Abstract
Background
Incisional hernias are one of the most frequent complications in abdominal surgery. Laparoscopic repair has been widely used since its first description but has not been standardized. A panel of hernia experts with expertise on the subject “incisional hernia” was established to review existing literature and define a standard approach to laparoscopic IPOM-repair for incisional hernia. All involved surgeons agreed to perform further IPOM-repairs of incisional hernia according to the protocol.
Methods/design
This article summarizes the development of an open prospective observational multicentre cohort study to analyse the impact of a standardization of laparoscopic IPOM-repair for incisional hernia on clinical outcome and quality of life (health care research study).
Discussion
Our literature search found that there is a lack of standardization in the surgical approach to incisional hernia and the use of medical devices. The possibility of different surgical techniques, various meshes and a variety of mesh fixation techniques means that the results on outcome after incisional hernia repair are often not comparable between different studies. We believe there is a need for standardization of the surgical procedure and the use of medical devices in order to make the results more comparable and eliminate confounding factors in interpreting the results of surgical hernia repair. This approach, in our view, will also illustrate the influence of the operative technique on the general quality of surgical treatment of incisional hernias better than a “highly selective” study and will indicate the “reality” of surgical treatment not only in specialist centres.
Trial registration
The LIPOM-trial is registered at www.clinicaltrials.gov, with identifier: NCT02089958.
Keywords: Laparoscopic incisional hernia repair, Clinical outcome, Quality of life, Prospective observational multicentre cohort trial, LIPOM-Trial
Abbreviations: LIPOM, Laparoscopic intraperitoneal onlay mesh augmentation; IPOM, Intraperitoneal onlay mesh augmentation; TM, Trade mark; EHS, European Hernia Society; W, Width; CCS, Carolina Comfort Scale; NRS, Numerical Rating Scale; CDC, Center for Disease Control and Prevention; IDEAL, Idea Development, Exploration, Assessment, Long-term Follow-up; ASA, American Society of Anesthesiologists; HP, Hernia Panel; DSMB, Data safety Management Board
1. Background/introduction
Incisional hernia is a frequent complication following abdominal surgery, with an expected occurrence rate of 15–20% of procedures performed in Germany [1]. Symptomatic incisional hernia is typically treated by elective surgery [2]. In addition to the wide variety of “traditionally” open surgical techniques for incisional hernia repair, LeBlanc described in 1993 the technique of laparoscopic intraperitoneal onlay mesh repair (IPOM) [3]. However, a standard protocol for the surgical technique of IPOM has never been developed, resulting in a huge number of variations, i.e. bridging alone in terms of tensionless repair or in combination with gap closure [4], the extent of mesh overlap covering the defect, which varies from 3 to 5 cm and more, and mesh fixation with a stapler device alone, transfascial sutures alone or a combination of these [5]. Furthermore, several study groups recommend a maximum incisional hernia size of 5–10 cm (EHS W 1–2) as the limit for IPOM because of unacceptable high rates of recurrence at larger sizes [6], [7], [8], [9], [10], [11], [12]. Selection bias may also be related to the fact that different surgeons perform laparoscopic IPOM without any adjustment for surgeon-related morbidity and the learning curve in different patients who may present with various, often not specified sizes of hernias and pre-morbid conditions [13]. A confounding factor is also the lack of differentiation between “primary ventral hernia” and “incisional hernia” along with the different medical devices such as mesh implants and fixation devices used in the majority of published studies [4], [14], [15]. However, this heterogeneity is still treated in the literature as “one surgical procedure for comparable indications”, meaning that available results are not completely comparable. Currently, it is impossible to provide standard recommendations for the management of incisional hernias by laparoscopic IPOM [2], [16].
The LIPOM-Trial (Laparoscopic Intraperitoneal Onlay Mesh Augmentation) was designed to investigate the impact of a consensus-driven standard protocol for both surgical technique and use of medical devices in laparoscopic IPOM on clinical outcome measures in a multicentre setting.
