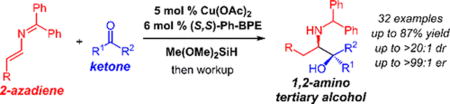
Abstract
We introduce a new strategy for synthesis of chiral amines: couplings of α-aminoalkyl nucleophiles generated by enantioselective migratory insertion of 2-azadienes to a Cu–H. In this report, we demonstrate its application in catalytic reductive coupling of 2-azadienes and ketones to furnish 1,2-amino tertiary alcohols with vicinal stereogenic centers.
Graphical abstract
New methods for the stereoselective synthesis of chiral amines are highly valuable as these units are found within numerous natural products, pharmaceuticals, ligands for metals, and fine chemicals. Classic C–C bond-forming approaches to chiral amines rely on nucleophilic addition to imines.1 Yet several classes of chiral amines, including 1,2-amino alcohols,2 are challenging to prepare by this normal polarity paradigm. A traditional reverse polarity strategy that addresses this issue utilizes nitroalkanes as a means of accessing N-substituted carbanions;3 however, this tactic requires subsequent nitro group reduction to form the amine, adversely affecting step/redox economy.4 A streamlined approach would enantioselectively assemble the desired amine building block via C–C bond formation with all atoms in the correct oxidation state.
Direct enantioselective α-lithiation of alkylamines for addition to electrophiles provides one path, but these methods rely on strong alkyllithium bases and often stoichiometric quantities of sparteine or its analogues.5 Catalytic formation of an α-aminoalkyl transition metal reagent (Scheme 1) is a powerful means of generating chiral amines with several established approaches. Deprotonation to form a 2-azaallyl anion and addition to a Pd catalyst6 or alternatively transmetalation of an α-amino zinc7 or α-amino borate8 to Ni or Pd has enabled aryl and alkyl cross-coupling reactions. Metal-catalyzed C–H functionalization at the N-α-position has also permitted aryl cross-coupling,9 allylic substitution reactions,10 or addition to olefins.11 Finally, catalytic formation of an α-amino radical, followed by recombination with a Ni or Fe catalyst, has allowed a variety of aryl, alkyl, or acyl cross-couplings,12 borylations,13 or conjugate additions to take place.14 In each approach, enantioselective reactions are uncommon.6b,7,10b,11b,c,12d
Scheme 1.
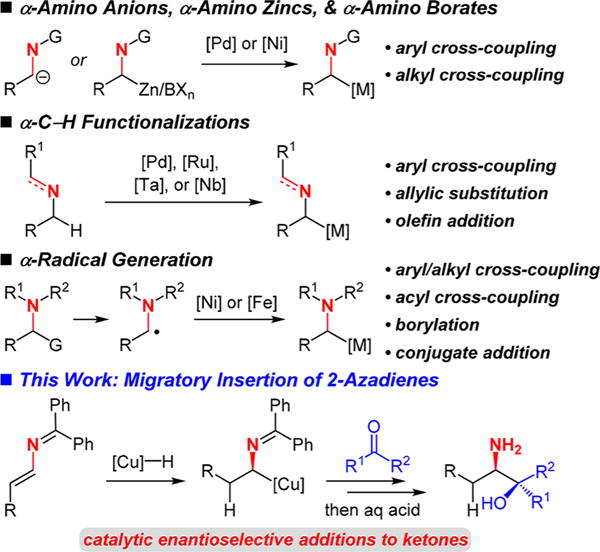
Methods and Uses for Catalytically-Generated α-Aminoalkyl-Substituted Transition Metals
