Abstract
Many plant species respond to herbivory with de novo production of a mixture of volatiles that attracts carnivorous enemies of the herbivores. One of the major components in the blend of volatiles produced by many different plant species in response to herbivory by insects and spider mites is the homoterpene 4,8-dimethyl-1,3(E),7-nonatriene. One study (J. Donath, W. Boland [1995] Phytochemistry 39: 785–790) demonstrated that a number of plant species can convert the acyclic sesquiterpene alcohol (3S)-(E)-nerolidol to this homoterpene. Cucumber (Cucumis sativus L.) and lima bean (Phaseolus lunatus L.) both produce 4,8-dimethyl-1,3(E),7-nonatriene in response to herbivory. We report the presence in cucumber and lima bean of a sesquiterpene synthase catalyzing the formation of (3S)-(E)-nerolidol from farnesyl diphosphate. The enzyme is inactive in uninfested cucumber leaves, slightly active in uninfested lima bean leaves, and strongly induced by feeding of the two-spotted spider mite (Tetranychus urticae Koch) on both plant species, but not by mechanical wounding. The activities of the (3S)-(E)-nerolidol synthase correlated well with the levels of release of 4,8-dimethyl-1,3(E),7-nonatriene from the leaves of the different treatments. Thus, (3S)-(E)-nerolidol synthase is a good candidate for a regulatory role in the release of the important signaling molecule 4,8-dimethyl-1,3(E),7-nonatriene.
Many plant species respond to arthropod herbivory with the production of a blend of volatiles that attracts carnivorous enemies of the herbivores, such as predators or parasitic wasps (Dicke et al., 1990b; Turlings et al., 1990, 1995; Vet and Dicke, 1992). For example, upon infestation with two-spotted spider mites (Tetranychus urticae), lima bean (Phaseolus lunatus L.) plants respond with the emission of a mixture of volatiles attracting the predatory mite Phytoseiulus persimilis (Dicke et al., 1990b), which effectively eliminates local populations of spider mites (Dicke et al., 1990a). The induced production of carnivore-attracting volatiles has been recorded for over 20 plant species in 13 families (Dicke, 1999), and these volatiles are usually either not emitted or emitted only in minor quantities in response to mechanical wounding. Examples are lima bean (Dicke et al., 1990b), corn (Turlings et al., 1990), and cucumber (Cucumis sativus L.) (Takabayashi et al., 1994). Each plant species emits its own specific blend of volatiles originating from several different biosynthetic pathways such as the isoprenoid, shikimic acid, and lipoxygenase pathways.
One of the compounds derived from the isoprenoid biosynthetic pathway, the homoterpene 4,8-dimethyl-1,3(E),7-nonatriene (Fig. 1), was shown to be an important constituent of the volatile blend emitted by the leaves of a large number of plant species in response to herbivory (e.g. lima bean [Dicke et al., 1990b]; cucumber [Dicke et al., 1990a; Takabayashi et al., 1994], corn [Turlings et al., 1990], cotton [Loughrin et al., 1994], and apple [Takabayashi et al., 1994]), but also of the fragrance produced by many flowers (Kaiser, 1991). The homoterpene is an important component of the volatile blend of lima bean and cucumber plants infested with the spider mite T. urticae, and attracts the predatory mite P. persimilis (Dicke et al., 1990a). A synthetic mixture of volatiles, including this homoterpene, that mimics the blend of volatiles that is emitted by corn plants infested with Spodoptera exigua caterpillars, attracts the parasitoid Cotesia marginiventris, which parasitizes the caterpillars (Turlings et al., 1991).
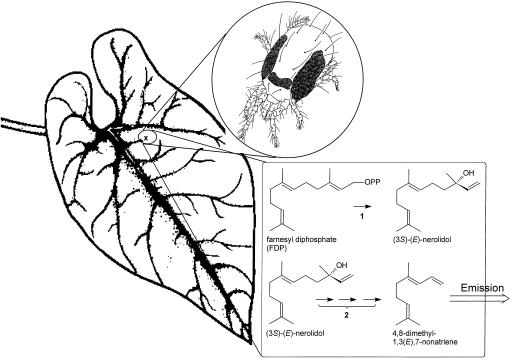
Figure 1.
Schematic representation of the release of 4,8-dimethyl-1,3(E),7-nonatriene, a volatile homoterpene released by many plant species after herbivory. Donath and Boland (1995) demonstrated the ability of leaves and flowers of several species to convert nerolidol to 4,8-dimethyl-1,3(E),7-nonatriene (2). We investigated the induction by herbivory in cucumber and lima bean of nerolidol synthase (1), the enzyme that should precede the sequence of reactions reported by Donath and Boland (1995).
Studies on the signaling cascade leading to the production of 4,8-dimethyl-1,3(E),7-nonatriene have recently been initiated, and it was demonstrated that herbivore oral secretions and components thereof can induce its formation in plants (Turlings et al., 1990; Alborn et al., 1997). Using 13CO2 pulse-labeling experiments, Paré and Tumlinson (1997) demonstrated that, among many other volatiles, 4,8-dimethyl-1,3(E),7-nonatriene is biosynthesized de novo in response to herbivory. Using 2H-labeled precursors, Donath and Boland (1994, 1995) elegantly demonstrated the ability of several plant species, such as lima bean, to produce 4,8-dimethyl-1,3(E),7-nonatriene from (3S)-(E)-nerolidol. The ability to convert (3S)-(E)-nerolidol to 4,8-dimethyl-1,3(E),7-nonatriene is not restricted to plants infested by herbivores: Uninfested leaves and flowers of several species convert exogenous nerolidol to 4,8-dimethyl-1,3(E),7-nonatriene (Gäbler et al., 1991; Donath and Boland, 1995), showing that the biosynthesis of 4,8-dimethyl-1,3(E),7-nonatriene from nerolidol is not regulated or induced by herbivory. Therefore, regulation of the release of 4,8-dimethyl-1,3(E),7-nonatriene must occur in the biosynthetic pathway upstream of the sesquiterpenoid nerolidol. The enzyme catalyzing the formation of a sesquiterpene skeleton from the ubiquitous precursor farnesyl diphosphate (FDP) is a good candidate for playing such a regulatory role (Gershenzon and Croteau, 1990; McGarvey and Croteau, 1995).