2. Methods/design
2.1. Study design
The LIPOM-trial is an open prospective multicentre cohort study. According to the proposal of the IDEAL Framework for Surgical Innovation the LIPOM-trial is an open prospective observational study at stage 2b (exploration), having a protocol that is driven by standardized eligibility and prospective data collection [17]. Health care outcome measures will be assessed in a consecutive cohort of patients with symptomatic incisional hernias, all of whom will receive a laparoscopic IPOM based on a standard protocol as developed in a consensus process. To avoid influences due to learning curve within the trial each surgeon is required to be board-certified, to have experience of more than 20 laparoscopic IPOM procedures and to have a yearly workload of more than 20 procedures. Inclusion criteria for participating centres and criteria to minimize bias are a center-specific caseload of >20 procedures per year, informed consent for the standard protocol, acceptance of monitoring (the monitor is a member of the hernia panel), digital imaging of each operation after adhesiolysis, outlining the hernia size with intraabdominal ruler and following mesh positioning and fixation, an uncut video of each procedure, and data source verification with 100% electronic monitoring during the study and local assessment by the monitor at the end of the study. The study protocol has been approved by the Ethics Committee of each local site, the Landesärztekammer Hessen, the Landesärztekammer Nordrhein, and the Landesärztekammer Schleswig.
2.2. Consensus process and development of a standard protocol
The structure of the consensus process was based on proposals from the National Institute of Health. Five board-certified surgeons with experience and readiness to be part in the development of a data-based standard protocol for surgical technique and use of medical devices in laparoscopic IPOM were included in the hernia panel (HP). Relevant studies were identified in Medline, Embase, Cochrane Library, Cochrane controlled trials register, and Science citation index (updated to May 2013) under the search terms “abdominal wall hernia” or “ventral hernia” or “incisional hernia”, and “laparoscopic” or “laparoscopy”, and “metaanalysis” or “randomized controlled trial” or “RCT” or “observational trial” or “register”, as well as by reviewing bibliographies from original research articles and reviews.
In the first step all HP members were asked in an interactive workshop to describe their specific surgical technique in order to identify possible variations in surgical technique and compile common elements of a complete procedure. In the second step, articles were reviewed for inclusion criteria as follows: “Incisional hernia”, “type of mesh”, “fixation technique”, “hernia size”, “mesh overlap”, and “surgical technique”. Based on these data each of the HP members was asked in a third step to propose a common step-wise surgical procedure and use of medical products. Each recommendation was discussed in a workshop. HP members achieved very high consensus (≥90%) with final approval. The results of this decision-making process were fixed in a consensus document containing the recommended standard procedure and selection of medical devices (Table 1). Proof of concept and safety of the underlying standardized technique and use of medical devices was demonstrated in a prospective pilot study with 20 patients enrolled (A. H., Fulda and G. P., Bamberg) without observation of adverse events (AEs) and/or severe adverse events (SAEs).
Table 1.
Summary of the consensus standard protocol for laparoscopic onlay mesh augmentation in incisional hernia repair.
