Abstract
Background
The accessibility for total joint arthroplasty often comes up against long wait lists, and may lead to deleterious effects for the awaiting patients. This pilot single blind randomized controlled trial aims to evaluate the impact of a telerehabilitation prehabilitation program before a hip or knee arthroplasty compared to in-person prehabilitation or to usual wait for surgery.
Methods/design
Thirty-six patients on a wait list for a total hip or knee arthroplasty will be recruited and randomly assigned to one of three groups. The in-person prehabilitation group (n = 12) will receive a 12-week rehabilitation program (2 sessions/week) including education, exercises of the lower limb and cardiovascular training. Patients in the tele-prehabilitation group (n = 12) will receive the same intervention using a telecommunication software. The control group (n = 12) will be provided with the hospital's usual documentation before surgery. The Lower Extremity Functional Scale (LEFS) will be the primary outcome measure taken at baseline and at 12 weeks. Secondary measures will include self-reported function and quality of life as well as performance tests. A mixed-model, 2-way repeated-measure ANOVA will be used to analyse the effects of the rehabilitation programs.
Discussion
This pilot study is the first to evaluate the feasibility and the impact of a telerehabilitation prehabilitation program for patients awaiting a total joint arthroplasty. The results of this pilot-RCT will set the foundations for further research in the fields of rehabilitation and tele-medicine for patients suffering from lower limb osteoarthritis.
Trial registration
ClinicalTrials.gov: NCT02636751.
Keywords: Prehabilitation, Telemedicine, Arthroplasty, Hip, Knee
Abbreviations: TJA, Total joint arthroplasty; TKA, Total knee arthroplasty; THA, Total hip arthroplasty; LEFS, Lower Extremity Functional Scale; WOMAC, Western Ontario & McMaster Universities Osteoarthritis Index; SF-36, The Short Form (36) Health Survey; GRS, Global Rating Scale; TUG, Timed Up and Go; SPW, Self-paced Walk; ST, Stair Test; FRSQ, Fonds de recherche du Québec – Santé; ANOVA, Analysis of variance; ICC, Intraclass correlation coefficient; RCT, Randomized clinical trial
1. Introduction
Osteoarthritis (OA) is a very common disorder that affects 1 in 8 Canadians among which more than half of the population over 65 years of age [1], [2]. Future estimations indicate that the incidence of OA will increase by at least 26% over the next 30 years in Canada because of inactivity, obesity and aging [2]. Hip and knee are the joints most affected by OA, incuring important disability [3]. Lower limb OA is initially treated conservatively with therapeutic interventions such as physical activity modification, exercise, weight control, and medication [4], [5]. Surgical interventions such as total joint arthroplasty (TJA) have been proven a treatment of choice for the most severe cases. However, the accessibility to such surgeries often come up against long wait lists, and prolonged wait times may lead to deleterious effects on the health status and quality of life of the awaiting patients [6].
Prehabilitation refers to education and exercising before a surgery. Attention to prehabilitation has increased in the last decade and a growing body of evidence suggests that it could have a positive effect on postoperative outcomes and may reduce disabilities before and after surgery for a number of conditions [7], [8], [9]. In the context of prehabilitation for TJA, trials have already shown that a rehabilitation exercise program before a TJA could lead to a shorter hospitalisation length of stay [10], in addition to increased muscle strength [11], [12] and range of motion following a total hip or knee arthroplasty [11], [13].
As the aging population and the constant increase in chronic diseases keep pressuring healthcare systems worldwide [14], lack of resources tends to lengthen wait time for surgery like TJA. Policymakers have therefore been searching for a care optimisation strategy to improve healthcare accessibility.
Among the solutions stands the use of technology to help improve accessibility to rehabilitation services. Telerehabilitation has gained increased recognition and is defined as the provision of rehabilitation services at a distance, using information and communication technologies [15]. Previous studies have already shown that telerehabilitation programs are feasible in a home-care setting [16], [17], [18].
Tousignant et al. demonstrated that a telerehabilitation program after a total knee arthroplasty (TKA) was as effective and less expensive than conventional physiotherapy [19], [20], [21]. Bedra et al. qualified as viable a home-based telerehabilitation program after a hip fracture [16], while Anton et al. demonstrated that a Kinect™-based system can be an adjuvant to physiotherapy after a total hip replacement [22], [23]. Such programs can optimise the delivery of care in community rehabilitation, especially by increasing the number of patients seen in a single day, by reducing health care costs and travel time, and by providing access to medical care otherwise unavailable in rural or remote areas [24]. However, no study, to our knowledge, analysed the outcome of telerehabilitation prior to a total joint replacement.
This pilot single blind randomized controlled trial therefore aims to evaluate the feasibility and impact on pain and disability of a telerehabilitation prehabilitation program for patients awaiting a total joint (hip or knee) arthroplasty compared to in-person prehabilitation or usual care. Our hypothesis is that a 12-week in-person or telerehabilitation prehabilitation program will significantly increase functional mobility and quality of life before the surgery, for the subjects in the experimental groups as compared to those in the control group.
2. Material and methods
2.1. Study design
Patients will be randomly assigned to one of three groups. The in-person prehabilitation group will receive a 12-week rehabilitation program including education, cardiovascular training using low-impact activities, as well as range of motion, strengthening and proprioceptive exercises of the lower limb, in addition to walking aid adjustment. General information about pain control, such as ice application and medication usage will also be provided. Patients in the tele-prehabilitation group will receive the same exercise program and advice through an Internet-based telecommunication software. The control group will be provided with the hospital's usual documentation before total joint arthroplasties, consisting of information regarding the pre- and post-surgery course and medication. The exercise components of the prehabilitation program are those commonly used with patients suffering from lower limb osteoarthritis or after TJA. The particularity lies in the timeframe when they will be held, i.e. the pre-operative phase of a TJA. All the prehabilitation sessions will we provided by licensed physiotherapists, members of the Ordre professionnel de la physiothérapie du Québec.
