Abstract
Objective
Pseudomonas aeruginosa is emerging as a highly multidrug-resistant (MDR) nosocomial pathogen. Data on the efficacy of infection control measures in endemic situations are lacking. We investigated the effect of antimicrobial stewardship (AMS) and infection control programs (ICPs) in controlling the resistance of P. aeruginosa at a tertiary hospital center.
Methods
Susceptibility and resistance were investigated using broth microdilution, as per the guidelines of the Clinical and Laboratory Standards Institute. Antibiotic use was restricted through AMS, which included a classification management system for antibiotic use. The ICPs included environmental cleaning and disinfection, hand hygiene, active surveillance of P. aeruginosa, and education about infection control.
Results
A total of 2,241 P. aeruginosa isolates were evaluated between 2012 and 2017. Sensitivity and resistance of the isolates to the antipseudomonal antimicrobials colistin and tigecycline were stable. The sensitivity and resistance to other antipseudomonal antimicrobials improved after 2014, after the AMS and ICPs were implemented in 2013. The use of alcohol-based hand gel significantly increased from 0.6 to 10.9 L per 1,000 patient-days (PD) during the study period (P=0.005). The incidence rates of extensively drug-resistant (XDR) and MDR P. aeruginosa showed a sustained decrease from 2013 (4.9 and 22%) to 2017 (1 and 15%), respectively. The yearly consumption of antimicrobial agents also showed a sustained and significant decrease from 45 defined daily doses (DDDs) per 1,000 PD to 38.15 DDDs per 1,000 PD (P=0.04). A significant correlation was found between the incidence rate of MDR P. aeruginosa and the consumption of antimicrobial agents (P=0.01).
Conclusion
Monitoring of P. aeruginosa, AMS, and comprehensive ICPs could be one of the best and effective methods to prevent the development of resistance in P. aeruginosa.
Keywords: Pseudomonas aeruginosa, antibiotic stewardship, infection control, antibiotic resistance
Introduction
Widespread antibiotic use has accelerated the incidence of antibiotic resistance (ABR). Although the exact magnitude of this global problem and its effect on human health are largely unknown, ABR against common bacterial pathogens has reached concerning levels in many parts of the world. As a result, many available treatment options are becoming ineffective.1 This situation was summarized by the World Health Day 2011 slogan “Combat antibiotic resistance: no action today, no cure tomorrow”. ABR is complex and driven by many interrelated factors, including knowledge, attitudes, perceptions, expectations, time constraints, economic incentives, cultural factors, health system characteristics, and regulations.2–6 A recognized key driver is the use, misuse, or overuse of antibiotics and unregulated consumer access to antibiotics.7,8 Although antibiotic overuse plays a pivotal role, underuse through inadequate dosing and poor adherence also plays an important role in ABR.3 In this regard, a complex association between outpatient antibiotic consumption and ABR has been observed in Europe.9,10 In addition, a strong link has been reported to exist between inpatient antibiotic consumption and the rate of ABR.9
Pseudomonas aeruginosa is one of the most important nosocomial pathogens.11 The emergence of multidrug-resistant (MDR) P. aeruginosa strains that are resistant to all the known classes of antimicrobials, except for one or two, is becoming a major public health concern.12 Unfortunately, new drugs are not being manufactured at a pace that comes anywhere close to the rate at which microbes are gaining resistance to the existing drugs. Several studies have shown that better hand hygiene, isolation of infected patients, environmental disinfection, and targeted surveillance can improve the success rate of controlling MDR Acinetobacter baumannii infection;13–15 however, there is no report on P. aeruginosa yet.
Although curbing the development of resistance in P. aeruginosa is important in hospitals, there are limited reports on MDR P. aeruginosa control measures in China. Antimicrobial stewardship (AMS) and infection control programs (ICPs) are coordinated strategies that are designed to promote and increase the appropriate use of antimicrobials, and they are important for conserving the effectiveness of antibiotics. In our study, we estimated the effects of AMS and ICPs on the resistance of P. aeruginosa, especially MDR P. aeruginosa, in a tertiary hospital center. To evaluate the efficacy of the infection control measures, the incidence of extensively drug-resistant (XDR) and MDR P. aeruginosa, the use of alcohol-based hand gel (ABHG), and the consumption of antibiotics during the study period were investigated.
Methods
The Fourth Affiliated Hospital of Harbin Medical University, a 2,600-bed teaching hospital located in Harbin, China, has a 30-bed medical intensive care unit (ICU), a 20-bed surgical ICU, and a 15-bed cardiac care unit (CCU). Due to an obvious increase in the incidence of ABR P. aeruginosa infections since 2013, different ICPs have been launched sequentially at this hospital. In this study, we retrospectively evaluate the efficacy of the intervention measures that were in effect between January 2012 and December 2017.
Bacterial isolates
Between January 2012 and December 2017, clinical isolates were obtained from emergency rooms, medical and surgical wards, clinics, and ICUs at the Fourth Affiliated Hospital of Harbin Medical University. Clinical isolates from blood; respiratory, urinary, and wound infections; and other sources (pleural effusion, bile, and so on) were obtained for every year. Isolate identification was executed at the reference site as required (ie, antimicrobial susceptibility patterns did not fit the reported identification). Isolates were transferred to Amies semisolid transport media, shipped to the coordinating laboratory, subcultured on suitable media, and reserved in skim milk at −80°C until the minimum inhibitory concentration (MIC) was determined.
