Abstract
Objectives
Previous research has reported associations between social relationships and carcinogenesis. Inflammation is a potential mediator of these associations. To clarify these links for one tumor site, we examined associations between social relationships, circulating inflammation markers, and breast cancer incidence.
Materials and Methods
Among 132,262 participants from the prospective Women’s Health Initiative, we used linear and logistic regression to evaluate associations between social relationship characteristics (social support, social strain, social network size) and inflammation markers of C-reactive protein (CRP) and white blood cell count (WBC). Cox regression was used to evaluate associations between inflammation markers and breast cancer incidence, as well as associations between social relationship characteristics and breast cancer incidence with and without adjustment for inflammation markers.
Results
Larger social networks were associated with lower continuous CRP (beta= −0.22, 95% CI −0.36, −0.08) and WBC (beta= −0.23, 95% CI −0.31, −0.16). Greater social strain was associated with higher continuous CRP (beta=0.24, 95% CI 0.14, 0.33) and WBC (beta=0.09, 95% CI 0.04, 0.14). When WBC was dichotomized at 10,000 cells/uL, high WBC was associated with greater hazards of in situ breast cancer (HR=1.65, 95% CI 1.17, 2.33) but not invasive breast cancer. Social relationship characteristics were not associated with incidence of invasive or in situ breast cancer.
Conclusion
Larger social networks were associated with lower inflammation and greater social strain was associated with higher inflammation. Higher inflammation might be associated with development of in situ breast cancer, but this appeared to be due to factors other than social relationships.
Keywords: Social relationship characteristics, inflammation, incidence, breast cancer, etiology, mediation
1. INTRODUCTION
Social gradients in health and illness have been widely documented [1]. Recent research in the social epidemiology of chronic disease has increasingly linked characteristics of social relationships, such as social networks and social strain, to cancer outcomes including quality of life [2] and survival [3–7]. Relatively little research has examined associations between social relationships and cancer incidence, although one study reported no association between caregiving stress and breast cancer incidence [8], while work on the related topic of job stress and risk of cancer has found inconsistent results [9–11]. Moreover, critical gaps remain in our understanding of the mechanisms underlying links between social relationships and cancer. Social relationships have been linked to inflammation [12,13], which is a potential mediator of associations between social relationships and cancer, providing one possible mechanism through which social interactions might “get under the skin” to influence health.
Social isolation, lack of social support, and high social strain have each been associated with higher systemic, low-grade, chronic inflammation [14–16]. Inflammation is also one major indicator of innate immunity and physiological stress response in the pathways to cancer [17]. In turn, chronic inflammation can contribute to different stages of carcinogenesis, including tumor initiation [18].
We evaluated the potential role of inflammation markers as mediators of associations between social relationships and breast cancer incidence in the Women’s Health Initiative (WHI). Breast cancer is an important tumor site in which to investigate these kinds of associations because of its high incidence and mortality, with over 250,000 new cases and 40,000 deaths expected in the United States in 2017 [19]. Previous WHI work has evaluated associations between characteristics of social relationships and breast cancer, but has not evaluated the role of inflammation [4,20,21]. We hypothesized that smaller social networks, lower social support, and higher social strain would each be associated with higher circulating concentrations of inflammation markers, that higher inflammation would be associated with greater hazards of subsequent diagnosis with breast cancer, and that associations between social relationships and breast cancer incidence would be attenuated after adjusting for inflammation markers.
2. MATERIALS AND METHODS
2.1 Study Population
WHI has been described previously [22]. Briefly, WHI is a large longitudinal study of United States women’s health (n=161,808) including Observational Study (OS; n=93,676) and Clinical Trial (CT; n=68,132) cohorts (CT registration identification number NCT00000611). Women aged 50–79 at baseline were enrolled during 1993–98. Those ineligible for the CT, typically due to prior health conditions or unwillingness to participate in a trial, were offered the opportunity to participate in the OS.
Starting from the overall WHI sample of 161,808, we applied the following exclusions sequentially: 1) self-reported history at baseline of any cancer except non-melanoma skin cancer (16,255 excluded), and 2) CT participants assigned to receive a hormone therapy intervention of either unopposed estrogen or a combination of estrogen and progesterone (13,291 excluded). CT participants assigned as controls in hormone therapy trials were not excluded. The final study sample for this analysis was 132,262 participants.
Procedures to ascertain incident breast cancer cases during the WHI observation period have been described [23,24]. Briefly, documents such as operative or oncology consultation reports were sent from the diagnosing clinic to the central WHI Clinical Coordinating Center, where trained coders working under the supervision of a physician and epidemiologist reviewed and coded the diagnostic information according to Surveillance, Epidemiology, and End Results Program coding guidelines [23]. Each participant was categorized as a case or non-case, with cases further subdivided into invasive and in situ cases.
2.2 Measures of Social Relationship Characteristics
Social relationship characteristics included social network size, social support, and social strain as assessed by self-report at baseline. We measured social network size on a scale of 0–3, the sum of three dichotomous indicators (0=no, 1=yes) for marital status, religious attendance in the past month, and social club or group attendance in the past month. Marital status was coded as “yes” if the participant indicated being presently married or in a marriage-like relationship, and “no” if widowed, divorced, separated, or never married. Social support was based on a previously validated measure rescaled to a range of 0–9, the sum of nine dichotomous indicators (0=no, 1=yes) for the availability of someone for the participant to talk to in various circumstances, for example, when she needed someone to listen or give good advice [25]. Social strain was based on a previously validated measure rescaled to a range of 0–4, the sum of four dichotomous indicators (0=no, 1=yes) for the presence of other people in the participant’s life who got on her nerves, asked too much, excluded her, or asked her to do things she did not want to do [26].
