Abstract
Background
Right ventricular (RV) remodeling involves changes in size, wall thickness, function, and shape. Previous studies have suggested that regional curvature indices (rCI) may be useful for RV shape analysis. The aim of this study was to establish normal three-dimensional echocardiographic values of rCI in a large group of healthy subjects to facilitate future three-dimensional echocardiographic study of adverse RV remodeling.
Methods
RV endocardial surfaces were reconstructed at end-diastole and end-systole in 245 healthy subjects (mean age, 42 ± 12 years) and analyzed using custom software to calculate mean curvature in six regions: RV inflow tract (RVIT) and RV outflow tract, apex, and body (both divided into free wall and septal regions). Associations with age and gender were studied.
Results
The apical free wall was convex, while the septum (apex and body) was more concave than the body free wall. Septal curvature did not change significantly from end-diastole to end-systole. The RV outflow tract and RVIT became flatter from end-diastole to end-systole. In keeping with the “bellows-like” action of RV contraction, the body free wall became flatter, while the apex free wall changed to a more convex surface. There were no intergender differences in rCI. In older subjects (≥55 years of age), the RV free wall and RV outflow tract were flatter, and from end-diastole to end-systole, the RVIT became less flattened and the apex less pointed. These changes suggest that the right ventricle is stiffer in older subjects, with less dynamic contraction of the RVIT and less bellows-like movement.
Conclusions
This study established normal three-dimensional echocardiographic values for RV rCI, which are needed to further study RV diastolic dysfunction and remodeling with disease.
Keywords: Right ventricle, Normal heart, Curvature, Three-dimensional echocardiography
It has been recognized that the right ventricle alters its shape in response to variations in pressure and volume loading.1,2 These shape alterations have been shown to be predictive of outcomes in several different clinical situations. Interventricular septal flattening, for instance, has been associated with prognosis in pulmonary hypertension and pulmonary embolism.3 In addition, less conical right ventricular (RV) remodeling in functional tricuspid regurgitation has been suggested as a potential factor for consideration in the methodology used for tricuspid valve repair.4 These morphologic changes, however, are difficult to characterize on two-dimensional (2D) echocardiography, because multiple imaging planes need to be integrated to appreciate the complex asymmetric crescent shape of the right ventricle, making quantitative evaluation complex and impractical. Recent advancements in three-dimensional (3D) transthoracic echocardiography allow the entire right ventricle to be contained in a single pyramidal data set lending itself to detailed analysis of RV size and function. After volumetric reconstruction, the endocardial surface of the right ventricle can be extracted and viewed from multiple vantage points, enabling, at least in theory, a description of dynamic changes in RV shape.
The normal left ventricle has a shape that resembles a prolate ellipsoid. In the presence of loading stressors or myocyte damage, the shape of the left ventricle often changes from conical to more spherical. Accordingly, left ventricular (LV) shape alterations have been quantitatively described by the degree of similarity between the left ventricle and a reference shape, such as a sphere or a cone.5 Furthermore, spherical LV shape has been associated with adverse outcomes.6–8 In contrast, the complex RV shape cannot be easily approximated by a predefined shape, such as a sphere or a cone, and thus RV remodeling has not been systematically studied by means of changes in its global shape. To date, most studies exploring the concept of RV shape have focused on changes in the interventricular septum, with quantification of these changes using 2D-derived eccentricity indices1,9 or interventricular septal curvature.10 A small number of studies have focused on changes in RV apical morphology.11
In this study, we sought to evaluate the dynamic changes that occur in regional RV curvature in a large population of healthy volunteers over a wide range of ages by generating regional color-coded parametric curvature maps from endocardial surfaces derived from 3D data sets of the right ventricle. This work was aimed at establishing a reference standard against which abnormal right ventricles could be compared and pathologic changes could be detected quantitatively.
METHODS
Patient Population
A total of 245 healthy volunteers (mean age, 42 ± 12 years; 44% men) with adequate image quality were enrolled at two university hospitals (University of Chicago and University of Padua, Italy). Recruited subjects were on no medications and did not have histories of cardiac or lung disease or cardiac risk factors. Inclusion criteria were normal results on 2D transthoracic echocardiography with normal RV and LV chamber size and function and not more than mild valvular regurgitation with normal pulmonary artery pressures (<35 mm Hg). The study was approved by the institutional review boards of both institutions.
Two-Dimensional and 3D Transthoracic Echocardiography
Comprehensive 2D transthoracic echocardiography was performed by an experienced sonographer using the iE33 system equipped with an S5 and X5 transducer (Philips Medical Imaging, Andover, MA) or the Vivid E9 system (GE Vingmed Ultrasound, Horten, Norway) equipped with an M5S transducer. Three-dimensional full-volume RV data sets were obtained from a RV-focused view by stitching together four to six consecutive electrocardiographically gated subvolumes taking care to avoid dropout of the anterior RV free wall and include the entire RV apex in the pyramidal data set. Image depth and sector were optimized to achieve frame rates ≥20 Hz. All images were analyzed offline.