For all participants, evaluations will be performed at the inclusion in the study (baseline) and after the completion of the 12-week rehabilitation program. The study will be approved by the Center intégré universitaire de santé et de services sociaux de l’Est de l’île de Montréal, site Maisonneuve-Rosemont (HMR) ethic committee and all study participants will sign an informed consent form. The study protocol will also be published on the https://clinicaltrials.gov website.
2.2. Participants
Thirty-six (36) patients on a wait list for a total arthroplasty of hip (18) or knee (18) at the Center intégré universitaire de santé et de services sociaux de l'est de l’île de Montréal, site Maisonneuve-Rosemont (HMR) and site Santa-Cabrini will be recruited. Maisonneuve-Rosemont is a tertiary care hospital with an orthopaedic department while Santa-Cabrini is a community hospital. Participants will need to fulfill the following eligibility criteria: 1- Age greater than 18 years; 2- Waiting for a TKA or a THA; 3- Suffering from severe OA of hip or knee; 4- Quebec resident covered by the Régie de l'assurance maladie du Québec (Quebec public healthcare insurance); 5- Speaks French; 6- Has access to a high-speed internet connection. The following exclusion criteria will be used: 1- Suffering from inflammatory arthritis; 2- Scheduled for a bilateral surgery; 3- Has had a lower limb surgery in the past 6 months; 4- Scheduled for a revision of a previous TKA or THA; 5- Planned for a wide acetabular head hip prosthesis or a hip articular resurfacing; 6- Receiving compensation from the Quebec Workers' Compensation Board (Commission des normes, de l'équité, de la santé et de la sécurité du travail); 7- Suffering from a severe psychiatric, neurologic or cardiac disorder, or other types of disorders that could interfere with the rehabilitation program.
Patients will be identified by the treating surgeons or by a research professional once patients are scheduled for surgery. Patients will be contacted by a research assistant by phone to receive further information about the trial and perform a preliminary screening. After obtaining preliminary consent, participants will be given an appointment at the Maisonneuve-Rosemont Research Center in order to validate their eligibility and a baseline evaluation and formal written consent will be sought. In case of refusal, sociodemographic data, such as age, sex and reason for refusal will be collected for further selection bias analysis.
Patients will then be randomly assigned to the control group or to one of the two experimental groups. An independent research assistant will open the sealed opaque randomization envelope indicating the participant's assignment to a group. A random number generator will be used to establish randomization lists prior to the initiation of the study. A member of the research team, not involved with data collection, will generate the randomization list. Blocked randomization of 6 will be used to make sure that three equal groups of 12 subjects participants are obtained.
2.3. Participant evaluation
Evaluations will take place at two points in time: at baseline and at the end of the 12 week intervention (or 12 weeks after baseline for the control group). During the baseline evaluation at the Maisonneuve-Rosemont Research Center, eligible participants will complete a questionnaire covering sociodemographic status, comorbidities, and medication usage. Patients will be asked to fill in four Canadian French validated self-reported questionnaires: the Lower Extremity Functional Scale, the Western Ontario and McMaster Universities Osteoarthritis Index, the Short Form Health Survey and a Global Rating of Change Scale. An online version of the questionnaires using the Survey Monkey® platform or a paper version will be provided to the participants. Three physical functional performance measures will also be collected: the self-paced walk (SPW), the timed up-and-go (TUG) and timed stair tests (ST). These tests are oriented toward activities of daily living and have been validated with a geriatric population [25], [26], [27], [28], [29]. They are reliable, reproducible, and responsive to change [30]. They also have widely been used for measuring the outcomes of patients undergoing a TJA [31]. They will be administered according to the pre-established standardized procedures. Finally, a logbook will be given to the participants. They will be asked to record the exercises executed at home, including the number of series and repetitions, in addition to the medication intake. This logbook will allow a monitoring of the adherence rate to the programs and the medication intake. The advent of any adverse effects will be noted by the treating physical therapist during the intervention.
At the 12-week follow-up session (i.e. end of the intervention), participants will be reassessed by a blind physiotherapist using the same evaluation tools as at baseline, adding a global rating of change scale. A satisfaction questionnaire about the telecommunication softwares experience will be filled by participants and therapists in the telerehabilitation group.
2.4. Outcome measures (dependent variables)
The Lower Extremity Functional Scale (LEFS) will be the primary outcome measure. It is a 20-item questionnaire [32] that has been shown to be highly reliable, correlates with other constructs, and is an independent predictor of patient and physician assessment of change [33]. The LEFS has outperformed other questionnaires in distinguishing between pain and function in patients following a hip or knee TJA [33]. Each item is rated on a five-point scale (0 = extreme difficulty or unable to perform activity, 4 = no difficulty); total scores range from 0 to 80, and lower scores represent greater difficulty. It has been shown to be highly reliable, correlates with other constructs, and is an independent predictor of patient and physician assessment of change in patients following total hip or knee arthroplasty [34]. The minimal clinically important difference of the LEFS for this population has been found to be a change of at least 9 points [32]. This questionnaire has been validated in a French-Canadian version [35].
The Western Ontario and McMaster Universities Arthritis Index (WOMAC) will be used to assess functional mobility. This self-administered questionnaire has been widely used to evaluate the effects of interventions after a TKA or a THA [36], [37], [38], [39], [40]. It consists of 24 items divided into 3 subscales: pain (5 items), stiffness (2 items) and physical function (17 items). Each item is scored on a five-point scale according to difficulty experienced (0 = none and 4 = extremely difficult [41]. Total score ranges from 0 to 96 with a minimal clinically important difference (MCID) established at −11.8 for hip OA and −14.8 for knee OA [42]. The reliability (Cronbach's alpha varying from 0.86 to 0.90; intra-class correlation coefficient varying from 0.70 to 0.90), convergent construct validity and responsiveness (Standardized Response Mean varying from 0.63 to 2.00) of the WOMAC scale have been found to be very good [39], [43], [44], [45], [46], [47], [48] and this scale has been used extensively for patients suffering from knee osteoarthritis or undergoing knee arthroplasty [40], [41]. Furthermore, this questionnaire has been validated in a French-Canadian version [41].
