Abstract
Background
Over the past several decades, there has been a significant increase in the incidence of Clostridium difficile infection (CDI) in patients suffering from inflammatory bowel disease (IBD). However, a wild-type animal model is not available to study these comorbid diseases.
Methods
We evaluated the susceptibility to CDI of mice with dextran sulfate sodium salt (DSS)-induced colitis (IBD mice) with or without antibiotic exposure; we examined the histopathology and cytokine response in the concomitant diseases after the model was created.
Results
No CDI occurs in healthy control mice, wherease the incidence of CDI in IBD mice is 40%; however, in IBD mice that received antibiotics, the incidence of CDI is 100% and the disease is accompanied by high levels of toxins in the mouse feces and sera. Compared to IBD and CDI alone, those IBD mice infected with C. difficile have more severe symptoms, toxemia, histopathological damage, and higher mortality. Moreover, several proinflammatory cytokines and chemokines are significantly elevated in the colon tissues from IBD mice infected with C. difficile.
Conclusions
We, for the first time, demonstrate in an animal model that mice with dextran sulfate sodium induced-inflammatory bowel disease are significantly more susceptible to C. difficile infection, and that the bacterial infection led to more severe disease and death. These findings are consistent with clinical observations, thus, the animal model will permit us to study the pathogenesis of these concurrent diseases and to develop therapeutic strategies against the comorbidity of IBD and CDI.
Keywords: inflammatory bowel diseases, dextran sulfate sodium, Clostridium difficile infection, comorbidity, cytokines, cytotoxicity
INTRODUCTION
The inflammatory bowel diseases (IBD), a category of diseases, of which the most common are ulcerative colitis (UC) and Crohn’s disease (CD), are chronic and frequently relapsing diseases with clinical presentations of bloody diarrhea, abdominal cramps, and pain that require long-term medical therapy, periodic hospitalization, and even surgery.1These diseases are characterized by dysregulation of the mucosal immune system and dysbiosis of the normal gut microbiota. The dysregulated mucosal immune responses, induced by the innate and the adaptive immune systems, result in elevated levels of cytokines and chemokines that are believed to cause intestinal inflammation in IBD, which subsequently alter the community composition of the gut microbiota.2, 3 The quantitative and qualitative changes in the composition of the gut microbiota include the increased numbers of select microorganisms and the decreased complexity of commensal bacteria.4, 5
Clostridium difficile, a Gram-positive anaerobic spore-forming bacterium that produces enterotoxins TcdA and TcdB, grows rapidly in the presence of disrupted intestinal microbiota and causes various diseases, ranging from asymptomatic carriage to mild diarrhea to life-threatening pseudomembranous colitis.6Disruption of normal intestinal microbiota is the main risk factor forC. difficileinfection (CDI) and plays an important role in the onset and progression of CDI. A rise in the frequency of CDI in patients with IBD has been noted and 40%–50% of these patients have documented antibiotic exposure before presentation with CDI.7, 8 Patients with IBD have higher rates of asymptomatic carriage of C. difficile at 8.2%, versus 1% in healthy volunteers; the asymptomatic carriage rate is 9.4% in patients with UC and 6.9% in patients with CD.9 A registry database suggests that 10% of IBD patients will develop a C. difficile infection at some point. In a multivariate analysis, IBD patients infected with C. difficile had a 4 times greater mortality than patients with either IBD or CDI alone, and a 3 days longer length of hospital stay.10C. difficile infection in IBD patients was associated with more severe clinical courses, including higher rates of endoscopies, higher rates of complications such as ileus and toxic megacolon, higher colectomy rates, and increased mortality compared with those patients with IBD alone.7, 11, 12
The underlying reasons for the increasing incidence of CDI and and severity in IBD patients remain elusive. Several common risk factors have been observed between CDI and IBD, such as the prevalent dysbiosis of the gut microbiota, immunosuppressive or antimicrobial therapies, and frequent hospitalizations.13, 14It is important to study how these factors contribute to the incidence and severity of the comorbidity of IBD and CDI. A major challenge to investigating the impact of IBD on the host susceptibility to C. difficile infection and the pathogenesis of the comorbidity is the deficiency of well-constructed animal models. Currently, several animal models have been developed to study the pathogenesis of either IBD or CDI and to evaluate therapeutic interventions against the individual diseases.15, 16 C57BL/6 mice are the most widely used strain in murine models of the 2 diseases. Mice, after exposure to a mixture of antibiotics and then challenge with C. difficile spores, develop clinical symptoms that closely resemble human disease, with similar intestinal histopathological features. Administration of dextran sulfate sodium (DSS) in the drinking water to C57BL/6 mice leads to the development of clinical signs of acute IBD such as bloody diarrhea and significant weight loss.
In this study, we describe the establishment of a CDI DSS-induced IBD model in C57BL/6 wild- type mice. We evaluate the susceptibility of IBD mice to C. difficile and the severity of IBD combined with CDI, testing the hypothesis that CDI aggravates colitis in DSS-induced IBD mice, and that the comorbidity is severely detrimental to survival.
MATERIALS AND METHODS
Animals
C57BL/6 mice (6–8 weeks old) were purchased from Envigo (Frederick, MD, USA). All mice used in the experiments were housed in groups of 5 per cage under the same conditions. Food, water, bedding, and cages were autoclaved. All procedures involving animals were conducted under protocols approved by the Institutional Animal Care and Use Committee at the University of Maryland, Baltimore.
