Abstract
Background
Cardiovascular (CV) disease due to atherosclerosis is a major cause of morbidity and mortality in adult patients with diabetes, either type 1 or type 2 diabetes. The aim of the study was to assess the association of the frequency and the burden of subclinical carotid atherosclerotic disease in patients with type 1 diabetes according to the presence and severity of diabetic retinopathy (DR).
Methods
A cross-sectional study was conducted in 340 patients with type 1 diabetes (41.5% with DR), and in 304 non-diabetic individuals. All participants were free from previous CV disease and chronic kidney disease (CKD). B-mode carotid ultrasound imaging was performed in all the study subjects. Patients with type 1 diabetes underwent a full eye examination, and DR patients were divided into two groups: mild disease and advanced disease.
Results
In the group of patients with type 1 diabetes, the percentage of patients with carotid plaques was higher in those with DR compared with those without DR (44.7% vs. 24.1%, p < 0.001). Patients with DR also presented a higher incidence of ≥ 2 carotid plaques (25.5% vs. 11.1%, p < 0.001). Apart from other traditional cardiovascular risk factors, the presence of advanced stages of DR was independently associated with the presence (p = 0.044) and the burden (≥ 2 carotid plaques; p = 0.009) of subclinical carotid atherosclerosis.
Conclusions
In patients with type 1 diabetes without previous CV disease or established CKD, the presence of advanced stages of DR is associated with a higher atherosclerotic burden in the carotid arteries. The presence of DR identifies patients at risk for carotid atherosclerotic disease.
Electronic supplementary material
The online version of this article (10.1186/s12933-018-0706-z) contains supplementary material, which is available to authorized users.
Keywords: Type 1 diabetes, Retinopathy, Subclinical carotid atherosclerosis
Background
Cardiovascular (CV) disease due to atherosclerosis is a major cause of morbidity and mortality in adult patients with diabetes, either type 1 or type 2 diabetes. Among the risk factors associated with the excess of cardiovascular risk in patients with type 1 diabetes are age, proteinuria, decreased glomerular filtration rate, hypertension, obesity, poor glycemic control, lipid levels, smoking and diabetes duration [1]. Even in patients with optimal glycemic control, the risk of death from CV causes is still more than twice that of non-diabetic individuals [2]. Unlike patients with type 2 diabetes, those with type 1 diabetes generally do not have excess rates of obesity, hypertension, or hypercholesterolemia compared with the general population [3]. Thus, the underlying mechanisms responsible for the increased risk of death from any cause and of death from CV causes among patients with type 1 diabetes who have good glycemic control are not fully understood. In recent years, a current line of thought has been that there is a “common soil” for the development of micro- and macrovascular complications of diabetes associated with common pathogenic pathways in both types of complications [4]. Diabetic retinopathy (DR) is a common and specific microvascular complication of diabetes that is identified in one-third of people with type 1 diabetes [5]. Glycemic control is the main factor involved in appearance and progression of DR in these patients. Interestingly, the presence of morphological abnormalities in retinal microvasculature is even observed in childhood type 1 diabetic patients with short-term poor glycemic control [6].
It has been shown in several studies that DR is an independent predictor of CV events and all-cause mortality in both patients with type 1 and type 2 diabetes [7]. Multiple potential mechanisms have been suggested to be involved in the pathophysiological link between diabetic micro- and macroangiopathy. These include oxidative and glycemic stress, chronic low-grade inflammation and impaired vascular tissue repair mechanisms [4]. More recently, diabetic microangiopathy of the artery wall microvessels, named vasa vasorum, has also been reported in patients with type 2 diabetes [8]. It is interesting to note that the main stimuli that drive diabetic microangiopathy angiogenesis are hypoxia and ischemia similar to what has been described in the retinal vascular changes that occur in DR [5]. Interestingly, the severity of retinopathy has been shown to be associated with the risk of incidental CV events in patients with type 2 diabetes in the action to control cardiovascular risk in diabetes (ACCORD) eye study, as this risk increased from 1.5 in people with mild nonproliferative retinopathy to 2.4 in people with severe retinopathy [9]. In patients with type 1 diabetes asymptomatic for coronary artery disease, severe forms of DR have been reported to be associated with the presence of coronary artery calcification [10], and also with increased carotid intima-media thickness [11].
On the other hand, established chronic kidney disease (CKD) is one of the main drivers of increased CV morbidity and mortality in patients with type 1 diabetes. Specifically, in patients with type 1 diabetes, the presence of albuminuric [12] and nonalbuminuric [13] CKD is associated with a higher risk of CV disease. In previous studies showing an increased CV risk in patients with DR, the inclusion of patients with CKD may have influenced the findings as DR is usually closely associated with diabetic nephropathy. Therefore, any studies considering the association of DR with CV disease should take this issue into consideration.
Carotid intima-media thickness (IMT) and the presence of carotid plaques are considered to be surrogate measures of atherosclerosis [14]. Both conditions are associated with CV risk factors [15], predict CV events independently of risk factors [16], and help to stratify patients into different risk categories [17]. However, it should be mentioned that the presence [18] and burden [19] of atherosclerotic plaque is a more powerful predictor of CV risk compared with IMT alone.
Both IMT and the frequency of plaques have been reported to be increased in children, adolescents and adults with type 1 diabetes compared with age- and sex-matched healthy control subjects [1], although data regarding atherosclerotic plaques in patients with type 1 diabetes are very scarce. A paucity of data exists on whether DR and subclinical atherosclerotic disease in the carotid artery territory in patients with type 1 diabetes are associated. Carotid plaque is recognized as an independent risk factor for ischemic stroke [20] with a risk that is markedly increased both in patients with type 1 and type 2 diabetes [21]. In patients with type 1 diabetes, this risk has been described to be associated with the presence of diabetic nephropathy and severe diabetic retinopathy [22]. In patients with type 2 diabetes, ours and other groups have previously described that DR is an independent risk factor associated with the presence of carotid plaques in subjects without previous CV disease [23, 24]. In these patients, it has recently been described that carotid atherosclerosis is also an independent risk factor for stroke [25].
