Abstract
Stomatal opening provides access to inner leaf tissues for many plant pathogens, so narrowing stomatal apertures may be advantageous for plant defense. We investigated how guard cells respond to elicitors that can be generated from cell walls of plants or pathogens during pathogen infection. The effect of oligogalacturonic acid (OGA), a degradation product of the plant cell wall, and chitosan (β-1,4-linked glucosamine), a component of the fungal cell wall, on stomatal movements were examined in leaf epidermis of tomato (Lycopersicon esculentum L.) and Commelina communis L. These elicitors reduced the size of the stomatal aperture. OGA not only inhibited light-induced stomatal opening, but also accelerated stomatal closing in both species; chitosan inhibited light-induced stomatal opening in tomato epidermis. The effects of OGA and chitosan were suppressed when EGTA, catalase, or ascorbic acid was present in the medium, suggesting that Ca2+ and H2O2 mediate the elicitor-induced decrease of stomatal apertures. We show that the H2O2 that is involved in this process is produced by guard cells in response to elicitors. Our results suggest that guard cells infected by pathogens may close their stomata via a pathway involving H2O2 production, thus interfering with the continuous invasion of pathogens through the stomatal pores.
Pathogens penetrate plant tissues by three different mechanisms: by digesting cell walls, by entering through wounds, and by invading natural openings (including stomata). Although many pathogens can force entry through closed stomata, some can infect plants only when the stomata are open (Agrios, 1997). Thus, narrow stomata may limit the penetration of some pathogens, thereby conferring resistance to them.
Upon pathogen infection, plants commonly activate a variety of defense mechanisms. Within a few minutes, the challenged plants frequently evolve reactive oxygen species (ROS) such as superoxide and H2O2, which in turn can facilitate the expression of a hypersensitive response (Levine et al., 1994). ROS can also damage pathogens directly by the oxidation of important biomolecules (including cell membranes; Adam et al., 1989; Keppler and Baker, 1989), and may induce additional defense mechanisms in plant cells, such as cell wall fortification (Bradley et al., 1992), defense-related gene expression (Levine et al., 1994; Jabs et al., 1997), and the synthesis of phytoalexins (Bradley et al., 1992; Jabs et al., 1997). Perhaps related to these functions is the observation that exogenously added superoxide and H2O2 inhibit stomatal opening and promote stomatal closing (McAinsh et al., 1996). The intriguing question, however, remains whether guard cells can perceive signals of pathogen infection directly and thus modify stomatal responses in vivo in a way that contributes to plant defense.
Pathogen attack can be mimicked by elicitor molecules produced during the infection process. Cell wall fragments from plants or pathogens can serve as elicitors in many plant species. Oligogalacturonic acid (OGA), a well-studied elicitor, is derived from plant cell walls (Nothnagel et al., 1983). When added to cultured plant cells, it induces an oxidative burst within minutes (Apostol et al., 1989), releasing ROS via a pathway that involves receptor binding (Horn et al., 1989), activation of a G-protein (Legendre et al., 1992), influx of Ca2+ (Chandra et al., 1997), stimulation of phospholipase C (Legendre et al., 1993b), and induction of a number of kinases (Chandra and Low, 1995). Chitosan (β-1,4-linked glucosamine) is a deacylated derivative of chitin that is a component of the cell walls of many fungi (Bartnicki-Garcia, 1970). It can be produced by the activity of the enzyme chitinase, one of the pathogenesis-related proteins that is activated during infection and is toxic to invading pathogens (Mauch et al., 1988). Chitosan induces phytoalexin production in pea pods, inhibiting fungal growth and promoting protection from further infection (Hadwiger and Beckman, 1980).
We investigated whether guard cells might be able to sense the presence of elicitors and then respond by changing stomatal apertures. For this purpose, we tested the effects of OGA and chitosan, common, non-species-specific elicitors, on stomatal movements in two different plant species, tomato (Lycopersicon esculentum L.) and Commelina communis L. Both OGA and chitosan were found to induce the production of ROS in guard cells and to reduce stomatal aperture either by inhibiting stomatal opening or by inducing stomatal closing.