In this work, we introduce a new strategy for catalytically forming an α-aminoalkyl transition metal for the enantioselective synthesis of amines. 2-Azadienes, which have rarely been used in synthesis,15 undergo migratory insertion at their least-hindered π-bond with a Cu–H to generate a 2-azaallyl-Cu intermediate, which may participate in stereoselective addition to a carbon electrophile. This reaction modality thus constitutes umpolung reactivity of an enamine. We demonstrate the feasibility of this approach in reductive coupling16–19 with ketones to furnish 1,2-amino tertiary alcohols in up to 87% yield, >20:1 dr, and >99:1 er.20–23
1,2-Amino tertiary alcohols are important building blocks for synthesis, but enantioselective construction of this functionality is all but unknown. Enantioselective Henry reactions with ketone electrophiles are few.24 The direct catalytic enantioselective synthesis of amino tertiary alcohols is limited.25,26 Furthermore, there are few examples where this functionality bears vicinal stereogenic centers. Typically this moiety is prepared by stepwise stereoselective addition of organometallics to α-amino acid derivatives.27
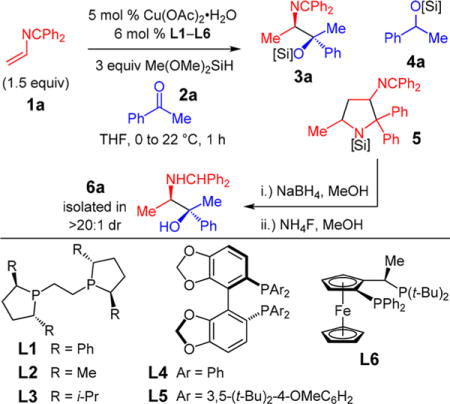
We envisioned that enantioselective Cu-catalyzed reductive coupling of 2-azadienes and ketones,16a,d followed by hydrolytic workup, would directly form a 1,2-amino tertiary alcohol (Scheme 1). We therefore began by examining the reaction of terminal azadiene 1a, acetophenone 2a, and a silane reducing agent (Table 1) and quickly identified Ph-BPE (L1) as uniquely effective at delivering desired product 3a (entry 1).16d Other ligands (entries 2–6) afford <2% 3a while generating significant amounts of ketone reduction product 4a and/or iminopyrrolidine 5, formed by reductive dimerization of 1a.28 In contrast, within 1.5 h L1 gives >98% conv to 3a (entry 7), which is formed in 4:1 dr as the major product (<10% ketone hydrosilylation). After imine reduction and ether desilylation (for ease of handling/assay purposes), the major diastereomer 6a is solely isolated in 73% yield and 99:1 er.29,30
Table 1.
Ligand Identification for Reductive Coupling of Acetophenone and 2-Azadiene 1aa
| |||||
|---|---|---|---|---|---|
|
| |||||
| entry | ligand | conv of 2a (%)b | 3a:4a:5c | dr of 3ac | er of 6ad |
| 1 | L1 | 82 (60)e | 9:1:0 | 5:1 | 99:1 |
| 2 | L2 | <2 | – | – | – |
| 3 | L3 | <2 | – | – | – |
| 4 | L4 | 60 | 0:6:1 | – | – |
| 5 | L5 | >98 | 0:1:0 | – | – |
| 6 | L6 | >96 | 0:1:0 | – | – |
| 7f | L1 | >98 (73)e | 11:1:0 | 4:1 | 99:1 |
Reaction under N2 with 0.1 mmol ketone 2a for 1 h.
Determined by 400 MHz 1H NMR spectroscopy according to remaining 2a in comparison to an internal standard.
Determined by 400 MHz 1H NMR spectroscopy of the unpurified mixture.
Determined by HPLC analysis of purified 6a.
Isolated yield of amino alcohol 6a (>20:1 dr).
Reaction with 0.2 mmol ketone 2a for 1.5 h. [Si] = Si(OMe)2Me.