The following 2D parameters were measured using the RV-focused apical four-chamber view in accordance with American Society of Echocardiography and European Association of Cardiovascular Imaging recommendations12: (1) basal and midventricular diameters and length; (2) fractional area change (FAC); and (3) maximal tricuspid annular plane systolic excursion (TAPSE) obtained using M-mode imaging from the lateral wall of the tricuspid annulus. Three-dimensional data sets were analyzed using dedicated 3D software to quantify RV end-diastolic and end-systolic volumes and RV ejection fraction and to reconstruct the RV endocardial surface for shape analysis (4D RV-Function 1.1; TomTec Imaging Systems, Unterschleissheim, Germany). RV contours were manually initialized in end-systolic and end-diastolic frames in the short- and long-axis planes, while including the trabeculae in the RV cavity.13,14 Side-by-side display of dynamic tracking of endocardial borders throughout the cardiac cycle enabled end-diastolic and end-systolic contour adjustments.
RV Shape Analysis
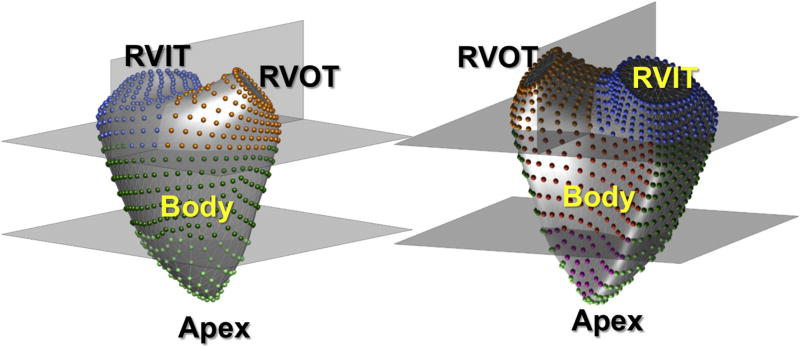
Three-dimensional RV endocardial surfaces were exported as a mesh of connected points and used as input into custom software for analysis of regional RV curvature as previously described.15 Zero curvature defines a flat surface, while a positive or negative curvature depicts convexity or concavity, respectively. The more positive or negative the curvature, the more convex or concave the surface. The curvature of a region was defined from a reference point outside the right ventricle looking onto the surface being described (Figure 1). To allow comparisons between right ventricles of different shapes, the 3D surface was automatically divided into four parts: one fourth apex, two fourths body, and one fourths RV outflow tract (RVOT) and RV inflow tract (RVIT; Figure 2). The free wall and septal components of both the RV apex and body were reported separately, as these regional curvatures were expected to be facing in opposing directions. Accordingly, the RV surface was divided into six regions: (1) RVIT, (2) RVOT, (3) septal free wall, (4) free wall body, (5) septal apex, and (6) free wall apex (Figure 2). Regional 3D curvature was derived by averaging the local curvature values of all control points within a particular region.
Figure 1.
RV surface wall curvature is described from a point of view outside the right ventricle looking onto the wall in question. For instance, when describing septal curvature, the eye is looking at the septum from a point in the left ventricle. When describing free wall curvature, the eye is looking at the free wall from a vantage point outside the ventricle.
Figure 2.
The 3D endocardial surface of the right ventricle was automatically divided into four parts: one-fourth apex, two-fourths body, and one-fourth RVOT and RVIT. The RV apex and body were further subdivided into free wall and septal components. Accordingly, the RV surface was divided into six regions: (1) RVIT, (2) RVOT, (3) septal free wall, (4) body free wall, (5) septal apex, and (6) apex free wall.
Statistical Analysis
Continuous variables are expressed as mean ± SD. Categorical variables are expressed as numerical values or percentages. P values < 0.05 were considered to indicate statistical significance. Subpopulations studied included men and women and age groups (<40, 40–55, and ≥55 years). To study differences between gender and age groups, a two-tailed unpaired Student’s t test was used for continuous variables. Chi-square analysis was used for categorical variables. When comparing end-diastolic and end-systolic values, paired t tests were used.
To determine inter- and intraobserver reproducibility of curvature and volume, RV endocardial surface tracing and shape analysis were repeated in 15 randomly selected patients by two independent observers (K.A. and D.M.), both blinded to prior measurements. The interoperator variability for RV volume and shape indices was computed using percentage variability, defined as the mean of the absolute differences between pairs of repeated measurements divided by their mean.
RESULTS
A total of 348 normal subjects were available for enrollment. Of these, 103 were eliminated because of poor image quality or inadequate attention to RV acquisition. Two hundred forty-five healthy subjects were therefore enrolled in the study. A summary of baseline RV geometry and gender differences is shown in Table 1. Normal subjects had a mean RV basal diameter of 37 ± 6 mm, a mean FAC of 46 ± 6%, and a mean TAPSE of 22 ± 4 mm. The mean 3D RV ejection fraction was 56 ± 5%. Compared with women, men had slightly but statistically significantly larger absolute RV dimensions, RV FAC, and 3D RV volumes, whereas women had higher 3D RV ejection fractions. TAPSE was not significantly different between genders. The apex had the smallest regional volume compared with all other regions (RVOT, RVIT, and body), while the RV body had the largest volume (Figure 3). The regional apical ejection fraction was highest (Table 1). Compared with women, men had significantly larger regional end-diastolic and end-systolic volumes. Women, however, had higher RV ejection fractions in all regions.
Table 1.