Establishment of the CDI Model and DSS-induced IBD Model in Mice
The CDI model was conducted as previously described.17 Briefly, C57BL/6 mice were administered an antibiotic cocktail (kanamycin, gentamicin, colistin, metronidazole, and vancomycin) for 3 days. Two days later, they were given clindamycin via intraperitoneal injection (10 mg/kg per dose) and then challenged 1 day later with C. difficile UK1 spores (105 CFU/mouse), a NAP1/BI/027 clinical strain.17 Mice were weighed every day to determine percentage weight changes. This was calculated as percent body weight change = (weight at day X / weight at day 0) ×100. Mice were also observed daily for the duration of the experiment for mortality and morbidity and the presence of diarrhea. Mice judged to be in a moribund state were killed; tissue samples from the colons were taken for histopathological analysis and cytokine/chemokine analysis.
IBD was induced by giving mice drinking water containing 3% (wt/vol) DSS (36,000–50,000 M.Wt, MP Biomedicals™, Solon, OH, USA) for 5 days (Fig. 1A). Body weight, stool consistency, and rectal bleeding were monitored daily after DSS administration. The disease activity index (DAI) was calculated by well-established and validated scores as described in Table 1.16, 18 On day 8, some mice were sacrificed by CO2 inhalation and then the colon was excised and fixed in formalin solution for histopathological assessment or prepared for cytokine/chemokine analysis.
FIGURE 1.
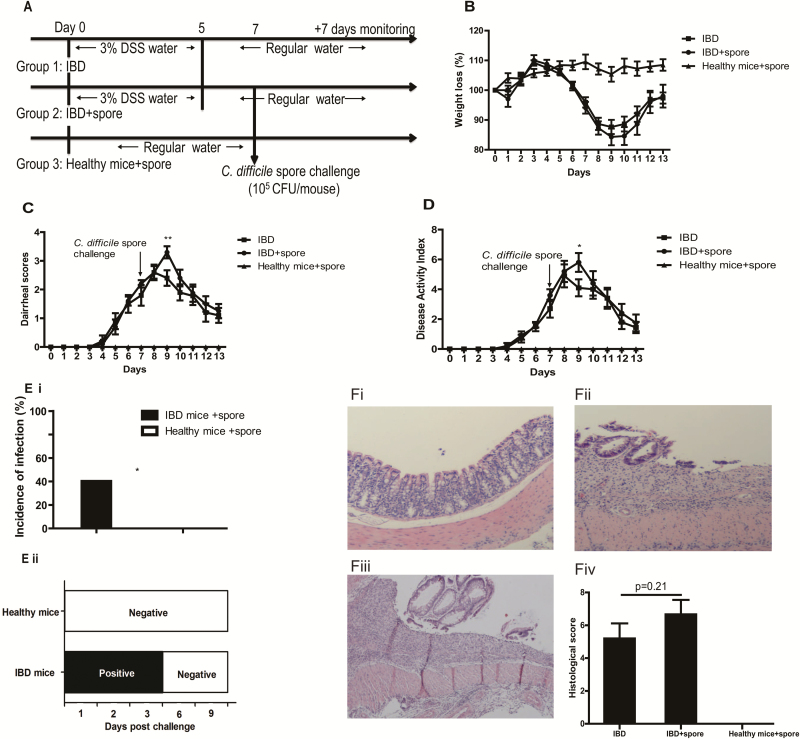
DSS-induced IBD mice are more susceptible to CDI. A, Experimental design schematics: IBD model and IBD with spore challenge. Group 1, IBD alone mice; group 2, IBD mice infected with C. difficile spores; group 3, healthy mice infected with C. difficile spore; n = 10 mice per group. B, Weight loss, (C) diarrhea scores, and (D) DAI of each group are shown. The data shown are mean ± SEM, and asterisks show significant differences between group 1 and group 2. *P < 0.05; **P < 0.01. E, After spore challenge, C. difficile toxins and spores in fecal samples are indicators of infection. CDI rate of IBD mice and healthy mice (Ei) according to the results of toxin-mediated cytotoxicity from feces sample extracts. Spore shedding in feces on day 1 (D1), D2, D3, D6, and D9 after spore challenge (Eii). *P < 0.05. F, The histopathologic changes in colon tissues of healthy mice challenged with C. difficile spore (Fi), IBD alone mice (Fii), IBD mice challenged with C. difficile spore (Fiii), and the histological scores of the 3 groups (Fiv). (H&E staining, X100).
Table 1:
DAI Score Parameters for IBD
| Weight Loss | Stool Consistency | Bleeding |
|---|---|---|
| 0 = None or gain | 0 = Formed (and Hard) | 0 = Normal color stool |
| 1 = 5%–10% weight loss | 1 = Mild-soft | |
| 2 = 10%–15% | 2 = Diarrhea | 2 = Reddish presence |
| 3 = 15%–20% | 3 = Mild diarrhea(Watery stool) | 3 = Bloody stool |
| 4 > 20% | 4 = Gross diarrhea | 4 = Gross bleeding |
Development of the IBD+CDI Comorbidity Mouse Model
The experimental schemes of the IBD-CDI models, wherein mice with established IBD are exposed to C. difficile, are illustrated in Figure 1A and Figure 2A. IBD mice were divided into 2 groups (n = 10/group), including an IBD group and a C. difficile spore challenged IBD group. There were no differences in DSS water intake between the 2 groups. In Figure 1A, to determine the susceptibility of IBD mice to C. difficile, a third group was added: healthy control mice that were challenged with C. difficile spores on the same day as the C. difficile spore challenged IBD (IBD+spore) group. Weights were measured and diarrhea scores were assessed every day. Feces on day 1 (D1), D2, D3, D6, and D9 after spore challenge were collected to detect C. difficile spore shedding by bacterial culture and C. difficile toxin by cytotoxicity. Colon tissues were collected for histopathological analysis from mice 2–3 days post spore challenge when CDI was the most severe.15
FIGURE 2.