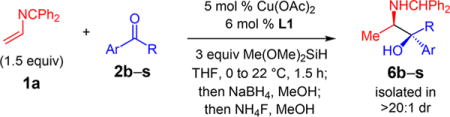
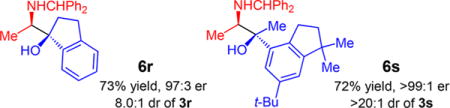
Several aryl/alkyl ketones undergo efficient reductive coupling with azadiene 1a under the optimized conditions (Table 2); the major diastereomer may be selectively isolated after the reductive/desilylative workup and chromatographic purification.29 A variety of substituents on the aromatic ring are tolerated (6b–k),31 including N-heterocycles (6d) and free hydroxyl (6g) functionality (entries 1–10). For aromatic rings bearing ortho groups (6h–i), diastereoselectivity is significantly higher. For example, 6h is formed as a single stereoisomer and 6i is generated in13:1 dr. Enantioselectivity is high in all cases (96.5:3.5 to >99:1 er) and the major product stereoisomer is isolated in 45–87% yield. Ketones containing aromatic heterocycles deliver amino alcohols 6l–n in good diastereoselectivity and excellent enantioselectivity (5–9:1 dr and 95:5 to >99:1 er, entries 11–13). Longer alkyl chains within the ketone (2o–p) generate amino alcohols with improved diastereoselectivity (8–10:1 dr, entries 14–15) and with high enantioselectivity. Diaryl ketones undergo efficient azadiene coupling but with poor diastereoselectivity. For example, fenofibrate adduct 6q is formed in only 1:1 dr. The isomers may be separately isolated; each is generated in 99:1 er (entry 16). 2-Indanone undergoes reductive coupling to form 6r in 73% yield, 8:1 dr, and 97:3 er. Azadiene addition to the fragrance celestolide delivers 6s as a single stereoisomer in 72% yield.
Table 2.
Ketone Variation for Cu-Catalyzed Enantioselective Reductive Couplings with Azadiene 1aa
| ||||
|---|---|---|---|---|
|
| ||||
| entry | product, Ar, R | dr of 3b | yield (%)c | er of 6d |
| 1e | 6b, 4-MeOC6H4, Me | 3.5:1 | 60 | 96.5:3.5 |
| 2 | 6c, 4-F3CC6H4, Me | 4.0:1 | 50 | >99:1 |
| 3 | 6d, 4-N-pyrazolylC6H4, Me | 4.0:1 | 62 | >99:1 |
| 4e | 6e, 3-BrC6H4, Me | 3.5:1 | 45 | >99:1 |
| 5e | 6f, 3-ClC6H4, Me | 4.5:1 | 57 | >99:1 |
| 6 | 6g, 3-HOC6H4, Me | 7.5:1 | 62 | >99:1 |
| 7e | 6h, 2-BrC6H4, Me | >20:1 | 77 | 99:1 |
| 8 | 6i, 2-MeOC6H4, Me | 13.0:1 | 87 | 97:3 |
| 9 | 6j, 2-napthyl, Me | 3.5:1 | 65 | 99:1 |
| 10 | 6k, 3,4-dioxolatoC6H3, Me | 5.5:1 | 61 | 98.5:1.5 |
| 11 | 6l, 2-furyl, Me | 5.0:1 | 55 | >99:1 |
| 12 | 6m, 3-thiophenyl, Me | 9.0:1 | 83 | 99:1 |
| 13 | 6n, 3-pyrrolyl(NTs), Me | 5.5:1 | 58 | 95:5 |
| 14 | 6o, Ph, Et | 10.0:1 | 67 | 99:1 |
| 15 | 6p, Ph, CH2CH2Ph | 8.0:1 | 63 | 98:2 |
| 16 | 6q, 4-ClC6H4, 4-(i-PrO2CCMe2O)C6H4 | 1.0:1 | 39, 37f | 99:1, 99:1g |
| ||||
Reaction with 0.2 mmol ketone 2.
Diastereomeric ratio of 3 determined by 400 MHz 1H NMR spectroscopy of the unpurified mixture prior to workup.
Isolated yield of purified 6 (>20:1 dr).
Enantiomeric ratio determined by HPLC analysis of 6.
Cu(OAc)2· H2O used.
Isolated yield of each diastereomer.
Enantiomeric ratio of each isomer.
A number of 4-alkyl-substituted 2-azadienes efficiently react with ketone 2i to afford α-alkyl chiral amines 7a–j as a single diastereomer in 43–59% yield (Table 3). The added steric hindrance imposed by the alkyl group necessitates a 12 h reaction time and leads to competitive ketone reduction, a pathway which is exacerbated with less-hindered ketones (e.g., acetophenone leads to >90% ketone reduction). A variety of functional groups are tolerated, such as thioether (entry 4), ether (entries 6–8), ester (entry 9), and halogen (entry 10).
