Abstract
The anionic peroxidase associated with the suberization response in potato (Solanum tuberosum L.) tubers during wound healing has been purified and partially characterized at the biochemical level. It is a 45-kD, class III (plant secretory) peroxidase that is localized to suberizing tissues and shows a preference for feruloyl (o-methoxyphenol)-substituted substrates (order of substrate preference: feruloyl > caffeoyl > p-coumaryl ≈ syringyl) such as those that accumulate in tubers during wound healing. There was little influence on oxidation by side chain derivatization, although hydroxycinnamates were preferred over the corresponding hydroxycinnamyl alcohols. The substrate specificity pattern is consistent with the natural substrate incorporation into potato wound suberin. In contrast, the cationic peroxidase(s) induced in response to wound healing in potato tubers is present in both suberizing and nonsuberizing tissues and does not discriminate between hydroxycinnamates and hydroxycinnamyl alcohols. A synthetic polymer prepared using E-[8-13C]ferulic acid, H2O2, and the purified anionic enzyme contained a significant amount of cross-linking through C-8, albeit with retention of unsaturation.
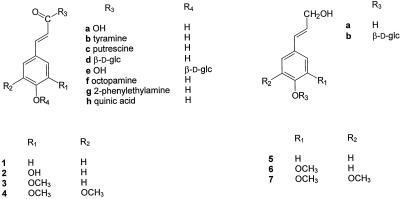
Suberization is a tissue-specific process whereby cell walls become impregnated with a poly(phenolic) matrix coincident with the deposition of a poly(aliphatic) matrix between the plasmalemma and carbohydrate cell wall (for review, see Bernards and Lewis, 1998). While the nature of the phenolic matrix remains incompletely defined, it has recently been shown that in potato (Solanum tuberosum L.) tubers it comprises primarily hydroxycinnamic acids (especially no. 3; see Scheme 1 for numbering), and their derivatives (especially no. 3b) (Bernards et al., 1995; Negrel et al., 1996). It has also been shown indirectly that in the suberized tissues of Quercus suber (Gil et al., 1997) and Clivia miniata (Schreiber, 1996; Zeier and Schreiber, 1997), there is a significant amount of hydroxycinnamic acid (especially nos. 1 and 3) present in the cell walls. These data suggest that the poly(phenolic) component of suberized cell walls is unique and distinct from cells that are lignified, where the poly(phenolic) matrix comprises oxidatively cross-linked hydroxycinnamyl alcohols (i.e. the monolignols 5a, 6a, and 7a) (Lewis and Yamamoto, 1990).
The macromolecular assembly process whereby monomeric hydroxycinnamic acids (and/or their derivatives) are transported to and subsequently incorporated (i.e. polymerized) into the carbohydrate cell wall matrix remains undefined. By analogy to the oxidative cross-linking model accepted for lignification, it has been hypothesized that the phenolic component of suberized cell walls is polymerized via a peroxidase/H2O2-mediated process (Kolattukudy, 1980). In this regard, an anionic peroxidase has been shown to be both temporally and spatially associated with the wound-induced suberization process in potato tubers (Borchert, 1978; Borchert and Decedue, 1978; Espelie and Kolattukudy, 1985; Espelie et al., 1986).
While the suberin-associated anionic peroxidase has not been fully characterized biochemically, it has been cloned and its molecular biology studied. Thus, a cDNA clone of the wound-induced anionic peroxidase of potato (Roberts et al., 1988; Roberts and Kolattukudy, 1989) was used to isolate a genomic clone (containing two tandemly oriented anionic peroxidase genes of 96% and 87% homology) from tomato (Lycopersicon esculentum Mill.) (Roberts et al., 1988). Expression studies of one of these genes, TAP 1 (tomato anionic peroxidase 1), demonstrated both a stress-induced and a developmentally regulated role for this peroxidase (Mohan and Kolattukudy, 1990; Mohan et al., 1993a, 1993b; Sherf and Kolattukudy, 1993). However, with its expression silenced in antisense tomato transformants, the incorporation of phenolics into the cell walls of wounded fruits (judged cytochemically by autofluorescence) continued unabated (Sherf et al., 1993). Thus, the suberin-specific role for the anionic peroxidase of potato and tomato remains tenuous, and more definitive evidence is required to unambiguously assign this specific function to it.
The specificity with which purified peroxidases oxidize different phenolic substrates (e.g. Converso and Fernandez, 1995; Marquez and Dunford, 1995; Pomar et al., 1997; Loukili et al., 1999), and characterization of the in vitro products formed when they are reacted with specific phenolic substrates (e.g. Lewis et al., 1987; Zimmerlin et al., 1994; Wallace and Fry, 1995) may provide clues to their in vivo role(s). However, for most peroxidase isoforms, these properties remain untested. We describe our progress in characterizing the wound-induced anionic peroxidase from potato at the biochemical level, particularly with respect to some of its basic biochemical and enzymological properties, including a major substrate specificity assessment.
MATERIALS AND METHODS
General
Solvents were of analytical or HPLC grade. Hydroxycinnamic acids (referred to by numbers as given in Scheme 1) 1a, 2a, 3a, and 4a, chlorogenic acid 2h, and coniferyl alcohol 6a were purchased from Aldrich. p-Coumaryl 5a and sinapyl 7a alcohols were a kind gift of Dr. Norman G. Lewis (Washington State University, Pullman). N-Feruloyloctopamine 3f was a kind gift of Dr. Jonathan Negrel (Institut National de la Recherche Agronomique, Dijon, France). All other substrates were either synthesized according to published protocols or isolated from natural sources (see below). Horseradish peroxidase type VIII (anionic) was purchased from Sigma.
Plant Material
Potato (Solanum tuberosum cv Russet Burbank) tubers were obtained from Monashee Mountain Seed Potatoes (Lumby, British Columbia, Canada), a member of the British Columbia Seed Potato Growers Association, and propagated in the Prince George, British Columbia, Canada, area. Tubers were harvested each fall and stored at 5°C in the dark until used. Suberization was induced by slicing surface-sterilized tubers into 0.5- to 1-cm-thick cross-sectional pieces, and incubating them in sterile Magenta boxes as described previously (Bernards and Lewis, 1992). For purification of the anionic peroxidase, acetone powders (Espelie and Kolattukudy, 1985) prepared from the mechanically removed suberized layers of 7-d wound-healed tubers were used.
Isoform Analysis
Total soluble protein extracts were prepared separately from 1 g each of suberized and nonsuberized (i.e. the tissue immediately underlying the suberized layer) tissues collected 7 d post wounding, for 30 min on ice in 10 mL of cold extraction buffer (50 mm potassium phosphate, pH 7.5, containing 300 mm Suc, 20 mm KCl, 10 mm DTT [added fresh at the time of extraction], 3 mm EDTA, and 0.1 mm MgCl2). After centrifugation at 13,500g, the supernatant was desalted (model P6-DG, Bio-Rad) into 25 mm Bis-Tris-iminodiacetate, pH 7.1, and chromatofocused on a Mono-P HR 5/5 column (Pharmacia) over a 7.1 to 3.5 pH range. The pH gradient was generated with buffer (Polybuffer 74, Pharmacia, pH adjusted to 3.5 with saturated iminodiacetate) at a flow rate of 0.5 mL min−1. Fractions (0.5 mL) were assayed spectrophotometrically using both guaiacol/H2O2 (20 mm/10 mm; 470 nm) and ferulic acid/H2O2 (0.15 mm/2 mm; 310 nm).
Purification of Anionic Peroxidase
All purification steps were performed at 4°C or on ice. Column fractions were assayed for peroxidase activity spectrophotometrically using 20 mm guaiacol and 20 mm H2O2 in acetate buffer (20 mm, pH 5.0) by following the oxidation of guaiacol at 470 nm. Column eluants were monitored at 280 nm.