The Short Form (36) Health Survey (SF-36) is a self-administered generic health status measure questionnaire. It calculates 8 multi-item scales (physical functioning, role physical, bodily pain, general health, vitality, social functioning, role emotional, and mental health) and 2 summary scales, the physical component summary (PCS) and the mental component summary (MCS). The use of the SF-36 has been extensive in populations suffering from osteoarthritis [49] and in particular in patients undergoing total joint arthroplasty [45], [47], [50], [51], [52], [53], [54], [55], [56], [57]. The reliability and validity of this self-administered questionnaire have been well established (concomitant construct validity has been demonstrated to be good [25]; test-retest reliability: ICC = 0.88–0.92 [25]; responsiveness: SMR varying from 1 to 1.1 [58], [59]. Total score of each scale ranges from 0 (poor health) to 100 (perfect health) [53], [56]. The MCID of SF-36 is established at 12% of baseline score or 6% of maximal score for rehabilitation studies [36]. A French-Canadian version exists as well as normative data according to age and gender for the Canadian population [60].
The Global Rating of Change scale (GRC) is designed to quantify a patient's perceived improvement or deterioration over time. Using an 11-point GRC scale, ranging from −5 (a great deal worse) to 0 (about the same) to +5 (a great deal better), participants will be asked to answer the following question: “Overall, has there been any change in your condition since the initial evaluation done at the beginning of your rehabilitation program? Please indicate if there has been any change in your condition by choosing one of the following options” [61]. The validity, reliability (ICC = 0.90) and responsiveness of GRC scales have been established [62].
The Timed Up and Go (TUG) is a test that assesses mobility, balance, walking ability, and fall risk in older adults. The patient sits in a chair with his/her back against the chair back. On the command “go”, the patient rises from the chair, walks 3 m at a comfortable and safe pace, turns, walks back to the chair and sits down. Timing begins at the instruction “go” and stops when the patient is seated. Time in seconds is the outcome of the test [63]. Minimal level of detectable change is 1.76s with the population suffering from osteoarthritis [30].
For the StairTest (ST), patients are asked to ascend and descend 8 stairs (step height 18.5 cm) in their usual manner, at a safe and comfortable pace. Use of a gait aide and/or of the handrail is allowed as needed [30]. Time in seconds is the outcome of the test. The minimal clinically important difference of the stair test for this population has been found to be a change of at least 3.88 s or 0.07 m/s [30].
For the Self-Paced Walk test, patients are required to walk two lengths of a 20 m indoor course in response to the instructions ‘‘walk as quickly as you can without overexerting yourself.’’ Usual gait aides are allowed as needed. The minimal clinically important difference of the self-paced walk test for this population has been found to be a change of at least 2.86 s or 0.07 m/s [30].
2.5. Independent variables
Age, height, weight, social status (married, single, widowed), employment situation, education level will be collected. The number of comorbidities will be documented using the Charlson Index validated questionnaire [25]. The score of the Charlson Index will be calculated from data found in the subjects' medical files. Data regarding the use of a walking aid before surgery (cane or walker) will be collected.
2.6. Intervention
Physical therapy interventions will be given to participants of the in-person prehabilitation and tele-prehabilitation groups at Maisonneuve-Rosemont Research Center according to a pre-established protocol, allowing a tailored prescription of exercises according to patient pain, strength and tolerance to activity.
Patients in the experimental groups will take part in a 12-week long program including strengthening of the hip and knee muscles, range of motion of the hip and knee as well as proprioceptive exercises, in addition to low-impact cardiovascular warm-up, education regarding medication usage and ice or heat application. After an initial evaluation visit at the research center, they will receive 2 supervised physiotherapy sessions per week and will be asked to repeat the same exercise program the other weekdays without supervision. Since exercise programs used in previous prehabilitation studies are heterogeneous, the prehabilitation protocol used in this trial will be based on the Osteoarthritis Research Society International (OARSI) recommendations for the management of hip and knee osteoarthritis and on programs detailed in previous prehabilitation intervention studies [4], [7], [8], [10], [11], [12], [13], [64]. For warm-up, participants will be asked to pedal for 15 min before beginning the exercise program. Strengthening exercises will be classified according to difficulty and muscles group solicited, from the easiest to the hardest. To avoid a workload bias, the total number of tasks executed for each physiotherapy session will be the same for all the participants. One intervention per targeted muscle group will be performed at each physiotherapy session. Patients will be asked to do 10 repetitions of the first exercise of each category twice. Resting time between series will be of 1 min. If the patient is unable to complete the task for the category, the number of repetitions will be noted and this task will be retried at the next physiotherapy session. In the case of the patient being able to complete the task, two more repetitions will be asked at the end of the last series. If the participant is able to perform 22 repetitions in total, without important pain and in a complete range of motion, the participant will progress to a more difficult exercise at the next physiotherapy session. In addition to the targeted strengthening exercises, 2 functional global exercises, squat and plantar flexion standing, will be executed at every session, if tolerated. The joint mobilisation exercises and proprioceptive exercises will be chosen by the physiotherapist according to the results of the biomechanical and clinical examination performed at the baseline evaluation and at each session. For proprioceptive exercises, participants will be asked to perform the task for 30 s. If he/she is able to complete the task, the participant will progress to another exercise at the next physiotherapy session. The participant's general health condition and tolerance to activity will be monitored at every physiotherapy session. Although unlikely, if pain significantly increases during the trial, participants will be met in person to further assess their condition and adjust the treatment.
