Abstract
H2O2 from the oxidative burst, cell death, and defense responses such as the production of phenylalanine ammonia lyase (PAL), salicylic acid (SA), and scopoletin were analyzed in cultured tobacco (Nicotiana tabacum) cells treated with three proteinaceous elicitors: two elicitins (α-megaspermin and β-megaspermin) and one glycoprotein. These three proteins have been isolated from Phytophthora megasperma H20 and have been previously shown to be equally efficient in inducing a hypersensitive response (HR) upon infiltration into tobacco leaves. However, in cultured tobacco cells these elicitors exhibited strikingly different biological activities. β-Megaspermin was the only elicitor that caused cell death and induced a strong, biphasic H2O2 burst. Both elicitins stimulated PAL activity similarly and strongly, while the glycoprotein caused only a slight increase. Only elicitins induced SA accumulation and scopoletin consumption, and β-megaspermin was more efficient. To assess the role of H2O2 in HR cell death and defense response expression in elicitin-treated cells, a gain and loss of function strategy was used. Our results indicated that H2O2 was neither necessary nor sufficient for HR cell death, PAL activation, or SA accumulation, and that extracellular H2O2 was not a direct cause of intracellular scopoletin consumption.
The hypersensitive response (HR) is a powerful defense mechanism used by plants against pathogen attack. Phenotypically, the HR results in plant cell death at the site of pathogen penetration. A set of defense responses is also rapidly induced in cells undergoing HR. For instance, genes encoding enzymes of the phenylpropanoid pathway, such as Phe ammonia lyase (PAL), the first committed enzyme of this pathway (Hahlbrock and Scheel, 1989; Dorey et al., 1997), are stimulated. PAL has been shown to play an important role in plant resistance (Mauch-Mani and Slusarenko, 1996). It is involved in the biosynthetic pathway providing scopoletin (Fritig et al., 1970), a coumarin with phytoalexin activity (Ahl-Goy et al., 1993), and precursors of lignin-like material thought to represent a defense reaction against pathogen invasion through its deposition into the cell wall. Transgenic tobacco (Nicotiana tabacum) plants with reduced PAL activity are more susceptible to infection by the fungal pathogen Cercospora nicotianae (Maher et al., 1994). PAL is also a key enzyme involved in the biosynthesis of the signal molecule salicylic acid (SA) (Mauch-Mani and Slusarenko, 1996), which was shown to accumulate in cells undergoing the HR (Enyedi et al., 1992; Dorey et al., 1997) and to be essential for local and systemic resistance (Gaffney et al., 1993; Delaney et al., 1994).
The production of reactive oxygen intermediates through an oxidative burst is a hallmark of plant defense responses (Doke and Ohashi, 1988; Baker et al., 1993; Legendre et al., 1993; Levine et al., 1994; Baker and Orlandi, 1995; Tavernier et al., 1995). Diphenylene iodonium (DPI) has been shown to block this oxidative burst in different cell culture systems, so the production of reactive oxygen intermediates seems to implicate a membrane-bound NADP(H) oxidase (Levine et al., 1994; Desikan et al., 1996; Xing et al., 1997; Keller et al., 1998). Other sources may also account for the production of reactive oxygen intermediates, for example, peroxidases (Bestwick et al., 1997) and amine oxidase-type enzyme(s) (Allan and Fluhr, 1997). NADP(H) oxidase generates superoxide anions (O2−), which are readily dismuted into H2O2 either spontaneously or by SOD (for review, see Sutherland, 1991).
H2O2, the most stable of the reactive oxygen intermediates, has been implicated in the cross-linking of cell wall proteins (Bradley et al., 1992), in signal transduction as a regulator of pathogenesis-related (PR-1) gene expression (Chen et al., 1995; Chamnongpol et al., 1998), in the plant cell death process (Levine et al., 1994), and in the direct killing of invading pathogens (Peng and Kuc, 1992). However, other reports did not implicate H2O2 as a key component in phytoalexin synthesis, one of the most common defense responses (Devlin and Gustine, 1992; Davis et al., 1993; Jabs et al., 1997), or as an initiator of plant cell death (Devlin and Gustine, 1992; Glazener et al., 1996).
There have been conflicting studies concerning PAL activation by H2O2. While the H2O2 generated by the reaction between Glc oxidase and Glc was not able to trigger PAL gene expression in soybean cell cultures (Levine et al., 1994), a 5 mm H2O2 dose induced PAL gene expression in cultured Arabidopsis cells (Desikan et al., 1998). However, the level of expression in Arabidopsis was much reduced compared with the treatment with harpin, a HR-inducing bacterial elicitor. H2O2 was also reported to activate benzoate-2-hydroxylase, an enzyme converting benzoate to SA (Leon et al., 1995). It was therefore hypothesized that H2O2 could activate the rapid synthesis of SA (Draper, 1997). The relationship between H2O2 and SA production is so far not clearly understood.
In this study, we assessed the role of H2O2 in elicitor-induced HR cell death, PAL activity stimulation, SA production, and scopoletin consumption. We analyzed tobacco cell suspensions treated with three different proteinaceous elicitors: two elicitins, α-megaspermin and β-megaspermin, and one 32-kD glycoprotein. These three proteins were isolated from the culture medium of Phytophthora megasperma H20 and were shown to display similar HR-inducing activity when infiltrated at a dose of 60 nm into tobacco leaves (Baillieul et al., 1996). When the elicitors were applied to the cultured cells, we observed that only β-megaspermin induced HR cell death, that all three elicitors triggered PAL activity (although with different intensity), and that only elicitins caused SA accumulation and a decrease in the scopoletin level (which was high in the cells prior to treatment). Using gain and loss of function experiments, we show that H2O2 from the oxidative burst was neither necessary nor sufficient for plant cell death, PAL activation, SA accumulation, or scopoletin consumption.
MATERIALS AND METHODS
Plant Material
The cell suspension derived from tobacco (Nicotiana tabacum cv Bright Yellow) was cultured in a modified Murashige-Skoog medium (Duchefa, Haarlem, The Netherlands) containing micro- and macroelements (4.3 g/L), Suc (30 g/L), myoinositol (100 mg/L), thiamine (1 mg/L), 2,4-D (0.2 mg/L), and KH2PO4 (200 mg/L). The pH of the medium was 5.8. Cells were maintained in the dark at 25°C under shaking at 120 rpm in 500-mL flasks. Subcultures were made weekly by transferring 10 mL of the cell suspension into 70 mL of fresh medium. For elicitation experiments, 5 mL of cells subcultured for 6 to 7 d were transferred into 50-mL flasks. Before any treatment, cells were allowed to adjust to the new conditions for a period of 2 to 3 h.
