Abstract
Background
Malnutrition and weight loss are commonly observed in patients with pancreatic cancer and contribute to poor survival. Pancreatic exocrine insufficiency (PEI), which can be caused by ductal obstruction by a tumor, causes maldigestion and malabsorption of nutrients, thus contributing to malnutrition in these patients. In this study, we evaluated the effects of pancreatic enzyme replacement therapy (PERT) on survival in patients with unresectable pancreatic cancer.
Methods
A retrospective analysis was conducted on a database of patients with unresectable, pathologically confirmed pancreatic cancer. All patients were evaluated for palliative chemotherapy and received the optimal palliative care. Patients were divided into two groups: Group 1 received standard therapy; Group 2 underwent additional evaluation of the pancreatic function and therapy with PERT, if needed. Survival (median and 95% confidence interval [CI]) was analyzed using Kaplan–Meier and Cox regression; groups were compared using the log-rank test.
Results
Overall, 160 patients with unresectable pancreatic cancer were included in the analysis (mean age: 70.5 years [range 28–100]; gender: 57.5% male; tumor stage: 78.7% Stage IV). Eighty-six patients (53.75%) were in Group 1 and 74 (46.25%) were in Group 2. Age, gender, tumor size, location and stage, weight loss, and serum CA 19–9 were similar between groups. Ninety-three (58.1%) patients received palliative chemotherapy; 46.5% in Group 1 and 71.6% in Group 2 (P < 0.001). Forty-nine (66.2%) patients in Group 2 and none in Group 1 received PERT. Survival in Group 2 (189 days, 95% CI 167.0–211.0 days) was significantly longer than in Group 1 (95.0 days, 95% CI 75.4–114.6 days) (HR 2.117, 95% CI 1.493–3.002; P < 0.001). Chemotherapy and PERT were significantly and independently associated with longer survival in a model controlled by age and tumor stage. In patients with significant weight loss at diagnosis (> 10% bodyweight within 6 months), PERT was associated with longer survival (HR 2.52, 95% CI 1.55–4.11; P < 0.001).
Conclusions
In patients with unresectable pancreatic cancer, PERT in patients with PEI was associated with longer survival compared with those not receiving PERT, especially in those experiencing significant weight loss. This finding should guide future prospective clinical trials of similar interventions.
Keywords: Pancreatic carcinoma, Pancreatic exocrine insufficiency, Malnutrition, Survival, Unresectable pancreatic cancer, Pancreatic enzyme replacement therapy
Background
Pancreatic cancer is the fourth-leading cause of cancer-related deaths in the United States [1] and is expected to become the second most common cancer by 2030 [2]. Patients presenting with pancreatic cancer typically have a poor prognosis, because of the advanced stage of the disease at the time of diagnosis. Pancreatic cancer is associated with a very high mortality rate [3]; an overall 5-year survival rate of 7% after diagnosis was reported over the 2004–2010 period [1]. This survival rate was further reduced to 2% for patients with advanced disease [1].
In patients with early-stage, resectable disease (15–20% of patients at time of diagnosis [4]), surgical resection of the tumor can be performed and, although associated with a low success rate, it represents the only potentially curative intervention. Median survival for resected patients is in the region of 14–20 months [5]. Five-year survival rates of up to 25% have been reported following pancreatectomy for ductal adenocarcinoma of the pancreas [5–8]; however, these values have been questioned and actual rates are believed to be much lower [9–12].
Approximately 80% of patients with pancreatic cancer present with either locally advanced or metastatic disease at time of diagnosis and are not suitable for surgery. Median survival for these patients is shorter (8–12 months for unresectable disease [13] and less than 6 months for metastatic disease [3, 10]).
Despite extensive research efforts over the last two decades, palliative treatment (e.g., chemotherapy and radiotherapy) has not significantly improved survival [14], quality of life and tumor progression for patients with unresectable pancreatic cancer. In recent years, however, the clinical relevance of malnutrition caused by pancreatic exocrine insufficiency (PEI) both in resected patients and in patients with advanced, unresectable pancreatic cancer, has been investigated to improve supportive care [15–17].
Malnutrition and significant weight loss are frequently observed in patients with pancreatic cancer [18] and influence survival, resection rate, tumor progression, and quality of life [5, 19]. Improvement of the nutritional status by parenteral nutrition enhances quality of life, and allows for tumor therapy to be administrated without interruption [20].
While malnutrition and weight loss may be partly attributed to anorexia, diarrhea, early satiety [21], chemotherapy, and/or tumor progression [20], PEI may also be a determinant, either due to postoperative changes in the digestion process after tumor resection, or to the tumor itself obstructing the pancreatic duct [22–24]. PEI causes digestion to be impaired, which commonly manifests as diarrhea and steatorrhea [25]. Reduced digestion results in malabsorption of nutrients, leading to malnourishment [18, 25]. A markedly reduced exocrine pancreatic secretion, as measured by low fecal elastase-1 (FE-1) levels, has been reported as an independent factor associated with poor prognosis in patients with advanced pancreatic cancer [26]. In this patient population, partial pancreatic resection for pancreatic malignancy leads to sustained PEI and reduced quality of life [27].
