Abstract
Renal cell carcinoma (RCC) denotes cancer originated from renal epithelium and accounts for >90% of cancers in the kidney. The disease encompasses >10 histological and molecular subtypes, of which clear cell RCC (ccRCC) is most common and accounts for most cancer-related deaths. Although somatic VHL mutations have been described for some time, more-recent cancer genomic studies have identified mutations in epigenetics regulatory genes and demonstrated marked intratumour heterogeneity, which could have prognostic, predictive and therapeutic relevance. Localized RCC can be successfully managed with surgery whereas metastatic RCC is refractory to conventional chemotherapy. However, over the past decade, marked advances in treatment of metastatic RCC have been made, with targeted agents including sorafenib, sunitinib, bevacizumab, pazopanib and axitini that inhibit vascular endothelial growth factor (VEGF) and its receptor(VEGFR) and everolimus and temsirolimus, which inhibit mTOR complex 1, being approved. Since 2015, agents with additional targets aside from VEGFR have been approved, such as cabozantinib and lenvatinib; immunotherapies such as nivolumab have also been added to the armamentarium for metastatic RCC. Here, we provide an overview of the molecular biology of RCC, with a focus on ccRCC, as well as updates to complement current clinical guidelines and an outline of potential future directions for RCC research and therapy.
INTRODUCTION
Renal cell carcinoma (RCC) encompasses a heterogeneous group of cancers derived from renal tubular epithelial cells1 and is among the 10 most common cancers worldwide. Key advances in histopathological and molecular characterization of RCC over the past two decades have led to major revisions in its classification2–5. Major subtypes6 with ≥5% incidence are clear cell RCC (ccRCC)7, papillary RCC (pRCC)8 and chromophobe RCC (chRCC)9 (FIG. 1). The remaining subtypes are very rare (each with ≤1% total incidence)5 and in cases where a tumour does not fit any subtype diagnostic criteria, it is designated as unclassified RCC (uRCC, ~4% total incidence)10. ccRCC is the most common subtype and accounts for the majority of kidney cancer deaths and is the focus of this Primer11. Indeed, owing to the predominance of clear cell histology in metastatic disease (83–88%)12,13, tumours with non-clear cell histology have been grouped as ‘nccRCC’ (Table 1) for feasibility in conducting clinical trials14–16. Furthermore, recent cancer genomic studies have revealed an overt complexity of intra-tumour17–19 and inter-tumour7,20 heterogeneity in ccRCC, which could contribute to the heterogeneous clinical outcomes observed21–23.
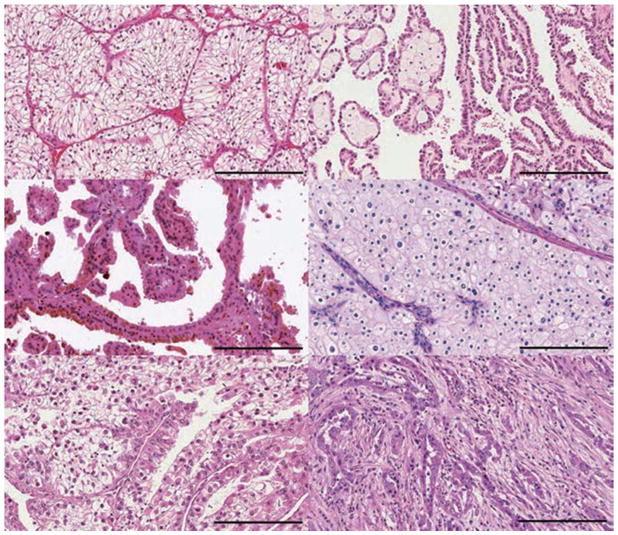
Figure 1. Distinct subtypes of RCC.
Approximately 75% of renal cell carcinomas (RCCs) are a | clear cell RCC (ccRCC). b | Papillary RCCs make up ~15% of all kidney cancers and are divided into two types based on staining features: b | type 1 (basophilic) and c | type 2 (eosinophilic). d | Chromophobe RCCs make up ~5% of kidney tumours. Other minor subtypes include e | MiT family translocation RCCs and f | collecting duct RCCs. Additional minor subtypes include medullary RCC, clear cell papillary RCC, acquired cystic disease-associated RCC, tubulocystic RCC, mucinous tubular and spindle RCC, succinate dehydrogenase-deficient RCC, hereditary leiomyomatosis, renal cell carcinoma-associated RCC and oncocytoma. Tumours not fitting into any of these categories are designated unclassified RCC. Scale bar = 200 μm.
Table 1.
Non-clear-cell renal cell carcinomas
| Tumour type | Subtype | Cytogenetic alterations | Genes mutated | Gross appearance | Histological features* |
|---|---|---|---|---|---|
| Papillary | Type 1 | Gains of 7, 8q, 12q, 16p, 17, 20, and loss of 9p | MET | • Mixed cystic/solid consistency • Often whitish in colour and may display haemorrhage and necrosis • Frequently with a well-demarcated pseudocapsule |
• Single layer of cuboid tumour cells • Thin, basophilic papillae with scant pale cytoplasm and low nuclear grade • Concentric lamellated calcifications (psamomma bodies) • Foamy macrophage infiltration |
| Type 2 | Gains of 8q, loss of 1p and 9p |
CDKN2A SETD2 NRF2 |
• Heterogeneous, thick papillae and eosinophilic cytoplasm, high nuclear grade, and pseudostratification • Concentric lamellated calcifications • Foamy macrophage infiltration |
||
| Chromophobe | Classic | Loss of chromosomes 1, 2, 6, 10, 13, 17 and 21 |
TP53 PTEN |
• Large, well-circumscribed grey to tan-brown coloured tumour • Occasional central scar |
• Tumour cells with prominent membrane and pale cytoplasm • Voluminous cytoplasm (cytoplasmic accumulation of acid mucopolysaccharides) |
| Eosinophilic | • Large tumor cells with fine eosinophilic granules • Distinct cell borders • Voluminous cytoplasm |
||||
| MiT family translocation | NA | Recurrent translocations involving Xp11.2 (TFE3) or 6p21(TFEB) |
TFE3 TFEB |
• Yellowish tissue • Often studded by haemorrhage and necrosis |
• Papillary or nested architecture • Abundant clear or eosinophilic cytoplasm |
| Collecting duct | NA | Losses at 8p, 16p, 1p, 9p, and gains at 13q | Unknown | • Partially cystic • White-grey appearance • Often exhibit invasion into the renal sinus |
• Tubulopapillary pattern • Often with cells taking columnar pattern with hobnail appearance • Presence of mucinous material • Desmoplastic stroma |
| Medullary | NA | Poorly described, but thought to be normal karyotype | SMARCB1 | • Tan/white appearance • Poorly defined • Capsule • Extensive haemorrhage and necrosis |
• Poorly differentiated, eosinophilic cells • Inflammatory infiltrative cells • Sheet-like or reticular pattern common |
| Oncocytoma | NA | Loss of chromosome 1 and Y and CCND1 rearrangement | Mitochondrial genes (COX1, COX2, ND4 and CYTB) | • Mahogany colour • Circumscribed • Occasional central scar • Rarely with necrosis |
• Polygonal cells with abundant eosinophilic cytoplasm • Uniform, round nuclei |
Adapted from Ref. 208. NA, no applicable.
No consensus is currently available describing the immune infilitration of non-clear-cell renal cell carcinomas.
Localized RCC can be treated with partial or radical nephrectomy (removal of the kidney)24, ablation25 (destruction of the malignant tissue with heat or cold) or active surveillance26 (monitoring of tumour growth with periodic radiographic studies). Despite nephrectomy with curative intent, ~30% of patients with ccRCC with localized disease eventually develop metastases27–30, which require systemic therapies and is associated with high mortality. Targeted therapy against vascular endothelial growth factor (VEGF) and mTOR pathways have been developed, but treatment response is varied and most patients eventually progress31. However, increased genomic and molecular understanding of metastatic ccRCC has contributed to an unprecedented number of drugs approvals in the United States and European Union (currently 12 approved drugs with six different effective mechanisms of action are approved). In this Primer, we discuss these new approvals and the major progress made in biology of ccRCC that led to their development. Furthermore, we present insights into genomics-based risk and treatment stratification and discuss treatment sequencing and combinations that are paving the way for the future design of personalized clinical management plans.
EPIDEMIOLOGY
Incidence and mortality
Kidney cancer accounts for approximately 2% of all cancer diagnoses and cancer deaths worldwide, with incidence rates generally higher in developed countries (FIG. 2)32. Annually, ~295,000 new kidney cancer cases are diagnosed and ~134,000 deaths are recorded worldwide33,34. Kidney cancer accounts for ~63,000 new cases and ~14,000 deaths yearly in the United States35, and for ~84,000 new cases and ~35,000 deaths in Europe36. Men are more affected than women (a 2:1 ratio of new diagnoses).
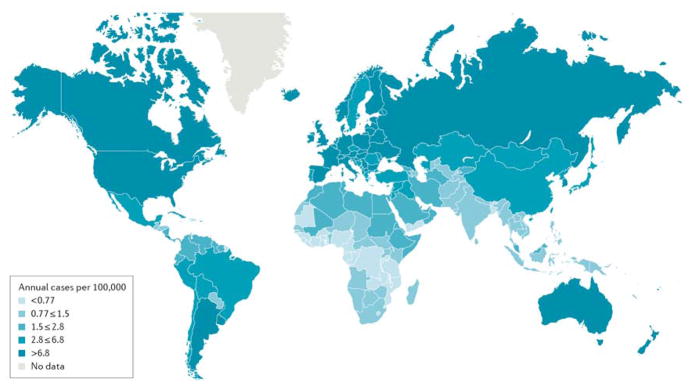
Figure 2. Globalkidney cancer incidence.
Estimated age-standardized rates (ASRs) of incidence for both sexes (per 100,000 persons) in 2012. Rates are generally higher in developed countries, with the highest incidence the Czech Republic (reasons unknown). Data from GLOBOCAN database; http://globocan.iarc.fr.
The median age of patients with RCC in the Surveillance, Epidemiology, and End Results (SEER) database in the United States was 64 years with a near normal distribution37. Accordingly, when RCC is diagnosed at younger ages (≤46 years, which represents the lowest decile of the age distribution)37,38, the possibility of an underlying hereditary kidney cancer syndrome — which accounts for 3–5% of all RCCs5 — should be considered (Table 2)39,40.
Table 2.
Hereditary syndromes associated with renal cell carcinoma
| Syndrome (Phenotype MIM reference) | Gene (position) | Protein | Incidence of developing kidney tumour (%) | Median age at diagnosis (years) | Other phenotypic features |
|---|---|---|---|---|---|
| Clear cell renal cell carcinoma* | |||||
| von Hippel Lindau disease (193300) | VHL (3p25–26) | pVHL | 25–45 | 40 | • Hemangioblastoma • Pancreatic neuroendocrine tumours • Pheochromocytoma • Renal cysts • Pancreatic cysts • Ovary cystadenoma • Epididymal cystadenoma |
| BAP1 mutant disease (614327) | BAP1 (3p21) | BRCA-associated protein | No data | No data | • Breast cancer • Uveal melanoma • Mesothelioma • Other cutaneous melanocytic tumors |
| SDH-associated kidney cancer (185470, 602413, 602690 and 115310) | SDHB (1p36), SDHC (1q21) and SDHD (11q23) | Succinate dehydrogenase subunits B, C and D | 5–15 | 30 | • Paraganglioma • Carotid body tumors • Pheochromocytoma • Gastrointestinal stromal tumour GIST |
| Papillary renal cell carcinoma | |||||
| Hereditary leiomyomatosis and renal cell cancer (150800)‡ | FH (1q43) | Fumarate hydratase | 2–21 | 46 | • Uterine leiomyosracomas • Breast cancer • Bladder Cancer • Cutaneous leiomyomas • Uterine leiomyomas |
| Hereditary papillary kidney cancer (605074)§ | MET (7q31) | Hepatocyte growth factor receptor | No data | <60 | • No additional features |
| Multiple tumour types | |||||
| Birt-Hogg-Dubé syndrome (135150)|| | FLCN (17p11.2) | Folliculin | 34 | 50 | • Fibrofolliculomas and trichodiscomas • Pulmonary cysts • Pneumothorax |
| Tuberous sclerosis complex (191100 and 191092) | TSC1 (9q34) and TSC2 (16p13) | Hamartin and tuberin | 2–4 | 30 | • Subependymal giant cell astrocytomas • Angiomyolipomas • Renal cysts • Facial angiofibroma • Ungal and periungal fibromas • Hypomelanotic macule, Forehead plaque • Cardiac rhabdomyomas • Connective tissue nevus |
| Cowden syndrome (multiple hamartoma syndrome; 158350)# | PTEN (10q23) | PTEN | 34 | 40 | • Breast Cancer • Endometrial cancer • Thyroid cancer • Prostate cancer • Macrocephaly • Intestinal hamartomatous polyps • Benign skin tumors (multiple trichilemmomas, papillomatous papules and acral keratoses) • Dysplastic gangliocytoma of the cerebellum |
| Hyperparathyroidism jaw tumour syndrome (145001)** | HRPT2 (1q31) | Parafibromin | No data | No data | • Parathyroid carcinomas • Uterine carcinomas • Renal cysts and hamartomas • Hyperparathyroidism • Parathyroid glands tumours • Jaw fibromas |
Familial clear cell kidney cancer with chromosome 3 translocation is another possible syndrome associated with clear cell renal cell carcinoma, but the genetic lesions and associated data are unknown.
Papillary renal cell carcinoma type 2.
Papillary renal cell carcinoma type 1.
Hybrid tumours; oncocytomas; and chromophobe, papillary and clear cell renal cell carcinomas. Angiomyolipomas, epithelioid angiomyolipomas, renal cysts, oncocytomas and papillary and clear cell renal cell carcinomas.
Clear cell, papillary and chromophone renal cell carcinomas.
Mixed tumours (epithelial and connective tissue), papillary renal cell carcinomas and nephroblastomas. MIM, Mendelian Inheritance in Man database.
The incidence of RCC highest in the Czech Republic, with age-standardized annual rates of 22.1 and 9.9 new cases per 100,000 men and women, respectively, over the period 2003–200741. The incidence is also very high in the Baltic and Eastern European countries, although the reasons for this excess are not known. Overall, incidence rates have been increasing over time in most populations, but mortality rates have levelled off or are decreasing since 1990s. This divergent pattern of increasing incidence and decreasing mortality is particularly evident in developed countries. For example, analyses within the SEER database indicate that the increase in RCC incidence is confined to small and localized tumours, likely due at least in part to increasingly frequent incidental detection of small renal masses (tumours ≤4 cm in size) that are unlikely to have metastasized from increased use of abdominal imaging42. The global increases in the prevalence of obesity, an established RCC risk factor, might also play a part in increasing incidence, as well as influencing clinical outcome41,43.