2.3 Inflammation Markers
Blood concentrations of inflammation markers were measured at baseline as continuous variables. High-sensitivity C-reactive protein (CRP; units: mg/L) was measured at the University of Minnesota (Minneapolis, MN) using an immunoturbidimetric assay on a Roche/Hitachi Modular P Chemistry Analyzer (Roche Diagnostics, Indianapolis, IN). Total white blood cell count (WBC; units: thousands of cells/uL) was measured using automated clinical hematology cell counters following standardized quality assurance procedures. Among the 132,262 participants eligible for this analysis, CRP was measured in 14,375 participants (11%) and WBC in 130,844 (99%).
2.4 Covariates
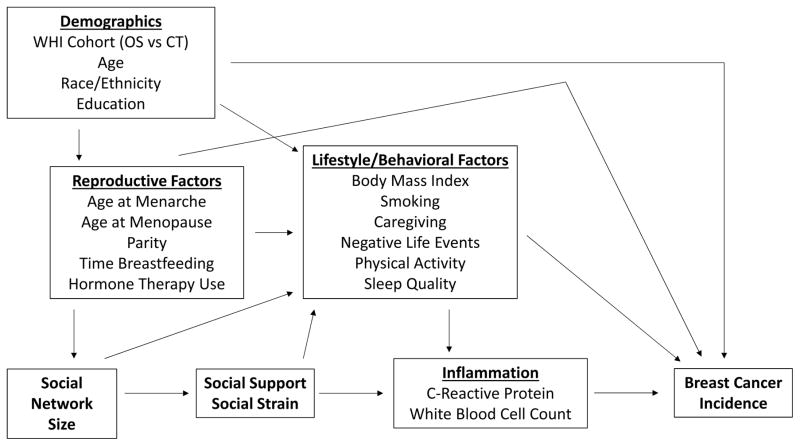
Based on the Berkman-Glass conceptual model of social networks on health outcomes [27], we created a directed acyclic graph (Figure 1) to identify potential sources of confounding of the associations of interest [28]. We identified three clusters of covariates: 1) demographic factors, including age (continuous), race (non-Hispanic white, other), education (0–12, 13+ years in school), and WHI enrollment (OS, CT); 2) reproductive factors, including hormone therapy use (ever, never), age at menarche (9 or less, 10, 11, 12, 13, 14, 15, 16, 17+), parity (0, 1, 2, 3, 4, 5+ term pregnancies), months breastfed (never, 1–6, 7–12, 13–23, 24+), and age at menopause (continuous); and 3) lifestyle and behavioral factors, including body mass index (continuous), smoking status (current, former, or never), caregiving (times a week: 0, <1, 1–2, 3–4, 5+), number of negative life events (0–11), physical activity (any, none), and level of sleep disturbance (0–20). Measurements of all covariates were taken at baseline.
Figure 1.
Directed acyclic graph of social relationship characteristics, circulating inflammation markers, and breast cancer incidence (CT=Clinical Trial, OS=Observational Study)
2.5 Statistical Analysis
Two versions of the analysis were run, the first using continuous inflammation marker measurements, the second using dichotomous inflammation marker status to assess possible threshold effects. Using prior literature, we identified cut points to dichotomize continuous inflammation marker measurements into variables that distinguished lower from higher concentrations. We dichotomized CRP at 3 mg/L [29] and WBC at 10,000 cells/uL [30].
We used linear regression to estimate associations between social relationship characteristics and outcomes of continuous inflammation markers. Logistic regression was used for the analogous models with outcomes of dichotomous inflammation marker status. Based on the conceptual model depicted in Figure 1, we evaluated the following sets of models: 1) a single social variable at a time, 2) social support and social strain simultaneously, and 3) all three social variables simultaneously. In models evaluating associations between social relationship characteristics and inflammation, we adjusted for demographic and reproductive covariates.
Associations between inflammation and breast cancer incidence, as well as associations between social relationship characteristics and breast cancer incidence, were estimated using Cox proportional hazards models. Breast cancer incidence was defined as time from baseline to breast cancer diagnosis, censored at 10 years post-baseline. Separate Cox models were run for invasive and in situ cases, that is, invasive cases were excluded from models of in situ cancer and vice versa. Based on Figure 1, models of inflammation and breast cancer incidence, as well as models of social relationship characteristics and breast cancer incidence, were adjusted for demographic, reproductive, and lifestyle/behavioral covariates.
Several sensitivity analyses were performed: 1) for time-to-event models, censoring at 5 years post-baseline as well as use of all available observation time, 2) use of continuous natural logarithm–transformed CRP to evaluate the impact of skewness in the distribution of continuous CRP, 3) for models of social relationship characteristics and CRP, coding the CRP outcome as a 3-level variable (<3 mg/L, 3-<10 mg/L, and >=10 mg/L) and modeling using ordinal logistic regression because CRP levels of 3 mg/L and 10 mg/L might each be clinically-relevant cut points for degree of inflammatory disease [31], and 4) evaluation of associations restricted to the OS or CT, as opposed to all eligible WHI participants. For this last sensitivity analysis, interaction terms were constructed for continuous social relationship characteristics and cohort enrollment. Likelihood ratio tests of interaction terms were used to evaluate heterogeneity between the OS and CT.