Oral pancreatic enzyme replacement therapy (PERT) is the standard treatment for PEI [21, 23]. Patients with advanced pancreatic cancer who received PERT have shown significant weight gain after PERT [16]. In addition, PERT was associated with significant improvement in fat and protein digestion in patients with a partial/total pancreatic resection [28]. Of note, a recent study reported low rates of PERT prescription (21%) in patients with metastatic pancreatic cancer, in which the vast majority of patients had tumors of the pancreatic head and were likely to have had a blocked pancreatic duct [29].
It is unclear whether treatment of PEI with PERT improves the survival of patients with either resectable or unresectable pancreatic cancer. Therefore, the aim of this analysis was to evaluate the impact of PEI diagnosis and treatment on the survival of patients with unresectable pancreatic cancer.
Methods
Study design
This retrospective analysis was conducted at the University Hospital of Santiago de Compostela, Spain, on a database of patients with unresectable, pathologically confirmed, pancreatic adenocarcinoma.
Study participants
Patients diagnosed with unresectable pancreatic cancer (locally advanced or metastatic disease) between January 2011 and October 2016, as evaluated by both computed tomography (CT) scan and endoscopic ultrasound (EUS), were eligible for inclusion in this analysis [30]. Confirmation of pancreatic adenocarcinoma by cytology or histology after EUS-guided fine needle aspiration (FNA) or fine needle biopsy (FNB) was required for final inclusion. EUS and EUS-guided FNA or FNB was performed in all patients by two experienced endosonographers using Pentax linear echoendoscopes and Hitachi Preirus (2011–2012) or Ascendus (2012–2016) equipment. All FNA and FNB samples were evaluated by a single highly experienced pathologist. Patients who survived less than 30 days after diagnosis, in whom no therapy could be started, and those who underwent neoadjuvant chemotherapy for downstaging, were excluded from the analysis.
Treatment
All patients were evaluated by medical oncologists for palliative chemotherapy and best palliative care, and the most appropriate tumor therapy according to age, performance status, liver and renal function, and general well-being was proposed to the patients. If obstructive jaundice was present, endoscopic biliary drainage was performed using a plastic or metal stent. In addition, the best supportive care for pain and symptoms, including nutritional supplements, was applied if needed.
Group 1 consisted of patients diagnosed with pancreatic cancer in the Department of Medical Oncology, General Surgery, or any department other than Gastroenterology, and who were treated according to well-accepted oncologic protocols [31]. Group 2 consisted of patients diagnosed with pancreatic cancer in the Department of Gastroenterology, and who were additionally followed-up at the Pancreas Unit of that Department and evaluated for symptoms of PEI, including significant weight loss (> 10% of body weight over less than 6 months before diagnosis), diarrhea and other maldigestion-related symptoms, and nutritional status. PERT was prescribed in patients in Group 2 if significant weight loss and/or other symptoms of PEI were present. Therefore, the management of patients was basically the same in the two groups except for evaluation of PEI and administration of PERT if needed in patients of Group 2.
Pancreatic exocrine insufficiency
As already mentioned, patients in Group 2 with symptoms suggestive of PEI were treated with PERT (Creon®, Abbott Laboratories GmbH, Germany), at an initial dose of 50,000 Eur.Ph.U with each main meal and 25,000 Eur.Ph.U. with snacks. This dose was individually titrated according to the evaluation of body weight, symptoms, and nutritional status. Omeprazole 20 mg was orally administered twice daily, before breakfast and dinner, in all patients with PEI. In addition, prokinetic drugs were prescribed three-times daily if dyspeptic symptoms (nausea, postprandial fullness, early satiety) were present.
Assessments
Demographics and clinical data including tumor stage, location and size, serum CA 19–9 levels, and weight loss (less or more than 10% of bodyweight) were recorded at diagnosis. Administration of chemotherapy and PERT were also recorded. Clinical and analytical follow-up of patients was performed according to medical needs. Patients in Group 2 were additionally assessed at the Department of Gastroenterology for: body weight; gastrointestinal symptoms, including those of PEI; nutritional status every 2–3 months. PERT was titrated if needed.
The survival of patients who received standard oncologic therapy alone (Group 1) was compared with that of patients who received the same therapy in addition to being evaluated and treated for PEI with PERT, if needed (Group 2). From this, the impact of PERT on survival was evaluated. All clinical information for each patient, including the data obtained for the study protocol, is accessible via a central electronic medical record, which is available to all the hospitals and health centers around the north-west region of Spain. All adverse events, newly diagnosed comorbidities, and deaths were prospectively recorded in this central electronic medical record.