A total of 100 g of acetone powder (obtained from approximately 530 g of suberized layers) was extracted in 20 separate 5-g batches. For each batch, proteins were extracted with 40 mL of cold extraction buffer (50 mm potassium-phosphate, pH 7.5, containing 300 mm Suc, 20 mm KCl, 10 mm DTT [added fresh at the time of extraction], 3 mm EDTA, and 0.1 mm MgCl2) on ice for 30 min with occasional stirring. After squeezing through eight layers of cheesecloth, the extract was centrifuged (10,000g, 20 min, 4°C) and the supernatant was immediately loaded onto a 2.5- × 28-cm Sephadex G25-M column (Pharmacia) pre-equilibrated with 25 mm Tris-HCl, pH 7.5, and gravity eluted with 25 mm Tris-HCl, pH 7.5, to separate the proteins from low-molecular-mass phenolics present in the extract. The protein fraction was collected and brought to 50% saturation with solid (NH4)2SO4. After centrifugation at 20,000g for 10 min at 4°C, the supernatant was brought to 90% saturation with solid (NH4)2SO4 and recentrifuged at 20,000g for 10 min at 4°C). The 50% to 90% (NH4)2SO4 pellets were reconstituted in a minimum volume of 25 mm Tris-HCl, pH 7.5, stored at −40°C. The 50% to 90% (NH4)2SO4 pellets were pooled, desalted into 25 mm Bis-Tris, pH 7.1 (Sephadex G25-M, 2.5- × 28-cm), concentrated by ultrafiltration (YM 10 membrane, Amicon, Beverly, MA), loaded onto a 2.5- × 100-cm Sephadex G100 column (Pharmacia) pre-equilibrated with 25 mm Bis-Tris, pH 7.1, in five 8- to 10-mL batches, and eluted with 25 mm Bis-Tris, pH 7.1, at 0.2 mL min−1.
Fractions from Sephadex G100 containing peroxidase activity were pooled, concentrated to 10 mL by ultrafiltration (YM 10 membrane, Amicon), and loaded onto a 1.5- × 18-cm polybuffer exchanger (PBE) column (Pharmacia) pre-equilibrated with 25 mm Bis-Tris, pH 7.1). Proteins were eluted first with equilibration buffer followed by a pH gradient (7.1−3.5) generated with buffer (Polybuffer 74 diluted 1:8 with water) at pH 3.5 at 0.5 mL min−1. Fractions containing peroxidase activity were pooled, desalted, and concentrated by ultrafiltration (YM 10 membrane, Amicon) into 25 mm Bis-Tris, pH 7.1.
The anionic peroxidase from PBE was loaded onto a 1.0- × 6.5-cm DEAE Sepharose Fast Flow column (Pharmacia) pre-equilibrated with 25 mm Bis-Tris, pH 7.1, and eluted with a salt gradient (0–300 mm NaCl in 25 mm Bis-Tris, pH 7.1, over 60 min) at 1 mL min−1. Fractions containing peroxidase activity were pooled, desalted, and concentrated by ultrafiltration (YM 10 membrane, Amicon) into 25 mm Bis-Tris, pH 7.1. The cationic peroxidase from PBE was retained without further purification.
Protein concentrations were estimated by the micro-method modification of the Bradford assay (Bradford, 1976) using commercially available dye reagent (Bio-Rad) and bovine-γ-globulins as standards, according to the manufacturer's instructions. The concentration of pure enzyme was estimated using a molar extinction coefficient (ε405) value of 105 mm−1 cm−1.
SDS-PAGE
SDS-PAGE was carried out using 14% acrylamide gels essentially as originally described (Laemmli, 1970) and modified for the Bio-Rad Mini Protean system according to the manufacturer's instructions but without boiling. Gels were silver-stained using a modified protocol of de Moreno et al. (1985). After successive fixing with 20% TCA (minimum 2 h) and MeOH:HOAc:H2O (4:1:5) (3 × 15 min), gels were rinsed with water (2 × 15 min) and treated with 0.1% AgNO3 (1 h). After rinsing (2 × 10 s), protein bands were visualized using successive washes (3 × 100 mL) with a developer solution (3% [w/v] Na2CO3 containing 0.0185% [w/v] formaldehyde). Color development was stopped using 2.3 m citric acid (7.5 mL/100 mL developer solution).
Gels were internally calibrated using a low-molecular-mass marker kit (Pharmacia) containing phosphorylase B (94 kD), BSA (67 kD), ovalbumin (43 kD), carbonic anhydrase (30 kD), soybean trypsin inhibitor (20.1 kD), and α-lactalbumin (14.1 kD). Peroxidase activity was visualized in gels without prior silver-staining using guaiacol/H2O2 (50 mm each in 50 mm acetate buffer, pH 5), after first rinsing the gels with water (2 × 15 min) to remove SDS. The reaction was stopped by removing the substrates and rinsing the gels with water.
Calibrated Molecular Sieving Chromatography
A Bio-Prep SE 100/17 column (Bio-Rad, molecular mass range 5–100 kD) was calibrated using thyroglobulin A (670 kD void volume estimate), IgG (150 kD), BSA (67 kD), ovalbumin (43 kD), carbonic anhydrase (29 kD), myoglobin (17 kD), RNase A (13.7 kD), and vitamin B12 (1.3 kD total volume estimate). Standard solutions (5 mg mL−1) of BSA, carbonic anhydrase, and RNase A were prepared in elution buffer (20 mm Tris-HCl, pH 7.5, containing 150 mm KCl). The remaining standards were part of a calibration kit (Bio-Rad) and were prepared according to the manufacturer's instructions. Samples were loaded individually (100 μL) and eluted with elution buffer at 0.25 mL min−1. For purified anionic peroxidase, 100 μL of a 5 μm solution in elution buffer was used. Fractions (0.25 mL) were collected and assayed for activity using guaiacol/H2O2.
Chemical Deglycosylation
Purified anionic peroxidase (35 μg) was deglycosylated according to the method of O'Donnell et al. (1992) and analyzed by SDS-PAGE followed by silver staining.
Enzyme Assays
Potato anionic peroxidase, potato cationic peroxidase, and horseradish type VIII (anionic) peroxidase were assayed at a final concentration of 0.5 nm. A molar extinction coefficient of 105 mm−1 cm−1 was used to adjust their concentrations. For ascorbate and guaiacol substrates, the method of Amako et al. (1994) was used essentially as described, except guaiacol was substituted for pyrogallol. For phenolic substrate specificity assays, solutions were preincubated at 40°C. The following quasi-rapid mixing method, based on that described by Rasmussen et al. (1995), was employed: anionic peroxidase (1 nm) and phenolic substrate (0–0.4 mm), in assay buffer (50 mm citrate buffer, pH 4.5 or 6.5, containing 1 mm CaCl2) was placed in one syringe (3 mL), while H2O2 (4 mm) in assay buffer (total 3 mL) was placed in another. Equal volumes (1 mL) of each solution were mixed by simultaneous injection into a flow cell (75 μL internal volume) and the initial rate of substrate disappearance was monitored for 30 s. Triplicate reactions were measured for each syringe filling and each substrate concentration, with each repeated at least three times. Slopes (in absorbance units min−1) were measured for the initial, linear phase of the reaction (usually over 5–10 s). The data were fitted to straight lines using Wolfe-Hanes transformations, and apparent maximum rates (Vmaxapp) values were extrapolated from intercepts.
Synthesis of Phenolic Substrates
N-(Hydroxycinnamoyl)tyramine derivatives 1b, 2b, 3b, and 4b and the 2-(phenyl)-ethylamine analog 3g were synthesized according to the method of Villegas and Brodelius (1990). N-(Hydroxycinnamoyl)putrescine derivatives 1c and 3c were synthesized according to the method of Malmberg (1984) and purified on a 1.5- × 30-cm polyamide column (model SC6, Machery-Nagel, Duren, Germany, pre-equilibrated with water) eluted with water. Hydroxycinnamyl alcohol-4-O-β-d-glucosides 5b, 6b, and 7b were synthesized via reduction of the corresponding hydroxycinnamoyl-ethyl esters using diisobutylaluminum hydride (Terashima et al., 1995). Hydroxycinnamate-4-O-β-d-glucosides 1e, 3e, and 4e were synthesized using the same basic procedure as for 5b, 6b, and 7b, but incorporating ester hydrolysis (10% [w/v] KOH in MeOH for 1 h followed by acidification [HCl] and extraction into ethylacetate) in place of the reduction with diisobutylaluminum hydride. The identity of each product was verified by NMR spectroscopy (1H and 13C) and comparison with published spectral data.