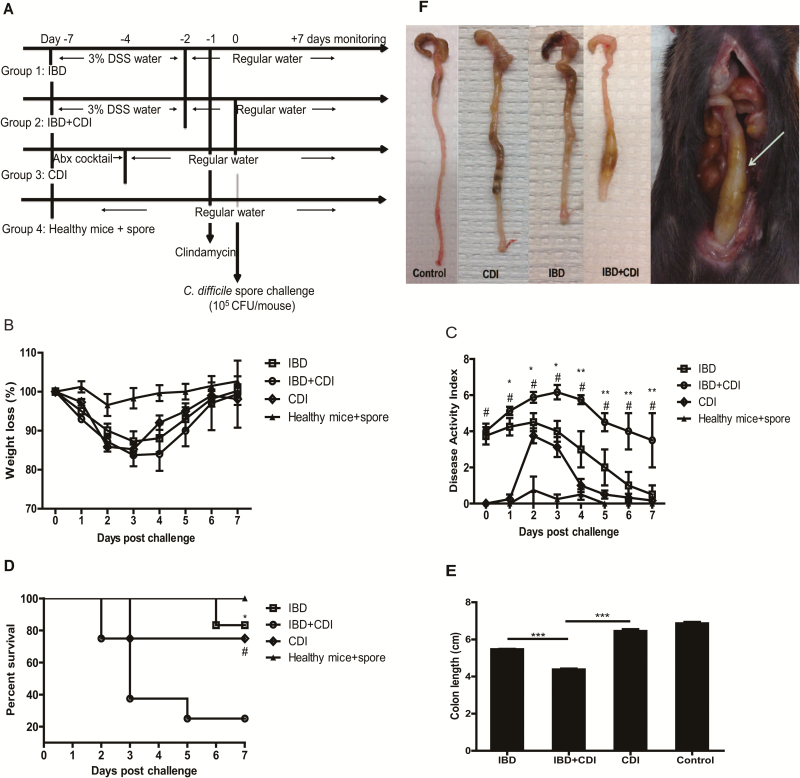
The disease severity of antibiotic-treated IBD comorbid with CDI is greater than IBD or CDI alone. A, Experimental schematic wherein all mice are treated with antibiotic before C. difficile spore challenge. Group 1, IBD alone; group 2, IBD and CDI comorbidity; group 3, CDI alone, wherein mice are pretreated with an antibiotic cocktail; group 4, healthy mice challenged with C. difficile spores (control); n = 10 mice per group. The weight loss (B), DAI (C), and survival curve (D) of the 4 groups of mice are shown. The values presented are the mean ± SEM. #indicates IBD+CDI vs CDI (#P < 0.05), * indicates IBD+CDI vs IBD (*P < 0.05; **P < 0.01). E, From the IBD group, colons were obtained 8 days after DSS administration; from the CDI and IBD+CDI groups, colons were obtained 3 days after spore challenge or from moribund mice. The length of these specimens (n = 6–10) were measured. The values presented are the mean ± SEM. ***P < 0.001. (F, The colon tissue of IBD and CDI mice showed thicker walls with shorter length compared with that of control mice. Toxic megacolon (arrow) was found in IBD+CDI comorbid mice.
In Figure 2A, the severity of IBD combined with CDI after exposure to antibiotics (IBD+CDI), was compared to IBD and CDI separately and included a control group (healthy mice+spore). As previously mentioned, up to 40% of UC and 50% of CD patients have documented antibiotic exposure before presentation with CDI. Hence, at day -1, IBD+CDI mice were given a clindamycin intraperitoneal injection and then challenged 1 day later with 105 CFU of C. difficile UK1 spores. For comparison, mice in the other 3 groups also were given a dose of clindamycin. The severity of colitis in each group was assessed using the DAI. Mice in a moribund state were killed. The colon tissue samples were taken for histopathological analysis and cytokine and chemokine detection. Colonic shortening was determined by measuring the length between the ileocecal junction and the proximal rectum. Also, feces and serum on D0 (4 hours post infection), D1, D2, D3, and D6 after spore challenge were collected to detect C. difficile toxin titer and C. difficile spore shedding.
Histopathological Analysis of Colon
Histopathological analysis was performed to evaluate mucosal damage and inflammation induced by the toxins and/or DSS in different groups. Resected colon tissues were collected and processed for routine histology with hematoxylin and eosin (H&E) stains on formalin-fixed, paraffin embedded sections. Standard light microscopic evaluation was then performed to assess pathologic changes within the sections using a previously published histologic scoring system based on 3 features: Inflammation (0 - no significant inflammation; 1- neutrophilic inflammation in epithelium or lamina propria; 2 - inflammatory cells extending into submucosa; 3 - transmural inflammation); Crypt injury (0 - no crypt injury; 1 - loss of basal one-third of crypts; 2 - loss of basal two-thirds of crypts; 3 - loss of full thickness crypts; 4 - full thickness crypt loss with surface erosion; 5 - diffuse/confluent surface erosion), and ulceration (0 - no ulceration; 1 - 2 or less foci of ulceration; 2 - 3 or 4 foci of ulceration; 3 - diffuse/confluent ulceration). The sum total of scores for each feature was calculated on a scale of 0-11.19
Serum and Fecal Cytotoxicity
After challenge with C. difficile spores, feces and blood were collected. Toxin titer was measured by cytotoxicity.20 Fecal samples were dissolved in an equal volume (g/ml) of sterile PBS and the supernatants were collected and filtered after centrifugation at 15,000 rpm for 5 minutes. Serum samples collected from whole mouse blood are stored at −80 °C until analysis. To measure toxin-mediated cytotoxicity, the serum samples and filtered fecal supernatants were 2-fold serially diluted before addition to Vero cell monolayers; cell rounding was observed under a phase-contrast microscope. Toxin titers were defined as the highest dilution to cause 100% cell rounding after 24 hours of incubation.