MATERIALS AND METHODS
Plant Materials and Elicitors
Commelina communis L. and tomato (Lycopersicon esculentum L.) plants were grown in a greenhouse or growth chamber at 23°C with 16-h/8-h light/dark cycles. Fully expanded leaves of 3- to 4-week-old plants were excised, and epidermal pieces were obtained either by peeling abaxial epidermis of C. communis or by blending tomato leaves in a solution containing 5 mm CaCl2, 10 mm MES (pH 6.0), and 0.1% (w/v) PVP-40. The blending process destroyed epidermal cells but guard cells were viable, as judged from staining with fluorescein diacetate. The preparation and isolation of OGA have been previously reported (Legendre et al., 1993a). Chitosan was purchased from Kumho Chemical Laboratories (Taejeon, Korea) and dissolved as previously described (Walker-Simmons et al., 1984).
Measurement of Stomatal Aperture
To study the effects of elicitors on light-induced stomatal opening, initially small apertures of stomata were obtained by keeping the plants in darkness overnight, and then floating the epidermal tissue on 10 mm K+-MES (pH 6.1) in darkness for 2 h. The tissues were then transferred to 10 mm K+-MES (pH 6.1) in a 50 mm (tomato) or 30 mm (C. communis) KCl solution, and illuminated with 250 to 500 μmol m−2 s−1 light with or without chemicals. To determine if elicitors can affect midday stomatal closing, initially open stomata were obtained by floating the epidermal pieces of leaves on 10 mm K+-MES (pH 6.1) containing 50 mm (tomato) or 30 mm (C. communis) KCl under light for 2 to 3 h, beginning at h 3 to 4 of the photoperiod. Chemicals were added alone or together to the same solution on which the epidermal tissues were floated. At the time of chemical or elicitor treatment, the control stomata began midday closing, possibly driven by the circadian clock. Stomatal apertures were measured using an eyepiece micrometer.
Microphotography of Production of H2O2 in Intact Guard Cells
The production of H2O2 by guard cells was examined by loading epidermal preparations with 2′,7′-dichlorofluorescin diacetate (H2DCF-DA). This nonfluorescent dye can cross the plasma membrane freely, and is then cleaved to its impermeable counterpart, dichlorofluorescin (H2DCF), by endogenous esterases. H2DCF, which accumulates in the cell, functions as a reporter of cytoplasmic H2O2 by converting upon oxidation to its fluorescent form, DCF (Ohba et al., 1994; Yahraus et al., 1995; Naton et al., 1996; Allan and Fluhr, 1997; Kang et al., 1998).
Tomato epidermal pieces were floated on 10 mm K+-MES (pH 6.1), 50 mm KCl with or without chitosan, and catalase or ascorbic acid for 30 min, then transferred to and floated on 10 mm K+-MES (pH 6.1) and 50 mm KCl solution containing 50 μm H2DCF-DA for 10 min. After a brief wash with 10 mm K+-MES (pH 6.1) and 50 mm KCl, the guard cells were observed under a fluorescent microscope (Optiphot-2, Nikon) equipped with filter blocks of a narrow band pass (excitation 465–495 nm, barrier 515–555 nm). Photographs were taken on T-MAX 400 film (Kodak) using a photographic attachment (Microflex UFX-DX, Nikon). H2DCF-DA was dissolved in dimethylformamide. The final concentration of the solvent was 0.5% (v/v), which did not induce any significant change in guard cell viability or stomatal aperture.