Antimicrobial sensitivity and resistance
The in vitro activity of regularly utilized antipseudomonal antimicrobials was determined by broth microdilution in line with the guidelines of the Clinical and Laboratory Standards Institute (CLSI).16,17 The antimicrobial activity of the antibiotics was determined using 96-well broth microdilution panels that were prepared in house. Colistin was examined in line with the recommendations of the CLSI–European Committee on Antimicrobial Susceptibility Testing (EUCAST).18 Polysorbate-80 was not added to the panels that were used to determine the MIC of colistin. The interpretive standards for MIC of the antibiotics were determined based on the CLSI breakpoints.17 MDR P. aeruginosa was defined as isolates that were not sensitive to at least one antibiotic from three or more different classes. XDR P. aeruginosa was defined as a subset of MDR P. aeruginosa isolates that were not sensitive to at least one antibiotic from five different classes. The XDR subset contained the total MDR isolates. For the purpose of our study, the five antibiotic classes included were aminoglycosides (gentamicin and amikacin), fluoroquinolones (ciprofloxacin), antipseudomonal cephalosporins (ceftazidime and cefoperazone/sulbactam), antipseudomonal penicillins (piperacillin/tazobactam), and carbapenems (meropenem and imipenem). Colistin and tigecycline were not utilized for the identification of MDR or XDR P. aeruginosa.
Infection control measures
A comprehensive and multifaceted six-point ICP was instituted in January 2013:
Instructions for fundamental hygiene (ie, hand washing and suitable use of gloves) were provided via an education plan. To sustain and promote fundamental hygiene, dispensers for ABHG were fixed in every room and aisle in 2013.
Instructions for contact with and seclusion of patients infected with MDR P. aeruginosa were provided, including placing patients in single rooms or in open-structured wards. These practices were enforced daily by infection control experts. Precautions for contact with infected patients were taken during the entire hospitalization period and enforced for every diagnostic or therapeutic course. A list of infected patients for seclusion and cautionary contact was also created. Moreover, these precautions were taken in patients admitted to the hospital again with a history of MDR P. aeruginosa infection. This program was enforced until colonization was removed.
Positive monitoring was performed via weekly rectal, perineal, and pharyngeal swabs and/or tracheal bronchus aspirate in all the patients who were admitted to the ICU for >2 days during times of ongoing transmission. Positive monitoring was strictly executed, especially in the ICU.
Cultures from the hands of the medical staffs who had cared for the infected patients and the environment were analyzed at three time points during their ICU stay to investigate cross-infection and spread of infection to the staff.
A rigid environmental cleaning plan for wards and for any equipment that infected patients might have touched was executed according to the suggestions of the Centers for Disease Control and Prevention.19 Some equipment (eg, stethoscopes) was used only for the source patient whenever possible and was kept within the confines of the ward.
Periodical conferences were held with the health care workers of the infected areas every 2–4 weeks by the infection control group and were compulsory for the ICU workers. Moreover, all health care workers were regularly notified about the development of infection rates as part of the educational plan (monthly in the ICU and CCU and quarterly in the other wards).
Antibiotic stewardship
From 2013 to 2017, antibiotic use was strictly restricted through AMS, which included the following classification management system for antibiotic use:
Unrestricted use of antimicrobial agents: this is applicable in the case of antibacterial drugs that have been proven to be safe, effective, and inexpensive in clinical practice for a long time and have little influence on bacterial resistance. The doctor is granted unrestricted use of the antimicrobial drug and can prescribe them after examination.
Restricted use of antimicrobial agents: there are limitations based on the efficacy, safety, cost, and so on, of the drug, in the case of antibiotics that have an obvious influence on bacterial resistance. Therefore, their use should be controlled. Doctors with an intermediate-level or above intermediate-level qualification are granted restricted use of the antimicrobial drug and can prescribe them after training and examination.
Special use of antimicrobial agents: this is applicable in the case of antibiotics for which the adverse effects are obvious. In such cases, the agents should be prescribed with caution to prevent the bacteria from becoming too resistant and producing severe consequences. Limited clinical data are available on the efficacy or safety of new antibiotics or their superiority to those currently in use. Furthermore, these drugs are expensive and their use must therefore be strictly controlled. Doctors qualified at the sub-senior or above sub-senior level are granted special use of such antimicrobial drugs and can prescribe them after training and examination.
Data collection and analysis
The data of all patients in whom P. aeruginosa infection was confirmed were reviewed between 2012 and 2017. Isolates were defined as susceptible, resistant, or intermediate to an antibiotic for statistical analysis. Multiple positive isolates from one patient were considered as a single sample. ABHG use was calculated as the number of liters per 1,000 patient-days (PD) and utilized as a marker for compliance with hand hygiene rules. Antibiotic consumption was calculated as the defined daily dose (DDD) per 1,000 PD in line with the suggestions of the World Health Organization.20 Data on antibiotic consumption between January 2012 and December 2017 were obtained from the Hospital Pharmacy Service. The seven types of antibiotic agents tested in this study were β-lactam/β-lactamase inhibitor combinations (piperacillin/tazobactam and cefoperazon/sulbactam), extended-spectrum cephalosporins (ceftazidime), carbapenems (imipenem and meropenem), quinolones (ciprofloxacin), aminoglycosides (gentamicin and amikacin), glycopeptides (colistin), and tetracyclines (tigecycline). Consumption of all the antibiotics was calculated on the basis of the sum of values of the aforementioned broad-spectrum antibiotics.
The χ2 test was utilized to determine the rate of susceptibility and resistance to P. aeruginosa and ABHG consumption during the study period. Segmented regression analysis was performed to test obvious changes in the susceptibility and resistance of P. aeruginosa. Spearman’s rank association was applied to confirm the correlation between ABHG use or antibiotic consumption and the rate of MDR P. aeruginosa. All examinations were two-tailed, and P<0.05 was considered to indicate statistical significance. Analyses were performed using SPSS v. 17 (SPSS Inc., Chicago, IL, NY).