Risk factors
RCC incidence increases markedly with age and is higher for men than women. In the United States, incidence varies by ethnic group, with rates highest among Native American, Indigenous Alaskans and African Americans, and lowest among Asian Americans and people of Pacific Island descent35. The major established risk factors for RCC include excess body weight, hypertension and cigarette smoking44, which were factors in approximately half of all diagnosed cases in one US study45. Other medical conditions that have been associated with RCC in epidemiological studies include chronic kidney disease, haemodialysis, kidney transplantation, acquired kidney cystic disease, a previous RCC diagnosis and, possibly, diabetes mellitus44. Many lifestyle, dietary, occupational and environmental factors have also been associated with RCC with varying levels of evidence46.
For example, contradictory reports exist on the association between red meat consumption and RCC risk47,48. Moderate alcohol consumption (≥11g per day) seems to reduce the risk for RCC48,49. In a case–control study on physical activity and the risk of RCC, inverse trends in risk were found, and the authors concluded that 9% of RCC cases could be avoided by increasing physical activity50. However, the inverse association might have involved other confounding factors such as BMI and social class correlates. Other studies have found no such inverse association51.
Genetic factors also contribute to RCC risk, as evidenced by individuals with a family history of renal cancer having an approximate twofold increased risk52. Investigations into familial RCC have uncovered mutations in at least 11 genes (namely BAP1, FLCN, FH, MET, PTEN, SDHB, SDHC, SDHD, TSC1, TSC2, and VHL), some of which have also been implicated in sporadic RCC development39. A notable example is VHL, the mutated gene underlying von Hippel-Lindau disease, which is characterized by a high risk of developing ccRCC53; inactivation of the VHL protein, leading to unchecked expression of oncogenic hypoxia-inducible factors (HIF-1 and HIF-2), is also a hallmark of sporadic ccRCC tumours (see Mechanisms, below)39,54. Genome-wide association studies (GWAS) of RCC have identified six susceptibility loci to date, on chromosome regions 2p21, 2q22.3, 8q24.21, 11q13.3, 12p11.23 and 12q24.3155–58. The 2p21 locus maps to EPAS1, a gene encoding the HIF-2α subunit 55 whereas the biological effects underlying the 11q13.3 locus seems to be attributable to changes in the regulation of CCND1 (encoding cyclin D1, which is involved in cell cycle regulation)59. The locus 12p11.23 probably maps to changes in BHLHE41 (encoding basic helix-loop-helix family member e41, which is thought to have a role in regulation of the circadian rhythm)60. The disease genes underlying the other GWAS susceptibility loci have yet to be identified.
MECHANISMS/PATHOPHYSIOLOGY
Genes and pathways
In ccRCC, the VHL tumour suppressor gene is the most frequently mutated gene7,54, and its complete loss through genetic (point mutations, indels and 3p25 loss) and/or epigenetic (promoter methylation) mechanisms constitutes the earliest, truncal oncogenic driving event61,62. VHL is the substrate recognition component of an E3 ligase complex that ubiquitinates HIF-1α and HIF-2α for proteasome-mediated degradation53,63,64. Loss of VHL, therefore, leads to aberrant accumulation of HIF proteins despite an adequately oxygenated tissue microenvironment, which in turn results in uncontrolled activation of HIF target genes that regulate angiogenesis, glycolysis and apoptosis (FIG. 3). Accordingly, human ccRCC tumours are rich in lipids and glycogens, and are highly vascular65,66 — which underlies why agents that primarily inhibit VEGF and its receptor VEGFR are effective treatments for metastatic ccRCC14,15,67. However, VHL loss alone is insufficient to induce ccRCC as evidenced by the long latency (>30 years) in individuals who harbour VHL germline mutations to develop ccRCC53 and by the observation that Vhl loss in mice is unable to induce ccRCC68. These results suggest that additional genetic and/or epigenetic events are probably needed for ccRCC to develop69.
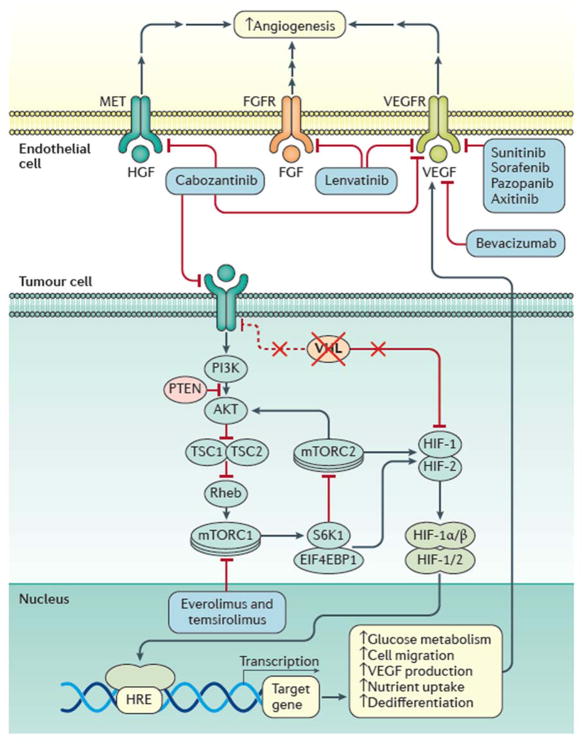
Figure 3. VHL inactivation in ccRCC and its implication in targeted therapy.
Loss of VHL is the most frequent genetic feature of clear cell renal cell carcinoma (ccRCC). Its loss relieves the cell of negative regulation of the hypoxia inducible factors (HIFs), which results in increase HIF target gene expression and ensuing changes in cellular metabolism and signalling that enhances cell survival. For example, increased vascular endothelial growth factor (VEGF) expression increases angiogenesis in concert with increased signalling from growth factor receptors in endothelial cells in the tumour microenvironment (including fibroblast growth factor (FGF) and hepatocyte growth factor (HGF)). Collectively, these changes provide the targets for therapeutic agents to impede tumour growth, as indicated. FGFR, FGF receptor VEGFR, VEGF; TSC, tuberous sclerosis complex; PI3K, phosphatidylinositol 4,5-bisphosphate 3-kinase; AKT, RAC-α serine/threonine-protein kinase; Rheb, GTP-binding protein Rheb; mTORC1, mTOR complex 1; mTORC2, mTOR complex 2; S6K1, ribosomal protein S6 kinase; 4EBP1, eukaryotic translation initiation factor 4E-binding protein 1; HRE, HIF response element; MET, hepatocyte growth factor receptor.
To identify these events, large-scale cancer genomic projects have been undertaken, and have revealed several novel prevalent mutations in ccRCC, including PBRM1 (29–41% of tumour samples), SETD2 (8–12%), BAP1 (6–10%), KDM5C (4–7%) and MTOR (5–6%)7,70–73. Interestingly, PBRM1, SETD2 and BAP1 encode chromatin and histone regulating proteins, are located at 3p21 and function as tumour suppressors7,70–72. As VHL resides at 3p25, a single copy loss of the short arm of chromosome 3 (3p) would result in haploinsufficiency of these four tumour suppressor genes, corroborating the fact that 3p loss (that is, loss of heterozygosity) is nearly a universal event in ccRCC61 and constitutes an early genetic event69. By contrast, MTOR mutations in ccRCC are generally missense and functionally activating73,74, which could explain the reason mTOR pathway inhibitors, including everolimus and temsirolimus, are effective75,76.
How individual mutations and their interactions contribute to the pathogenesis and their values as prognostic or predictive biomarkers in ccRCC are largely unknown. Nevertheless, a few studies have demonstrated interesting clinical correlations that warrant future validation. As inactivation of VHL is the founding event of ccRCC, its mutation status has no effect on clinical outcome, whereas mutations involved in disease progression such as PBRM1, SETD2 and BAP1 as well as KDM5C (which is also involved in chromatin modification) were shown to associate with aggressive clinical features77–79. Small renal masses harbouring PBRM1 mutations were associated with stage III pathological features (that is, extrarenal growth but not extending beyond Gerota’s fascia see below)71, whereas BAP1 mutations were associated with larger tumour sizes, higher Fuhrman nuclear grade (large nucleus with prominent nucleolus) and worse cancer-specific survival77,78,80. Interestingly, mutations in BAP1 and PBRM170 or KDM5C20 seem to occur mutually exclusively in ccRCC, offering a molecular subclassification of ccRCC. Furthermore, mutations of KDM5C, which is located at Xp.11, were predominantly detected in male patients and correlated with long-term therapeutic benefit from sunitinib20; and mutations of SETD2 were associated with reduced relapse-free survival80.
Tumour heterogeneity and cancer evolution
As Nowell first described 40 years ago81, genetic diversity within tumours is thought to provide the substrate upon which selection can act, to enable tumours to adapt to new microenvironmental pressures and metabolic demands during the natural history of the cancer (FIG. 4A). Such genetic diversity has been studied extensively in ccRCC. For example, in a study of four patients with ccRCC who had multiple tumours were subjected to multi-region genetic analysis, VHL mutation and 3p loss of heterozygosity were found to be ubiquitous events across all regions sampled17. By contrast, common driver events such as SETD2, PBRM1, MTOR, PIK3CA, PTEN and KDM5C mutations were present heterogeneously within the primary tumour and metastatic sites — in some regions but not others. Such genetic characteristics enable the construction of tumour phylogenies, whereby the ‘trunk’ of the evolutionary tree depicts mutations found in the most recent common ancestor (MRCA) that are present in every tumour cell. ‘Branched’ mutations are found in some subclones but not others; these mutations may be regionally distributed across the tumour, occupying distinct regional niches within the primary tumour or different niches between the primary and metastatic sites of disease.
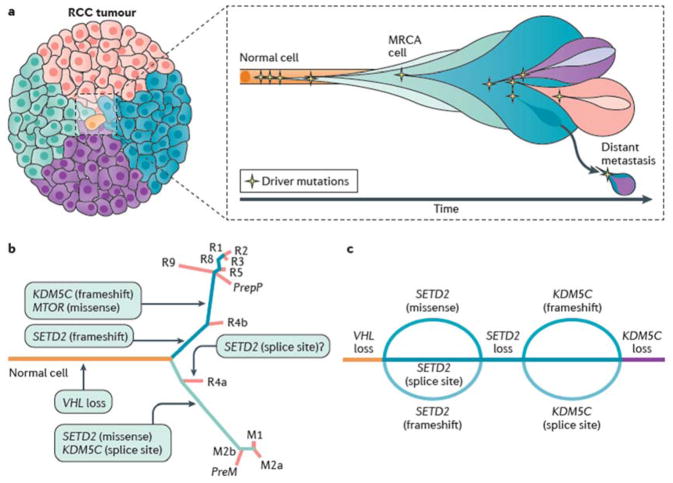
Figure 4. Cancer evolution and tumour heterogeneity in ccRCC.
Although VHL mutation and 3p loss of heterozygosity are early events that are evident in all clear cell renal cell carcinoma (ccRCC) cells regardless of the region of the tumour sampled, common driver mutations (for example, SETD2, MTOR and KDM5C mutations) are present heterogeneously — suggestive of subclonal evolution of the tumour. a | Cancer subclones originate from the most recent common ancestor cell (MRCA) in which a normal cell acquires all functional capacities to become cancer cell. b | Genomic heterogeneity can result from the sequential, parallel accumulation of mutations, contributing to the heterogeneity and the evolution of ccRCC. In this example, ‘R’ represents the genomic characteristics of the primary tumour and ‘M’ represents the genomic characteristics of the metastatic sites, numbered accordingly. The major genetic lesions acquired after VHL mutation feature in different samples and are indicated on the branches. c | However, some evidence suggests that tumours can converge by way of parallel evolution. Here, a hypothetical beaded river model depicts the sequential convergence of SETD2 and KDM5C mutations through different spatiotemporally distinct genetic events.
Furthermore, parallel evolution has also been observed, whereby recurrent branch alterations in subclones affect the same gene, signal transduction pathway or protein complex (FIG. 4B). In some cases — such as BAP1, PBRM1 and SETD2 mutations — such recurrent but distinct alterations can be readily explained as the ‘second hit’ event in the evolution of the tumour. In other cases, parallel evolution suggests considerable selection pressures for disruption of the same signalling pathway or protein complex. Additionally, convergence of genetic characteristics has been noted in several studies of ccRCC19,23,82, whereby mutations in genes occur at different time points but result in similar overall genomic and phenotypic profiles; a ‘braided river’ model has been conceived to illustrate this phenomenon (FIG. 4C)69. Regardless of the modality, a follow up study of ccRCC samples for eight patients demonstrated evidence for branched evolution in which 73–75% of driver alterations were found to be subclonal18.
Multi-region tumour analyses suggest the intriguing possibility that evolutionary trajectories are remarkably constrained in ccRCC, which — as our knowledge of microenvironmental, therapeutic and host selection pressures grows — could render the evolutionary routes predictable and, therefore, therapeutically tractable. For example, it has been shown that patients who responded well to mTOR inhibition harbour recurrent regionally separated aberrations in components of the mTOR pathway75. Furthermore, some subclonal alterations might be involved in the initiation and maintenance of cell-to-cell variation necessary for clonal selection. For example, SETD2 loss of function has been shown to impair nucleosome compaction, minichromosome maintenance complex component 7 (MCM7) function and DNA polymerase delta loading to chromatin, resulting in impaired DNA replication fork progression. Additionally, failure to load lens epithelium-derived growth factor p75 splice variant (LEDGF) and DNA repair protein RAD51 homolog 1 (RAD51) — which are involved in DNA break repair — has also been observed upon SETD2 loss, resulting in homologous recombination repair deficiency83. These events are, accordingly, plausible genomic biomarkers in ccRCC dispersed within distinct regional niches within each tumour19,84.
Immune infiltration and the tumour microenvironment
In addition to genetic alterations, gene expression, metabolic and immunological analyses of ccRCC have also yielded important mechanistic and clinical insights 20,85–87. Of these, perhaps the immune infiltration characteristics of ccRCC is of increasing interest, given the rise of immune checkpoint-blocking therapies in this disease (see below, Management). Notably, among 19 cancer types examined by The Cancer Genome Atlas research programme, ccRCC has the highest T cell infiltration score 87. Furthermore, higher nuclear grade and stage in ccRCC was correlated with an increase in T helper 2 and T regulatory cell infiltration87,88.