RESULTS
Effects of OGA on Stomatal Movements in Tomato Leaves
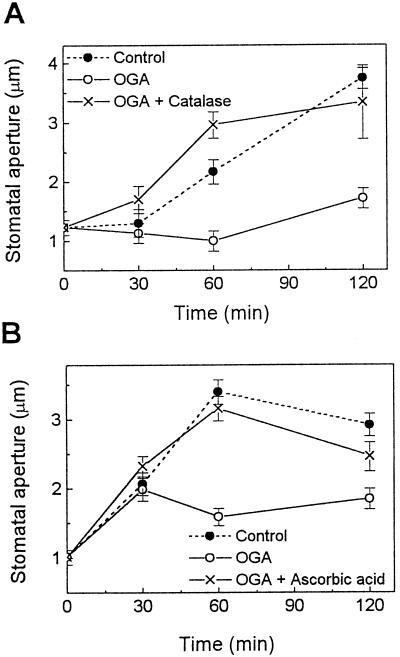
The effects of OGA, a plant cell wall-derived elicitor, on stomatal movements were tested in tomato epidermal tissues. Tomato is one of the best-characterized plant systems employed in studying plant-pathogen interactions. Treatment of leaf epidermal tissues with 5 μg/mL OGA, a concentration known to induce an oxidative burst in cultured soybean and tomato cells (Legendre et al., 1992), inhibited light-induced stomatal opening (Fig. 1). Following 120 min of treatment with OGA, the stomatal aperture was 46% (Fig. 1A) and 63% (Fig. 1B) of that of control plants illuminated without OGA. To determine whether the effect of OGA on stomatal movements might involve ROS known to participate in elicitor-induced defense responses, tomato epidermal tissues were treated with OGA in the presence of either catalase or ascorbic acid, both of which remove or reduce the level of ROS (Bradley et al., 1992; Levine et al., 1994). As noted in Figure 1, both agents reversed the inhibitory effects of OGA on stomatal opening, indicating that OGA inhibits light-induced stomatal opening via a mechanism involving ROS. Treatment of the epidermis with catalase or ascorbic acid alone did not induce any change of stomatal aperture (data not shown).
Figure 1.
Effects of OGA on light-induced stomatal opening in tomato leaf epidermis. Note that 3 mg/mL catalase (A) and 5 mm ascorbic acid (B), which reduce the level of ROS, reversed the inhibitory effect of OGA (5 μg/mL) on stomatal opening. Experimental procedures are described in Methods. Results are the averages ± se (n = 120) of four independent experiments.
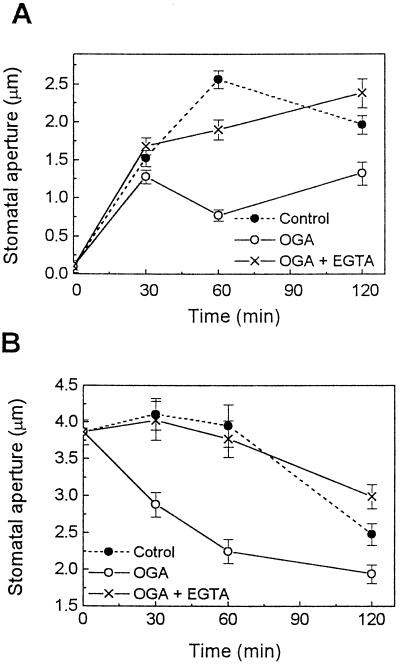
ROS has been reported to reduce stomatal aperture via a pathway involving a Ca2+ increase in cytosol (McAinsh et al., 1996). Therefore, we addressed whether Ca2+ is also involved in elicitor-induced changes in stomatal movements. Stomatal opening was inhibited by 5 μg/mL OGA (Figs. 1 and 2A), while midday stomatal closing was accelerated by 50 μg/mL OGA (Fig. 2B). In the presence of EGTA, however, the effects of OGA on both stomatal opening (Fig. 2A) and closing (Fig. 2B) were completely reversed, suggesting an important role of Ca2+ in the OGA-induced reduction of stomatal aperture. EGTA alone induced a slight stomatal opening over untreated controls (data not shown), as was reported previously (Schwartz, 1985).
Figure 2.