Bacterial Culture
Fecal samples were dissolved in an equal volume (g/ml) of sterile PBS. Each sample was 2-fold serially diluted, plated on chromID C. difficile agar plates (bioMérieux Inc., Durham, NC, USA), and cultured anaerobically for 48 hours at 37 °C; C. difficile colonies were counted. For strain typing, C. difficile was isolated and DNA was extracted and digested with HindIII. The fragments were separated on 1.0% agarose, and DNA fragment patterns were compared with those of the C. difficile strain used for infection.
Cytokine and Chemokine Aanalysis
Based on literature demonstrating a correlation between the host inflammatory response and disease severity, 12 cytokines and chemokines [(interleukin-1 beta (IL-1β), IL-10, IL-17, IL-6, IL-25, IL-22, IL-23, IL-4, IL-13, Tumor Necrosis Factor alpha (TNF-α), IL-8 (KC), granulocyte-macrophage colony-stimulating factor (GM-CSF))] related either to IBD or CDI were assayed in colonic tissue samples with MCYTOMAG-70k and MTH17MAG-47k kits by Luminex 100 system (EMD Millipore Corp, Billericia, MA, USA).21–24Briefly, colonic samples were separately weighed and homogenized in PBS containing 1× HALT protease inhibitor (Pierce, Rockford, IL, USA) and 5 mM HEPES (Sigma-Aldrich, St. Louis, MO, USA), then sonicated for 30 seconds to get a homogenous suspension. The sonicated colonic samples were centrifuged at 4 ºC, 12,000 rpm for 15 minutes and supernatants were collected and stored at −80 °C until assay. The total protein concentration was assessed by BCA assay according to the manufacturer’s instructions (Pierce). All cytokine and chemokine concentrations were shown relative to total protein concentration.
Ethical Considerations
All procedures involving animals were conducted under protocols approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Maryland, Baltimore.
Statistical Analysis
Data were subjected to Kaplan-Meier survival analysis, single factor analysis of variance using SPSS, version 16.0 (SPSS, Cary, NC, USA). A P value 0.05 was defined as statistically significant. Quantitative results were expressed with error bars as means ± SEM unless otherwise specified. All data presented are representative of at least 2 repeated experiments. All statistical analysis was performed using GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA).
RESULTS
DSS-induced IBD Mice are More Susceptible to CDI
To test the susceptibility of mice with IBD to C. difficile, we determined the incidence of CDI in IBD mice and healthy mice. Mice were treated with 3% DSS for only 5 days to induce moderate to severe colitis albeit without the severe weight loss (> 20%) considered as moribund by IACUC protocol. At day 4 of DSS treatment, the IBD group mice began to lose body weight and produce bloody stool (Fig. 1B). After being given C. difficile spores, the healthy mice group exhibited no signs of disease as measured by appearance, body weight, and diarrhea throughout the experiment. All the mice in both the IBD and the IBD+spore groups produced diarrhea; however, the diarrhea score and the overall DAI in the IBD+spore group was higher than the IBD group (Fig. 1C, 1D). After spore challenge, feces were collected to perform toxin-mediated cytotoxicity and for C. difficile culture. The results showed that CDI+IBD mice shed C. difficile toxins and spore, whereas neither toxins nor spores were detected in samples from healthy mice (Fig. 1E). The incidence of CDI in the IBD group was 40%, compared with the asymptomatic healthy group. The IBD+spore group had a slightly higher average histological score than the IBD alone group, but the difference was not significant (Fig 1F).
The Severity of Antibiotic-treated IBD in Presence of CDI is Greater than IBD or CDI Alone
As reported previously, 40%–50% of IBD patients have documented antibiotic exposure before presentation with CDI.8 To determine the susceptibility to CDI of IBD mice that received antibiotics before spore challenge and to evaluate disease severity, mice were given 1 intraperitoneal injection of clindamycin 1 day after withdrawal of DSS treatment and/or 1 day before inoculation with spores (Fig. 2A). The severity of IBD in the presence of CDI was assessed by DAI, survival, and colonic shortening. Our results showed that the IBD mice exposed to a single dose of clindamycin treatment were significantly more susceptible to C. difficile challenge, as all these mice developed CDI according to the results of C. difficile spore culture from feces (Fig. 3) as compared with the 40% rate of infection when IBD mice were not exposed to antibiotic (Fig. 1E). DAIs were significantly increased in the IBD+CDI group compared with the IBD and CDI alone groups (Fig. 2C). By day 3 of infection, only those mice infected with C. difficile, without established IBD, began to recover from weight loss and clinical symptoms. On the contrary, the IBD+CDI group continued losing weight and experiencing diarrhea (Fig 2B-C). IBD+CDI mice displayed significantly higher mortality than those mice with either CDI or IBD alone (25% survival rate vs 75% and 83.3%, respectively, Fig. 2D). We also observed that CDI and IBD comorbidity led to an increased shortening of the colon compared with control mice, indicating more severe colitis in those mice (Fig. 2E). In addition, obvious megacolon was frequently identified only in the IBD+CDI group (Fig. 2F).
FIGURE 3.
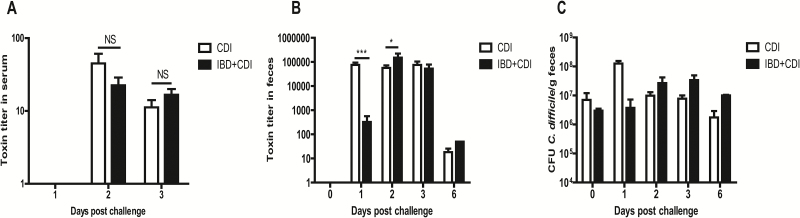
Toxemia in the serum and C. difficile toxin and spore shedding in the feces of CDI and IBD+CDI mice. All mice in CDI (n = 20) and IBD+CDI (n = 20) groups developed toxemia (A) on day 2 and day 3, and shed toxins in feces (B) for 6 days. (C)C. difficile spore shedding in feces of the two groups. *P < 0.05; **P < 0.01; ***P < 0.001. NS: not significant.