Patients in the in-person prehabilitation group (N = 12) will attend physiotherapy sessions at Maisonneuve-Rosemont research center, while patients in the tele-prehabilitation group (N = 12) will perform the exercise protocol at home. For the tele-prehabilitation group, only the first session will be in person and the supervision of the home-based program will be provided by a physiotherapist through various telecommunication softwares. The REACTS® medical consultation platform will be used. It is an affordable new technology from Technologies innovatrices d'imagerie inc, Montreal, Canada. It allows for one-on-one audiovisual interactions between the therapist and the patient on a web platform through high-quality secure audio-video communication, which includes an interactive screen, and file, application and desktop sharing. It requires commonly-used hardware, such as a desktop, laptop, tablet or smartphone. An Internet connection of 500 KBPS is required to allow virtual conversations. It is available in French, English, Spanish and Portuguese on many operating systems: PC windows 7 or higher, iOS for Apple mobile devices and MAC OS on Apple computers. Other generic telecommunication software such as Skype (Microsoft corporation, Redmond, USA) and Facetime (Apple, Cuppertino, USA) will be used to assess their ability to deliver a similar experience to participants. iPads will be used by physiotherapist and participants for the telerehabilitation sessions. Participants who already own an iPad will use it and have the different software installed. In the other case, a preconfigured iPad will be lent to the participants during the trial.
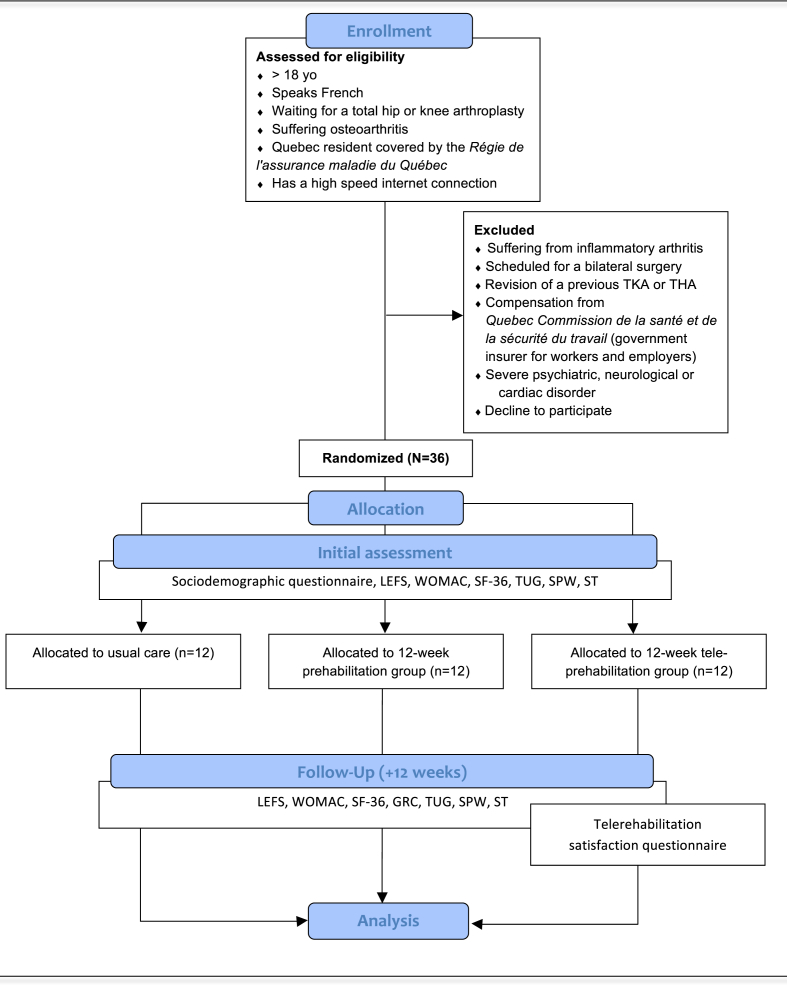
Patients from the control (N = 12) group will receive usual care from Maisonneuve-Rosemont and Santa-Cabrini hospitals, without any prehabilitation. Usual care consists of a single home visit from a community physiotherapist before the surgery. During the visit, the patient is given a booklet containing general information about the surgery, the use of medication and rehabilitation (see Fig. 1).
Fig. 1.
Flow Diagram of the randomized controlled trial. TKA: Total knee arthroplasty, THA: Total hip arthroplasty, LEFS: Lower Extremity Functional Scale, WOMAC: Western Ontario & McMaster Universities Osteoarthritis Index, SF-36: The Short Form (36) Health Survey, GRS: Global Rating Scale, TUG: Timed Up and Go, SPW: Self-paced Walk, ST; Stair Test.
2.7. Sample size and analyses
This is a pilot study to assess the feasibility of a multicenter randomized controlled trial comparing a telerehabilitation prehabilitation program for patients awaiting a total joint (hip or knee) arthroplasty compared to in-person prehabilitation or to usual care. The sample size calculation that would be required for a full randomized controlled trial is based on the primary outcome measure, the LEFS. The LEFS has a clinically important difference of 9 points for patients presenting with hip or knee OA. The standard deviation reported in the literature for this population is 16.2 [65]. The considered parameters are 0.05 for type I error (α) with a power of 0.80 (1-β). For an analysis of variance (ANOVA), the sample size required is 51 subjects per group. This sample size will provide sufficient power to detect a clinically important difference between any of the three groups.
Descriptive statistics (mean, standard deviation, median, frequency counts) will be calculated for all outcome measures at the different measurement times (week 0, 12) to summarise results. Baseline demographic data will be compared (independent Student t-tests and Chi-squared tests) across groups to establish the comparability of covariables. If needed, statistical adjustments will be made for baseline characteristics that are significantly different between groups. All data will be tested to ensure they meet the assumptions for the inferential statistical analyses. If they do not meet the necessary assumptions, appropriate non-parametric procedures will be used. An intention-to-treat analysis will be used in which all participants will be analysed in the group to which they were originally assigned.