| General considerations |
| 1. Use of a classification to improve the possibility of comparing different populations/studies |
| 2. Separation of the two entities “Ventral Hernia” and “Incisional Hernia” |
| 3. Standardized operative technique (see surgical technique) |
| 4. Standardized use of medical products (see surgical technique) |
| 5. Technique of “bridging” is limited to hernia sizes up to a maximum of 10 cm |
| 6. Technique of “gap closure” is limited to hernia sizes up to a maximum of 10 cm |
| 7. Perform a Health Care Research Study |
| Surgical technique |
| 1. Preoperative estimation of hernia classification by physical examination and/or ultrasound and/or CT scan/MRI according to EHS guidelines |
| 2. Access by mini-laparotomy or optical trocar |
| 3. Capnoperitoneum up to 20 mmHg (see cardiac and respiratory function) |
| 4. Three trocar technique, additional trocars to specify |
| 5. Adhesiolysis/preparation of the spatium recii or the Lig. teres hepatis to insure mesh overlap of 5 cm |
| 6. Resection of hernia sac (recommendation) |
| 7. Measurement of hernia size/defect size under low pressure capnoperitoneum (≤8 mmgH) according to EHS guidelines |
| 8. Calculating of mesh (Physiomesh™, CE marked) size with overlap of 5 cm (gap + overlap) |
| 9. Arming of mesh with one suture (nonabsorbable) each at the edges of the mesh (transfascial fixation of mesh) |
| 10. Alternative to “bridging”: “gap closure” with nonabsorbable sutures according to Chelala [4], calculation of mesh size as mentioned above |
| 11. Positioning of the mesh in low pressure capnoperitoneum during fixation (≤8 mmHg) |
| 12. Transfascial fixation of mesh with the four nonabsorbable sutures at the edges of the mesh (epifascial nodes, see 9.) |
| 13. Fixation of mesh by SecureStrap™ (CE marked) in double crown technique (outer line: distance to the edges 0.5 cm, distance between tacs 2 cm, inner line: distance 1 cm to gap edge, distance between tacs 2 cm) |
| 14. Transfascial closure of trocar incisions >5 mm |
| 15. Standardized postoperative analgesia based on NRS, medication according to WHO scheme |
2.3. Patients' inclusion/exclusion criteria
Patients will be consecutively enrolled in the study if they met the following eligibility criteria: (1) age ≥ 18 years, (2) primary incisional hernia, (3) symptomatic/progressive hernia, (4) hernia size ≤ 10 cm (EHS W 1–2), and (5) hernia location according to EHS of M 1–5 and L 1–3.
Patients will be excluded if they met any one of the following criteria: (1) recurrent incisional hernia, (2) primary ventral hernia, (3) hernia size > 10 cm (EHS W 3), (4) hernia location classified as EHS L 4, (5) simultaneous surgical intervention, e.g. appendectomy, (6) mesh overlap < 5 cm, (6) ASA score > 3, (7) malignant disease, (8) liver cirrhosis, (9) peritoneal carcinosis, (10) lack of study agreement, and (11) intraoperative lesion of the colon.
2.4. Data collection, objectives and endpoints
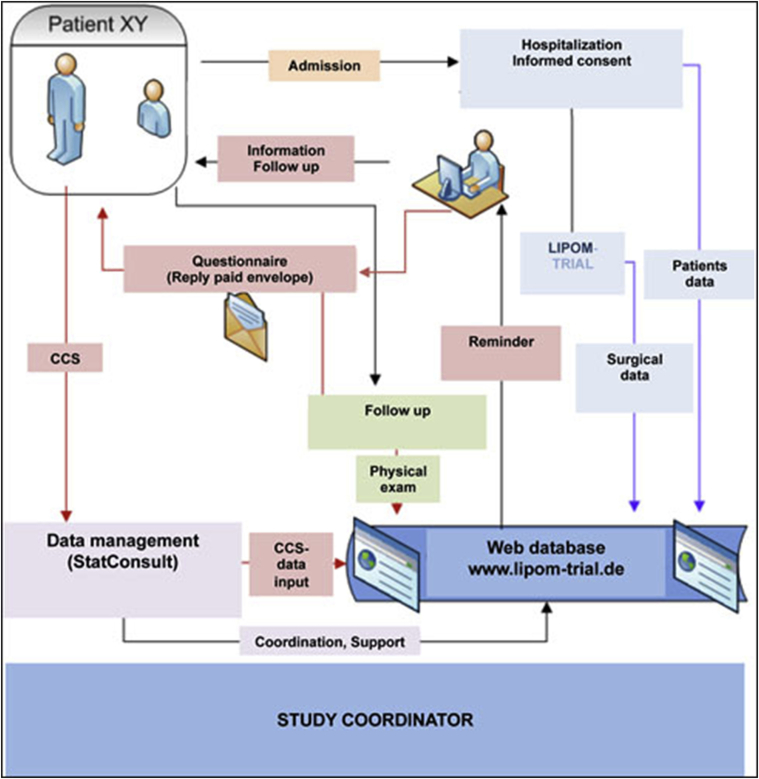
Research staff at the study sites will collect the data in an organized electronic ongoing data collection program (Patient Registry) according to the definition of the Agency for Healthcare Research and Quality (AHRQ), (Fig. 1). Documentation will be conducted via the web-based database “LIPOM” using an electronic case report form (eCRF), ClinWise Version 1.0 (ClinWise HealthCare, StatConsult, Germany). The number of patients lost to follow-up will be recorded.
