Abstract
Purpose
Currently, treatment-emergent adverse events (TEAEs) during a clinical study are summarized over the entire study period.
Objective
Develop and validate a novel methodology, BURDEN OF THERAPY©∗ (BOTh©∗), to quantify presence and severity of TEAEs on each day of study.
Methods
BOTh utilizes patient-level safety data to derive a quantitative estimate for the burden of TEAEs that all or individual patients experience on each day of a clinical study. Burden estimate for each day is based on number and severity of TEAEs. A chart displays the total burden experienced by patients on each day throughout the study and statistical analyses may be performed with the area under curve. Methodology was applied to two validated and published clinical studies and statistically analyzed.
Results
In a peripheral neuropathic pain study, the topical group had a greater incidence of TEAEs than the oral anticonvulsant group when evaluated using current methodology. Utilizing BOTh, TEAEs with the topical agent were of short duration and occurred for three days after application, whereas TEAEs with the oral agent increased during dose titration and persisted to study end. In an overactive bladder study there was a minimal difference in overall TEAEs between groups, but BOTh revealed a higher burden related to dry mouth in the antimuscarinic versus β3 adrenergic agonist group.
Conclusions
BOTh is a highly sensitive method to evaluate the comparative burden experienced by patients during treatment, and can facilitate better informed treatment selection. We propose BOTh as the new standard for analyzing safety during clinical studies.
Keywords: Burden, Methodology, Quantify, Safety, Severity, Treatment-emergent adverse events
1. Introduction
Clinical studies offer a unique opportunity to assess the frequency and severity of treatment-emergent adverse events (TEAEs) in a controlled and objective setting. The safety and efficacy data of drugs investigated during clinical studies is one of the most important pieces of information available to physicians and patients to determine the choice of treatment. From the perspective of the patient, survey data suggests that over 75% of outpatients desired to be told of every single possible TEAE associated with their medication [1]. Assessing the tolerability of medications is important from the perspective of healthcare organizations, as it may have a negative impact on the level of compliance with treatment, cost of healthcare, as well as patient quality of life [2], [3]. Although it is important to present a detailed analysis of the tolerability of any medication, detailed safety discussion may be frequently under-represented in published literature and the quality and quantity of safety reporting across a range of studies has been found to be inadequate [4].
A substantial amount of detailed information on the incidence and presence of TEAEs is recorded during the course of any clinical study. TEAEs are classified into various categories to include common, serious, unexpected, of special interest and drug-related effects, as well as severity grades such as mild, moderate, severe, life threatening or mortality [5], [6]. Although a wealth of information is available in a clinical study database, the focus when reporting safety is on the overall incidence of TEAEs (an absolute number) during a study. One major drawback to this is that patients who experience more than one TEAE and their severity on each day of a study, either contemporaneously or in a consecutive manner, are not identified or considered using current methods.
Significant improvements can be made in the reporting of safety information during clinical studies and to further utilize collected data. However, analyzing TEAEs from the patient perspective and taking into account all tolerability issues experienced by that patient on each day of the study, may more accurately represent the safety burden experienced by patients. In this manuscript, a method to report the safety burden experienced by patients on each day of a clinical study is described. Using data from validated clinical study databases, graphical and quantitative statistical tools were integrated in this novel method to analyze patient-level safety data. The utility of modifying this methodology to incorporate useful efficacy endpoints is also outlined. This method of reporting safety from a patient perspective is described as “BURDEN OF THERAPY© (BOTh©).”
2. Methods
2.1. Clinical trial databases
Data from two validated and published non-inferiority clinical studies were used to investigate the utility of the BURDEN OF THERAPY methodology: peripheral neuropathic pain database (ClinicalTrials.gov Identifier: NCT01713426) with a study of topical vanilloid receptor subtype 1 (TRPV1) agonist and an oral anticonvulsant agent; overactive bladder study database (NCT01638000) with a β3 adrenergic receptor agonist and an antimuscarinic agent [7], [8]. General names for each class of drug treatment are used as the objective of this manuscript was to primarily investigate the utility of the BURDEN OF THERAPY methodology.
