was a reason for their participation in the randomized or observational cohorts of the study, and this was particularly pronounced for those who chose the observational arm. Our observations suggest that information provided by study personnel – regarding the known risks and benefits of the treatments under investigation, and the extent to which true uncertainty is expressed - has the potential to significantly influence who and how quickly people are recruited into a trial.
Our results suggest that participants in both cohorts did not regard the protocol or follow-up as a concern. This may explain why all patients who were eligible to participate in CROSSBAT consented to be part of either the randomized or observational cohort. Previous studies have shown that keeping the protocol simple, with minimal follow-up burden can lead to improved recruitment [2], [6]. In the context of our study, these factors may have helped overall recruitment, regardless of which arm of the study a patient consented to be involved in.
The study has strengths and limitations. It was nested in a large-scale, multi-centre trial both of which enhance the generalizability of the findings. As all those screened for participation consented to be involved in either the randomized or observational component, our results arguably relate to why people participate or not in a RCT compared to being involved in an observational study. This not withstanding, as our findings echo previous studies exploring patient participation in randomized trials in the absence of an observational arm, we contend our results usefully add to the small body of work that currently exists in this area. In terms of limitations, our study does not provide data regarding why people may or may not participate in a surgical trial per se. Consequently, we cannot provide recommendations regarding how to improve patient recruitment to clinical studies overall.
5. Conclusion
People who decline randomization in a surgical vs. non-surgical trial tend to be more worried about the uncertainty of treatment and have a stronger preference for a particular treatment. In essence, they tend to be risk averse. Further, it appears clinicians influence what potential participants decide. To avoid protracted recruitment periods, investigators can increase the number of study sites and ensure personnel involved have equipoise and are trained to provide a balanced view of both treatment arms.
Acknowledgements
This trial was supported in part by a grant from the Australian Orthopaedic Association Research Foundation. RM was supported with: a postgraduate scholarship from the National Health and Medical Research Council, Avant Doctors-in-training research scholarship and the Foundation for Surgery John Loewenthal Research Fellowship from the Royal Australasian College of Surgeons. The funding organisations of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
References
- 1.Wright J.G., Katz J.N., Losina E. Clinical trials in orthopaedics research. Part I. Cultural and practical barriers to randomized trials in orthopaedics*. J. Bone Jt. Surg. Am. 2011;93:e151–e157. doi: 10.2106/JBJS.J.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abraham N.S., Young J.M., Solomon M.J. A systematic review of reasons for nonentry of eligible patients into surgical randomized controlled trials. Surgery. 2006;139:469–483. doi: 10.1016/j.surg.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 3.Cook J.A. The challenges faced in the design, conduct and analysis of surgical randomised controlled trials. Trials. 2009;10:9. doi: 10.1186/1745-6215-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Creel A.H., Losina E., Mandl L.A., Marx R.J., Mahomed N.N., Martin S.D. An assessment of willingness to participate in a randomized trial of arthroscopic knee surgery in patients with osteoarthritis. Contemp. Clin. Trials. 2005;26:169–178. doi: 10.1016/j.cct.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 5.McLeod R.S. Issues in surgical randomized controlled trials. World J. Surg. 1999;23:1210–1214. doi: 10.1007/s002689900649. [DOI] [PubMed] [Google Scholar]
- 6.Ross S., Grant A., Counsell C., Gillespie W., Russell I., Prescott R. Barriers to participation in randomised controlled trials: a systematic review. J. Clin. Epidemiol. 1999;52:1143–1156. doi: 10.1016/s0895-4356(99)00141-9. [DOI] [PubMed] [Google Scholar]