Involvement of Ca2+ in OGA-induced narrowing of stomata in tomato leaves. In light-induced opening (A) and midday closing (B) experiments, the inhibitory effect of OGA (5 μg/mL) on opening and the enhancing effect of OGA (50 μg/mL) on closing of stomata were reversed by EGTA (2 mm). Experimental procedures are described in Methods. Results are the averages ± se (n = 120) of four independent experiments.
Effects of Chitosan on Stomatal Movements of Tomato Leaves
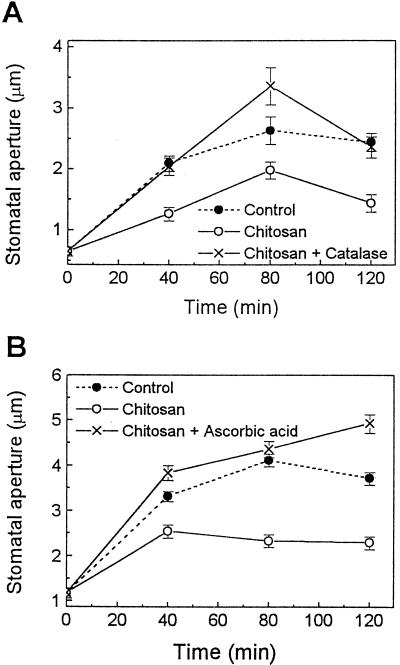
Because OGA is a plant-derived elicitor, we questioned whether pathogen-derived elicitors may have similar effects on stomatal movements. Chitosan (100 or 200 μg/mL), an elicitor that can be generated from the degradation of fungal cell walls, inhibited light-induced opening (Fig. 3). Following 120 min of chitosan treatment, the stomatal aperture was 59% (Fig. 3A) and 62% (Fig. 3B) of that of the controls illuminated in the absence of chitosan. This effect of chitosan on stomatal opening was also suppressed by catalase and ascorbic acid (Fig. 3), as seen previously with OGA (Fig. 1).
Figure 3.
Effect of chitosan on light-induced stomatal opening in tomato leaf epidermis. Note that 3 mg/mL catalase (A) and 10 mm ascorbic acid (B) suppressed the inhibitory effect of chitosan (100 μg/mL in A or 200 μg/mL in B) on stomatal opening. Experimental procedures are described in Methods. Results are the averages ± se (n = 120) of three to four independent experiments.
Although the initial and/or final apertures of stomata varied between experiments, the effects of elicitors and other chemicals on stomatal movements were consistent (Figs. 1 and 3). The variation in stomatal aperture may have been due to seasonal changes in growth conditions or to a slight difference in the preparation of epidermal tissue pieces.
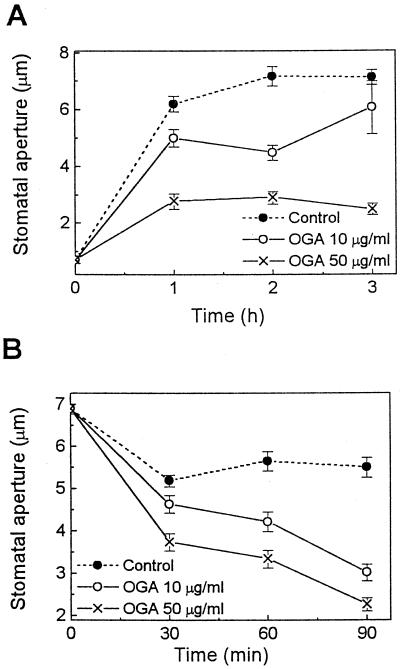
Effects of OGA on Stomatal Movements in C. communis Leaves
If the regulation of stomatal movements is an important plant defense mechanism, then this response should be exhibited by many species. Therefore, we also tested whether the OGA elicitor might affect stomatal movements in C. communis leaf epidermal tissues. C. communis is the most widely used plant for studying guard cell physiology. We found that OGA inhibited light-induced stomatal opening and enhanced stomatal closing in a concentration-dependent manner in C. communis (Fig. 4). In the opening experiment, after 3 h of illumination, stomatal apertures of epidermis treated with 10 and 50 μg/mL OGA were 85% and 35% of control, respectively. In the closing experiment, stomatal apertures after 90 min of treatment with 10 and 50 μg/mL OGA were 55% and 41% of control. Therefore, our data suggest that the effects of OGA on stomatal movements are not unique for tomato, but may have also evolved in other species.