2.2. Statistical analysis
The mathematical rules applied to derive the BURDEN OF THERAPY graph and an example calculation are described below.
Assume a subject i has k adverse events in a study, and subject i was in the study for n days. For this study, h weight elements are identified and it is known on which days adverse events (AEs) are present and on which days they are not. The burden on each day for an individual subject is burdenij:; AEvij = 1 if an AE is present for subject i on day j; AEvij = 0 if an AE is not present for subject i on day j and Weight_elementuvij = 1 if no additional weight should be added for AE number v of subject i on day j; Weight elementuvij>1 if extra weight should be added for AE number v of subject i on day j.
Burdenij is the burden of subject i on day j, based on the k adverse events that this subject experienced. Weightvij is the weight of the vth AE that subject i experienced on day j. Weightvij is the product of the different weight elements (weight_elementuvij), such as severity, drug discontinuation and seriousness. h in the equation is the number of weight_element factors taken into account. So weight_elementuvij is the uth weight element of weight v for subject i on day j.
Each bar in the BURDEN OF THERAPY graph represents the total burden of all subjects in the study divided by the subjects at risk, and can be derived by the sum of the burden of each individual subject divided by the subjects in the study on day j (nj). The graph is generated with each bar with length barj, with the order as day number.
The corrected area under the curve (AUC) for each individual subject can also be calculated. The corrected AUC represents the total burden that an individual subject experienced during the trial and is the sum of the burden, for all burdens of an individual subject. The AUC of subject i is, where ti is the total number of study days of subject i. The AUC can be used in an ANCOVA as a response variable such that the impact of different covariates and factors on the AUC can be investigated. Possible factors of interest in a clinical study may include prior treatment, medication history, age, gender or body mass index.
2.3. Example BURDEN OF THERAPY analysis
For example, take a five day study wherein a subject had k = 2 adverse events.
Adverse event 1 (a mild headache) was present on day 2 and day 3 of the study. Adverse event 2 (severe and ongoing dizziness) was present from day 1 to day 5. On day 3 and day 4 the study treatment was discontinued because of the dizziness and on day 5 the subject started using the study treatment again. The weight_elements in this example are severity (mild = 1, moderate = 2 and severe = 3) and study drug discontinuation (1 = not discontinued, 2 = temporary discontinued and 3 = permanent discontinued). As it is known in this study on which days the study drug was discontinued, it is decided that the weighting for discontinuation is only done on the days that the study drug is discontinued else the weighting = 1.
For day one, only the severe dizziness was present but the drug was not discontinued yet. The weight factor for day one for dizziness is = severity * discontinuation = 3 × 1 = 3.
The weight factor for day 1 for headache is severity * discontinuation = 1 × 1 = 1. And the burden on day one for this subject is = weight headache*presence indicator + weight dizziness*presence indicator = 1 × 0 + 3 × 1 = 3.
For day 2 the mild headache (weight = 1), started and the severe dizziness was still present but the drug was still not discontinued so the burden for day 2 = weight headache*presence indicator + weight dizziness*presence indicator = 1 × 1 + 3 × 1 = 4.
For day 3 the mild headache was still present and the drug was discontinued because of the dizziness, weight element for discontinuation increased to 2 this gives the burden for day 3 = weight headache*presence indicator + weight dizziness*presence indicator = 1 × 1 + 3 × 2 = 7.
Similarly, the burden for day 4 = weight headache*presence indicator + weight dizziness*presence indicator = 1 × 0 + 3 × 2 = 6; and for day 5 = weight headache*presence indicator + weight dizziness*presence indicator = 1 × 0 + 3 × 1 = 3.
The total burden is the sum of the burdens per day = 3 + 3+7 + 6+3 = 22. Dividing 22 by the number of days this subject was in the study gives the corrected AUC = 22/5 = 4.4.