E-[8-13C]Ferulic Acid
Piperidine (50 μL) was added to a suspension of vanillin (97.3 mg, 0.64 mmol) and [2-13C]malonic acid (120.2 mg, 1.14 mmol, 1.8 equivalents) in freshly distilled pyridine (1 mL). The resulting yellow solution was stirred at 55°C for 17 h. The yellow pyridine solution was cooled to room temperature, poured into a 6 m solution of HCl (6 mL), and stirred vigorously for 15 min. The aqueous mixture was extracted with EtOAc (4 × 10 mL) and the organic solubles were combined, dried (MgSO4), concentrated in vacuo, and chromatographed on silica gel (EtOAc:CH2Cl2:MeOH, 5:5:1) to produce a yellow solid (94.6 mg, 76%). 1H-NMR (300 MHz, acetone-d6): δ 3.92 (3H, s, Ar-OMe), 6.38 (1H, dd, JH7-H8 = 15.9 Hz, JC7-H8 = 160.8 Hz, H-8), 6.87 (1H, d, JH5-H6 = 8.2 Hz, H-5), 7.14 (1H, dd, JH2-H6 = 1.8 Hz, JH5-H6 = 8.2 Hz, H-6), 7.34 (1H, d, JH2-H6 = 1.8 Hz, H-2), 7.59 (1H, dd, JH7-H8 = 15.9 Hz, JC7-H7 = 2.7 Hz, H-7), 8.2 (1H, br s, exchangeable with D2O, Ar-OH). 13C-NMR (75 MHz, acetone-d6): δ 116.3 (C-8).
Isolation of Phenolic Substrates
The 9-O-β-d-Glc esters 1d and 3d were isolated from young tomato leaves after first feeding the appropriate hydroxycinnamate precursor (10 mm in water) for 2 to 3 d (Harborne and Corner, 1961). For sinapoyl Glc 4d, 4-d-old radish seedlings were used. In either case, a total phenolic extract was prepared (80% aqueous MeOH) from 35 to 100 g fresh weight of plant material, concentrated in vacuo (<40°C) until aqueous, filtered, and applied to a 2.5- × 25-cm polyamide SC6 column (Machery-Nagel) pre-equilibrated with water. The hydroxycinnamoyl glucosides were eluted from the polyamide column with water and concentrated in vacuo (<40°C). Free sugars were precipitated at 4°C by repeatedly adding (four times) cold MeOH (to 80% [v/v]) to the aqueous fraction, followed by filtration and concentration in vacuo to remove the MeOH. Crude, sugar-free samples were loaded onto a water-equilibrated 1.5- × 20-cm Sephadex LH20 column (Pharmacia), and the phenolic glucosides eluted with a stepwise gradient of MeOH (100 mL each of 0%, 25%, 50%, 75%, and 100% [v/v] MeOH). Fractions containing hydroxycinnamoyl conjugates were selected using their distinctive UV spectra as a marker. Final purification was achieved using a semipreparative 25- × 100-mm HPLC C18 column (NovaPak, Waters). Glucosides were eluted with an isocratic gradient (3% [v/v] acetonitrile in water) at 9 mL min−1 and identified on the basis of their 1H-NMR spectra.
p-Coumaroylglucose 1d
Isolated as an amorphous powder (5 mg). UV (MeOH, λmax) 330 nm. 1H-NMR (300 MHz, MeOH-d4): δ 3.37–3.46 (4H, m, Glc protons 2′, 3prime], 4′, 5′), 3.69 (1H, dd, J = 12.0 Hz, 4.8 Hz, H-6′B), 3.85 (1H, dd, J = 1.0 Hz, 12.0 Hz, H-6′A), 5.57 (1H, d, JH1′-H2′ = 7.6 Hz, H-1′), 6.38 (1H, d, JH7-H8 = 15.9 Hz, H-8), 6.38 (2H, d, JH5-H6 = 8.7 Hz, H-3, H-5) 7.49 (2H, d, JH2-H3 = 8.7 Hz, H-2, H-6), 7.73 (1H, d, JH7-H8 = 16.0 Hz, H-7).
Feruloylglucose 3d
Isolated as an amorphous powder (38 mg). UV (MeOH, λmax) 330 nm. 1H-NMR (300 MHz, MeOH-d4): δ 3.31–3.43 (4H, m, Glc protons 2′, 3′, 4′, 5′), 3.66 (1H, dd, J = 12.1 Hz, 4.5 Hz, H-6′B), 3.82 (1H, dd, J = 1.6 Hz, 12.0 Hz, H-6′A), 3.86 (3H, s, Ar-OMe), 5.54 (1H, d, JH1′-H2′ = 7.6 Hz, H-1′), 6.38 (1H, d, JH7-H8 = 15.9 Hz, H-8), 6.79 (1H, d, JH5-H6 = 8.2 Hz, H-5), 7.07 (1H, dd, J H2-H6 = 1.6 Hz, JH5-H6 = 8.2 Hz, H-6), 7.18 (1H, d, JH2-H6 = 1.8 Hz, H-2), 7.70 (1H, d, JH7-H8 = 16.1 Hz, H-7).
Sinapoylglucose 4d
Isolated as pale yellow needles from water (28 mg). UV (MeOH, λmax) 330 nm. 1H-NMR (300 MHz, MeOH-d4): δ 3.34–3.50 (4H, m, Glc protons 2′, 3′, 4′, 5′), 3.70 (1H, dd, J = 8.0 Hz, 4.5 Hz, H-6′B), 3.84 (1H, d, J = 1.8 Hz, H-6′A), 3.88 (6H, s, Ar-OMe), 5.59 (1H, d, JH1′-H2′ = 7.9 Hz, H-1′), 6.44 (1H, d, JH7-H8 = 15.9 Hz, H-8), 6.93 (2H, s, H-2, H-6), 7.72 (1H, d, JH7-H8 = 15.9 Hz, H-7).
Product Formation
Polymeric products were prepared by the slow addition (0.8 mL h−1) of H2O2 (50 mm, 10 mL, in 10 mm phosphate buffer, pH 7) to a stirring solution (10 mL, 10 mm phosphate buffer, pH 7) of pure potato anionic peroxidase (0.14 mg) and either ferulic acid 3a (19.4 mg, 0.1 mmol) or E-[8-13C]ferulic acid (19.5 mg, 0.1 mmol) in a 40°C water bath. All solutions were bubbled with N2 gas prior to use. After 24 h, the reaction mixture was deep red, and the product was precipitated with the addition of a few drops of concentrated HCl, collected by centrifugation (1250g, 10 min, room temperature), and washed with water (two times), collecting the precipitate by centrifugation as above. The final pellet was freeze-dried to yield a dark orange powder, reconstituted in 1 mL of 0.1 m NaOH, loaded onto a 1.5- × 25-cm Sephadex G25-M column (Pharmacia) pre-equilibrated with 0.1 m NaOH, and eluted with 0.1 m NaOH at 1.8 mL min−1. The UV-absorbing eluant (A280) was collected, acid precipitated with HCl, washed with water, and freeze-dried as above to yield 10 mg (52%). BSA and ferulic acid were used to estimate the void and total volumes, respectively, of the column used. For NMR, equal amounts of either natural abundance or 13C-enriched reaction product were dissolved separately in 1 mL of 0.1 m KOH in D2O. A drop of DMSO-d6 was added as an internal standard.
RESULTS
Isoform Analysis
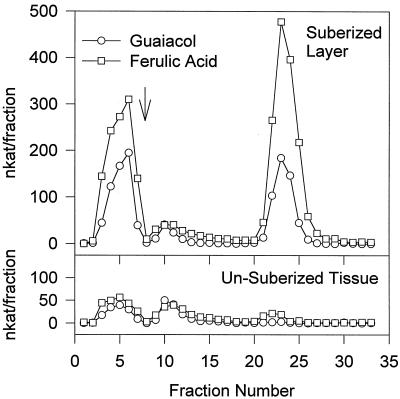
Wounding of potato tubers induced at least three groups of peroxidase isoforms, cationic, neutral, and anionic, in the suberizing tissue isolated from a 7-d-old wound site (Fig. 1). By contrast, the nonsuberized tissue underlying the suberized layer contained predominantly cationic and neutral forms, with only trace amounts of the anionic forms. All three groups of isoforms oxidized both ferulic acid 3a and guaiacol, albeit with different specific activity. For example, the cationic isoforms oxidized ferulic acid 3a approximately 1.5 times faster than guaiacol, while the anionic form oxidized ferulic acid 3a approximately 2.5 times faster than guaiacol. The neutral peroxidase oxidized both substrates equally well.
Figure 1.