Disease models
Although RCC cell lines have been used for mechanistic studies, 89 ccRCC tumours in patients are highly vascular — a feature that cannot be recapitulated with in vitro cell studies. Furthermore, such cell lines can acquire additional genetic and/or epigenetic changes during passages such that in vitro drug screens do not yield specific, translatable insights90. Nevertheless, when these cell lines were injected subcutaneously into laboratory animals, xenografted tumours largely respond to anti-VEGF therapy91 and can be used to investigate resistance mechanisms92,93.
More recently, patient-derived xenograft (PDX) models have been established and have been shown to recapitulate the patient’s documented clinical response to targeted therapies, which could be used in pre-clinical drug trials94. At the same time, efforts to develop mouse models that truly reflect human ccRCC genomics and morphology have been hampered by the fact that homozygous inactivation of the Vhl gene in mice does not result in ccRCC68. However, the identification of additional recurrent, prevalent mutations in human ccRCC have rekindled efforts to generate such models. For example, homozygous deletion of Vhl and Pbrm1 in a mouse model resulted in multifocal, lipid-rich, glycogen-rich, transplantable ccRCC (J.J.H., unpublished data). Interestingly, homozygous deletion of Vhl and Bap1 in a mouse model resulted in early lethality (<1 month), and some mice (within a cohort of 7) carrying homozygous deletion of Vhl and heterozygous deletion of Bap1 developed tumour micronodules (0.25–1.8mm) with unknown tumour incidence and molecular characteristics 95. Overall, animal models of RCC are currently limited but being eagerly pursued.
DIAGNOSIS, SCREENING AND PREVENTION
Diagnosis
Historically, patients were diagnosed with RCC after presenting with flank pain, gross haematuria and a palpable abdominal mass. Nowadays, the majority of diagnoses result from incidental findings. This shift is a consequence of the widespread use of non-invasive radiological techniques such as ultrasonography or abdominal CT imaging performed for another reason. That being said, paraneoplasic syndromes — symptoms caused by hormones or cytokines excreted by tumour cells or by an immune response against the tumour — are not uncommon in RCC 96 and symptoms include hypercalcaemia, fever and erythrocytosis. Most of these symptoms are usually reversed after tumour resection11. Diagnosis is usually strongly suspected by imaging studies although RCCs can display variable radiographic appearances97. Typical radiological features for ccRCC include exophytic (outward) growth, heterogeneity due to intratumoral necrosis or haemorrhage and high uptake of contrast-enhancement agents98.
Staging
The stage of RCC reflects the tumour size, extent of invasion outside of the kidney, the involvement of lymph nodes and whether the tumour has metastasized (FIG. 5). CT imaging with contrast enhancement of the chest, abdominal cavity and pelvis is required for optimal staging. Such imaging enables assessment of primary tumour (size and whether the tumour is organ-confined or extends to perinephric fat or kidney hilum), regional spread (lymph node involvement) and distant metastases (lung, bone and distant lymph nodes). MRI can also provide additional information, especially to determine whether the tumour extends into the vasculature (vena cava tumour thrombus). Bone scan, 18F-fluorodeoxyglucose PET and imaging of the brain are not systematically recommended for initial staging14,15. Prognostic assessment will require further laboratory testing that includes, but is not limited to, haemoglobin, leukocyte and platelet counts; serum-corrected calcium levels; and lactate dehydrogenase levels99,100.
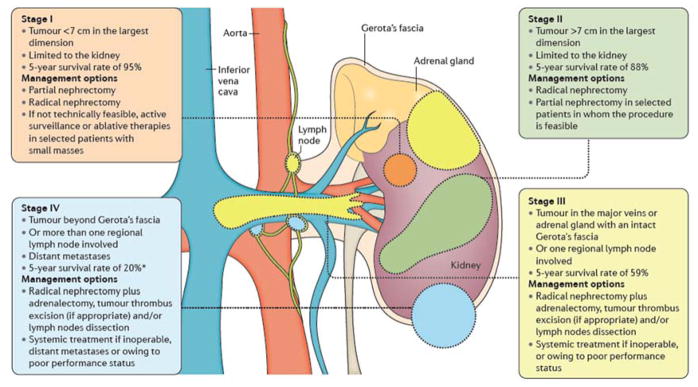
Figure 5. Stages of kidney cancer and recommended treatments.
Staging renal cell carcinoma (RCC) is based on size, position and lymph node involvement15. For example, a stage I or II tumour is enclosed wholly in the kidney. Stage III tumours can extend into major veins or adrenal glands within Gerota’s fascia (the layer of connective tissue encapsulating the kidneys and adrenal glands) or can involve one regional lymph node involvement. Stage IV tumours can invade beyond Gerota’s fascia and/or have distant metastases. *Until the introduction of newer targeted therapies beginning in 2005, the 5-year survival of stage IV RCC was <10%. Treatment is largely guided by stage15,24. For example, those with stage I RCC who are fit for surgery are recommended partial nephrectomy. However, radical nephrectomy is also an option; for elderly patients or those who cannot undergo surgery owing to comorbidities, active surveillance or ablative therapies are recommended. In patients with stage III RCC, radical nephrectomy is recommended with lymph node dissection in those with clinical enlarged lymph nodes, but systemic therapies might be the only available option for those with extensive disease and poor performance status.
Genomic implications
An age of onset of ≤46 years raises the possibility of a hereditary syndrome (Table 2) and, according to the American Society of Clinical Oncology, should trigger consideration for genetic counselling and might serve as a useful cut-off age when establishing genetic testing guidelines37. Indeed, awareness of the non-renal malignancies and non-neoplastic features associated with RCC is of interest to the physician to identify hereditary syndromes40. Furthermore, specific therapeutic options driven by the underlying biology are now being developed for these different RCC related to cancer susceptibility syndromes101. Upon confirmation, patients and their families harbouring mutations are subject to specialized monitoring and treatment plans to minimize morbidity and prevent mortality.
Histopathological confirmation
Histopathological confirmation of malignancy is obtained either with renal core biopsy or on the partial or radical nephrectomy specimen. Initial biopsy is recommended before ablative therapy is undertaken (in those for whom surgery is not an option) or before initiating systemic therapy (in those who have metastatic disease)102. In 2016, the WHO classification of RCC was updated5 from previous (2004) WHO1 and International Society of Urological Pathology (ISUP) Consensus Conference4 (2013) systems. Although most RCCs can be easily classified on the basis of histological criteria, some tumours pose a diagnostic problem because they display a combination of features characteristic of different subtypes. For instance, the presence of clear cells is not unique to ccRCC but can be observed in pRCC, chRCC and MiT family translocation RCC (tRCC)66. Similarly, papillary structures, characteristic of pRCC, can be present in other RCC types103. In challenging cases, careful evaluation of cytological features, growth pattern, immunophenotype and genetic alterations usually enables the proper diagnosis. However, a subset of RCCs (~4%) cannot be assigned to any specific category because they either present combined morphologies or display unusual features and are, therefore, designated uRCC3,104,105. Nevertheless, a recent molecular characterization of 62 aggressive uRCC revealed distinct subsets including NF2 loss (26%), mTORC1 pathway activation (21%) and mutations in chromatin and DNA damage regulators (21%)10.
At macroscopic examination, the cut surface of the ccRCC tumours is golden yellow with frequent haemorrhagic, necrotic and cystic areas. Microscopically, ccRCC usually consists of tumour cells with clear cytoplasm arranged in nests or tubules surrounded by a rich vascular network. The clear appearance of the cytoplasm is due to the accumulation of glycogen and lipids. A variable proportion of tumour cells with granular eosinophilic cytoplasm can be observed and, in some cases, these cells constitute the entire tumour mass3,104,105. The most widely used grading system for ccRCC is the Fuhrman grading system, which defines four nuclear grades (1–4) in order of increasing nuclear size, irregularity and nucleolar prominence106. The Fuhrman nuclear grade has been shown to have prognostic value in ccRCC30,107,108.
It should be noted that all RCC types can contain foci of high-grade malignant spindle cells (that is, sarcomatoid differentiation). Thus, sarcomatoid RCC is no longer considered as an entity but rather as a progression of any RCC type109. Of note, recent genomic insights from sequencing matched sarcomatous and carcinomatous RCC demonstrated enrichment in TP53 and CDKN2A mutations, implicating these genetic defects as underlying causes of sarcomatoid differentiation in RCC110–112.
Screening
Owing to the relatively low incidence of RCC, universal screening (such as that for asymptomatic micro-hematuria) has not demonstrated a positive effect on outcomes in RCC113. Furthermore, other biomarkers have not yet been established for screening114,115. Imaging remains the primary tool for RCC detection and screening. An ultrasonography screening study in 45,905 participants reported a 10-fold higher RCC-incidence than expected for a general population with improved cancer-free survival when compared with symptomatic patients116.
Although most cases are sporadic62, the majority of patients with RCC might have a genetic predisposition38,117. Although, no guideline is available regarding the selection of patients for germline mutation testing, guidelines for monitoring those with confirmed hereditary syndromes that increase the risk of RCC are available37.
Prevention: modifiable risk factors
Smoking, obesity and hypertension are associated with increased risks of developing RCC whereas exercise and moderate consumption of alcohol and flavonoids reduce RCC risks.
Tobacco
When compared to never smokers, a relative risk for ever smokers of 1.38 (95%CI=1.27–1.50) was reported in a meta-analysis including 8,032 cases and 13,800 controls from 5 cohort studies118. A dose-dependent increase in risk in both men and women was found; individuals who had quit smoking >10 years prior had a lower risk when compared to those who had quit <10 years prior. Other studies have confirmed smoking as a risk factor for RCC119.
Obesity
A 5 kg/m2 increase in body mass index (BMI) was found to be strongly associated with RCC120. Similarly, a strong association between weight gain in early and mid-adulthood (18–35 years of age) with RCC was reported121. Moreover, central adiposity (relative risk 1.8, 95%CI 1.2–2.5) and the waist-to-hip ratio (0.86–2.88) was positively associated with RCC in women122. The impact of BMI on overall survival was also studied in 1,975 patients treated with targeted agents. The authors reported on a median overall survival of 25.6 months (95%CI 23.2–28.6) in patients with high BMI versus 17.1 months (95%CI 15.5–18.5) in patients with low BMI (adjusted hazard ratio of 0.84, 95%CI 0.73–0.95)123. Compared with stable weight, neither steady gain in weight nor weight loss was significantly associated with risk of RCC121.
Hypertension and medications
Higher BMI and hypertension were independently shown to increase the long-term risk of RCC in men whereas a reduction in blood pressure lowered the risk124. Aspirin use was found to be associated with an increased RCC risk in one out of five studies125; by contrast, paracetamol (acetaminophen) exposure showed no increased risk126. The role of phenacetin (acetophenetidin) exposure has been inconclusive127. Statins were reported to significantly reduce the risk of RCC in a large analysis (n=483,733), with a 48% risk reduction (adjusted odds ratio 0.52, 95%CI 0.45–0.60)128. However, owing to the sporadic and low frequency nature, current guideline does not support the role of empiric treatment for prevention of RCC in general population; patients with hereditary syndromes should be monitored more closely and treated accordingly.
MANAGEMENT
For patients with surgically resectable RCC, the standard of care is surgical excision by either partial or radical nephrectomy with a curative intent. By contrast, those with inoperable or metastatic RCC typically undergo systemic treatment with targeted agents and/or immune checkpoint inhibitors. Deciding on which treatment has been largely guided by various nomograms30. For example, the UCLA Integrated Staging System (UISS) and Stage Size Grade and Necrosis (SSIGN) score integrate clinical (1997 TNM stage) and pathological (Fuhrman nuclear grade) information to recommend the length and frequency of clinical follow-up and the selection of high-risk patients for adjuvant studies129–131. Similarly, key prognostic factors have been identified, validated and adopted to guide and stratify patients with metastatic RCC for systemic treatment, including performance status, time from diagnosis to systemic treatment and blood levels of haemoglobin, neutrophils, platelets, calcium and lactate dehydrogenase99,132,133.
Surgery
Surgical treatment of RCC is related to the clinical stage of the disease and to the general condition of the patient (FIG. 5). Although typically reserved for localized disease, both partial and radical nephrectomy can also be used with cytoreductive intent in patients with metastatic disease. Indeed, randomized controlled trials (RCTs) demonstrating the benefit of this approach date from the 1990s, when cytokine-based therapies dominated the systemic therapy landscape. Furthermore, although most patients included in RCTs of targeted therapies also underwent cytoreductive nephrectomy, the current role of excision of the primary tumour in these patients has yet validated. However, according to main international guidelines many centres in offer cytoreductive nephrectomy if there is a substantial disease volume at the primary site but only a low burden of metastatic disease134
Partial nephrectomy
The goal of partial nephrectomy is to completely remove the primary tumour while preserving the largest possible amount of healthy renal parenchyma. Partial nephrectomy is indicated for patient with T1 tumours (according to the Union for International Cancer Control TNM staging system) and a normal contralateral kidney (elective indication). Moreover, partial nephrectomy is strongly recommended (imperative absolute indications) in patients with RCC who have only one kidney (anatomically or functionally), in those with bilateral synchronous RCC and in those with von Hippel-Lindau syndrome. Similarly, imperative relative indications include conditions that can impair renal function (for example, kidney stones, hypertension, diabetes and pyelonephritis). Indeed, partial nephrectomy offers lower renal function impairment135–137 and equivalent oncological survival outcomes compared with radical nephrectomy in those with T1 tumours138,139. More controversial is the favourable impact of partial nephrectomy on overall survival140,141 because conventional wisdom dictates that removal of the whole kidney is better in terms of oncological outcome. In this scenario, surgical feasibility remains the main factor influencing the final decision making process.
In the past decade, nephrometry scoring systems have been proposed to predict the complexity of the partial nephrectomy procedure and predict perioperative outcomes according to the anatomical and topographical tumour characteristics (Table 3)142. The R.E.N.A.L. and PADUA nephrometry systems are still the most popular and most used tools to preoperatively classify tumours 143. These first-generation systems, along with the Centrality Index system, mainly factor in tumour-related anatomical parameters, including face location (that is, anterior or posterior faces, accordingly to their coverage by the anterior or posterior layers of the renal fascia, respectively), longitudinal polar location, rim location (that is, whether the tumour is located at the lateral or medial rim of the kidney), degree of tumour extension into the parenchyma, renal sinus involvement, upper urinary collecting system involvement and clinical maximal diameter of the tumour. Clinical studies demonstrated that such nephrometry systems were able to predict the risk of bleeding and post-operative complications in patients who underwent partial nephrectomy 142. Thus, they represent valid tools for counselling patients and selecting the ideal candidate for partial nephrectomy according to surgeon experience 143. Second-generation nephrometry systems, such as Diameter-Axial-Polar system, Zonal NePhRo scoring system and Arterial Based Complexity System, should be externally validated and tested head-to-head against a first-generation system before being introduced in the clinical practice.