Results
A total of 2,241 P. aeruginosa clinical isolates were collected between January 2012 and December 2017. The number of isolates obtained annually varied between 221 and 486. Table 1 presents data related to antibiotic susceptibility and resistance of the isolates. Colistin and tigecycline were the most effective antipseudomonal antibiotics assessed: 99.6 and 98.83% of the isolates showed in vitro susceptibility to colistin and tigecycline, respectively, and 0.4 and 1.17% showed resistance to colistin and tigecycline, respectively. In contrast, gentamicin and amikacin were the least effective antibiotics evaluated: only 68.4% of the isolates were susceptible and 29.01% were resistant to gentamicin and 76.07% were susceptible and 22.22% were resistant to amikacin. Colistin and tigecycline remained the most effective antipseudomonal antibiotics assessed, with 98.41 and 95.6% of the MDR P. aeruginosa isolates showing in vitro susceptibility to colistin and tigecycline, respectively. Furthermore, colistin and tigecycline were the most effective antipseudomonal antibiotics assessed, with 94 and 91% of the XDR P. aeruginosa isolates showing in vitro susceptibility to colistin and tigecycline, respectively.
Table 1.
Antimicrobial susceptibility of P. aeruginosa clinical isolates obtained from patients at a hospital center in China, 2012–2017
| Antimicrobial | All isolates (n=2,241)
|
MDR (n=414)
|
XDR (n=78)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| MIC (μg/mL)
|
Range of values
|
Breakpoint interpretationsa
|
|||||||
| MIC50 | MIC90 | Min | Max | %S | %I | %R | %S | %S | |
| Amikacin | 4 | 16 | ≤1 | >64 | 76.07 | 1.71 | 22.22 | 56 | 24.5 |
| Ceftazidime | 4 | 32 | ≤0.25 | >32 | 74.82 | 7.7 | 17.48 | 20.4 | 0 |
| Ciprofloxacin | 0.25 | 4 | ≤0.06 | >16 | 73 | 10.1 | 16.9 | 23 | 0 |
| Colistin | 1 | 2 | ≤0.06 | >16 | 99.6 | 0 | 0.4 | 98.41 | 95.6 |
| Gentamicin | 2 | 8 | ≤0.5 | >32 | 68.4 | 2.59 | 29.01 | 45.1 | 1.3 |
| Meropenem | 0.5 | 8 | ≤0.03 | >32 | 76.55 | 6.2 | 17.25 | 71 | 67 |
| Piperacillin/tazobactam | 4 | 64 | ≤1 | >128 | 81.07 | 5.11 | 13.82 | 22.3 | 0 |
| Cefoperazone/sulbactam | 4 | 64 | ≤1 | >128 | 76.4 | 17.49 | 6.11 | 70.35 | 69 |
| Imipenem | 0.5 | 8 | ≤0.03 | >32 | 71.23 | 8.85 | 19.92 | 60 | 65 |
| Tigecycline | 1 | 2 | ≤0.06 | >16 | 98.83 | 0 | 1.17 | 94 | 91 |
Notes:
%S = %susceptible, %I = %intermediate, %R = %resistant; breakpoint interpretation: amikacin S≤16 μg/mL, I=32 μg/mL, R≥64 μg/mL; ceftazidime S≤8 μg/mL, I=16 μg/mL, R≥32 μg/mL; ciprofloxacin S≤1 μg/mL, I=2 μg/mL, R≥4 μg/L; colistin S≤2 μg/mL, I=4 μg/mL, R≥8 μg/mL; gentamicin S≤4 μg/mL, I=8 μg/mL, R≥16 μg/mL; meropenem S≤2 μg/mL, I=4 μg/mL, R≥8 μg/mL; piperacillin/tazobactam S≤16/4 μg/mL, I=32/4 μg/mL, R≥128/4 μg/mL; cefoperazone/sulbactam S≤16/4 μg/mL, I=32/4 μg/mL, R≥128/4 μg/mL; imipenem S≤2 μg/L, I=4 μg/mL, R≥8 μg/mL; tigecycline S≤2 μg/mL, I=4 μg/mL, R≥8 μg/mL.
Abbreviations: Max, maximum; MDR, multidrug resistant; Min, minimum; MIC, minimum inhibitory concentration; P. aeruginosa, Pseudomonas aeruginosa; XDR, extensively drug resistant.
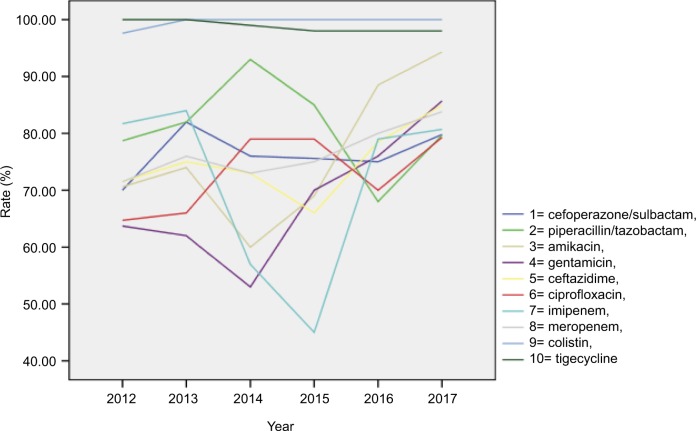
The sensitivity of the isolates, stratified by study year, is presented in Table 2 and Figure 1. Over the 6-year study period, colistin and tigecycline were found to be stable and to have the highest susceptibility among the antipseudomonal antimicrobials. After the interventions were implemented in 2013, the susceptibility of the other antipseudomonal antimicrobials, except for piperacillin/tazobactam, obviously improved. Only susceptibility to piperacillin/tazobactam was found to be reduced.