Fig. 1.
Data flow chart.
The primary outcome measure is the 1-year recurrence rate, according to the recommendations for reporting outcome results in abdominal wall repair (Results of a consensus meeting in Palermo, Italy, 28–30 June 2012 [18]) as a time-to-event analysis for “freedom-of-recurrence” as determined by physical examination and ultrasound, or in the case of diagnostic failure of these techniques, MRI or CT-scan.
Secondary outcome measures are perioperative complications such as bleeding, haematoma, seroma, wound infection (classified according to CDC A1–3), mesh infection, bowel injury, bowel fistula, reoperation, patient-reported pain (measured by Numeric Rating Scale, NRS), patient-reported health-adapted quality of life measured by California Comfort Scale (questionnaire translated into German language; CCS [11]), and mortality.
2.5. Data safety
For statistical analysis personal data will be pseudonymised, and for publication the data will be anonymized. Data will be documented via web access (www.lipom-trial.de) in the database “LIPOM” using an eCRF in ClinWise® Version 1.0 with automatic pseudonymization of the patient's personal data. Access to the database is limited to authentification via user name and password with free access limited to personal records. The database features a reminder function regarding survey of follow up data and automatically generates correspondence to the patient. Follow up of NRS and CCS is patient-related. Data management including documentation and analysis of follow up questionnaires and statistical analysis will be performed by StatConsult. Data safety is guaranteed by StatConsult. ClinWise® is connected via an interface to the Herniamed registry.
2.6. Monitoring and statistical analysis
The following adverse events will be monitored: (1) wound infection, (2) intraabdominal abscess, (3) mesh infection, (4) sepsis, (5) intestinal or organ injury, (6) postoperative bleeding, (7) pneumonia, (8) urinary tract infection, (9) deep vein thrombosis, (10) bowel obstruction, (11) recurrent hernia, (12) vomiting, and (13) pain at rest. Severe adverse events include (1) reoperation, (2) ICU admission, (3) acute incarceration, (4) rehospitalization, and (5) death. The principal investigator will be informed about SAEs within 3 days. An independent Data Safety Management Board (DSMB) consisting of two independent, external clinical experts (Bernd Stechemesser; MD, PAN Klinik, Köln, Germany, and Andreas Koch, MD, FACS, Chirurgische Praxis, Cottbus, Germany) in hernia surgery has been established to address patient safety and perform risk-benefit analysis. In accordance with its standard operative procedures, the DSMB reviews the incoming data to ensure continued patient safety. The DSMB assesses study aspects such as progress, integrity, and design and makes recommendations to the study coordinator regarding modification, continuation or termination of the study. The statistical analyses will be conducted by StatConsult. The primary endpoint will be evaluated with the Kaplan-Meier estimate and the related confidence interval. For the secondary endpoint “perioperative complications” rates and related confidence intervals will be reported. Further descriptive statistics will be reported for (quasi-)continuous variables (mean, standard deviation, minimum, Q1, median, Q3, maximum) and categorical variables (absolute and relative frequencies). Additionally Box-Whisker-Plots will be presented for patient-reported pain (NRS) and patient-reported health-adapted quality of life (CCS).
2.7. Implementation of the study and trial status
All patients with incisional hernias presenting to the participating clinics will be screened for trial eligibility. After physical examination and ultrasound examination (and if necessary CT or MRI scan) the indication for an operation will be determined. In the case of an existing indication the inclusion and exclusion criteria will be checked and informed consent will be obtained. The operative procedure will be performed according to the standard protocol as described above to allow a structured exploration with comparable results.