To conduct experiments with cells displaying a similar responsiveness to elicitor treatment, we performed a medium alkalinization test (Boller, 1995), which has been described as a very sensitive test of elicitor perception. A typical experiment was conducted using two independent cell batches treated similarly. Each typical experiment was repeated two to six times to check for reproducibility. Figures describe the results of a typical experiment. For PAL, SA, and scopoletin assays, cells were harvested by filtration, frozen in liquid nitrogen, and stored at −80°C until use. For cell death and H2O2 assays, cells were analyzed immediately after harvest.
Chemicals
Aspergillus niger Glc oxidase was purchased from ICN, and DPI, bovine catalase, horseradish peroxidase (type II), 5-amino-2,3-dihydro-1,4-phthalazine dione (luminol), and Evans blue were from Sigma. DPI was dissolved in DMSO as a 50 mm stock solution. A total of 10 μm DPI was applied to the cells, corresponding to a final solvent concentration of 0.02%.
Cell Death Assay
Cell death was monitored as described by Levine et al. (1994). For each sample, a 400-μL aliquot of cells was incubated with 0.05% Evans blue for 30 min and then washed extensively. The dye bound to dead cells was solubilized in 50% methanol with 1% SDS for 30 min at 50°C and quantified by A600.
PAL, SA, Scopoletin, and H2O2 Analysis
To measure PAL activity, 0.5 g of cells was ground at 4°C in the presence of quartz sand and activated charcoal in 1.5 mL of 0.1 m borate buffer, pH 8.8, containing 17 mm β-mercaptoethanol. The mixture was centrifuged at 13,000 rpm for 20 min, and 50 to 100 μL of the supernatant was used for enzymatic assays. PAL activity was assayed as described previously (Pellegrini et al., 1994). Total SA and total scopoletin (free and conjugated forms) were extracted from 0.5 g of cells and analyzed by HPLC according to the method of Dorey et al. (1997). H2O2 accumulating in the culture medium was measured as the chemiluminescence of luminol (Glazener et al., 1991) using a microplate luminometer (TR717, Perkin-Elmer). Luminescence, expressed as relative luminescence units (RLU), is proportional to H2O2 according to [H2O2] (μm) = RLU/5.2 × 104 (linearity range 1 μm–1 mm). For each sample, cell aliquots of 100 μL were transferred to microplate wells for the assay. Peroxidase (60 units) and luminol (25 μL of a 400 μm stock solution in 300 mm MES buffer, pH 7.0) were automatically dispensed and the measurements were integrated over a 10-s period just after the addition of luminol/peroxidase.
RESULTS
Cell Death Induction
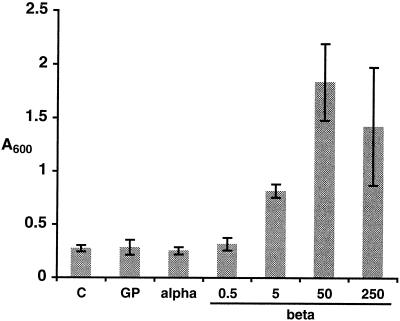
The ability of α-megaspermin, β-megaspermin, and the glycoprotein to cause death of tobacco suspension cells was investigated using Evans blue as a vital dye. Treatments with increasing concentrations of β-megaspermin resulted in increasing cell death (Fig. 1). Maximum cell death was observed with a 50 nm concentration, and affected about 40% of the cells. Analysis performed 3 d after the treatments did not reveal any further increase in cell death (data not shown). Surprisingly, treatments with 250 nm α-megaspermin or glycoprotein did not cause increased cell death compared with control cells (Fig. 1), even when the cell culture was incubated for 3 d (data not shown). A 500 nm concentration of either elicitor was also ineffective, whereas a 60 nm concentration was sufficient to induce HR in tobacco leaves (Baillieul et al., 1996). These data pointed to very striking differences in cell death between tobacco cells in culture and tobacco leaf tissues challenged with two closely related elicitins.
Figure 1.
Cell death of cultured tobacco cells treated with the glycoprotein, α-megaspermin, or β-megaspermin. Cells were incubated in the presence of 250 nm glycoprotein (GP), or 250 nm α-megaspermin (alpha), or various concentrations (in nm) of β-megaspermin (beta). C, Control cells. Cell death was measured after 24 h of incubation using the Evans blue method. Bars represent the means ± sd of two independent experiments.
PAL Activation
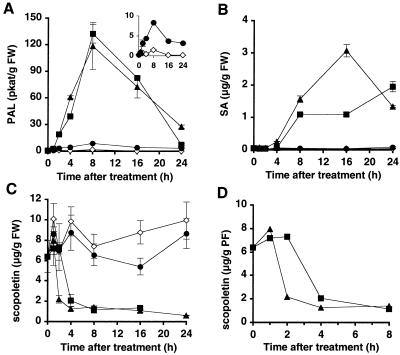
All three elicitors induced a rapid and transient increase in PAL activity, with the maximum induction occurring 8 h after treatment (Fig. 2A). Similar kinetics and amplitudes were observed whether cells were supplied with 250 nm α-megaspermin or 50 nm β-megaspermin (Fig. 2A). PAL stimulation induced by the glycoprotein treatment (Fig. 2A) was much less pronounced. At 8 h, the glycoprotein triggered PAL activity 4-fold over control, while the elicitins caused an 80-fold augmentation. These results indicated that both the α-megaspermin and the glycoprotein were perceived by the cells, although they did not induce cell death, and that the elicitor-stimulated PAL activity was not correlated with the ability of the elicitor to induce cell death.
Figure 2.
Changes in PAL activity and levels of SA and scopoletin after treatment of cultured tobacco cells with the glycoprotein, α-megaspermin, or β-megaspermin. Cells were incubated in the presence of 250 nm glycoprotein (•), 250 nm α-megaspermin (▪), or 50 nm β-megaspermin (▴). PAL activity (A), SA (B), and scopoletin (C and D) were measured from untreated (⋄) and elicitor-treated cells. Each time point represents the mean ± sd of two independent experiments. D is a close view using the values (without sd values) from C. It allows a better reading of the scopoletin consumption after α-megaspermin and β-megaspermin treatments. FW, Fresh weight.
SA Production and Scopoletin Consumption
PAL is upstream in the SA biosynthetic pathway (Mauch-Mani and Slusarenko, 1996). Although all three elicitors were able to stimulate PAL activity, only the two elicitins caused SA accumulation (Fig. 2B). Unexpectedly (but reproducibly), treatment with 50 nm β-megaspermin caused a higher SA accumulation than treatment with 250 nm α-megaspermin. Thus, there was no close parallel between PAL activity and SA level.