Statistical analyses
Descriptive data are shown as absolute numbers and percentages, median and range, and mean ± standard deviation (SD) as appropriate. Survival is shown as median and 95% confidence interval (CI). Baseline characteristics that could influence survival rates, such as age, gender, tumor stage, tumor location and size, serum CA 19–9 levels (dichotomized as > 1000 U/mL or < 1000 U/mL at diagnosis, or after biliary drainage in patients with obstructive jaundice), palliative chemotherapy and PERT (both dichotomized as “yes” or “no”), were studied as independent variables using a Cox proportional hazard regression model and expressed as hazard ratios (HR) (95% CI). Multivariate analysis was conducted using the forward-stepwise method, with P < 0.1 as a cut-off to enter into the model. Survival of different subgroups of patients was compared by the log-rank test and it was plotted as Kaplan–Meier curves using log-rank Mantel–Cox tests.
Results
Demographics
A total of 191 patients diagnosed with unresectable, pathologically-confirmed, pancreatic adenocarcinoma was identified during the study period. Thirty-one patients died within 30 days of diagnosis before any therapy could be started; thus, 160 patients were included in the final analysis. Mean age was 70.5 years (range 28–100); 92 patients were male (57.5%) and 68 were female (42.5%). Tumor staging revealed that 34 patients (21.2%) had locally advanced tumor and 126 (78.7%) had metastatic disease. The tumor was more frequently located in the head of the pancreas (58.1% of the cases, compared with 33.7% in the body and 8.1% in the tail of the pancreas). Tumor size was 4.2 ± 1.5 cm. Serum CA 19–9 levels at diagnosis and after biliary drainage were > 1000 U/mL in 73 patients (45.6%). Ninety-five patients (59.4%) had lost more than 10% of bodyweight within 1–6 months before diagnosis.
Of the 160 patients included, 86 (53.75%) were in Group 1 and 74 (46.25%) were in Group 2. Age, gender, tumor size, location and stage, weight loss, and serum CA 19–9 levels were similar in both groups (Table 1).
Table 1.
Demographics and clinical data of patients with unresectable pancreatic cancer according to the study group
| Group 1 | Group 2 | P value | |
|---|---|---|---|
| n (%) | 86 (53.75%) | 74 (46.25%) | |
| Age at diagnosis, median (range), years | 71.5 (43–100) | 69.5 (28–90) | 0.470c |
| Gender (M/F) | 52/34 | 40/34 | 0.427 |
| Tumor Stage (locally advanced/metastatic) | 16/70 | 18/56 | 0.440 |
| Tumor location (head/body/tail) | 46/33/7 | 47/21/6 | 0.396 |
| Tumor size, mean ± SD (cm) | 4.5 ± 1.5 | 4.1 ± 1.5 | 0.828d |
| Patients with CA 19–9 > 1000 U/mL at diagnosisa | 40 (55.5%) | 33 (47.8%) | 0.401 |
| Weight loss > 10% BW at diagnosis, n (%) | 47 (54.7%) | 48 (64.9%) | 0.220 |
| Patients receiving palliative chemotherapy, n (%) | 40 (46.5%) | 53 (71.6%) | 0.001 |
| Number of chemotherapy cycles, median (range)b | 3 (0.3–20) | 4 (1–40) | 0.004c |
| Survival, median (range), days | 95.0 (33–768) | 189 (31–997) | < 0.001c |
| Patients receiving PERT, n (%) | 0 (0%) | 49 (66.2%) | < 0.001 |
aAfter biliary drainage in patients with obstructive jaundice (data available in 88.1% of patients)
bPatients receiving chemotherapy (n = 93)
cMann–Whitney U test
dStudent-t test
Treatment
Ninety-three patients (58.1%) were clinically judged to be suitable for, and accepted, palliative chemotherapy; 46.5% in Group 1 and 71.6% in Group 2 (P < 0.001). Of the 53 patients in Group 2 receiving chemotherapy, 13 were initially considered unsuitable, but were later suitable and treated with chemotherapy after receiving other supportive therapies, including PERT. Chemotherapy regimens were selected following standard guidelines [31] according to age and performance status of the patients, and included gemcitabine monotherapy, gemcitabine-Nab-paclitaxel, folfirinox, and folfox. Sixty-seven patients were unfit for chemotherapy and only received best palliative clinical care.
Pancreatic enzyme replacement therapy was prescribed for 49 patients in Group 2 (30.6% of the total patient population; 66.2% of the patients in Group 2), at a median daily dose of 325,000 Ph.Eur. (75,000 Ph.U. with main meals, 50,000 Ph.U. with snacks), ranging from 200.000 to 400,000 Ph.Eur. daily. Forty of the 49 patients receiving PERT (81.6%) had reported significant weight loss (> 10% bodyweight within 6 months) at diagnosis.