All participating study sites have received a study synopsis and signed a Declaration of Commitment. Hereby, all study centres have agreed to act in strict accordance with the defined surgical procedure, which has to be documented by an uncut video of each procedure and observed by an authorized monitor. According to the study protocol at least 100 patients will be included over 12 months. Primary medical centres of different levels of health care as well as university medical centres are involved in the LIPOM-Trial. The time between the first patient recruited and last patient treated will be 24 months, which includes a recruitment period of 12 months and a follow up period of 12 months. Time points for the collection of patient-reported and functional outcome data are given in Table 2. The estimated duration of the entire study is 30 months including data analysis. The trial started in September 2013 with recruitment of the first patients.
Table 2.
Time points for the collection of patient-reported and functional outcome data (AE, adverse event; SAE, severe adverse event; NRS, numeric rating scale; CCS, Carolina Comfort Scale); *without questionnaire on mesh implant.
| Criteria | Time |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| −1 d | 0 | 1 | 2 | 3 | 4 | 5 | Dis charge | 6 wks | 6 mth | 12 mth | |
| Patient's data | x | ||||||||||
| Case history | x | ||||||||||
| Comorbidity | x | ||||||||||
| Physical exam/diagnostics | x | x | x | x | x | ||||||
| Inclusion/exclusion criteria | x | ||||||||||
| Preop discussion | x | ||||||||||
| Informed consent | x | ||||||||||
| Surgical data | x | ||||||||||
| AEs/SAEs | x | x | x | x | x | x | x | x | x | x | |
| NRS | x | x | x | x | x | x | x | x | x | x | x |
| CCS | X(*) | x | x | x | |||||||
| Follow up questionnaire | x | x | x | ||||||||
| Follow up interview | x | x | x | ||||||||
3. Discussion
This article describes the development of an open prospective observational multicentre cohort study analysing the influence of a standard protocol for surgical technique and use of medical devices for laparoscopic IPOM on clinical outcome and quality of life. The standard protocol was developed on the basis of current literature and the results of a stepwise consensus process performed by a group of experienced surgeons. Our literature search found that there is a lack of standardization in the surgical approach to incisional hernia and the use of medical devices. The possibility of different surgical techniques, various meshes and a variety of mesh fixation techniques means that the results on outcome after incisional hernia repair are often not comparable between different studies. Given these variations in parameters and the individual differences in every hernia we believe there is a need for standardization of the surgical procedure and the use of medical devices in order to make the results more comparable and eliminate confounding factors in interpreting the results of surgical hernia repair. This approach, in our view, will also illustrate the influence of the operative technique on the general quality of surgical treatment of incisional hernias better than a “highly selective” study and will indicate the “reality” of surgical treatment not only in specialist centres.
4. Conclusion
The LIPOM-trial was designed to explore the impact of a strictly defined stepwise surgical technique for laparoscopic incisional hernia repair on clinical outcome in order to reach a standard recommendation for this procedure. To our opinion this standardization is a prerequisite to reduce surgical variation and consequently improve the comparability of data.
Authors'contributions
1. Substantial contribution to conception and design: Hellinger A., Wotzlaw F., Fackeldey V., Pistorius G., Jünemann,R., Zdichavsky M., Buia A.
2. Substantial contribution to data management and analysis: Jünemann,R.
3. Involvement in drafting the manuscript: Hellinger A., Fackeldey V., Buia A.
4. Involvement in revising the manuscript critically for content: Hellinger A., Wotzlaw F., Fackeldey V., Pistorius G., Jünemann,R., Zdichavsky M., Buia A.
5. Final approval of the version to be published: Hellinger A., Wotzlaw F., Fackeldey V., Pistorius G., Jünemann,R., Zdichavsky M., Buia A.
6. Agreement to be accountable for all aspects of the work ensuring that questions related to the accuracy or integrity of any part of the work are appropriate portions of content: Hellinger A., Wotzlaw F., Fackeldey V., Pistorius G., Jünemann,R., Zdichavsky M., Buia A.