Table 3.
Substituted 2-Azadienes for Enantioselective Additions to Ketonesa
| ||||
|---|---|---|---|---|
|
| ||||
| entry | product, R | dr of 3b | yield (%)c | erd |
| 1 | 7a, n-Bu | >20:1 | 43 | 96.5:3.5 |
| 2e | 7b, (CH2)2Ph | >20:1 | 54 | 98.5:1.5 |
| 3 | 7c, (CH2)2(3-thiophenyl) | >20:1 | 52 | >99:1 |
| 4f | 7d, (CH2)2SMe | >20:1 | 52 | 98.5:1.5 |
| 5 | 7e, (CH2)3Ph | >20:1 | 45 | 98:2 |
| 6 | 7f, (CH2)3OBn | >20:1 | 47 | 98:2 |
| 7 | 7g, (CH2)3OPh | >20:1 | 59 | 99:1 |
| 8 | 7h, (CH2)3OTBS | >20:1 | 45 | 98.5:1.5 |
| 9 | 7i, (CH2)4OBz | >20:1 | 46 | 99:1 |
| 10 | 7j, (CH2)4C1 | >20:1 | 48 | 98.5:1.5 |
Reaction of (E)-azadiene 1 unless otherwise noted.
Determined by 400 MHz 1H NMR spectroscopy of the unpurified mixture prior to workup.
Isolated yield of purified 7.
Enantiomeric ratio determined by HPLC analysis of 7.
(E)- and (Z)-azadienes 1c deliver identical results.
From (Z)-1e.
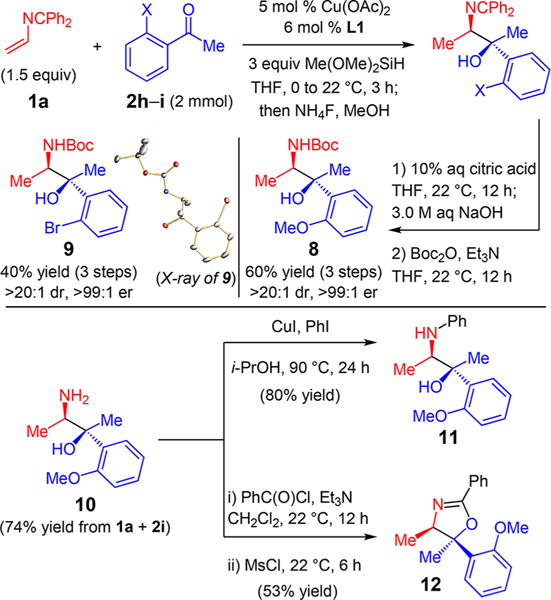
Carbamates 8–9 (Scheme 2) may be obtained by the sequential reductive coupling of azadiene 1a and ketones 2h–i with desilylative workup, imine hydrolysis under mildly acidic conditions,27 and Boc protection of the resulting primary amine (40–60% overall yield for the three-step sequence). The stereochemistry of the major isomer of 9 has been assigned as (R) at the amino center and (S) at the hydroxyl center. The free amine (10) may also be utilized for C–N cross-coupling reactions such as the Ullman coupling to generate aniline 11. The amine and hydroxyl group can instead both be engaged to form heterocycles such as oxazoline 12.
Scheme 2.