Table 3.
Nephrometry scoring systems to predict partial nephrectomy complexity and outcomes.
| Nephrometry system | Parameters included | Outcomes prediction | External validation |
|---|---|---|---|
| R.E.N.A.L. nephrometry 25 | Tumour size Exophytic rate Polar location Renal sinus involvement UCS involvement Face location |
Blood loss Warm ischaemia time UCS lesion Overall complications Functional outcomes Benign or malignant tumour Tumour grade |
Yes |
| PADUA classification 153 | Tumour size Exophytic rate Polar location Rim location Renal sinus involvement UCS involvement Face location |
Blood loss Ischaemia time UCS lesion Overall complications Functional outcomes |
Yes |
| Centrality Index 135 | Tumour radius Tumour depth (horizontal and vertical distances) |
Ischaemia time Functional outcomes |
Yes |
| Diameter–Axial–Polar system 136 | Diameter Axial distance Polar distance |
Blood loss Ischaemia time Functional outcomes |
No |
| Zonal NePhRo scoring system 137 | Nearness Physical zone Tumour radius Organization of the tumour |
Perioperative complications | No |
| Arterial Based Complexity Scoring System 138 | Size of the renal arterial branches needing to be dissected or transected to achieve complete excision of the renal tumour | Ischaemia time Urinary fistula |
No |
UCS, upper collecting system.
Laparoscopic partial nephrectomy (LPN) and robot-assisted partial nephrectomy (RAPN) are the main alternative to classical open partial nephrectomy (OPN). However, RAPN and OPN are more appropriate in the treatment of more-complex cases (based on expert opinion). Conversely, LPN should be reserved for small tumours (usually ≤4 cm in size) in patients without complex features as defined according to nephrometry systems (low- or intermediate-risk categories). Available meta-analyses have demonstrated that RAPN provides equivalent perioperative outcomes to LPN, but a significantly shorter warm ischaemia time144,145. Moreover, RAPN seems to be significantly better than OPN in terms of perioperative complications, estimated blood loss and hospital stay146,147. Conversely, transfusion rate, ischaemia time, estimated glomerular filtration rate change and early cancer outcomes are similar between the two approaches147. International guidelines recommended the use of both approaches according to the surgeon and patient preferences.
Finally, partial nephrectomy can also involve simple enucleation — entirely sparing the healthy parenchyma around the tumour. Alternatively, classic enucleoresection whereby a thin layer of healthy parenchyma is removed or polar or wedge resection whereby a wider excision of healthy parenchyma is performed are also viable options. A minimal tumour-free surgical margin following partial nephrectomy seems appropriate to avoid the increased risk of local recurrence24. Positive surgical margins have been reported in 1–6% of cases regardless the type of used surgical technique148. Haematuria, perirenal haematoma and urinary fistulas are the most common complications of partial nephrectomy procedures. Less frequent postoperative complications can be represented by acute renal impairment and infection149.
Radical nephrectomy
Classical radical nephrectomy consists in the removal of kidney, perirenal fat tissue, adrenal gland and regional lymph nodes. However, in patients with tumour ≤5 cm in size, located at the inferior pole, the adrenal gland can be spared. Similarly, regional lymph nodes dissection can be reserved for patients with clinically positive nodes detected by CT or during the surgical procedure 150. Radical nephrectomy can be considered in cases with multiple small renal tumours, in cases in which the tumour extends into the vasculature and can be a laparoscopic or open procedure (FIG. 6). In most patients with stage I and II tumours, radical nephrectomy is currently performed using a traditional laparoscopic approach; the open approach remains the gold standard for the treatment of more complex cases. In experienced hands, the robot-assisted approach can represent a potential alternative to open surgery in cases with venous tumour thrombus.
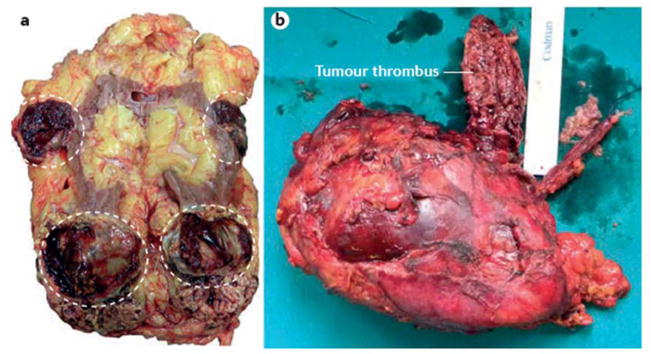
Figure 6. Indications for radical nephrectomy.
a | Radical nephrectomy could be considered in cases with multiple small renal tumours (circled). b | Conversely, radical nephrectomy and contextual excision of neoplastic thrombus into renal vein or cava vein tumour thrombus is the gold standard treatment for patients with venous involvement.
Data recently extracted from the US National Cancer Data Base support the use of cytoreductive nephrectomy in those with metastatic disease even while they receive systemic targeted therapies. Indeed, the median overall survival was 17.1 months in cytoreductive nephrectomy cases versus 7.7 months in non-cytoreductive nephrectomy group151.
Active surveillance and ablative therapies
Active surveillance and ablative techniques such as cryotherapy or radiofrequency ablation are alternative strategies for elderly patients and/or those with competing health risks and limited life expectancy that renders them unsuitable for surgery15,24.
A definite protocol for active surveillance has yet to be defined. The most common approach consists of alternating between ultrasonography imaging and CT or MRI every 3 months in the first year, every 6 months in the second year and annually thereafter. Intervention should be considered for growth to >3–4 cm or by >0.4–0.5 cm per year152. Data from the Delayed Intervention and Surveillance for Small Renal Masses (DISSRM) registry in the United States showed that in a well-selected cohort of patients with up to 5 years of prospective follow-up, active surveillance was not inferior to primary intervention in terms of both overall survival and cancer-specific survival 26.
Ablative technology must be able to completely destroy all viable tumour tissue with no area of viable tumour left. Both cryotherapy and radiofrequency ablation can be performed using a laparoscopic or percutaneous approach under a CT or ultrasound guidance. A meta-analysis of case series showed 89% and 90% of efficacy for cryoablation and radiofrequency ablation, respectively25; complication rates are 20% and 19%. Available low quality studies suggest a higher local recurrence rate for ablative therapies compared with partial nephrectomy153.
Medical management
The past 10 years have seen the approval of a number of targeted therapeutic agents and one immunotherapy agent for the treatment of metastatic RCC (FIG. 7). However, in the adjuvant setting after surgery, the situation is less clear and a randomised trial (ASSURE) of sunitinib versus sorafenib versus placebo showed no benefit for either drug therapy in terms of disease-free survival131. Notably, a recent study in the adjuvant setting reported a disease-free survival benefit for 1 year of sunitinib therapy in comparison with observation in the S-TRAC trial130. A number of other trials of adjuvant targeted therapies (such as PROTECT and SORCE) have completed accrual and will report outcomes in the next 12 months.
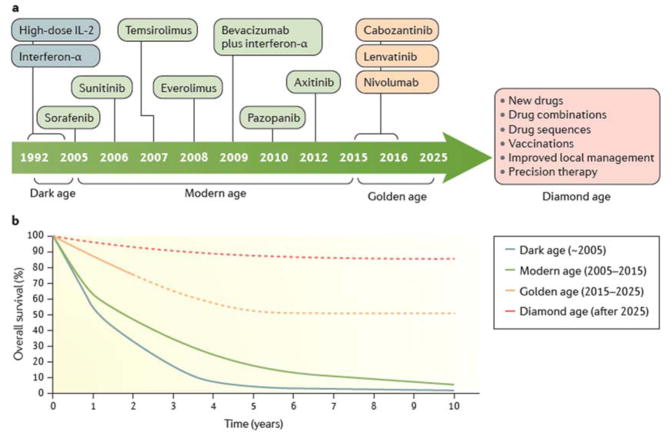
Figure 7. Therapeutic evolution and survival outcome of metastatic ccRCC through the four different eras.
a | Prior to 2004, two drugs were available to treat RCC (with a median survival of ~15 months). This so-called dark age of treatments was followed by the modern age (2005–2014), which saw seven additional regimens gain approval (increasing median survival to ~30 months). Currently, the golden age has already witnessed the introduction of three drugs, with more anticipated over the next decade. b | These advances promise to be translated to a significant number of patients (~50%) achieving durable remissions under active surveillance by 2025 with a median survival of ~5 years. The ultimate goal is the future diamond age of drug approvals is >80% of patients with metastatic ccRCC long-term survival. Dashed lines represent predicted survival.
Targeted therapies
Given the highly vascular nature of RCCs, it is unsurprising that several therapies are available to exploit this feature. Indeed, tyrosine kinase inhibitors targeting the VEGF signalling axis approved in the first-line and second-line settings for the treatment of metastatic RCC in United States and European Union are sorafenib, sunitinib, pazopanib, axitinib, lenvatinib and cabozantinib154–159. All approvals have been as single agents except the combinations of lenvatinib with everolimus; additionally, the anti-VEGF monoclonal antibody bevacizumab is approved for use with interferon-α160,161. Broadly speaking, sunitinib, pazopanib and the combination of bevacizumab and interferon-α are approved as first-line options whereas axitinib and cabozantinib are approved in the second line. The mTOR inhibitors everolimus and temsirolimus are approved as single agents in the second-line setting and in the first line in patients with poor risk status162,163. Indeed, arguably the landmark trial of first-line systemic therapy of metastatic RCC was the phase 3 study of sunitinib versus interferon-α reported in 2007 in which the superiority of sunitinib in terms of response rate, progression free and overall survival was reported155. This trial established sunitinib as the standard of care and the drug remains the comparator for all currently recruiting phase 3 studies of new drugs.
No clinically usable markers are available to select patients for particular therapies, despite intensive efforts. As such, the average duration of disease control with these drugs is 8–9 months in the first line setting and 5–6 months in the second line setting. Most of the phase 3 RCTs leading to the approval of these agents have excluded patients with nccRCC (Box 1) and as such this evidence base relates largely to ccRCC. Furthermore all of these agents are given continuously until disease progression in the absence of major toxicity. Furthermore, alternative schedules such as those electively interrupting therapy for prolonged periods have not been reported from RCTs.
Box 1. Limitations in the management of nccRCC.
From the perspective of surgical management, the presence of non-clear cell histology rarely has a bearing on treatment and, in fact, histological subtype is often unknown pre-operatively. Limited data are available to guide medical management of non-clear cell renal cell carcinoma (nccRCC) as a consequence of the exclusion in general of non-clear cell histologies from registration trials of targeted agents over the past 10 years. Importantly, the tumours classed as nccRCC are fundamentally different; there is no reason to suppose that a therapy effective for papillary RCC would be effective for chromophobe or indeed any other subtype of kidney cancer. Nevertheless, some trials have been carried out and have broadly established sunitinib as a reasonable first line option in nccRCC, although the efficacy is less than for clear cell renal carcinoma (ccRCC). Most patients with metastatic nccRCC are treated with targeted agents approved for ccRCC, with the data favouring VEGF inhibitors over mTORC1 inhibitors204,22,205. Unfortunately, most patients with nccRCC succumb to their diseases within 18 months despite systemic treatment12,13,204,205,206, and currently there is no evidence base for the treatment of nccRCC with checkpoint inhibitors. Encouragingly, a recent phase 2 trial reported everolimus plus bevacizumab as an effective combination in treating nccRCC in patients whose tumours display papillary features, achieving an overall response rate at 43% and a median progression free survival at 12.9 months194. Arguably, everolimus plus bevacizumab should be considered as the comparison arm in trials in rare RCC subtypes displaying predominant papillary morphology (papillary RCC type I and type II, and unclassified RCC with papillary features). Overall, the advances made are encouraging, but drug therapies tailored specifically to subtype remains an unmet need. Initiatives such as rarekidneycancer.org set up by experts and patient advocates are important steps to encourage rapid communication among patients with rare kidney cancer, doctors specialized in nccRCC and trialists.
Immunotherapy
Cytokines such as interferon-α and high dose IL-2 that enhance anti-tumour immune activity have been used since the 1990s to treat metastatic RCC and were standards of care prior to the introduction of sunitinib164. Both drugs typically benefit only a small subset of patients (generally those with intrinsically favourable disease biology) and are associated with significant toxicity, particularly in the case of high-dose IL-2. Many studies are currently investigating combinations of anti-VEGF therapy with new-generation of immunotherapy agents in the form of T-cell immune checkpoint inhibitors such as the antibodies against programmed cell death protein 1 ligand 1 (PDL1), which include avelumab and atezolizumab, and antibodies against programmed cell death protein 1 (PD1), which include nivolumab and pembrolizumab). PD1 negatively regulates T cell function and its ligand PDL1 is highly expressed by cancer cells; accordingly, blockade of the PD1–PDL1 axis promotes T cell activation and immune killing of the cancer. Another combination under investigation (Checkmate 214, NCT02231749) is nivolumab with ipilimumab, an inhibitor of the T-cell checkpoint cytotoxic T-lymphocyte-associated protein 4 (CTLA-4). CTLA-4 also downregulates T cell function; its inhibition by these antibodies promotes T cell activation.
Nivolumab was approved in United States and European Union after the Checkmate 025 RCT showed an overall survival benefit compared with everolimus in patients who had failed prior therapy with sunitinib and pazopanib165. However, the response rate to nivolumab was only 25% (5% for everolimus) and most patients treated did not experience significant tumour shrinkage. Although these check point inhibitors show promise, predicting response is difficult. In Checkmate 025, for example, PDL1 expression did not correlate with response, as had been reported in other trials in other cancer types 165. The reason for this observation is unknown, but PD-L1 expression is dynamic in space and time and archival (paraffin-embedded) material from the primary tumour used in Checkmate 025 might not have been representative of PD-L1 expression at metastatic tumour sites.
Finally, nivolumab is well tolerated compared with everolimus. Furthermore, it has been possible to combine nivolumab (and other anti-PD1 or anti-PDL1 therapies) with ‘clean’ (that is, more-specific, less-toxic and easier to combine) anti-VEGF therapies such as axitinib and bevacizumab, leading to a number of phase 3 studies of such combinations in metastatic RCC.