Although nerolidol, the sesquiterpene analog of the monoterpenoid linalool, is a component of many essential oils and flower headspaces (Bauer et al., 1990; Knudsen et al., 1993), enzymes catalyzing the formation of nerolidol from FDP have not to our knowledge been reported. The products of many of the enzymes catalyzing the formation of a terpenoid skeleton from the respective diphosphate substrates are mostly cyclic hydrocarbons (Bohlmann et al., 1998; Bouwmeester et al., 1998; De Kraker et al., 1998), but there are some exceptions to this rule. For example, Munck and Croteau (1990) purified a patchoulol synthase from Pogostemon cablin. The enzyme catalyzing the formation of the acyclic terpenol linalool from geranyl diphosphate was purified and the corresponding gene cloned from Clarkia breweri (Dudareva et al., 1996).
In the present study we investigated whether a nerolidol synthase catalyzes the first dedicated step in the biosynthesis of 4,8-dimethyl-1,3(E),7-nonatriene in response to herbivory (Fig. 1). To that end we infested lima bean and cucumber plants with T. urticae to induce volatile production and measured the induction of sesquiterpene synthase activity.
MATERIALS AND METHODS
Plant Material
Cucumber (Cucumis sativus L. cv Corona) plants were grown from seeds in 1-L pots containing potting compost in a greenhouse at a 20°C/18°C, 12-h/12-h light/dark cycle. Plants of lima bean (Phaseolus lunatus L. cv Sieva) were grown from seeds in 1-L plastic pots containing potting compost in a greenhouse at a 20°C to 30°C, 16-h/8-h light/dark cycle, with natural daylight supplemented with high-pressure mercury lights. Two-spotted spider mites (Tetranychus urticae Koch) were reared on lima bean plants under the same greenhouse conditions. Cucumber and lima bean plants were infested with spider mites at 30 and 21 d after germination, respectively, by placing leaves of spider mite-infested lima bean plants on the leaves of plants to be infested. Mechanical damage to cucumber plants was applied by rubbing the entire surface of all expanded leaves with carborundum powder (180 grit) on a wet cotton wool swab and spraying with water 0 and 5 d after infection of spider mite-treated plants. The plants were kept under the same greenhouse conditions until harvest. For each treatment two plants were used and leaves were harvested at d 5 after infestation. One sample of leaves was enclosed in a 1-L jar for headspace analysis (see below); another sample was frozen in liquid N2 and stored at −80°C for analysis of sesquiterpene synthase activity (see below).
Headspace Analysis
Glass jars (1 L) containing the detached leaves of the respective treatments were closed with a Teflon-lined lid equipped with an inlet and an outlet, and placed upside down in a climate room at 25°C and a light intensity of 210 μmol m−2 s−1 provided by 400-W lights (HPI-T, Philips, Eindhoven, The Netherlands). A vacuum pump was used to draw off air through the glass jar at approximately 100 mL min−1, with the incoming air purified through a glass cartridge (140 × 4 mm) containing 150 mg of Tenax TA (20/35-mesh, Alltech). At the outlet the volatiles emitted by the detached leaves were trapped on a similar cartridge. Volatiles were sampled for 3 h. Cartridges were eluted using 3 × 1 mL of re-distilled pentane:diethyl ether (4:1). Of the (non-concentrated) samples, 2 μL was analyzed by GC-MS using a gas chromatograph (5890 series II, Hewlett-Packard) equipped with a 30-m × 0.25-mm i.d., 0.25-μm film thickness column (5MS, Hewlett-Packard) and a mass-selective detector (model 5972A, Hewlett-Packard). The GC was programmed at an initial temperature of 45°C for 1 min, with a ramp of 10°C min−1 to 220°C and final time of 5 min. The injection port (splitless mode), interface, and MS source temperatures were 250°C, 290°C, and 180°C, respectively, and the He inlet pressure was controlled with an electronic pressure control to achieve a constant column flow of 1.0 mL min−1. The ionization potential was set at 70 eV, and scanning was performed from 30 to 250 atomic mass units.
Enzyme Isolation and Product Identification
During enzyme isolation and preparation of the assays, all operations were carried out on ice or at 4°C. Frozen leaves (1.0 g) were ground in a pre-chilled mortar and pestle in 8 mL of buffer containing 50 mm MOPSO (pH 6.8), 20% (v/v) glycerol, 50 mm sodium ascorbate, 50 mm NaHSO3, 10 mm MgCl2, and 5 mm DTT (buffer A) slurried with polyvinylpolypyrrolidone (0.1 g g−1 tissue) and a spatula tip of purified sea sand. Polystyrene resin (0.5 g g−1 tissue, Amberlite XAD-4, Sigma) was added and the slurry was stirred carefully for 10 min and then filtered through cheesecloth. The filtrate was centrifuged at 20,000g for 20 min, the pellet discarded, and the supernatant centrifuged at 100,000g for 90 min. Three milliliters of the 100,000g supernatant were desalted to buffer B containing 15 mm MOPSO (pH 7.0), 10% (v/v) glycerol, 10 mm MgCl2, 1 mm sodium ascorbate, and 2 mm DTT. For experiments with the phosphohydrolase inhibitor vanadate, buffer B was supplemented with 5 mm sodium orthovanadate (Janssen Chimica, Geels, Belgium).