Table 2.
Antimicrobial susceptibility of P. aeruginosa clinical isolates obtained from patients at a hospital center in China
| Antimicrobial | %Susceptible
|
P-value | |||||
|---|---|---|---|---|---|---|---|
| 2012 (n=370) | 2013 (n=344) | 2014 (n=406) | 2015 (n=414) | 2016 (n=486) | 2017 (n=221) | ||
| Amikacin | 70.6 | 74 | 60 | 69 | 88.5 | 94.3 | <0.001 |
| Ceftazidime | 71.5 | 75 | 73 | 66 | 78.5 | 84.9 | <0.001 |
| Ciprofloxacin | 64.7 | 66 | 79 | 79 | 70 | 79.3 | <0.001 |
| Colistin | 97.6 | 100 | 100 | 100 | 100 | 100 | NS |
| Gentamicin | 63.7 | 62 | 53 | 70 | 76 | 85.7 | <0.001 |
| Meropenem | 71.5 | 76 | 73 | 75 | 80 | 83.8 | <0.001 |
| Piperacillin/tazobactam | 78.7 | 82 | 93 | 85 | 68 | 79.7 | <0.001 |
| Cefoperazone/sulbactam | 70 | 82 | 76 | 75.6 | 75 | 79.8 | <0.001 |
| Imipenem | 81.7 | 84 | 57 | 45 | 79 | 80.7 | <0.001 |
| Tigecycline | 100 | 100 | 99 | 98 | 98 | 98 | NS |
| % of isolates with the MDR or XDR phenotype | |||||||
| MDR | 20 | 22 | 18.5 | 17 | 17.4 | 15 | 0.04 |
| XDR | 5.8 | 4.9 | 3.5 | 2.1 | 1.2 | 1 | nd |
Note: nd, statistical analysis not performed for the XDR subset due to the small number of isolates.
Abbreviations: MDR, multidrug resistant; nd, no data; NS, not significant; P. aeruginosa, Pseudomonas aeruginosa; XDR, extensively drug resistant.
Figure 1.
Antimicrobial susceptibility of Pseudomonas aeruginosa clinical isolates obtained from patients at a hospital center in China, 2012–2017.
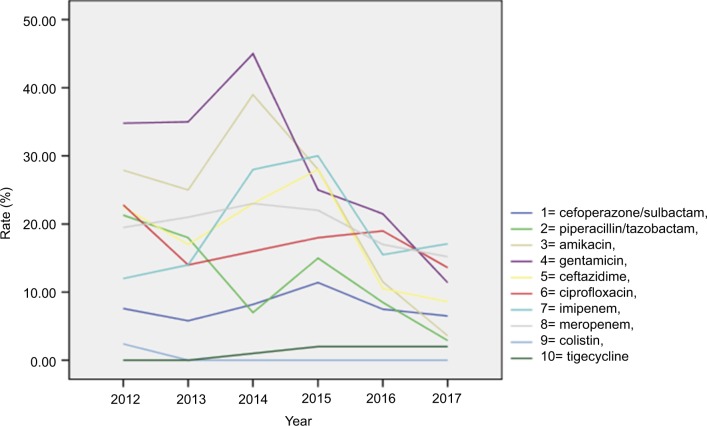
Resistance to the isolates, stratified by study year, is presented in Table 3 and Figure 2. Colistin and tigecycline were found to be stable and to have the lowest resistance as antipseudomonal antimicrobials. The resistance to other antipseudomonal antimicrobials clearly decreased after the interventions were implemented in 2013.
Table 3.
Antimicrobial resistance of Pseudomonas aeruginosa clinical isolates obtained from patients at a hospital center in China
| Antimicrobial | %Resistance
|
P-value | |||||
|---|---|---|---|---|---|---|---|
| 2012 (n=370) | 2013 (n=344) | 2014 (n=406) | 2015 (n=414) | 2016 (n=486) | 2017 (n=221) | ||
| Amikacin | 27.9 | 25 | 39 | 28 | 11.5 | 3.6 | <0.001 |
| Ceftazidime | 22.5 | 17 | 23 | 28 | 10.5 | 8.6 | <0.001 |
| Ciprofloxacin | 22.8 | 14 | 16 | 18 | 19 | 13.6 | <0.001 |
| Colistin | 2.4 | 0 | 0 | 0 | 0 | 0 | NS |
| Gentamicin | 34.8 | 35 | 45 | 25 | 21.5 | 11.4 | <0.001 |
| Meropenem | 19.5 | 21 | 23 | 22 | 17 | 15.2 | <0.001 |
| Piperacillin/tazobactam | 21.3 | 18 | 7 | 15 | 8.5 | 2.9 | <0.001 |
| Cefoperazone/sulbactam | 7.6 | 5.8 | 8.2 | 11.4 | 7.5 | 6.5 | <0.001 |
| Imipenem | 12 | 14 | 28 | 30 | 15.5 | 17.1 | <0.001 |
| Tigecycline | 0 | 0 | 1 | 2 | 2 | 2 | NS |
Abbreviation: NS, not significant.
Figure 2.
Antimicrobial resistance of P. aeruginosa clinical isolates obtained from patients at a hospital center in China, 2012–2017
Abbreviation: P. aeruginosa, Pseudomonas aeruginosa.