Therefore, we hypothesized that DR is associated with the presence of carotid plaques in subjects with type 1 diabetes without previous CV disease and established chronic kidney disease. In the present study, we sought to assess the association of the frequency and burden of subclinical carotid atherosclerotic disease in patients with type 1 diabetes according to presence and severity of DR. As a secondary outcome, we also evaluated the differences in the presence and burden of subclinical carotid atherosclerosis compared with non-diabetic subjects.
Methods
Subjects
This study was designed as a cross-sectional study in subjects with type 1 diabetes recruited from the diabetic outpatient clinics at two university hospitals in the North-Western region of Spain (Catalonia). All potential participants were identified from the electronic clinical records from the two participating institutions that belong to the same health care organization.
The inclusion criteria for type 1 diabetic subjects were as follows: age > 18 years; diabetes for at least 1 year; normal renal function [estimated glomerular filtration rate (eGFR > 60 mL/min)]; no previous cardiovascular (CV) disease defined as any form of clinical coronary heart disease, stroke or peripheral vascular disease; and any form of diabetic foot disease. We excluded patients with a urine albumin excretion ratio > 300 mg/g and any conditions that precluded a proper full eye examination. A subject was considered to have previous hypertension or dyslipidaemia if he/she was taking medication for the given condition. The selection of non-diabetic control participants was based on the same criteria, except for the criteria that concerned the diabetic specific microvascular complications (retinopathy and albuminuria). Additionally, subjects in the control group had fasting glucose and HbA1c values below 100 mg/dL and 5.7% HbA1c, respectively.
Clinical assessment
For each subject, the age, sex, weight, height, body mass index and waist circumference were obtained by standardized methods. Serum and spot urine samples were collected in the fasting state, and all serum and urine tests were performed using standard laboratory methods as previously described [23].
Ophthalmic examination
In subjects with type 1 diabetes, an experienced ophthalmologist performed a complete eye evaluation and defined DR according to the ETDRS classification in five stages: (1) no apparent retinopathy, (2) mild non-proliferative retinopathy (NPDR) (defined by the presence of microaneurysms), (3) moderate NPDR (presence of microaneurysms, intraretinal hemorrhages or venous beading that did not reach the severity of the next stage), (4) severe NPDR (more than 20 intraretinal hemorrhages in four quadrants, venous beading in two quadrants or intraretinal microvascular abnormalities in one quadrant), and (5) proliferative diabetic retinopathy (PRD) defined by the presence of disc/retina/angle/iris neovascularization, vitreous hemorrhage or tractional retinal detachment [26]. For the purpose of the analysis, patients with DR were classified into two groups according to the severity of diabetic retinopathy: mild diabetic retinopathy and advanced DR (stages 3, 4 and 5 of the ETDRS classification).
Carotid ultrasound imaging
All the study participants underwent the same carotid ultrasound examination. Carotid ultrasonography imaging was performed using a LOGIQ® E9-General Electric (Wauwatosa, WI 53226, USA) equipped with a 15 MHz linear array probe or a Sequoia 512, Siemens, (North Rhine, Westphalia, Germany) equipped with a 15 MHz linear array probe. All measures and ultrasound studies were assessed by the same researcher at each participating hospital. The detailed carotid ultrasound protocol performed to evaluate the presence of carotid plaques has been previously described [23]. Plaques were identified using B-mode and color Doppler examinations in both the longitudinal and transverse planes to consider circumferential asymmetry and were defined as a “focal structure that encroaches into the arterial lumen of at least 0.5 mm or 50% of the surrounding carotid IMT value or demonstrates a thickness of 1.5 mm, as measured from the media-adventitia interference to the intima-lumen surface” according to the Mannheim consensus [27]. The arterial territories explored included the left and right carotid arteries; for each of them, the common and internal carotid territories and their bifurcation were examined.
The Local Ethics Committee of both participating centers approved the protocol, and all the participants signed informed consent forms.
Statistical analysis
The descriptive statistics of the mean (standard deviation) or median [interquartile range] were estimated for quantitative variables with a normal or non-normal distribution, respectively. For the qualitative variables, absolute and relative frequencies were used. The normal distribution was analyzed by the Shapiro–Wilks test. The differences between groups were assessed by Student’s test, analysis of variance (ANOVA) or Mann–Whitney test, and Kruskal–Wallis test depending on the distributions of the quantitative variables. The significance of the differences in qualitative variables was assessed by Chi squared test or Fisher’s exact test. Logistic regression and multinomial logistic regression models were performed to study the prevalence and burden of subclinical carotid atherosclerotic disease and its association with DR. All variables of the bivariate analysis with a p < 0.2 were used. Only the main effects with a significant contribution to the final model according to the likelihood ratio test were included in the final model. For logistic regression model calibration and discrimination, the Homer–Lemeshow test and the area under the ROC curve were used. The R statistical software, version 3.3.1, was used for all the analyses, using a significance level of 0.05.
Sample size
Based on a predefined prevalence of DR in patients with type 1 diabetes of 33%, we estimated a sample size of 309 patients with type 1 diabetes as being sufficient to detect a difference in the frequency of carotid atherosclerotic plaques of 15% between patients with type 1 diabetes with and without DR, assuming frequencies of plaques of 35 and 20% in patients with and without DR, respectively. This assumption yielded an overall frequency of plaques of 25% in this sample of subjects with type 1 diabetes. A comparison with a group with the same number of non-diabetic subjects would then allow the detection of a difference of 9% between them and type 1 diabetes.
Results
From the initial number of type 1 diabetic patients recruited (n = 397) in the study, five patients were excluded for presenting an eGFR < 60 mL/min, two patients were excluded for presenting a urine albumin/creatinine ratio > 300 mg/g, three patients were excluded for presenting cardiovascular disease and 11 patients were excluded for presenting with one or more ophthalmic exclusion criteria. Additionally, six patients did not attend the carotid ultrasound examination, and 30 patients did not complete all the determinations of the main study outcomes. Therefore, 340 patients with type 1 diabetes were finally enrolled in the study. We enrolled more subjects in the type 1 diabetes group as we allowed recruitment in the two centers until the control group was completed. A total of 332 individuals were contacted for inclusion in the control group. From this initial number, nine individuals did not accept participation, and 19 were excluded because of the presence of exclusion criteria or for not completing all the study procedures. Therefore, from the expected size of the group of non-diabetic individuals (n = 309), a total of 304 individuals were recruited.