Survival
The overall mortality rate was 86.9% (139/160 patients) at the end of the study period. Median survival was 139 days (95% CI 110–168 days), and was longer in patients with locally advanced tumors (257 days, 95% CI 125–389 days) than in those with metastatic disease (126 days, 95% CI 95–157 days) (p = 0.007). Survival in Group 2 (189 days, 95% CI 167.0–211.0 days), in which presence of PEI and need of PERT was evaluated additionally to standard oncologic therapy, was significantly longer than in Group 1 (95.0 days, 95% CI 75.4–114.6 days), in which only standard oncologic therapy was given (HR 2.12, 95% CI 1.49–3.00; P < 0.001) (Fig. 1).
Fig. 1.
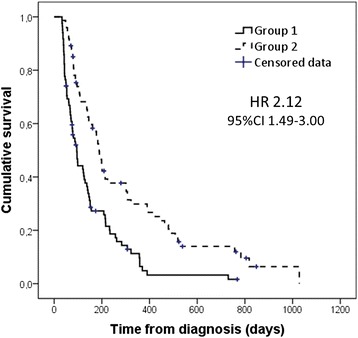
Kaplan–Meier survival curves of patients with unresectable pancreatic cancer in Group 1 (standard oncologic therapy, n = 86) and Group 2 (evaluation of symptoms of pancreatic exocrine insufficiency [PEI] and the need for pancreatic enzyme replacement therapy [PERT] in addition to standard oncologic therapy, n = 74). The hazard ratio (HR) and 95% confidence interval (CI) associated with Group 2 are shown. Log-rank test (Mantel–Cox) P < 0.001
In the univariate and multivariate analysis, chemotherapy and PERT were significantly and independently associated with longer survival in a model that included age and tumor stage (Table 2). In Group 2, the survival of patients treated with PERT due to symptoms suggestive of PEI was similar to that of those who did not receive PERT due to absence of PEI (HR 1.12, 95% CI 0.67–1.89). In contrast, if all patients from Groups 1 and 2 with significant weight loss at diagnosis (> 10% bodyweight within 6 months) were considered (n = 95), the survival of those who received PERT (n = 40) was significantly longer than that of those who did not (median survival 199 days [95% CI 152–246] versus 99 days [95% CI 68–130 days]; HR 2.52, 95% CI 1.55–4.11, P < 0.001) (Fig. 2).
Table 2.
Analysis of factors associated with survival among patients with unresectable pancreatic cancer (n = 160) (Cox proportional hazard regression analysis)
| Univariate analysis | P value | Multivariate analysis | P value | |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||
| Age at diagnosis | 1.03 (1.01–1.05) | < 0.001 | 1.02 (1.00–1.03) | 0.072 |
| Gender | ||||
| Female | 1.00 | 0.517 | ||
| Male | 0.89 (0.63–1.26) | |||
| Tumor stage | ||||
| Locally advanced tumor | 1.00 | 0.008 | 1.00 | 0.001 |
| Metastatic disease | 0.56 (0.37–0.86) | 0.47 (0.30–0.72) | ||
| Tumor location | ||||
| Head | 1.00 | 0.202 | ||
| Body/tail | 1.25 (0.89–1.76) | |||
| Tumor size (cm) | 0.97 (0.84–1.13) | 0.733 | ||
| CA 19–9 levels at diagnosis | ||||
| < 1000 U/mL | 1 | 0.143 | ||
| > 1000 U/mL | 0.76 (0.53–1.10) | |||
| Weight loss at diagnosis | ||||
| < 10% BW | 1 | 0.420 | ||
| > 10% BW | 0.87 (0.62–1.22) | |||
| Chemotherapy | 4.68 (3.18–6.89) | < 0.001 | 3.69 (2.33–5.85) | < 0.001 |
| PERT | 1.80 (1.24–2.62) | 0.002 | 1.81 (1.23–2.66) | 0.002 |
BW body weight, CI confidence interval, HR hazard ratio, PERT pancreatic enzyme replacement therapy
Fig. 2.
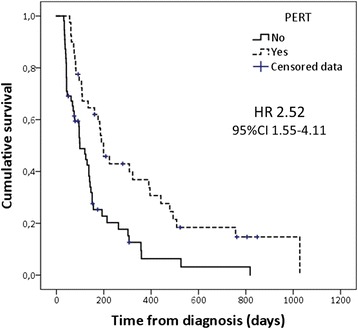
Kaplan–Meier survival curves of patients with unresectable pancreatic cancer and a significant weight loss (> 10% bodyweight within 6 months) at diagnosis (n = 95) according to administration of pancreatic enzyme replacement therapy (PERT). The hazard ratio (HR) and 95% confidence interval (CI) associated with PERT are shown. Log-rank test (Mantel–Cox) P < 0.001
Discussion
This retrospective analysis conducted in patients with unresectable pancreatic cancer (locally advanced or metastatic disease) showed that the evaluation and treatment of PEI, in addition to standard chemotherapy and best supportive care, was associated with significantly prolonged survival compared with standard chemotherapy alone (189 versus 95 days, respectively, P < 0.001). This is especially true for patients with significant weight loss of more than 10% of their bodyweight at diagnosis. Palliative chemotherapy and PERT were independently associated with longer survival.
