Abstract
Introduction
Actual weight-based (AWB) chemotherapy dosing is recommended for obese patients in the 2012 ASCO Clinical Practice Guideline. CALGB 49907, which utilized ABW-based adjuvant chemotherapy dosing, was a phase 3 trial in women age ≥65 years with early stage breast cancer, providing the opportunity to examine impact of such dosing on toxicities and outcome in older patients with breast cancer.
Patients and Methods
Adverse event data were available for 615 of 633 enrolled patients. Objectives were to assess grade ≥3 hematologic/non-hematologic toxicities by treatment arm, age, study entry BSA/BMI, and relapse-free (RFS) and overall survival (OS) by BSA/BMI.
Results
The 615 patients were sub-grouped by BSA (quartiles) and standard BMI categories, with BMI underweight/normal weight categories combined. Overall, grade ≥3 non-hematologic and hematologic toxicities occurred in 39.8% and 28.3% of patients, respectively. There were no significant differences in grade ≥3 toxicities among BSA quartiles. However, more grade ≥3 hematologic toxicities occurred in the underweight/normal weight BMI subgroup compared to overweight/obese subgroups (p=0.048). Type of chemotherapy and age had no impact on toxicity occurrence by BSA/BMI categories. RFS was superior in the 25th–50th BSA percentile patients in univariate analysis (p=0.042), as was OS in both univariate and multivariate analyses (p=0.007, p=0.009, respectively). No differences in RFS or OS were found by BMI categories.
Conclusion
Obesity was not correlated with adverse relapse or survival outcome, and grade ≥3 toxicities were not greater with ABW-based dosing. This supports safety and efficacy of ABW-based dosing as per the 2012 ASCO clinical practice guideline.
Keywords: Elderly, breast cancer, actual body weight-based chemotherapy dosing, toxicity, outcome
Introduction
The American Society of Clinical Oncology (ASCO) clinical practice guideline for appropriate chemotherapy dosing for obese adult patients with cancer was published in 2012 [1]. Guideline recommendations were based on a two-decade systematic review of trials utilizing cytotoxic chemotherapy, primarily in breast, ovarian, colon, and non-small cell lung cancer, with no data specific to an elderly population. Trials involving novel targeted agents were not examined. Practice pattern studies had demonstrated that up to 40% of obese patients received reduced chemotherapy doses based not on actual body weight (ABW) but on ideal body weight (IBW) or “capped” doses, generally due to toxicity concerns [2,3]. However, the panel found no evidence that short- or long-term toxicities, including myelosuppression, were increased in obese patients receiving actual weight-based doses. It was recommended that actual weight-based dosing be considered for obese patients, regardless of age, and especially in the potentially curative setting such that disease-free (DFS) and overall survival (OS) rates are not compromised.
Cancer and Leukemia Group B (CALGB) 49907 was a phase three randomized study in which women ≥65 years of age with early stage (stages I-IIIB) breast cancer were randomly assigned with equal probability to standard adjuvant chemotherapy (either doxorubicin plus cyclophosphamide [AC] or cyclophosphamide, methotrexate, and 5-fluorouracil [CMF]) or capecitabine [C]. Eligible women had operable, histologically confirmed adenocarcinoma of the breast, Eastern Cooperative Oncology Group performance status (PS) 0–2, tumor diameter ≥1 cm, adequate hematologic/renal/hepatic function, clear surgical margins, and expected survival ≥5 years. The trial demonstrated the superior efficacy of standard adjuvant chemotherapy [4]. The primary endpoint was relapse-free survival (RFS); overall survival (OS) was a secondary endpoint. A total of 633 patients were enrolled (standard therapy, n=326; capecitabine, n=307). Actual weight-based chemotherapy dosing based upon body surface area (BSA) was utilized in this trial. This trial’s exclusive focus on a large group of older adults provided the opportunity to examine the impact of ABW (full)-based chemotherapy dosing on the occurrence of hematologic and non-hematologic toxicities, as well as outcome (RFS, OS), in older patients with breast cancer. CALGB is now a part of the Alliance for Clinical Trials in Oncology (Alliance).
Objectives
The objectives of this retrospective study of CALGB 49907 (Alliance A151436) were based upon adverse event assessment and potential relationship to actual weight-based dosing, age, and survival. The frequency of Common Toxicity Criteria version 2.0 grade three/four hematologic and non-hematologic adverse events among enrolled patients was determined. Toxicities were assessed by: 1) treatment arm (capecitabine versus standard therapy); 2) age at study entry (continuous, and by age category: 65-69, 70-79, ≥80 years [yrs]); and 3) study entry BSA, divided into quartiles, and BMI, divided as underweight/normal weight, overweight, and obese. Survival outcome (RFS, OS) was assessed by BSA and BMI categories.