Competing interests
All authors declare that they have partial funding by Ethicon UK, a division of Johnson & Johnson Medical Limited.
Principle investigator
A. Hellinger, Klinikum Fulda, Universitätsmedizin Marburg – Campus Fulda, Fulda, Germany.
Advisory Board/Hernia Panel
Alexander Buia, Asklepios Klinik Langen, Langen, Germany.
Volker Fackeldey, Klinik Kitzinger Land, Kitzingen, Germany.
Achim Hellinger, Klinikum Fulda, Universitätsmedizin Marburg – Campus Fulda, Fulda, Germany.
Georg Pistorius, Sozialstiftung Bamberg, Bamberg, Germany.
Michael Zachert, Steigerwaldklinik Burbach, Bamberg, Germany.
Collaboration Group
A. Buia, Asklepios Klinik Langen, Langen, Germany.
A. Beulecke, KRH Klinikum Grossburgwedel, Grossburgwedel, Germany.
V. Fackeldey, Klinik Kitzinger Land, Kitzingen, Germany.
A. Hämmerle, Sana Klinikum Hameln, Hameln, Germany.
A. Hellinger and F. Wotzlaw, Klinikum Fulda, Universitätsmedizin Marburg – Campus Fulda, Fulda, Germany.
G. Pistorius, Sozialstiftung Bamberg, Bamberg, Germany.
O. Stern, Asklepios Klinik Wandsbek, Hamburg, Germany.
A. Türler, Evangelische Kliniken Bonn, Bonn, Germany.
R. Wilke, Luisenhospital Aachen, Aachen, Germany.
M. Zachert, Steigerwaldklinik Burgerbach, Bamberg, Germany.
M. Zdichavsky, Universitätsklinikum Tübingen, Tübingen, Germany.
Acknowledgments
The LIPOM-Trial was partly funded by Ethicon UK (IIS 12-103), a division of Johnson & Johnson Medical Limited.
We thank all members of the Collaboration Group for assistance and critical comments.
References
- 1.Seiler C.M., Bruckner T., Diener M.K., Papyan A., Golcher H., Seidlmayer C., Franck A., Kieser M., Büchler M.W., Knaebel H.-P. Interrupted or continuous slowly absorbable sutures for closure of primary elective midline abdominal incisions: a multicenter randomized trial (INSECT: ISRCTN24023541) Ann. Surg. 2009;249:576–582. doi: 10.1097/SLA.0b013e31819ec6c8. [DOI] [PubMed] [Google Scholar]
- 2.Berger D. Laparoskopische IPOM-Technik. Chirurg. 2010;81:211–215. doi: 10.1007/s00104-009-1819-4. [DOI] [PubMed] [Google Scholar]
- 3.LeBlanc K.A., Booth W.V. Laparoscopic repair of incisional abdominal hernias using expanded polytetrafluoroethylene: preliminary findings. Surg. Laparosc. Endosc. 1993;3:39–41. [PubMed] [Google Scholar]
- 4.Chelala E., Gaede F., Douillez V., Dessily M., Alle J.L. The suturing concept for laparoscopic mesh fixation in ventral and incisional hernia repair: preliminary results. Hernia. 2003;7:191–196. doi: 10.1007/s10029-003-0143-z. [DOI] [PubMed] [Google Scholar]
- 5.LeBlanc K.A. Laparoscopic incisional hernia repair: are transfascial sutures necessary? A review of the literature. Surg. Endosc. Other Interv. Tech. 2007;21:508–513. doi: 10.1007/s00464-006-9032-8. [DOI] [PubMed] [Google Scholar]
- 6.Bageacu S., Blanc P., Breton C., Gonzales M., Porcheron J., Chabert M., Balique J.G. Laparoscopic repair of incisional hernia: a retrospective study of 159 patients. Surg. Endosc. Other Interv. Tech. 2002;16:345–348. doi: 10.1007/s00464-001-0018-2. [DOI] [PubMed] [Google Scholar]
- 7.Dumanian G.A., Denham W. Comparison of repair techniques for major incisional hernias. Am. J. Surg. 2003;185:61–65. doi: 10.1016/s0002-9610(02)01122-4. [DOI] [PubMed] [Google Scholar]