QUALITY OF LIFE
Quality of life and patient reported outcomes have become an important way to assess therapeutic strategies in the treatment of patients with RCC. Adverse events are important to consider and these are summarized in Table 4. Although oncological outcomes such as survival are more objective, validated quality of life measures have been developed to help assess the patient experience.
Table 4.
Selected adverse events and quality of life of the approved agents
| Drug | Adverse events | Improvement inquality of life? | Reference |
|---|---|---|---|
| Axitinib | Hypertension, diarrhoea, hypothyroidism and hand–foot syndrome | Yes versus sorafenib | 157 |
| Bevacizumab | Proteinuria, hypertension and bleeding | Not reported | 160 |
| Cabozantinib | Diarrhoea, hand–foot syndrome, hypertension, nausea and hypothyroidism | Not reported | |
| Everolimus | Stomatitis, hypercholesterolaemia, hyperglycaemia and pneumonitis | No versus placebo | 209 |
| Nivolumab | Colitis, pneumonitis and endocrinopathies | Yes versus everolimus | 165 |
| Pazopanib | Diarrhoea, hypertension, liver function test abnormalities and hand–foot syndrome | No versus placebo, Yes versus sunitinib | 156 |
| Sorafenib | Hypertension, diarrhea, hand–foot syndrome and rash | Yes versus placebo | 154 |
| Sunitinib | Diarrhoea, hand–foot syndrome, mucositis and hypertension | Yes versus IFN | 155 |
| Temsirolimus | Stomatitis, hyperglycaemia, hypercholesterolaemia and oedema | Yes versus IFN | 210 |
For localized RCC, a systematic review was performed which included data from 29 studies that included randomized and non-randomized studies149. It noted that quality of life outcomes after partial nephrectomy were superior to those of radical nephrectomy regardless of approach or technique. Interestingly, no good evidence suggested that cryotherapy or radiofrequency ablation had better quality of life outcomes compared to nephrectomy.
For metastatic RCC, quality of life measures become more important as treatment is usually palliative and patients continually balance quality versus quantity of life. A validated 15-question tool called the Functional Assessment of Cancer Therapy (FACT)–Kidney Cancer Symptom Index (FKSI) is the most specific to kidney cancer 166. A subscale of this, the FKSI-DRS (disease-related symptoms) has nine kidney cancer-specific questions on the topics of lack of energy, pain, weight loss, bone pain, fatigue, shortness of breath, cough, fever and haematuria. Other more-general questionnaires exist and have been used in RCC clinical trials, including the Functional Assessment of Cancer Therapy General (FACT-G), the EuroQOL EQ-5D and Visual Analogue Scale (VAS)167. These tools enable investigators to assess quality of life; however, limitations including questionnaire burden, incomplete answering and defining a truly clinically significant minimal difference in quality of life scores remain.
In the phase 3 registration trial of first-line sunitinib versus interferon-α in the metastatic setting, the FKSI, FKSI-DRS, FACT-G, EQ-5D and EQ-VAS demonstrated a consistent favourable difference in quality of life for sunitinib 155. This finding can probably be attributed to the favourable adverse effect profile of sunitinib, which is associated with less fatigue than interferon-α, and higher efficacy of sunitinib (31% response rate) compared with interferon-α (6%).
Quality of life was assessed with FKSI-19, the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), Cancer Therapy Satisfaction Questionnaire (CTSQ) and Seville Quality of Life Questionnaire (SQLQ) in the COMPARZ clinical trial comparing first line sunitinib versus pazopanib156. Measurements were taken at baseline and at day 28 of each treatment cycle, which is typically the point of highest sunitinib toxicity (including soreness in mouth, throat, hands and feet). Improved quality of life scores were observed in those patients taking pazopanib versus those taking sunitinib.
The immune checkpoint inhibitors have also had quality of life analyses reported167. In the Checkmate 025 study of nivolumab used the FKSI-DRS score, these were performed at baseline and every 4 weeks up to study week 104 after which assessments were reduced. Median time to health-related quality of life improvement was shorter in patients given nivolumab (4.7 months, 95% CI 3.7–7.5) than in patients given everolimus (median not reached). The overall survival of patients was longer in those who had high baseline health-related quality of life scores who then improved than those with similar baseline whose scores then deteriorated. The shortest overall survival was observed in those with low baseline scores who then deteriorated.
OUTLOOK
With the considerable advances in the molecular biology and management of RCC over the past several decades, it is not without reason that one could describe the current era of knowledge and available treatments as the ‘golden age’ of research. If we are to progress further, advances in diagnosis, local management and systemic therapy are needed to achieve >80% long-term survival that might define the future ‘diamond age’ of kidney cancer research and therapy (FIG. 7). Areas that currently show promise include developing strategies for treating high-risk patients, biomarkers to guide treatment and preventing and overcoming drug resistance.
Biomarkers to guide treatment
Although wide ranging clinical outcomes can be attributed to tumour heterogeneity in RCC, opportunities to further improve clinical outcomes on the basis of individual tumour characteristics (so called precision medicine) is an emerging field. Given that nivolumab, cabozantinib and lenvatinib were only recently approved, and few correlative studies have been reported, potential biomarkers for VEGF and mTOR inhibitors currently have the most promise.
Biomarkers can range from clinical parameters (such as blood pressure) and endogenous substances (such as plasma proteins) to pathobiological features specific to individual tumours (such as mutations). For example, as an on-target clinical biomarker, hypertension (systolic blood pressure ≥140mmHg) in patients receiving VEGF inhibitors has been shown to be associated with longer progression free survival and overall survival168. Additionally, many studies have looked into circulating biomarkers169, among which high levels of IL-6, IL-8, hepatocyte growth factor and osteopontin were associated with shorter progression free survival in patients receiving pazopanib and sunitinib170,171 whereas high levels of lactate dehydrogenase were associated with better overall survival in those receiving temsirolimus but not interferon-α172.
Genetic biomarkers are also beginning to be studied for associations with treatment outcome in various metastatic settings173–175. For example, RECORD-3, a large randomized phase 2 trial (n=471), demonstrated the better first-line efficacy of sunitinib (progression free survival of 10.7 months) over first-line everolimus (progression free survival of 7.9 months)22. Interestingly, genomic biomarker analysis of patients enrolled in RECORD-3 showed that BAP1 mutations were associated with 8.1 month progression free survival with first line sunitinib but 5.5 month with first-line everolimus — a significant difference. By contrast PBRM1 mutations showed no such association20, which is consistent with a VEGF inhibitor outlier study173 and warrants further validation. That BAP1 mutations were associated with inferior outcomes on everolimus20 is surprising given their reported higher mTORC1 activity than PBMR1 mutant tumours70. Furthermore, patients with KDM5C mutations were associated with a much longer first-line progression-free survival with sunitinib (20.6 months) than everolimus (9.8 months)20. As mutual exclusivity was detected between mutations of BAP1 and PBRM1 or KDM5C20, molecular subgrouping of metastatic ccRCC based on these three genes could be of clinical value in the future. In addition, case-based mTOR inhibitor outlier studies recognized activation mutations of MTOR and bi-allelic inactivation of TSC1 or TSC2 as potential biomarkers for long-term responders69,73,75,76.
Managing high-risk patients
A significant number (~30%) of patients with non-metastatic disease (based on clinical and pathological evaluation at the initial diagnosis) have occult metastases that will eventually become clinically evident. How to identify and better manage these high-risk patients presents a major challenge for operating urologists. As we begin to appreciate the impact that prevalent RCC mutations (in PBRM1, SETD2, BAP1, KDM5C, PTEN, and TP53) have on clinical outcomes, incorporating specific mutational information into prognostic nomograms will become increasingly useful. For example, transcription signatures such as ClearCode3486, and other biomarkers in the blood and urine, might be incorporated into validated predictive biomarkers for RCC recurrence after surgery. Similarly, predicting treatment response to systemic therapy might be plausible and will reduce cost and improve RCC cancer patient care. Our improving ability to identify high-risk patients with RCC and formulate personalized treatment and follow-up plans based on multi-omics holds the promise to quickly reduce the incidence of patients developing overt metastatic disease and render long-term survival.
Emerging therapies and changes to treatment
Several promising new drugs with novel mechanisms of action are in various stages of clinical trials. For immunotherapeutics, ipilimumab, an anti-CTLA-4 antibody, in combination with nivolumab has shown remarkable response rate of ~40% in the Checkmate 016 trial176. Additionally, the efficacy of autologous dendritic cell-based immunotherapy, which consists of expanding patient’s own dendritic cells in vitro followed by the introduction of tumour RNA before re-infusion back to the patient, in combination with sunitinib has been examined and showed early promise177.
In the realm of targeted therapeutics, inhibitors specifically targeting HIF-2 have been developed178,179. As kidney cancer is characterized by aberrant glycolysis (with aberrant glutamine and tryptophan metabolism65,180,181), it is of interest to learn if the glutaminase inhibitor CB-839182 and the indolemaine-2,3-dioxygenase inhibitor INCB024360183 could yield additional clinical benefits when added to existing therapies. Finally, as many of these novel therapeutic agents act on modulating the anti-cancer response in patients, further understanding of the intricate relationship between individual an kidney cancer cell and its respective immune microenvironment would be critical for the future success in designing combination treatment to improve survival87,184–187.
Given the increasing understanding of tumour biology and the increasing number of treatment options, how treatments are selected in the future will undoubtedly change (FIG. 8). As well as those already discussed, potential measures of high values personalized vaccination188, targeted radiotherapy to enhance anti-tumour immune response189 and selective cytoreductive nephrectomy in patients who were initially inoperable but later showed marked shrinkage of tumours after systemic treatments. Additionally, neoadjuvant or adjuvant190 immunotherapy or targeted therapy could become integrated into the current treatment algorithms.
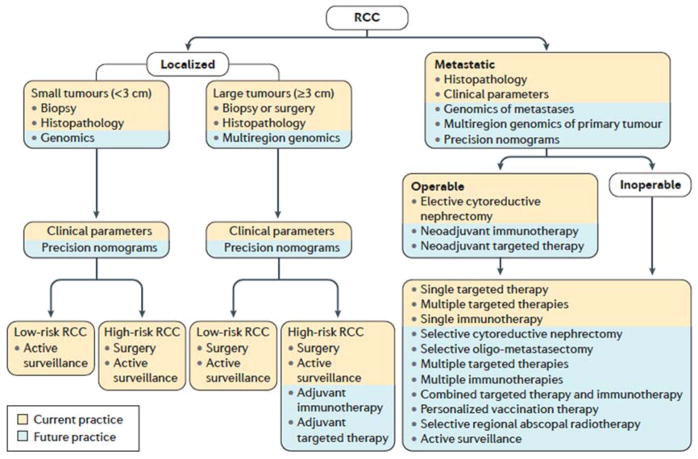
Figure 8. Treatment algorithms for renal cell carcinoma.
Given the advances in renal cell carcinoma (RCC) research, how patients are treated — based on their individual tumour characteristics — will likely change in the future.
Preventing and overcoming drug resistance
Model systems and clinical experience have shown that inhibiting RCC activity with multiple drugs specific to different targets is superior to single-agent approaches23,191,192. However, such approaches tend to produce more toxicities — on and off-target. For example, the combination of sunitinib and everolimus in treating metastatic RCC subjected patients to severe toxicity193. Nevertheless, bevacizumab, a more tolerable VEGF pathway inhibitor than sunitinib, plus everolimus is well tolerated and has been shown to be efficacious in treating nccRCC with papillary features194. The success of polypharmacy relies on efficient and correct targeting of both primary and secondary (bypass) pathways69,195. In ccRCC, VEGF is the primary pathway due to the universal VHL loss; secondary targets can include mTORC1, MET and IL-8 but not EGFR or PI3K pathways when one takes into consideration of available clinical158,159,169,196,197 and preclinical studies92,198–200.
Given the availability of targeted therapies (FIG. 7), immediate challenge is to design the most effective and specific regimen through combining or sequencing drugs to prevent resistance in individual patients201. Interestingly, a recent study in melanoma patients who relapsed after the initial treatment response on PD-1 blockade revealed invaluable insights on how tumour cells might develop resistance to immunotherapies, including defects in interferon-receptor signalling and in antigen presentation 202. As immune checkpoint inhibitors functions independently of specific oncogenic pathways and incur distinct resistance mechanisms202, the combination of these drugs with targeted therapies is of great clinical interests203 and theoretically can prevent the emergence of escape mechanisms from either agent.
Acknowledgments
J.J.H. is supported by the J. Randall & Kathleen L. MacDonald Family Research Fund, the Tom and Mila Tuttle Family Research Fund, the Jill and Rafic Dahan Family Research Fund, and the Jill and Jeffrey Weiss Family Research Fund for the cure of metastatic kidney cancer. J.L. is supported by the NIHR Royal Marsden/Institute of Cancer Research Biomedical Research Centre for Cancer.
Footnotes
AUTHOR CONTRIBUTIONS
Introduction (J.J.H); Epidemiology (M.P.); Mechanisms/pathophysiology (S.S., J.J.H. and C.S.); Diagnosis screening and prevention (L.A. and M.S.); Management (V.F. and J.L.); Quality of life (D.Y.H.); Outlook (J.J.H.); and Overview of Primer (J.J.H.).
COMPETING INTERESTS
J.J.H. is a consultant for Novartis, Eisai and Chugai and received research funding from Pfizer, Novartis, Eisai and Cancer Genomics Inc. C.S. is a consultant for Roche, Pfizer, Boehringer Ingelheim, Novartis, Celgene, Servler, Eli Lilly, and Glaxo Smithkline, and owns stock options from Achilles Therapeutics, Epic Biosciences, Grail, and Apogen Biotech. L.A. is a consultant for Pfizer, Novartis, Sanofi, Amgen, Bristol-Myers Squibb, Bayer and Cerulean, and received research funding from Pfizer and Novartis. M.S. is a consultant for Pfizer, Bristol-Myers Squibb, Ipsen, Exelixis, Eisai, Roche, Novartis and Astellas. D.Y.H. is a consultant for Pfizer, Novartis and Bristol-Myers Squibb. J.L. received research funding from Novartis, Pfizer, Bristol-Myers Squibb, and Merck Sharp & Dohme. M.P.P., S.S. and V.F. declare no competing interests.