Depending on the experiment, 10 or 50 μm [3H]FDP (at 100 or 20 Ci mol−1, respectively) ([3H]FDP from Amersham; unlabeled FDP from Sigma; De Kraker et al., 1998) was added to 0.5 or 1 mL of the enzyme preparations. Assays without MgCl2 (omitted from buffer B) were run to test the effect of Mg2+. After the addition of a 1-mL redistilled pentane overlay, the assays were incubated for 30 or 60 min at 30°C. Controls that had been boiled for 5 min showed no enzymatic activity. After the assay, the tubes were vortexed and the pentane layer was removed and passed over a short column of grade III aluminum oxide overlaid with anhydrous MgSO4. The assay was extracted again with 1 mL of redistilled diethyl ether, which was also passed over the aluminum oxide column, and the column was washed with 1.5 mL of diethyl ether. The total volume of the pentane/diethyl ether extract was determined and 50 or 100 μL of the extract was removed for liquid-scintillation counting in 4.5 mL of scintillation cocktail (Ultima Gold, Packard Bioscience, Groningen, The Netherlands). The distribution of the radiolabel over different products in the pentane/diethyl ether extract was determined using radio-GLC (i.e. the ratio between the peak areas in the radiosignal).
Before radio-GLC analysis, unlabeled reference compounds (Z)- and (E)-nerolidol and (E,E)-farnesol were added to the extract, which was then slowly concentrated under a stream of N2. Radio-GLC was performed on a gas chromatograph (4160 series, Carlo-Erba, Milano, Italy) equipped with a radioactivity detector (RAGA-90, Raytest, Straubenhardt, Germany). Sample components eluting from the column were quantitatively reduced before radioactivity was measured by passage through a conversion reactor filled with platinum chips at 800°C. Samples of 1 μL were injected in the cold, on-column mode. The GC was equipped with a 30-m × 0.32-mm i.d., 0.25-μm film thickness EC-WAX column (EconoCap, Alltech) or an enantioselective column coated with heptakis(6-O-tert-butyldimethylsilyl-2,3-di-O-methyl) -β-cyclodextrin (50% in OV17, w/w) (25-m × 0.25-mm i.d.; König et al., 1994) and operated with a He flow of 1.2 mL min−1. For use with the EC-WAX column, the oven temperature was programmed to 70°C for 5 min, followed by a ramp of 5°C min−1 to 210°C and a final time of 5 min. For use with the enantioselective column, the oven temperature was programmed to 116°C for 2 min, followed by a ramp of 0.5°C min−1 to 125°C and a final time of 10 min. About 20% of the column effluent was split with an adjustable splitter to a flame ionization detector (310°C). The remainder was directed to the conversion reactor and radiodetector. H2 was added prior to the reactor at 3 mL min−1, and CH4 as a quench gas prior to the radioactivity detector (5-mL-counting tube) to give a total flow of 36 mL min−1. A sample containing the four isomers of nerolidol was a kind gift of W.A. König, who also determined their elution order on the enantioselective column (König et al., 1992).
GC-MS identification of the products was carried out as described above in “Headspace Analysis,” but scanning was performed in both the scanning mode and the selected ion monitoring mode: m/z 93, 107, 136, and 161.
Linearity of the enzymatic reaction was tested on 100-μL enzyme preparations (undiluted and 2-fold diluted) in buffer B supplemented with 5 mm sodium orthovanadate in Eppendorf tubes to which 50 μm [3H]FDP was added. The reaction mixture was overlaid with 1 mL of hexane to trap volatile products, and the contents were carefully mixed and centrifuged briefly to separate phases. After incubation at 30°C, the vials were immediately cooled, vigorously mixed, and centrifuged to separate phases. A 750-μL portion of the hexane phase was removed for liquid scintillation counting in 4.0 mL of scintillation cocktail. All assays were performed in duplicate; controls that had been boiled for 5 min and incubated for 45 min were used to determine nonenzymatic hexane-soluble products. The ratio between nerolidol and farnesol produced was determined in 1.0-mL enzyme assays run for 30, 60, and 90 min in buffer B under the same conditions as the 100-μL assays. These assays were worked up and analyzed by GLC as described above. The ratio between nerolidol and farnesol in the 1-mL assays was used to calculate the contribution of nerolidol to the total hexane-soluble radiolabeled products of the 100-μL assays.
For quantitative comparison of enzymatic activities between treatments, duplicate 0.5-mL assays were run under the conditions giving a linear assay, i.e. with undiluted enzyme, for 60 min, as well as in the presence of 50 μm [3H]FDP and 5 mm sodium orthovanadate. The assays were worked up and analyzed by GLC as described above.
RESULTS AND DISCUSSION
Headspace Analysis
Undamaged leaves of cucumber emitted only traces of volatiles (Fig. 2A). Spider mite infestation caused a dramatic change in the production of volatile compounds by cucumber leaves (Fig. 2B), with the major compounds being (Z)-3-hexen-1-yl acetate (1), (E)-β-ocimene (2), and the C11 homoterpene 4,8-dimethyl-1,3(E),7-nonatriene (3). These compounds have been reported previously to be induced in cucumber by spider mite infestation (Takabayashi et al., 1994), and are also important constituents of the herbivore-induced blends of volatiles in corn and cotton (Turlings et al., 1991; Röse et al., 1996). Mechanical wounding of cucumber leaves did not induce these volatiles (Fig. 2C).
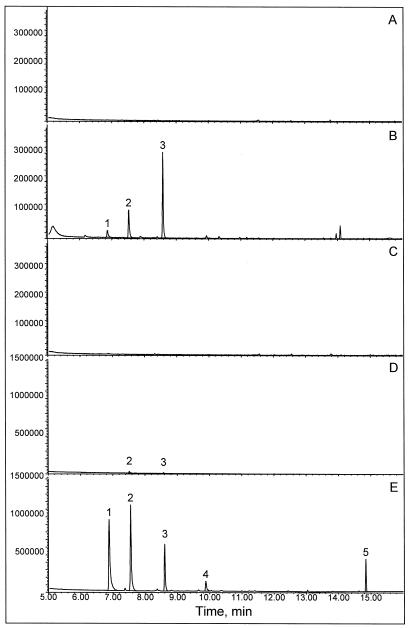
Figure 2.