The incidence of pancreatic cancer is projected to increase to become the second most common cancer by 2030 [2], yet the overall survival rate remains low [1]. Around 80% of patients present with unresectable, advanced disease, which has a median survival of just 6–12 months [4, 13]. Therefore, for this patient population, in which curative surgery is not possible, palliative and supportive care is key to prolonging survival and improving quality of life.
Cachexia can directly reduce the quality of life of patients with cancer, and pancreatic cancer often causes a high prevalence of severe cachexia [32]. Cachexia has previously been shown to be associated with short survival and poor quality of life in patients with pancreatic cancer [5, 19]. Furthermore, tolerance to chemotherapy is reduced in patients with cachexia and malnutrition [33].
In patients with pancreatic cancer, weight loss is obviously multifactorial, but PEI plays a major role [5, 34]. PEI can develop after surgery or may be caused by the tumor obstructing the pancreatic duct [22, 24]. PEI, the presence of metastases, anemia, and hypoalbuminemia are all independent predictors of survival in patients with advanced pancreatic cancer [26]. Furthermore, PEI has been shown to negatively impact the quality of life of patients with pancreatic cancer, mainly due to its associated difficulties in managing diet and gastrointestinal symptoms [35]. The positive impact of PERT on survival reported in the present study in patients with unresectable pancreatic cancer and associated significant weight loss supports the relevant role of PEI in these patients. Conversely, PERT may have no benefit in patients with no significant weight loss.
Pancreatic enzyme replacement therapy, the standard treatment for PEI [23], has been shown to improve weight gain in patients with advanced pancreatic cancer by improving digestion and nutrient absorption [16]. This analysis shows that these improvements are reflected in improved survival rates in patients with unresectable pancreatic cancer who received PERT for PEI, together with standard oncologic therapy, compared with those who received oncologic therapy alone. Interestingly, the rate of survival of patients with PEI can be increased to the level of patients without PEI by giving PERT; this suggests that the enzyme doses used in this study (starting at 200000 Ph.Eur. per day, followed by titration up to a median dose of 325,000 Ph.U. per day according to body weight, symptoms, and nutritional status during follow-up) are effective in treating PEI in these patients. It should be underlined that PERT is well tolerated with mild and infrequent side effects [36], which is especially important for patients with pancreatic cancer. In this analysis, no patient discontinued PERT due to side effects or intolerance.
In the present study, omeprazole was consistently administered to patients with PEI to improve the efficacy of PERT due to the resultant inhibition of gastric acid secretion. It is well known that PEI is not only the consequence of reduced pancreatic secretion of enzymes, but also of reduced bicarbonate secretion [37]. Pancreatic enzymes in enteric-coated preparations require a pH higher than 5.5 for them to be released [38]. Intestinal pH is frequently acidic in patients with PEI, thus avoiding the release of exogenous enzymes within the proximal gut [39]. Addition of a proton pump inhibitor to PERT has been shown to be effective in improving fat digestion in patients with PEI [40]. This is especially important in patients with unresectable pancreatic cancer in whom pancreatic secretion is markedly reduced due to the obstruction of the main pancreatic duct.
Palliative chemotherapy was strongly associated with a longer survival in this study. Nevertheless, only patients who were clinically judged to be suitable for chemotherapy were administered this treatment, whereas those who were unsuitable with a poor performance status were not administered chemotherapy. Thus, there is a very relevant bias in the selection of patients for chemotherapy, and it is not appropriate to use this study to evaluate the efficacy of this form of therapy. Interestingly, the proportion of patients in this study that were suitable for chemotherapy and the number of therapy cycles that these patients tolerated increased with PERT, thus suggesting that PERT may increase tolerance to chemotherapy.
The retrospective study design is the main limitation of the present study; however, the analysis was limited to hard objective variables that are well-recorded in clinical practice. In addition, this study used data from a single central electronic medical record of mandatory use for all the physicians in hospitals and health centers in the north-west region of Spain. This electronic medical record includes information on any medical assistance, as well as any laboratory tests, imaging, and any other examination performed. The use of this central electronic medical record markedly reduces the possibility of missed data. The limited number of patients included is also a limitation in this study, and is mainly due to the single-center study design and the need for pathological confirmation of pancreatic adenocarcinoma for inclusion. The single-center design limits the variability in patients’ care and therapy, and the requirement of pathological confirmation for inclusion avoids biases related to the inclusion of other malignant lesions. These two features could be therefore considered strengths of the study.
Prospective clinical trials are needed to confirm the impact of PERT in patients with unresectable pancreatic cancer, not only on survival, but also on their quality of life and tolerance to chemotherapy. A recent Phase II trial found that the mean percentage change in body weight of patients with unresectable pancreatic cancer did not differ significantly between those randomized to receive PERT or placebo for 8 weeks [41]; however, this study had several limitations. The limitations included: a small sample size; short follow-up period; low enzyme dose (50,000 Ph.Eur. with main meal and 25,000 Ph.Eur. with snacks); tablets were used and not minimicrospheres; and finally, FE-1 levels were above the normal threshold of 200 μg/g stool in 44.1% of patients in the PERT group and 21.2% of those in the placebo group [41]. Larger, well-designed, randomized trials are needed to identify patients who may benefit from PERT.