Clinical variables
The results of the study variables for each study group are shown in Table 1. Among the 340 study patients with type 1 diabetes, 141 (41.5%) had DR. The patients with DR were older, had a higher prevalence of hypertension and dyslipidemia and a longer diabetes duration. These patients also exhibited higher systolic blood pressure, pulse pressure, BMI, waist circumference, plasma triglycerides and HbA1c compared with those without DR (Table 1). The distribution of the stages of DR in the 141 patients with DR was as follows: 89 patients had mild DR, 18 patients had moderate DR, 15 patients had severe DR, and 19 patients had proliferative DR. For further analysis, we classified the status of retinopathy into non-DR (n = 199, 58.5%), mild DR (n = 89, 26.2%), and advanced DR (including moderate, severe and proliferative disease) (n = 52, 15.3%). The clinical characteristics of these 3 groups of patients are shown in Table 2.
Table 1.
Clinical characteristics of the study groups
| Control | Type 1 diabetes | p. control vs. type 1 diabetes | p. no DR vs. DR | |||
|---|---|---|---|---|---|---|
| All | No retinopathy | Retinopathy | ||||
| N = 307 | N = 340 | N = 199 | N = 141 | |||
| Age, years | 44.0 [37.0; 51.0] | 45.0 [37.0; 53.0] | 43.4 (11.1) | 48.6 (12.1) | 0.164 | < 0.001 |
| Sex, men | 139 (45.7%) | 155 (45.6%) | 89 (44.7%) | 66 (46.8%) | 1 | 0.787 |
| Non-caucasian | 9 (2.96%) | 4 (1.18%) | 3 (1.51%) | 1 (0.71%) | 0.185 | 0.645 |
| Current or former smoker | 167 (55.3%) | 174 (51.3%) | 97 (49.0%) | 77 (54.6%) | 0.354 | 0.363 |
| Dyslipidemia | 39 (12.8%) | 144 (42.4%) | 72 (36.2%) | 72 (51.1%) | < 0.001 | 0.009 |
| Hypertension | 26 (8.55%) | 88 (25.9%) | 36 (18.1%) | 52 (36.9%) | < 0.001 | < 0.001 |
| Systolic BP, mmHg | 120 [112; 129] | 129 [114; 139] | 127 [112; 135] | 132 [119; 145] | < 0.001 | < 0.001 |
| Diastolic BP, mmHg | 76.0 [70.0; 81.0] | 75.0 [68.0; 80.0] | 74.5 (10.1) | 73.7 (9.98) | 0.009 | 0.447 |
| Pulse pressure, mmHg | 44.0 [38.0; 51.8] | 52.0 [42.5; 62.0] | 50.0 [40.0; 58.5] | 56.0 [46.8; 70.0] | < 0.001 | < 0.001 |
| BMI kg/m2 | 25.2 [23.2; 28.4] | 25.6 [22.8; 28.4] | 25.0 [22.5; 27.4] | 26.3 [23.5; 29.4] | 0.827 | 0.004 |
| Waist circumference, cm | 93.0 [84.2; 100] | 88.0 [80.0; 98.5] | 87.0 [79.0; 95.0] | 90.0 [82.0; 102] | < 0.001 | 0.003 |
| HbA1c, % | 5.40 [5.20; 5.60] | 7.50 [7.00; 8.10] | 7.40 [6.80; 7.90] | 7.60 [7.20; 8.40] | <0.001 | < 0.001 |
| HbA1c, mmol/mol | 36.0 [33.0; 38.0] | 58.0 [53.0; 65.0] | 57.0 [51.0; 63.0] | 60.0 [55.0; 68.0] | < 0.001 | < 0.001 |
| Total-c, mg/dL | 192 [170; 220] | 176 [160; 200] | 175 [161; 200] | 177 [156; 201] | < 0.001 | 0.903 |
| HDL, mg/dL | 56.5 [47.0; 68.0] | 62.0 [53.0; 74.0] | 63.0 [54.0; 74.0] | 60.5 [55.0; 72.8] | < 0.001 | 0.197 |
| LDL, mg/dL | 116 [94.8; 138] | 99.4 [83.0; 116] | 99.4 [84.6; 116] | 99.5 [79.9; 117] | < 0.001 | 0.684 |
| Triglycerides, mg/dL | 85.0 [63.0; 122] | 68.0 [53.0; 86.0] | 65.0 [53.0; 83.5] | 70.0 [55.0; 89.0] | < 0.001 | 0.071 |
| Creatinine, mg/dL | 0.78 [0.68; 0.90] | 0.76 [0.65; 0.87] | 0.77 [0.66; 0.88] | 0.76 [0.65; 0.87] | 0.044 | 0.890 |
| Urine albumin/creatinine ratio, mg/g | – | 3.96 [2.08; 6.84] | 3.58 [1.82; 5.95] | 4.70 [2.76; 8.34] | – | 0.030 |
| Diabetes duration, years | – | 20.0 [14.0; 29.0] | 16.0 [11.0; 22.0] | 27.0 [20.0; 33.0] | – | < 0.001 |
Variables are expressed as median and interquartile range, unless otherwise specified
BMI body mass index, BP blood pressure, HDL high-density lipoprotein cholesterol, LDL low-density lipoprotein cholesterol, Total-c total cholesterol
Table 2.