We defined statistical significance as alpha=0.05. Missing data were handled in all models using the complete–case approach. Statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC). Every participant provided informed consent. The Institutional Review Board at the University of North Carolina at Chapel Hill approved the analysis.
3. RESULTS
Table 1 presents participant characteristics for invasive cases, in situ cases, and non-cases. Among the 132,262 participants, 6,583 incident cases of invasive breast cancer (5%) and 1,595 incident cases of in situ breast cancer (1%) were ascertained during up to 18.6 years of observation. Median time from baseline to breast cancer diagnosis was 5.9 years for invasive cases (range: 0–17.1 years) and 6.2 years for in situ cases (range: 0–17.9 years). Median observation time for non-cases was 14.0 years (range: 0–18.6 years). Participant characteristics and amounts of missing data were generally similar across invasive cases, in situ cases, and non-cases.
Table 1.
Characteristics of breast cancer incident cases and non-cases (N=132,262)
| Invasive Cases (n=6,583) | In Situ Cases (n=1,595) | Non-Cases (n=124,084) | ||||
|---|---|---|---|---|---|---|
| Characteristic | Mean (SD) or N (%)a | N (%) Missingb | Mean (SD) or N (%)a | N (%) Missingb | Mean (SD) or N (%)a | N (%) Missingb |
| Inflammation Markers | ||||||
| CRP (mg/L) | 5.64 (7.67) | 5,974 (91) | 5.12 (5.18) | 1,428 (90) | 5.35 (7.32) | 110,485 (89) |
| CRP>3mg/L | 323 (53%) | 5,974 (91) | 94 (56%) | 1,428 (90) | 6,902 (51%) | 110,485 (89) |
| WBC (thousand cells/uL) | 6.15 (8.46) | 72 (1) | 7.52 (31.09) | 18 (1) | 6.15 (11.53) | 1,328 (1) |
| WBC>10,000 cells/uL | 138 (2%) | 72 (1) | 48 (3%) | 18 (1) | 2,648 (2%) | 1,328 (1) |
| Social Relationships | ||||||
| Social Network Size (0–3) | 1.46 (0.88) | 356 (5) | 1.50 (0.89) | 91 (6) | 1.45 (0.89) | 6,327 (5) |
| Social Support (0–9) | 6.74 (2.80) | 125 (2) | 6.94 (2.72) | 37 (2) | 6.62 (2.89) | 3,096 (3) |
| Social Strain (0–4) | 1.50 (1.30) | 107 (2) | 1.44 (1.29) | 17 (1) | 1.57 (1.34) | 2,838 (2) |
| Demographics | ||||||
| Age (years) | 63.17 (6.94) | 0 (0) | 62.36 (6.93) | 0 (0) | 63.03 (7.22) | 0 (0) |
| Race | 10 (0) | 4 (0) | 310 (0) | |||
| American Indian/Alaskan Native | 18 (0%) | 3 (0%) | 543 (0%) | |||
| Asian/Pacific Islander | 137 (2%) | 38 (2%) | 3,463 (3%) | |||
| Black/African-American | 430 (7%) | 139 (9%) | 11,445 (9%) | |||
| Hispanic/Latino | 150 (2%) | 39 (2%) | 5,021 (4%) | |||
| White, not of Hispanic origin | 5,783 (88%) | 1,361 (86%) | 101,843 (82%) | |||
| Not one of above | 55 (1%) | 11 (1%) | 1,459 (1%) | |||
| Education | 47 (1) | 12 (1) | 921 (1) | |||
| 0–11 years | 208 (3%) | 46 (3%) | 6,380 (5%) | |||
| 12 years/GED | 971 (15%) | 250 (16%) | 21,069 (17%) | |||
| 13+ years | 5,357 (82%) | 1,287 (81%) | 95,714 (78%) | |||
| Enrolled in WHI OS | 3,965 (60%) | 0 (0) | 913 (57%) | 0 (0) | 75,971 (61%) | 0 (0) |
| Health, Lifestyle, & Behaviors | ||||||
| Smoking | 73 (1) | 15 (1) | 1,499 (1) | |||
| Never | 3,149 (48%) | 804 (51%) | 63,195 (52%) | |||
| Past | 2,959 (45%) | 701 (44%) | 51,222 (42%) | |||
| Current | 402 (6%) | 75 (5%) | 8,168 (7%) | |||
| Alcohol (not current drinker) | 1,672 (26%) | 42 (1) | 383 (24%) | 9 (1) | 36,509 (30%) | 800 (1) |
| Physical Activity (no activity) | 904 (14%) | 310 (5) | 240 (16%) | 79 (5) | 18,473 (16%) | 5,163 (4) |
| Body Mass Index (kg/m2) | 28.08 (5.79) | 55 (1) | 27.57 (5.73) | 10 (1) | 27.86 (5.92) | 1,128 (1) |
| Number of Chronic Illnessesc | 0.88 (0.93) | 0 (0) | 0.86 (0.91) | 0 (0) | 0.93 (0.98) | 0 (0) |
| Negative Life Events (0–11) | 1.60 (1.36) | 107 (2) | 1.57 (1.38) | 24 (2) | 1.70 (1.44) | 2,609 (2) |
| Weekly Care Giving (1+ times/week) | 1,917 (29%) | 37 (1) | 488 (31%) | 8 (1) | 38,099 (31%) | 999 (1) |
| Sleep Disturbance (0–20) | 6.50 (4.33) | 117 (2) | 6.38 (4.20) | 26 (2) | 6.59 (4.46) | 2,596 (2) |
| Reproductive History | ||||||
| Hormone Therapy Use | 7 (0) | 1 (0) | 113 (0) | |||
| Never | 2,418 (37%) | 511 (32%) | 51,561 (42%) | |||
| Past | 827 (13%) | 208 (13%) | 17,932 (14%) | |||
| Current | 3,331 (51%) | 875 (55%) | 54,478 (44%) | |||
| Age at Menarche (<=12 years old) | 3,267 (50%) | 16 (0) | 758 (48%) | 2 (0) | 59,264 (48%) | 322 (0) |
| Parity (no term pregnancies) | 900 (14%) | 41 (1) | 222 (14%) | 6 (0) | 14,503 (12%) | 626 (1) |
| Breastfeeding (<=6 months total) | 4,808 (74%) | 64 (1) | 1,159 (74%) | 20 (1) | 91,645 (75%) | 1,563 (1) |
| Age at Menopause (years) | 48.80 (6.05) | 300 (5) | 48.67 (6.34) | 62 (4) | 48.16 (6.37) | 6,482 (5) |
Table includes WHI participants with non-missing baseline data on history of breast cancer and having baseline data available for at least one inflammation marker. All characteristics were measured at baseline unless otherwise noted.