It is important to note that PERT is generally accepted for the treatment of PEI of any etiology, and until new data are available, it should be considered as part of the therapeutic armamentarium in patients with pancreatic cancer. This is important not only in clinical practice, but also in clinical trials for new chemotherapy agents. The possibility of PEI and its treatment with PERT in patients with pancreatic cancer has been consistently ignored in previous clinical trials of chemotherapy agents. The present study suggests that PERT increases tolerance to chemotherapy, and opens the question of whether the efficacy of standard chemotherapeutic agents could be improved by the inclusion of the evaluation and treatment of PEI with PERT as part of the best standard of care in these patients.
Conclusions
This study showed that evaluation of PEI and its treatment with PERT can prolong survival in patients with unresectable pancreatic cancer. PEI and its treatment should be taken into consideration as part of the best standard of care in these patients, and when assessing future oncology treatment to increase patient survival and quality of life in this population.
Acknowledgements
The authors would like to thank Dr. John Timney of Alpharmaxim Healthcare Communications for medical writing support, which was funded by Abbott Products Operations, Allschwil, Switzerland.
Funding
Funding for editorial assistance and writing support was provided by Abbott Products Operations, Allschwil, Switzerland. The funders were not involved in the study design and collection, analysis or interpretation of data.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the Ethics and Clinical Research Committee of Santiago de Compostela-Lugo, Spain.
Abbreviations
- BW
Body weight
- CI
Confidence interval
- CT
Computed tomography
- EUS
Endoscopic ultrasound
- FE-1
Fecal elastase-1
- FNA
Fine needle aspiration
- FNB
Fine needle biopsy
- HR
Hazard ratio
- PEI
Pancreatic exocrine insufficiency
- PERT
Pancreatic enzyme replacement therapy
- SD
Standard deviation
Authors’ contributions
JEDM: contributed to study concept and design, supervised the study, drafted the manuscript and provided statistical analysis. LNG and JLD contributed to study concept and design, acquired data and drafted the manuscript. IA, JLN, JIG contributed to study concept and design, revised the histological data and imaging records for tumor diagnosis/staging and drafted the manuscript. All authors read and approved the final manuscript.
Authors’ information
J.E.D.-M. is expert pancreatologist, Director of the Department of Gastroenterology and Hepatology and Professor of Medicine at the University Hospital of Santiago de Compostela, Spain. J.L.-N. and J.I.-G. are experts endosonographers and clinical pancreatologists. I.A. is an expert cytopathologist for EUS-guided FNA-FNB samples of pancreatic lesions.
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki and its amendments, and with Good Clinical Practice guidelines. Ethical approval for this study was obtained from the Ethics and Clinical Research Committee of Santiago de Compostela-Lugo, Spain. The need for consent for this study was waived by the institutional review board (Ethics and Clinical Research Committee of Santiago de Compostela-Lugo, Span) due to its retrospective design.
Competing interests
J.E.D.-M. has worked as advisor and speaker for Mylan and Abbott. J.L.-N. and J.I.-G. have worked as speaker for Mylan and Abbott. The remaining authors declare that they have nothing to disclose.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Juan Enrique Domínguez-Muñoz, Email: enriquedominguezmunoz@hotmail.com.
Laura Nieto-Garcia, Email: Laura.Nieto.Garcia@sergas.es.
Javier López-Díaz, Email: Javier.Lopez.Diaz@sergas.es.
Jose Lariño-Noia, Email: joselarnoi@outlook.es.
Ihab Abdulkader, Email: ihab.abdulkader.nallib@sergas.es.