Isoform analysis of wound-induced peroxidases of potato tubers. Total soluble proteins were extracted from either the (mechanically removed) suberized layer or the unsuberized tissue immediately below the suberized layer of 7-d wound-healed potato tubers, and chromatofocused on a Mono-P HR 5/5 column (Pharmacia). Proteins were loaded at pH 7.1, and the pH gradient (7.1–3.5 over approximately 15 min) started after all of the unbound protein had been washed through (indicated by an arrow). Fractions were assayed separately for activity using both ferulic acid and guaiacol.
Purification of a Wound-Induced Anionic Peroxidase
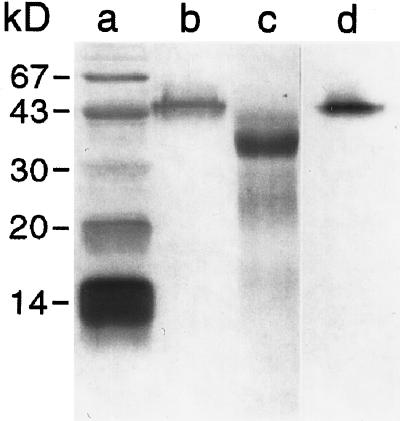
Potato anionic peroxidase was readily purified to apparent electrophoretic homogeneity (Fig. 2, lane b) from wound-induced tubers through a combination of size-exclusion and anion-exchange chromatography (Table I). Enzyme activity (using guaiacol as the substrate) was used as the basis for selection at each step. The final product, representing approximately 20% of the original (i.e. total) activity (measured using ferulic acid as substrate) was recovered in a total yield of 3.5 mg and had a Reinheitszahl value (ratio of heme A405 to protein A280) of 2.7. Typical values reported for purified peroxidases range from 1.6 (Kwak et al., 1995) to 4.1 (Converso and Fernandez, 1995), with most in the 2.6 to 3.3 range (Zimmerlin et al., 1994; Padiglia et al., 1995; Rasmussen et al., 1995). The relatively low pI of the protein (approximately 3.5) and its small size (approximately 45 kD) facilitated purification. Remarkably, the enzyme retained its H2O2-dependent activity in the SDS-PAGE gel (Fig. 2, lane d), confirming that the isolated protein was a peroxidase.
Figure 2.
SDS-PAGE analysis of purified anionic peroxidase from potato electrophoresed in a 14% acrylamide gel under denaturing conditions, before (lane b) and after (lane c) treatment with TFMS. Lane a, Molecular mass markers, including BSA (67 kD), ovalbumin (43 kD), carbonic anhydrase (30 kD), soybean trypsin inhibitor (20.1 kD), and α-lactalbumin (14.1 kD). Lane d, Activity stain using guaiacol/H2O2. Proteins in lanes a to c were visualized by silver staining. After staining, gels were dried onto cellulose acetate sheets (Bio-Rad) and scanned to generate digital images.
Table I.
Purification summary for the anionic peroxidase isolated from potato tubers during wound healing
| Purification Step | Total Protein | Total Activitya | Purification | Recovery | RZbValue | |
|---|---|---|---|---|---|---|
| mg | μkat | μkat/mg protein | fold | % | ||
| Crude | 2,552 | 12,731 | 5 | 1 | 100.0 | NDc |
| AS pelletd | 790 | 8,578 | 11 | 2.2 | 67.4 | ND |
| SG 100 | 108 | 4,775 | 44 | 8.9 | 37.5 | 0.4 |
| PBE | 11.2 | 2,803 | 250 | 50.2 | 22.0 | 1.6 |
| DEAE | 3.5 | 2,517 | 719 | 144.2 | 19.8 | 2.7 |
Anionic peroxidase was purified from potato tubers 7 d after wounding.
Using ferulic acid as a substrate.
RZ, Reinheitszahl value (A405/A280) in deionized water.
ND, Not determined.
50% to 90% ammonium sulfate pellet.
Potato Anionic Peroxidase Characterization
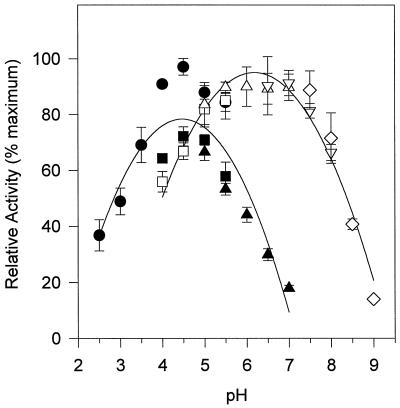
The purified potato anionic peroxidase has a molecular mass of 45.8 kD, based on SDS-PAGE (Fig. 2, lane b). Deglycosylation with trifluoromethane sulfonic acid (TFMS) yielded a 35.3-kD protein (Fig. 2, lane c). Calibrated molecular-sieving chromatography predicted a molecular mass of 44.9 kD for the purified protein (data not shown). The enzyme displayed a broad temperature optimum between 40°C and 60°C (data not shown) and a pH optimum of 4.5 for phenolic acids and 6.5 for monolignols (Fig. 3). In both cases, the pH optima were broad, and neutral conjugates (e.g. 3b) were equally good substrates at either pH (data not shown). For convenience, all substrates except the hydroxycinnamyl alcohols (5a, 5b, 6a, 6b, 7a, and 7b) were assayed at pH 4.5.
Figure 3.
Dependence of phenolic oxidation rate by potato anionic peroxidase on pH. The rate of oxidation of ferulic acid 3a (black symbols) and coniferyl alcohol 6a (white symbols) was measured spectrophotometrically in buffers of differing pH. The buffers used (all at 50 mm) were citrate (•), acetate (▪, □), His (▴, ▵), phosphate (▿), and Tris (⋄). Oxidation rates were measured in triplicate. Error bars represent ±1 sd.
Substrate Specificity of Potato Peroxidases
Anionic Peroxidase
Twenty-five different phenolic compounds were tested as substrates (Table II; Scheme 1). The potato anionic peroxidase showed a strong preference for substrates with o-methoxyphenol-substituted aromatic ring systems. Thus the hydroxycinnamates 3a, 3b, 3c, 3d, 3f, and 3g were all excellent substrates, while (in decreasing order) the caffeoyl (2a, 2b, and 2h), p-coumaroyl (1a–1d), and sinapoyl (4a–4d) compounds were less effective. The hydroxycinnamyl alcohols (5a, 6a, and 7a) were poorer substrates than the corresponding hydroxycinnamates, but still showed the same pattern of maximal activity with the o-methoxyphenol-substituted coniferyl alcohol 6a. As expected, protection of the phenolic hydroxyl groups (i.e. the initial site of oxidation by peroxidase) with Glc moieties (e.g. 1e, 3e, 4e, 5b, 6b, and 7b) prevented their oxidation by the enzyme. The potato anionic peroxidase readily oxidized guaiacol, both in the presence and absence of p-chloromercuribenzoic acid (pCMB) (up to 200 μm), while ascorbate was a very poor substrate (data not shown).
Table II.
Substrate specificity of potato anionic peroxidase
| Substrate (refer to Scheme 1) | Vmaxapp | Relative Activitya |
|---|---|---|
| pkat | ||
| 1ap-Coumaric acid | 9.5 | 3.1 |
| 1bN-(p-Coumaroyl)tyramine | 70.6 | 22.9 |
| 1cN-(p-Coumaroyl)putrescine | 51.1 | 16.6 |
| 1dp-Coumaroylglucose | 50.0 | 16.2 |
| 1ep-Coumarate-4-O-β-d-glucoside | <1b | 0.3 |
| 2a Caffeic acid | 163.4 | 53.1 |
| 2bN-Caffeoyltyramine | 129.6 | 42.1 |
| 2h Chlorogenic acid | 137.7 | 44.7 |
| 3a Ferulic acid | 307.8 | 100 |
| 3bN-Feruloyltyramine | 318.6 | 103.5 |
| 3cN-Feruloylputrescine | 366.0 | 118.9 |
| 3d Feruloylglucose | 379.9 | 123.5 |
| 3e Ferulate-4-O-β-d-glucoside | <1b | 0.3 |
| 3fN-Feruloyloctopamine | 258.1 | 83.8 |
| 3gN-Feruloyl-(2-phenyl)-ethylamine | 376.0 | 122.2 |
| 4a Sinapic acid | 34.6 | 11.2 |
| 4bN-Sinapoyltyramine | 32.2 | 10.5 |
| 4d Sinapoylglucose | 15.9 | 5.2 |
| 4e Sinapate-4-O-β-d-glucoside | <1b | 0.3 |
| 5ap-Coumaryl alcohol | 3.6b | 1.2 |
| 5bp-Coumaryl alcohol-4-O-β-d-glucoside | 2.2b | 0.7 |
| 6a Coniferyl alcohol | 174.6 | 56.7 |
| 6b Coniferin | <1b | 0.3 |
| 7a Sinapyl alcohol | 12.5b | 4.1 |
| 7b Syringin | 2.2b | 0.7 |
Maximum catalytic rates were determined for each substrate by measuring the initial rate of their consumption over a range of concentrations (0–0.2 mm) with a fixed concentration of H2O2 (2 mm). The rate values were predicted from the intercepts of Wolfe-Hanes plots. For all assays, the purified potato anionic peroxidase was used at a 0.5 nm final concentration.