Derivatization of Coupled Products
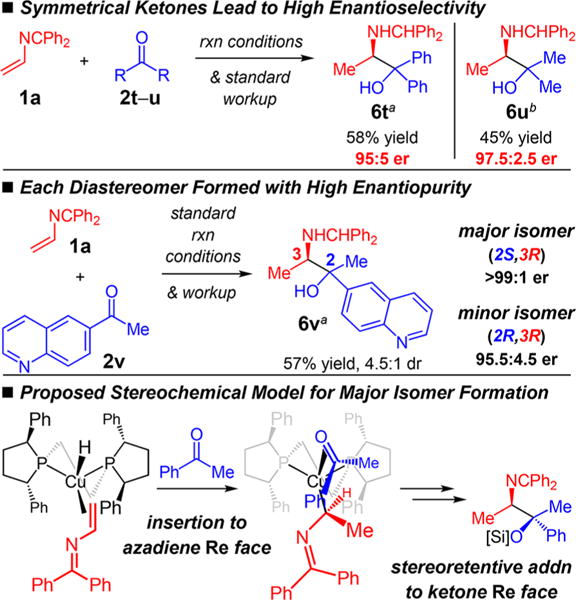
Although we have been able in most cases to separate the two product diastereomers, we have not successfully isolated the minor stereoisomer of aryl/alkyl ketone addition in order to secure its stereochemical assignment. Experiments with symmetrical ketones (Scheme 3), however, suggest that the minor isomer differs in its stereochemistry at the hydroxyl-containing center. Both benzophenone and acetone32 undergo coupling with 1a with significantly higher enantioselectivity (6t–u formed in 95:5 to 97.5:2.5 er) than the diastereoselectivity observed in most other reactions (Tables 1–2). Additionally, unlike for 6q, where each diastereomer is formed in equal enantiopurity, in the case of 6v (Scheme 3), the major (2S,3R)-diastereomer is formed in >99:1 er but the minor, likely (2R,3R)-isomer, is furnished in only 95.5:4.5 er.
Scheme 3.
Implications for Stereochemistry of the Minor Diastereomer and Stereochemical Model
aStandard catalysis conditions; see Table 2. b3.0 equiv acetone, 5.0 equiv silane, 5 mol % Cu(OAc)2, 6 mol % L1, THF, 22 °C, 1 h.
Based on the available data, a working model that accounts for the stereochemical outcome of the azadiene/ketone couplings is proposed in Scheme 3. Coordination of azadienes to [(S,S)-Ph-BPE]Cu–H occurs to place the benzophenone imine portion in the least hindered quadrant, leading to insertion into the Re-face, consistent with previous models.16d,e Stereorententive addition of the alkyl–Cu to the ketone’s Re-face delivers the major stereoisomer. The minor isomer arises from addition to the ketone’s Si-face, and all other stereoisomers are generated by stereoinvertive alkyl–Cu addition.
2-Azadienes are a promising class of reagents for preparation of chiral amines. Here, through reductive coupling with ketones, they have enabled catalytic enantioselective construction of 1,2-amino tertiary alcohols that have previously been inaccessible. Application of 2-azadienes for preparing other challenging amine scaffolds is underway.
Supplementary Material
Acknowledgments
We thank the NIH (GM124286), ACS Petroleum Research Fund (56575-DNI1), and Duke University for financial support. K. L. is grateful to the Duke Chemistry Department for a Burroughs-Welcome Fellowship. We thank Dr. Roger Sommer (NC State) for X-ray crystallographic analysis.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.7b12213.
Experimental procedures (PDF)
Analytical data for new compounds (PDF)
X-ray crystallographic data (CIF)
ORCID
Steven J. Malcolmson: 0000-0003-3229-0949
Notes
The authors declare no competing financial interest.