Julio Iglesias-Garcia, Email: julioiglesiasgarcia@gmail.com.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 3.Spanknebel K, Conlon KC. Advances in the surgical management of pancreatic cancer. Cancer J. 2001;7(4):312–323. [PubMed] [Google Scholar]
- 4.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363(9414):1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 5.Bachmann J, Ketterer K, Marsch C, Fechtner K, Krakowski-Roosen H, Buchler MW, Friess H, Martignoni ME. Pancreatic cancer related cachexia: influence on metabolism and correlation to weight loss and pulmonary function. BMC Cancer. 2009;9:255. doi: 10.1186/1471-2407-9-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trede M, Schwall G, Saeger HD. Survival after pancreatoduodenectomy. 118 consecutive resections without an operative mortality. Ann Surg. 1990;211(4):447–458. doi: 10.1097/00000658-199004000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geer RJ, Brennan MF. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg. 1993;165(1):68–72. doi: 10.1016/S0002-9610(05)80406-4. [DOI] [PubMed] [Google Scholar]
- 8.Sener SF, Fremgen A, Menck HR, Winchester DP. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985-1995, using the National Cancer Database. J Am Coll Surg. 1999;189(1):1–7. doi: 10.1016/S1072-7515(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 9.Carpelan-Holmstrom M, Nordling S, Pukkala E, Sankila R, Luttges J, Kloppel G, Haglund C. Does anyone survive pancreatic ductal adenocarcinoma? A nationwide study re-evaluating the data of the Finnish Cancer registry. Gut. 2005;54(3):385–387. doi: 10.1136/gut.2004.047191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann Surg. 1996;223(3):273–279. doi: 10.1097/00000658-199603000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris GJ, Gaskill HV, III, Cruz AB., Jr Carcinoma of the pancreas: a retrospective review. J Surg Oncol. 1990;45(3):184–189. doi: 10.1002/jso.2930450311. [DOI] [PubMed] [Google Scholar]
- 12.Cooperman AM. Pancreatic cancer: the bigger picture. Surg Clin North Am. 2001;81(3):557–574. doi: 10.1016/S0039-6109(05)70143-2. [DOI] [PubMed] [Google Scholar]
- 13.Golden DW, Novak CJ, Minsky BD, Liauw SL. Radiation dose >/=54 Gy and CA 19-9 response are associated with improved survival for unresectable, non-metastatic pancreatic cancer treated with chemoradiation. Radiat Oncol. 2012;7:156. doi: 10.1186/1748-717X-7-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ridwelski K, Meyer F. Current options for palliative treatment in patients with pancreatic cancer. Dig Dis. 2001;19(1):63–75. doi: 10.1159/000050655. [DOI] [PubMed] [Google Scholar]
- 15.Ockenga J. Importance of nutritional management in diseases with exocrine pancreatic insufficiency. HPB (Oxford) 2009;11(Suppl 3):11–15. doi: 10.1111/j.1477-2574.2009.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruno MJ, Haverkort EB, Tijssen GP, Tytgat GN, van Leeuwen DJ. Placebo controlled trial of enteric coated pancreatin microsphere treatment in patients with unresectable cancer of the pancreatic head region. Gut. 1998;42(1):92–96. doi: 10.1136/gut.42.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saito T, Hirano K, Isayama H, Nakai Y, Saito K, Umefune G, Akiyama D, Watanabe T, Takagi K, Hamada T, Takahara N, et al. The role of pancreatic enzyme replacement therapy in unresectable pancreatic cancer: a prospective cohort study. Pancreas. 2017;46(3):341–346. doi: 10.1097/MPA.0000000000000767. [DOI] [PubMed] [Google Scholar]
- 18.Perez MM, Newcomer AD, Moertel CG, Go VL, DiMagno EP. Assessment of weight loss, food intake, fat metabolism, malabsorption, and treatment of pancreatic insufficiency in pancreatic cancer. Cancer. 1983;52(2):346–352. doi: 10.1002/1097-0142(19830715)52:2<346::AID-CNCR2820520228>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 19.Davidson W, Ash S, Capra S, Bauer J. Weight stabilisation is associated with improved survival duration and quality of life in unresectable pancreatic cancer. Clin Nutr. 2004;23(2):239–247. doi: 10.1016/j.clnu.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Richter E, Denecke A, Klapdor S, Klapdor R. Parenteral nutrition support for patients with pancreatic cancer--improvement of the nutritional status and the therapeutic outcome. Anticancer Res. 2012;32(5):2111–2118. [PubMed] [Google Scholar]
- 21.Freelove R, Walling AD. Pancreatic cancer: diagnosis and management. Am Fam Physician. 2006;73(3):485–492. [PubMed] [Google Scholar]
- 22.Dominguez-Munoz JE. Pancreatic enzyme replacement therapy: exocrine pancreatic insufficiency after gastrointestinal surgery. HPB (Oxford). 2009;11(Suppl 3):3–6. doi: 10.1111/j.1477-2574.2009.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dominguez-Munoz JE. Pancreatic enzyme therapy for pancreatic exocrine insufficiency. Curr Gastroenterol Rep. 2007;9(2):116–122. doi: 10.1007/s11894-007-0005-4. [DOI] [PubMed] [Google Scholar]