Presence of C. difficile Toxins in Serum
Clostridium difficile toxins A and B present in the sera is significantly correlated with severe disease outcomes in CDI as it is indicative of toxins escaping the damaged intestinal tract and disseminating into systemic circulation.25 Serum samples from mice that experienced either IBD, CDI, or both were examined for the presence of C. difficile toxins from day 1 to day 3 postinfection when the diseases were the most severe. We found that 100% of the serum samples collected from CDI alone or IBD+CDI groups on day 2 and day 3 were positive for toxin, both with peak toxin titers seen on day 2. The titers of the toxins present in serum samples were not significantly different between CDI and IBD+CDI groups (Fig. 3A). As expected, no toxins were detected in serum samples from mice that only experienced IBD (data not shown).
C. difficile Toxin and Spore Shedding in Feces
After challenge with C. difficile spores, feces from infected mice were collected from each group on D0 (4 hours postinfection), D1, D2, D3 to D6; C. difficile toxin and spore shedding were examined by cytotoxicity assay and bacterial culture. Toxins rapidly peaked on day 1 in the CDI group whereas the IBD+CDI group shed the highest amount of toxins on day 2. At the same time points, the toxin titers in feces were drastically higher than those in the sera. We also found that the peak spore shedding of the CDI group and the IBD+CDI group was seen on day 1 and day 3, respectively (Fig. 3C), suggesting that the antibiotic cocktail treatment in the CDI group allows a rapid establishment of C. difficile colonization. Although, C. difficile established a quicker colonization when mice were exposed to mixed antibiotic cocktail, both groups of mice continued to shed spores similarly from day 2 to day 6 (Fig. 3C) and gradually stopped shedding on day 9 (data not shown), which is in agreement with recent findings.17
Histopathologic Changes
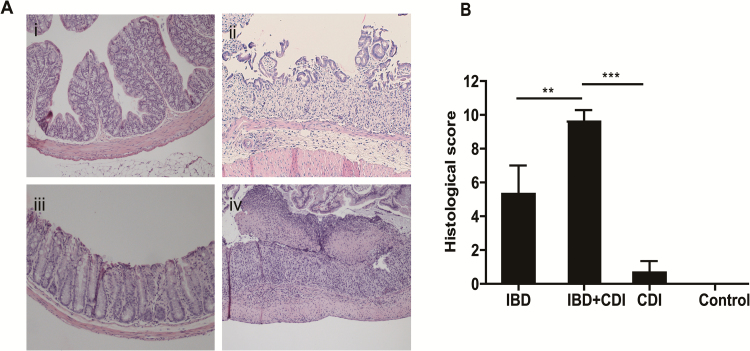
In the present study, the severity of colitis was further evaluated by histopathological analysis as histopathologic changes also are an index of the response to toxin-mediated inflammation. Colon sections from mice with IBD showed extensive ulceration, neutrophilic infiltration, and edema (Fig. 1Fii, 4Aii) in comparison with those of the control mice, as histopathologic changes were not found in mice from the healthy mice+spore group (Fig. 1Fi, 4Ai). Colon tissues from mice with CDI displayed mild-to-moderate colitis (Fig. 4Aiii). Compared with the IBD and CDI alone groups, mice with both IBD and CDI exhibited more severe damage in surface epithelia, loss of full thickness crypts, and confluent ulceration (Fig. 4Aiv). The colon tissue damages of the IBD+CDI mice also were more severe than those of the IBD+spore group (Fig. 1Fiii), consistent with the histologic scores of the 2 groups. Pseudomembranous colitis, regarded as the main cause of death in CDI patients in the clinic,26 was also seen in sections from the IBD+CDI mice. Forty percent of the IBD+CDI group’s sections revealed pseudomembranes, whereas none were found in the IBD+spore group’s sections. The histopathological injury scores were significantly increased in the IBD+CDI group compared to either IBD alone or CDI alone (Fig. 4B).
FIGURE 4.
The histopathologic changes in colon tissues of IBD, CDI, and IBD+CDI mice. (H&E staining X100). Colon tissue of control group (Ai), the IBD group (Aii), the CDI group (Aiii), the IBD+CDI group with pseudomembrane (arrow) (Aiv), and the histological scores of the 4 groups (B). *P < 0.05; **P < 0.01; ***P < 0.001.