A mixed-model, 2-way repeated-measure ANOVA (Groups × Evaluation time point) will be used to analyse the effects of the rehabilitation programs. Separate analyses will be conducted on each of the primary and secondary outcomes and will include a stratification for hip or knee surgeries. If an interaction is detected (p < 0.05), simple effects will be examined. The Kolmogorov-Smirnov test (p < 0.05) will be conducted on the different scores to ensure normality for all variables with significant main effects. The sphericity of the data will be verified by Mauchly's test. For normally distributed variables with significant main effects, post hoc dependent Student t tests will be conducted and effect sizes (Cohen's d) will be calculated. For any variables that will not be normally distributed, the Wilcoxon signed-rank test and Glass's delta (effect size) will be used for post hoc contrasts. Patients' perceived change following the programs will also be categorised as either success or failure. Success will be defined as a GRC score rated as (+2) or higher. Patients will be classified as failure if the change on the GRC is (+1) or at any level below this. The proportions of success/failure will be compared across groups (Chi-squared tests).
3. Discussion
Long wait times can lead to deteriotation in function in patients awaiting a TJA, and prehabilitation offers a low cost and highly feasible solution for optimising the delivery of healthcare for this population. As the application of such programs encounter obstacles from a geographical, material and human resources point of view, finding effective and innovative ways for providing rehabilitation services is a must. Although more attention has been driven toward prehabilitation programs during the last decades, the optimal exercise components still need to be identified. This pilot study is the first to evaluate the feasibility and impact of a telerehabilitation prehabilitation program for patients awaiting a total joint arthroplasty compared to in-person prehabilitation or to usual care. The results of this pilot-RCT will set the foundations for further research in the fields of rehabilitation and telemedicine for patients suffering of OA. It will also contribute to the current evidence on tele-rehabilitation and prehabilitation with this population.
Authors' contributions
The primary authors for this protocol are PDC, FD & DK. PDC & FD wrote the manuscript. PDC, FD & VL created the protocol. PDC led the publication. PV, SP & FD did the methodological quality assessment. All the authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
This project will be financially supported by François Desmeules‘ Fond de recherche du Québec – Santé (FRQS) (grant number: 32647) research scholar junior 1 start-up fund and by the Ordre professionnel de la physiothérapie du Québec. The funding agencies have no role in the study design, writing the manuscript, or in the decision to submit for publication.
References
- 1.Lawrence R.C., Helmick C.G., Arnett F.C., Deyo R.A., Felson D.T., Giannini E.H., Heyse S.P., Hirsch R., Hochberg M.C., Hunder G.G., Liang M.H., Pillemer S.R., Steen V.D., Wolfe F. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheumatism. 1998;41(5):778–799. doi: 10.1002/1529-0131(199805)41:5<778::AID-ART4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 2.Canada T.A.A.O. 2011. The Impact of Arthritis in Canada: Today and over the Next 30 Years, Toronto. [Google Scholar]
- 3.Cross M., Smith E., Hoy D., Nolte S., Ackerman I., Fransen M., Bridgett L., Williams S., Guillemin F., Hill C.L., Laslett L.L., Jones G., Cicuttini F., Osborne R., Vos T., Buchbinder R., Woolf A., March L. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann. Rheumatic Dis. 2014;73(7):1323–1330. doi: 10.1136/annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- 4.Bhatia D., Bejarano T., Novo M. Current interventions in the management of knee osteoarthritis. J. Pharm. Bioallied Sci. 2013;5(1):30–38. doi: 10.4103/0975-7406.106561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manyanga T., Froese M., Zarychanski R., Abou-Setta A., Friesen C., Tennenhouse M., Shay B.L. Pain management with acupuncture in osteoarthritis: a systematic review and meta-analysis. BMC Complementary Altern. Med. 2014;14:312. doi: 10.1186/1472-6882-14-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Derrett S., Paul C., Morris J.M. Waiting for elective surgery: effects on health-related quality of life, International journal for quality in health care. J. Int. Soc. Qual. Health Care/ISQua. 1999;11(1):47–57. doi: 10.1093/intqhc/11.1.47. [DOI] [PubMed] [Google Scholar]
- 7.Barbay K. Research evidence for the use of preoperative exercise in patients preparing for total hip or total knee arthroplasty. Orthop. Nurs. 2009;28(3):127–133. doi: 10.1097/NOR.0b013e3181a46a09. [DOI] [PubMed] [Google Scholar]
- 8.Brown K., Topp R., Brosky J.A., Lajoie A.S. Prehabilitation and quality of life three months after total knee arthroplasty: a pilot study. Percept. Mot. Ski. 2012;115(3):765–774. doi: 10.2466/15.06.10.PMS.115.6.765-774. [DOI] [PubMed] [Google Scholar]