Ferulic acid set to 100%.
Maximum rate measured; substrate did not show typical saturation kinetics, precluding the use of the Wolfe-Hanes transformation.
Cationic Peroxidase
A subset of the phenolics tested as substrates for the anionic peroxidase, including the hydroxycinnamates 1a, 2a, 3a, and 4a and coniferyl alcohol 6a, were also tested with the partially purified cationic peroxidase(s) of potato (Table III; Scheme 1). In contrast to the specificity apparent for the anionic peroxidase, the cationic peroxidase(s) oxidized ferulic acid 3a and coniferyl alcohol 6a equally well. A similar trend in preference for aromatic substitution patterns was observed. The (descending) order of substrate preference for the cationic isoform(s) was feruloyl > caffeoyl > syringyl > p-coumaryl.
Table III.
Substrate specificity for selected peroxidase isoforms
| Plant Source | Isoform | Substrate
|
Reference | ||||
|---|---|---|---|---|---|---|---|
| p-Coumarate 1a | Caffeate 2a | Ferulate 3a | Sinapate 4a | Coniferyl Alcohol 6a | |||
| Cationic peroxidases | |||||||
| Potato | 4.9 | 33 | 100 | 12 | 105 | Present work | |
| Horseradish | IX | 121 | NAa | 100 | 1.7 | NA | Takahama (1995) |
| V. myrtillus | C1 | 14 | 64 | 100 | NA | 150 | Melo et al. (1997) |
| C2 | 61 | 69 | 100 | NA | 128 | Melo et al. (1997) | |
| Soybean | Leaf | NA | 174 | 100 | NA | NA | Schmitz et al. (1997) |
| Stem | NA | 466 | 100 | NA | NA | Schmitz et al. (1997) | |
| Anionic peroxidases | |||||||
| Potato | 3.1 | 53 | 100 | 11 | 57 | Present work | |
| Horseradish | VII | 44 | NA | 100 | 3.6 | NA | Takahama (1995) |
| VIII | 18 | NA | 100 | 6.6 | NA | Takahama (1995) | |
| VIII | 6.3 | 64 | 100 | 5.1 | 64 | Present work | |
| Soybean | Leaf-1 | NA | 160 | 100 | NA | NA | Schmitz et al. (1997) |
| Leaf-2 | NA | 0b | 100 | NA | NA | Schmitz et al. (1997) | |
| Stem | NA | 0 | 100 | NA | NA | Schmitz et al. (1997) | |
Values represent relative rates of oxidation of the substrates listed, with ferulic acids arbitrarily set to 100%. Isoforms are grouped as either cationic or anionic.
NA, Data not available.
0, No measurable activity.
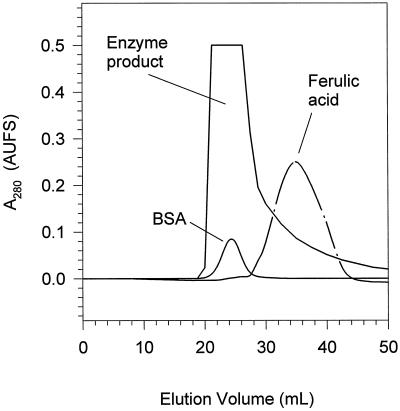
Potato Anionic Peroxidase Reaction Products
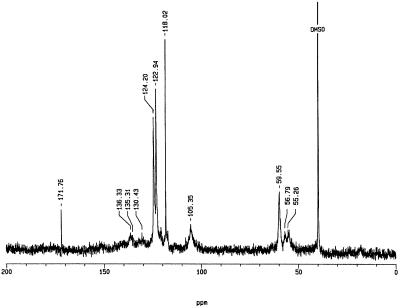
The acid-insoluble product(s) obtained from the slow addition of H2O2 to an enzyme/ferulic acid solution appeared to be polymeric (Mr > 5,000), on the basis of its elution in the void volume of a Sephadex G25-M column (Fig. 4). The natural abundance polymer had only a single weak resonance in its 13C-NMR spectrum, corresponding to the methoxyl carbon (δ 56.8 ppm), owing to the heterogeneous nature of the polymer as well as the low abundance of sample (data not shown). In the 13C-NMR spectrum obtained for the polymer prepared from E-[8-13C]ferulic acid (Fig. 5), however, major resonances wereapparent at δ 171.8, 124.2, 122.9, 118.0, 105.4, and 59.6 ppm, with minor resonances observed at δ 136.3, 135.3, 130.4, and 55.3 ppm.
Figure 4.
Mr characterization of the polymeric product prepared by the incubation of ferulic acid/H2O2 with purified anionic peroxidase. The acid-insoluble precipitate collected after incubation of anionic peroxidase with ferulic acid/H2O2 was dissolved in 0.1 m NaOH and eluted from a Sephadex G25-M column. BSA was used to mark the void volume of the column. The ferulic acid monomer was used to mark the total volume of the column.
Figure 5.
Solution-state 13C-NMR spectroscopic analysis of the polymeric product prepared by the incubation of E-[8-13C]ferulic acid/H2O2 with purified anionic peroxidase. Ten milligrams of the polymeric product collected from a Sephadex G25-M column was dissolved in 0.1 m KOH prepared in deuterated water. A drop of DMSO was added as an internal standard. With the exception of the resonance at 56.79 ppm, all resonances are due to the enhanced C-8 of the initial substrate.
DISCUSSION
The Anionic Potato Peroxidase and Suberization
Wounding of potato tubers results in a gradual increase in total soluble peroxidase activity over a period of 5 to 7 d (Borchert, 1978; Roberts et al., 1988). This involves the synthesis of new protein and is preceded by the accumulation of mRNA (Roberts et al., 1988). However, plants typically contain multiple peroxidase isoforms and it is not surprising that several (i.e. cationic, neutral, and anionic) are induced in potato under wound healing conditions (Borchert, 1978; Borchert and Decedue, 1978; Fig. 1), with the cationic and anionic forms predominating. Since suberization is restricted to the two to three cell layers immediately below the wound site (Borchert, 1978; Borchert and Decedue, 1978; Kolattukudy, 1980), and the anionic isoform(s) is immunocytochemically (Espelie et al., 1986) and biochemically (Fig. 1) localized to this region, it is strongly implicated in the suberization process.
Potato Anionic Peroxidase Characterization
Heme peroxidases are classified as either class I (intracellular), class II (fungal secretory), or class III (plant secretory), largely based on their structure (i.e. carbohydrate content, number of bound Ca2+ atoms, number of disulfide bridges, etc.) (Welinder, 1985; O'Donnell et al., 1992), substrate preference (i.e. ascorbate versus guaiacol), and sensitivity to pCMB (Amako et al., 1994). For example, class I intracellular peroxidases, typified by ascorbate peroxidase, prefer ascorbate as substrate and are inhibited by pCMB, while class III peroxidases (the so-called guaiacol or secretory peroxidases) prefer guaiacol as substrate and show no sensitivity to pCMB (Amako et al., 1994). For potato anionic peroxidase, the large (10.7 kD) shift in molecular mass after treatment with TFMS, its preference for guaiacol over ascorbate as substrate, and its insensitivity to pCMB clearly distinguish it as a class III peroxidase.