Headspace analysis of cucumber and lima bean leaves. A, Undamaged cucumber leaves; B, cucumber leaves infested with spider mites for 5 d; C, cucumber leaves mechanically damaged with carborundum powder at 0 and 5 d before sampling; D, uninfested lima bean leaves; and E, lima bean leaves infested with spider mites for 5 d. Headspace samples were analyzed by GC-MS. The peaks are: (Z)-3-hexen-1-yl acetate (1); (E)-β-ocimene (2); 4,8-dimethyl-1,3(E),7-nonatriene (3); methyl salicylate (4); and 4,8,12-trimethyl-1,3(E),7(E),11-tridecatetraene (5).
Uninfested lima bean leaves produced traces of several volatiles, including some normally associated with herbivore induction, such as (E)-β-ocimene (2) and 4,8-dimethyl-1,3(E),7-nonatriene (3) (Fig. 2D). However, spider mite infestation dramatically increased the production of these volatiles by lima bean leaves and induced the formation of a number of compounds not detectable in the headspace of control leaves, such as methyl salicylate (4) and the C16 homoterpene 4,8,12-trimethyl-1,3(E),7(E),11-tridecatetraene (5) (Fig. 2E), which have been reported previously to be induced by spider mites in lima bean (Dicke et al., 1990b). The C16 homoterpene 4,8,12-trimethyl-1,3(E),7(E),11-tridecatetraene has been suggested to be derived from the diterpene analog of nerolidol, geranyllinalool (Gäbler et al., 1991), and is also a constituent of herbivore-induced volatile blends of a number of other plant species such as corn and cotton (Turlings et al., 1991; Paré and Tumlinson, 1997). Methyl salicylate attracts the predatory mite P. persimilis to spider mite-infested lima bean plants (Dicke et al., 1990b). In addition, methyl salicylate is supposed to play an important role in the activation of plant defense responses (Yang et al., 1997), such as the induction in tobacco plants of systemic acquired resistance against tobacco mosaic virus reported by Shulaev et al. (1997).
Sesquiterpene Synthase Activity and Product Identification
In both cucumber and lima bean, the C11 homoterpene 4,8-dimethyl-1,3(E),7-nonatriene showed a strong induction after spider mite infestation (Fig. 2; Dicke et al., 1990a, 1990b; Takabayashi et al., 1994). To investigate whether this coincides with an increase in nerolidol synthase activity, undamaged, mechanically wounded (cucumber only) and spider mite-infested leaves were assayed for nerolidol synthase activity. Enzyme assays on control and mechanically damaged cucumber leaves showed only the formation of (E,E)-farnesol, produced from [3H]FDP by phosphohydrolase activity, and some unknown minor compounds (Fig. 3, B and D). In assays on spider mite-infested leaves, a second radiolabeled product was detected in addition to (E,E)-farnesol, which co-eluted on radio-GLC with (E)-nerolidol (Fig. 3C). The product also co-eluted with an authentic standard of (E)-nerolidol on the more apolar column used for GC-MS analysis, and had the same mass spectrum as the standard (data not shown). In lima bean, (E)-nerolidol synthase activity was also detected in control leaves (Fig. 4B), but the activity increased strongly upon spider mite feeding (Fig. 4C).
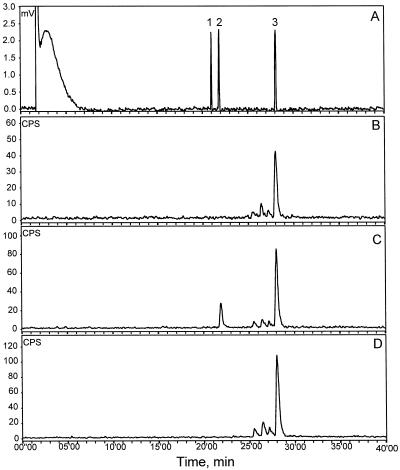
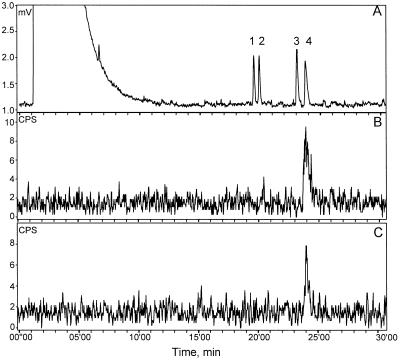
Figure 3.
Radio-GLC analysis of radiolabeled products formed from 10 μm [3H]FDP (60-min assay in buffer B) by enzyme preparations of cucumber leaves. A, Flame ionization detection (FID) signal of co-injected, unlabeled standards of (Z)-nerolidol (1), (E)-nerolidol (2), and (E,E)-farnesol (3). B to D, Radiotraces showing radiolabeled products of assays with undamaged cucumber leaves (B), cucumber leaves infested with spider mites for 5 d (C), and cucumber leaves mechanically damaged with carborundum powder 0 and 5 d before sampling (D).
Figure 4.
Radio-GLC analysis of radiolabeled products formed from 50 μm [3H]FDP (30-min assay in buffer B) by enzyme preparations of lima bean leaves. A, FID signal of co-injected, unlabeled standards of (Z)-nerolidol (1), (E)-nerolidol (2), and (E,E)-farnesol (3). B to C, Radiotraces showing radiolabeled products of assays with undamaged lima bean leaves (B) and lima bean leaves infested with spider mites for 5 d (C).