- 9.Nunez M., Nunez E., Segur J.M., Macule F., Quinto L., Hernandez M.V., Vilalta C. The effect of an educational program to improve health-related quality of life in patients with osteoarthritis on waiting list for total knee replacement: a randomized study. Osteoarthr. Cartil./OARS, Osteoarthr. Res. Soc. 2006;14(3):279–285. doi: 10.1016/j.joca.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Williamson L., Wyatt M.R., Yein K., Melton J.T. Severe knee osteoarthritis: a randomized controlled trial of acupuncture, physiotherapy (supervised exercise) and standard management for patients awaiting knee replacement. Rheumatology. 2007;46(9):1445–1449. doi: 10.1093/rheumatology/kem119. [DOI] [PubMed] [Google Scholar]
- 11.Ferrara P.E., Rabini A., Maggi L., Piazzini D.B., Logroscino G., Magliocchetti G., Amabile E., Tancredi G., Aulisa A.G., Padua L., Aprile I., Bertolini C. Effect of pre-operative physiotherapy in patients with end-stage osteoarthritis undergoing hip arthroplasty. Clin. Rehabil. 2008;22(10–11):977–986. doi: 10.1177/0269215508094714. [DOI] [PubMed] [Google Scholar]
- 12.Oosting E., Jans M.P., Dronkers J.J., Naber R.H., Dronkers-Landman C.M., Appelman-de Vries S.M., van Meeteren N.L. Preoperative home-based physical therapy versus usual care to improve functional health of frail older adults scheduled for elective total hip arthroplasty: a pilot randomized controlled trial. Arch. Phys. Med. Rehabil. 2012;93(4):610–616. doi: 10.1016/j.apmr.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Aoki O., Tsumura N., Kimura A., Okuyama S., Takikawa S., Hirata S. Home stretching exercise is effective for improving knee range of motion and gait in patients with knee osteoarthritis. J. Phys. Therapy Sci. 2009;21(2):113–119. [Google Scholar]
- 14.World Health Organisation . WHO; Geneva, Switzerland: 2014. World Health Statistics 2014: a Wealth of Information on Global Public Health. [Google Scholar]
- 15.Kairy D., Lehoux P., Vincent C., Visintin M. A systematic review of clinical outcomes, clinical process, healthcare utilization and costs associated with telerehabilitation. Disabil. Rehabilitation. 2009;31(6):427–447. doi: 10.1080/09638280802062553. [DOI] [PubMed] [Google Scholar]
- 16.Bedra M., Finkelstein J. Feasibility of post-acute hip fracture telerehabilitation in older adults. Stud. health Technol. Inf. 2015;210:469–473. [PubMed] [Google Scholar]
- 17.Pineau G., Moqadem K., St-Hilaire C., Levac E., Hamel B. ETMIS, Agence d’évaluation des technologies et des modes d’intervention en santé (AETMIS); Québec, Québec: 2006. Télésanté : lignes directrices cliniques et normes technologiques en téléréadaptation; pp. 1–74. [Google Scholar]
- 18.Tousignant M., Boissy P., Corriveau H., Moffet H. In home telerehabilitation for older adults after discharge from an acute hospital or rehabilitation unit: a proof-of-concept study and costs estimation, Disability and rehabilitation. Assist. Technol. 2006;1(4):209–216. doi: 10.1080/17483100600776965. [DOI] [PubMed] [Google Scholar]
- 19.Moffet H., Tousignant M., Nadeau S., Merette C., Boissy P., Corriveau H., Marquis F., Cabana F., Ranger P., Belzile E.L., Dimentberg R. In-home telerehabilitation compared with face-to-face rehabilitation after total knee arthroplasty: a noninferiority randomized controlled trial. J. Bone Jt. Surg. Am. 2015;97(14):1129–1141. doi: 10.2106/JBJS.N.01066. [DOI] [PubMed] [Google Scholar]
- 20.Piqueras M., Marco E., Coll M., Escalada F., Ballester A., Cinca C., Belmonte R., Muniesa J.M. Effectiveness of an interactive virtual telerehabilitation system in patients after total knee arthoplasty: a randomized controlled trial. J. Rehabilitation Med. 2013;45(4):392–396. doi: 10.2340/16501977-1119. [DOI] [PubMed] [Google Scholar]
- 21.Tousignant M., Moffet H., Nadeau S., Merette C., Boissy P., Corriveau H., Marquis F., Cabana F., Ranger P., Belzile E.L., Dimentberg R. Cost analysis of in-home telerehabilitation for post-knee arthroplasty. J. Med. Internet Res. 2015;17(3):e83. doi: 10.2196/jmir.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anton D., Goni A., Illarramendi A. Exercise recognition for Kinect-based telerehabilitation. Methods Inf. Med. 2015;54(2):145–155. doi: 10.3414/ME13-01-0109. [DOI] [PubMed] [Google Scholar]
- 23.Anton D., Nelson M., Russell T., Goni A., Illarramendi A. Validation of a Kinect-based telerehabilitation system with total hip replacement patients. J. Telemed. Telecare. 2016;22(3):192–197. doi: 10.1177/1357633X15590019. [DOI] [PubMed] [Google Scholar]
- 24.Rogante M., Grigioni M., Cordella D., Giacomozzi C. Ten years of telerehabilitation: a literature overview of technologies and clinical applications. NeuroRehabilitation. 2010;27(4):287–304. doi: 10.3233/NRE-2010-0612. [DOI] [PubMed] [Google Scholar]
- 25.Aaronson N., Acquadro C., Alonso J., Apolone G., Bucquet D., Bullinger M., Bungay K., Fukuhara S., Gandek B., Keller S. International quality of life assessment (IQOLA) project. Qual. Life Res. 1992;1(5):349–351. doi: 10.1007/BF00434949. [DOI] [PubMed] [Google Scholar]
- 26.Bassey E.J., Fentem P.H., MacDonald I.C., Scriven P.M. Self-paced walking as a method for exercise testing in elderly and young men. Clin. Sci. Mol. Med. 1976;51(6):609–612. doi: 10.1042/cs0510609. [DOI] [PubMed] [Google Scholar]
- 27.Cunningham D.A., Rechnitzer P.A., Pearce M.E., Donner A.P. Determinants of self-selected walking pace across ages 19 to 66. J. Gerontology. 1982;37(5):560–564. doi: 10.1093/geronj/37.5.560. [DOI] [PubMed] [Google Scholar]