Figure 4.
Effects of OGA on stomatal movements in C. communis. OGA effects on both stomatal opening (A) induced by white light of 500 μmol m−2 s−1 and midday stomatal closing (B) were tested. Note that OGA inhibited stomatal opening (A) and enhanced stomatal closing (B) in a concentration-dependent manner. Results are the averages ± se (n = 120) of four independent experiments.
H2O2 Production by Guard Cells in Response to OGA and Chitosan
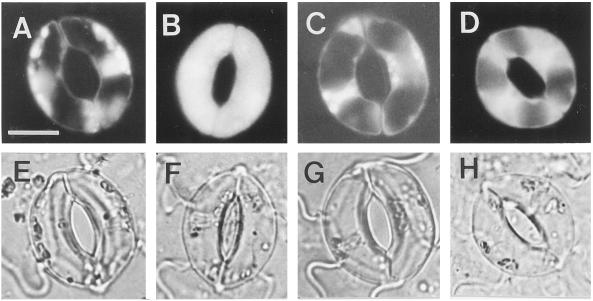
Reversal of the effects of OGA and chitosan by catalase and ascorbic acid suggests that ROS, especially H2O2, are involved in elicitor-inhibited stomatal opening and elicitor-enhanced stomatal closing. We questioned whether guard cells themselves could produce H2O2 using the dye H2DCF-DA. H2O2 evolution was evident throughout the entire surface of tomato guard cells treated with chitosan for 30 min (Fig. 5, B and F). In contrast, control guard cells showed fluorescence only in regions where chloroplasts were located (Fig. 5, A and E). Chloroplasts are well known for the formation of ROS during photosynthetic electron transport. In the present study, chitosan-induced H2O2 evolution became apparent from 10 to 20 min after the onset of the treatment and continued until 30 to 60 min, after which time the dye was bleached. In the presence of catalase (Fig. 5C) and ascorbic acid (Fig. 5D), chitosan-induced H2O2 production was suppressed and the fluorescence existed only in regions where chloroplasts were enriched (Fig. 5, G and H), as was also seen in control cells. OGA also induced the production of H2O2 in tomato guard cells (data not shown).
Figure 5.
Chitosan-induced production of H2O2 by guard cells of tomato leaf. Epidermal pieces of tomato leaf without (control) (A and E) or with 30 min of treatment with chitosan alone (B and F), with chitosan and catalase (C and G), or with chitosan and ascorbic acid (D and H) were loaded with 50 μm of H2DCF-DA for 10 min. After a brief wash with 50 mm KCl and 10 mm K+-MES (pH 6.1), photographs were taken for a representative pair of guard cells from each treatment using fluorescence microscopy (A–D) or light microscopy (E–H). The bar in A is 10 μm, and applies to all photographs.
DISCUSSION
Some pathogens penetrate into plant tissues only through open stomata (Agrios, 1997). Since guard cells govern access to this port of entry, they are likely to be the first cells to come into contact with these pathogens. Our results suggest that guard cells might have evolved the capability to detect and respond to signal molecules generated during such contact. We have shown that guard cells can recognize common, non-species-specific elicitors of both plant and pathogen origin, can generate ROS, and can respond to ROS by narrowing stomatal apertures. Furthermore, OGA was shown to stimulate the same stomatal changes in two different plant species. These observations suggest the possibility that elicitor-induced changes in stomatal properties may not be isolated responses but, rather, are a characteristic of many plants following the detection of pathogen attack.