For both plant species, assays in the presence of vanadate and 50 μm [3H]FDP on enzyme extracts of spider mite-induced leaves were linear with protein concentration and time (for over 60 min) (Fig. 5). In cucumber, (E)-nerolidol synthase activity increased from being undetectable in control leaves to 10.7 nmol h−1 g−1 (fresh weight) in spider mite-infested leaves (Table I). In the absence of vanadate, phosphohydrolase activity increased 10- to 15-fold in cucumber and lima bean, whereas (E)-nerolidol synthase activity slightly decreased (Table I). This small decrease was probably due to the much higher substrate utilization by phosphohydrolases in the absence of vanadate and the corresponding lower substrate availability for (E)-nerolidol synthase (Table I).
Figure 5.
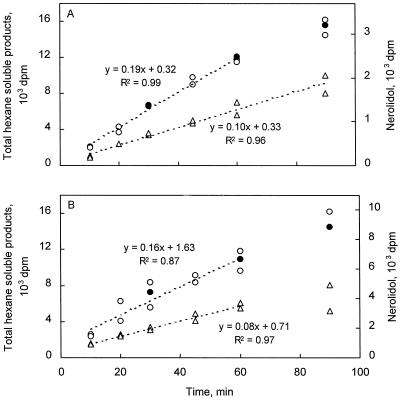
Time-dependent formation (dpm per 100-μL assay) of nerolidol (•, right y axis) and total hexane-soluble products (left y axis) for undiluted (○) and 2-fold-diluted (▵) enzyme preparations of spider mite-infested cucumber (A) and lima bean (B). Enzyme extracts of spider mite-infested leaves were desalted to assay buffer B supplemented with 5 mm sodium orthovanadate, and assayed with 50 μm [3H]FDP as a substrate. Total hexane-soluble products were determined in duplicate 100-μL assays, and nerolidol formation was analyzed by radio-GLC on 1-mL enzyme assays run for 30, 60, and 90 min.
Table I.
Nerolidol synthase and phosphohydrolase (production of [3H]-(E,E)-farnesol) activities in leaves of lima bean and cucumber with and without spider mite infestation
| Species | Spider Mites | Vanadate | Mg2+ | Substrate Converted | Enzyme Activity
|
|
|---|---|---|---|---|---|---|
| Nerolidol synthase | Phosphohydrolase | |||||
| % | nmol h−1 g−1 leaves (fresh wt) | |||||
| Cucumber | − | + | + | 1.8 | 0a | 8.4 ± 0.5 |
| + | + | + | 4.4 | 10.7 ± 4.3 | 13.0 ± 0.8 | |
| + | − | + | 29.5 | 7.4 ± 0.7 | 149.9 ± 12.5 | |
| + | + | − | 3.7 | 3.6 ± 1.2 | 16.2 ± 7.6 | |
| Lima bean | − | + | + | 2.6 | 5.2 ± 2.1 | 8.5 ± 3.2 |
| + | + | + | 7.3 | 30.7 ± 2.3 | 8.5 ± 3.4 | |
| + | − | + | 30.1 | 22.9 ± 0.5 | 137.6 ± 20.9 | |
| + | + | − | 5.0 | 4.7 ± 0.4 | 22.0 ± 0.5 | |
Results are means ± sd of two replicates. Enzyme extracts were prepared as described in Methods. Duplicate 0.5-mL enzyme preparations (in buffer B) were incubated for 60 min at 30°C with 50 μm [3H]FDP (20 Ci mol−1) with or without 5 mm sodium orthovanadate and with or without 10 mm MgCl2. Total radioactivity in the pentane/diethyl ether extracts of the assays (containing nerolidol, if present, as well as farnesol) was determined on a 50-μL subsample using liquid-scintillation counting. The distribution of the radiolabel over nerolidol and farnesol was determined using radio-GLC (ratio of peak areas in radiotrace). For further details, see Methods.
Below detection limit.
In lima bean, (E)-nerolidol synthase activity was detected in both control and spider mite-infested leaves (Fig. 4, Table I), but the activity of (E)-nerolidol synthase increased approximately 6-fold upon spider mite infestation (Table I). In both species the absence of Mg2+ in the assay buffer decreased (E)-nerolidol synthase activity by 65% to 85% (Table I). The requirement for Mg2+ is in line with reports on other sesquiterpene synthases (Munck and Croteau, 1990; De Kraker et al., 1998). Furthermore, the lack of an effect of vanadate on nerolidol synthase activity, in contrast to the strong inhibition of farnesol formation, supports the involvement of a specific sesquiterpene synthase. The absence of (E)-nerolidol synthase activity in control and mechanically wounded cucumber leaves, the low background activity in lima bean leaves, and the strong induction of the enzyme upon spider mite infestation in both species correlate well with the emission of 4,8-dimethyl-1,3(E),7-nonatriene, as shown in the corresponding headspace profiles in Figure 2.
The absolute configuration of the (E)-nerolidol produced was established by radio-GLC using an enantioselective column. Figure 6 shows that for both species the enzymatically produced nerolidol was (3S)-(E)-nerolidol. For a number of plant species, it has been shown that leaves and flowers, which have also been shown to emit 4,8-dimethyl-1,3(E),7-nonatriene (Kaiser, 1991; Knudsen et al., 1993), were able to convert exogenous nerolidol to 4,8-dimethyl-1,3(E),7-nonatriene (Gäbler et al., 1991; Donath and Boland, 1995). The conversion in plant leaves occurred with a high degree of enantioselectivity, and in all species investigated (3S)-(E)-nerolidol was the preferred substrate compared with (3R)-(E)-nerolidol (Donath and Boland, 1995).
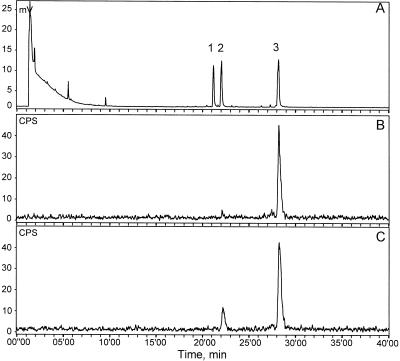
Figure 6.