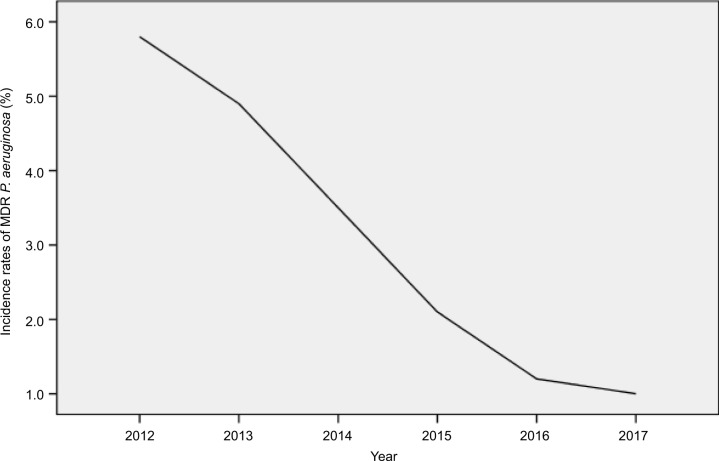
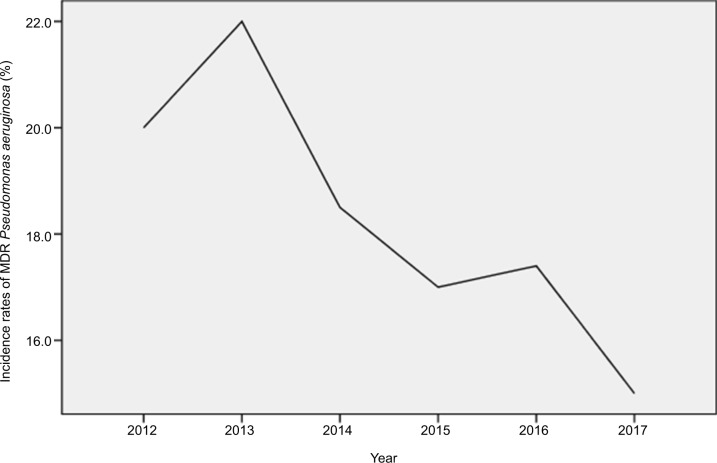
Overall, 18.5% of the P. aeruginosa isolates assessed were MDR and 3.5% of the P. aeruginosa isolates assessed were XDR. As shown in Table 2 and Figure 3, the percentage of MDR isolates ranged between 15 and 22 across the study years. AMS and ICPs were strictly implemented in 2013. The incidence rate of MDR P. aeruginosa decreased from 22 to 15%, and a statistically significant decline was observed in the MDR P. aeruginosa isolates over time (P=0.04). Segmented regression analysis showed a significant change in the trend of incidence rate (r=−0.018, P=0.032). The incidence rate of XDR P. aeruginosa decreased from 5.8 to 1%, and an obvious reduction trend was observed in the XDR isolates over time (Table 2 and Figure 4).
Figure 3.
Incidence rates of MDR P. aeruginosa during the study period at a single hospital center in China.
Abbreviations: MDR, multidrug-resistant; P. aeruginosa, Pseudomonas aeruginosa.
Figure 4.
Incidence rates of XDR P. aeruginosa during the study period at a single hospital center in China.
Abbreviation: XDR, extensively drug-resistant.
ABHG use increased gradually from 2013 to 2017 (Table 4), and the consumption per 1000 PD was as follows: 0.6 L in 2012, 3.8 L in 2013, 5.7 L in 2014, 8.5 L in 2015, 9.8 L in 2016, and 10.9 L in 2017 (P=0.005). ABHG use was not significantly correlated to the incidence rate of MDR P. aeruginosa (r=0.14; P=0.3).
Table 4.
Consumption of antimicrobial agents and ABHG during the study period at a hospital center in China
| Year
|
P-value | ||||||
|---|---|---|---|---|---|---|---|
| 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | ||
| DDD (g/1,000 patient-days) | 45 | 44.19 | 43.37 | 39.52 | 38.55 | 38.15 | 0.04 |
| ABHG (L/1,000 patient-days) | 0.6 | 3.8 | 5.7 | 8.5 | 9.8 | 10.9 | 0.005 |
Abbreviations: ABHG, alcohol-based hand gel; DDD, daily defined dose.
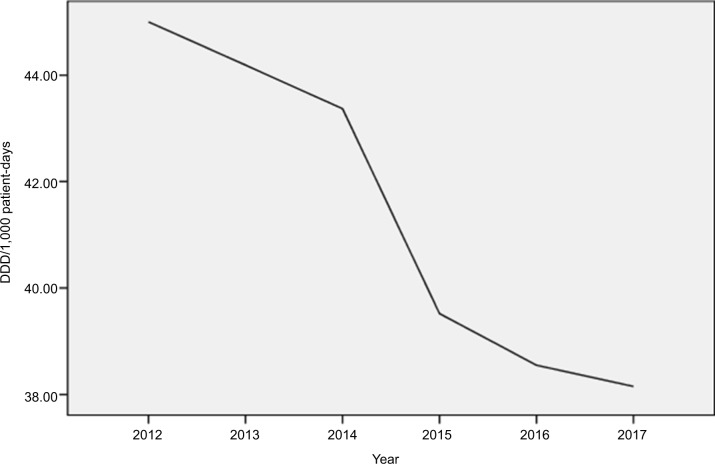
The consumption pattern of antimicrobials is shown in Figure 5 and Table 4. AMS and ICPs were strictly implemented from 2013 to 2017. The DDD per 1000 PD of total antibiotics decreased gradually from 44.19 to 38.15, and the reduction was significant (P=0.04). Furthermore, antibiotic usage was significantly correlated to the incidence rate of MDR P. aeruginosa (r=0.63, P=0.025).