Patients and Methods
The primary results of CALGB 49907, which accrued patients from September 2001 through December 2006, have been previously reported [4]. National cancer Institute Common Toxicity Criteria (NCI-CTC) adverse events across all cycles of delivered therapy (standard therapy [AC or CMF] or capecitabine) and outcome (RFS, OS) data were previously collected. Each participant signed an IRB-approved, protocol-specific informed consent in accordance with federal and institutional guidelines.
Body size at study entry was assessed by two measures. Body surface area (BSA) was calculated per Dubois formulation and stratified into BSA quartiles. Body mass index (BMI) was calculated by dividing body mass in kilograms (kg) by body height in meters squared (kg/m2). BMI categories were: underweight <18.5; normal weight 18.5-24.9; overweight 25-29.9; obese ≥30 [5]. As only nine patients were in the BMI underweight category, they were combined with normal weight patients (n=151) for analytic purposes. For a separate analysis, based upon prior reports of underweight BMI individuals having poorer outcome, the first two BMI categories were subdivided as: underweight/low normal weight <23; normal weight 23-25. The BSA and BMI categories were assessed by baseline stratification factors including age (65-69, 70-79, ≥80 years), ECOG PS (0-1, 2), and race (white, black, Asian, other), as well as treatment arm, to determine if the parameters were balanced among these categories. The primary endpoint of RFS was defined as time from study entry until local recurrence, distant metastases, or death from any cause, whichever occurred first. OS was defined as time from study entry until death from any cause.
Per protocol, all chemotherapy doses were determined by BSA as calculated from actual body weight, and were centrally reviewed. BMI was not utilized in dose calculations. Capecitabine doses were capped in the 22 patients with BSA ≥2.18 m2. Protocol-specific dose adjustments for methotrexate and capecitabine based on creatinine clearance were delineated. Cycle one planned and delivered doses were collected. Delivered doses within 10% of planned doses were considered “full dose” therapy. Myeloid growth factor and prophylactic antimicrobial usage were per ASCO guidelines and at the investigator’s discretion.
Statistical Analysis
Categorical variables were summarized as frequencies and percentages. Comparison of categorical variables between groups was made with a chi-square test or Fisher’s exact test if expected cell sizes were too small for chi-square tests. Overall survival events were defined as death due to any cause, and patients who were alive at the time of analysis were censored. Recurrence-free survival events were defined as recurrence (any location) or death due to any cause, and patients still alive and recurrence-free at the time of analysis were censored. Kaplan-Meier survival curves for OS and RFS were compared by log-rank test. Univariable and multivariable Cox models were used to generate hazard ratios with 95% confidence intervals (Cis). Multivariable models included BMI or BSA plus treatment arm. All tests were two-sided, and p values <0.05 were considered to be statistically significant. Analyses were performed using Statistical Analysis System (SAS) version 9.3 (SAS Institute Inc., Cary, NC). Data were frozen on March 3, 2015.
Results
Of the 633 enrolled patients, eighteen never received the assigned therapy and 615 were assessable for the occurrence of adverse events. Minimum and maximum study entry BSA values were 1.28 and 2.71 m2, respectively (median, 1.80 m2), with the 25th percentile BSA value of 1.66 m2 and 75th percentile BSA value of 1.96 m2. Minimum and maximum study entry BMI values were 18.0 and 53.0, respectively (median 28.6) with the 25th percentile BMI of 25.0, and 75th percentile BMI of 33.2. By BMI category, patients were normal or underweight (n=160; 26%), overweight (n=200; 32.5%), or obese (n=255; 41.5%). By age strata, 35% of patients were 65-69 years, 61% were 70-79 years, and 4% were ≥80 years of age. The majority of patients were Caucasian (85%), and had an ECOG PS of 0-1 (97%). BSA and BMI categories were balanced for these demographics, as well as for treatment arm.
Grade ≥3 non-hematologic and hematologic toxicities occurred in 39.8% and 28.3% of patients, respectively. Grade ≥3 anemia and thrombocytopenia were distinctly uncommon (2.1% and 2.4%, respectively). However, 16.3% of patients had grade ≥3 neutropenia. Eight patients had grade five toxicities: colitis, infection with grade three/four neutropenia (both probably related); intracranial hemorrhage/bleeding, constitutional symptoms-other, cardiac ischemia/infarction, upper airway infection with normal absolute neutrophil count (ANC), adult respiratory distress syndrome, cardiovascular/general-other (all unrelated or unlikely related). Drug-related deaths occurred in two patients on capecitabine, and in no standard chemotherapy patients.