Unlike healthy tobacco leaf tissues, in which scopoletin levels are very low, cultured tobacco cells already contain high amounts of scopoletin (about 10 μg/g cells). The application of 50 nm β-megaspermin or 250 nm α-megaspermin triggered a decrease in scopoletin levels, which then remained very low during the course of the experiment (Fig. 2, C and D). The decrease induced by β-megaspermin occured earlier (after 1 h) than that induced by α-megaspermin (after 2 h; Fig. 2D), and this effect was reproducible. The effect of glycoprotein was less clear (Fig. 2C). Although the slight decrease in scopoletin levels (compared with control) between 8 and 16 h after glycoprotein treatment was reproduced in different experiments, it was not clear whether it reflected a real decrease followed by an increase. The reasons behind the scopoletin consumption are speculative. Scopoletin is a potent antioxidant substance and is used as a substrate to measure the oxidative burst in plant cell suspensions (Levine et al., 1994). Thus, the decrease in scopoletin levels could be explained in terms of scopoletin consumption during the elicitor-induced oxidative burst.
The H2O2 Burst
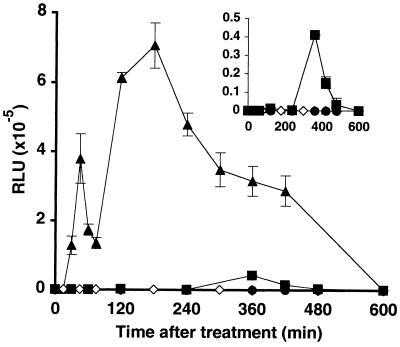
H2O2 from the cell suspension culture was measured using a luminol-peroxidase assay. β-Megaspermin induced a strong and sustained H2O2 accumulation of about 15 μm (Fig. 3). Similar H2O2 amounts were reported for tobacco cell suspensions treated with cryptogein, an elicitin closely related to β-megaspermin (Rustérucci et al., 1996). The addition of SOD did not increase the amount of H2O2 detected, indicating that it was mostly H2O2 that was measured and not O2−. The kinetics were biphasic: phase I peaked at 0.75 h and phase II at 3 h. In contrast, only a low increase in H2O2 was measured after application of 250 nm α-megaspermin, and no burst at all occurred after treatment with 250 nm glycoprotein (Fig. 3 and inset). Our data indicated: (a) an apparent correlation between the strong H2O2 burst and cell death induced by β-megaspermin treatment; (b) no clear correlation between H2O2 levels and PAL activity or SA accumulation (by comparing the differential effects of the two elicitins); and (c) a possible role of scopoletin as a H2O2 scavenger, which could account for the transient H2O2 decrease after β-megaspermin treatment, for the low H2O2 levels found after α-megaspermin treatment, and for no detectable H2O2 burst found after glycoprotein treatment.
Figure 3.
The H2O2 burst in tobacco cell suspensions treated with the glycoprotein, α-megaspermin, or β-megaspermin. Cells were incubated in the presence of 250 nm glycoprotein (•), 250 nm α-megaspermin (▪), or 50 nm β-megaspermin (▴). H2O2 in the culture medium was measured from untreated (◊) and elicitor-treated cell suspensions. Each time point represents the mean ± sd of two independent experiments.
Assessment of the Role of H2O2 in Elicitor Activity
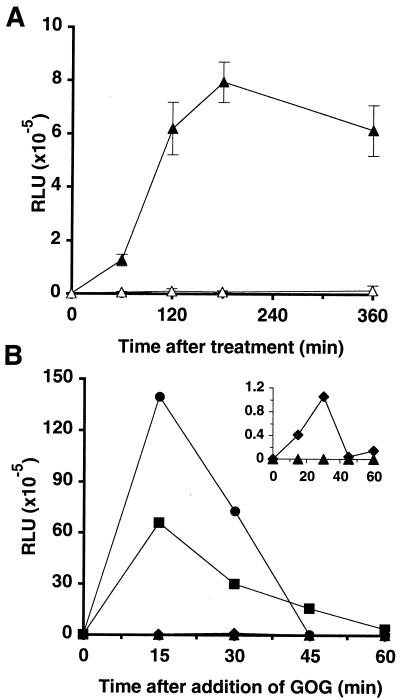
To assess the role of H2O2 in the defense responses induced by elicitor treatment, we performed gain and loss of function experiments based on the blocking or the mimicking of the H2O2 burst. DPI is a “suicide substrate inhibitor” of the mammalian NADPH oxidase, and has also been shown to block the oxidative burst in plant cells (Levine et al., 1994). DPI (10 μm) was added to the cell suspensions 10 min prior to elicitor treatment. This concentration of DPI was not toxic to the cells. Under these conditions, the DPI treatment completely abolished phase I (data not shown) and phase II (Fig. 4A) of the oxidative burst induced by 50 nm β-megaspermin. Catalase treatment (100 units/mL) inhibited only 70% of the H2O2 burst (data not shown). Application to the cell culture of 5 mm Glc and various amounts of Glc oxidase, a H2O2-generator system often used (Jabs et al., 1997; Alvarez et al., 1998), triggered an accumulation of H2O2 over a period of about 45 min (Fig. 4B). The addition of 5 units of enzyme/mL of culture caused a 20-fold higher H2O2 burst than treatment with 50 nm β-megaspermin. Therefore, the experiments described below were performed with 5 units of Glc oxidase.
Figure 4.
Blocking and mimicking the H2O2 burst. A, Inhibition of the H2O2 burst. Cells were incubated in the presence of 50 nm β-megaspermin (▴) or 50 nm β-megaspermin plus 10 μm DPI (▵). H2O2 in the culture medium was measured, and each time point represents the mean ± sd of two independent experiments. B, Production of H2O2 in the medium of cultured tobacco cells incubated with Glc oxidase and Glc. Glc (5 mm) and various amounts of Glc oxidase (•, 5 units/mL; ▪, 0.5 unit/mL; ♦, 0.05 unit/mL; ▴, 0.005 unit/mL) were added to the culture, and the amount of H2O2 accumulating in the medium was measured.