In the present study, we found that the highly anionic peroxidase from potato had a molecular mass of approximately 45 kD, as determined by both SDS-PAGE and calibrated molecular-sieving chromatography. While this molecular mass is consistent with that reported earlier (Espelie and Kolattukudy, 1985), and with that for other anionicperoxidases of solanaceous species (e.g. Pomar et al., 1997), it is not consistent with that predicted by the published amino acid sequence (Roberts et al., 1988). Since the latter was deduced from a cDNA showing three possible start sites, it could not be used with confidence to predict the molecular mass of the native protein. Instead, as a tightly folded, globular protein, its elution from a molecular-sieving column can be taken as a good first approximation. In this case, both the SDS-PAGE (45.8 kD) and molecular-sieving chromatography (44.9 kD) predictions were in close agreement. While the influence of the carbohydrate side chains on sieving behavior cannot be discounted, the deglycosylated protein still had a molecular mass approximately 6 kD greater than that predicted (Roberts et al., 1988) for the unglycosylated protein.
Substrate Specificity
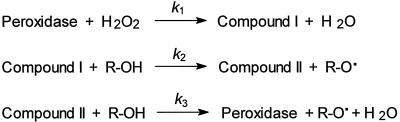
The reaction catalyzed by peroxidase is both complex (Scheme 2) and fast, and does not follow simple Michaelis-Menten kinetics (e.g. Nakajima et al., 1991). The initial step involves the binding of H2O2 by the Fe(III) heme, followed by its oxidation, cleavage of the O-O bond, and the subsequent formation of a ferryl (Fe[IV]=O)-porphorin π-cation radical (referred to as compound I), accompanied by the release of water. Next, the first of two reducing substrates (e.g. R-OH in Scheme 2) binds and donates one electron to compound I, reducing the porphorin cation and resulting in a ferryl (Fe[IV]=O) enzyme (referred to as compound II). The reducing substrate is released as a radical (e.g. R-O· in Scheme 2). In the last step of the cycle, a second reducing substrate binds and donates an electron to Fe(IV)=O, resulting in the reduction of the heme to Fe(III) and, with the addition of two protons, the release of water. A second radical is generated in the process. Thus, the stoichiometry of the reaction involves 2 mol phenolic substrate oxidized for each mole of H2O2 reduced, with different binding affinities for the phenolics for compounds I and II.
Coupled with the apparent inhibition observed when the concentration of H2O2 is disproportionately large, simple Michaelis-Menten constant determinations become increasingly difficult. While it is possible to measure rate constants for the individual reactions shown in Scheme 2 (Rasmussen et al., 1995; Converso and Fernandes, 1996; Rodriguez-Lopez et al., 1996), it requires special rapid kinetics equipment. Therefore, in determining the substrate preference of the potato anionic peroxidase, we have employed a relatively simple, quasi-rapid mixing spectrophotometric assay in which the initial rate of phenolic substrate depletion was used as an indicator of relative activity under conditions of saturating H2O2 (i.e. saturation or steady-state kinetics). Rapid mixing is essential to monitor the reaction, since the initial rate is only linear for about 5 to 10 s (depending on substrate concentration). Our procedure allowed monitoring within 1 to 2 s of mixing. Under the conditions used, the rate of ferulic acid oxidation was linear with respect to enzyme concentration (data not shown), indicating that the reaction was being monitored at saturating concentrations. For most substrates, saturation kinetics were observed, but for others (especially 1e, 3e, 4e, 5a, 5b, 6b, 7a, and 7b) saturation was not achieved. For these compounds, oxidation rates were very low relative to ferulic acid 3a (Table II), and the maximum rate observed is reported.
The reduction of compound II (step 3 in Scheme 2) is rate limiting when there is sufficient H2O2 present (Rodriguez-Lopez et al., 1996); however, there are substantial differences in the rate of compound II formation (step 2 in Scheme 2) depending on the reducing substrate (Takahama, 1995; Takahama et al., 1996). For example, the rate of sinapic acid 4a oxidation by horseradish peroxidase isoforms can be greatly enhanced by the addition of small amounts of either p-coumaric 1a or ferulic 3a acids to the reaction mixture (Takahama, 1995), presumably because the latter (especially 3a) react to form compound II more readily than sinapic 4a acid alone. Consequently, the “rates” shown in Table II reflect the relative efficiency with which the enzyme can use each substrate for its complete catalytic cycle.
The substrates used were selected because many (i.e. 2c, 2h, 3b, 3c, and 3f) are known to accumulate in potato tubers during would healing (e.g. Malmberg, 1984; Bernards and Lewis, 1992; Borg-Olivier and Monties, 1993; Negrel et al., 1996), and are potential “natural” substrates for the enzyme. Other compounds (e.g. the hydroxycinnamoyl-9-O-β-d-glucosides 1d, 3d, and 4d) are common in solanaceous plants (Harborne and Corner, 1961). The monolignols 5 to 7 were also included since they are known to be incorporated into suberizing cell walls, albeit in minute amounts (Borg-Olivier and Monties, 1989, 1993). The remaining substrates represent the free acids, analogs, and/or modifications of the “natural” substrates. For example, N-feruloyl-(2-phenyl)-ethylamine 3g represents a dehydro analog of N-feruloyltyramine 3b.
The highest rate of enzyme activity was measured with feruloyl derivatives as substrate (Table II). Indeed, the enzyme seems particularly sensitive to the aromatic substitution pattern of its substrates, and showed a marked preference for those that are o-methoxyphenol substituted. The (descending) order of substrate preference is feruloyl > caffeoyl > p-coumaryl ≈ syringyl, with little influence by side chain derivatization. Coincidentally, the major compounds accumulating in potato tubers induced to suberize via wounding (with the exception of chlorogenic acid 2h) are ferulic acid derivatives, and their ready oxidation by the potato anionic peroxidase implicates them (and the enzyme) in the suberization process. Interestingly, the oxidation of N-feruloyl-(2-phenyl)-ethylamine 3g was more rapid than that of N-feruloyltyramine 3b, indicating that the enzyme discriminates between the hydroxycinnamate and tyramine ends of the substrate.
In order to place the substrate specificity of the potato anionic peroxidase in context, we measured the relative oxidation of the hydroxycinnamic acids 1a, 2a, 3a, and 4a, as well as coniferyl alcohol 6a by a partially purified cationic peroxidase preparation from wounded potato tubers, as well as a commercially available anionic horseradish peroxidase (Table III). In addition, literature data for nine other peroxidase isoforms for which comparative data are available are included. (Note that the literature contains many reports in which only one hydroxycinnamate substrate was used, usually ferulic acid 3a, but these do not provide comparative values and are not considered here.) Whereas most isoforms show a similar preference for o-methoxyphenol substituted substrates (i.e. ferulic acid 3a), some differences are apparent. For example, the descending order of substrate preference for the anionic horseradish peroxidase isoforms VII and VIII is feruloyl > caffeoyl > p-coumaroyl > syringyl, while that for the cationic isoform IX is p-coumaryl > feruloyl > syringyl (Takahama, 1995).
The two cationic peroxidases from Vaccinium myrtillus also preferentially oxidize ferulic acid 3a over other hydroxycinnamic acids, although they differ in their ability to oxidize p-coumaric acid 1a (Melo et al., 1997). In contrast, three soybean peroxidase isoforms (two cationic and one anionic) more readily oxidized caffeic acid 2a than ferulic acid 3a, while two others (both anionic) were unable to oxidize caffeic acid 2a at all (Schmitz et al., 1997). In general, the cationic peroxidases listed in Table III oxidized coniferyl alcohol 6a equally well or better than ferulic acid 3a, while anionic ones more readily oxidized ferulic acid 3a. For the wound-induced anionic peroxidase of potato, all hydroxycinnamates appeared to be more readily oxidized than their corresponding hydroxycinnamyl alcohols (Table II). Thus, of the peroxidase isoforms induced upon wounding of potato tubers, the anionic one appears predisposed to favor oxidation of the major phenolic compounds that accumulate coincidentally. Since some of these (especially 3c) become oxidatively cross-linked in the suberized cell walls of tubers, the anionic peroxidase is implicated in the process.
Product Analysis
Horseradish peroxidase has often been used to generate dehydrogenation polymers of coniferyl alcohol (e.g. Lewis et al., 1987; Lewis and Yamamoto, 1990). The type of product obtained depends on the rate at which the substrates (i.e. coniferyl alcohol and H2O2) are added to the enzyme (see Saake et al., 1996; Guan et al., 1997). In the present study, the slow addition of H2O2 to a stirring solution of purified anionic peroxidase and ferulic acid 3a yielded a polymer of Mr > 5,000, as judged by chromatography on a Sephadex G25-M column (Fig. 4). More than half (i.e. 52%) of the original monomer was recovered in this polymeric fraction. Based on a monomer molecular mass of approximately 194 g mol−1, the recovered material represents a polymer with a minimal degree of polymerization of 26. By contrast, either the rapid addition of H2O2 or the addition of both substrates at once to a stirring solution of purified anionic peroxidase yielded mainly low-Mr, acid-soluble products, not unlike those reported by Zimmerlin et al. (1994) (data not shown).