Baseline characteristics
| Overall | Women | Men | |
|---|---|---|---|
| n (%) | 245 (100%) | 108 (44%) | 137 (56%) |
| Age (y) | 42 ± 12 | 43 ± 12 | 41 ± 13 |
| BSA (m2) | 1.8 ± 0.2 | 1.7 ± 0.2 | 1.9 ± 0.1 |
| 2D echocardiographic parameters | |||
| RVEDb (mm) | 37 ± 6 | 35 ± 5 | 40 ± 6 |
| RVEDm (mm) | 28 ± 6 | 25 ± 5 | 31 ± 6 |
| RV length (mm) | 72 ± 9 | 45 ± 33 | 52 ± 35 |
| RV FAC (%) | 46 ± 6 | 47 ± 6 | 45 ± 6 |
| TAPSE (mm) | 22 ± 4 | 22 ± 3 | 22 ± 4 |
| 3D echocardiographic parameters | |||
| Mean frame rate (Hz) | 27 ± 7 | ||
| RV EDV (indexed, mL)* | |||
| Total | 124 ± 33 (69 ± 16) | 110 ± 25 (65 ± 14) | 142 ± 32 (74 ± 17) |
| Apical | 19 ± 5 (11 ± 3) | 17 ± 4 (10 ± 2) | 22 ± 5 (12 ± 3) |
| Body | 47 ± 13 (26 ± 6) | 41 ± 10 (25 ± 5) | 54 ± 13 (28 ± 7) |
| RVOT | 23 ± 6 (13 ± 3) | 21 ± 7 (12 ± 3) | 27 ± 6 (14 ± 3) |
| RVIT | 34 ± 9 (19 ± 4) | 31 ± 5 (18 ± 4) | 39 ± 9 (21 ± 5) |
| RV ESV (indexed, mL)* | |||
| Total | 55 ± 17 (30 ± 8) | 47 ± 13 (28 ± 7) | 64 ± 13 (33 ± 8) |
| Apical | 8 ± 3 (8 ± 3) | 7 ± 2 (4 ± 1) | 9 ± 2 (5 ± 1) |
| Body | 21 ± 6 (12 ± 3) | 18 ± 5 (11 ± 3) | 25 ± 6 (13 ± 3) |
| RVOT | 10 ± 3 (6 ± 1) | 9 ± 2 (5 ± 1) | 12 ± 3 (6 ± 1) |
| RVIT | 15 ± 5 (8 ± 2) | 13 ± 4 (8 ± 2) | 18 ± 4 (9 ± 2) |
| RVEF (%)† | |||
| Total | 56 ± 5 | 57 ± 5 | 55 ± 5 |
| Apical | 59 ± 5 | 60 ± 5 | 58 ± 5 |
| Body | 55 ± 6 | 56 ± 6 | 53 ± 6 |
| RVOT | 56 ± 6 | 57 ± 6 | 56 ± 6 |
| RVIT | 56 ± 5 | 57 ± 5 | 55 ± 5 |
BSA, Body surface area; EDV, end-diastolic volume; ESV, end-systolic volume; RVEDb, RV end-diastolic dimension at the base; RVEDm, RV end-diastolic dimension at the midlevel; RVEF, RV ejection fraction.
Global and regional volumes were significantly smaller in women than in men (P < .05).
Global and regional RVEFs were significantly higher in women than in men (P < .05).
Figure 3.
Graph representing the mean total RV volume throughout the cardiac cycle for the entire cohort of 245 healthy subjects with SD (left). The right panel depicts the regional RV volumes throughout the cardiac cycle. Body volumes are the largest, followed by RVOT, RVIT, and apex.
Static Analysis of RV Curvature
Mean regional curvature values for the normal right ventricle are shown in Table 2. An example of an individual regional RV color-coded curvature map is shown in Figure 4. The apical region had the highest curvature values, denoting that is it is the most convex (pointed) region. On the color-coded curvature map, this corresponds to the red-orange color, which likewise represents high convexity. The septum (both apical and body) is predominantly characterized by concavity, so the mean curvature values in this region are close to zero. Multiple nodal values in these regions are negative, hence the intense blue color on the color-coded curvature map, describing a more concave surface in the region of the septum. Mean body free wall curvature values were noted to be above zero (Table 2), suggesting convexity, less in magnitude compared with the apex and therefore resulting in a blue-green-yellow hue on the color-coded curvature map. Both the RVOT and RVIT had curvature values > 1, depicting rounded regions. The complex color-coded map of this region (red, yellow, and green hues) describes multiple degrees of regional convexity and roundness. No gender differences were noted in regional RV curvature values.
Table 2.
Normal values for regional curvature
| Overall | Women | Men | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Region | ED | ES | P | ED | ES | P | ED | ES | P |
| Apical free wall | 2.38 ± 0.24 | 2.45 ± 0.34 | <.01 | 2.36 ± 0.23 | 2.43 ± 0.36 | <.01 | 2.39 ± 0.24 | 2.48 ± 0.31 | .04 |
|
| |||||||||
| Body free wall | 1.17 ± 0.06 | 1.13 ± 0.10 | <.01 | 1.17 ± 0.07 | 1.13 ± 0.11 | <.01 | 1.17 ± 0.06 | 1.13 ± 0.09 | <.01 |
|
| |||||||||
| Apical septum | 0.51 ± 0.34 | 0.52 ± 0.43 | .55 | 0.53 ± 0.34 | 0.57 ± 0.46 | .10 | 0.50 ± 0.35 | 0.46 ± 0.38 | .54 |
|
| |||||||||
| Body septum | 0.24 ± 0.15 | 0.25 ± 0.18 | .11 | 0.24 ± 0.15 | 0.26 ± 0.20 | .05 | 0.24 ± 0.16 | 0.24 ± 0.16 | .96 |
|
| |||||||||
| RVOT | 1.42 ± 0.11 | 1.31 ± 0.12 | <.01 | 1.43 ± 0.11 | 1.31 ± 0.12 | <.01 | 1.41 ± 0.11 | 1.31 ± 0.12 | <.01 |
|
| |||||||||
| RVIT | 1.27 ± 0.16 | 1.20 ± 0.13 | <.01 | 1.26 ± 0.20 | 1.20 ± 0.14 | <.01 | 1.28 ± 0.11 | 1.22 ± 0.12 | <.01 |
ED, End-diastole; ES, end-systole.