Figure 5.
Consumption of antimicrobial agents during the study period.
Note: Data are presented as DDD per 1,000 patient-days.
Abbreviation: DDD, daily defined dose.
Discussion
The increasing incidence of MDR P. aeruginosa is a consequence of the ability of this microorganism to develop resistance to almost all antimicrobial agents during antimicrobial chemotherapy, either by chromosomal gene mutation selection or by horizontal gene transfer.21,22 The escalating situation of MDR P. aeruginosa should be addressed promptly, and the medical profession should undertake most responsibilities, to save one of the antibiotic’s most precious and long-term resources.23 Cutting down on the unreasonable usage of antibiotics has been shown to improve patient prognosis and contain the adverse effects of antimicrobial usage (including antimicrobial resistance, side effects of antibiotics, and medical costs).24 Although this is not a simple task especially given the associated cultural factors,25,26 strategic interventions for behavior change to promote prudent antibiotic use and prescription are urgently required and attainable, as demonstrated in many European countries.25,27 Simple solutions that consider local cultures and that can be scaled up to become self-sustainable should be proposed.28 However, changing antibiotic prescription behavior is a complex initiative that requires multifaceted interventions.29
AMS is one the measures that was planned to impel and enhance the reasonable usage of antibiotics and is important for ensuring the continued effectiveness of antimicrobials. Australia’s first National Antimicrobial Resistance policy for 2015–2019 mentions the demand for resources to sustain the implementation of AMS for all hospitals.30 In particular, it is important for medical workers to make patients aware that they are twice as likely to carry resistant bacteria after antimicrobial use as someone who has not used the antimicrobial before.31–33 One study showed that providing education about ICPs, hand hygiene, and the judicious use of carbapenems may decrease the nosocomial incidence of carbapenem-resistant A. baumannii.34 Another study showed that apart from infection control measures, the removal of key antibiotic selection pressures during a national antibiotic stewardship intervention can result in large and sustained reduction in hospital-associated and community-associated methicillin-resistant Staphylococcus aureus infection.35 Improved monitoring of hospital-acquired infections and effective infection control measures may be the best way to solve the present problem.36
In our study, 2,241 P. aeruginosa clinical isolates were collected between January 2012 and December 2017. AMS and ICPs were strictly implemented in 2013, and the incidence rate of MDR P. aeruginosa decreased from 22 to 15%. A statistically obvious reduction in MDR P. aeruginosa isolates was found over time. The incidence rate of XDR P. aeruginosa decreased from 5.8 to 1%, and an obvious reduction was found in the number of XDR isolates. ABHG use was not significantly related to the incidence of MDR P. aeruginosa, whereas antibiotic consumption was significantly related to the incidence of MDR P. aeruginosa.
AMS and ICPs were strictly implemented from 2013 to 2017. The DDD per 1,000 PD of total antibiotics decreased gradually from 44.19 to 38.15, and the reduction was statistically significant. The DDD is ~23/1,000 population/day in Australia and 11/1,000 population/day in the Netherlands.37,38 Clearly, our values are higher than those reported in Australia and the Netherlands. This may be related to the insufficient of administration of antibiotic application.
A hospital center study in Southwest China reported that piperacillin/tazobactam was the most effective antibiotic against P. aeruginosa isolates and that antibiotic use prior to admission was an independent risk factor for P. aeruginosa infection.39 In our study, however, colistin and tigecycline were found to be the most effective antipseudomonal antibiotics assessed. Gentamicin and amikacin were the least effective antibiotics evaluated, and susceptibility to only piperacillin/tazobactam was found to be deteriorated. The differences in the reports of these two hospitals in the same country could be explained by differences in the habit, frequency, and region of antibiotic use.
Widespread transmissible colistin resistance is a major concern in the current era of MDR gram-negative infections because colistin is commonly used to treat infections caused by these organisms, despite its nephrotoxicity and limitations in determining susceptibility and appropriate dosing regimens.40,41 In spite of the above shortcomings of the two drugs, in our study, however, colistin and tigecycline were the most effective antipseudomonal antibiotics assessed.
Our study findings indicated that the trends in the resistance of P. aeruginosa to antimicrobial agents were influenced by the antibiotic stewardship and ICPs implemented at the Chinese university hospital and that antibiotic usage was significantly related to the incidence rate of MDR P. aeruginosa. Currently, new antimicrobials with resistance to P. aeruginosa are not being developed at a fast enough pace. Therefore, controlling the resistance of P. aeruginosa by combining antibiotic stewardship and ICPs is necessary. The monitoring of P. aeruginosa, AMS, and comprehensive ICPs may be one of the best and most effective ways to solve the developing resistance of P. aeruginosa.