- 8.Kingsnorth A.N., Sivarajasingham N., Wong S., Butler M. Open mesh repair of incisional hernias with significant loss of domain. Ann. R. Coll. Surg. Engl. 2004;86:363–366. doi: 10.1308/147870804236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreno-Egea A., Carrillo-Alcaraz A., Aguayo-Albasini J.L. Is the outcome of laparoscopic incisional hernia repair affected by defect size? A prospective study. Am. J. Surg. 2012;203:87–94. doi: 10.1016/j.amjsurg.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 10.Sauerland S., Walgenbach M., Habermalz B., Seiler C.M., Miserez M. Laparoscopic versus open surgical techniques for ventral or incisional hernia repair (Review) Cochrane Database Syst. Rev. 2011 doi: 10.1002/14651858.CD007781.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Baccari P., Nifosi J., Ghirardelli L., Staudacher C. Short- and mid-term outcome after laparoscopic repair of large incisional hernia. Hernia. 2013;17:567–572. doi: 10.1007/s10029-012-1026-y. [DOI] [PubMed] [Google Scholar]
- 12.Dietz U.A., Winkler M.S., Härtel R.W., Fleischhacker A., Wiegering A., Isbert C., Jurowich C., Heuschmann P., Germer C.T. Importance of recurrence rating, morphology, hernial gap size, and risk factors in ventral and incisional hernia classification. Hernia. 2014;18:19–30. doi: 10.1007/s10029-012-0999-x. [DOI] [PubMed] [Google Scholar]
- 13.Salvilla S., Thusu S., Panesar S. Analysing the benefits of laparoscopic hernia repair compared to open repair: a meta-analysis of observational studies. J. Minim. Access Surg. 2012;8:111. doi: 10.4103/0972-9941.103107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heniford B.T., Ramshaw B.J. Laparoscopic ventral herma repair: a report of 100 consecutive cases. Surg. Endosc. 2000;14:419–423. doi: 10.1007/s004640000179. [DOI] [PubMed] [Google Scholar]
- 15.Köckerling F., Schug-Paß C., Adolf D., Reinpold W., Stechemesser B. Is pooled data analysis of ventral and incisional hernia repair acceptable? Front. Surg. 2015;2(May):2014–2016. doi: 10.3389/fsurg.2015.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pawlak M., Bury K., Śmietański M. The management of abdominal wall hernias - in search of consensus. Wideochirurgia i inne Tech. mało inwazyjne = Videosurgery other miniinvasive Tech./Kwart. Pod patronatem Sekc. Wideochirurgii TChP oraz Sekc. Chir. Bariatrycznej TChP. 2015;10:49–56. doi: 10.5114/wiitm.2015.49512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ergina P.L., Barkun J.S., McCulloch P., Cook J.A., Altman D.G. IDEAL framework for surgical innovation 2: observational studies in the exploration and assessment stages. BMJ. 2013;346:f3011. doi: 10.1136/bmj.f3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muysoms F.E., Deerenberg E.B., Peeters E., Agresta F., Berrevoet F., Campanelli G., Ceelen W., Champault G.G., Corcione F., Cuccurullo D., Debeaux A.C., Dietz U.A., Fitzgibbons R.J., Gillion J.F., Hilgers R.D., Jeekel J., Kyle-Leinhase I., Köckerling F., Mandala V., Montgomery A., Morales-Conde S., Simmermacher R.K.J., Schumpelick V., Śmietański M., Walgenbach M., Miserez M. Recommendations for reporting outcome results in abdominal wall repair: results of a consensus meeting in Palermo, Italy, 28-30 June 2012. Hernia. 2013;17:423–433. doi: 10.1007/s10029-013-1108-5. [DOI] [PubMed] [Google Scholar]