There were no significant differences in ED or ES curvature between women and men.
Figure 4.
Example of a color-coded curvature map for a normal subject is shown from the free wall perspective (left) and from the septal perspective (right). The color scale bar on the far right matches the colors on the map, with curvature values ranging from −1.5 to 2.5. The deepest red signifies the most convex surface (and most positive curvature values); the deepest blue corresponds to the most concave surface (and most negative curvature values). The green midpoint signifies a horizontal (flat) surface with curvature value 0.
Dynamic Analysis of RV Curvature
The shape changes associated with RV contraction can be seen in Figure 5. The base of the right ventricle (RVIT) exhibits a downward movement, associated with a decrease in curvature changing from a convex to a flatter shape from end-diastole to end-systole. The RV free wall shows a lateral displacement as it transitions from a convex to a flatter shape. This motion of the apical free wall also causes the RV apex to become more pointed. The latter occurs without a significant longitudinal displacement of the apex. The motion of the RVOT, which is frequently not appreciated on 2D echocardiography, also has a downward displacement, becoming flatter at end-systole. Overall, the mean change in septal curvature was not significant between end-diastole and end-systole (Table 2, Figure 5). These regional RV dynamic changes were similar between genders (Table 2).
Figure 5.
Diagrammatic representation of the transition in curvature values from end-diastole to end-systole. The gray mesh represents the right ventricle in end-diastole and the blue solid frame the right ventricle in end-systole in a representative normal subject. The apical free wall becomes more pointed (black arrows at apex), and the body free wall flattens, as do the RVOT and RVIT (see arrows at respective positions). There was no change in septal curvature during the transition from end-diastole to end-systole.
Age-Related Changes in Dynamic Behavior of the Right Ventricle
Dynamic RV changes (i.e., differences between end-diastole and end-systole) were most pronounced in the youngest cohort (<40 years of age). Global and regional RV ejection fractions decreased with increasing age in our cohort. Significant differences were seen between those <40 and 40 to 55 years of age and those <40 and ≥55 years of age. Global RV ejection fraction for age groups <40, 40 to 55, and ≥55 years were 57 ± 5%, 56 ± 6%, and 54 ± 6%, respectively. Regional ejection fractions for the respective age groups were as follows: apex: 60 ± 5%, 59 ± 5%, and 57 ± 5%; body: 56 ± 5%, 54 ± 6%, and 53 ± 7%; RVOT: 55 ± 5%, 55 ± 6%, and 54 ± 7%; and RVIT: 58 ± 5%, 56 ± 5%, and 54 ± 6%, respectively. There were no significant differences between age groups in total or regional volumes.
With respect to age-related changes in regional curvature, the apical free wall was significantly less convex in the older subjects (≥55 years of age) compared with the younger age group (<40 years of age). The body free wall was flatter or “less rounded” in the older group compared with the younger group. No significant differences in septal curvature were noted with age. Also, in the oldest age group, the RVIT was rounder, whereas the RVOT was flatter (Figure 6).
Figure 6.
Age-related changes in curvature for each of the regions (apical free wall, top left; apical septum, top middle; RVIT, top right; body free wall, bottom left; body septum, bottom middle; and RVOT, bottom right). Solid bars represent mean curvature values in end-diastole, while hatched bars represent mean curvature values in end-systole. Stars represent statistically significant differences between groups denoted by the horizontal color-coded brackets. See text for details.
When regional curvature changes throughout the cardiac cycle were compared between age groups, age-related differences were noted in the apical and RVIT regions (Table 3). Both the body free wall and RVOT transitioned to become significantly flatter in end-systole, irrespective of age group. However, in the older age groups (>40 years of age) the apical free-wall, while continuing to transition to a more convex (pointed) shape from end-diastole to end-systole, the overall increase in convexity was less pronounced in the older age group. Similarly, although the RVIT still progressed to a flatter shape in end-systole, the curvature difference between end-diastole and end-systole was less pronounced compared with the younger age group (Table 3, Figure 6).
Table 3.
Regional curvature changes during the transition from end-diastole to end-systole by gender and age groups
| Region | All ages | Men | Women | <40 y | 40–55 y | ≥55 y |
|---|---|---|---|---|---|---|
| n | 245 | 108 | 137 | 114 | 83 | 48 |
| Apical free wall | * | * | * | * | ||
| Body free wall | * | * | * | * | * | * |
| Apical septum | ||||||
| Body septum | * | * | ||||
| RVOT | * | * | * | * | * | * |
| RVIT | * | * | * | * |
The change in regional curvature between end-diastole and end-systole in the specified region is significant (P < .05).
Interobserver Variability
Analysis of repeated measurements showed fair to excellent reproducibility. The intraclass correlation coefficients for interoperator variability of 3D curvature for the RVOT, RVIT, septum, and free wall were 0.94, 0.91, 0.74, and 0.93 in end-diastole and 0.95, 0.94, 0.65, and 0.92 in end-systole, respectively. The values for intraoperator variability for the same regions were 0.97, 0.91, 0.80, and 0.97 in end-diastole and 0.97, 0.96, 0.77, and 0.95 in end-systole, respectively.