Clinical characteristics of patients with type 1 diabetes according to the presence and severity of DR
| No DR | Mild DR | > Mild DR | p overall | |
|---|---|---|---|---|
| N = 199 | N = 89 | N = 52 | ||
| Age, years | 43.4 (11.1) | 47.1 (12.2) | 51.1 (11.6) | < 0.001 |
| Sex, men | 89 (44.7%) | 41 (46.1%) | 27 (51.9%) | 0.906 |
| Non-caucasian | 3 (1.51%) | 1 (1.12%) | 0 (0.00%) | 1 |
| Current or former smoker | 97 (49.0%) | 49 (55.1%) | 28 (53.8%) | 0.588 |
| Antiplatelet | 46 (23.1%) | 23 (25.8%) | 23 (44.2%) | 0.009 |
| Dyslipidemia | 72 (36.2%) | 41 (46.1%) | 31 (59.6%) | 0.007 |
| Hypertension | 36 (18.1%) | 25 (28.1%) | 27 (51.9%) | < 0.001 |
| Systolic BP, mmHg | 127 [112; 135] | 130 [119; 140] | 136 [116; 150] | 0.001 |
| Diastolic BP, mmHg | 74.5 (10.1) | 74.4 (8.92) | 72.4 (11.6) | 0.395 |
| Pulse pressure, mmHg | 50.0 [40.0; 58.5] | 53.0 [45.0; 66.0] | 62.0 [50.5; 75.0] | < 0.001 |
| BMI, kg/m2 | 25.0 [22.5; 27.4] | 26.2 [23.6; 29.4] | 26.3 [23.3; 30.2] | 0.013 |
| Waist circumference, cm | 87.0 [79.0; 95.0] | 90.0 [82.0; 100] | 91.5 [82.0; 103] | 0.009 |
| HbA1c, % | 7.40 [6.80; 7.90] | 7.50 [7.10; 8.20] | 7.90 [7.30; 8.60] | < 0.001 |
| HbA1c, mmol/mol | 57.0 [51.0; 63.0] | 58.0 [54.0; 66.0] | 63.0 [56.0; 70.0] | < 0.001 |
| Total-c, mg/dL | 175 [161; 200] | 177 [154; 200] | 184 [157; 201] | 0.989 |
| HDL, mg/dL | 63.0 [54.0; 74.0] | 60.5 [51.8; 71.0] | 60.5 [50.8; 76.0] | 0.422 |
| LDL, mg/dL | 99.4 [84.6; 116] | 98.0 [81.8; 121] | 105 [78.1; 115] | 0.913 |
| Triglycerides, mg/dL | 65.0 [53.0; 83.5] | 69.0 [53.0; 93.0] | 74.0 [56.8; 88.0] | 0.166 |
| Creatinine, mg/dL | 0.77 [0.66; 0.88] | 0.75 [0.64; 0.85] | 0.78 [0.68; 0.92] | 0.340 |
| Urine albumin/creatinine ratio mg/g | 3.58 [1.82; 5.95] | 4.00 [1.98; 5.62] | 5.71 [3.12; 16.3] | 0.001 |
| Diabetes duration | 16.0 [11.0; 22.0] | 25.0 [18.0; 30.0] | 30.5 [22.0; 38.0] | < 0.001 |
Variables are expressed as median and interquartile range, unless otherwise specified
BMI body mass index, BP blood pressure, HDL high-density lipoprotein cholesterol, LDL low-density lipoprotein cholesterol, Total-c total cholesterol
Ultrasound examination
The percentage of patients with carotid plaques was higher in patients with type 1 diabetes compared with control subjects (32.6% vs. 23%, p = 0.009). The patients with type 1 diabetes also presented with a higher percentage of ≥ 2 carotid plaques (17.1% vs. 11.2%, p = 0.023) compared with non-diabetic subjects. In the group of patients with type 1 diabetes, the percentage of patients with carotid plaques was higher in those with DR compared with those without DR (44.7% vs. 24.1%, p < 0.001). The patients with DR also presented a higher percentage of ≥ 2 carotid plaques (25.5% vs. 11.1%, p < 0.001) compared to those without DR (Fig. 1).
Fig. 1.
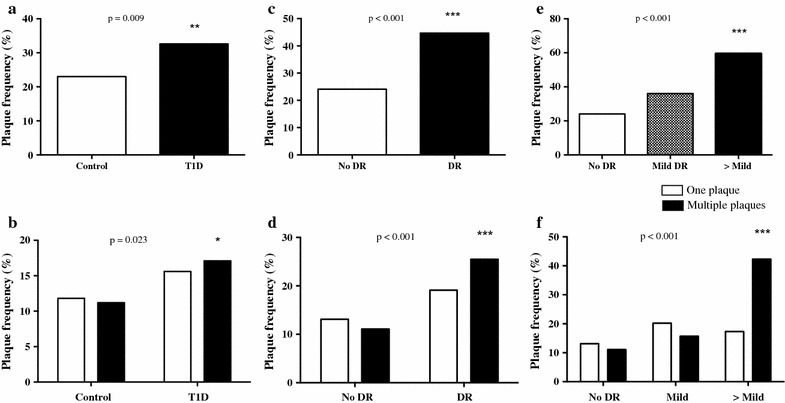
Percentage of patients with the presence of one atherosclerotic plaque (a) or multiple plaques (b) in the control group and in patients with type 1 diabetes. Percentage of patients with type 1 diabetes with the presence of one atherosclerotic plaque or multiple plaques according to the presence or absence of DR (c, d). Percentage of patients with type 1 diabetes with the presence of one atherosclerotic plaque (e) or multiple plaques (f) according to the degree of DR
Frequency and burden of atherosclerotic plaques in patients with type 1 diabetes
In the group of patients with type 1 diabetes, as the frequency of subclinical carotid atherosclerosis was mainly at the expense of patients with advanced DR, we performed a multiple regression analysis that used the group of patients without DR as the reference group. This analysis revealed that the variables associated with the presence of carotid plaques were age (p < 0.001), advanced DR (p = 0.044), smoking (p = 0.013), dyslipidemia (p < 0.001), pulse pressure (p = 0.014), and albumin creatinine ratio (p = 0.013) (Fig. 2a).
Fig. 2.
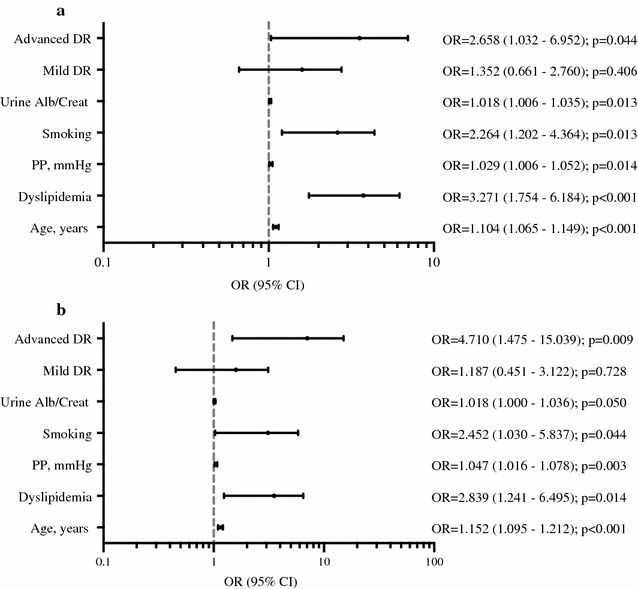
Multivariate logistic regression models: a model for the presence or absence of carotid plaque in patients with type 1 diabetes. b Model for absence vs presence of multiple (≥ 2) plaques in patients with type 1 diabetes. Models adjusted by sex, age, diabetes duration, smoking, diastolic BP, dyslipidemia, diabetic retinopathy, urine albumin/creatinine ratio, BMI, pulse pressure and LDL. Smoking refers to current and former smokers
Next, we performed the multivariate logistic regression analysis for the presence of one plaque or multiple (≥ 2) plaques (Additional file 1: Table S1). The variables associated with multiple plaques were age, smoking, dyslipidemia, advanced stages of retinopathy, pulse pressure and albumin creatinine ratio (Fig. 2b).