Means and percentages based on non-missing data for the respective breast cancer case status.
Denominator for percentage of data missing is number of participants with the respective breast cancer case status.
Number of chronic illnesses is sum of 13 dichotomous flags (0=never, 1=ever) for whether participant reported a history of each of angina, arthritis, asthma, emphysema, myocardial infarction, heart failure, cancer, broken hip, osteoporosis, broken back/spine, broken lower arm, stroke, or diabetes.
CRP=C-reactive protein, GED=General Educational Development, OS=Observational Study, WBC=white blood cell count
Table 2 presents associations between social relationship characteristics and outcomes of inflammation markers, with separate models of continuous and dichotomous inflammation markers. All estimates are per 1-unit change in the respective social relationship characteristic. For continuous inflammation marker outcomes, larger social networks were associated with lower concentrations of both CRP (beta= −0.22 mg/L, 95% CI −0.36, −0.08) and WBC (beta= −230 cells/uL, 95% CI −310, −160). Greater social strain was associated with higher concentrations of CRP (beta=0.24 mg/L, 95% CI 0.14, 0.33) and WBC (beta=90 cells/uL, 95% CI 40, 140). Greater social support was not associated with CRP concentration and correlated with a slightly lower WBC concentration (beta= −30 cells/uL, 95% CI −50, 0).
Table 2.
Associations between social relationship characteristics and inflammation markers
| Inflammation Marker Outcome | Social Relationship Characteristic | Dichotomous Marker | Continuous Marker | |||
|---|---|---|---|---|---|---|
| N | OR | 95% CI | Beta | 95% CI | ||
| C-Reactive Protein (mg/L) | Social Network Sizea | 12,291 | 0.96 | 0.92, 1.00 | −0.22 | −0.36, −0.08 |
| Social Supporta | 12,240 | 1.00 | 0.98, 1.01 | 0.00 | −0.04, 0.05 | |
| Social Straina | 12,135 | 1.04 | 1.01, 1.06 | 0.24 | 0.14, 0.33 | |
| Social Supportb | 11,802 | 1.00 | 0.99, 1.01 | 0.03 | −0.01, 0.08 | |
| Social Strainb | 1.03 | 1.00, 1.06 | 0.26 | 0.16, 0.36 | ||
| Social Network Sizec | 11,484 | 0.96 | 0.92, 1.00 | −0.24 | −0.40, −0.09 | |
| Social Supportc | 1.00 | 0.99, 1.01 | 0.06 | 0.01, 0.10 | ||
| Social Strainc | 1.03 | 1.00, 1.06 | 0.26 | 0.16, 0.36 | ||
| White Blood Cell Count (thousands of cells/uL) | Social Network Sizea | 116,444 | 0.78 | 0.75, 0.82 | −0.23 | −0.31, −0.16 |
| Social Supporta | 117,880 | 0.97 | 0.96, 0.99 | −0.03 | −0.05, 0.00 | |
| Social Straina | 118,215 | 1.06 | 1.03, 1.10 | 0.09 | 0.04, 0.14 | |
| Social Supportb | 115,926 | 0.98 | 0.96, 0.99 | −0.02 | −0.04, 0.01 | |
| Social Strainb | 1.04 | 1.01, 1.08 | 0.07 | 0.02, 0.12 | ||
| Social Network Sizec | 112,070 | 0.80 | 0.76, 0.84 | −0.21 | −0.29, −0.13 | |
| Social Supportc | 0.99 | 0.98, 1.01 | 0.00 | −0.03, 0.02 | ||
| Social Strainc | 1.05 | 1.02, 1.08 | 0.08 | 0.02, 0.13 | ||
Dependent variable was C-Reactive Protein or White Blood Cell Count. Two sets of models were run: 1) logistic regression models using dichotomous marker status as the dependent variable, 2) linear regression models using continuous marker measurements as the dependent variable. Dichotomous C-Reactive Protein was coded as >=3 mg/L vs <3 mg/L and dichotomous White Blood Cell Count was coded as >=10,000 cells/uL vs <10,000 cells/uL. For each model, the social relationship variable was categorical Social Network Size (0–3), Social Support (0–9), and/or Social Strain (0–4). All models adjusted for age, race/ethnicity, education, WHI cohort enrollment, age at menarche, age at menopause, parity, amount of time breastfeeding, and hormone therapy use. All variables were measured at baseline.