DISCUSSION
In this study, we quantified regional curvature indices from 3D endocardial surfaces in a large group of normal right ventricles over a wide spectrum of ages. We demonstrated that (1) RV shape can be depicted using a color-coded surface curvature map, and regional curvature indices can be quantified; (2) the normal right ventricle is angulated at the apex and convex at the level of the free wall, and both the RVOT and the RVIT are rounded; (3) the normal right ventricle follows a consistent and dynamic pattern when transitioning from end-diastole to end-systole, whereby the apex becomes more narrowly angulated, while the RV free wall, RVOT, and RVIT become flatter; (4) there are no gender differences in regional RV curvature; and (5) with aging, the RV free wall and RVOT become less rounded (i.e., flatter), whereas the transition from end-diastole to end-systole produces a less pointed apex. The findings of this study provide the basis for characterization of morphologic changes in RV shape with increasing or decreasing loading conditions, as a result of disease progression or therapy.
Lessons Learned from the Analysis of LV Shape
Changes in LV shape have been linked to both adaptive and maladaptive remodeling in a number of disease states, including ischemic and nonischemic cardiomyopathy and valvular heart disease.7,16–20 Global shape parameters, including sphericity and conicity, have been studied using 3D transthoracic data sets of the left ventricle.5 Severe mitral regurgitation has been shown to negatively affect LV shape, rendering the ventricle more spherically shaped despite having a preserved ejection fraction in the initial stages of regurgitation. Spherical LV shape has been associated with greater morbidity and mortality.17,21 Favorable LV remodeling patterns after mitral valve repair or percutaneous mitral valve surgery can be detected using indices of sphericity and conicity.22 Because of the complexity of its shape, however, the right ventricle cannot be characterized using these same indices. We have recently shown that the right ventricle in patients with severe pulmonary arterial hypertension can be differentiated from normal right ventricles using regional curvature–based analysis.15
Describing RV Shape Using Regional Curvature
We segmented the right ventricle into regions (apex, body, RVOT, and RVIT) to quantify the regional shape of this complex structure. Furthermore, by individually analyzing the free wall and septum, it was possible to differentiate between distortions of these unique regions. For instance, in our cohort of healthy volunteers, the RV septum (both apical and body components) did not change significantly in shape during the transition between end-diastole and end-systole. In contrast, the body free wall became flatter, while the apical free wall became more “pointed” during this transition. This transition from end-diastole to end-systole describes the “bellows-like” pattern during RV contraction. Interestingly, both men and women had similarly shaped right ventricles, with similar changes in regional curvature values between end-diastole and end-systole. Age, in contrast, had a significant impact on regional curvature values. In the older age group, both free wall segments (apex and body) were flatter, and the progression of the apex free wall from a less convex surface to a more pointed surface was significantly less pronounced compared with the younger age group. These age-related changes in RV shape could suggest that the right ventricle becomes less compliant with age, resulting in a less pronounced pattern of contraction.
Impact of the Quantification of Regional RV Shape
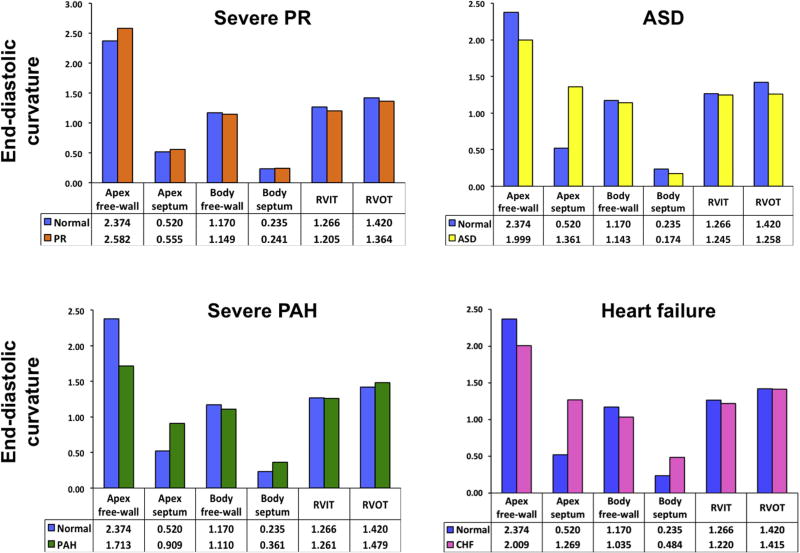
Figures 7 and 8 illustrate four different RV pathologies that were evaluated using the 3D regional curvature–based analysis method described in this study. Each individual patient’s regional end-diastolic curvature values were compared against the regional normal reference values generated in this study. The bar graphs in Figure 7 plot the mean regional curvature values from the normal population (blue bars) juxtaposed against the regional RV curvature values obtained for each individual patient (colored bars). A parametric display of regional curvature indices was produced for each pathology (Figure 8). The scale for this curvature map is located on the far right, with red hues depicting positive curvature values (convexity) and blue hues negative curvature values (concavity). The green color reflects a curvature of zero (flat surface). Table 4 reports the 2D echocardiographic parameters that were measured in each patient.
Figure 7.