We also performed a similar multivariate logistic regression analysis for the presence and absence of DR as a microvascular complication of atherosclerosis. In this analysis, we did not find a significant contribution of retinopathy (p = 0.140) for the presence of subclinical carotid atherosclerosis (Additional file 1: Table S2).
Frequency and burden of atherosclerotic plaques in the whole study group
To analyze the variables associated with the presence and burden of atherosclerotic plaques in the whole group (type 1 diabetes and non-diabetic participants), we performed a logistic regression analysis. We first analyzed the variables associated with the presence of atherosclerotic plaques. In this model, we found an interaction of diabetes with smoking. The variables associated with presence of any plaque were age (p < 0.001), dyslipidemia (p < 0.001), pulse pressure (p < 0.001) and smoking if type 1 diabetes (p = 0.003) (Additional file 1: Table S3).
Next, we performed the multivariate logistic regression analysis for the presence of one plaque or multiple (≥ 2) plaques (Additional file 1: Table S3). There were several variables that were consistently associated with the presence of one or multiples plaques: age, dyslipidemia, pulse pressure and smoking if type 1 diabetes. In contrast, only one variable was associated with the presence of multiple plaques, the sex.
Discussion
In the present study, that included a large sample of patients with type 1 diabetes and an age- and sex-matched control group of participants without diabetes, the frequency of carotid plaques was significantly higher in patients with type 1 diabetes. Interestingly, apart from other well-known cardiovascular risk factors, the presence of advanced DR was an independent predictor of the presence of carotid plaques and of the atherosclerotic burden in type 1 diabetes.
The natural history of atherosclerosis involves a protracted subclinical phase, with the atherosclerotic disease often being detected only at an advanced stage or after a CV event. Non-invasive methods, such as arterial ultrasonography, are used to detect and quantify atherosclerosis and have shown to be a useful screening test for the prediction of future CV events. It is now generally accepted that presence and burden of carotid plaques compared with that of carotid IMT has a higher diagnostic accuracy for the prediction of future CV events [17, 18]. Several studies showed that patients with type 1 diabetes had a significantly increased carotid IMT compared with age- and sex-matched non-diabetic subjects [28]. Less information is available regarding the presence of atherosclerotic plaques in patients with type 1 diabetes, as well as its association with the presence of DR. In a study by Distiller et al. in 148 patients with type 1 diabetes (mean age 48 years, and mean diabetes duration of 26 years), the prevalence of plaques was 18.9%. In this study, DR was reported to be independently associated with the presence of plaques [29]. Additionally, Ogawa et al. [30] described a plaque frequency of 28.8% in a group of 73 patients with type 1 diabetes (mean age 38 years) with early-onset long-duration type 1 diabetes. This study reported an association between proliferative DR with a maximal carotid IMT of the whole carotid artery. Patients with proliferative DR from that study also showed a non-significant higher frequency of plaques compared with those without this condition (42.9% vs. 23.1%). In the current study, the presence of plaques was significantly higher in patients with type 1 diabetes compared with non-diabetic subjects. Moreover, a higher proportion of patients with type 1 diabetes presented with ≥ 2 carotid plaques compared with controls. The factors independently associated with carotid atherosclerotic plaques in patients with type 1 diabetes were different depending on whether only one plaque or ≥ 2 carotid plaques were present. In both cases, well-known cardiovascular risk factors and, more importantly, advanced stages of DR were independently associated with subclinical atherosclerosis. Interestingly, in a recent study, we have reported that the frequency of preclinical carotid atherosclerosis in patients with latent autoimmune diabetes of the adults (LADA) is similar, or even greater in those with longer disease duration, to subjects of similar age with classic type 1 diabetes and type 2 diabetes [31]. In LADA, preclinical carotid atherosclerosis was also associated with classical risk factors like hypertension and tobacco exposure.
The data related to the presence and extent of carotid plaques in patients with type 2 diabetes are more abundant [32]. In these patients, a high prevalence of carotid plaque presence and burden compared with healthy subjects has been described [33]. In patients with type 2 diabetes, ours and other groups have previously described that DR is an independent risk factor associated with the presence of carotid plaques in subjects without previous cardiovascular disease [23, 24]. Specifically, the proportion of carotid plaques in type 2 diabetes patients with DR was higher than in type 2 diabetes patients without DR; additionally, patients with DR had a higher burden of atherosclerosis (≥ 2 carotid plaques) [23]. Further, patients with type 2 diabetes and advanced stages of retinopathy have stenotic atherosclerotic plaques more frequently than patients with mild retinopathy [24].
Another proposed measure to evaluate the presence and progression of microvascular complications in patients with type 1 diabetes is the analysis of advanced glycation end-products (AGEs). In these patients, it has been described that accumulation of AGEs in the skin is independently associated with progression of retinopathy as well as with presence and progression of subclinical cardiovascular disease (i.e. the severity of coronary artery calcification and the IMT carotid progression) [34, 35]. Other authors have suggested factors, other than the presence and burden of atherosclerotic plaques, that may be involved in the increased cardiovascular risk in patients with type 1 diabetes, i.e. increased arterial stiffness [36, 37], and HDL dysfunction [38].