Radio-GLC analysis of sesquiterpene synthase activities in spider mite-infested cucumber leaves using heptakis(6-O-tert-butyldimethylsilyl-2,3-di-O-methyl)-β-cyclodextrin (50% in OV17, w/w) as the enantioselective stationary phase. A, FID signal of co-injected, unlabeled (3R)- and (3S)-(Z)-nerolidol (1 and 2, elution order of the two enantiomers is unknown), (3R)-(E)-nerolidol (3), and (3S)-(E)-nerolidol (4). B and C, Radiotraces showing radiolabeled product formed from 50 μm [3H]FDP (30-min assay in buffer B) by enzyme preparations from cucumber leaves infested with spider mites for 5 d (B) and lima bean leaves infested with spider mites for 5 d (C).
In all of the plant species tested in the above studies, the conversion to 4,8-dimethyl-1,3(E),7-nonatriene was achieved without herbivory or elicitor treatment, suggesting that the enzymes required for this conversion are constitutively expressed. Therefore, the specific release of 4,8-dimethyl-1,3(E),7-nonatriene after herbivory must be regulated upstream of nerolidol (Paré and Tumlinson, 1997). This could be achieved either by release of nerolidol from a stored intermediate or by de novo biosynthesis, but Paré and Tumlinson (1997) demonstrated that 4,8-dimethyl-1,3(E),7-nonatriene, among other volatiles, is synthesized de novo after herbivory in cotton, excluding release from a stored intermediate as a possibility for regulation.
Our results show that in cucumber and lima bean, (3S)-(E)-nerolidol synthase is induced upon spider mite infestation, which supports the de novo biosynthesis of nerolidol. An interesting aspect of the regulation of 4,8-dimethyl-1,3(E),7-nonatriene formation is that the volatile nerolidol is not released from cucumber and lima bean leaves, whereas both (E)-nerolidol and 4,8-dimethyl-1,3(E),7-nonatriene are important constituents of the volatile blend produced in corn upon feeding by beet army worm larvae (Turlings et al., 1990) and in gerbera in response to feeding by spider mites (Krips et al., 1999). Furthermore, in the headspace of several flowers, nerolidol is an important constituent, often together with 4,8-dimethyl-1,3(E),7-nonatriene (Kaiser, 1991; Knudsen et al., 1993). It seems likely that there are differences between plant species and organs in the relative activities of the enzymes involved in the 4,8-dimethyl-1,3(E),7-nonatriene pathway up- and downstream of nerolidol that determine how much of each compound is released. However, regulation through a controlled release of the two compounds cannot be excluded.
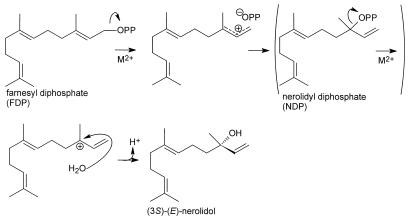
Although nerolidol is a constituent of many essential oils and the headspace of a large number of flowers, this is the first report (to our knowledge) of an enzyme catalyzing the formation of the acyclic sesquiterpene alcohol nerolidol from FDP. Ionization of FDP to the farnesyl cation is the first step in the biosynthesis of a large number of sesquiterpenes. This cation can attack either of the two double bonds, leading to cyclic sesquiterpenoids such as germacranes, eudesmanes, and caryophyllanes (McCaskill and Croteau, 1997). For a large number of other sesquiterpenoids, the enzymatic reaction is initiated by isomerization of FDP to the isomer nerolidyl diphosphate, which is ionized to generate the nerolidyl cation (Fig. 7). This enzyme-bound carbocation can undergo electrophilic cyclizations, rearrangements, hydride shifts, and deprotonation to yield cyclic sesquiterpenoid constituents such as the cadinanes, bergamotanes, and bisabolanes (McCaskill and Croteau, 1997). In the case of nerolidol formation, the nerolidyl carbocation is quenched by the addition of water, which is analogous to the formation of patchoulol described by Munck and Croteau (1990). In principle, the formation of the enzyme-bound nerolidyl diphosphate is not even required (Fig. 7), and whether this intermediate is formed or not probably depends on the evolution of the enzyme.
Figure 7.
Proposed enzymatic mechanism of the formation of (3S)-(E)-nerolidol from FDP.
The induction of terpene cyclases in response to attacking organisms is widespread. Croteau and coworkers demonstrated a dramatic increase in mono- and diterpene cyclase activity upon wounding of grand fir (Funk et al., 1994). These enzymes are involved in the production of oleoresin, a mixture of mono- and diterpenes produced to deter attacking insects and fungi. The induction partly represented an enhancement of constitutive activities, and partly the appearance of new activities. The latter is also reported for glandless cotton in response to inoculation with the bacterial pathogen Xanthomonas campestris cv malvacearum (Davis and Essenberg, 1995). The glandless cotton line lacks the constitutive terpene production of glanded cotton, but does respond to the pathogen with a strong induction of a sesquiterpene synthase catalyzing the formation of (+)-δ-cadinene, an intermediate in the biosynthesis of cotton phytoalexins. In tobacco and potato, microorganisms and elicitors induce the activity of sesquiterpene synthases catalyzing the first committed step in the biosynthesis of phytoalexins (Vögeli et al., 1990; Zook et al., 1992).