Bar graphs comparing end-diastolic regional curvature values for the normal cohort analyzed in this study (blue bars) and patients with select RV pathologies (colored bars): top left, chronic severe pulmonary regurgitation (PR); top right, secundum-type ASD with moderate pulmonary hypertension; bottom left, severe pulmonary arterial hypertension (PAH); bottom right, acute, decompensated heart failure (CHF). See text for details.
Figure 8.
Regional color-coded parametric end-diastolic regional curvature maps for each right ventricle plotted in Figure 6. The colored bar on the far right represents the color-curvature legend. Red and orange hues represent varying degrees of convexity, with the reddest hues representing the most convex surface. Green represents a flat surface (curvature = 0). Blue hues represent varying degrees of concavity, with the darkest blue representing the most concave surface. Each right ventricle (except for the one on the bottom right) is displayed from the free wall perspective and the septal perspective. The pathologies are labeled: top left (A), chronic severe pulmonary regurgitation (PR); top right (B), secundum-type ASD with moderate pulmonary hypertension; bottom left (C), severe pulmonary arterial hypertension (PAH); bottom right (D), patient with acute, decompensated heart failure. The yellow arrows point to the apex and the white arrows to the septum. See text for details.
Table 4.
Two-dimensional echocardiographic parameters in four different RV pathologies
| Dimensions | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Case | RV pathology | RVEDb (mm) | RVEDm (mm) | RV length (mm) | Systolic PA pressure (mm Hg) | TR vena contracta |
| Normal | 38 ± 6 | 28 ± 6 | 72 ± 9 | NA | NA | |
|
| ||||||
| 1 | Chronic severe PR | 51 | 49 | 91 | 36 | 0.23 |
|
| ||||||
| 2 | ASD | 63 | 48 | 108 | 58 | 0.52 |
|
| ||||||
| 3 | PAH | 55 | 47 | 88 | 103 | 0.57 |
|
| ||||||
| 4 | Heart failure | 49 | 42 | 89 | 62 | 0.52 |
NA, Not applicable; PA, pulmonary artery; PAH, pulmonary arterial hypertension; PR, pulmonary regurgitation; RVEDb, RV end-diastolic dimension at the base; RVEDm, RV end-diastolic dimension at the mid-level; TR, tricuspid regurgitation.
The first case (Figure 7, top left, and Figure 8A) is a patient with severe chronic severe pulmonary regurgitation. The bar graph shows that the apex free wall is more pointed in this patient. From the 2D echocardiographic measurements (Table 4), it can be appreciated that the RV length dimension is >2 SDs above the length for the normal cohort, suggesting that the increased RV length without a concomitant change in RV width likely accounts for the more pointed apex (Figure 8A, yellow arrow).
The second case (Figure 7, top right, and Figure 8B) illustrates a patient with a secundum atrial septal defect (ASD). The bar graphs show that compared with the normal group, the patient with ASD has a more rounded apex free wall, a flatter RVOT, and a more convex septum that bulges more into the left ventricle. These changes can also be appreciated on the parametric color map, as the septum is less “blue” and therefore more convex in the patient with an ASD (white arrow), and the apex is less pointed (yellow arrow). The relative convexity of the septum in the patient with an ASD is due in part to the presence of least moderate pulmonary hypertension (systolic pulmonary artery pressure 58 mm Hg; see Table 4).
The third case (Figure 7, bottom left, and Figure 8C) illustrates a patient with severe pulmonary arterial hypertension. The bar graph shows that in this patient, the apex free wall is rounded and the septum (both apical and body segments) more convex. This shift in the septal curvature in pulmonary arterial hypertension is synonymous with the septal “flattening” that is seen on 2D echocardiography in these patients. In this case, systolic pulmonary pressure was 103 mm Hg (Table 4).
The fourth case (Figure 7, bottom right, and Figure 8D) depicts the right ventricle of a patient with acute decompensated heart failure with reduced ejection fraction (congestive heart failure). The bar graph shows that the patient with heart failure has a more rounded apex, a more convex septum, and a flatter body free wall compared with the control group. This case illustrates the impact of loading conditions on the right ventricle.
These clinical examples demonstrate the potential utility of our proposed method for quantifying RV regional curvature. In all these situations, changes in volume and pressure overload appear to be the predominant driving forces for changes in RV shape. Further studies are needed to better characterize unique changes in RV shape secondary to different combinations of loading conditions. Furthermore, the parametric display of regional RV curvature could be useful as a quantitative visual aide to objectively follow RV remodeling and the impact of therapeutic interventions. The normal values of RV shape reported in this study are integral to fully understanding the shape changes related to a particular disease process. Thereafter, we envision that each patient could be used as his or her own control.
Limitations
Curvature-based analysis of 3D data sets is experience dependent because it requires acquisition of good-quality images and experienced endocardial tracing. A learning curve is anticipated before the tools described in this study can be used by other investigators. Acquisition of 3D RV data sets is challenging and is often hampered by varying degrees of anterior wall dropout. With a combination of image optimization during suspended expiration or inspiration as well as judicious use of nonstandard RV views and specific attention to patient position, it is possible to obtain reasonable-quality RV data sets, especially in subjects with normal-sized right ventricles. Additionally, endocardial tracings of the right ventricle using 3D data sets have become easier in the past few years13,14 with the availability of new and more user-friendly software that allows tracings to be performed in short- and long-axis planes. Given this new method for RV assessment, the difficulty associated with RV analysis lies mainly with adequacy of the 3D acquisition. In the future, automated programs may make this methodology even more accessible. The method for RV segmentation used in this study was decided on the basis of consensus of the authors. There are conceivably other ways to segment the right ventricle, but we felt that the methodology proposed here was the easiest to implement. Curvature measurements, like volume data, are influenced by the frame rate of the 3D data set. In our study we tried to ensure optimal frame rates by decreasing sector width and optimizing depth during a four- to six-beat breath hold. Despite this, frame rates varied in response to patient and machine-related factors. Future improvements in transducers should help rectify some of these limitations.