- 28.Fiatarone M.A., Evans W.J. The etiology and reversibility of muscle dysfunction in the aged. J. Gerontology. 1993;48:77–83. doi: 10.1093/geronj/48.special_issue.77. Spec No. [DOI] [PubMed] [Google Scholar]
- 29.Langlois J.A., Keyl P.M., Guralnik J.M., Foley D.J., Marottoli R.A., Wallace R.B. Characteristics of older pedestrians who have difficulty crossing the street. Am. J. Public Health. 1997;87(3):393–397. doi: 10.2105/ajph.87.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kennedy D.M., Stratford P.W., Wessel J., Gollish J.D., Penney D. Assessing stability and change of four performance measures: a longitudinal study evaluating outcome following total hip and knee arthroplasty. BMC Musculoskelet. Disord. 2005;6:3. doi: 10.1186/1471-2474-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gill S.D., McBurney H. Does exercise reduce pain and improve physical function before hip or knee replacement surgery? A systematic review and meta-analysis of randomized controlled trials. Arch. Phys. Med. Rehabil. 2013;94(1):164–176. doi: 10.1016/j.apmr.2012.08.211. [DOI] [PubMed] [Google Scholar]
- 32.Binkley J.M., Stratford P.W., Lott S.A., Riddle D.L. The lower extremity functional scale (LEFS): scale development, measurement properties, and clinical application. North American orthopaedic rehabilitation research network. Phys. Ther. 1999;79(4):371–383. [PubMed] [Google Scholar]
- 33.Stratford P.W., Kennedy D.M., Hanna S.E. Condition-specific western Ontario McMaster osteoarthritis index was not superior to region-specific lower extremity functional scale at detecting change. J. Clin. Epidemiol. 2004;57(10):1025–1032. doi: 10.1016/j.jclinepi.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Stratford P.W., Kennedy D.M., Woodhouse L.J. Performance measures provide assessments of pain and function in people with advanced osteoarthritis of the hip or knee. Phys. Ther. 2006;86(11):1489–1496. doi: 10.2522/ptj.20060002. [DOI] [PubMed] [Google Scholar]
- 35.René F., Casimiro L., Tremblay M., Brosseau L., Lefebvre A., Beaudouin M., Belliveau V., Bergeron L.-P. Une version canadienne française du Lower Extremity Functional Scale (LEFS): L'Échelle fonctionnelle des membres inférieurs (ÉFMI), partie I. Physiother. Can. 2011;63(2):242–248. doi: 10.3138/ptc.2010-11F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Angst F., Aeschlimann A., Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheumatism. 2001;45(4):384–391. doi: 10.1002/1529-0131(200108)45:4<384::AID-ART352>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 37.Bourne R.B. Measuring tools for functional outcomes in total knee arthroplasty. Clin. Orthop. Relat. Res. 2008;466(11):2634–2638. doi: 10.1007/s11999-008-0468-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ethgen O., Bruyere O., Richy F., Dardennes C., Reginster J.Y. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J. Bone Jt. Surg. Am. 2004;86-A(5):963–974. doi: 10.2106/00004623-200405000-00012. [DOI] [PubMed] [Google Scholar]
- 39.McConnell S., Kolopack P., Davis A.M. The western Ontario and McMaster Universities osteoarthritis index (WOMAC): a review of its utility and measurement properties. Arthritis Rheumatism. 2001;45(5):453–461. doi: 10.1002/1529-0131(200110)45:5<453::aid-art365>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 40.Parent E., Moffet H. Comparative responsiveness of locomotor tests and questionnaires used to follow early recovery after total knee arthroplasty. Arch. Phys. Med. Rehabil. 2002;83(1):70–80. doi: 10.1053/apmr.2002.27337. [DOI] [PubMed] [Google Scholar]
- 41.Bellamy N. WOMAC: a 20-year experimental review of a patient-centered self-reported health status questionnaire. J. Rheumatology. 2002;29(12):2473–2476. [PubMed] [Google Scholar]
- 42.Tubach F., Ravaud P., Baron G., Falissard B., Logeart I., Bellamy N., Bombardier C., Felson D., Hochberg M., van der Heijde D. Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: the minimal clinically important improvement. Ann. Rheumatic Dis. 2005;64(1):29–33. doi: 10.1136/ard.2004.022905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hashimoto H., Hanyu T., Sledge C.B., Lingard E.A. Validation of a Japanese patient-derived outcome scale for assessing total knee arthroplasty: comparison with Western Ontario and McMaster Universities osteoarthritis index (WOMAC) J. Orthop. Sci. 2003;8(3):288–293. doi: 10.1007/s10776-002-0629-0. [DOI] [PubMed] [Google Scholar]
- 44.Kirschner S., Walther M., Böhm D., Matzer M., Heesen T., Faller H., König A. German short musculoskeletal function assessment questionnaire (SMFA-D): comparison with the SF-36 and WOMAC in a prospective evaluation in patients with primary osteoarthritis undergoing total knee arthroplasty. Rheumatol. Int. 2003;23(1):15–20. doi: 10.1007/s00296-002-0253-4. [DOI] [PubMed] [Google Scholar]
- 45.Fortin P.R., Penrod J.R., Clarke A.E., St-Pierre Y., Joseph L., Belisle P., Liang M.H., Ferland D., Phillips C.B., Mahomed N., Tanzer M., Sledge C., Fossel A.H., Katz J.N. Timing of total joint replacement affects clinical outcomes among patients with osteoarthritis of the hip or knee. Arthritis Rheumatism. 2002;46(12):3327–3330. doi: 10.1002/art.10631. [DOI] [PubMed] [Google Scholar]