Only social relationship characteristic variable in model.
Model included both Social Support and Social Strain.
Model included Social Network Size, Social Support, and Social Strain
Table 3 presents associations between inflammation markers and an outcome of breast cancer incidence, with separate models of continuous and dichotomous inflammation markers. These estimates are per 1-unit change in the respective continuous inflammation marker. CRP concentration was not associated with incidence of either invasive or in situ breast cancer, regardless of whether CRP was modeled as continuous or dichotomous. Greater continuous WBC concentration was associated with greater hazards of both invasive and in situ breast cancer, but the magnitudes of these associations were negligible. For dichotomous WBC, compared to WBC less than 10,000 cells per uL, WBC greater than 10,000 cells per uL was associated with a 65% higher hazard of in situ breast cancer (HR=1.65, 95% CI 1.17, 2.33) but was not associated with incidence of invasive breast cancer (HR=1.06, 95% CI 0.87, 1.30).
Table 3.
Associations between inflammation markers and time to breast cancer diagnosis
| Dichotomous Marker | Continuous Marker | ||||||
|---|---|---|---|---|---|---|---|
| Breast Cancer Outcome | Inflammation Marker | N, Cases | N, Non-Cases | HR | 95% CI | HR | 95% CI |
| Invasive | C-Reactive Protein | 394 | 10,939 | 1.03 | 0.83, 1.27 | 1.00 | 0.99, 1.02 |
| White Blood Cell Count | 4,328 | 104,741 | 1.06 | 0.87, 1.30 | 1.00 | 1.00, 1.00 | |
| In Situ | C-Reactive Protein | 100 | 10,863 | 1.02 | 0.67, 1.55 | 0.99 | 0.96, 1.02 |
| White Blood Cell Count | 1,049 | 103,794 | 1.65 | 1.17, 2.33 | 1.00 | 1.00, 1.01 | |
Dependent variable was time from baseline to breast cancer diagnosis, censored at 10 years after baseline. Two sets of Cox models were run, one using dichotomous marker status as the inflammation marker independent variable and the second using continuous marker measurements as the inflammation marker independent variable. Dichotomous C-Reactive Protein was coded as >=3 mg/L vs <3 mg/L and dichotomous White Blood Cell Count was coded as >=10,000 cells/uL vs <10,000 cells/uL. Estimates of continuous markers are per 1 mg/L for CRP or per 1,000 cells/uL for WBC. All models adjusted for age, race/ethnicity, education, WHI cohort enrollment, age at menarche, age at menopause, parity, amount of time breastfeeding, hormone therapy use, body mass index, smoking, caregiving, negative life events, physical activity, and sleep quality. Models of invasive breast cancer excluded those who developed in situ breast cancer, and vice versa. Inflammation markers and confounders were measured at baseline.
Table 4 shows associations between social relationship characteristics and breast cancer incidence, with and without adjustment for a dichotomous inflammation marker. Estimates are per 1-unit change in the respective social relationship characteristic. Without adjusting for inflammation markers, the social variables were at most weakly correlated with incidence of either invasive or in situ breast cancer. Adjustment for dichotomous or continuous inflammation markers led to negligible changes in estimates (not shown for continuous inflammation markers).
Table 4.
Associations between social relationship characteristics and time to breast cancer diagnosis, with and without adjustment for inflammation markers
| Breast Cancer Outcome | Social Relationship Characteristic | Inflammation Marker Mediator | N Cases | N Non-Cases | HR | 95% CI |
|---|---|---|---|---|---|---|
| Invasive | Social Support | None | 4,318 | 103,807 | 1.00 | 0.99, 1.01 |
| Social Support | C-Reactive Protein | 388 | 10,635 | 0.98 | 0.95, 1.02 | |
| Social Support | White Blood Cell Count | 4,266 | 102,721 | 1.00 | 0.99, 1.01 | |
| Social Strain | None | 4,320 | 104,136 | 0.99 | 0.97, 1.02 | |
| Social Strain | C-Reactive Protein | 379 | 10,553 | 1.04 | 0.96, 1.12 | |
| Social Strain | White Blood Cell Count | 4,265 | 103,049 | 0.99 | 0.97, 1.02 | |
| Social Network Size | None | 4,358 | 105,189 | 0.99 | 0.96, 1.03 | |
| Social Network Size | C-Reactive Protein | 391 | 10,839 | 1.02 | 0.91, 1.14 | |
| Social Network Size | White Blood Cell Count | 4,303 | 104,092 | 0.99 | 0.96, 1.03 | |
| In Situ | Social Support | None | 1,038 | 102,869 | 1.03 | 1.00, 1.05 |
| Social Support | C-Reactive Protein | 98 | 10,562 | 1.01 | 0.94, 1.08 | |
| Social Support | White Blood Cell Count | 1,026 | 101,787 | 1.03 | 1.00, 1.05 | |
| Social Strain | None | 1,047 | 103,190 | 0.95 | 0.91, 1.00 | |
| Social Strain | C-Reactive Protein | 97 | 10,479 | 0.98 | 0.84, 1.14 | |
| Social Strain | White Blood Cell Count | 1,035 | 102,107 | 0.95 | 0.91, 1.00 | |
| Social Network Size | None | 1,057 | 104,240 | 1.06 | 0.99, 1.14 | |
| Social Network Size | C-Reactive Protein | 99 | 10,764 | 1.04 | 0.83, 1.30 | |
| Social Network Size | White Blood Cell Count | 1,045 | 103,147 | 1.06 | 0.99, 1.14 |
Dependent variable was time from baseline to breast cancer diagnosis, censored at 10 years after baseline. Social relationships were measured as categorical Social Support (0–9), Social Strain (0–4), or Social Network Size (0–3). All models adjusted for age, race/ethnicity, education, WHI cohort enrollment, age at menarche, age at menopause, parity, amount of time breastfeeding, hormone therapy use, body mass index, smoking, caregiving, negative life events, physical activity, and sleep quality. Models further adjusted for dichotomous inflammation marker included C-Reactive Protein (>=3 mg/L vs <3 mg/L) or White Blood Cell Count (>=10,000 cells/uL vs <10,000 cells/uL). Models of invasive breast cancer excluded those who developed in situ breast cancer, and vice versa. Social relationship characteristics, inflammation markers, and confounders were measured at baseline.