Conclusion
Our study showed that the concomitant implementation of strict AMS and comprehensive ICPs could effectively control the resistance of P. aeruginosa at the current tertiary hospital center over a 6-year period. The yearly consumption of antimicrobial agents with AMS significantly decreased, and the use of ABHG significantly increased over the study period. Furthermore, the incidence rates of MDR and XDR P. aeruginosa showed a sustained decrease from 2013 to 2017. Among the common antipseudomonal antimicrobials examined, colistin and tigecycline were found to be the most stable in terms of the susceptibility and resistance of the isolates, while the other antipseudomonal antimicrobials showed significant fluctuation and improvement after 2014. A statistically obvious correlation was found between the incidence rate of MDR P. aeruginosa and the consumption of antimicrobial agents. However, endemic MDR P. aeruginosa was effectively controlled with strict AMS and comprehensive ICPs, including hospital staff education, active surveillance of cultures, limited contact with and isolation of patients, environmental cleaning, hand hygiene promotion, and a classification management system for antibiotic use, among others. Without the application of these interventions simultaneously, successful control of the resistance of MDR P. aeruginosa would have been difficult. Thus, the monitoring of P. aeruginosa, AMS and comprehensive ICPs may be one of the best and effective ways to resolve the problem of resistance of P. aeruginosa at the current tertiary hospital center.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.World Health Organization . Antimicrobial Resistance: Global Report on Surveillance. Geneva: World Health Organization; 2014. [Google Scholar]
- 2.Vallin M, Polyzoi M, Marrone G, Rosales-Klintz S, Tegmark Wisell K, Stålsby Lundborg C. Knowledge and attitudes towards antibiotic use and resistance - a latent class analysis of a Swedish population-based sample. PLoS One. 2016;11(4):e0152160. doi: 10.1371/journal.pone.0152160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . The Evolving Threat of Antimicrobial Resistance: Options for Action. Geneva: World Health Organization; 2012. [Google Scholar]
- 4.Björkman I, Berg J, Röing M, Erntell M, Lundborg CS. Perceptions among Swedish hospital physicians on prescribing of antibiotics and antibiotic resistance. Qual Saf Health Care. 2010;19(6):e8. doi: 10.1136/qshc.2008.029199. [DOI] [PubMed] [Google Scholar]
- 5.Sahoo KC, Tamhankar AJ, Johansson E, Lundborg CS. Antibiotic use, resistance development and environmental factors: a qualitative study among healthcare professionals in Orissa, India. BMC Public Health. 2010;10:629. doi: 10.1186/1471-2458-10-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization . WHO Global Strategy for Containment of Antimicrobial Resistance. Geneva: World Health Organization; 2001. [Google Scholar]
- 7.Laxminarayan R, Duse A, Wattal C, et al. Antibiotic resistance the need for global solutions. Lancet Infect Dis. 2013;13(12):1057–1098. doi: 10.1016/S1473-3099(13)70318-9. [DOI] [PubMed] [Google Scholar]
- 8.Morgan DJ, Okeke IN, Laxminarayan R, Perencevich EN, Weisenberg S. Non-prescription antimicrobial use worldwide: a systematic review. Lancet Infect Dis. 2011;11(9):692–701. doi: 10.1016/S1473-3099(11)70054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goossens H, Ferech M, Vander Stichele R, Elseviers M, ESAC Project Group Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005;365(9459):579–587. doi: 10.1016/S0140-6736(05)17907-0. [DOI] [PubMed] [Google Scholar]
- 10.Mölstad S, Lundborg CS, Karlsson AK, Cars O. Antibiotic prescription rates vary markedly between 13 European countries. Scand J Infect Dis. 2002;34(5):366–371. doi: 10.1080/00365540110080034. [DOI] [PubMed] [Google Scholar]
- 11.Hoiby N, Ciofu O, Bjarnsholt T. Pseudomonas. In: Jorgensen JH, Pfaller MA, Carroll KC, Funke G, Landry ML, Richter SS, Warnock DW, editors. Manual of Clinical Microbiology. 11th ed. Washington, DC: ASM Press; 2015. pp. 773–790. [Google Scholar]
- 12.Ciofi Degli Atti M, Bernaschi P, Carletti M, et al. An outbreak of extremely drug-resistant Pseudomonas aeruginosa in a tertiary care pediatric hospital in Italy. BMC Infect Dis. 2014;14:494. doi: 10.1186/1471-2334-14-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karageorgopoulos DE, Falagas ME. Current control and treatment of multidrug-resistant Acinetobacter baumannii infections. Lancet Infect Dis. 2008;8(12):751–762. doi: 10.1016/S1473-3099(08)70279-2. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez-Bano J, Garcia L, Ramirez E, et al. Long-term control of hospital-wide, endemic multidrug-resistant Acinetobacter baumannii through a comprehensive “bundle” approach. Am J Infect Control. 2009;37(9):715–722. doi: 10.1016/j.ajic.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Apisarnthanarak A, Warren DK, Fraser VJ. Creating a cohort area to limit transmission of pandrug-resistant Acinetobacter baumannii in a Thai tertiary care center. Clin Infect Dis. 2009;48(10):1487–1488. doi: 10.1086/598512. [DOI] [PubMed] [Google Scholar]
- 16.Clinical and Laboratory Standards Institute . M07-A10 Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard. 10th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2015. [Google Scholar]
- 17.Clinical and Laboratory Standards Institute . M100S Performance Standards for Antimicrobial Susceptibility Testing. 26th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2016. [Google Scholar]
- 18.European Committee on Antimicrobial Susceptibility Testing [homepage on the Internet] Recommendations for MIC Determination of Colistin (Polymyxin E) as Recommended by the Joint CLSI-EUCAST Polymyxin Breakpoints Working Group. [Accessed March 22, 2016]. Available from: www.eucast.org.