2.4. Data analysis
The application of the BURDEN OF THERAPY methodology involves the use of a clinical study database with individual data entries for TEAEs per patient per day. The presence of TEAEs may be plotted against study day, such that if a patient reported a TEAE from Days 3–5, this TEAE was considered present on Days 3, 4 and 5. If that patient reported TEAEs of differing severity on a single day, the worst severity was taken into account in the analysis. Also, if an end date was not present in the database, or a TEAE was ongoing, the last study day of the patient was taken as the end day of the TEAE. The subsequent graph that is produced displays the number of patients with a TEAE on each study day. The addition of coloring that corresponds with TEAE severity allows easy discrimination of TEAEs of differing severity in the graph.
In order to visually represent the safety burden experienced by a patient, varied weighting was applied to TEAE severity in each arm in a consistent manner. The graph therefore displays the weighted TEAEs per day. For the purposes of example graphs presented in this manuscript, TEAEs that were recorded as mild, moderate, and severe were weighted to represent 1x, 2x, and 3x TEAEs per event per day, respectively.
By summing up the burden of each day for each patient, with or without the weighting of events, one can derive an Area Under Curve (AUC) per patient. This will give a burden estimate for each patient (see above). Using, for example, an ANCOVA model with the burden estimate as dependent variable and treatment as independent variable, one can estimate the treatment effect of the BOTh. By adding baseline characteristics as covariates, that might have influenced the BOTh and the treatment difference of the BOTh, one can adjust the treatment difference estimates for these covariates.
In addition to the presentation of TEAEs, the BURDEN OF THERAPY graph can also be integrated with an efficacy analysis. By displaying both the efficacy outcome and weighted TEAEs over time in one graph, it is possible to directly compare the effectiveness of treatments and their burden on the patient in a single analysis. Any efficacy endpoint that has been recorded by time may be used for this type of analysis. Such integrated efficacy and safety graphs visually describe the tolerability burden and efficacy benefit of treatment from the patient point of view. An example of an integrated graph is included in this manuscript.
2.5. BURDEN OF THERAPY graph design