Regarding sensitivity analyses, time-to-event models were stable when varying the censoring time (not shown). Results for log-transformed continuous CRP were qualitatively similar to those for untransformed continuous CRP (not shown). In models of social relationships and CRP when CRP was modeled as a 3-category variable, results were virtually identical to those for dichotomous CRP (not shown). Confidence intervals for the OS and CT generally overlapped (Supplemental Tables 1–4 in Supplemental Results File), and likelihood ratio tests of heterogeneity between the OS and CT were not statistically significant.
4. DISCUSSION
In a large prospective study of postmenopausal women from the United States, we found evidence that social relationship characteristics were associated with circulating inflammation marker levels. Most notably, larger social networks were associated with lower inflammation, and greater social strain was associated with higher inflammation. Social relationship characteristics and inflammation markers were not associated with breast cancer incidence, except that women with elevated WBC had a greater hazard of in situ breast cancer. This suggests at most a minor role of social relationship characteristics in the development of breast cancer.
We were especially interested in the possibility that circulating inflammation markers might mediate associations between social relationships and breast cancer incidence. Such a finding would suggest a mechanism by which social relationships influence internal biology to affect a person’s risk of cancer. In our analysis, social relationship characteristics were not related to breast cancer incidence regardless of whether we adjusted for inflammation markers. Nevertheless, our positive findings for each link in the putative pathway—between social relationships and inflammation, and between inflammation and breast cancer incidence—suggest that further work in this area is warranted. For example, inflammation might mediate associations between social relationships and forms of cancer other than breast cancer, or for specific subtypes of breast cancer. Another possibility is that inflammation markers other than the ones we had available may mediate associations between social relationships and breast cancer incidence.
Associations sometimes differed depending on whether we modeled inflammation as continuous or dichotomous. For example, for models of WBC and in situ breast cancer, we found associations for both continuous and dichotomous WBC, but the magnitude of the association was larger for dichotomous WBC (Table 3). This finding highlighted the importance of considering the relationship between the biology of inflammation and the modeling of inflammation markers. Inflammation marker concentrations are naturally continuous. Modeling a continuous variable treats every 1-unit change as equivalent, but which concentrations of an inflammation marker are normal and which are pathological might involve threshold effects [32]. Categorical or dichotomous variables permit evaluation of threshold effects, though at the cost of lower statistical precision and coarsened measures compared to continuous measurements. These trade-offs and our results suggest the value of evaluating inflammation markers as both continuous and categorical variables.
Our analysis had several strengths. It included a large prospective study in which inflammation markers were measured in thousands of individuals and several thousand incident breast cancer cases were observed during the follow-up period. The analysis was designed based on a well-established conceptual model of how social networks impact health [27]. Based on the conceptual model, we created a directed acyclic graph that guided identification of appropriate adjustment sets to control for confounding [28].
Regarding limitations of the analysis, first, while WBC was measured in nearly all WHI participants, CRP was measured in far fewer participants, thereby reducing the precision of those models. Second, as suggested earlier, the number of available inflammation markers was limited. Finally, our measure of social network size was motivated by the Social Network Index (SNI), which incorporates information on a large number of factors such as marital status, religious attendance, number of living children, and frequency of contacts with each component of the social network [33]. Not all of the information used in the SNI was available in WHI, making it impossible to construct the validated measure. Thus, while our measure of social network size is similar, it should not be considered equivalent to the SNI. In addition, the scale of our measure of social network size (0–3) was coarser than the scale of the SNI (0–12), meaning our measure had relatively lower variability and therefore lower power to detect effects. However, previous studies of social network size have used similar procedures to ours [34,35], and those studies and ours detected notable associations involving social network size. This suggests that the lower variability in our measure compared to the original scale of the SNI probably did not qualitatively influence our findings.
In sum, social relationship characteristics were related to inflammation levels, but appeared to have little influence on development of breast cancer. Our findings are consistent with prior work on social relationship characteristics and inflammation [12,13], though the present study had a much larger sample size and placed the findings in the context of subsequent development of a specific form of cancer.
Future research should examine the relative importance of social relationships and inflammation markers for specific breast cancer subtypes (e.g. luminal A, basal-like) and at different points along the cancer trajectory (e.g. incidence, survival, recurrence). Such research would contribute to our understanding of the links between social relationships, inflammation, and cancer, and thereby clarify whether or how encouraging healthy improvements in social relationships can contribute to improving cancer prevention and cancer outcomes.