McAinsh et al. (1996) showed that externally applied H2O2 induces stomatal closing in C. communis, as well as an increase in cytosolic free Ca2+ in guard cells. We observed that the elicitor-stimulated inhibition of stomatal opening and the elicitor-enhanced stomatal closing were both inhibited by extracellular EGTA. Ca2+ is an important regulator of guard cell movements (for review, see Mansfield et al., 1990; Assmann, 1993), and an increase in cytosolic Ca2+ from intracellular or extracellular sources is closely correlated with stomatal closing (Gilroy et al., 1990; McAinsh et al., 1995). Our data support the idea that Ca2+ serves as a common component of signal transduction pathways induced by many different stomatal closing signals.
Treatment with elicitors generally renders a plant more resistant to subsequent pathogen attack. Indeed, elicitor stimulation has been observed to induce the hypersensitive response (Bradley et al., 1992), PR gene expression (Broekaert and Peumans, 1988; Jabs et al., 1997), phytoalexin biosynthesis (Nürnberger et al., 1994; Jabs et al., 1997), and cell wall stabilization (Bruce and West, 1989; Bradley et al., 1992), all of which are plant defense responses. All of these defense mechanisms depend at least in part on ROS (Bradley et al., 1992; Jabs et al., 1997). Our data now suggest that stomatal closure is similarly triggered by elicitor-induced ROS, which may also contribute to plant defense.
Although Figure 5 clearly shows that ROS were present inside the elicitor-treated guard cells, inhibition of both the elicitor-induced stomatal narrowing responses (Figs. 1 and 3) and H2O2 evolution of guard cells (Fig. 5) by externally applied catalase (240 kD), which is not likely to cross the plasma membrane, indicates that H2O2 may also act outside of the plasma membrane of guard cells. This is consistent with studies showing high permeability of the membrane to H2O2 (Yamasaki et al., 1997). It was recently reported that epidermal cells of tobacco leaves produce H2O2 in response to a peptide elicitor, cryptogein, and that the product can diffuse to neighboring cells (including guard cells) (Allan and Fluhr, 1997). Because the tomato leaf preparation employed in our study did not maintain the viability of the epidermal cells, it was not possible to evaluate whether epidermal cells of tomato also participate directly in elicitor-stimulated ROS production. Nevertheless, the guard cells in the same preparations were highly viable and responded to elicitation with a significant oxidative burst. Further studies are necessary to understand the site of H2O2 production in guard cells.
As shown previously, H2O2 biosynthesis involves the induction of a sequence of second messengers, including G-proteins, Ca2+ influx, phospholipase C, and protein kinases (Legendre et al., 1992, 1993b; Schwacke and Hager, 1992; Chandra and Low, 1995; Chandra et al., 1997). In view of the mobilization of these signaling components, it may seem surprising that guard cells still use H2O2 as a signal to trigger their elicitor-stimulated closure. However, one distinct advantage of relying on H2O2 to promote stomatal closure is that if initially infected guard cells or adjacent epidermal cells can respond to pathogen attack with an oxidative burst, then the product, H2O2, rapidly diffusing in the apoplastic pathway (as shown in tobacco epidermal tissue; Allan and Fluhr, 1997) might induce narrowing of neighboring stomata before more of the same or similar pathogens can find other open stomata. In this manner, elicitor-stimulated stomatal closure might provide one of the most rapid mechanisms to thwart pathogen invasion.
ACKNOWLEDGMENTS
We thank Dr. Ro-Dong Park for information concerning chitosan and Shi-In Kim for management of plants.
Footnotes
This work was supported by a Korea-United States cooperative research grant from the Korea Science and Engineering Foundation (no. 966–0500–007–2 awarded to Y.L.) and by a grant from the National Science Foundation of the United States (nos. INT–9600183 and MCB–9725934 awarded to P.S.L.).