The BURDEN OF THERAPY graph is produced using the SAS® program. The suggested format for the graph is a mirrored display bar chart with study day on the y-axis and number of TEAEs on the x-axis. This design allows convenient comparison of the adverse event profile per day of each treatment along the horizontal x-axis. Alternatively, study day can also be presented on the x-axis and TEAE profiles can be compared along the vertical y-axis. The addition of an efficacy outcome in the graph may be in a line graph format and in a different color to that used for TEAEs. An alternative format for the graph is to overlay two treatments on top of each other. This can be done to compare two treatments that have a similar safety profile and in conjunction with a comparison of the mean burden per day. In addition, the difference in TEAEs per day between treatments can also be plotted.
3. Results
3.1. Peripheral neuropathic pain study
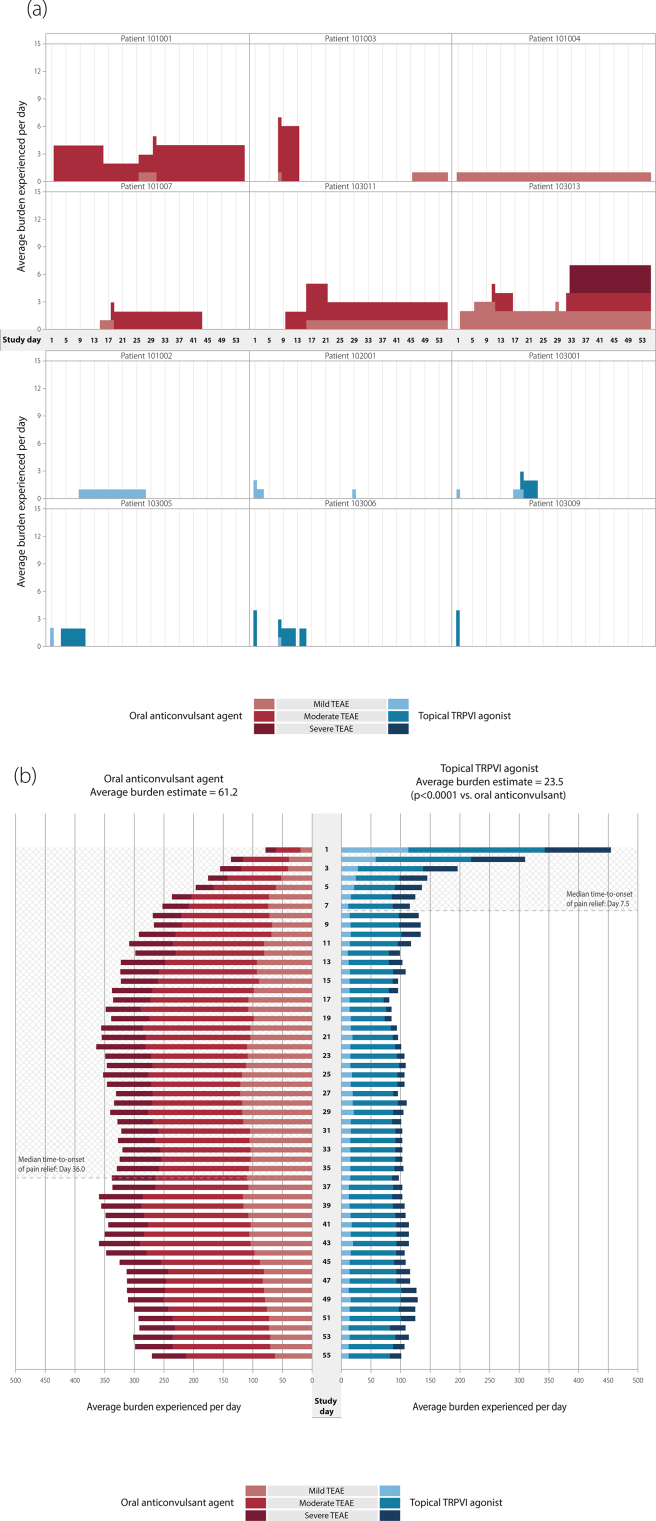
In this study, the total number of patients with TEAEs was 210 (74.5%) in the topical TRPV1 agonist arm and 177 (63.9%) in the oral anticonvulsant agent arm [7]. A BURDEN OF THERAPY analysis can be performed on an individual patient level and such an analysis during this study showed that the presence of TEAEs was different in the two treatment arms. A greater number of TEAEs were experienced for a greater number of days with the oral anticonvulsant versus the topical TRPV1 agonist (Fig. 1a).
Fig. 1.
a. BURDEN OF THERAPY assessment of individual patients in a peripheral neuropathic pain study. b. BURDEN OF THERAPY in a peripheral neuropathic pain study. TEAE, treatment-emergent adverse event; TRPV1, topical vanilloid receptor subtype 1.
The overall graph of TEAEs shows that there was a noticeable difference in the presence of events in the two groups as the study progressed. In the topical TRPV1 agonist group there was an initial peak followed by a rapid decline in TEAEs per day (Fig. 1b), as they were primarily transient application site reactions [7]. From Days 1–4, the proportion of patients reporting a TEAE declined (Day 1, 58%; Day 2, 39%; Day 3, 23%; Day 4, 17%). Less than 15% of the topical group reported TEAEs for the remainder of the study. In the oral anticonvulsant group there was a gradual increase and relatively consistent burden and then minor decrease of burden (Fig. 1b). Patients reporting TEAEs rose from 11% at Day 1–39% at Day 22, during the period of dose-titration of the oral agent. For the remainder of the study up to Day 55, TEAEs were reported by 20–39% of the oral group. The overall burden estimate was 61.2 for the oral anticonvulsant and 23.5 for the topical TRPV1 agonist (p < 0.0001 for between-group comparison; ANOVA model with treatment and sex as categorical variables, and sex by treatment as interaction). The BURDEN OF THERAPY graph highlighted the contrasting severity of TEAEs in the oral versus topical groups. There were more weighted moderate and severe TEAEs in the oral compared with topical group from Days 5–55. TEAE weighting in both arms allowed this to be more easily visualized as the representation of all moderate or severe TEAEs increased in both arms.