Diabetic retinopathy has been described to be associated with macrovascular disease [39], as well as with increased CV morbidity and mortality [7]. It is interesting to note that the presence of severe DR is among the independent risk factors for stroke both in patients with type 1 diabetes [7, 22] and in patients with type 2 diabetes [40]. Thus, the presence and extent of carotid atheromatous disease described in the present study in patients with type 1 diabetes and DR may be one of the factors associated with the higher risk of stroke described in patients with type 1 diabetes given that the presence of carotid plaque is an accepted independent risk factor for stroke. Lipid concentrations are strongly related to the risk of cardiovascular disease.
In patients with type 1 diabetes, it has been reported that in the context of good glycemic control HDL cholesterol is often similar or higher, and triglycerides and LDL cholesterol are often lower in type 1 diabetes in comparison with non-diabetic subjects [41]. This is consistent with the fair glycemic control observed in type 1 diabetes patients in our study. In the current study, the increased HDL concentration observed in type 1 diabetes compared with control subjects may be related to the increased waist circumference in control subjects. The latter has already been found to be associated with reduced HDL concentrations in patients with type 1 diabetes [42].
The current study has several limitations. First, the study design does not allow for the identification of a pathophysiological link between DR and atherosclerosis. Additionally, the initial sample size calculation was primarily based on the presence of DR and not on the severity of this microvascular complication; given our findings, a larger study may be warranted to confirm the current results.
Conclusions
The present study performed in a large cohort of patients with type 1 diabetes free from CV disease shows a higher prevalence and burden of carotid atherosclerotic disease in these patients. In a similar way to what has been described in patients with type 2 diabetes, the presence of advanced stages of DR is independently associated with the presence of carotid plaques and the burden of atherosclerotic disease in patients with type 1 diabetes. Given the association described between the atherosclerotic plaque burden and the risk of future CV events in the general population, the patients with type 1 diabetes and DR may be a subgroup of patients who should be regarded at increased risk for future CV events and should be followed more closely to prevent CV disease. The follow-up of the whole cohort of our patients with type 1 diabetes may help to address these issues. The current findings should lead to an increased awareness of DR as a non-classic cardiovascular risk factor for type 1 diabetes and to its proper consideration for preventive treatment among clinicians.
Additional file
Additional file 1: Table S1. Multivariate and multinomial logistic regression models of the presence/absence of atherosclerotic plaque, none vs one plaque, and none vs multiple plaques in patients with type 1 diabetes with DR as a variable introduced as stages No/Mild/>Mild. Table S2. Multivariate and multinomial logistic regression models of the presence/absence of atherosclerotic plaque, none vs one plaque, and none vs multiple (≥ 2) plaques in patients with type 1 diabetes with DR as a variable introduced as presence/absence. Table S3. Multivariate and multinomial logistic regression models of the presence/absence of atherosclerotic plaque, none vs one plaque, and none vs multiple (≥ 2) plaques in the whole study group.
Authors’ contributions
MC and EC contributed to the study design, conducting the study, data analysis, and writing of the manuscript. NA and DM contributed to the study design and coordination, conducting the study, data analysis, and writing of the manuscript. XV, MG, MH, FV, MM, AT, and ER contributed to the data collection and conducting the study. AA, AB, AL, MP-D. and JF-N. contributed to data interpretation and discussion. DM is the guarantor of this work and, as such, has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript.
Acknowledgements
The authors thank Montserrat Martinez (Statistics Unit, IRB Lleida) for her valuable assistance in conducting the statistical analysis. We particularly acknowledge the patients, IGTP-HUGTP and IRB Lleida (B.0000682) Biobanks (part of the Spanish National Biobanks Network of ISCIII PT13/0010/0009 and PT13/0010/0014, respectively), and the Tumor Bank Network of Catalonia for their collaboration.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Primary material is held by the authors.
Consent for publication
Authors give full consents for publication of the present article.
Ethics approval and consent to participate
The Local Ethics Committee of both participating centers approved the protocol, and all the participants signed informed consent forms.
Funding
This research was supported by grants from the Spanish Ministry of Health, the Carlos III National Institute of Health (PI12/0183 and PI15/0625) and European Regional Development Fund (ERDF). CIBER for Diabetes and Associated Metabolic Diseases (CIBERDEM) is an initiative of ISCIII, Spain. The Health Sciences Research Institute Germans Trias i Pujol is part of the CERCA Programme/Generalitat de Catalunya.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- CV
cardiovascular
- DR
diabetic retinopathy
- CKD
chronic kidney disease
- IMT
intima-media thickness
- eGFR
estimated glomerular filtration rate
Footnotes
Marc Carbonell and Esmeralda Castelblanco contributed equally to this work
Electronic supplementary material
The online version of this article (10.1186/s12933-018-0706-z) contains supplementary material, which is available to authorized users.
Contributor Information
Marc Carbonell, Email: marc_carbonell@hotmail.com.
Esmeralda Castelblanco, Email: esmeraldacas@gmail.com.
Xavier Valldeperas, Email: xvalldeperas@gmail.com.
Àngels Betriu, Email: angels.betriu.bars@gmail.com.
Alícia Traveset, Email: atravessetm@gmail.com.
Minerva Granado-Casas, Email: mgranado@igtp.cat.
Marta Hernández, Email: martahernandez@gmail.com.
Federico Vázquez, Email: fvazquez@igtp.cat.
Mariona Martín, Email: marionamarting@gmail.com.
Esther Rubinat, Email: rubinatesther@gmail.com.
Albert Lecube, Email: alecube@gmail.com.
Josep Franch-Nadal, Email: josep.franch@gmail.com.
Elvira Fernández, Email: efernandez@irblleida.cat.
Manel Puig-Domingo, Email: mpuigd@gmail.com.
Angelo Avogaro, Email: angelo.avogaro@unipd.it.
Núria Alonso, Phone: +34 934978860, Email: nalonso32416@yahoo.es.
Dídac Mauricio, Phone: +34 934978860, Email: didacmauricio@gmail.com.