Supplementary Material
Tables of associations between social relationship characteristics, inflammation markers, and breast cancer incidence stratified by Women’s Health Initiative cohort (Observational Study or Clinical Trial).
Highlights.
Social relationships and breast cancer have been associated
The potential role of inflammation as a mediator of these associations was evaluated
Larger social networks were associated with lower inflammation
Greater social strain was associated with higher inflammation
Greater inflammation was associated with greater hazards of in situ breast cancer
Acknowledgments
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller
Clinical Coordinating Center: Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg
Investigators and Academic Centers: (Brigham and Women's Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker
Women’s Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker
FUNDING SOURCES
The WHI program was supported by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services [HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C]. ELB was supported by the National Cancer Institute (5T32CA009001). CHK was supported by the National Cancer Institute (K07 CA187403). YCY was supported by the National Institute of Aging (K01AG036745–01) and the UNC-Chapel Hill University Cancer Research Fund. The Carolina Population Center at UNC-Chapel Hill provided general research support.
None of the funding sources had any role in study design; collection, analysis, and interpretation of data; in the writing of the report; nor in the decisions to submit the article for publication.
ABBREVIATIONS
| Abbreviation | Definition |
|---|---|
| CRP | C-Reactive Protein |
| CT | Clinical Trial |
| OS | Observational Study |
| WBC | White Blood Cell Count |
| WHI | Women’s Health Initiative |
Footnotes
COMPLIANCE WITH ETHICAL STANDARDS
The authors declare that they have no conflict of interest. Informed consent was obtained from all individual participants included in the study. The Institutional Review Board at the University of North Carolina at Chapel Hill approved the analysis. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marmot MG. Status syndrome: a challenge to medicine. JAMA : the journal of the American Medical Association. 2006;295(11):1304–1307. doi: 10.1001/jama.295.11.1304. [DOI] [PubMed] [Google Scholar]
- 2.Kroenke CH, Kwan ML, Neugut AI, Ergas IJ, Wright JD, Caan BJ, Hershman D, Kushi LH. Social networks, social support mechanisms, and quality of life after breast cancer diagnosis. Breast cancer research and treatment. 2013;139(2):515–527. doi: 10.1007/s10549-013-2477-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kroenke CH, Kubzansky LD, Schernhammer ES, Holmes MD, Kawachi I. Social networks, social support, and survival after breast cancer diagnosis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(7):1105–1111. doi: 10.1200/jco.2005.04.2846. [DOI] [PubMed] [Google Scholar]
- 4.Kroenke CH, Michael Y, Tindle H, Gage E, Chlebowski R, Garcia L, Messina C, Manson JE, Caan BJ. Social networks, social support and burden in relationships, and mortality after breast cancer diagnosis. Breast cancer research and treatment. 2012;133(1):375–385. doi: 10.1007/s10549-012-1962-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kroenke CH, Quesenberry C, Kwan ML, Sweeney C, Castillo A, Caan BJ. Social networks, social support, and burden in relationships, and mortality after breast cancer diagnosis in the Life After Breast Cancer Epidemiology (LACE) study. Breast cancer research and treatment. 2013;137(1):261–271. doi: 10.1007/s10549-012-2253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stringhini S, Berkman L, Dugravot A, Ferrie JE, Marmot M, Kivimaki M, Singh-Manoux A. Socioeconomic status, structural and functional measures of social support, and mortality: The British Whitehall II Cohort Study, 1985-2009. American journal of epidemiology. 2012;175(12):1275–1283. doi: 10.1093/aje/kwr461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang YC, McClintock MK, Kozloski M, Li T. Social isolation and adult mortality: the role of chronic inflammation and sex differences. Journal of health and social behavior. 2013;54(2):183–203. doi: 10.1177/0022146513485244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kroenke CH, Hankinson SE, Schernhammer ES, Colditz GA, Kawachi I, Holmes MD. Caregiving stress, endogenous sex steroid hormone levels, and breast cancer incidence. American journal of epidemiology. 2004;159(11):1019–1027. doi: 10.1093/aje/kwh148. [DOI] [PubMed] [Google Scholar]
- 9.Jansson C, Jeding K, Lagergren J. Job strain and risk of esophageal and cardia cancers. Cancer epidemiology. 2009;33(6):473–475. doi: 10.1016/j.canep.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Schernhammer ES, Hankinson SE, Rosner B, Kroenke CH, Willett WC, Colditz GA, Kawachi I. Job stress and breast cancer risk: the nurses' health study. American journal of epidemiology. 2004;160(11):1079–1086. doi: 10.1093/aje/kwh327. [DOI] [PubMed] [Google Scholar]
- 11.van Loon AJ, Tijhuis M, Surtees PG, Ormel J. Lifestyle risk factors for cancer: the relationship with psychosocial work environment. International journal of epidemiology. 2000;29(5):785–792. doi: 10.1093/ije/29.5.785. [DOI] [PubMed] [Google Scholar]