Finally, in our cohort of healthy subjects, RV ejection fractions increased with age and RV volumes showed no significant changes with age. These findings appear to be in conflict with some of the published findings from our group and others.23–25 However, putting our results in context, our cohort was smaller than the cohorts previously studied. The RV software used in this study was also different from the one used in the previous studies. Furthermore, although one study found increasing RV ejection fraction with age and decreasing volumes, another did not report significant changes in RV function and volumes with age. Larger studies are under way to help resolve these issues.
CONCLUSIONS
Regional curvature analysis, as described in this study, can be used to characterize the dynamic behavior of the normal right ventricle, providing additive information not otherwise available through conventional parameters such as TAPSE, FAC, or even 3D volumes and ejection fraction. With regional curvature analysis, it is possible to generate a color-coded parametric map of regional RV shape and describe in a quantitative manner regional changes in RV shape during the transition from end-diastole to end-systole.
HIGHLIGHTS.
RV shape can be quantified using regional curvature.
Parametric overlays of curvature on the RV endocardial surface help depict RV shape.
Positive curvature indicates a convex surface; negative values imply a concave surface.
During the cardiac cycle, the normal RV free wall flattens and the apex becomes convex.
During the cardiac cycle, the normal RV outflow and inflow tracts flatten.
Knowledge of normal RV shape allows characterization of the abnormal RV.
Acknowledgments
The authors received research grants from Philips Healthcare (Dr. Lang) and Astellas Pharma (Dr. Mor-Avi) for other unrelated studies. Dr. Badano and Dr. Muraru have received equipment grants from GE Vingmed (Horten, N) and TomTec Imaging Systems (Unterschleissen, D), and received Speakers’ honoraria from GE Vingmed (Horten, N). Thomas Ryan, MD, FASE, served as guest editor for this report.
Abbreviations
- 2D
Two-dimensional
- 3D
Three-dimensional
- ASD
Atrial septal defect
- FAC
Fractional area change
- LV
Left ventricular
- RV
Right ventricular
- RVIT
Right ventricular inflow tract
- RVOT
Right ventricular outflow tract
- TAPSE
Tricuspid annular plane systolic excursion
Footnotes
Conflicts of Interest: None.
References
- 1.Ryan T, Petrovic O, Dillon JC, Feigenbaum H, Conley MJ, Armstrong WF. An echocardiographic index for separation of right ventricular volume and pressure overload. J Am Coll Cardiol. 1985;5:918–27. doi: 10.1016/s0735-1097(85)80433-2. [DOI] [PubMed] [Google Scholar]
- 2.Sheehan FH, Ge S, Vick GW, III, Urnes K, Kerwin WS, Bolson EL, et al. Three-dimensional shape analysis of right ventricular remodeling in repaired tetralogy of Fallot. Am J Cardiol. 2008;101:107–13. doi: 10.1016/j.amjcard.2007.07.080. [DOI] [PubMed] [Google Scholar]
- 3.Kang DK, Thilo C, Schoepf UJ, Barraza JM, Jr, Nance JW, Jr, Bastarrika G, et al. CT signs of right ventricular dysfunction: prognostic role in acute pulmonary embolism. JACC Cardiovasc Imaging. 2011;4:841–9. doi: 10.1016/j.jcmg.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Topilsky Y, Khanna A, Le Tourneau T, Park S, Michelena H, Suri R, et al. Clinical context and mechanism of functional tricuspid regurgitation in patients with and without pulmonary hypertension. Circ Cardiovasc Imaging. 2012;5:314–23. doi: 10.1161/CIRCIMAGING.111.967919. [DOI] [PubMed] [Google Scholar]
- 5.Maffessanti F, Lang RM, Corsi C, Mor-Avi V, Caiani EG. Feasibility of left ventricular shape analysis from transthoracic real-time 3-D echocardiographic images. Ultrasound Med Biol. 2009;35:1953–62. doi: 10.1016/j.ultrasmedbio.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Douglas PS, Morrow R, Ioli A, Reichek N. Left ventricular shape, afterload and survival in idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 1989;13:311–5. doi: 10.1016/0735-1097(89)90504-4. [DOI] [PubMed] [Google Scholar]
- 7.St John Sutton M, Linde C, Gold MR, Abraham WT, Ghio S, Cerkvenik J, et al. Left ventricular architecture, long-term reverse remodeling, and clinical outcome in mild heart failure with cardiac resynchronization: results from the REVERSE trial. JACC Heart Fail. 2017;5:169–78. doi: 10.1016/j.jchf.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Wong SP, French JK, Lydon AM, Manda SO, Gao W, Ashton NG, et al. Relation of left ventricular sphericity to 10-year survival after acute myocardial infarction. Am J Cardiol. 2004;94:1270–5. doi: 10.1016/j.amjcard.2004.07.110. [DOI] [PubMed] [Google Scholar]