Levels of Cytokines and Chemokines in Colonic Tissue
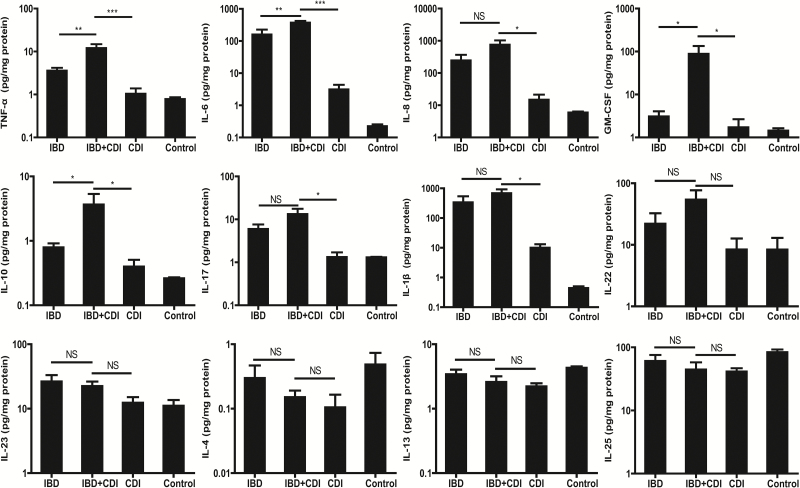
As both diseases are characterized by dysregulated mucosal immunity, 12 inflammatory cytokines and chemokines (related either to IBD or CDI) were quantified. We found that samples from the IBD+CDI group induced significantly more colonic TNF-α and IL-6 than IBD or CDI alone, indicating a potential role of the proinflammatory cytokines in the pathogenesis of the comorbidity in mice (Fig. 5). Similarly, we observed significantly elevated colonic IL-10 and GM-CSF in IBD+CDI mice. These results suggest that IBD combined with CDI results in a stronger local inflammatory response within the murine host than each disease alone. The amounts of IL-1β, IL-8 (KC), and IL-17 were higher in the colon tissues of IBD+CDI mice than in those with CDI, but not significantly higher than in those with IBD alone. In addition, there was no significant difference between IBD+CDI, IBD, or CDI groups in the levels of cytokines IL-4, IL-13, IL-22, IL-23, and IL-25.
FIGURE 5.
Cytokines and chemokines expression in colon tissues. The expression levels of 12 cytokines and chemokines [(interleukin (IL)-1β, IL-10, IL-17, IL-6, IL-25, IL-22, IL-23, IL-4, IL-13, Tumor Necrosis Factor alpha (TNF-α), IL-8(KC), granulocyte-macrophage colony-stimulating factor (GM-CSF))] were examined in colon tissues obtained from IBD, IBD+CDI, CDI, and control mice. *P < 0.05; **P < 0.01; ***P < 0.001. NS: not significant.
DISCUSSION
IBD is a chronic and relapsing disease, characterized by dysregulation of the mucosal immune system and dysbiosis of the normal gut microbiota. C. difficile, the major infectious cause of nosocomial diarrhea and colitis, proliferates and colonizes the gut when the composition of the gut microbiota is disrupted by antimicrobial drug therapy. The CDI rate in IBD patients is increasing significantly and becoming a serious issue faced by clinicians in the management of CDI and IBD patients.
Various mouse models have been used to investigate IBD, the majority of which are based either on chemical induction (with DSS or 2,4,6-trinitrobenzenesulfonic acid), immune cell transfer, or gene targeting. Mice induced by DSS develop IBD, characterized by increased inflammatory cytokine production and microbiota dysbiosis. In CDI models, mice challenged with C. difficile spores rapidly develop severe C. difficile-associated colitis after treatment with a mixture of antibiotics, which disrupt the normal gut microbiota. Although animal models of IBD and Clostridium-associated disease have been reported,15, 16 for now, only Kim et al have reported the establishment of C. difficile-aggravated colitis in an IBD animal model using IL-10-knockout mice.27 Because these mice do not express IL-10, a critical anti-inflammatory cytokine, it is difficult to assess normal host immune responses and evaluate immunotherapies using this model.
In this study, we reported the establishment of a mouse model of CDI in the presence of IBD in conventional immune competent mice. We observed the development of fatal pseudomembranous colitis and megacolon in mice experiencing IBD and CDI simultaneously. These findings are in accordance with clinical observations that IBD+CDI causes significant mortality.8, 28 CDI in mice with IBD resulted in histopathological features typical of C. difficile-associated colitis in humans, such as epithelial cell damage, inflammatory cells infiltration, and submucosal edema. However, the comorbidity aggravated the histopathological changes such that they were significantly more severe as compared with mice with either IBD or CDI alone. We also evaluated CDI susceptibility during different phases of DSS-induced inflammation. Mice treated with DSS for 3 days failed to consistently develop typical IBD symptoms, such diarrhea and bleeding, and there was no appreciable weight loss compared to the control group. Most reports suggested 5–7 days of DSS treatment as a good starting point to construct an IBD mouse model.16, 18 Mice treated with DSS for 7 days, however, lost greater than 20% of their body weight and were considered moribund. Therefore in this study, we treated the mice with DSS for 5 days to establish IBD with a moderate severity and then challenged with C. difficile spore to develop the IBD and CDI comorbidity model. This is the first report of an IBD+CDI model in normal immune competent mice, which provides a useful tool to investigate the relationship between CDI and IBD in pathogenesis and host immune response and to develop novel immunotherapies against the diseases.
Commensal bacteria are critically involved in various physiological functions in the gut, and microbial imbalance might cause intestinal pathology such as IBD and allow infection by opportunistic pathogens such as C. difficile.29 In the present study, we compared a group of healthy mice with IBD mice for susceptibility to C. difficile spore challenge and found that only IBD mice developed CDI. We also gave IBD mice an antibiotic before spore challenge and found that the incidence of CDI was higher and that the infection leads to more severe disease outcomes, with higher mortality and life-threatening pseudomembranous colitis. We believe that the altered gut microbiota ecology in patients with IBD is strongly associated with the increased C. difficile colonization and infection. Clinical data showed that antibiotics, which can disrupt gut microbiota, was a risk factor and likely increased the CDI rate in IBD patients.8 Moreover, many studies reported that fecal microbiota transplantation is useful for treating IBD and CDI.30 Therefore, gut microbiota may be an attractive target for the control of concomitant CDI and IBD. Some pre- and probiotics, advocated for their potential benefits in IBD and CDI, can be evaluated in this model.