References
- 1.Kobayashi S, Mori Y, Fossey JS, Salter MM. Chem Rev. 2011;111:2626. doi: 10.1021/cr100204f. [DOI] [PubMed] [Google Scholar]
- 2.For reviews on preparing 1,2-amino alcohols, see:; (a) Bergmeier SC. Tetrahedron. 2000;56:2561. [Google Scholar]; (b) Burchak ON, Py S. Tetrahedron. 2009;65:7333. [Google Scholar]; (c) Karjalainen OK, Koskinen AMP. Org Biomol Chem. 2012;10:4311. doi: 10.1039/c2ob25357g. [DOI] [PubMed] [Google Scholar]
- 3.For reviews, see:; (a) Palomo C, Oiarbide M, Laso A. Eur J Org Chem. 2007;2007:2561. [Google Scholar]; (b) Blay G, Hernández-Olmos V, Pedro JR. Synlett. 2011;2011:1195. [Google Scholar]; (c) Matsunaga S, Shibasaki M. Chem Commun. 2014;50:1044. doi: 10.1039/c3cc47587e. [DOI] [PubMed] [Google Scholar]
- 4.Burns NZ, Baran PS, Hoffmann RW. Angew Chem, Int Ed. 2009;48:2854. doi: 10.1002/anie.200806086. [DOI] [PubMed] [Google Scholar]
- 5.For reviews, see:; (a) Beak P, Basu A, Gallagher DJ, Park YS, Thayumanavan S. Acc Chem Res. 1996;29:552. [Google Scholar]; (b) O’Brien P. Chem Commun. 2008;44:655. doi: 10.1039/b711420f. [DOI] [PubMed] [Google Scholar]
- 6.(a) Li M, Yücel B, Adrio J, Bellomo A, Walsh PJ. Chem Sci. 2014;5:2383. doi: 10.1039/C3SC53526F. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhu Y, Buchwald SL. J Am Chem Soc. 2014;136:4500. doi: 10.1021/ja501560x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Li M, Berritt S, Walsh PJ. Org Lett. 2014;16:4312. doi: 10.1021/ol502043j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Campos KR, Klapars A, Waldman JH, Dormer PG, Chen C-y. J Am Chem Soc. 2006;128:3538. doi: 10.1021/ja0605265. [DOI] [PubMed] [Google Scholar]; (b) Cordier CJ, Lundgren RJ, Fu GC. J Am Chem Soc. 2013;135:10946. doi: 10.1021/ja4054114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Molander GA, Hiebel MA. Org Lett. 2010;12:4876. doi: 10.1021/ol102039c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Awano T, Ohmura T, Suginome M. J Am Chem Soc. 2011;133:20738. doi: 10.1021/ja210025q. [DOI] [PubMed] [Google Scholar]; (c) Hong K, Morken JP. J Am Chem Soc. 2013;135:9252. doi: 10.1021/ja402569j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Pastine SJ, Gribkov DV, Sames D. J Am Chem Soc. 2006;128:14220. doi: 10.1021/ja064481j. [DOI] [PubMed] [Google Scholar]; (b) Spangler JE, Kobayashi Y, Verma P, Wang DH, Yu JQ. J Am Chem Soc. 2015;137:11876. doi: 10.1021/jacs.5b06740. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Li M, González-Esguevillas M, Berritt S, Yang X, Bellomo A, Walsh PJ. Angew Chem, Int Ed. 2016;55:2825. doi: 10.1002/anie.201509757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Trost BM, Mahapatra S, Hansen M. Angew Chem, Int Ed. 2015;54:6032. doi: 10.1002/anie.201501322. [DOI] [PubMed] [Google Scholar]; (b) Trost BM, Li X. Chem Sci. 2017;8:6815. doi: 10.1039/c7sc02899g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Herzon SB, Hartwig JF. J Am Chem Soc. 2008;130:14940. doi: 10.1021/ja806367e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Eisenberger P, Ayinla RO, Lauzon JMP, Schafer LL. Angew Chem, Int Ed. 2009;48:8361. doi: 10.1002/anie.200903656. [DOI] [PubMed] [Google Scholar]; (c) Reznichenko AL, Hultzsch KC. J Am Chem Soc. 2012;134:3300. doi: 10.1021/ja211945m. [DOI] [PubMed] [Google Scholar]