NMR spectroscopic analysis (Fig. 5) revealed two important features of the in vitro polymeric product(s) formed by the slow addition of H2O2 to a stirring solution of E-[8-13C]ferulic acid and the purified enzyme. First, only a small proportion of the total enhanced resonances (approximately 30%) correspond to that of the original E-[8-13C]ferulic acid (i.e. δ 118.0 ppm), indicating that the majority of carbons originating from C-8 of the monomer were found in a modified electronic environment, including their involvement in cross-linking. Second, the majority of resonances were found in the olefinic region of the spectrum (i.e. δ 110–150 ppm), indicating that C-8 of the original monomer retains its unsaturation, despite being cross-linked with other monomeric units. This feature of in vitro peroxidase/H2O2-generated polymers represents a deviation from polymers generated using horseradish peroxidase and monolignols (e.g. Lewis et al., 1987; Guan et al., 1997), where the side chain carbons were reduced during coupling. It is consistent, however, with the retention of side chain unsaturation noted when [2-13C]Phe was administered to suberizing potato tubers (Bernards et al., 1995). Recently, Ralph et al. (1994) described a number of ferulic acid dehydrodimers present in grass cell walls, some of which show carbon resonances consistent with those observed in the polymeric product generated by the anionic peroxidase. Curiously, the enhanced signal at 171.8 ppm (Fig. 5) suggests that a minor proportion of the ferulic acid monomers underwent oxidation at C-8 to a carbonyl during the reaction with anionic peroxidase. Notwithstanding this interpretation, the analysis of the polymers generated in vitro by the anionic peroxidase of potato requires further study.
CONCLUSIONS
The macromolecular assembly of the aromatic domain in suberized tissues is hypothesized to involve a peroxidase/H2O2-mediated free radical coupling process. One candidate peroxidase in potato tubers is the highly anionic isoform that is induced by wounding. The biochemical evidence presented here supports this contention on two counts. First, the anionic peroxidase is restricted to the suberizing tissues in the immediate vicinity of the wound site. Second, the anionic peroxidase of potato prefers o-methoxyphenol-substituted hydroxycinnamates (typical of those that accumulate in tubers during wound healing and incorporated into the suberized cell wall) to other phenolic substrates (order of substrate preference: guaiacyl > caffeoyl > p-coumaryl ≈ syringyl) including hydroxycinnamyl alcohols. This contrasts with the cationic peroxidase(s) of potato, which is found in the tissues underlying the wound site (in addition to the suberizing tissues), and does not discriminate between ferulic acid 3a and coniferyl alcohol 6a. The purified anionic enzyme readily formed dehydrogenative polymers from ferulic acid 3a in the presence of H2O2 that are characterized by a high level of cross-linking (potentially through side chain C-8) and a high degree of retention of side chain unsaturation. In general, the data presented in this paper are consistent with the involvement of the anionic peroxidase isoform of potato in the polymerization of the poly(aromatic) domain during suberization, although definitive proof awaits further investigation.
Scheme 1.
Hydroxycinnamic acid and hydroxycinnamyl alcohol derivatives used as substrates in this study. The aromatic ring substitution pattern is denoted by a number (i.e. hydroxycinnamates 1−4 and hydroxycinnamyl alcohols 5−7), while derivatives are denoted by lowercase letters. Not every possible derivative was used; refer to Table II for a complete listing of the 25 phenolics used in the substrate specificity study.
Scheme 2.
General reaction mechanism for peroxidase. See text for detailed description of each step. R-OH, Phenolic substrate; R-O·, phenolic radical.
ACKNOWLEDGMENT
The authors gratefully acknowledge the assistance of Dr. David Dick (University of Northern British Columbia) in acquiring NMR spectra.
Footnotes
This research was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) operating grant to M.A.B. D.B.L. was supported in part by a NSERC undergraduate scholarship.
LITERATURE CITED
- Amako K, Chen G-X, Asada K. Separate assays specific for ascorbate peroxidase and guaiacol peroxidase and for the chloroplastic and cytosolic isozymes of ascorbate peroxidase in plants. Plant Cell Physiol. 1994;35:497–504. [Google Scholar]
- Bernards MA, Lewis NG. Alkyl ferulates in wound healing potato tubers. Phytochemistry. 1992;31:3409–3412. doi: 10.1016/0031-9422(92)83695-u. [DOI] [PubMed] [Google Scholar]
- Bernards MA, Lewis NG. The macromolecular aromatic domain in suberized tissue: a changing paradigm. Phytochemistry. 1998;47:915–933. doi: 10.1016/s0031-9422(98)80052-6. [DOI] [PubMed] [Google Scholar]
- Bernards MA, Lopez ML, Zajicek J, Lewis NG. Hydroxycinnamic acid-derived polymers constitute the polyaromatic domain of suberin. J Biol Chem. 1995;270:7382–7386. doi: 10.1074/jbc.270.13.7382. [DOI] [PubMed] [Google Scholar]
- Borchert R. Time course and spatial distribution of phenylalanine ammonia-lyase and peroxidase activity in wounded potato tuber tissue. Plant Physiol. 1978;62:789–793. doi: 10.1104/pp.62.5.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchert R, Decedue CJ. Simultaneous separation of acidic and basic isoperoxidases in wounded potato tissue by acrylamide gel electrophoresis. Plant Physiol. 1978;62:794–797. doi: 10.1104/pp.62.5.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg-Olivier O, Monties B. Characterization of lignins, phenolic acids and tyramine in the suberized tissues of natural and wound induced potato periderm. C R Acad Sci Paris. 1989;308:141–147. [Google Scholar]
- Borg-Olivier O, Monties B. Lignin, suberin, phenolic acids and tyramine in the suberized, wound-induced potato periderm. Phytochemistry. 1993;32:601–606. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Converso DA, Fernandez ME. Peroxidase isozymes from wheat germ: purification and properties. Phytochemistry. 1995;40:1341–1346. [Google Scholar]
- Converso DA, Fernandez ME. Ca2+ activation of wheat peroxidase: a possible physiological mechanism of control. Arch Biochem Biophys. 1996;333:59–65. doi: 10.1006/abbi.1996.0364. [DOI] [PubMed] [Google Scholar]
- de Moreno MR, Smith JF, Smith RV. Silver staining of proteins in polyacrylamide gels: increased sensitivity through a combined Coomassie blue-silver stain procedure. Anal Biochem. 1985;151:466–470. doi: 10.1016/0003-2697(85)90206-4. [DOI] [PubMed] [Google Scholar]
- Espelie KE, Franceschi VR, Kolattukudy PE. Immunocytochemical localization and time course of appearance of an anionic peroxidase associated with suberization in wound-healing potato tuber tissue. Plant Physiol. 1986;81:487–492. doi: 10.1104/pp.81.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espelie KE, Kolattukudy PE. Purification and characterization of an abscisic acid-inducible anionic peroxidase associated with suberization in potato (Solanum tuberosum) Arch Biochem Biophys. 1985;240:539–545. doi: 10.1016/0003-9861(85)90060-8. [DOI] [PubMed] [Google Scholar]
- Gil AM, Lopes M, Rocha J, Neto CP. A 13C solid state nuclear magnetic resonance spectroscopic study of cork cell wall structure: the effect of suberin removal. Int J Biol Macromol. 1997;20:293–305. doi: 10.1016/s0141-8130(97)00029-9. [DOI] [PubMed] [Google Scholar]
- Guan S-Y, Mlynar J, Sarkanen S. Dehydrogenative polymerization of coniferyl alcohol on macromolecular lignin templates. Phytochemistry. 1997;45:911–918. [Google Scholar]