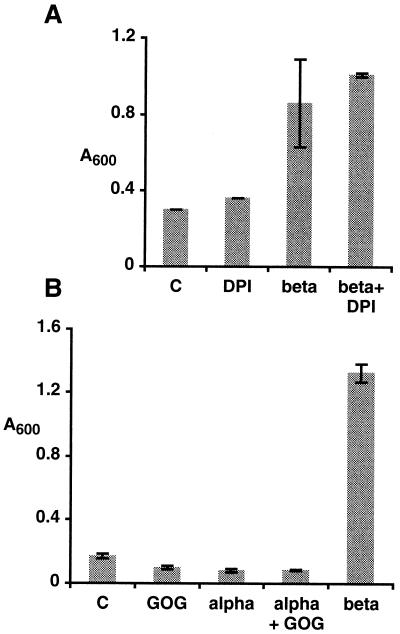
The above results showed a correlation between the presence of high amounts of H2O2 and β-megaspermin-induced cell death. The addition of DPI, however, did not suppress cell death induced by 50 nm β-megaspermin (Fig. 5A). Mimicking the H2O2 burst through Glc-oxidase-Glc (GOG) activity did not induce cell death (Fig. 5B). The addition of GOG and 250 nm α-megaspermin, which induced PAL activity and SA accumulation in a manner similar to the 50 nm β-megaspermin treatment, did not result in cell death (Fig. 5B). Overall, the data strongly suggest that H2O2 from the oxidative burst did not cause cell death.
Figure 5.
Effect of DPI and of H2O2 generated by GOG on cell death. Death of treated and control (C) cells was measured after 24 h of incubation. Bars represent the means ± sd of two independent experiments. A, Cells treated with 50 nm β-megaspermin (beta), 10 μm DPI (DPI), or both (beta+DPI). B, Cells treated with 50 nm β-megaspermin (beta), 5 units/mL Glc oxidase and 5 mm Glc (GOG), 250 nm α-megaspermin (alpha), or 250 nm α-megaspermin and 5 units/mL Glc oxidase and 5 mm Glc (alpha+GOG).
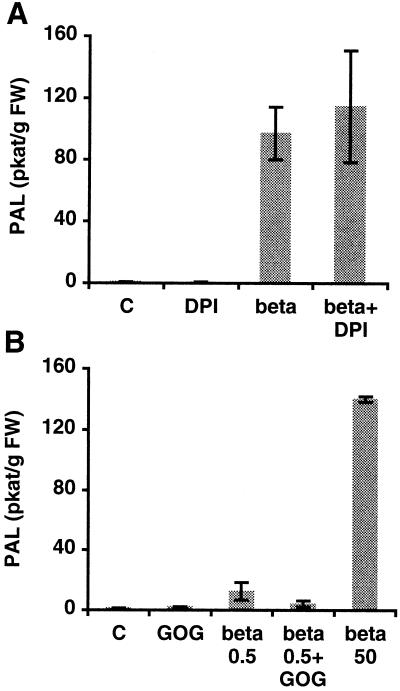
DPI treatment did not block PAL activation induced by the application of 50 nm β-megaspermin (Fig. 6A), and treatment with GOG did not induce PAL activity (Fig. 6B). PAL activation was not triggered by H2O2 from the oxidative burst. To investigate the possibility that H2O2 can enhance the elicitor-induced PAL stimulation, we treated cell suspensions with 0.5 nm β-megaspermin and GOG. While the elicitor alone induced a 4-fold stimulation of PAL activity over control (Fig. 6B), the application of both β-megaspermin and GOG did not cause increased PAL activity (Fig. 6B). These results reinforce the view that PAL stimulation resulting from elicitor treatment is independent of H2O2.
Figure 6.
Effect of DPI and of H2O2 generated by GOG on PAL activity. PAL activity from treated and control (C) cells was measured after 8 h of incubation. Bars represent the means ± sd of two independent experiments. A, Cells treated with 50 nm β-megaspermin (beta), 10 μm DPI (DPI), or both (beta+DPI). B, Cells treated with 0.5 nm (beta 0.5) or 50 nm (beta 50) β-megaspermin, 5 units/mL Glc oxidase and 5 mm Glc (GOG), or 0.5 nm β-megaspermin and 5 units/mL Glc oxidase and 5 mm Glc (beta 0.5+GOG).
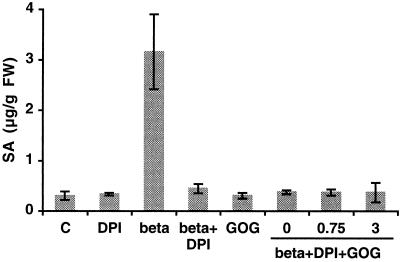
Preventing the H2O2 burst via DPI application completely abolished SA accumulation induced by β-megaspermin treatment (Fig. 7), suggesting that H2O2 (or O2−) is involved in SA synthesis. However, the production of exogenous H2O2 via GOG did not cause increased SA production (Fig. 7, GOG), indicating that H2O2 is not sufficient to cause SA accumulation. To determine whether the combined action of elicitor activity and H2O2 is necessary for SA synthesis, we pretreated cells for 10 min with DPI, then applied 50 nm β-megaspermin and GOG. GOG was either added at the same time (time 0) as elicitin or 0.75 or 3 h later. Whatever the experimental condition, no increased SA levels (compared with control) were measured (Fig. 7), showing that exogenously furnished H2O2 cannot overcome the block due to DPI treatment. Therefore, H2O2 was neither necessary nor sufficient for SA accumulation in our system.
Figure 7.
Effect of DPI and of H2O2 generated by GOG on SA accumulation. Cells were treated with 50 nm β-megaspermin (beta), 10 μm DPI (DPI), or both (beta+DPI), or with 5 units/mL Glc oxidase and 5 mm Glc (GOG), 50 nm β-megaspermin and 10 μm DPI and 5 units/mL Glc oxidase and 5 mm Glc (beta+DPI+GOG). For the latter treatment, GOG was added at the same time as the elicitor (0), 0.75 h later (0.75), or 3 h later (3). SA was measured after 14 h of incubation. C, Control cells. Bars represent the means ± sd of two independent experiments.
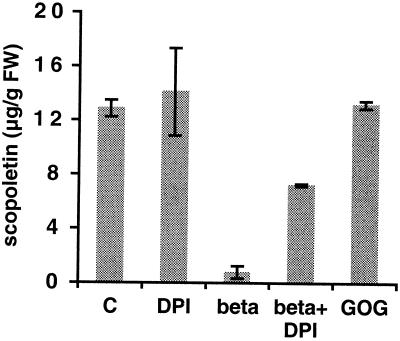
We hypothesized that the strong and rapid decrease in intracellular scopoletin levels after elicitin treatment could be caused by the oxidative burst. When the latter was abolished by treatment with DPI, we observed a partial inhibition of the scopoletin decrease (Fig. 8): a 50% reduction in scopoletin levels compared with control cells occured after elicitor plus DPI treatment, while elicitor application alone caused a 95% decrease. Therefore, intracellular scopoletin consumption must be explained by a mechanism different from oxidation via H2O2 (or O2−) from the extracellular oxidative burst. This conclusion was confirmed by the fact that the addition of GOG (which generated high H2O2 levels under our experimental conditions) to the cells did not cause a decrease in scopoletin (Fig. 8).