- 12.(a) Zuo Z, Ahneman DT, Chu L, Terrett JA, Doyle AG, MacMillan DWC. Science. 2014;345:437. doi: 10.1126/science.1255525. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) El Khatib M, Serafim RAM, Molander GA. Angew Chem, Int Ed. 2016;55:254. doi: 10.1002/anie.201506147. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Joe CL, Doyle AG. Angew Chem, Int Ed. 2016;55:4040. doi: 10.1002/anie.201511438. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Zuo Z, Cong H, Li W, Choi J, Fu GC, MacMillan DWC. J Am Chem Soc. 2016;138:1832. doi: 10.1021/jacs.5b13211. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Qin T, Cornella J, Li C, Malins LR, Edwards JT, Kawamura S, Maxwell BD, Eastgate MD, Baran PS. Science. 2016;352:801. doi: 10.1126/science.aaf6123. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Johnston CP, Smith RT, Allmendinger S, MacMillan DWC. Nature. 2016;536:322. doi: 10.1038/nature19056. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) McCarver SJ, Qiao JX, Carpenter J, Borzilleri RM, Poss MA, Eastgate MD, Miller MM, MacMillan DWC. Angew Chem, Int Ed. 2017;56:728. doi: 10.1002/anie.201608207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li C, Wang J, Barton LM, Yu S, Tian M, Peters DS, Kumar M, Yu AW, Johnson KA, Chatterjee AK, Yan M, Baran PS. Science. 2017;356 doi: 10.1126/science.aam7355. No. eaam7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin T, Malins LR, Edwards JT, Merchant RR, Novak AJE, Zhong JZ, Mills RB, Yan M, Yuan C, Eastgate MD, Baran PS. Angew Chem, Int Ed. 2017;56:260. doi: 10.1002/anie.201609662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.(a) Govindan CK, Taylor G. J Org Chem. 1983;48:5348. [Google Scholar]; (b) Movassaghi M, Hill MD. J Am Chem Soc. 2006;128:4592. doi: 10.1021/ja060626a. [DOI] [PubMed] [Google Scholar]; (c) Leijendekker LH, Weweler J, Leuther TM, Streuff J. Angew Chem, Int Ed. 2017;56:6103. doi: 10.1002/anie.201702310. [DOI] [PubMed] [Google Scholar]
- 16.For pioneering examples of enantioselective Cu-catalyzed reductive C–C couplings, see:; (a) Saxena A, Choi B, Lam HW. J Am Chem Soc. 2012;134:8428. doi: 10.1021/ja3036916. [DOI] [PubMed] [Google Scholar]; (b) Wang YM, Buchwald SL. J Am Chem Soc. 2016;138:5024. doi: 10.1021/jacs.6b02527. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Bandar JS, Ascic E, Buchwald SL. J Am Chem Soc. 2016;138:5821. doi: 10.1021/jacs.6b03086. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Yang Y, Perry IB, Lu G, Liu P, Buchwald SL. Science. 2016;353:144. doi: 10.1126/science.aaf7720. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Yang Y, Perry IB, Buchwald SL. J Am Chem Soc. 2016;138:9787. doi: 10.1021/jacs.6b06299. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Han JT, Jang WJ, Yun J, Kim N. J Am Chem Soc. 2016;138:15146. doi: 10.1021/jacs.6b11229. [DOI] [PubMed] [Google Scholar]; (g) Lee J, Torker S, Hoveyda AH. Angew Chem, Int Ed. 2017;56:821. doi: 10.1002/anie.201611444. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Zhou Y, Bandar JS, Buchwald SL. J Am Chem Soc. 2017;139:8126. doi: 10.1021/jacs.7b04937. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Gui YY, Hu N, Chen XW, Liao LL, Ju T, Ye JH, Zhang Z, Li J, Yu DG. J Am Chem Soc. 2017;139:17011. doi: 10.1021/jacs.7b10149. [DOI] [PubMed] [Google Scholar]
- 17.For Cu–H reviews, see:; (a) Rendler S, Oestreich M. Angew Chem, Int Ed. 2007;46:498. doi: 10.1002/anie.200602668. [DOI] [PubMed] [Google Scholar]; (b) Lipshutz BH. Synlett. 2009;2009:509. [Google Scholar]; (c) Jordan AJ, Lalic G, Sadighi JP. Chem Rev. 2016;116:8318. doi: 10.1021/acs.chemrev.6b00366. [DOI] [PubMed] [Google Scholar]