- 46.Fortin P.R., Clarke A.E., Joseph L., Liang M.H., Tanzer M., Ferland D., Phillips C., Partridge A.J., Belisle P., Fossel A.H., Mahomed N., Sledge C.B., Katz J.N. Outcomes of total hip and knee replacement: preoperative functional status predicts outcomes at six months after surgery. Arthritis Rheum. 1999;42(8):1722–1728. doi: 10.1002/1529-0131(199908)42:8<1722::AID-ANR22>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 47.Jones C.A., Voaklander D.C., Suarez-Alma M.E. Determinants of function after total knee arthroplasty. Phys. Ther. 2003;83(8):696–706. [PubMed] [Google Scholar]
- 48.Davis A.M., Badley E.M., Beaton D.E., Kopec J., Wright J.G., Young N.L., Williams J.I. Rasch analysis of the western Ontario McMaster (WOMAC) osteoarthritis index: results from community and arthroplasty samples. J. Clin. Epidemiol. 2003;56(11):1076–1083. doi: 10.1016/s0895-4356(03)00179-3. [DOI] [PubMed] [Google Scholar]
- 49.Kosinski M., Keller S.D., Ware J.E., Jr., Hatoum H.T., Kong S.X. The SF-36 Health Survey as a generic outcome measure in clinical trials of patients with osteoarthritis and rheumatoid arthritis: relative validity of scales in relation to clinical measures of arthritis severity. Med. Care. 1999;37(5):MS23–MS39. doi: 10.1097/00005650-199905001-00003. [DOI] [PubMed] [Google Scholar]
- 50.Bachmeier C.J., March L.M., Cross M.J., Lapsley H.M., Tribe K.L., Courtenay B.G., Brooks P.M. A comparison of outcomes in osteoarthritis patients undergoing total hip and knee replacement surgery. Osteoarthr. Cartil. 2001;9(2):137–146. doi: 10.1053/joca.2000.0369. [DOI] [PubMed] [Google Scholar]
- 51.Bombardier C., Melfi C.A., Paul J., Green R., Hawker G., Wright J., Coyte P. Comparison of a generic and a disease-specific measure of pain and physical function after knee replacement surgery. Med. Care. 1995;33(4 Suppl) AS131–44. [PubMed] [Google Scholar]
- 52.Jones C.A., Voaklander D.C., Johnston D.W.C., Suarez-Almazor M.E. Health related quality of life outcomes after total hip and knee arthroplasties in a community based population. J. Rheumatology. 2000;27(7):1745–1752. [PubMed] [Google Scholar]
- 53.Jones C.A., Voaklander D.C., Johnston D.W.C., Suarez-Almazor M.E. The effect of age on pain, function, and quality of life after total hip and knee arthroplasty. Archives Intern. Med. 2001;161:454–460. doi: 10.1001/archinte.161.3.454. [DOI] [PubMed] [Google Scholar]
- 54.Keller S.D., Majkut T.C., Kosinski M., Ware J.E., Jr. Monitoring health outcomes among patients with arthritis using the SF-36 Health Survey: overview. Med. Care. 1999;37(5 Suppl):MS1–9. doi: 10.1097/00005650-199905001-00001. [DOI] [PubMed] [Google Scholar]
- 55.March L.M., Cross M.J., Lapsley H., Brnabic A.J., Tribe K.L., Bachmeier C.J., Courtenay B.G., Brooks P.M. Outcomes after hip or knee replacement surgery for osteoarthritis. A prospective cohort study comparing patients' quality of life before and after surgery with age-related population norms. Med. J. Aust. 1999;171(5):235–238. [PubMed] [Google Scholar]
- 56.Ware J.E., Jr., Sherbourne C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 57.Williams J.I., Llewellyn-Thomas H.A., Arshinoff R., Young N., Naylor C.D. The burden of waiting for hip and knee replacements in Ontario. J. Eval. Clin. Pract. 1997;3(1):59–68. doi: 10.1111/j.1365-2753.1997.tb00068.x. [DOI] [PubMed] [Google Scholar]
- 58.Brazier J.E., Harper R., Munro J., Walters S.J., Snaith M.L. Generic and Condition-specific Outcome Measures for People with Osteoarthritis of the Knee. Rheumatology. 1999;38(9):870–877. doi: 10.1093/rheumatology/38.9.870. (Oxford) [DOI] [PubMed] [Google Scholar]
- 59.Lingard E.A., Katz J.N., Wright E.A., Sledge C.B. Predicting the outcome of total knee arthroplasty. J. Bone Jt. Surg. Am. 2004;86-A(10):2179–2186. doi: 10.2106/00004623-200410000-00008. [DOI] [PubMed] [Google Scholar]
- 60.Hopman W.M., Towheed T., Anastassiades T., Tenenhouse A., Poliquin S., Berger C., Joseph L., Brown J.P., Murray T.M., Adachi J.D. Canadian normative data for the SF-36 health survey. Can. Med. Assoc. J. 2000;163(3):265–271. [PMC free article] [PubMed] [Google Scholar]
- 61.Scopaz K.A., Piva S.R., Wisniewski S., Fitzgerald G.K. Relationships of fear, anxiety, and depression with physical function in patients with knee osteoarthritis. Arch. Phys. Med. Rehabil. 2009;90(11):1866–1873. doi: 10.1016/j.apmr.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kamper S.J., Maher C.G., Mackay G. Global rating of change scales: a review of strengths and weaknesses and considerations for design. J. Man. Manip. Ther. 2009;17(3):163–170. doi: 10.1179/jmt.2009.17.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Podsiadlo D., Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J. Am. Geriatrics Soc. 1991;39(2):142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 64.Zhang W., Moskowitz R., Nuki G., Abramson S., Altman R., Arden N., Bierma-Zeinstra S., Brandt K., Croft P., Doherty M. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthr. Cartil. 2008;16(2):137–162. doi: 10.1016/j.joca.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 65.Desmeules F., Hall J., Woodhouse L.J. Prehabilitation improves physical function of individuals with severe disability from hip or knee osteoarthritis, Physiotherapy Canada. Physiother. Can. 2013;65(2):116–124. doi: 10.3138/ptc.2011-60. [DOI] [PMC free article] [PubMed] [Google Scholar]