C. difficile colonization of mouse intestines leads to the production of the major virulence factors TcdA and TcdB that target intestinal epithelial cells of the hosts. This activates a cascade of proinflammatory cytokines such as TNF-α, IL-23, IL-1β, and interferon-γ, leading to apoptosis of gut epithelial cells and increased permeability of the intestinal mucosa, preceding disease worsening and progression.31, 32 In the active state of IBD, reports have shown that the innate immune system is activated and responds by secreting a host of proinflammatory cytokines such as IL-1β, IL-6, IL-8, and TNF-α, amplifying the inflammatory response and ultimately resulting in tissue damage.33, 34 In this study, the amounts of TNF-α in the tissue were elevated in the IBD group, but were significantly increased in the CDI and IBD group, which may be associated with the increased severity of the concurrent diseases. In clinics, anti-TNF-α agents, such as infliximab, infliximab biosimilar CTP13, and adalimumab, have been approved for the treatment of patients with IBD. The application of anti-TNF-α agents have been shown to induce clinical responses in up to 60% of CD patients and long-term maintenance of remission in a large number of patients.35, 36 This may suggest that anti-TNF-α agents can be used to treat CDI and IBD patients. Increased levels of IL-6 in colon tissues corresponded to the severity of colitis that is consist ent with other reported data that IL-6 is highly upregulated during CDI and influences the type of immune response that develops.37, 38 A case-control study of participants enrolled in 2 population-based, nationwide, prospective cohort studies reported that levels of IL-6 before diagnosis are associated with risk of incident CD and UC.39 IL-6 may be a plausi ble target for future investigations. In addition, IL-10 and GM-CSF, which may contribute to the development of IBD and CDI comorbidity as indicated by our data, may warrant further investigation.
A limitation of this study is that the pathological changes of DSS -induced IBD model more closely approximate human UC.40 This fact makes the DSS model well suited for research on UC, given the occurrence of C. difficile among UC patients was 3 times higher than that among CD patients.41 However, other mouse IBD models, such as the TNBS (2,4,6-trinitrobenzene sulfonic acid) model that simulates the symptoms of human CD, are needed to confirm the role of CDI in IBD. Another limitation is that whereas pathological mechanisms of IBD and CDI are both associated with dysbiosis of the normal gut microbiota, this study lacks microbiome characterization of each group. Further studies are needed to better understand the pathogenic role of CDI in IBD exacerbation to evaluate the efficacy of newer treatments such as anti-TNF-α antibody in CDI and IBD comorbid mice.
CONCLUSION
This is the first study that uses a DSS-induced IBD animal model to study the role of CDI in IBD. The results of the present study confirm that IBD mice with prior antibiotic exposure are more susceptible to CDI and demonstrate that the severity of the concurrent diseases is significantly greater than IBD or CDI alone. We believe that this model provides a much needed tool to investigate the relationship between CDI and IBD in pathogenesis and host immune response and to evaluate new strategies for interventions against these diseases.
Conflicts of Interest
No potential conflicts of interest relevant to this article were reported.
Supported by
Shanghai committee of Science and Technology Fund (No. 16140902400) and NIH grants U19 AI109776, R43AI129044, and R01AI132207.
REFERENCES
- 1. Colombel JF, Narula N, Peyrin-Biroulet L. Management strategies to improve outcomes of patients with inflammatory bowel diseases. Gastroenterology. 2017;152:351–361 e355. [DOI] [PubMed] [Google Scholar]
- 2. Cader MZ, Kaser A. Recent advances in inflammatory bowel disease: mucosal immune cells in intestinal inflammation. Gut. 2013;62:1653–1664. [DOI] [PubMed] [Google Scholar]
- 3. Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tamboli CP, Neut C, Desreumaux P, Colombel JF. Dysbiosis in inflammatory bowel disease. Gut. 2004;53:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dupaul-Chicoine J, Dagenais M, Saleh M. Crosstalk between the intestinal microbiota and the innate immune system in intestinal homeostasis and inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:2227–2237. [DOI] [PubMed] [Google Scholar]
- 6. Surawicz CM, Brandt LJ, Binion DG, et al. . Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478–98; quiz 499. [DOI] [PubMed] [Google Scholar]
- 7. Bossuyt P, Verhaegen J, Van Assche G, et al. . Increasing incidence of Clostridium difficile-associated diarrhea in inflammatory bowel disease. J Crohns Colitis. 2009;3:4–7. [DOI] [PubMed] [Google Scholar]
- 8. Zhang T, Lin QY, Fei JX, et al. . Clostridium difficile infection worsen outcome of hospitalized patients with inflammatory bowel disease. Sci Rep. 2016;6:29791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clayton EM, Rea MC, Shanahan F, et al. . The vexed relationship between Clostridium difficile and inflammatory bowel disease: an assessment of carriage in an outpatient setting among patients in remission. Am J Gastroenterol. 2009;104:1162–1169. [DOI] [PubMed] [Google Scholar]
- 10. Ananthakrishnan AN, McGinley EL, Binion DG. Excess hospitalisation burden associated with Clostridium difficile in patients with inflammatory bowel disease. Gut. 2008;57:205–210. [DOI] [PubMed] [Google Scholar]
- 11. Ananthakrishnan AN, McGinley EL, Saeian K, et al. . Temporal trends in disease outcomes related to Clostridium difficile infection in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:976–983. [DOI] [PubMed] [Google Scholar]
- 12. Berg AM, Kelly CP, Farraye FA. Clostridium difficile infection in the inflammatory bowel disease patient. Inflamm Bowel Dis. 2013;19:194–204. [DOI] [PubMed] [Google Scholar]