Figure 8.
Effect of DPI and of H2O2 generated by GOG on scopoletin amounts. Cells were treated with 50 nm β-megaspermin (beta), 10 μm DPI (DPI), or both (beta+DPI), or 5 units/mL of Glc oxidase and 5 mm Glc (GOG). Scopoletin was measured after 14 h of incubation. C, Control cells. Bars represent the means ± sd of two independent experiments.
DISCUSSION
Our results provide two major findings. First, among the three elicitors we used, α-megaspermin and the glycoprotein showed a strikingly different biological activity on suspension-cultured tobacco cells compared with that observed on tobacco leaves. Second, in tobacco cell suspensions elicited with elicitins, H2O2 from the oxidative burst was not a necessary or a sufficient signal for HR cell death induction, PAL activity stimulation, SA accumulation, or intracellular scopoletin consumption.
The glycoprotein, α-megaspermin, and β-megaspermin were isolated from the same Phytophthora megasperma isolate, and were shown to be equally efficient in inducing HR when infiltrated into tobacco leaves (Dorey et al., 1999). The reason(s) behind their different biological activities toward suspension-cultured cells is unclear. A lack of perception by the cells can be ruled out, since all three elicitors were able to trigger responses, among them PAL activation and medium alkalinization (Dorey et al., 1999). Medium alkalinization has been described as a highly sensitive assay of elicitor perception (Boller, 1995). The glycoprotein and α-megaspermin are acidic proteins while β-megaspermin is basic (Baillieul et al., 1995). Different pIs and the presence of the polysaccharide moiety in the glycoprotein may explain, at least in part, the differences in biological activities, since the plant cell wall is a negatively charged matrix. This hypothesis suggests that an altered accessibility of the elicitors to the targeted cells, rather than a different capacity of cells to respond to different elicitors, is the causal element. Differential defense response induction has also been described with other elicitors. For example, AVR9 elicitor is active on Cf9 tomato plants but not on Cf9 suspension-cultured tomato cells (Honée et al., 1998). In this case, it was inferred that defense response induction was developmentally regulated.
We have taken advantage of the differences in the biological activity of the three elicitors on cell suspensions to assess the role of H2O2 from the oxidative burst in HR cell death, PAL stimulation, SA accumulation, and scopoletin consumption. The glycoprotein turned out to be the less-active elicitor, triggering only a 4-fold stimulation of PAL activity and no SA accumulation. Apparently, a threshold level of PAL activation is necessary to provide enough metabolic flux into the biosynthetic loop to SA, and this threshold was achieved by treatment with the elicitins.
α-Megaspermin, which displays more than 80% amino acid sequence identity with β-megaspermin, caused a PAL stimulation as strong as that due to β-megaspermin, but was slightly less effective in triggering SA accumulation. This suggests that another step farther downstream of PAL in the SA pathway is an important control point. Benzoic acid-2-hydroxylase could be this control point, as it converts benzoic acid into SA and has been shown to be stimulated by H2O2 (Lee et al., 1995; Leon et al., 1995). However, β-megaspermin induced a strong H2O2 burst, while α-megaspermin caused only a low and delayed burst. This latter observation should be interpreted with caution, however, because the experiments described here were performed using unwashed 6- to 7-d-old cells. When the same cells were washed and replaced in a minimal medium containing no carbon source and only 175 mm mannitol, 0.5 mm CaCl2, and 0.5 m K2SO4 (these conditions were applied by others [Simon-Plas et al., 1997; Bourque et al., 1998] to study the oxidative burst triggered by different elicitins), α- and β-megaspermin induced the same rapid (within the first 20 min) oxidative burst (Dorey et al., 1999). These observations suggest that some antioxidant compounds present in the culture medium of unwashed cells may somehow scavenge H2O2 from the oxidative burst.
The oxidative burst was measured from unwashed cells for two reasons. First, since one of the goals of this study was to investigate the relationships and connections between the early H2O2 burst and later defense responses, the same experimental conditions of suspended cell cultivation had to be used for H2O2 assays and measurements of the defense responses. Second, when measurements have to be recorded over several hours, cells have to be maintained in a medium allowing regular growth. Using these experimental conditions, we were able to measure a biphasic (phase I and phase II) oxidative burst after treatment with β-megaspermin. The oxidative burst triggered by the elicitin cryptogein was reported to be rapid (within minutes) and transient (Viard et al., 1994; Pugin et al., 1997; Simon-Plas et al., 1997). However, only the H2O2 produced by cultured tobacco cells maintained in the minimal medium was analyzed and only over a 1-h period.
A biphasic oxidative burst was also reported to occur in parsley cell suspensions treated with a crude Phytophthora soja cell wall preparation (Jabs et al., 1997) and in ozone-exposed Bel W3 tobacco, which is known as an ozone biomonitor (Schraudner et al., 1998). Analyzing the oxidative burst induced by compatible, incompatible, and saprophytic bacteria, Baker and Orlandi (1995) hypothesized that phase I and II resulted from the activity of two different elicitors. This possibility can be excluded in our system. A possible explanation of the transient decrease in H2O2 between phase I and II could be H2O2 degradation via an antioxidant mechanism. Phase I was also reported as a biologically nonspecific reaction (Lamb and Dixon, 1997), since the treatment of cultured tobacco cells with the compatible bacteria Pseudomonas syringae pv tabaci induced only phase I, while the incompatible P. syringae pv glycinea induced both phase I and II (Baker and Orlandi, 1995). Recently, it was suggested that the occurrence of phase I may result from the suspension cultures not being allowed enough time to adjust to altered conditions before the addition of elicitors (Able et al., 1998). Whether this possibility applies to our system is not clear, since we used unwashed cells that were transferred to small flasks 3 h before treatments.
The oxidative burst has often been described as causing the HR cell death (Levine et al., 1994; Wojtaszek, 1997; Desikan et al., 1998; Kazan et al., 1998). In our system, there was a correlation between high levels of H2O2 and cell death. Rustérucci et al. (1996) have also reported a correlation between a cryptogein-induced oxidative burst in tobacco cell cultures and the capacity of cryptogein to induce tissue necrosis on tobacco leaves. However, our gain and loss of function experiments clearly indicated that H2O2 from the oxidative burst was neither necessary nor sufficient to induce cell death (Fig. 5).