The integration of safety and efficacy into the BURDEN OF THERAPY graph allowed the safety burden to be directly compared with the median time to treatment response in both arms during the clinical study. In this study, median time to treatment response was compared, which was a secondary efficacy endpoint defined as the first of three consecutive days in which the patient reported a ≥30% reduction in average daily pain score [7]. With the topical TRPV1 agonist, patients rapidly achieved this median treatment response (Day 7.5), and this overlapped with the period that transient application site reactions were observed and then stabilized to a low background level (reported by <5%). With the oral anticonvulsant agent, the median treatment response was more gradual (Day 36.0) and this period coincided with dose-titration and a gradual increase in reporting of drug-related TEAEs to a moderate-to-high background level (reported by 20–39%) (Fig. 1b).
3.2. Overactive bladder study
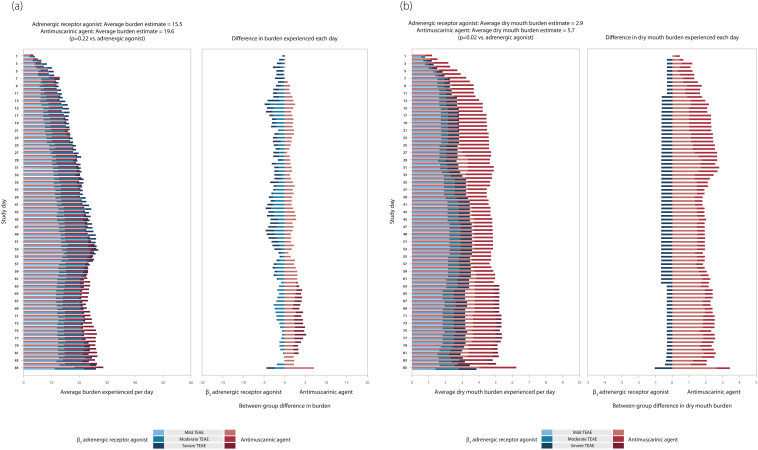
In the overactive bladder study, the total number of patients with TEAEs was 274 (29.3%) in the β3 adrenergic receptor agonist group and 282 (30.2%) in the antimuscarinic group [8]. Using the BURDEN OF THERAPY methodology, the presence of weighted TEAEs per day during the study was observed as similar in both groups and differences between the groups were not immediately noticeable in the chart (Fig. 2a). The overall burden estimate (prior antimuscarinic use as a covariate) was 19.6 for the antimuscarinic agent and 15.5 for the adrenergic receptor agonist (p = 0.22 for between-group comparison). In order to help visualize the difference between treatment groups, the percentage of patients with a TEAE per day in each group was overlaid in a single plot and the difference was also plotted (Fig. 2a). Using this variation of the BURDEN OF THERAPY methodology, it was possible to identify that during the study there was a small increase in the proportion of patients with TEAEs in the antimuscarinic group, which gradually increased from 0.1% at Day 10 to 5.0% by study end at Day 85.
Fig. 2.
a. BURDEN OF THERAPY in an overactive bladder study. b. Burden of dry mouth in an overactive bladder study. TEAE, treatment-emergent adverse event.
The antimuscarinic class are known for specific side effects such as dry mouth, constipation and headache [9]. In this study, dry mouth was reported by 29 patients (3.1%) in the adrenergic agonist group and 54 patients (5.8%) in the antimuscarinic group [8]. An analysis of patients who reported dry mouth was performed to investigate whether there was a difference between arms and approximately 1.5–2.0% of patients reported dry mouth in the antimuscarinic group from Days 17–85 (Fig. 2b). The burden estimate (prior antimuscarinic use as a covariate) from dry mouth was 5.7 with the antimuscarinic agent and 2.9 for the adrenergic receptor agonist (p = 0.02 for between-group comparison). There was a statistically significant difference between treatment groups and it was more likely that patients reported dry mouth in the antimuscarinic group than in the β3 adrenergic receptor agonist group.
4. Discussion
The BURDEN OF THERAPY methodology is a novel tool to report the presence and severity of TEAEs on each day of any clinical study, providing a visual representation of the comparative burden of any medication on the patient. It also permits statistical comparisons between treatment arms, safety analyses in individual patients or subgroups of interest and the combined presentation of tolerability and efficacy. The methodology is highly sensitive and permits a number of more detailed and clinically relevant analyses than current safety reporting, which is frequently an incomplete summary over an entire study and does not assess patients who experience more than one TEAE, or differences in the frequency or severity of TEAEs on each day during a study. Therefore, the methodology is a valuable new tool for the quantification, presentation and comparison of TEAEs during a clinical study, as well as for the assessment of medication safety.