- 13. Morgan XC, Tickle TL, Sokol H, et al. . Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012;13:R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang H, Wu S, Chen R, et al. . Risk factors of Clostridium difficile infections among patients in a university hospital in shanghai, china. Anaerobe. 2014;30:65–69. [DOI] [PubMed] [Google Scholar]
- 15. Best EL, Freeman J, Wilcox MH. Models for the study of clostridium difficile infection. Gut Microbes. 2012;3:145–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bang B, Lichtenberger LM. Methods of inducing inflammatory bowel disease in mice. Curr Protoc Pharmacol. 2016;72:5.58.1–5.5842. [DOI] [PubMed] [Google Scholar]
- 17. Sun X, Wang H, Zhang Y, et al. . Mouse relapse model of Clostridium difficile infection. Infect Immun. 2011;79:2856–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. De Fazio L, Spisni E, Cavazza E, et al. . Dietary geraniol by oral or enema administration strongly reduces dysbiosis and systemic inflammation in dextran sulfate sodium-treated mice. Front Pharmacol. 2016;7:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Laroui H, Ingersoll SA, Liu HC, et al. . Dextran sodium sulfate (DSS) induces colitis in mice by forming nano-lipocomplexes with medium-chain-length fatty acids in the colon. Plos One. 2012;7:e32084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Keessen EC, Hopman NE, van Leengoed LA, et al. . Evaluation of four different diagnostic tests to detect Clostridium difficile in piglets. J Clin Microbiol. 2011;49:1816–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vazeille E, Buisson A, Bringer MA, et al. . Monocyte-derived macrophages from crohn’s disease patients are impaired in the ability to control intracellular adherent-invasive escherichia coli and exhibit disordered cytokine secretion profile. J Crohns Colitis. 2015;9:410–420. [DOI] [PubMed] [Google Scholar]
- 22. Hirota SA, Iablokov V, Tulk SE, et al. . Intrarectal instillation of Clostridium difficile toxin A triggers colonic inflammation and tissue damage: development of a novel and efficient mouse model of Clostridium difficile toxin exposure. Infect Immun. 2012;80:4474–4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yao J, Wang JY, Lai MG, et al. . Treatment of mice with dextran sulfate sodium-induced colitis with human interleukin 10 secreted by transformed bifidobacterium longum. Mol Pharm. 2011;8:488–497. [DOI] [PubMed] [Google Scholar]
- 24. McDermott AJ, Frank CR, Falkowski NR, et al. . Role of GM-CSF in the inflammatory cytokine network that regulates neutrophil influx into the colonic mucosa during Clostridium difficile infection in mice. Gut Microbes. 2014;5:476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Steele J, Chen K, Sun X, et al. . Systemic dissemination of Clostridium difficile toxins A and B is associated with severe, fatal disease in animal models. J Infect Dis. 2012;205:384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kelly CP, Pothoulakis C, LaMont JT. Clostridium difficile colitis. N Engl J Med. 1994;330:257–262. [DOI] [PubMed] [Google Scholar]
- 27. Kim MN, Koh SJ, Kim JM, et al. . Clostridium difficile infection aggravates colitis in interleukin 10-deficient mice. World J Gastroenterol. 2014;20:17084–17091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nitzan O, Elias M, Chazan B, et al. . Clostridium difficile and inflammatory bowel disease: role in pathogenesis and implications in treatment. World J Gastroenterol. 2013;19:7577–7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. [DOI] [PubMed] [Google Scholar]
- 30. Fischer M, Kao D, Kelly C, et al. . Fecal microbiota transplantation is safe and efficacious for recurrent or refractory clostridium difficile infection in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2016;22:2402–2409. [DOI] [PubMed] [Google Scholar]
- 31. Cowardin CA, Kuehne SA, Buonomo EL, et al. . Inflammasome activation contributes to interleukin-23 production in response to Clostridium difficile. MBio. 2015;6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cowardin CA, Buonomo EL, Saleh MM, et al. . The binary toxin CDT enhances Clostridium difficile virulence by suppressing protective colonic eosinophilia. Nat Microbiol. 2016;1:16108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Egger B, Bajaj-Elliott M, MacDonald TT, et al. . Characterisation of acute murine dextran sodium sulphate colitis: cytokine profile and dose dependency. Digestion. 2000;62:240–248. [DOI] [PubMed] [Google Scholar]
- 34. Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1756–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Panaccione R, Colombel JF, Sandborn WJ, et al. . Adalimumab maintains remission of crohn’s disease after up to 4 years of treatment: data from CHARM and ADHERE. Aliment Pharmacol Ther. 2013;38:1236–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schreiber S, Rutgeerts P, Fedorak RN, et al. ; CDP870 Crohn’s Disease Study Group A randomized, placebo-controlled trial of certolizumab pegol (CDP870) for treatment of crohn’s disease. Gastroenterology. 2005;129:807–818. [DOI] [PubMed] [Google Scholar]
- 37. Mitsuyama K, Sata M, Rose-John S. Interleukin-6 trans-signaling in inflammatory bowel disease. Cytokine Growth Factor Rev. 2006;17:451–461. [DOI] [PubMed] [Google Scholar]
- 38. Czepiel J, Biesiada G, Brzozowski T, et al. . The role of local and systemic cytokines in patients infected with Clostridium difficile. J Physiol Pharmacol. 2014;65:695–703. [PubMed] [Google Scholar]
- 39. Lochhead P, Khalili H, Ananthakrishnan AN, et al. . Association between circulating levels of C-reactive protein and interleukin-6 and risk of inflammatory bowel disease. Clin Gastroenterol Hepatol. 2016;14:818–824.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chassaing B, Aitken JD, Malleshappa M, et al. . Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol. 2014;104:Unit 15.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nguyen GC, Kaplan GG, Harris ML, et al. . A national survey of the prevalence and impact of Clostridium difficile infection among hospitalized inflammatory bowel disease patients. Am J Gastroenterol. 2008;103:1443–1450. [DOI] [PubMed] [Google Scholar]