Using another approach based on mutant bacteria, Glazener et al. (1996) reported that the reactive oxygen intermediate response of cultured tobacco cells to incompatible bacteria was not sufficient to cause HR cell death. Furthermore, there are several reports in which elicitors and pathogens were shown to trigger a strong oxidative burst without causing HR cell death (Baker and Orlandi, 1995; Jabs et al., 1997; Rouet-Mayer et al., 1997). Although H2O2 did not appear as an essential signal for cell death induction, it could act as a molecule initiating the phenomenon. Our data do not support such a role. Treatment of cells with GOG and a sublethal dose of β-megaspermin or a high dose of α-megaspermin, which does not trigger cell death but does induce PAL activity and SA accumulation in a manner similar to the 50 nm β-megaspermin treatment, did not cause HR death.
Pugin et al. (1997) showed that, when applied to tobacco cells, cryptogein, an elicitin closely related to β-megaspermin, activated a plasma membrane redox system, resulting in the oxidation of NADPH. Activation of this redox system resulted in extracellular H2O2 accumulation. The consequence of NADPH oxidation was a large decrease in the NADPH to NADP+ ratio in the elicitor-treated cells, which may result in a change in the redox status of the cell. Whether this change may explain the observed intracellular scopoletin consumption remains to be established. Another consequence of this redox system activation was extracellular alkalinization and cytoplasmic acidification; the latter was abolished when cells were treated with DPI (Pugin et al., 1997). It was proposed that cytoplasm acidification induced by cryptogein in tobacco cells could be a component of the signal cascade leading to the HR response. In our system, DPI did not abolish the elicitor-induced cell death, but strongly reduced the elicitor-induced SA accumulation. The implication of cytoplasm acidification and/or of the change in the redox status of the cell in the synthesis of SA remains to be demonstrated.
There have been conflicting results concerning the relationship between H2O2 and PAL gene induction. In soybean cell suspensions, H2O2 was not a signal for PAL activation (Levine et al., 1994; Delledonne et al., 1998), while in Arabidopsis and tobacco cultured cells, the addition of exogenous H2O2 triggered PAL gene expression (Mehdy, 1994; Desikan et al., 1998). Our own results do not support a key role for H2O2 in PAL activity induction. Mimicking the H2O2 burst did not induce PAL activity. Elicitor-induced PAL activity was not enhanced by exogenous H2O2. Inhibiting the H2O2 burst did not abolish or even decrease PAL activity triggered by elicitin treatment. Mehdy (1994) proposed that, depending on the elicitor and the plant, the degree of inhibition of phytoalexin accumulation by antioxidant mechanisms can vary, suggesting that pathways independent of the oxidative burst contribute to the regulation of phytoalexin accumulation. The same hypothesis could apply to other defense responses, such as the PAL response.
A model was proposed in which H2O2 generated during phase I would activate benzoate-2-hydroxylase, resulting in rapid SA accumulation, which would potentiate HR cell death and defense gene expression (Draper, 1997). In our system, H2O2 was not able to induce SA accumulation, suggesting that H2O2 is not sufficient to cause SA production. Inhibition of the oxidative burst prevented the elicitin-induced SA production. Similar results were obtained when oligosaccharidic elicitors such as linear β-1,3-glucans and oligogalacturonides were applied to cultured tobacco cells (O. Klarzynski, B. Plesse, M. Kopp, and B. Fritig, unpublished data). The DPI block experiment suggested that H2O2 is necessary for the elicitor-induced SA accumulation. If this inference is correct, then the exogenous addition of H2O2 via GOG activity to cells treated with the elicitor and DPI should cause SA accumulation. Such experimental conditions did not result in increased SA production; in fact, the SA level was even lower than that induced by elicitin treatment. Consequently, one possible candidate as the active molecule is O2−, since DPI blocks NADPH oxidases, which generates O2− that is readily dismuted into H2O2. Studies have involved O2− rather than H2O2 as an essential component involved in defense activation in parsley (Jabs et al., 1997) and cell death induction in the lsd1 mutant of Arabidopsis (Jabs et al., 1996). Whether the lack of O2− production through DPI activity explains the inhibition of elicitin-induced SA accumulation remains to be demonstrated.
The suspension-cultured tobacco cells used in this study contained rather high constitutive amounts of scopoletin. The reason(s) behind the presence of scopoletin in undifferentiated cells remains elusive. The decrease in scopoletin amount after elicitor treatment is also intriguing. Since scopoletin can be readily oxidized by peroxidase and H2O2 (Levine et al., 1994), the observed scopoletin decrease could be interpreted as an indirect measure of the oxidative burst. However, gain and loss of function experiments did not support the hypothesis that the extracellular H2O2 burst was causally linked to the intracellular scopoletin decrease (Fig. 8). Indeed, the extracellular production of H2O2 via GOG did not cause a decrease in the intracellular scopoletin levels.
H2O2 is able to freely diffuse across plasma membranes. Thus, if under our experimental conditions some H2O2 diffused inside the cells, it did not react with scopoletin. Allan and Fluhr (1997) reported the occurrence of an intracellular source for reactive oxygen intermediates of the oxidative burst in cryptogein-treated epidermal tobacco cells. That study analyzed reactive oxygen intermediate production during the first 30 to 40 min after elicitor application, whereas the scopoletin decrease in our system occurred after 1 h of incubation with β-megaspermin. That such an intracellular burst may explain our data concerning the scopoletin decrease remains to be established.
ACKNOWLEDGMENT
We thank Patrick Saindrenan for helpful discussions and critical reading of the manuscript.