LITERATURE CITED
- Adam A, Farkas T, Somlyai G, Hevesi M, Kiraly Z. Consequence of O2− generation during a bacterially induced hypersensitive reaction in tobacco: deterioration of membrane lipids. Physiol Mol Pathol. 1989;34:13–26. [Google Scholar]
- Agrios GN (1997) Plant Pathology, Ed 4. Academic Press, San Diego, pp 46–52
- Allan AC, Fluhr R. Two distinct sources of elicited reactive oxygen species in tobacco epidermal cells. Plant Cell. 1997;9:1559–1572. doi: 10.1105/tpc.9.9.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostol I, Heinstein PF, Low PS. Rapid stimulation of an oxidative burst during elicitation of cultured plant cells: role in defense and signal transduction. Plant Physiol. 1989;90:109–116. doi: 10.1104/pp.90.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann SM. Signal transduction in guard cells. Annu Rev Cell Biol. 1993;9:345–375. doi: 10.1146/annurev.cb.09.110193.002021. [DOI] [PubMed] [Google Scholar]
- Bartnicki-Garcia S. Cell wall composition and other biochemical markers in fungal phylogeny. In: Harborne JB, editor. Phytochemical Phylogeny. London: Academic Press; 1970. pp. 81–101. [Google Scholar]
- Bradley DJ, Kjellbom P, Lamb CJ. Elicitor- and wound-induced oxidative cross-linking of a proline-rich plant cell wall protein: a novel, rapid defense response. Cell. 1992;70:21–30. doi: 10.1016/0092-8674(92)90530-p. [DOI] [PubMed] [Google Scholar]
- Broekaert WF, Peumans WJ. Pectic polysaccharides elicit chitinase accumulation in tobacco. Physiol Plant. 1988;74:740–744. [Google Scholar]
- Bruce RJ, West CA. Elicitation of lignin biosynthesis and isoperoxidase activity by pectic fragments in suspension cultures of castor bean. Plant Physiol. 1989;91:889–897. doi: 10.1104/pp.91.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Low PS. Role of phosphorylation in elicitation of the oxidative burst in cultured soybean cells. Proc Natl Acad Sci USA. 1995;92:4120–4123. doi: 10.1073/pnas.92.10.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Stennis M, Low PS. Measurement of Ca2+ fluxes during elicitation of the oxidative burst in aequorin-transformed tobacco cells. J Biol Chem. 1997;272:28274–28280. doi: 10.1074/jbc.272.45.28274. [DOI] [PubMed] [Google Scholar]
- Gilroy S, Read ND, Trewavas AJ. Elevation of cytoplasmic calcium by caged calcium or caged inositol trisphosphate initiates stomatal closure. Nature. 1990;346:769–771. doi: 10.1038/346769a0. [DOI] [PubMed] [Google Scholar]
- Hadwiger LA, Beckman JM. Chitosan as a component of pea-Fusarium solani interactions. Plant Physiol. 1980;66:205–211. doi: 10.1104/pp.66.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn MA, Heinstein PF, Low PS. Receptor-mediated endocytosis in plant cells. Plant Cell. 1989;1:1003–1009. doi: 10.1105/tpc.1.10.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabs T, Tschöpe M, Colling C, Hahlbrock K, Scheel D. Elicitor-stimulated ion fluxes and O2− from the oxidative burst are essential components in triggering defense gene activation and phytoalexin synthesis in parsley. Proc Natl Acad Sci USA. 1997;94:4800–4805. doi: 10.1073/pnas.94.9.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SW, Chae HZ, Seo MS, Kim K, Baines IC, Rhee SG. Mammalian peroxiredoxin isoforms can reduce hydrogen peroxide generated in response to growth factors and tumor necrosis factor. J Biol Chem. 1998;273:6297–6302. doi: 10.1074/jbc.273.11.6297. [DOI] [PubMed] [Google Scholar]
- Keppler LD, Baker CJ. O2− initiated lipid peroxidation in a bacteria-induced hypersensitive reaction in tobacco cell suspensions. Phytopathology. 1989;79:555–562. [Google Scholar]
- Legendre L, Heinstein PF, Low PS. Evidence for participation of GTP-binding proteins in elicitation of the rapid oxidative burst in cultured soybean cells. J Biol Chem. 1992;267:20140–20147. [PubMed] [Google Scholar]