The BURDEN OF THERAPY methodology was applied to two different validated clinical study databases and several new safety conclusions were made and quantitatively analyzed in greater detail. With the peripheral neuropathic pain study, the methodology allowed the observation that the burden estimate was significantly higher with the oral anticonvulsant compared with the topical TRPV1 agonist. In particular, patients experienced TEAEs in the topical group immediately after treatment was applied, compared to the anticonvulsant group as the agent was titrated and at its maintenance dose. By combining safety with efficacy into the BURDEN OF THERAPY graph, it was possible to track the tolerability burden and efficacy benefit of treatment during the peripheral neuropathic pain study. This analysis showed that the topical agent was associated with a rapid treatment response that coincided with transient application site reactions immediately after application. However, with the anticonvulsant agent, TEAEs gradually increased during dose titration and persisted until study end, and the efficacy benefit was delayed until patients received their optimal dose. In a variation of the methodology with the overactive bladder study, it was found that there was a significantly higher burden associated with dry mouth with the antimuscarinic agent versus β3 adrenergic agonist, which was not apparent with traditional reporting methods. Such a formal statistical analysis shows that by deriving an AUC with the BURDEN OF THERAPY methodology, the profile of any TEAE can be compared, and the influence of any exploratory variable can also be assessed.
A simple scale of TEAE weighting (1 for mild, 2 for moderate and 3 for severe) was applied in the BURDEN OF THERAPY methodology in order to more accurately reflect the clinical impact and burden of TEAEs on a patient during a study. In clinical practice, TEAEs of different severities can have a very different level of impact on a patient. By not applying weighting criteria, a weighting of one is assumed for all TEAEs and it is also assumed that all TEAEs have an equal impact or burden on the patient. In theory, additional weighting could be used in order to reflect the seriousness in addition to the severity of TEAEs. Also, a weighting method could in the future be standardized for a particular development program, therapeutic area, or patient population, or particular diseases. Despite this, the relative advantages and disadvantages of the weighting scale of TEAEs used in this paper may be a point of consideration when this methodology is utilized and other means or criteria of weighting TEAEs may be considered.
The BURDEN OF THERAPY can also be utilized in a personalized manner as the datasets for individual patients of interest, specific patient populations, TEAEs that affect specific body systems, or within specific timelines of a study, can all be analyzed individually using the methodology. Therefore, the BURDEN OF THERAPY methodology may help to achieve personalized treatment strategies that may be well tolerated in specific patient subgroups. In addition, this methodology can be utilized to assess disease progression, especially when “treatment failure” outcome measures are used, in order to provide valuable insight into the TEAEs experienced by patients, as well as the toxicity related to treatments such as chemotherapy that may continue after patients had reached such endpoints.
The BURDEN OF THERAPY as described in this manuscript uses patient level data as collected in clinical studies and with no assumptions regarding pre- or post-study medication burden were made. However, in the event that one compared a drug that is more toxic and is associated with more drug-related discontinuations than its comparator, this method may need adjustment. Possible solutions may include the simulation of any post-dropout period based on the pre-dropout period, and the analysis of tolerability findings of patients who completed the study.
5. Conclusion
BURDEN OF THERAPY analyses the daily presence and severity of TEAEs experienced by patients during a clinical study. The methodology clearly provides a more accurate reflection of the burden of treatment from a patient perspective than current safety reporting. It is a highly sensitive and versatile tool and may also be applied in a personalized manner to the datasets of individual patients of interest, on TEAEs that affect specific body systems, or during specific treatment periods. By integrating efficacy and safety into the analysis, it is possible to present the risk and benefit of a therapeutic agent throughout a clinical study. BURDEN OF THERAPY takes advantage of the wealth of information that is gathered during a clinical study and does not require additional data collection. The visual representation of BURDEN OF THERAPY aids in better informed treatment selection by clinicians and patients. We therefore propose this novel BURDEN OF THERAPY methodology to be considered as a new standard for analyzing safety during clinical studies.