LITERATURE CITED
- Able AJ, Guest DI, Sutherland MW. Use of a new tetrazolium-based assay to study the production of superoxide radicals by tobacco cell cultures challenged with avirulent zoospores of Phytophthora parasitica var nicotianae. Plant Physiol. 1998;117:491–499. doi: 10.1104/pp.117.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahl-Goy P, Signer H, Reist R, Aichholz R, Blum W, Schmidt E, Kessmann H. Accumulation of scopoletin is associated with the high disease resistance of the hybrid Nicotiana glutinosa × Nicotiana debneyi. Planta. 1993;191:200–206. [Google Scholar]
- Allan AC, Fluhr R. Two distinct sources of elicited reactive oxygen species in tobacco epidermal cells. Plant Cell. 1997;9:1559–1572. doi: 10.1105/tpc.9.9.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez ME, Pennell RI, Meijer P, Ishikawa A, Dixon RA, Lamb C. Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell. 1998;92:773–784. doi: 10.1016/s0092-8674(00)81405-1. [DOI] [PubMed] [Google Scholar]
- Baillieul F, Fritig B, Kauffmann S. Occurrence among Phytophthora species of a glycoprotein eliciting a hypersensitive response in tobacco and its relationships with elicitins. Mol Plant-Microbe Interact. 1996;9:214–216. [Google Scholar]
- Baillieul F, Genetet I, Kopp M, Saindrenan P, Fritig B, Kauffmann S. A new elicitor of the hypersensitive response in tobacco: a fungal glycoprotein elicits cell death, expression of defence genes, production of salicylic acid, and induction of systemic acquired resistance. Plant J. 1995;8:551–560. doi: 10.1046/j.1365-313x.1995.8040551.x. [DOI] [PubMed] [Google Scholar]
- Baker CJ, Orlandi EW. Active oxygen in plant pathogenesis. Annu Rev Phytopathol. 1995;33:299–321. doi: 10.1146/annurev.py.33.090195.001503. [DOI] [PubMed] [Google Scholar]
- Baker CJ, Orlandi EW, Mock NM. Harpin, an elicitor of the hypersensitive response in tobacco caused by Erwinia amylovora, elicits active oxygen production in suspension cells. Plant Physiol. 1993;102:1341–1344. doi: 10.1104/pp.102.4.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestwick CS, Brown IR, Bennet MHR, Mansfield JW. Localization of hydrogen peroxide accumulation during the hypersensitive reaction of lettuce cells to Pseudomonas syringae pv phaseolicola. Plant Cell. 1997;9:209–221. doi: 10.1105/tpc.9.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T. Chemoperception of microbial signals in plant cells. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:189–214. [Google Scholar]
- Bourque S, Ponchet M, Binet MN, Ricci P, Pugin A, Lebrun-Garcia A. Comparison of binding properties and early biological effects of elicitins in tobacco cells. Plant Physiol. 1998;118:1317–1326. doi: 10.1104/pp.118.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley DJ, Kjellbom P, Lamb CJ. Elicitor- and wound-induced oxidative cross-linking of a proline-rich plant cell wall protein: a novel, rapid defense response. Cell. 1992;70:21–30. doi: 10.1016/0092-8674(92)90530-p. [DOI] [PubMed] [Google Scholar]
- Chamnongpol S, Willekens H, Moeder W, Langebartels C, Sandermann H, Montagu MV, Inzé D, Camp WV. Defense activation and enhanced pathogen tolerance induced by H2O2 in transgenic tobacco. Proc Natl Acad Sci USA. 1998;95:5818–5823. doi: 10.1073/pnas.95.10.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Malamy J, Henning J, Conrath U, Sanchez-Casas P, Silva H, Ricigliano J, Klessig DF. Induction, modification, and transduction of the salicylic acid signal in plant defense responses. Proc Natl Acad Sci USA. 1995;92:4134–4137. doi: 10.1073/pnas.92.10.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D, Merida J, Legendre L, Low PS, Heinstein P. Independent elicitation of the oxidative burst and phytoalexin formation in cultured plant cells. Phytochemistry. 1993;32:607–611. [Google Scholar]
- Delaney TP, Ukness S, Vernooij B, Friedrich L, Weymann K, Negrotto N, Gaffney T, Gut-Rella M, Kessmann H, Ward E, Ryals J. A central role of salicylic acid in plant disease resistance. Science. 1994;266:1247–1250. doi: 10.1126/science.266.5188.1247. [DOI] [PubMed] [Google Scholar]
- Delledonne M, Xia YJ, Dixon RA, Lamb C. Nitric oxide functions as a signal in plant disease resistance. Nature. 1998;394:585–588. doi: 10.1038/29087. [DOI] [PubMed] [Google Scholar]
- Desikan R, Hancock JT, Coffey MJ, Neill SJ. Generation of active oxygen in elicited cells of Arabidopsis thaliana is mediated by a NADPH oxidase-like enzyme. FEBS Lett. 1996;382:213–217. doi: 10.1016/0014-5793(96)00177-9. [DOI] [PubMed] [Google Scholar]
- Desikan R, Reynolds A, Hancock JT, Neill SJ. Harpin and hydrogen peroxide both initiate programmed cell death but have differential effects on defence gene expression in Arabidopsis suspension cultures. Biochem J. 1998;330:115–120. doi: 10.1042/bj3300115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin WS, Gustine DL. Involvement of the oxidative burst in phytoalexin accumulation and hypersensitive reaction. Plant Physiol. 1992;100:1189–1195. doi: 10.1104/pp.100.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doke N, Ohashi N. Involvement of an O2− generating system in the induction of necrotic lesions on tobacco leaves infected with tobacco mosaic virus. Physiol Mol Plant Pathol. 1988;32:163–175. [Google Scholar]
- Dorey S, Baillieul F, Pierrel MA, Saindrenan P, Fritig B, Kauffmann S. Spatial and temporal induction of cell death, defense genes, and accumulation of salicylic acid in tobacco leaves reacting hypersensitively to a fungal glycoprotein elicitor. Mol Plant-Microbe Interact. 1997;10:646–655. [Google Scholar]
- Dorey S, Kopp M, Fritig B, Kauffmann S. Induced defense mechanisms in plant-fungus interactions: differences between cells in culture and leaf tissue. In: Altman A, Izhar S, Ziv M, editors. Current Plant Science and Biotechnology in Agriculture: Plant Biotechnology and in Vitro Biology in the 21st Century, Vol 36. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1999. pp. 473–476. [Google Scholar]
- Draper J. Salicylate, superoxide synthesis and cell suicide in plant defence. Trends Plant Sci. 1997;2:162–165. [Google Scholar]
- Enyedi AJ, Yalpani N, Silverman P, Raskin I. Localization, conjugation, and function of salicylic acid in tobacco during the hypersensitive reaction to tobacco mosaic virus. Proc Natl Acad Sci USA. 1992;89:2480–2484. doi: 10.1073/pnas.89.6.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritig B, Hirth L, Ourisson G. Biosynthesis of the coumarins: scopoletin formation in tobacco tissue cultures. Phytochemistry. 1970;9:1963–1975. [Google Scholar]
- Gaffney T, Friedrich L, Vernooij B, Negrotto D, Nye G, Uknes S, Ward E, Kessmann H, Ryals J. Requirement of salicylic acid in systemic acquired resistance. Science. 1993;261:754–756. doi: 10.1126/science.261.5122.754. [DOI] [PubMed] [Google Scholar]