- Legendre L, Rueter S, Heinstein PF, Low PS. Characterization of the oligogalacturonide-induced oxidative burst in cultured soybean (Glycine max) cells. Plant Physiol. 1993a;102:233–240. doi: 10.1104/pp.102.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre L, Yueh YG, Crain R, Haddock N, Heinstein PF, Low PS. Phospholipase C activation during elicitation of the oxidative burst in cultured plant cells. J Biol Chem. 1993b;268:24559–24563. [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Mansfield TA, Hetherington AM, Atkinson CJ. Some current aspects of stomatal physiology. Annu Rev Plant Physiol Plant Mol Biol. 1990;41:55–75. [Google Scholar]
- Mauch F, Mauch-Mani B, Boller T. Antifungal hydrolases in pea tissue. II. Inhibition of fungal growth by combinations of chitinase and β-1,3-glucanase. Plant Physiol. 1988;88:936–942. doi: 10.1104/pp.88.3.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinsh MR, Clayton H, Mansfield TA, Hetherington AM. Changes in stomatal behavior and guard cell cytosolic free calcium in response to oxidative stress. Plant Physiol. 1996;111:1031–1042. doi: 10.1104/pp.111.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinsh MR, Webb AAR, Taylor JE, Hetherington AM. Stimulus-induced oscillation in guard cell cytosolic free calcium. Plant Cell. 1995;7:1207–1219. doi: 10.1105/tpc.7.8.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naton B, Hahlbrock K, Schmelzer E. Correlation of rapid cell death with metabolic changes in fungus-infected, cultured parsley cells. Plant Physiol. 1996;112:433–444. doi: 10.1104/pp.112.1.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothnagel EA, McNeil M, Ahbersheim P, Dell A. Host-pathogen interactions. XXII. A galacturonic acid oligosaccharide from plant cell walls elicits phytoalexins. Plant Physiol. 1983;71:916–926. doi: 10.1104/pp.71.4.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nürnberger T, Nennstiel D, Jabs T, Sacks WR, Hahlbrock K, Scheel D. High affinity binding of a fungal oligopeptide elicitor to parsley plasma membranes triggers multiple defense responses. Cell. 1994;78:449–460. doi: 10.1016/0092-8674(94)90423-5. [DOI] [PubMed] [Google Scholar]
- Ohba M, Shibanuma M, Kuroki T, Nose K. Production of hydrogen peroxide by transforming growth factor-beta 1 and its involvement in induction of egr-1 in mouse osteoblastic cells. J Cell Biol. 1994;126:1079–1088. doi: 10.1083/jcb.126.4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwacke R, Hager A. Fungal elicitors induce a transient release of active oxygen species from cultured spruce cells that is dependent on Ca2+ and protein-kinase activity. Planta. 1992;187:136–141. doi: 10.1007/BF00201635. [DOI] [PubMed] [Google Scholar]
- Schwartz A. Role of Ca2+ and EGTA on stomatal movements in epidermal peels of Commelina communis L. Plant Physiol. 1985;79:1003–1005. doi: 10.1104/pp.79.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker-Simmons M, Jin D, West CA, Hadwiger L, Ryan CA. Comparison of proteinase inhibitor-inducing activities and phytoalexin elicitor activities of a pure fungal endopolygalacturonase, pectic fragments, and chitosans. Plant Physiol. 1984;76:833–836. doi: 10.1104/pp.76.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahraus T, Chandra S, Legendre L, Low PS. Evidence for a mechanically induced oxidative burst. Plant Physiol. 1995;109:1259–1266. doi: 10.1104/pp.109.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H, Sakihama Y, Ikehara N. Flavonoid-peroxidase reaction as a detoxification mechanism of plant cells against H2O2. Plant Physiol. 1997;115:1405–1412. doi: 10.1104/pp.115.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]