Role of the funding source
The two clinical studies that were analyzed in this paper (NCT01713426 and NCT01638000) were designed, initiated, funded and conducted by Astellas Pharma Europe Ltd [7], [8].
Acknowledgments
The two clinical studies that were analyzed in this paper (ClinicalTrials.gov Identifier: NCT01713426 and NCT01638000) were sponsored by Astellas Pharma Europe. Faysal K. Riaz and Andreas J. Karas are employed by Astellas Pharma Europe Ltd. Robert J. Snijder is employed by Astellas Pharma Europe B.V. Ayad K. Abdulahad and Moeen K. Panni were employed by Astellas Pharma Europe Ltd. at time of study and are now employed by BOTh Analytics GmbH and MSD respectively.
Ayad K. Abdulahad developed the concept of the BURDEN OF THERAPY methodology and Robert J. Snijder developed the statistical and mathematical rules, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. In addition, all authors were involved in planning the manuscript, data interpretation, drafting of the manuscript, approved the final version of the manuscript, and take responsibility for the integrity of the data.
Footnotes
©∗ Intellectual property and Copyrights, BOTh Analytics GmbH, 2016.
Contributor Information
Ayad K. Abdulahad, Email: ayad.abdulahad@BOTh-analytics.com.
Robert J. Snijder, Email: robert.snijder@astellas.com.
Moeen K. Panni, Email: moeen.panni@merck.com.
Faysal K. Riaz, Email: faysal.riaz@astellas.com.
Andreas J. Karas, Email: andreas.karas@astellas.com.
References
- 1.Ziegler D.K., Mosier M.C., Buenaver M., Okuyemi K. How much information about adverse effects of medications do patients want from physicians? Arch. Intern. Med. 2001;16:706–713. doi: 10.1001/archinte.161.5.706. [DOI] [PubMed] [Google Scholar]
- 2.Puschner B., Angermeyer M.C., Leese M.M. Course of adherence to medication and quality of life in people with schizophrenia. Psychiatry Res. 2009;165:224–233. doi: 10.1016/j.psychres.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 3.Brown M.T., Bussell J.K. Medication adherence: WHO cares? Mayo. Clin. Proc. 2011;86:304–314. doi: 10.4065/mcp.2010.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ioannidis J., Lau J. Completeness of safety reporting in randomized trials: an evaluation of 7 medical areas. JAMA. 2001;285:437–443. doi: 10.1001/jama.285.4.437. [DOI] [PubMed] [Google Scholar]
- 5.European Medicines Agency . June 2012. Module VI – Management and Reporting of Adverse Reactions to Medicinal Products, European Medicines Agency.http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500129135.pdf EMA/873138/2011. (Accessed 04.April.2016) [Google Scholar]
- 6.Food and Drug Administration . December 2012. Guidance for Industry and Investigators - Safety Reporting Requirements for INDs and BA/BE Studies.http://www.fda.gov/downloads/Drugs/.../Guidances/UCM227351.pdf (Accessed 04.April.2016) [Google Scholar]
- 7.Haanpää M., Crucco G., Nurmikko T.J. Capsaicin 8% patch versus oral pregabalin in patients with peripheral neuropathic pain. Eur. J. Pain. 2016;20:316–328. doi: 10.1002/ejp.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Batista J.E., Kölbl H., Herschorn S. The efficacy and safety of mirabegron compared with solifenacin in overactive bladder patients dissatisfied with previous antimuscarinic treatment due to lack of efficacy: results of a non-inferiority, randomized, phase IIIb trial. Ther. Adv. Urol. 2015;7:167–179. doi: 10.1177/1756287215589250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sacco E., Bientinesi R. Mirabegron: a review of recent data and its prospects in the management of overactive bladder. Ther. Adv. Urol. 2012;4:315–324. doi: 10.1177/1756287212457114. [DOI] [PMC free article] [PubMed] [Google Scholar]