References
- 1.Eble JN, Sauter G, Epstein JI, Sesterhenn IA. World health organization classification of tumours. IARC Press; Lyon: 2004. [Google Scholar]
- 2.Kovacs G, et al. The Heidelberg classification of renal cell tumours. The Journal of pathology. 1997;183:131–133. doi: 10.1002/(SICI)1096-9896(199710)183:2<131::AID-PATH931>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 3.Lopez-Beltran A, Scarpelli M, Montironi R, Kirkali Z. 2004 WHO classification of the renal tumors of the adults. Eur Urol. 2006;49:798–805. doi: 10.1016/j.eururo.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 4.Srigley JR, et al. The International Society of Urological Pathology (ISUP) Vancouver Classification of Renal Neoplasia. The American journal of surgical pathology. 2013;37:1469–1489. doi: 10.1097/PAS.0b013e318299f2d1. [DOI] [PubMed] [Google Scholar]
- 5.Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur Urol. 2016;70:93–105. doi: 10.1016/j.eururo.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 6.Chen FJ, et al. Multilevel Genomics-Based Taxonomy of Renal Cell Carcinoma. Cell Rep. 2016;14:2476–2489. doi: 10.1016/j.celrep.2016.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cancer Genome Atlas Research, N. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 2013;499:43–49. doi: 10.1038/nature12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cancer Genome Atlas Research, N., et al. Comprehensive Molecular Characterization of Papillary Renal-Cell Carcinoma. The New England journal of medicine. 2016;374:135–145. doi: 10.1056/NEJMoa1505917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis CF, et al. The somatic genomic landscape of chromophobe renal cell carcinoma. Cancer Cell. 2014;26:319–330. doi: 10.1016/j.ccr.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen YB, et al. Molecular analysis of aggressive renal cell carcinoma with unclassified histology reveals distinct subsets. Nat Commun. 2016;7:13131. doi: 10.1038/ncomms13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet. 2009;373:1119–1132. doi: 10.1016/S0140-6736(09)60229-4. [DOI] [PubMed] [Google Scholar]
- 12.Vera-Badillo FE, et al. Systemic therapy for non-clear cell renal cell carcinomas: a systematic review and meta-analysis. Eur Urol. 2015;67:740–749. doi: 10.1016/j.eururo.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Kroeger N, et al. Metastatic non-clear cell renal cell carcinoma treated with targeted therapy agents: characterization of survival outcome and application of the International mRCC Database Consortium criteria. Cancer. 2013;119:2999–3006. doi: 10.1002/cncr.28151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Escudier B, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2016;27:v58–v68. doi: 10.1093/annonc/mdw328. [DOI] [PubMed] [Google Scholar]
- 15.Motzer RJ, et al. Kidney cancer, version 3.2015. Journal of the National Comprehensive Cancer Network: JNCCN. 2015;13:151–159. doi: 10.6004/jnccn.2015.0022. [DOI] [PubMed] [Google Scholar]
- 16.Sankin A, Hakimi AA, Hsieh JJ, Molina AM. Metastatic non-clear cell renal cell carcinoma: an evidence based review of current treatment strategies. Frontiers in oncology. 2015;5:67. doi: 10.3389/fonc.2015.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerlinger M, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. The New England journal of medicine. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerlinger M, et al. Genomic architecture and evolution of clear cell renal cell carcinomas defined by multiregion sequencing. Nat Genet. 2014;46:225–233. doi: 10.1038/ng.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sankin A, et al. The impact of genetic heterogeneity on biomarker development in kidney cancer assessed by multiregional sampling. Cancer Med-Us. 2014;3:1485–1492. doi: 10.1002/cam4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsieh JJ, et al. Genomic Biomarkers of a Randomized Trial Comparing First-line Everolimus and Sunitinib in Patients with Metastatic Renal Cell Carcinoma. European Urology. 2016 doi: 10.1016/j.eururo.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molina AM, et al. Sunitinib objective response in metastatic renal cell carcinoma: analysis of 1059 patients treated on clinical trials. Eur J Cancer. 2014;50:351–358. doi: 10.1016/j.ejca.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 22.Motzer RJ, et al. Phase II randomized trial comparing sequential first-line everolimus and second-line sunitinib versus first-line sunitinib and second-line everolimus in patients with metastatic renal cell carcinoma. J Clin Oncol. 2014;32:2765–2772. doi: 10.1200/JCO.2013.54.6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsieh JJ, et al. Overcome Tumor Heterogeneity-Imposed Therapeutic Barriers through Convergent Genomic Biomarker Discovery: A Braided Cancer River Model of Kidney Cancer. Semin Cell Dev Biol. 2016 doi: 10.1016/j.semcdb.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ljungberg B, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67:913–924. doi: 10.1016/j.eururo.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 25.El Dib R, Touma NJ, Kapoor A. Cryoablation vs radiofrequency ablation for the treatment of renal cell carcinoma: a meta-analysis of case series studies. BJU Int. 2012;110:510–516. doi: 10.1111/j.1464-410X.2011.10885.x. [DOI] [PubMed] [Google Scholar]
- 26.Pierorazio PM, et al. Five-year analysis of a multi-institutional prospective clinical trial of delayed intervention and surveillance for small renal masses: the DISSRM registry. Eur Urol. 2015;68:408–415. doi: 10.1016/j.eururo.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Frank I, et al. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol. 2002;168:2395–2400. doi: 10.1097/01.ju.0000035885.91935.d5. [DOI] [PubMed] [Google Scholar]
- 28.Patard JJ, et al. Use of the University of California Los Angeles integrated staging system to predict survival in renal cell carcinoma: an international multicenter study. J Clin Oncol. 2004;22:3316–3322. doi: 10.1200/JCO.2004.09.104. [DOI] [PubMed] [Google Scholar]
- 29.Wolff I, et al. Do we need new high-risk criteria for surgically treated renal cancer patients to improve the outcome of future clinical trials in the adjuvant setting? Results of a comprehensive analysis based on the multicenter CORONA database. Eur J Surg Oncol. 2016;42:744–750. doi: 10.1016/j.ejso.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Meskawi M, et al. A review of integrated staging systems for renal cell carcinoma. Eur Urol. 2012;62:303–314. doi: 10.1016/j.eururo.2012.04.049. [DOI] [PubMed] [Google Scholar]
- 31.Motzer RJ, Hutson TE, McCann L, Deen K, Choueiri TK. Overall survival in renal-cell carcinoma with pazopanib versus sunitinib. New England Journal of Medicine. 2014;370:1769–1770. doi: 10.1056/NEJMc1400731. [DOI] [PubMed] [Google Scholar]
- 32.Ferlay J, et al. GLOBOCAN 2012 v1. 0, Cancer incidence and mortality worldwide: IARC CancerBase No. 11. 2013. [accessed on Aug 4, 2016];International Agency for Research on Cancer Web site. 2016 Available online: http://globocan.iarc.fr.
- 33.Torre LA, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 34.Global Burden of Disease Cancer, C., et al. The Global Burden of Cancer 2013. JAMA Oncol. 2015;1:505–527. doi: 10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 36.Ferlay J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 37.Shuch B, et al. Defining early-onset kidney cancer: implications for germline and somatic mutation testing and clinical management. J Clin Oncol. 2014;32:431–437. doi: 10.1200/JCO.2013.50.8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nielsen SM, et al. Von Hippel-Lindau Disease: Genetics and Role of Genetic Counseling in a Multiple Neoplasia Syndrome. J Clin Oncol. 2016;34:2172–2181. doi: 10.1200/JCO.2015.65.6140. [DOI] [PubMed] [Google Scholar]
- 39.Haas NB, Nathanson KL. Hereditary kidney cancer syndromes. Adv Chronic Kidney Dis. 2014;21:81–90. doi: 10.1053/j.ackd.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adeniran AJ, Shuch B, Humphrey PA. Hereditary Renal Cell Carcinoma Syndromes: Clinical, Pathologic, and Genetic Features. The American journal of surgical pathology. 2015;39:e1–e18. doi: 10.1097/PAS.0000000000000562. [DOI] [PubMed] [Google Scholar]
- 41.Znaor A, Lortet-Tieulent J, Laversanne M, Jemal A, Bray F. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol. 2015;67:519–530. doi: 10.1016/j.eururo.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 42.Sun M, et al. Age-adjusted incidence, mortality, and survival rates of stage-specific renal cell carcinoma in North America: a trend analysis. Eur Urol. 2011;59:135–141. doi: 10.1016/j.eururo.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 43.Hakimi AA, et al. An epidemiologic and genomic investigation into the obesity paradox in renal cell carcinoma. J Natl Cancer Inst. 2013;105:1862–1870. doi: 10.1093/jnci/djt310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McLaughlin J, Lipworth L, Tarone R, Blot W. Cancer Epidemiology and Prevention. Oxford University Press; Oxford: 2006. Kidney cancer; pp. 1087–1100. [Google Scholar]
- 45.Benichou J, Chow WH, McLaughlin JK, Mandel JS, Fraumeni JF., Jr Population attributable risk of renal cell cancer in Minnesota. Am J Epidemiol. 1998;148:424–430. doi: 10.1093/oxfordjournals.aje.a009667. [DOI] [PubMed] [Google Scholar]
- 46.Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol. 2010;7:245–257. doi: 10.1038/nrurol.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Faramawi MF, Johnson E, Fry MW, Sall M, Zhou Y. Consumption of different types of meat and the risk of renal cancer: meta-analysis of case-control studies. Cancer Causes Control. 2007;18:125–133. doi: 10.1007/s10552-006-0104-9. [DOI] [PubMed] [Google Scholar]
- 48.Lee JE, et al. Fat, protein, and meat consumption and renal cell cancer risk: a pooled analysis of 13 prospective studies. J Natl Cancer Inst. 2008;100:1695–1706. doi: 10.1093/jnci/djn386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Setiawan VW, Stram DO, Nomura AM, Kolonel LN, Henderson BE. Risk factors for renal cell cancer: the multiethnic cohort. Am J Epidemiol. 2007;166:932–940. doi: 10.1093/aje/kwm170. [DOI] [PubMed] [Google Scholar]
- 50.Tavani A, et al. Lifetime physical activity and the risk of renal cell cancer. Int J Cancer. 2007;120:1977–1980. doi: 10.1002/ijc.22438. [DOI] [PubMed] [Google Scholar]
- 51.Bergstrom A, et al. Physical activity and risk of renal cell cancer. Int J Cancer. 2001;92:155–157. [PubMed] [Google Scholar]
- 52.Karami S, et al. Family history of cancer and renal cell cancer risk in Caucasians and African Americans. Br J Cancer. 2010;102:1676–1680. doi: 10.1038/sj.bjc.6605680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaelin WG. Von Hippel-Lindau disease. Annu Rev Pathol. 2007;2:145–173. doi: 10.1146/annurev.pathol.2.010506.092049. [DOI] [PubMed] [Google Scholar]
- 54.Gnarra JR, et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet. 1994;7:85–90. doi: 10.1038/ng0594-85. [DOI] [PubMed] [Google Scholar]
- 55.Purdue MP, et al. Genome-wide association study of renal cell carcinoma identifies two susceptibility loci on 2p21 and 11q13.3. Nat Genet. 2011;43:60–65. doi: 10.1038/ng.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu X, et al. A genome-wide association study identifies a novel susceptibility locus for renal cell carcinoma on 12p11.23. Hum Mol Genet. 2012;21:456–462. doi: 10.1093/hmg/ddr479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Henrion M, et al. Common variation at 2q22.3 (ZEB2) influences the risk of renal cancer. Hum Mol Genet. 2013;22:825–831. doi: 10.1093/hmg/dds489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gudmundsson J, et al. A common variant at 8q24.21 is associated with renal cell cancer. Nat Commun. 2013;4:2776. doi: 10.1038/ncomms3776. [DOI] [PubMed] [Google Scholar]
- 59.Schodel J, et al. Common genetic variants at the 11q13.3 renal cancer susceptibility locus influence binding of HIF to an enhancer of cyclin D1 expression. Nat Genet. 2012;44:420–425. S421–422. doi: 10.1038/ng.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bigot P, et al. Functional characterization of the 12p12.1 renal cancer-susceptibility locus implicates BHLHE41. Nat Commun. 2016;7:12098. doi: 10.1038/ncomms12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hakimi AA, Pham CG, Hsieh JJ. A clear picture of renal cell carcinoma. Nat Genet. 2013;45:849–850. doi: 10.1038/ng.2708. [DOI] [PubMed] [Google Scholar]
- 62.Linehan WM, Srinivasan R, Schmidt LS. The genetic basis of kidney cancer: a metabolic disease. Nat Rev Urol. 2010;7:277–285. doi: 10.1038/nrurol.2010.47. nrurol.2010.47 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Masson N, Ratcliffe PJ. Hypoxia signaling pathways in cancer metabolism: the importance of co-selecting interconnected physiological pathways. Cancer Metab. 2014;2:3. doi: 10.1186/2049-3002-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Semenza GL. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. The Journal of clinical investigation. 2013;123:3664–3671. doi: 10.1172/JCI67230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hakimi AA, et al. An Integrated Metabolic Atlas of Clear Cell Renal Cell Carcinoma. Cancer Cell. 2016;29:104–116. doi: 10.1016/j.ccell.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reuter VE, Tickoo SK. Differential diagnosis of renal tumours with clear cell histology. Pathology. 2010;42:374–383. doi: 10.3109/00313021003785746. [DOI] [PubMed] [Google Scholar]
- 67.Lee CH, et al. Bevacizumab Monotherapy as Salvage Therapy for Advanced Clear Cell Renal Cell Carcinoma Pretreated With Targeted Drugs. Clinical Genitourinary Cancer. 2016;14:56–62. doi: 10.1016/j.clgc.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kapitsinou PP, Haase VH. The VHL tumor suppressor and HIF: insights from genetic studies in mice. Cell death and differentiation. 2008;15:650–659. doi: 10.1038/sj.cdd.4402313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wei EY, Hsieh JJ. A river model to map convergent cancer evolution and guide therapy in RCC. Nat Rev Urol. 2015;12:706–712. doi: 10.1038/nrurol.2015.260. [DOI] [PubMed] [Google Scholar]
- 70.Pena-Llopis S, et al. BAP1 loss defines a new class of renal cell carcinoma. Nat Genet. 2012;44:751–759. doi: 10.1038/ng.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hakimi AA, et al. Clinical and pathologic impact of select chromatin-modulating tumor suppressors in clear cell renal cell carcinoma. Eur Urol. 2013;63:848–854. doi: 10.1016/j.eururo.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sato Y, et al. Integrated molecular analysis of clear-cell renal cell carcinoma. Nat Genet. 2013;45:860–867. doi: 10.1038/ng.2699. [DOI] [PubMed] [Google Scholar]