Grade ≥3 toxicities were assessed by BSA quartile and BMI categories (Table 1). As only nine patients were in the BMI underweight (<18.5) category, they were combined with the normal weight patients (n=151) for analytic purposes. There were no significant differences in occurrence of any grade ≥3 adverse events (all, non-hematologic, hematologic, anemia, neutropenia, thrombocytopenia) among the BSA quartiles. However, among the three BMI categories, differences in occurrence of grade ≥3 hematologic toxicities were seen, with more adverse events in the underweight/normal weight subgroup (p=0.048). Specifically, more grade ≥3 anemia and neutropenia were seen in the underweight/normal weight subgroup (p=0.019, p=0.043, respectively), although grade ≥3 anemia occurrence was uncommon, overall (≤5% in all BMI categories). Similar findings were noted when the underweight/normal weight BMI category was divided into two groups (BMI ≤23, BMI 23-25, Table 2). There were no differences in grade ≥3 non-hematologic toxicities and thrombocytopenia by BMI category. The occurrence of grade ≥3 toxicities was also assessed by the type of chemotherapy received and BSA quartile and BMI categories (see supplementary data). The type of chemotherapy received had no impact on grade ≥3 toxicity occurrence in the BSA/BMI categories, except that anemia was more common in underweight/low normal BMI patients receiving standard chemotherapy, and neutropenia was more common in overweight BMI patients receiving capecitabine, although patient numbers were quite small. Grade three adverse event occurrence by age strata (65-69, 70-79 ≥80 years), as well as by age and chemotherapy received, was also assessed. No correlation of toxicity by age strata, or age strata and chemotherapy received, was found (see supplementary data).
Table 1.
Adverse Events Grade ≥3 by BSA and BMI Category (n=615)
| Type of AEs and number | BSA Category (no. pts., %) | BMI Category (no. pts., %) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| <25th Percentile (≤1.663) n=151 | 25th-50th Percentile (1.663-1.801) n=152 | 50th-75th Percentile (1.801-1.956) n=154 | >75th Percentile (>1.956) n=158 | p | Normal or Underweight (≤25) n=160 | Overweight (25-30) n=200 | Obese (≥30) n=255 | p | |
| Number of Grade ≥3 AEs (Any AEs) | 0.39 | 0.062 | |||||||
| 0 | 66 (43.7%) | 69 (45.4%) | 76 (49.4%) | 70 (44.3%) | 60 (37.5%) | 98 (49.0%) | 123 (48.2%) | ||
| 1 | 36 (23.8%) | 35 (23.0%) | 36 (23.4%) | 53 (33.5%) | 41 (25.6%) | 45 (22.5%) | 74 (29.0%) | ||
| 2-3 | 34 (22.5%) | 32 (21.0%) | 31 (20.1%) | 24 (15.2%) | 41 (25.6%) | 40 (20.0%) | 40 (15.7%) | ||
| 4 or more | 15 (9.9%) | 16 (10.5%) | 11 (7.1%) | 11 (7.0%) | 18 (11.2%) | 17 (8.5%) | 18 (7.1%) | ||
| Number of Grade ≥3 AEs (Non- Heme AEs only) | 0.63 | 0.20 | |||||||
| 0 | 96 (63.6%) | 90 (59.2%) | 95 (61.7%) | 89 (56.3%) | 92 (57.5%) | 128 (64.0%) | 150 (58.8%) | ||
| 1 | 31 (20.5%) | 34 (22.4%) | 40 (26.0%) | 45 (28.5%) | 38 (23.8%) | 39 (19.5%) | 73 (28.6%) | ||
| 2-3 | 17 (11.3%) | 18 (11.8%) | 15 (9.7%) | 19 (12.0%) | 22 (13.8%) | 26 (13.0%) | 21 (8.2%) | ||