- 9.Reynertson SI, Kundur R, Mullen GM, Costanzo MR, McKiernan TL, Louie EK. Asymmetry of right ventricular enlargement in response to tricuspid regurgitation. Circulation. 1999;100:465–7. doi: 10.1161/01.cir.100.5.465. [DOI] [PubMed] [Google Scholar]
- 10.Maffessanti F, Lang RM, Niel J, Steringer-Mascherbauer R, Caiani EG, Nesser HJ, et al. Three-dimensional analysis of regional left ventricular endocardial curvature from cardiac magnetic resonance images. Magn Reson Imaging. 2011;29:516–24. doi: 10.1016/j.mri.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Leary PJ, Kurtz CE, Hough CL, Waiss MP, Ralph DD, et al. Three-dimensional analysis of right ventricular shape and function in pulmonary hypertension. Pulm Circ. 2012;2:34–40. doi: 10.4103/2045-8932.94828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Medvedofsky D, Addetia K, Patel AR, Sedlmeier A, Baumann R, Mor-Avi V, et al. Novel approach to three-dimensional echocardiographic quantification of right ventricular volumes and function from focused views. J Am Soc Echocardiogr. 2015;28:1222–31. doi: 10.1016/j.echo.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 14.Muraru D, Spadotto V, Cecchetto A, Romeo G, Aruta P, Ermacora D, et al. New speckle-tracking algorithm for right ventricular volume analysis from three-dimensional echocardiographic data sets: validation with cardiac magnetic resonance and comparison with the previous analysis tool. Eur Heart J Cardiovasc Imaging. 2016;17:1279–89. doi: 10.1093/ehjci/jev309. [DOI] [PubMed] [Google Scholar]
- 15.Addetia K, Maffessanti F, Yamat M, Weinert L, Narang A, Freed BH, et al. Three-dimensional echocardiography-based analysis of right ventricular shape in pulmonary arterial hypertension. Eur Heart J Cardiovasc Imaging. 2016;17:564–75. doi: 10.1093/ehjci/jev171. [DOI] [PubMed] [Google Scholar]
- 16.Di Donato M, Dabic P, Castelvecchio S, Santambrogio C, Brankovic J, Collarini L, et al. Left ventricular geometry in normal and post-anterior myocardial infarction patients: sphericity index and ‘new’ conicity index comparisons. Eur J Cardiothorac Surg. 2006;29(suppl 1):S225–30. doi: 10.1016/j.ejcts.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Di Mauro M, Iaco AL, Bencivenga S, Clemente D, Marcon S, Asif M, et al. Left ventricular surgical remodelling: is it a matter of shape or volume? Eur J Cardiothorac Surg. 2015;47:473–9. doi: 10.1093/ejcts/ezu186. [DOI] [PubMed] [Google Scholar]
- 18.Dor V, Montiglio F, Sabatier M, Coste P, Barletta G, Di Donato M, et al. Left ventricular shape changes induced by aneurysmectomy with endoventricular circular patch plasty reconstruction. Eur Heart J. 1994;15:1063–9. doi: 10.1093/oxfordjournals.eurheartj.a060629. [DOI] [PubMed] [Google Scholar]
- 19.Gaudron P, Eilles C, Ertl G, Kochsiek K. Adaptation to cardiac dysfunction after myocardial infarction. Circulation. 1993;87:IV83–9. [PubMed] [Google Scholar]
- 20.Maffessanti F, Caiani EG, Tamborini G, Muratori M, Sugeng L, Weinert L, et al. Serial changes in left ventricular shape following early mitral valve repair. Am J Cardiol. 2010;106:836–42. doi: 10.1016/j.amjcard.2010.04.044. [DOI] [PubMed] [Google Scholar]
- 21.Castelvecchio S, Menicanti L, Ranucci M, Di Donato M. Impact of surgical ventricular restoration on diastolic function: implications of shape and residual ventricular size. Ann Thorac Surg. 2008;86:1849–54. doi: 10.1016/j.athoracsur.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Gripari P, Tamborini G, Bottari V, Maffessanti F, Carminati MC, Muratori M, et al. Three-dimensional transthoracic echocardiography in the comprehensive evaluation of right and left heart chamber remodeling following percutaneous mitral valve repair. J Am Soc Echocardiogr. 2016;29:946–54. doi: 10.1016/j.echo.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 23.Kawut SM, Lima JA, Barr RG, Chahal H, Jain A, Tandri H, et al. Sex and race differences in right ventricular structure and function: the multiethnic study of atherosclerosis-right ventricle study. Circulation. 2011;123:2542–51. doi: 10.1161/CIRCULATIONAHA.110.985515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maffessanti F, Muraru D, Esposito R, Gripari P, Ermacora D, Santoro C, et al. Age-, body size-, and sex-specific reference values for right ventricular volumes and ejection fraction by three-dimensional echocardiography: a multicenter echocardiographic study in 507 healthy volunteers. Circ Cardiovasc Imaging. 2013;6:700–10. doi: 10.1161/CIRCIMAGING.113.000706. [DOI] [PubMed] [Google Scholar]
- 25.Tamborini G, Marsan NA, Gripari P, Maffessanti F, Brusoni D, Muratori M, et al. Reference values for right ventricular volumes and ejection fraction with real-time three-dimensional echocardiography: evaluation in a large series of normal subjects. J Am Soc Echocardiogr. 2010;23:109–15. doi: 10.1016/j.echo.2009.11.026. [DOI] [PubMed] [Google Scholar]