For biosynthetic pathways that are not induced by pathogens or insects, a regulatory role for terpene synthases has also been suggested (Gershenzon and Croteau, 1990; McCarvey and Croteau, 1995). For example, in Clarkia breweri there is a close correlation between linalool synthase activity and the production of linalool and linalool oxides (Pichersky et al., 1994). Croteau and coworkers demonstrated the enzymatic cyclization of geranylgeranyl diphosphate to taxa-4(5),11(12)-diene and suggested that this enzyme catalyzes the regulatory step in the biosynthesis of taxol in Taxus brevifolia (Koepp et al., 1995). The first dedicated step in the biosynthesis of the antimalarial drug artemisinin in Artemisia annua also has the typical characteristics of a regulatory role (e.g. no accumulation of the enzyme's direct product) (Bouwmeester et al., 1999).
The results of the present study suggest that the enzyme (3S)-(E)-nerolidol synthase plays an important role in regulating the formation of 4,8-dimethyl-1,3(E),7-nonatriene, a key signal molecule in induced plant defense mediated by the attraction of enemies of herbivores. The identification of this enzyme is an important step forward in the elucidation of the signaling cascade that leads from herbivory to the release of plant volatiles that attract the natural enemies of herbivores.
ACKNOWLEDGMENTS
The authors thank Wilfried König for the gift of the nerolidol isomers, Unifarm (Wageningen, The Netherlands) for growing the plants, Herman Dijkman for rearing spider mites, Jan-Willem de Kraker for helpful suggestions, Jacques Davies for technical assistance, and Hans Helsper for helpful comments concerning the manuscript.
Footnotes
This work was supported by the Dutch Ministry of Agriculture, Nature Management, and Fisheries (H.J.B., F.W.A.V.) and the Uyttenboogaart-Eliasen Foundation (M.D.).
LITERATURE CITED
- Alborn T, Turlings TCJ, Jones TH, Stenhagen G, Loughrin JH, Tumlinson JH. An elicitor of plant volatiles from beet armyworm oral secretion. Science. 1997;276:945–949. [Google Scholar]
- Bauer K, Garbe D, Surburg H (1990) Common Fragrance and Flavor Materials. Preparations, Properties and Uses, Ed 2. VCH Verlaggesellschaft, Weinheim, Germany
- Bohlmann J, Meyer-Gauen G, Croteau R. Plant terpenoid synthases: molecular biology and phylogenetic analysis. Proc Natl Acad Sci USA. 1998;95:4126–4133. doi: 10.1073/pnas.95.8.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwmeester HJ, Gershenzon J, Konings MCJM, Croteau R. Biosynthesis of limonene and carvone in fruits of caraway (Carum carvi l.). I. Developmental changes in the activities of three monoterpenoid biosynthetic enzymes. Plant Physiol. 1998;117:901–912. doi: 10.1104/pp.117.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwmeester HJ, Wallaart TE, Janssen MHA, Van Loo B, Jansen BJM, Posthumus MA, Schmidt CO, De Kraker J-W, König WA, Franssen MCR (1999) Amorpha-4,11-diene synthases catalyses the first probable step in artemisinin biosynthesis. Phytochemistry (in press) [DOI] [PubMed]
- Davis GD, Essenberg M. (+)-δ-Cadinene is a product of sesquiterpene cyclase activity in cotton. Phytochemistry. 1995;39:553–567. [Google Scholar]
- De Kraker JW, Bouwmeester HJ, Franssen MCR, König WA, De Groot Ae (+)-Germacrene A biosynthesis: the first committed step in the biosynthesis of bitter sesquiterpene lactones in chicory (Cichorium intybus L.) Plant Physiol. 1998;117:1381–1392. doi: 10.1104/pp.117.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicke M. Local and systemic production of volatile herbivore-induced terpenoids: their role in plant-carnivore mutualism. J Plant Physiol. 1994;143:465–472. [Google Scholar]
- Dicke M (1999) Evolution of induced indirect defence of plants. In R Tollrian, CD Harvell, eds, The Ecology and Evolution of Inducible Defenses. Princeton University Press, Princeton, pp 62–88
- Dicke M, Sabelis MW, Takabayashi J, Bruin J, Posthumus MA. Plant strategies of manipulating predator-prey interactions through allelochemicals: prospects for application in pest control. J Chem Ecol. 1990a;16:3091–3118. doi: 10.1007/BF00979614. [DOI] [PubMed] [Google Scholar]
- Dicke M, Van Beek TA, Posthumus MA, Ben Dom N, Van Bokhoven H, De Groot Ae Isolation and identification of volatile kairomone that effects acarine predator-prey interactions: involvement of host plant in its production. J Chem Ecol. 1990b;16:381–396. doi: 10.1007/BF01021772. [DOI] [PubMed] [Google Scholar]
- Donath J, Boland W. Biosynthesis of acyclic homoterpenes in higher plants parallels steroid hormone metabolism. J Plant Physiol. 1994;143:473–478. [Google Scholar]
- Donath J, Boland W. Biosynthesis of acyclic homoterpenes: enzyme selectivity and absolute configuration of the nerolidol precursor. Phytochemistry. 1995;39:785–790. [Google Scholar]
- Dudareva N, Cseke L, Blanc VM, Pichersky E. Evolution of floral scent in Clarkia: novel patterns of S-linalool synthase gene expression in the C. breweri flower. Plant Cell. 1996;8:1137–1148. doi: 10.1105/tpc.8.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk C, Lewinsohn E, Vogel BS, Steele CL, Croteau R. Regulation of oleoresinosis in grand fir (Abies grandis): coordinate induction of monoterpene and diterpene cyclases and two cytochrome P450-dependent diterpenoid hydroxylases by stem wounding. Plant Physiol. 1994;106:999–1005. doi: 10.1104/pp.106.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gäbler A, Boland W, Preiss H, Simon H. Stereochemical studies on homoterpene biosynthesis in higher plants: mechanistic, phylogenetic, and ecological aspects. Helv Chim Acta. 1991;74:1773–1789. [Google Scholar]