- Glazener JA, Orlandi EW, Baker CJ. The active oxygen response of cell suspensions to incompatible bacteria is not sufficient to cause hypersensitive cell death. Plant Physiol. 1996;110:759–763. doi: 10.1104/pp.110.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazener JA, Orlandi EW, Harmon GL, Baker CJ. An improved method for monitoring active oxygen in bacteria-treated suspension cells using luminol-dependent chemiluminescence. Physiol Mol Plant Pathol. 1991;39:123–133. [Google Scholar]
- Hahlbrock K, Scheel D. Physiology and molecular biology of phenylpropanoid metabolism. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:347–369. [Google Scholar]
- Honée G, Buitink J, Jabs T, De Kloe J, Sijbolts F, Apotheker M, Weide R, Sijen T, Stuiver M, De Wit PJGM. Induction of defense-related responses in Cf9 tomato cells by the AVR9 elicitor peptide of Cladosporium fulvum is developmentally regulated. Plant Physiol. 1998;117:809–820. doi: 10.1104/pp.117.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabs T, Dietrich RA, Dangl JL. Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science. 1996;273:1853–1856. doi: 10.1126/science.273.5283.1853. [DOI] [PubMed] [Google Scholar]
- Jabs T, Tschöpe M, Colling C, Hahlbrock K, Scheel D. Elicitor-stimulated ion fluxes and O2.− from the oxidative burst are essential components in triggering defense gene activation and phytoalexin synthesis in parsley. Proc Natl Acad Sci USA. 1997;94:4800–4805. doi: 10.1073/pnas.94.9.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazan K, Murray FR, Goulter KC, Llewellyn DJ, Manners JM. Induction of cell death in transgenic plants expressing a fungal glucose oxidase. Mol Plant-Microbe Interact. 1998;11:555–562. [Google Scholar]
- Keller T, Damude HG, Werner D, Doerner P, Dixon R, Lamb C. A plant homolog of the neutrophil NADPH oxidase gp91phox subunit gene encodes a plasma membrane protein with Ca2+ binding motifs. Plant Cell. 1998;10:255–266. doi: 10.1105/tpc.10.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb C, Dixon RA. The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:251–275. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- Lee H, Leon J, Raskin I. Biosynthesis and metabolism of salicylic acid. Proc Natl Acad Sci USA. 1995;92:4076–4079. doi: 10.1073/pnas.92.10.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre L, Rueter S, Heinstein PF, Low PS. Characterization of the oligogalacturonide-induced oxidative burst in cultured soybean (Glycine max) cells. Plant Physiol. 1993;102:233–240. doi: 10.1104/pp.102.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon J, Shulaev V, Yalpani N, Lawton MA, Raskin I. Benzoic acid 2-hydroxylase, a soluble oxygenase from tobacco, catalyses salicylic acid biosynthesis. Proc Natl Acad Sci USA. 1995;92:10413–10417. doi: 10.1073/pnas.92.22.10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Maher EA, Bate NJ, Ni W, Elkind Y, Dixon RA, Lamb CJ. Increased disease susceptibility of transgenic tobacco plants with suppressed levels of preformed phenylpropanoid products. Proc Natl Acad Sci USA. 1994;91:7802–7806. doi: 10.1073/pnas.91.16.7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauch-Mani B, Slusarenko AJ. Production of salicylic acid precursors is a major function of phenylalanine ammonia-lyase in the resistance of Arabidopsis to Perenospora parasitica. Plant Cell. 1996;8:203–212. doi: 10.1105/tpc.8.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehdy MC. Active oxygen species in plant defense against pathogens. Plant Physiol. 1994;105:467–472. doi: 10.1104/pp.105.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini L, Rohfritsch O, Fritig B, Legrand M. Phenylalanine ammonia-lyase in tobacco: molecular cloning and gene expression during the hypersensitive reaction to tobacco mosaic virus and the response to a fungal elicitor. Plant Physiol. 1994;106:877–886. doi: 10.1104/pp.106.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng M, Kuc J. Peroxidase-generated hydrogen peroxide as a source of antifungal activity in vitro and on tobacco leaf disks. Phytopathology. 1992;82:696–699. [Google Scholar]
- Pugin A, Franchisse J-M, Tavernier E, Bligny R, Gout E, Douce R, Guern J. Early events induced by the elicitor cryptogein in tobacco cells: involvement of a plasma membrane NADPH oxidase and activation of glycolysis and the pentose phosphate pathway. Plant Cell. 1997;9:2077–2091. doi: 10.1105/tpc.9.11.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouet-Mayer M-A, Mathieu Y, Cazalé A-C, Guern J, Laurière C. Extracellular alkalinization and oxidative burst induced by fungal pectin lyase in tobacco cells are not due to the perception of oligogalacturonide fragments. Plant Physiol Biochem. 1997;35:321–330. [Google Scholar]
- Rustérucci C, Stallaert V, Milat M-L, Pugin A, Ricci P, Blein J-P. Relationship between active oxygen species, lipid peroxidation, necrosis, and phytoalexin production induced by elicitins in Nicotiana. Plant Physiol. 1996;111:885–891. doi: 10.1104/pp.111.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraudner M, Moeder W, Wiese C, Van Camp W, Inzé D, Langebartels C, Sandermann H. Ozone-induced oxidative burst in the ozone biomonitor plant, tobacco Bel W3. Plant J. 1998;16:235–245. doi: 10.1046/j.1365-313x.1998.00294.x. [DOI] [PubMed] [Google Scholar]
- Simon-Plas F, Rustérucci C, Milat M-L, Humbert C, Montillet J-L, Blein JP. Active oxygen species production in tobacco cells elicited by cryptogein. Plant Cell Environ. 1997;20:1573–1579. [Google Scholar]
- Sutherland MW. The generation of oxygen radicals during host plant responses to infection. Physiol Mol Plant Pathol. 1991;39:79–93. [Google Scholar]
- Tavernier E, Wendehenne J-P, Blein J-P, Pugin A. Involvement of free calcium in action of cryptogein, a proteinaceous elicitor of a hypersensitive reaction in tobacco cells. Plant Physiol. 1995;109:1025–1031. doi: 10.1104/pp.109.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viard MP, Martin F, Pugin A, Blein JP. Protein phosphorylation is induced in tobacco cells by the elicitor cryptogein. Plant Physiol. 1994;104:1245–1249. doi: 10.1104/pp.104.4.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtaszek P. Oxidative burst: an early plant response to pathogen infection. Biochem J. 1997;322:681–692. doi: 10.1042/bj3220681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing T, Higgins VJ, Blumwald E. Race-specific elicitors of Cladosporium fulvum promote translocation of cytosolic components of NADPH oxidase to plasma membrane of tomato cells. Plant Cell. 1997;9:249–259. doi: 10.1105/tpc.9.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]