- 18.For examples of 1,3-diene/aldehyde reductive couplings, see:; (a) Zbieg JR, Yamaguchi E, McInturff EL, Krische M. Science. 2012;336:324. doi: 10.1126/science.1219274. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) McInturff EL, Yamaguchi E, Krische MJ. J Am Chem Soc. 2012;134:20628. doi: 10.1021/ja311208a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.For reviews of enantioselective reductive C–C couplings, see:; (a) Montgomery J. Angew Chem, Int Ed. 2004;43:3890. doi: 10.1002/anie.200300634. [DOI] [PubMed] [Google Scholar]; (b) Bower JF, Kim IS, Patman RL, Krische MJ. Angew Chem, Int Ed. 2009;48:34. doi: 10.1002/anie.200802938. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Standley EA, Tasker SZ, Jensen KL, Jamison TF. Acc Chem Res. 2015;48:1503. doi: 10.1021/acs.accounts.5b00064. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Nguyen KD, Park BY, Luong T, Sato H, Garza VJ, Krische MJ. Science. 2016;354 doi: 10.1126/science.aah5133. No. aah5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.For a review on enantioselective Cu-catalyzed synthesis of tertiary alcohols, see:; Shibasaki M, Kanai M. Chem Rev. 2008;108:2853. doi: 10.1021/cr078340r. [DOI] [PubMed] [Google Scholar]
- 21.For enantioselective aldehyde/ketone reductive couplings, see:; (a) Horwitz MA, Tanaka N, Yokosaka T, Uraguchi D, Johnson JS, Ooi T. Chem Sci. 2015;6:6086. doi: 10.1039/c5sc02170g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Horwitz MA, Zavesky BP, Martinez-Alvarado JI, Johnson JS. Org Lett. 2016;18:36. doi: 10.1021/acs.orglett.5b03127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.For enantioselective aldehyde/ketone cross-benzoin reactions, see:; (a) Goodman CG, Johnson JS. J Am Chem Soc. 2014;136:14698. doi: 10.1021/ja508521a. [DOI] [PMC free article] [PubMed] [Google Scholar]; For enantioselective cross-aza-benzoin reactions, see:; (b) DiRocco DA, Rovis T. Angew Chem, Int Ed. 2012;51:5904. doi: 10.1002/anie.201202442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murray SA, Green JC, Tailor SB, Meek SJ. Angew Chem, Int Ed. 2016;55:9065. doi: 10.1002/anie.201603465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.(a) Christensen C, Juhl K, Hazell RG, Jørgensen KA. J Org Chem. 2002;67:4875. doi: 10.1021/jo025690z. [DOI] [PubMed] [Google Scholar]; (b) Tosaki S-y, Hara K, Gnanadesikan V, Morimoto H, Harada S, Sugita M, Yamagiwa N, Matsunaga S, Shibasaki M. J Am Chem Soc. 2006;128:11776. doi: 10.1021/ja064858l. [DOI] [PubMed] [Google Scholar]
- 25.(a) Silverio DL, Fu P, Carswell EL, Snapper ML, Hoveyda AH. Tetrahedron Lett. 2015;56:3489. doi: 10.1016/j.tetlet.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wang C, Qin J, Shen X, Riedel R, Harms K, Meggers E. Angew Chem, Int Ed. 2016;55:685. doi: 10.1002/anie.201509524. [DOI] [PubMed] [Google Scholar]
- 26.For non-enantioselective allenamide/aldehyde reductive coupling to generate 1,2-amino secondary alcohols, see:; Skucas E, Zbieg JR, Krische MJ. J Am Chem Soc. 2009;131:5054. doi: 10.1021/ja900827p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ooi T, Takeuchi M, Kato D, Uematsu Y, Tayama E, Sakai D, Maruoka K. J Am Chem Soc. 2005;127:5073. doi: 10.1021/ja0459328. [DOI] [PubMed] [Google Scholar]
- 28.For additional ligand and other screening results, see the Supporting Information.
- 29.The minor diastereomer is removed by chromatography and its fate is unclear at this time.
- 30.t-BuOH addition increases the quantity of 4a relative to 3a.
- 31.Ketones 2c and 2e undergo a more competitive reduction compared to C–C bond formation, which adversely affects yield.
- 32.>90% ketone reduction is observed with other dialkyl ketones.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