- Gershenzon J, Croteau R (1990) Regulation of monoterpene biosynthesis in higher plants. In GHN Towers, HA Stafford, eds, Recent Advances in Phytochemistry, Vol 24: Biochemistry of the Mevalonic Acid Pathway to Terpenoids. Plenum Press, New York, pp 99–160
- Kaiser R. Trapping, investigation and reconstitution of flower scents. In: Müller PM, Lamparsky D, editors. Perfumes: Art, Science and Technology. Essex, UK: Elsevier Science Publishers; 1991. pp. 213–250. [Google Scholar]
- Knudsen JT, Tollsten L, Bergström LG. Floral scents: a checklist of volatile compounds isolated by head-space techniques. Phytochemistry. 1993;33:253–280. [Google Scholar]
- Koepp AE, Hezari M, Zajicek J, Stofer-Vogel B, LaFever RE, Lewis NG, Croteau R. Cyclization of geranylgeranyl diphosphate to taxa-4(5),11(12)-diene is the committed step of taxol biosynthesis in Pacific yew. J Biol Chem. 1995;270:8686–8690. doi: 10.1074/jbc.270.15.8686. [DOI] [PubMed] [Google Scholar]
- König WA, Gehrcke B, Icheln D, Evers P, Dönnecke J, Wang W. New, selectively substituted cyclodextrins as stationary phases for the analysis of chiral constituents of essential oils. J High Resolut Chromatogr. 1992;15:367–372. [Google Scholar]
- König WA, Rieck A, Hardt I, Gehrcke B, Kubeczka K-H, Muhle H. Enantiomeric composition of the chiral constituents of essential oils. Part 2. Sesquiterpene hydrocarbons. J High Resolut Chromatogr. 1994;17:315–320. [Google Scholar]
- Krips OE, Willems PEL, Gols R, Posthumus MA, Dicke M (1999) The response of Phytoseiulus persimilis to spider-mite induced volatiles from gerbera: influence of starvation and experience. J Chem Ecol (in press) [DOI] [PubMed]
- Loughrin JH, Manukian A, Heath RR, Turlings TCJ, Tumlinson JH. Diurnal cycle of emission of induced volatile terpenoids by herbivore-injured cotton plants. Proc Natl Acad Sci USA. 1994;91:11836–11840. doi: 10.1073/pnas.91.25.11836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarvey DJ, Croteau R. Terpenoid metabolism. Plant Cell. 1995;7:1015–1026. doi: 10.1105/tpc.7.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaskill D, Croteau R. Prospects for bioengineering of isoprenoid biosynthesis. In: Berger RG, de Bont JAM, Cheetham PSJ, Croteau R, editors. Advances in Biochemical Engineering/Biotechnology. Berlin: Springer-Verlag; 1997. pp. 107–146. [DOI] [PubMed] [Google Scholar]
- Munck SL, Croteau R. Purification and characterization of the sesquiterpene cyclase patchoulol synthase from Pogostemon cablin. Arch Biochem Biophys. 1990;282:58–64. doi: 10.1016/0003-9861(90)90086-e. [DOI] [PubMed] [Google Scholar]
- Paré PW, Tumlinson JH. De novo biosynthesis of volatiles induced by insect herbivory in cotton plants. Plant Physiol. 1997;114:1161–1167. doi: 10.1104/pp.114.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichersky E, Raguso RA, Lewinsohn E, Croteau R. Floral scent production in Clarkia (Onagraceae). I. Localization and developmental modulation of monoterpene emission and linalool synthase activity. Plant Physiol. 1994;106:1533–1540. doi: 10.1104/pp.106.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röse USR, Manukian A, Heath RR, Tumlinson JH. Volatile semiochemicals released from undamaged cotton leaves. Plant Physiol. 1996;111:487–495. doi: 10.1104/pp.111.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulaev V, Silverman P, Raskin I. Airborne signalling by methyl salicylate in plant pathogen resistance. Nature. 1997;385:718–721. [Google Scholar]
- Takabayashi J, Dicke M, Posthumus MA. Volatile herbivore-induced terpenoids in plant-mite interactions: variation caused by biotic and abiotic factors. J Chem Ecol. 1994;20:1329–1354. doi: 10.1007/BF02059811. [DOI] [PubMed] [Google Scholar]
- Turlings TCJ, Loughrin JH, McCall PJ, Rose USR, Lewis WJ, Tumlinson JH. How caterpillar-damaged plants protect themselves by attracting parasitic wasps. Proc Natl Acad Sci USA. 1995;92:4169–4174. doi: 10.1073/pnas.92.10.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turlings TCJ, Tumlinson JH, Heath RR, Proveaux AT, Doolittle RE. Isolation and identification of allelochemicals that attract the larval parasitoid, Cotesia marginiventris (Cresson), to the microhabitat of one of its hosts. J Chem Ecol. 1991;17:2235–2251. doi: 10.1007/BF00988004. [DOI] [PubMed] [Google Scholar]
- Turlings TCJ, Tumlinson JH, Lewis WJ. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science. 1990;250:1251–1253. doi: 10.1126/science.250.4985.1251. [DOI] [PubMed] [Google Scholar]
- Vet LEM, Dicke M. The ecology of infochemical use by natural enemies of herbivores in a tritrophic context. Annu Rev Entomol. 1992;37:141–172. [Google Scholar]
- Vögeli U, Freeman JW, Chappell J. Purification and characterization of an inducible sesquiterpene cyclase from elicitor-treated tobacco cell suspension cultures. Plant Physiol. 1990;93:182–187. doi: 10.1104/pp.93.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Shah J, Klessig DF. Signal perception and transduction in plant defense responses. Genes Dev. 1997;11:1621–1639. doi: 10.1101/gad.11.13.1621. [DOI] [PubMed] [Google Scholar]
- Zook MN, Chappell J, Kuc JA. Characterization of elicitor-induction of sesquiterpene cyclase activity in potato tuber tissue. Phytochemistry. 1992;31:3441–3445. [Google Scholar]