- Harborne JB, Corner JJ. Plant polyphenols. 4. Hydroxycinnamic acid-sugar derivatives. Biochem J. 1961;81:242–250. doi: 10.1042/bj0810242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolattukudy PE. Biopolyester membranes of plants: cutin and suberin. Science. 1980;208:990–1000. doi: 10.1126/science.208.4447.990. [DOI] [PubMed] [Google Scholar]
- Kwak S-S, Kim S-K, Lee M-S, Jung K-H, Park I-H, Liu J-R. Acidic peroxidases from suspension cultures of sweet potato. Phytochemistry. 1995;39:981–984. [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis NG, Just G, Ripmeister J. Determination of bonding patterns of 13C specifically enriched DHP lignin in solution and solid state. Macromolecules. 1987;20:1752–1756. [Google Scholar]
- Lewis NG, Yamamoto E. Lignin: occurrence, biogenesis and biodegradation. Annu Rev Plant Physiol Plant Mol Biol. 1990;41:455–496. doi: 10.1146/annurev.pp.41.060190.002323. [DOI] [PubMed] [Google Scholar]
- Loukili A, Liman F, Ayadi A, Boyer N, Ouelhazi L. Purification and characterization of a neutral peroxidase induced by rubbing tomato internodes. Physiol Plant. 1999;105:24–31. [Google Scholar]
- Malmberg A. N-Feruloylputrescine in infected potato tubers. Acta Chem Scand. 1984;38:153–155. [Google Scholar]
- Marquez LA, Dunford HB. Transient and steady-state kinetics of the oxidation of scopoletin by horseradish peroxidase compounds I, II and III in the presence of NADH. Eur J Biochem. 1995;233:364–371. doi: 10.1111/j.1432-1033.1995.364_1.x. [DOI] [PubMed] [Google Scholar]
- Melo NS, Larsen E, Welinder KG, Fevereiro PS. Characterization of two major cationic peroxidases from cell suspension cultures of Vaccinium myrtillus. Plant Sci. 1997;122:1–10. [Google Scholar]
- Mohan R, Bajar MA, Kolattukudy PE. Induction of a tomato anionic peroxidase gene (tap1) by wounding in transgenic tobacco and activation of tap1/GUS and tap2/GUS chimeric gene fusions in transgenic tobacco by wounding and pathogen attack. Plant Mol Biol. 1993a;21:341–354. doi: 10.1007/BF00019949. [DOI] [PubMed] [Google Scholar]
- Mohan R, Kolattukudy PE. Differential activation of expression of a suberization-associated anionic peroxidase gene in near-isogenic resistant and susceptible tomato lines by elicitors of Verticillium albo-atrum. Plant Physiol. 1990;921:276–280. doi: 10.1104/pp.92.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan R, Vijayan P, Kolattukudy PE. Developmental and tissue-specific expression of a tomato anionic peroxidase (tap1) gene by a minimal promoter, with wound and pathogen induction by an additional 5′-flanking region. Plant Mol Biol. 1993b;22:475–490. doi: 10.1007/BF00015977. [DOI] [PubMed] [Google Scholar]
- Negrel J, Pollet B, Lapierre C. Ether-linked ferulic acid amides in natural and wound periderms of potato tuber. Phytochemistry. 1996;43:1195–1199. [Google Scholar]
- Nakajima R, Hoshino N Yamazaki I (1991) Oxidative decomposition of oxoperoxidases during peroxidase reactions: effect of localization of the enzyme. In J Lobarzewski, H Greppin, C Penel, T Gaspar, eds, Biochemical, Molecular and Physiological Aspects of Peroxidases. Impremiere Nationale, Geneva, pp 89–97
- O'Donnell JP, Wan L, van Huystee RB. Characterization of two forms of cationic peroxidase from cultured peanut cells. Biochem Cell Biol. 1992;70:166–169. doi: 10.1139/o92-024. [DOI] [PubMed] [Google Scholar]
- Padiglia A, Cruciana E, Pazzaglia G, Medda R, Floris G. Purification and characterization of Opuntia peroxidase. Phytochemistry. 1995;38:295–297. [Google Scholar]
- Pomar F, Bernal MA, Diaz J, Merino F. Purification, characterization and kinetic properties of pepper fruit acidic peroxidase. Phytochemistry. 1997;46:1313–1317. [Google Scholar]
- Ralph J, Quideau S, Grabber JH, Hatfield RD (1994) Identification and synthesis of new ferulic acid dehydrodimers present in grass cell walls. J Chem Soc Perkin Trans, pp 3485–3498
- Rasmussen CB, Dunford HB, Welinder KG. Rate enhancement of compound I formation of barley peroxidase by ferulic acid, caffeic acid, and coniferyl alcohol. Biochemistry. 1995;34:4022–4029. doi: 10.1021/bi00012a021. [DOI] [PubMed] [Google Scholar]
- Roberts E, Kolattukudy PE. Molecular cloning, nucleotide sequence, and abscisic acid induction of a suberization-associated highly anionic peroxidase. Mol Gen Genet. 1989;217:223–232. doi: 10.1007/BF02464885. [DOI] [PubMed] [Google Scholar]
- Roberts E, Kutchan T, Kolattukudy PE. Cloning and sequencing of cDNA for a highly anionic peroxidase from potato and the induction of its mRNA in suberizing potato tubers and tomato fruits. Plant Mol Biol. 1988;11:15–26. doi: 10.1007/BF00016010. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Lopez JN, Smith AT, Thorneley RNF. Role of arginine 38 in horseradish peroxidase: a critical residue for substrate binding and catalysis. J Biol Chem. 1996;271:4023–4030. doi: 10.1074/jbc.271.8.4023. [DOI] [PubMed] [Google Scholar]
- Saake B, Argyropoulos DS, Beinhoff O, Faix O. A comparison of lignin polymer models (DHPs) and lignins by 31P NMR spectroscopy. Phytochemistry. 1996;43:499–507. [Google Scholar]
- Schmitz N, Gijzen M, van Huystee R. Characterization of anionic soybean (Glycine max) seed coat peroxidase. Can J Bot. 1997;75:1336–1341. [Google Scholar]
- Schreiber L. Chemical composition of Casparian strips isolated from Clivia miniata Reg. roots: evidence for lignin. Planta. 1996;199:596–601. [Google Scholar]
- Sherf BA, Bajar MA, Kolattukudy PE. Abolition of an inducible highly anionic peroxidase activity in transgenic tomato. Plant Physiol. 1993;101:201–208. doi: 10.1104/pp.101.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherf BA, Kolattukudy PE. Developmentally regulated expression of the wound and pathogen-responsive tomato anionic peroxidase in green fruits. Plant J. 1993;3:829–833. [Google Scholar]
- Takahama U. Oxidation of hydroxycinnamic acid and hydroxycinnamyl alcohol derivatives by laccase and peroxidase: interactions among p-hydroxyphenyl, guaiacyl and syringyl groups during the oxidation reactions. Physiol Plant. 1995;93:61–68. [Google Scholar]
- Takahama U, Oniki T, Shimokawa H. A possible mechanism for the oxidation of sinapyl alcohol by peroxidase-dependent reactions in the apoplast: enhancement of the oxidation by hydroxycinnamic acids and components of the apoplast. Plant Cell Physiol. 1996;37:499–504. [Google Scholar]
- Terashima N, Ralph SA, Landucci LL. New facile syntheses of monolignol glucosides: p-glucocoumaryl alcohol, coniferin and syringin. Holzforschung. 1995;50:151–155. [Google Scholar]
- Villegas M, Brodelius PE. Elicitor-induced hydroxycinnamoyl coenzyme A:tyramine hydroxycinnamoyltransferase in plant cell suspension cultures. Physiol Plant. 1990;78:414–420. [Google Scholar]
- Wallace G, Fry SC. In vitro peroxidase-catalyzed oxidation of ferulic acid esters. Phytochemistry. 1995;39:1293–1299. [Google Scholar]
- Welinder KG. Plant peroxidases: their primary, secondary and tertiary structures, and relation to cytochrome c peroxidase. Eur J Biochem. 1985;151:497–450. doi: 10.1111/j.1432-1033.1985.tb09129.x. [DOI] [PubMed] [Google Scholar]
- Zeier J, Schreiber L. Chemical composition of hypodermal and endodermal cell walls and xylem vessels isolated from Clivia miniata: identification of the biopolymers lignin and suberin. Plant Physiol. 1997;113:1223–1231. doi: 10.1104/pp.113.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerlin A, Wojtaszek P, Bolwell GP. Synthesis of dehydrogenation polymers of ferulic acid with high specificity by a purified cell-wall peroxidase from French bean (Phaseolus vulgaris L.) Biochem J. 1994;299:747–753. doi: 10.1042/bj2990747. [DOI] [PMC free article] [PubMed] [Google Scholar]