References
- 1.de Ferranti SD, de Boer IH, Fonseca V, et al. Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American heart association and American diabetes association. Circulation. 2014;130:1110–1130. doi: 10.1161/CIR.0000000000000034. [DOI] [PubMed] [Google Scholar]
- 2.Lind M, Svensson AM, Kosiborod M, et al. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med. 2014;371:1972–1982. doi: 10.1056/NEJMoa1408214. [DOI] [PubMed] [Google Scholar]
- 3.Livingstone SJ, Looker HC, Hothersall EJ, et al. Risk of cardiovascular disease and total mortality in adults with type 1 diabetes: Scottish registry linkage study. PLoS Med. 2012;9:e1001321. doi: 10.1371/journal.pmed.1001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 5.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 6.Li LJ, Lamoureux E, Wong TY, Lek N. Short-term poor glycemic control and retinal microvascular changes in pediatric type 1 diabetes patients in Singapore: a pilot study. BMC Ophthalmol. 2017;17:60. doi: 10.1186/s12886-017-0449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kramer CK, Rodrigues TC, Canani LH, Gross JL, Azevedo MJ. Diabetic retinopathy predicts all-cause mortality and cardiovascular events in both type 1 and 2 diabetes: meta-analysis of observational studies. Diabetes Care. 2011;34:1238–1244. doi: 10.2337/dc11-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arcidiacono MV, Traveset A, Rubinat E, et al. Microangiopathy of large artery wall: a neglected complication of diabetes mellitus. Atherosclerosis. 2013;228:142–147. doi: 10.1016/j.atherosclerosis.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Gerstein HC, Ambrosius WT, Danis R, et al. Diabetic retinopathy, its progression, and incident cardiovascular events in the ACCORD trial. Diabetes Care. 2013;36:1266–1271. doi: 10.2337/dc12-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Almeida FK, Esteves JF, Gross JL, Biavatti K, Rodrigues TC. Severe forms of retinopathy predict the presence of subclinical atherosclerosis in type 1 diabetes subjects. Arq Bras Cardiol. 2011;97(4):346–349. doi: 10.1590/S0066-782X2011005000101. [DOI] [PubMed] [Google Scholar]
- 11.Araszkiewicz A, Rogowicz-Frontczak A, Zozulinska-Ziolkiewicz D, Pilacinski S, Wykretowicz A, Wierusz-Wysocka B. Presence of retinopathy in type 1 diabetic patients is associated with subclinical macroangiopathy. Scand J Clin Lab Investig. 2011;71(7):563–568. doi: 10.3109/00365513.2011.593268. [DOI] [PubMed] [Google Scholar]
- 12.de Boer IH, Gao X, Cleary PA, et al. Diabetes control and complications trial/epidemiology of diabetes interventions and complications (DCCT/EDIC) research group. Albuminuria changes and cardiovascular and renal outcomes in type 1 diabetes: the DCCT/EDIC study. Clin J Am Soc Nephrol. 2016;11:1969–1977. doi: 10.2215/CJN.02870316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thorn LM, Gordin D, Harjutsalo V, et al. The presence and consequence of nonalbuminuric chronic kidney disease in patients with type 1 diabetes. Diabetes Care. 2015;38:2128–2133. doi: 10.2337/dc15-0641. [DOI] [PubMed] [Google Scholar]
- 14.Weber LA, Cheezum MK, Reese JM, et al. Cardiovascular imaging for the primary prevention of atherosclerotic cardiovascular disease events. Curr Cardiovasc Imaging Rep. 2015;8:36. doi: 10.1007/s12410-015-9351-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Leary DH, Polak JF, Kronmal RA, et al. Thickening of the carotid wall. A marker for atherosclerosis in the elderly? Cardiovascular health study collaborative research group. Stroke. 1996;27:224–231. doi: 10.1161/01.STR.27.2.224. [DOI] [PubMed] [Google Scholar]
- 16.Chambless LE, Heiss G, Folsom AR, et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the atherosclerosis risk in communities (ARIC) study, 1987–1993. Am J Epidemiol. 1997;146:483–494. doi: 10.1093/oxfordjournals.aje.a009302. [DOI] [PubMed] [Google Scholar]
- 17.Nambi V, Chambless L, Folsom AR, et al. Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: the ARIC (atherosclerosis risk in communities) study. J Am Coll Cardiol. 2010;55:1600–1607. doi: 10.1016/j.jacc.2009.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gepner AD, Young R, Delaney JA, et al. Comparison of coronary artery calcium presence, carotid plaque presence, and carotid intima-media thickness for cardiovascular disease prediction in the multi-ethnic study of atherosclerosis. Circ Cardiovasc Imaging. 2015;8:e002262. doi: 10.1161/CIRCIMAGING.114.002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sillesen H, Muntendam P, Adourian A, et al. Carotid plaque burden as a measure of subclinical atherosclerosis: comparison with other tests for subclinical arterial disease in the high risk plaque BioImage study. JACC Cardiovasc Imaging. 2012;5:681–689. doi: 10.1016/j.jcmg.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Touboul PJ, Labreuche J, Vicaut E, Amarenco P, GENIC Investigators Carotid intima-media thickness, plaques, and Framingham risk score as independent determinants of stroke risk. Stroke. 2005;36:1741–1745. doi: 10.1161/01.STR.0000174490.23495.57. [DOI] [PubMed] [Google Scholar]
- 21.Sundquist K, Li X. Type 1 diabetes as a risk factor for stroke in men and women aged 15–49: a nationwide study from Sweden. Diabet Med. 2006;23:1261–1267. doi: 10.1111/j.1464-5491.2006.01959.x. [DOI] [PubMed] [Google Scholar]
- 22.Hägg S, Thorn LM, Putaala J, et al. Incidence of stroke according to presence of diabetic nephropathy and severe diabetic retinopathy in patients with type 1 diabetes. Diabetes Care. 2013;36:4140–4146. doi: 10.2337/dc13-0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alonso N, Traveset A, Rubinat E, et al. Type 2 diabetes-associated carotid plaque burden is increased in patients with retinopathy compared to those without retinopathy. Cardiovasc Diabetol. 2015;14:33. doi: 10.1186/s12933-015-0196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Kreutzenberg SV, Coracina A, Volpi A, et al. Microangiopathy is independently associated with presence, severity and composition of carotid atherosclerosis in type 2 diabetes. Nutr Metab Cardiovasc Dis. 2011;21:286–293. doi: 10.1016/j.numecd.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Wu Y, He J, Sun X, et al. Carotid atherosclerosis and its relationship to coronary heart disease and stroke risk in patients with type 2 diabetes mellitus. Medicine (Baltimore) 2017;96:e8151. doi: 10.1097/MD.0000000000008151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilkinson CP, Ferris FL, 3rd, Klein RE, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677–1682. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 27.Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim carotid intima-media thickness consensus (2004–2006). An update on behalf of the advisory board of the 3rd and 4th watching the risk symposium, 13th and 15th European stroke conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis. 2007;23:75–80. doi: 10.1159/000097034. [DOI] [PubMed] [Google Scholar]