- 24.Mossner J, Keim V. Pancreatic enzyme therapy. Dtsch Arztebl Int. 2010;108(34–35):578–582. doi: 10.3238/arztebl.2011.0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toouli J, Biankin AV, Oliver MR, Pearce CB, Wilson JS, Wray NH. Management of pancreatic exocrine insufficiency: Australasian Pancreatic Club recommendations. Med J Aust. 2010;193(8):461–467. doi: 10.5694/j.1326-5377.2010.tb04000.x. [DOI] [PubMed] [Google Scholar]
- 26.Partelli S, Frulloni L, Minniti C, Bassi C, Barugola G, D'Onofrio M, Crippa S, Falconi M. Faecal elastase-1 is an independent predictor of survival in advanced pancreatic cancer. Dig Liver Dis. 2012;44(11):945–951. doi: 10.1016/j.dld.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 27.Halloran CM, Cox TF, Chauhan S, Raraty MG, Sutton R, Neoptolemos JP, Ghaneh P. Partial pancreatic resection for pancreatic malignancy is associated with sustained pancreatic exocrine failure and reduced quality of life: a prospective study. Pancreatology. 2011;11(6):535–545. doi: 10.1159/000333308. [DOI] [PubMed] [Google Scholar]
- 28.Seiler CM, Izbicki J, Varga-Szabó L, Czakó L, Fiók J, Sperti C, Lerch MM, Pezzilli R, Vasileva G, Pap A, Varga M, et al. Randomised clinical trial: a 1-week, double-blind, placebo-controlled study of pancreatin 25 000 Ph. Eur. minimicrospheres (Creon 25000 MMS) for pancreatic exocrine insufficiency after pancreatic surgery, with a 1-year open-label extension. Aliment Pharmacol Ther. 2013;37(7):691–702. doi: 10.1111/apt.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landers A, Muircroft W, Brown H. Pancreatic enzyme replacement therapy (PERT) for malabsorption in patients with metastatic pancreatic cancer. BMJ Support Palliat Care. 2016;6(1):75–79. doi: 10.1136/bmjspcare-2014-000694. [DOI] [PubMed] [Google Scholar]
- 30.American Joint Committee on Cancer . Pancreas cancer staging. 2009. [Google Scholar]
- 31.Vera R, Dotor E, Feliu J, Gonzalez E, Laquente B, Macarulla T, Martinez E, Maurel J, Salgado M, Manzano JL. SEOM Clinical Guideline for the treatment of pancreatic cancer (2016) Clin Transl Oncol. 2016;18(12):1172–1178. doi: 10.1007/s12094-016-1586-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wigmore SJ, Plester CE, Richardson RA, Fearon KC. Changes in nutritional status associated with unresectable pancreatic cancer. Br J Cancer. 1997;75(1):106–109. doi: 10.1038/bjc.1997.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dintinjana RD, Redzovic A, Cubranic A, Dintinjana M, Vanis N. Nutrition in cancer patients. Coll Antropol. 2014;38(4):1271–1275. [PubMed] [Google Scholar]
- 34.Vujasinovic M, Valente R, Del Chiaro M, Permert J, Löhr JM. Pancreatic exocrine insufficiency in pancreatic cancer. Nutrients. 2017;9(3):183. doi: 10.3390/nu9030183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gooden HM, White KJ. Pancreatic cancer and supportive care--pancreatic exocrine insufficiency negatively impacts on quality of life. Support Care Cancer. 2013;21(7):1835–1841. doi: 10.1007/s00520-013-1729-3. [DOI] [PubMed] [Google Scholar]
- 36.Thorat V, Reddy N, Bhatia S, Bapaye A, Rajkumar JS, Kini DD, Kalla MM, Ramesh H. Randomised clinical trial: the efficacy and safety of pancreatin enteric-coated minimicrospheres (Creon 40000 MMS) in patients with pancreatic exocrine insufficiency due to chronic pancreatitis - a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2012;36(5):426–436. doi: 10.1111/j.1365-2036.2012.05202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trang T, Chan J, Graham DY. Pancreatic enzyme replacement therapy for pancreatic exocrine insufficiency in the 21(st) century. World J Gastroenterol. 2014;20(33):11467–11485. doi: 10.3748/wjg.v20.i33.11467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Case CL, Henniges F, Barkin JS. Enzyme content and acid stability of enteric-coated pancreatic enzyme products in vitro. Pancreas. 2005;30(2):180–183. doi: 10.1097/01.mpa.0000151577.91790.b1. [DOI] [PubMed] [Google Scholar]
- 39.Layer P, Groger G. Fate of pancreatic enzymes in the human intestinal lumen in health and pancreatic insufficiency. Digestion. 1993;54(Suppl 2):10–14. doi: 10.1159/000201097. [DOI] [PubMed] [Google Scholar]
- 40.Dominguez-Munoz JE, Iglesias-Garcia J, Iglesias-Rey M, Vilarino-Insua M. Optimising the therapy of exocrine pancreatic insufficiency by the association of a proton pump inhibitor to enteric coated pancreatic extracts. Gut. 2006;55(7):1056–1057. doi: 10.1136/gut.2006.094912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woo SM, Joo J, Kim SY, Park SJ, Han SS, Kim TH, Koh YH, Chung SH, Kim YH, Moon H, Hong EK, et al. Efficacy of pancreatic exocrine replacement therapy for patients with unresectable pancreatic cancer in a randomized trial. Pancreatology. 2016;16(6):1099–1105. doi: 10.1016/j.pan.2016.09.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the Ethics and Clinical Research Committee of Santiago de Compostela-Lugo, Spain.