- 7.McCann S.K., Campbell M.K., Entwistle V.A. Reasons for participating in randomised controlled trials: conditional altruism and considerations for self. Trials. 2010;11:31. doi: 10.1186/1745-6215-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrison J.D., Solomon M.J., Young J.M., Meagher A., Hruby G., Salkeld G. Surgical and oncology trials for rectal cancer: who will participate? Surgery. 2007;142:94–101. doi: 10.1016/j.surg.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Bower P., King M., Nazareth I., Lampe F., Sibbald B. Patient preferences in randomised controlled trials: conceptual framework and implications for research. Soc. Sci. Med. 2005;61:685–695. doi: 10.1016/j.socscimed.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Naylor J.M., Mittal R., Carroll K., Harris I.A. Introductory insights into patient preferences for outpatient rehabilitation after knee replacement: implications for practice and future research. J. Eval. Clin. Pract. 2012;18:586–592. doi: 10.1111/j.1365-2753.2010.01619.x. [DOI] [PubMed] [Google Scholar]
- 11.Peterson E.D., Lytle B.L., Biswas M.S., Coombs L. Willingness to participate in cardiac trials. Am. J. Geriatr. Cardiol. 2004;13:11–15. doi: 10.1111/j.1076-7460.2004.01709.x. [DOI] [PubMed] [Google Scholar]
- 12.Jenkins V., Farewell D., Batt L., Maughan T., Branston L., Langridge C. The attitudes of 1066 patients with cancer towards participation in randomised clinical trials. Br. J. Cancer. 2010;103:1801–1807. doi: 10.1038/sj.bjc.6606004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diener-West M., Earle J.D., Fine S.L., Hawkins B.S., Moy C.S., Reynolds S.M. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma, II: characteristics of patients enrolled and not enrolled. COMS Report No. 17. Arch. Ophthalmol. 2001;119:951–965. doi: 10.1001/archopht.119.7.951. [DOI] [PubMed] [Google Scholar]
- 14.Group COMS The Collaborative Ocular Melanoma Study (COMS) randomized trial of pre-enucleation radiation of large choroidal melanoma I: characteristics of patients enrolled and not enrolled COMS report no. 9. Am. J. Ophthalmol. 2016;125:767–778. doi: 10.1016/s0002-9394(98)00038-5. n.d. [DOI] [PubMed] [Google Scholar]
- 15.Corbett F., Oldham J., Lilford R. Offering patients entry in clinical trials: preliminary study of the views of prospective participants. J. Med. Ethics. 1996;22:227–231. doi: 10.1136/jme.22.4.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moody L.E., Gregory S.J., Bocanegra T., Vasey F. Factors influencing post-menopausal African-American Women’s participation in a clinical trial. J. Am. Acad. Nurse Pract. 1995;7:483–488. doi: 10.1111/j.1745-7599.1995.tb01116.x. [DOI] [PubMed] [Google Scholar]
- 17.Newburg S.M., Holland A.E., Pearce L.A. Motivation of subjects to participate in a research trial. Appl. Nurs. Res. 1992;5:89–93. doi: 10.1016/s0897-1897(05)80020-5. [DOI] [PubMed] [Google Scholar]
- 18.Penn Z.J., Steer P.J. Reasons for declining participation in a prospective randomized trial to determine the optimum mode of delivery of the preterm breech. Control Clin. Trials. 1990;11:226–231. doi: 10.1016/0197-2456(90)90038-4. [DOI] [PubMed] [Google Scholar]
- 19.Ross M.W., Jeffords K., Gold J. Reasons for entry into and understanding of HIV/AIDS clinical trials: a preliminary study. AIDS Care. 1994;6:77–82. doi: 10.1080/09540129408258027. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz C.E., Fox B.H. Who says yes? Identifying selection biases in a psychosocial intervention study of multiple sclerosis. Soc. Sci. Med. 1995;40:359–370. doi: 10.1016/0277-9536(94)e0092-7. [DOI] [PubMed] [Google Scholar]
- 21.Siminoff L.A., Fetting J.H., Abeloff M.D. Doctor-patient communication about breast cancer adjuvant therapy. J. Clin. Oncol. 1989;7:1192–1200. doi: 10.1200/JCO.1989.7.9.1192. [DOI] [PubMed] [Google Scholar]
- 22.Plaisier P.W., Berger M.Y., van der Hul R.L., Nijs H.G., Toom den R., Terpstra O.T. Unexpected difficulties in randomizing patients in a surgical trial: a prospective study comparing extracorporeal shock wave lithotripsy with open cholecystectomy. World J. Surg. 1994;18:769–772. doi: 10.1007/BF00298927. discussion773. [DOI] [PubMed] [Google Scholar]
- 23.Yeomans-Kinney A., Vernon S.W., Frankowski R.F., Weber D.M., Bitsura J.M., Vogel V.G. Factors related to enrollment in the breast cancer prevention trial at a comprehensive cancer center during the first year of recruitment. Cancer. 1995;76:46–56. doi: 10.1002/1097-0142(19950701)76:1<46::aid-cncr2820760107>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 24.Henzlova M.J., Blackburn G.H., Bradley E.J., Rogers W.J. Patient perception of a long-term clinical trial: experience using a close-out questionnaire in the studies of left ventricular dysfunction (SOLVD) trial. SOLVD close-out working group. Control Clin. Trials. 1994;15:284–293. doi: 10.1016/0197-2456(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 25.Wilcox M., Schroer S. The perspective of patients with vascular disease on participation in clinical trials. J. Vasc. Nurs. 1994;12:112–116. [PubMed] [Google Scholar]