- 73.Xu J, et al. Mechanistically distinct cancer-associated mTOR activation clusters predict sensitivity to rapamycin. The Journal of clinical investigation. 2016;126:3526–3540. doi: 10.1172/JCI86120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grabiner BC, et al. A diverse array of cancer-associated MTOR mutations are hyperactivating and can predict rapamycin sensitivity. Cancer discovery. 2014;4:554–563. doi: 10.1158/2159-8290.CD-13-0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Voss MH, et al. Tumor genetic analyses of patients with metastatic renal cell carcinoma and extended benefit from mTOR inhibitor therapy. Clin Cancer Res. 2014;20:1955–1964. doi: 10.1158/1078-0432.CCR-13-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kwiatkowski DJ, et al. Mutations in TSC1, TSC2, and MTOR are associated with response to rapalogs in patients with metastatic Renal Cell Carcinoma. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-15-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hakimi AA, et al. Adverse Outcomes in Clear Cell Renal Cell Carcinoma with Mutations of 3p21 Epigenetic Regulators BAP1 and SETD2: A Report by MSKCC and the KIRC TCGA Research Network. Clin Cancer Res. 2013;19:3259–3267. doi: 10.1158/1078-0432.CCR-12-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kapur P, et al. Effects on survival of BAP1 and PBRM1 mutations in sporadic clear-cell renal-cell carcinoma: a retrospective analysis with independent validation. Lancet Oncol. 2013;14:159–167. doi: 10.1016/S1470-2045(12)70584-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nam SJ, Lee C, Park JH, Moon KC. Decreased PBRM1 expression predicts unfavorable prognosis in patients with clear cell renal cell carcinoma. Urol Oncol. 2015;33:340e349–316. doi: 10.1016/j.urolonc.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 80.Manley BJ, et al. Integration of Recurrent Somatic Mutations with Clinical Outcomes: A Pooled Analysis of 1049 Patients with Clear Cell Renal Cell Carcinoma. European Urology Focus. 2016 doi: 10.1016/j.euf.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194:23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 82.Venkatesan S, Swanton C. Tumor Evolutionary Principles: How Intratumor Heterogeneity Influences Cancer Treatment and Outcome. American Society of Clinical Oncology educational book / ASCO. American Society of Clinical Oncology. Meeting. 2016;35:e141–149. doi: 10.14694/EDBK_158930. [DOI] [PubMed] [Google Scholar]
- 83.Kanu N, et al. SETD2 loss-of-function promotes renal cancer branched evolution through replication stress and impaired DNA repair. Oncogene. 2015;34:5699–5708. doi: 10.1038/onc.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gulati S, et al. Systematic evaluation of the prognostic impact and intratumour heterogeneity of clear cell renal cell carcinoma biomarkers. Eur Urol. 2014;66:936–948. doi: 10.1016/j.eururo.2014.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Beuselinck B, et al. Molecular subtypes of clear cell renal cell carcinoma are associated with sunitinib response in the metastatic setting. Clin Cancer Res. 2015;21:1329–1339. doi: 10.1158/1078-0432.CCR-14-1128. [DOI] [PubMed] [Google Scholar]
- 86.Brooks SA, et al. ClearCode34: A Prognostic Risk Predictor for Localized Clear Cell Renal Cell Carcinoma. Eur Urol. 2014;66:77–84. doi: 10.1016/j.eururo.2014.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Senbabaoglu Y, et al. Tumor immune microenvironment characterization in clear cell renal cell carcinoma identifies prognostic and immunotherapeutically relevant messenger RNA signatures. Genome Biol. 2016;17:231. doi: 10.1186/s13059-016-1092-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Geissler K, et al. Immune signature of tumor infiltrating immune cells in renal cancer. Oncoimmunology. 2015;4:e985082. doi: 10.4161/2162402X.2014.985082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sinha R, et al. Evaluating renal cell carcinoma cell lines as tumor models. Nat Commun. 2017 in press. [Google Scholar]
- 90.Winter S, et al. Methylomes of renal cell lines and tumors or metastases differ significantly with impact on pharmacogenes. Sci Rep. 2016;6:29930. doi: 10.1038/srep29930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huang D, et al. Sunitinib acts primarily on tumor endothelium rather than tumor cells to inhibit the growth of renal cell carcinoma. Cancer research. 2010;70:1053–1062. doi: 10.1158/0008-5472.CAN-09-3722. [DOI] [PubMed] [Google Scholar]
- 92.Huang D, et al. Interleukin-8 mediates resistance to antiangiogenic agent sunitinib in renal cell carcinoma. Cancer research. 2010;70:1063–1071. doi: 10.1158/0008-5472.CAN-09-3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhou L, et al. Targeting MET and AXL overcomes resistance to sunitinib therapy in renal cell carcinoma. Oncogene. 2016;35:2687–2697. doi: 10.1038/onc.2015.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dong YY, et al. Development of a Clear Cell Renal Cell Carcinoma Xenograft Model: A Case for the Use of Biopsy Tissue over Surgical Tissue. J Urology. 2016;195:E916–E916. [Google Scholar]
- 95.Wang SS, et al. Bap1 is essential for kidney function and cooperates with Vhl in renal tumorigenesis. Proc Natl Acad Sci U S A. 2014;111:16538–16543. doi: 10.1073/pnas.1414789111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Palapattu GS, Kristo B, Rajfer J. Paraneoplastic syndromes in urologic malignancy: the many faces of renal cell carcinoma. Rev Urol. 2002;4:163–170. [PMC free article] [PubMed] [Google Scholar]
- 97.Low G, Huang G, Fu W, Moloo Z, Girgis S. Review of renal cell carcinoma and its common subtypes in radiology. World J Radiol. 2016;8:484–500. doi: 10.4329/wjr.v8.i5.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Karlo CA, et al. Radiogenomics of Clear Cell Renal Cell Carcinoma: Associations between CT Imaging Features and Mutations. Radiology. 2014;270:464–471. doi: 10.1148/radiol.13130663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20:289–296. doi: 10.1200/JCO.2002.20.1.289. [DOI] [PubMed] [Google Scholar]
- 100.Heng DY, et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol. 2013;14:141–148. doi: 10.1016/S1470-2045(12)70559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Linehan WM, Srinivasan R, Garcia JA. Non-clear cell renal cancer: disease-based management and opportunities for targeted therapeutic approaches. Seminars in oncology. 2013;40:511–520. doi: 10.1053/j.seminoncol.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Marconi L, et al. Systematic Review and Meta-analysis of Diagnostic Accuracy of Percutaneous Renal Tumour Biopsy. Eur Urol. 2016;69:660–673. doi: 10.1016/j.eururo.2015.07.072. [DOI] [PubMed] [Google Scholar]
- 103.Tickoo SK, Reuter VE. Differential diagnosis of renal tumors with papillary architecture. Adv Anat Pathol. 2011;18:120–132. doi: 10.1097/PAP.0b013e31820cb3dd. [DOI] [PubMed] [Google Scholar]
- 104.Moch H. An overview of renal cell cancer: pathology and genetics. Semin Cancer Biol. 2013;23:3–9. doi: 10.1016/j.semcancer.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 105.Algaba F, et al. Current pathology keys of renal cell carcinoma. Eur Urol. 2011;60:634–643. doi: 10.1016/j.eururo.2011.06.047. [DOI] [PubMed] [Google Scholar]
- 106.Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. The American journal of surgical pathology. 1982;6:655–663. doi: 10.1097/00000478-198210000-00007. [DOI] [PubMed] [Google Scholar]
- 107.Cheville JC, Blute ML, Zincke H, Lohse CM, Weaver AL. Stage pT1 conventional (clear cell) renal cell carcinmoa: pathological features associated with cancer specific survival. J Urol. 2001;166:453–456. doi: 10.1016/s0022-5347(05)65962-9. [DOI] [PubMed] [Google Scholar]
- 108.Grignon DJ, Che M. Clear cell renal cell carcinoma. Clin Lab Med. 2005;25:305–316. doi: 10.1016/j.cll.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 109.de Peralta-Venturina M, et al. Sarcomatoid differentiation in renal cell carcinoma: a study of 101 cases. The American journal of surgical pathology. 2001;25:275–284. doi: 10.1097/00000478-200103000-00001. [DOI] [PubMed] [Google Scholar]
- 110.Bi M, et al. Genomic characterization of sarcomatoid transformation in clear cell renal cell carcinoma. Proc Natl Acad Sci U S A. 2016;113:2170–2175. doi: 10.1073/pnas.1525735113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Malouf GG, et al. Genomic Characterization of Renal Cell Carcinoma with Sarcomatoid Dedifferentiation Pinpoints Recurrent Genomic Alterations. Eur Urol. 2016 doi: 10.1016/j.eururo.2016.01.051. [DOI] [PubMed] [Google Scholar]
- 112.Manley BJ, Hsieh JJ. Sarcomatoid renal cell carcinoma: genomic insights from sequencing of matched sarcomatous and carcinomatous components. Translational Cancer Research. 2016;5:S160–S165. doi: 10.21037/tcr.2016.07.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Thompson IM. The evaluation of microscopic hematuria: a population-based study. J Urol. 1987;138:1189–1190. doi: 10.1016/s0022-5347(17)43545-2. [DOI] [PubMed] [Google Scholar]
- 114.Zhang L, et al. iTRAQ-based quantitative proteomic analysis reveals potential early diagnostic markers of clear-cell Renal cell carcinoma. Biosci Trends. 2016;10:210–219. doi: 10.5582/bst.2016.01055. [DOI] [PubMed] [Google Scholar]
- 115.Morrissey JJ, et al. Evaluation of Urine Aquaporin-1 and Perilipin-2 Concentrations as Biomarkers to Screen for Renal Cell Carcinoma: A Prospective Cohort Study. JAMA Oncol. 2015;1:204–212. doi: 10.1001/jamaoncol.2015.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tosaka A, et al. Incidence and properties of renal masses and asymptomatic renal cell carcinoma detected by abdominal ultrasonography. J Urol. 1990;144:1097–1099. doi: 10.1016/s0022-5347(17)39667-2. [DOI] [PubMed] [Google Scholar]
- 117.Gudbjartsson T, et al. A population-based familial aggregation analysis indicates genetic contribution in a majority of renal cell carcinomas. Int J Cancer. 2002;100:476–479. doi: 10.1002/ijc.10513. [DOI] [PubMed] [Google Scholar]
- 118.Hunt JD, van der Hel OL, McMillan GP, Boffetta P, Brennan P. Renal cell carcinoma in relation to cigarette smoking: meta-analysis of 24 studies. Int J Cancer. 2005;114:101–108. doi: 10.1002/ijc.20618. [DOI] [PubMed] [Google Scholar]
- 119.Theis RP, Dolwick Grieb SM, Burr D, Siddiqui T, Asal NR. Smoking, environmental tobacco smoke, and risk of renal cell cancer: a population-based case-control study. BMC Cancer. 2008;8:387. doi: 10.1186/1471-2407-8-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 121.Adams KF, et al. Body size and renal cell cancer incidence in a large US cohort study. Am J Epidemiol. 2008;168:268–277. doi: 10.1093/aje/kwn122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Luo J, et al. Body size, weight cycling, and risk of renal cell carcinoma among postmenopausal women: the Women’s Health Initiative (United States) Am J Epidemiol. 2007;166:752–759. doi: 10.1093/aje/kwm137. [DOI] [PubMed] [Google Scholar]
- 123.Albiges L, et al. Body Mass Index and Metastatic Renal Cell Carcinoma: Clinical and Biological Correlations. J Clin Oncol. 2016 doi: 10.1200/JCO.2016.66.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chow WH, Gridley G, Fraumeni JF, Jr, Jarvholm B. Obesity, hypertension, and the risk of kidney cancer in men. The New England journal of medicine. 2000;343:1305–1311. doi: 10.1056/NEJM200011023431804. [DOI] [PubMed] [Google Scholar]
- 125.Gago-Dominguez M, Yuan JM, Castelao JE, Ross RK, Yu MC. Regular use of analgesics is a risk factor for renal cell carcinoma. Br J Cancer. 1999;81:542–548. doi: 10.1038/sj.bjc.6690728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McCredie M, Stewart JH, Day NE. Different roles for phenacetin and paracetamol in cancer of the kidney and renal pelvis. Int J Cancer. 1993;53:245–249. doi: 10.1002/ijc.2910530212. [DOI] [PubMed] [Google Scholar]
- 127.Dhote R, Thiounn N, Debre B, Vidal-Trecan G. Risk factors for adult renal cell carcinoma. Urol Clin North Am. 2004;31:237–247. doi: 10.1016/j.ucl.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 128.Khurana V, Caldito G, Ankem M. Statins might reduce risk of renal cell carcinoma in humans: case-control study of 500,000 veterans. Urology. 2008;71:118–122. doi: 10.1016/j.urology.2007.08.039. [DOI] [PubMed] [Google Scholar]
- 129.Stewart-Merrill SB, et al. Oncologic Surveillance After Surgical Resection for Renal Cell Carcinoma: A Novel Risk-Based Approach. J Clin Oncol. 2015;33:4151–4157. doi: 10.1200/JCO.2015.61.8009. [DOI] [PubMed] [Google Scholar]
- 130.Ravaud A, et al. Adjuvant Sunitinib in High-Risk Renal-Cell Carcinoma after Nephrectomy. The New England journal of medicine. 2016 doi: 10.1056/NEJMoa1611406. [DOI] [PubMed] [Google Scholar]
- 131.Haas NB, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet. 2016;387:2008–2016. doi: 10.1016/S0140-6736(16)00559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Negrier S, et al. Prognostic factors of survival and rapid progression in 782 patients with metastatic renal carcinomas treated by cytokines: a report from the Groupe Francais d’Immunotherapie. Ann Oncol. 2002;13:1460–1468. doi: 10.1093/annonc/mdf257. [DOI] [PubMed] [Google Scholar]
- 133.Heng DY, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27:5794–5799. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 134.Bex A, Ljungberg B, van Poppel H, Powles T European Association of U. The Role of Cytoreductive Nephrectomy: European Association of Urology Recommendations in 2016. Eur Urol. 2016;70:901–905. doi: 10.1016/j.eururo.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 135.Huang WC, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006;7:735–740. doi: 10.1016/S1470-2045(06)70803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Scosyrev E, Messing EM, Sylvester R, Campbell S, Van Poppel H. Renal function after nephron-sparing surgery versus radical nephrectomy: results from EORTC randomized trial 30904. Eur Urol. 2014;65:372–377. doi: 10.1016/j.eururo.2013.06.044. [DOI] [PubMed] [Google Scholar]
- 137.Pignot G, et al. Nephron-sparing surgery is superior to radical nephrectomy in preserving renal function benefit even when expanding indications beyond the traditional 4-cm cutoff. Urol Oncol. 2014;32:1024–1030. doi: 10.1016/j.urolonc.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 138.Van Poppel H, et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol. 2011;59:543–552. doi: 10.1016/j.eururo.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 139.MacLennan S, et al. Systematic review of oncological outcomes following surgical management of localised renal cancer. European urology. 2012;61:972–993. doi: 10.1016/j.eururo.2012.02.039. [DOI] [PubMed] [Google Scholar]