- 12.Li A, Tu MT, Sousa AC, Alvarado B, Kone GK, Guralnik J, Zunzunegui MV. Early life adversity and C-reactive protein in diverse populations of older adults: a cross-sectional analysis from the International Mobility in Aging Study (IMIAS) BMC geriatrics. 2015;15:102. doi: 10.1186/s12877-015-0104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang YC, Schorpp K, Harris KM. Social support, social strain and inflammation: evidence from a national longitudinal study of U.S. adults. Social science & medicine (1982) 2014;107:124–135. doi: 10.1016/j.socscimed.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lutgendorf SK, Sood AK, Antoni MH. Host factors and cancer progression: biobehavioral signaling pathways and interventions. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(26):4094–4099. doi: 10.1200/jco.2009.26.9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McClintock MK, Conzen SD, Gehlert S, Masi C, Olopade F. Mammary cancer and social interactions: identifying multiple environments that regulate gene expression throughout the life span. The journals of gerontology Series B, Psychological sciences and social sciences. 2005;60(Spec No 1):32–41. doi: 10.1093/geronb/60.special_issue_1.32. [DOI] [PubMed] [Google Scholar]
- 16.Penwell LM, Larkin KT. Social support and risk for cardiovascular disease and cancer: a qualitative review examining the role of inflammatory processes. Health Psychology Review. 2009;4(1):42–55. doi: 10.1080/17437190903427546. [DOI] [Google Scholar]
- 17.Finch CE. The biology of human longevity: Inflammation, nutrition, and aging in the evolution of lifespans. Burlington Academic Press; 2007. [Google Scholar]
- 18.Vendramini-Costa DB, Carvalho JE. Molecular link mechanisms between inflammation and cancer. Current pharmaceutical design. 2012;18(26):3831–3852. doi: 10.2174/138161212802083707. [DOI] [PubMed] [Google Scholar]
- 19.NCI Breast Cancer. [Accessed 05 December 2014]; http://www.cancer.gov/cancertopics/types/breast.
- 20.Messina CR, Lane DS, Glanz K, West DS, Taylor V, Frishman W, Powell L. Relationship of social support and social burden to repeated breast cancer screening in the women's health initiative. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2004;23(6):582–594. doi: 10.1037/0278-6133.23.6.582. [DOI] [PubMed] [Google Scholar]
- 21.Michael YL, Carlson NE, Chlebowski RT, Aickin M, Weihs KL, Ockene JK, Bowen DJ, Ritenbaugh C. Influence of stressors on breast cancer incidence in the Women's Health Initiative. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2009;28(2):137–146. doi: 10.1037/a0012982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Controlled clinical trials. 1998;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 23.Curb JD, McTiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt M, Johnson KC, Proulx-Burns L, Pastore L, Criqui M, Daugherty S. Outcomes ascertainment and adjudication methods in the Women's Health Initiative. Annals of epidemiology. 2003;13(9 Suppl):S122–128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 24.Vogtmann E, Levitan EB, Hale L, Shikany JM, Shah NA, Endeshaw Y, Lewis CE, Manson JE, Chlebowski RT. Association between sleep and breast cancer incidence among postmenopausal women in the Women's Health Initiative. Sleep. 2013;36(10):1437–1444. doi: 10.5665/sleep.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stewart AL, SC, Hays R, et al. Summary and discussion of MOS measures. In: SAaW JE, editor. Measuring functioning and well-being: The Medical Outcomes Study approach. Duke University Press; Durham, NC: 1992. pp. 345–371. [Google Scholar]
- 26.Antonucci TA, KR, Akiyama H. Psychosocial factors and the response to cancer symptoms. In: Yanick RYJ, editor. Cancer in the Elderly: Approaches to Early Detection and Treatment. Springer Publishing Company; New York, NY: 1989. pp. 40–52. [Google Scholar]
- 27.Berkman LG, TA . Social integration, social networks, social support, and health. In: Berkman LKI, editor. Social Epidemiology. Oxford University Press; New York: 2000. [Google Scholar]
- 28.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology (Cambridge, Mass) 1999;10(1):37–48. [PubMed] [Google Scholar]
- 29.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez-Pacheco H, Amezcua-Guerra LM, Vazquez-Rangel A, Martinez-Sanchez C, Perez-Mendez O, Verdejo J, Bojalil R. Levels of High-Density Lipoprotein Cholesterol are Associated With Biomarkers of Inflammation in Patients With Acute Coronary Syndrome. The American journal of cardiology. 2015 doi: 10.1016/j.amjcard.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 31.Macy EM, Hayes TE, Tracy RP. Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clinical chemistry. 1997;43(1):52–58. [PubMed] [Google Scholar]
- 32.Kushner I, Samols D, Magrey M. A unifying biologic explanation for "high-sensitivity" C-reactive protein and "low-grade" inflammation. Arthritis care & research. 2010;62(4):442–446. doi: 10.1002/acr.20052. [DOI] [PubMed] [Google Scholar]
- 33.Berkman LF, Syme SL. Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. American journal of epidemiology. 1979;109(2):186–204. doi: 10.1093/oxfordjournals.aje.a112674. [DOI] [PubMed] [Google Scholar]
- 34.Yang YC, Boen C, Mullan Harris K. Social relationships and hypertension in late life: evidence from a nationally representative longitudinal study of older adults. Journal of aging and health. 2015;27(3):403–431. doi: 10.1177/0898264314551172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang YC, Li T, Ji Y. Impact of social integration on metabolic functions: evidence from a nationally representative longitudinal study of US older adults. BMC public health. 2013;13:1210. doi: 10.1186/1471-2458-13-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables of associations between social relationship characteristics, inflammation markers, and breast cancer incidence stratified by Women’s Health Initiative cohort (Observational Study or Clinical Trial).