- 28.Sun YP, Cai YY, Li HM, Deng SM, Leng RX, Pan HF. Increased carotid intima-media thickness (CIMT) levels in patients with type 1 diabetes mellitus (T1DM): a meta-analysis. J Diabetes Complicat. 2015;29:724–730. doi: 10.1016/j.jdiacomp.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 29.Distiller LA, Joffe BI, Melville V, Welman T, Distiller GB. Carotid artery intima-media complex thickening in patients with relatively long-surviving type 1 diabetes mellitus. J Diabetes Complicat. 2006;20:280–284. doi: 10.1016/j.jdiacomp.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 30.Ogawa Y, Uchigata Y, Iwamoto Y. Progression factors of carotid intima-media thickness and plaque in patients with long-term, early-onset type 1 diabetes mellitus in Japan: simultaneous comparison with diabetic retinopathy. J Atheroscler Thromb. 2009;16:821–828. doi: 10.5551/jat.1701. [DOI] [PubMed] [Google Scholar]
- 31.Hernández M, López C, Real J, Valls J, Ortega-Martinez de Victoria E, Vázquez F, Rubinat E, Granado-Casas M, Alonso N, Molí T, Betriu A, Lecube A, Fernández E, Leslie RD, Mauricio D. Preclinical carotid aterosclerosis in patients with latent autoimmune diabetes in adults (LADA), type 2 diabetes and classical type 1 diabetes. Cardiovasc Diabetol. 2017;16:94. doi: 10.1186/s12933-017-0576-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernard S, Sérusclat A, Targe F, et al. Incremental predictive value of carotid ultrasonography in the assessment of coronary risk in a cohort of asymptomatic type 2 diabetic subjects. Diabetes Care. 2005;28:1158–1162. doi: 10.2337/diacare.28.5.1158. [DOI] [PubMed] [Google Scholar]
- 33.Catalan M, Herreras Z, Pinyol M, et al. Prevalence by sex of preclinical carotid atherosclerosis in newly diagnosed type 2 diabetes. Nutr Metab Cardiovasc Dis. 2015;25:742–748. doi: 10.1016/j.numecd.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 34.Sell DR, Sun W, Gao X, Strauch C, Lachin JM, Cleary PA, Genuth S, Vincent M, Monnier VM, The DCCT/EDIC Research Group Skin collagen fluorophore LW-1 versus skin fluorescence as markers for the long-term progression of subclinical macrovascular disease in type 1 diabetes. Cardiovasc Diabetol. 2016;15:30. doi: 10.1186/s12933-016-0343-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Osawa S, Katakami N, Kuroda A, Takahara M, Sakamoto F, Kawamori D, Matsuoka T, Matsuhisa M, Shimomura I. Skin autofluorescence is associated with early-stage atherosclerosis in patients with type 1 diabetes. J Atheroscler Thromb. 2017;24:312–326. doi: 10.5551/jat.35592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bradley TJ, Slorach C, Mahmud FH, Dunger DB, Deanfield J, Deda L, Elia Y, Har RLH, Hui W, Moineddin R, Reich HN, Scholey JW, Mertens L, Sochett E, Cherney DZI. Early changes in cardiovascular structure and function in adolescents with type 1 diabetes. Cardiovasc Diabetol. 2016;15:31. doi: 10.1186/s12933-016-0351-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peeters SA, Engelen L, Buijs J, Chaturvedi N, Fuller JH, Jorsal A, Parving HH, Tarnow L, Theilade S, Rossing P, Schalkwijk CG, Stehouwer CDA. Circulating matrix metalloproteinases are associated with arterial stiffness in patients with type 1 diabetes: pooled analysis of three cohort studies. Cardiovasc Diabetol. 2017;16:139. doi: 10.1186/s12933-017-0620-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heier M, Borja MS, Brunborg C, Seljeflot I, Margeirsdottir HD, Hanssen KF, Dahl-Jørgensen K, Oda MN. Reduced HDL function in children and young adults with type 1 diabetes. Cardiovasc Diabetol. 2017;16:85. doi: 10.1186/s12933-017-0570-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li LX, Zhao CC, Ren Y, et al. Prevalence and clinical characteristics of carotid atherosclerosis in newly diagnosed patients with ketosis-onset diabetes: a cross-sectional study. Cardiovasc Diabetol. 2013;12:18. doi: 10.1186/1475-2840-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xie J, Ikram MK, Cotch MF, et al. Association of diabetic macular edema and proliferative diabetic retinopathy with cardiovascular disease: a systematic review and meta-analysis. JAMA Ophthalmol. 2017;135:586–593. doi: 10.1001/jamaophthalmol.2017.0988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guy J, Ogden L, Wadwa RP, Hamman RF, Mayer-Davis EJ, Liese AD, D’Agostino R, Jr, Marcovina S, Dabelea D. Lipid and lipoprotein profiles in youth with and without type 1 diabetes: the SEARCH for diabetes in youth case-control study. Diabetes Care. 2009;32(3):416–420. doi: 10.2337/dc08-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernández-Miró M, Chillarón JJ, Albareda M, Fontserè S, Colom C, Vila L, Pedro-Botet J, Flores JLR, TEST-T1D study group Hypertriglyceridemic waist in type 1 diabetes patients: prevalence and related factors. Minerva Endocrinol. 2017;42(1):1–7. doi: 10.23736/S0391-1977.16.02561-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Multivariate and multinomial logistic regression models of the presence/absence of atherosclerotic plaque, none vs one plaque, and none vs multiple plaques in patients with type 1 diabetes with DR as a variable introduced as stages No/Mild/>Mild. Table S2. Multivariate and multinomial logistic regression models of the presence/absence of atherosclerotic plaque, none vs one plaque, and none vs multiple (≥ 2) plaques in patients with type 1 diabetes with DR as a variable introduced as presence/absence. Table S3. Multivariate and multinomial logistic regression models of the presence/absence of atherosclerotic plaque, none vs one plaque, and none vs multiple (≥ 2) plaques in the whole study group.
Data Availability Statement
Primary material is held by the authors.