- 140.Weight CJ, et al. Partial nephrectomy is associated with improved overall survival compared to radical nephrectomy in patients with unanticipated benign renal tumours. Eur Urol. 2010;58:293–298. doi: 10.1016/j.eururo.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 141.Sun M, et al. A non-cancer-related survival benefit is associated with partial nephrectomy. Eur Urol. 2012;61:725–731. doi: 10.1016/j.eururo.2011.11.047. [DOI] [PubMed] [Google Scholar]
- 142.Klatte T, et al. A Literature Review of Renal Surgical Anatomy and Surgical Strategies for Partial Nephrectomy. Eur Urol. 2015;68:980–992. doi: 10.1016/j.eururo.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Crestani A, et al. Introduction to small renal tumours and prognostic indicators. Int J Surg. 2016 doi: 10.1016/j.ijsu.2016.03.038. [DOI] [PubMed] [Google Scholar]
- 144.Aboumarzouk OM, et al. Robotic versus laparoscopic partial nephrectomy: a systematic review and meta-analysis. Eur Urol. 2012;62:1023–1033. doi: 10.1016/j.eururo.2012.06.038. [DOI] [PubMed] [Google Scholar]
- 145.Zhang X, et al. Comparison of peri-operative outcomes of robot-assisted vs laparoscopic partial nephrectomy: a meta-analysis. BJU international. 2013;112:1133–1142. doi: 10.1111/bju.12255. [DOI] [PubMed] [Google Scholar]
- 146.Ficarra V, et al. A multicentre matched-pair analysis comparing robot-assisted versus open partial nephrectomy. BJU Int. 2014;113:936–941. doi: 10.1111/bju.12570. [DOI] [PubMed] [Google Scholar]
- 147.Wu Z, et al. Robotic versus open partial nephrectomy: a systematic review and meta-analysis. PLoS One. 2014;9:e94878. doi: 10.1371/journal.pone.0094878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Marszalek M, et al. Positive surgical margins after nephron-sparing surgery. Eur Urol. 2012;61:757–763. doi: 10.1016/j.eururo.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 149.MacLennan S, et al. Systematic review of perioperative and quality-of-life outcomes following surgical management of localised renal cancer. Eur Urol. 2012;62:1097–1117. doi: 10.1016/j.eururo.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 150.Blom JH, et al. Radical nephrectomy with and without lymph-node dissection: final results of European Organization for Research and Treatment of Cancer (EORTC) randomized phase 3 trial 30881. Eur Urol. 2009;55:28–34. doi: 10.1016/j.eururo.2008.09.052. [DOI] [PubMed] [Google Scholar]
- 151.Hanna N, et al. Survival Analyses of Patients With Metastatic Renal Cancer Treated With Targeted Therapy With or Without Cytoreductive Nephrectomy: A National Cancer Data Base Study. J Clin Oncol. 2016;34:3267–3275. doi: 10.1200/JCO.2016.66.7931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lane BR, Tobert CM, Riedinger CB. Growth kinetics and active surveillance for small renal masses. Curr Opin Urol. 2012;22:353–359. doi: 10.1097/MOU.0b013e328355ecdf. [DOI] [PubMed] [Google Scholar]
- 153.Campbell SC, et al. Guideline for management of the clinical T1 renal mass. J Urol. 2009;182:1271–1279. doi: 10.1016/j.juro.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 154.Escudier B, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. The New England journal of medicine. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 155.Motzer RJ, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. The New England journal of medicine. 2007;356:115–124. doi: 10.1056/NEJMoa065044. 356/2/115 [pii] [DOI] [PubMed] [Google Scholar]
- 156.Motzer RJ, McCann L, Deen K. Pazopanib versus sunitinib in renal cancer. The New England journal of medicine. 2013;369:1970. doi: 10.1056/NEJMc1311795. [DOI] [PubMed] [Google Scholar]
- 157.Rini BI, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378:1931–1939. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 158.Motzer RJ, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 2015;16:1473–1482. doi: 10.1016/S1470-2045(15)00290-9. [DOI] [PubMed] [Google Scholar]
- 159.Choueiri TK, et al. Cabozantinib versus Everolimus in Advanced Renal-Cell Carcinoma. The New England journal of medicine. 2015;373:1814–1823. doi: 10.1056/NEJMoa1510016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Escudier B, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 161.Rini BI, et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol. 2008;26:5422–5428. doi: 10.1200/JCO.2008.16.9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Motzer RJ, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 163.Hudes G, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. The New England journal of medicine. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 164.McDermott DF, et al. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol. 2005;23:133–141. doi: 10.1200/JCO.2005.03.206. [DOI] [PubMed] [Google Scholar]
- 165.Motzer RJ, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. The New England journal of medicine. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cella D. Quality of life in patients with metastatic renal cell carcinoma: the importance of patient-reported outcomes. Cancer Treat Rev. 2009;35:733–737. doi: 10.1016/j.ctrv.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 167.Cella D, et al. Quality of life in patients with advanced renal cell carcinoma given nivolumab versus everolimus in CheckMate 025: a randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17:994–1003. doi: 10.1016/S1470-2045(16)30125-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Donskov F, et al. Sunitinib-associated hypertension and neutropenia as efficacy biomarkers in metastatic renal cell carcinoma patients. Br J Cancer. 2015;113:1571–1580. doi: 10.1038/bjc.2015.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Funakoshi T, Lee CH, Hsieh JJ. A systematic review of predictive and prognostic biomarkers for VEGF-targeted therapy in renal cell carcinoma. Cancer Treat Rev. 2014;40:533–547. doi: 10.1016/j.ctrv.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 170.Tran HT, et al. Prognostic or predictive plasma cytokines and angiogenic factors for patients treated with pazopanib for metastatic renal-cell cancer: a retrospective analysis of phase 2 and phase 3 trials. Lancet Oncol. 2012;13:827–837. doi: 10.1016/S1470-2045(12)70241-3. [DOI] [PubMed] [Google Scholar]
- 171.Voss MH, et al. Circulating biomarkers and outcome from a randomised phase II trial of sunitinib vs everolimus for patients with metastatic renal cell carcinoma. Brit J Cancer. 2016;114:642–649. doi: 10.1038/bjc.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Armstrong AJ, George DJ, Halabi S. Serum lactate dehydrogenase predicts for overall survival benefit in patients with metastatic renal cell carcinoma treated with inhibition of mammalian target of rapamycin. J Clin Oncol. 2012;30:3402–3407. doi: 10.1200/JCO.2011.40.9631. [DOI] [PubMed] [Google Scholar]
- 173.Fay AP, et al. Whole-Exome Sequencing in Two Extreme Phenotypes of Response to VEGF-Targeted Therapies in Patients With Metastatic Clear Cell Renal Cell Carcinoma. Journal of the National Comprehensive Cancer Network: JNCCN. 2016;14:820–824. doi: 10.6004/jnccn.2016.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ho TH, et al. Correlation Between Molecular Subclassifications of Clear Cell Renal Cell Carcinoma and Targeted Therapy Response. Eur Urol Focus. 2016;2:204–209. doi: 10.1016/j.euf.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 175.Hsieh J, et al. ASCO Annual Meeting Proceedings. :4509. [Google Scholar]
- 176.Hammers HJ, et al. ASCO Annual Meeting Proceedings. 4516 [Google Scholar]
- 177.Amin A, et al. Survival with AGS-003, an autologous dendritic cell-based immunotherapy, in combination with sunitinib in unfavorable risk patients with advanced renal cell carcinoma (RCC): Phase 2 study results. J Immunother Cancer. 2015;3:14. doi: 10.1186/s40425-015-0055-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chen W, et al. Targeting Renal Cell Carcinoma with a HIF-2 antagonist. Nature. 2016 doi: 10.1038/nature19796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cho H, et al. On-Target Efficacy of a HIF2alpha Antagonist in Preclinical Kidney Cancer Models. Nature. 2016 doi: 10.1038/nature19795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wettersten HI, et al. Grade-Dependent Metabolic Reprogramming in Kidney Cancer Revealed by Combined Proteomics and Metabolomics Analysis. Cancer research. 2015;75:2541–2552. doi: 10.1158/0008-5472.CAN-14-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rodrigues D, et al. Renal cell carcinoma: a critical analysis of metabolomic biomarkers emerging from current model systems. Transl Res. 2016 doi: 10.1016/j.trsl.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 182.Harding JJ, et al. ASCO Annual Meeting Proceedings. :2512. [Google Scholar]
- 183.Beatty GL, et al. ASCO Annual Meeting Proceedings. :3025. [Google Scholar]
- 184.Tartour E, et al. Angiogenesis and immunity: a bidirectional link potentially relevant for the monitoring of antiangiogenic therapy and the development of novel therapeutic combination with immunotherapy. Cancer Metastasis Rev. 2011;30:83–95. doi: 10.1007/s10555-011-9281-4. [DOI] [PubMed] [Google Scholar]
- 185.Remark R, et al. Characteristics and clinical impacts of the immune environments in colorectal and renal cell carcinoma lung metastases: influence of tumor origin. Clin Cancer Res. 2013;19:4079–4091. doi: 10.1158/1078-0432.CCR-12-3847. [DOI] [PubMed] [Google Scholar]
- 186.Giraldo NA, et al. Orchestration and Prognostic Significance of Immune Checkpoints in the Microenvironment of Primary and Metastatic Renal Cell Cancer. Clin Cancer Res. 2015;21:3031–3040. doi: 10.1158/1078-0432.CCR-14-2926. [DOI] [PubMed] [Google Scholar]
- 187.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 188.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 189.Demaria S, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–870. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 190.Janowitz T, Welsh SJ, Zaki K, Mulders P, Eisen T. Adjuvant therapy in renal cell carcinoma-past, present, and future. Seminars in oncology. 2013;40:482–491. doi: 10.1053/j.seminoncol.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 192.Bozic I, et al. Evolutionary dynamics of cancer in response to targeted combination therapy. Elife. 2013;2:e00747. doi: 10.7554/eLife.00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Molina AM, et al. Phase 1 trial of everolimus plus sunitinib in patients with metastatic renal cell carcinoma. Cancer. 2012;118:1868–1876. doi: 10.1002/cncr.26429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Voss MH, et al. Phase II Trial and Correlative Genomic Analysis of Everolimus Plus Bevacizumab in Advanced Non-Clear Cell Renal Cell Carcinoma. J Clin Oncol. 2016 doi: 10.1200/JCO.2016.67.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hsieh JJ, Cheng EH. A braided cancer river connects tumor heterogeneity and precision medicine. Clinical and Translational Medicine. 2016;5:42. doi: 10.1186/s40169-016-0123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Powles T, et al. Randomized Open-Label Phase II Trial of Apitolisib (GDC-0980), a Novel Inhibitor of the PI3K/Mammalian Target of Rapamycin Pathway, Versus Everolimus in Patients With Metastatic Renal Cell Carcinoma. J Clin Oncol. 2016;34:1660–1668. doi: 10.1200/JCO.2015.64.8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Carlo MI, et al. A Phase Ib Study of BEZ235, a Dual Inhibitor of Phosphatidylinositol 3-Kinase (PI3K) and Mammalian Target of Rapamycin (mTOR), in Patients With Advanced Renal Cell Carcinoma. Oncologist. 2016;21:787–788. doi: 10.1634/theoncologist.2016-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Nakaigawa N, et al. Inactivation of von Hippel-Lindau gene induces constitutive phosphorylation of MET protein in clear cell renal carcinoma. Cancer research. 2006;66:3699–3705. doi: 10.1158/0008-5472.CAN-05-0617. [DOI] [PubMed] [Google Scholar]
- 200.Mizukami Y, et al. Induction of interleukin-8 preserves the angiogenic response in HIF-1alpha-deficient colon cancer cells. Nature medicine. 2005;11:992–997. doi: 10.1038/nm1294. [DOI] [PubMed] [Google Scholar]
- 201.Albiges L, et al. A systematic review of sequencing and combinations of systemic therapy in metastatic renal cancer. Eur Urol. 2015;67:100–110. doi: 10.1016/j.eururo.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 202.Zaretsky JM, et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. The New England journal of medicine. 2016;375:819–829. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Massari F, et al. PD-1 blockade therapy in renal cell carcinoma: current studies and future promises. Cancer Treat Rev. 2015;41:114–121. doi: 10.1016/j.ctrv.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 204.Armstrong AJ, et al. Everolimus versus sunitinib for patients with metastatic non-clear cell renal cell carcinoma (ASPEN): a multicentre, open-label, randomised phase 2 trial. Lancet Oncol. 2016;17:378–388. doi: 10.1016/S1470-2045(15)00515-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Tannir NM, et al. Everolimus Versus Sunitinib Prospective Evaluation in Metastatic Non-Clear Cell Renal Cell Carcinoma (ESPN): A Randomized Multicenter Phase 2 Trial. Eur Urol. 2015 doi: 10.1016/j.eururo.2015.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Dutcher JP, et al. Effect of temsirolimus versus interferon-alpha on outcome of patients with advanced renal cell carcinoma of different tumor histologies. Med Oncol. 2009;26:202–209. doi: 10.1007/s12032-009-9177-0. [DOI] [PubMed] [Google Scholar]
- 207.Yates LR, Campbell PJ. Evolution of the cancer genome. Nature reviews. Genetics. 2012;13:795–806. doi: 10.1038/nrg3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Shuch B, et al. Understanding Pathologic Variants of Renal Cell Carcinoma: Distilling Therapeutic Opportunities from Biologic Complexity. Eur Urol. 2014 doi: 10.1016/j.eururo.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 209.Beaumont JL, et al. Patient-reported outcomes in a phase iii study of everolimus versus placebo in patients with metastatic carcinoma of the kidney that has progressed on vascular endothelial growth factor receptor tyrosine kinase inhibitor therapy. Oncologist. 2011;16:632–640. doi: 10.1634/theoncologist.2010-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Yang S, de Souza P, Alemao E, Purvis J. Quality of life in patients with advanced renal cell carcinoma treated with temsirolimus or interferon-alpha. Br J Cancer. 2010;102:1456–1460. doi: 10.1038/sj.bjc.6605647. [DOI] [PMC free article] [PubMed] [Google Scholar]