- 19.Sehulster L, Chinn RY, Centers for Disease Control and Prevention. HICPAC Guidelines for environmental infection control in healthcare facilities: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC) MMWR Recomm Rep. 2003;52(RR–10):1–42. [PubMed] [Google Scholar]
- 20.WHO Collaborating Centre for Drug Statistics Methodology . Guidelines for ATC Classification and DDD Assignment. Oslo, Norway: Norwegian Institute of Public Health; 2009. [Google Scholar]
- 21.Xavier DE, Picao RC, Girardello R, et al. Efflux pumps expression and its association with porin down-regulation and b-lactamase production among Pseudomonas aeruginosa causing bloodstream infections in Brazil. BMC Microbiol. 2010;10:1–7. doi: 10.1186/1471-2180-10-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zavascki AP, Gaspareto PB, Martins AF, Gonçalves AL, Barth AL. Outbreak of carbapenem-resistant Pseudomonas aeruginosa producing SPM-1 metallo-{beta}-lactamase in a teaching hospital in southern Brazil. J Antimicrob Chemother. 2005;56(6):1148–1151. doi: 10.1093/jac/dki390. [DOI] [PubMed] [Google Scholar]
- 23.Carlet J, Collignon P, Goldmann D, et al. Society’s failure to protect a precious resource: antibiotics. Lancet. 2011;378(9788):369–371. doi: 10.1016/S0140-6736(11)60401-7. [DOI] [PubMed] [Google Scholar]
- 24.MacDougall C, Polk RE. Antimicrobial stewardship programs in health care systems. Clin Microbiol Rev. 2005;18(4):638–656. doi: 10.1128/CMR.18.4.638-656.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borg MA. National cultural dimensions as drivers of inappropriate ambulatory care consumption of antibiotics in Europe and their relevance to awareness campaigns. J Antimicrob Chemother. 2012;67(3):763–767. doi: 10.1093/jac/dkr541. [DOI] [PubMed] [Google Scholar]
- 26.Deschepper R, Grigoryan L, Lundborg CS, et al. Are cultural dimensions relevant for explaining cross-national differences in antibiotic use in Europe? BMC Health Serv Res. 2008;8:123. doi: 10.1186/1472-6963-8-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edwards R, Charani E, Sevdalis N, et al. Optimisation of infection prevention and control in acute health care by use of behaviour change: a systematic review. Lancet Infect Dis. 2012;12(4):318–329. doi: 10.1016/S1473-3099(11)70283-3. [DOI] [PubMed] [Google Scholar]
- 28.Charani E, Castro-Sánchez E, Holmes A. The role of behavior change in antimicrobial stewardship. Infect Dis Clin North Am. 2014;28(2):169–175. doi: 10.1016/j.idc.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Stalsby Lundborg C, Tamhankar AJ. Understanding and changing human behaviour-antibiotic mainstreaming as an approach to facilitate modification of provider and consumer behaviour. Ups J Med Sci. 2014;119(2):125–133. doi: 10.3109/03009734.2014.905664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferri M, Ranucci E, Romagnoli P, Giaccone V. Antimicrobial resistance: A global emerging threat to public health systems. Crit Rev Food Sci Nutr. 2017;57(13):2857–2876. doi: 10.1080/10408398.2015.1077192. [DOI] [PubMed] [Google Scholar]
- 31.Nasrin D, Collignon PJ, Roberts L, Wilson EJ, Pilotto LS, Douglas RM. Effect of beta lactam antibiotic use in children on pneumococcal resistance to penicillin: prospective cohort study. BMJ. 2002;324(7328):28–30. doi: 10.1136/bmj.324.7328.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung A, Perera R, Brueggemann AB, et al. Effect of antibiotic prescribing on antibiotic resistance in individual children in primary care: prospective cohort study. BMJ. 2007;335(7617):429. doi: 10.1136/bmj.39274.647465.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096. doi: 10.1136/bmj.c2096. [DOI] [PubMed] [Google Scholar]
- 34.Cho OH, Bak MH, Baek EH, Park KH, Kim S, Bae IG. Successful control of carbapenem-resistant Acinetobacter baumannii in a Korean university hospital: a 6-year perspective. Am J Infect Control. 2014;42(9):976–979. doi: 10.1016/j.ajic.2014.05.027. [DOI] [PubMed] [Google Scholar]
- 35.Lawes T, Lopez-Lozano JM, Nebot CA, et al. Effects of national antibiotic stewardship and infection control strategies on hospital-associated and community-associated meticillin-resistant Staphylococcus aureus infections across a region of Scotland: a non-linear time-series study. Lancet Infect Dis. 2015;15(12):1438–1449. doi: 10.1016/S1473-3099(15)00315-1. [DOI] [PubMed] [Google Scholar]
- 36.Zhu X, Tong A, Wang D, Sun H, Chen L, Dong M. Antibiotic resistance patterns of Gram-negative and Gram-positive strains isolated from inpatients with nosocomial infections in a tertiary hospital in Beijing, China from 2011 to 2014. J Chemother. 2016;29(5):317–320. doi: 10.1080/1120009X.2016.1157946. [DOI] [PubMed] [Google Scholar]
- 37.Butler CC. Antibiotics: responding to a global challenge. Antibiotics (Basel) 2012;1(1):14–6. doi: 10.3390/antibiotics1010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.NethMap 2014 Consumption of antimicrobial agents and antimicrobial resistance among medically important bacteria in the Netherlands. SWAB, the Dutch Foundation of the Working Party on Antibiotic Policy. National Institute for Public Health and the Environment, Ministry of Health, Welfare and Sport; Nijmegen, Netherlands: 2014. [Google Scholar]
- 39.Feng W, Sun F, Wang Q, et al. Epidemiology and resistance characteristics of Pseudomonas aeruginosa isolates from the respiratory department of a hospital in China. J Glob Antimicrob Resist. 2017;8:142–147. doi: 10.1016/j.jgar.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 40.Landersdorfer CB, Nation RL. Colistin: how should it be dosed for the critically ill? Semin Respir Crit Care Med. 2015;36(1):126–135. doi: 10.1055/s-0034-1398390. [DOI] [PubMed] [Google Scholar]
- 41.Humphries RM. Susceptibility testing of the polymyxins: where are we now? Pharmacotherapy. 2015;35(1):22–27. doi: 10.1002/phar.1505. [DOI] [PubMed] [Google Scholar]