| 4 or more | 7 (4.6%) | 10 (6.6%) | 4 (2.6%) | 5 (3.2%) | 8 (5.0%) | 7 (3.5%) | 11 (4.3%) | ||
| Number of Grade ≥3 AEs (Heme AEs only) | 0.63 | 0.048 | |||||||
| 0 | 102 (67.6%) | 105 (69.1%) | 111 (72.1%) | 123 (77.8%) | 103 (64.4%) | 139 (69.5%) | 199 (78.0%) | ||
| 1 | 25 (16.6%) | 23 (15.1%) | 24 (15.6%) | 21 (13.3%) | 27 (16.9%) | 35 (17.5%) | 31 (12.2%) | ||
| 2-3 | 22 (14.6%) | 23 (15.1%) | 18 (11.7%) | 14 (8.9%) | 28 (17.5%) | 25 (12.5%) | 24 (9.4%) | ||
| 4 or more | 2 (1.3%) | 1 (0.7%) | 1 (0.6%) | 0 | 2 (1.2%) | 1 (0.5%) | 1 (0.4%) | ||
| Anemia | 0.16 | 0.019 | |||||||
| Yes | 7 4.6%) | 2 (1.3%) | 2 (1.3%) | 2 (1.3%) | 8 (5.0%) | 3 (1.5%) | 2 (0.8%) | ||
| No | 144 (95.4%) | 150 (98.7%) | 152 (98.7 %) | 156 (98.7%) | 152 (95.0%) | 197 (98.5%) | 253 (99.2%) | ||
| Neutropenia | 0.36 | 0.043 | |||||||
| Yes | 29 (19.2%) | 28 (18.4%) | 23 (14.9%) | 20 (12.7%) | 34 (21.2%) | 35 (17.5%) | 31 (12.2%) | ||
| No | 122 (80.7%) | 124 (81.6%) | 131 (85.1%) | 138 (87.3%) | 126 (78.8%) | 165 (82.5%) | 224 (87.8%) | ||
| Thrombocytopenia | 0.95 | 0.71 | |||||||
| Yes | 4 (2.6%) | 4 (2.6%) | 4 (2.6%) | 3 (1.9%) | 5 (3.1%) | 5 (2.5%) | 5 (2.0%) | ||
| No | 147 (97.4%) | 148 (97.4%) | 150 (97.4 %) | 155 (98.1%) | 155 (96.9%) | 195 (97.5%) | 250 (98.0%) | ||
AE=adverse event
BSA=body surface area
BMI=body mass index
No. pts.=number of patients
N=number
Heme=hematologic
Table 2.
Adverse Events by 4-Level BMI Category (n=615)
| Type of AEs and number | BMI Category (no. pts., %) | p | ||||
|---|---|---|---|---|---|---|
| Underweight/Low Normal weight (<23) (n=82) | Normal weight (23-25) (n=78) | Overweight (25-30) (n=200) | Obese (≥30) (n=255) | |||
| Number of Grade ≥3 AEs (Any AEs) | 0.043 | |||||
| 0 | 31 (37.8%) | 29 (37.2%) | 98 (49.0%) | 123 (48.2%) | ||
| 1 | 20 (24.4%) | 21 (26.9%) | 45 (22.5%) | 74 (29.0%) | ||
| 2-3 | 18 (22.0%) | 23 (29.5%) | 40 (20.0%) | 40 (15.7%) | ||
| 4 or more | 13 (15.8%) | 5 (6.4%) | 17 (8.5%) | 18 (7.1%) | ||
| Number of Grade ≥3 AEs (Non-Heme AEs only) | 0.082 | |||||
| 0 | 42 (51.2%) | 50 (64.1%) | 128 (64.0%) | 150 (58.8%) | ||
| 1 | 22 (26.8%) | 16 (20.5%) | 39 (19.5%) | 73 (28.6%) | ||
| 2-3 | 11 (13.4%) | 11 (14.1%) | 26 (13.0%) | 21 (8.2%) | ||
| 4 or more | 7 (8.5%) | 1 (1.3%) | 7 (3.5%) | 11 (4.3%) | ||
| Number of Grade ≥3 AEs (Heme AEs only) | 0.053 | |||||
| 0 | 55 (67.0%) | 48 (61.5%) | 139 (69.5%) | 199 (78.0%) | ||
| 1 | 13 (15.8%) | 14 (18.0%) | 35 (17.5%) | 31 (12.2%) | ||
| 2-3 | 12 (14.6%) | 16 (20.5%) | 25 (12.5%) | 24 (9.4%) | ||
| 4 or more | 2 (2.4%) | 0 | 1 (0.5%) | 1 (0.4%) | ||
| Anemia | 0.008 | |||||
| Yes | 6 (7.3%) | 2 (2.6%) | 3 (1.5%) | 2 (0.8%) | ||
| No | 76 (92.7%) | 76 (97.4%) | 197 (98.5%) | 253 (99.2%) | ||
| Neutropenia | 0.037 | |||||
| Yes | 14 (17.1%) | 20 (25.6%) | 35 (17.5%) | 31 (12.2%) | ||
| No | 68 (82.9%) | 58 (74.4%) | 165 (82.5%) | 224 (87.8%) | ||
| Thrombocytopenia | 0.49 | |||||
| Yes | 4 (4.9%) | 1 (1.3%) | 5 (2.5%) | 5 (2.0%) | ||
| No | 78 (95.1%) | 77 (98.7%) | 195 (97.5%) | 250 (98.0%) | ||
AE=adverse event
BMI=body mass index
No. pts.=number of patients
N=number
Heme=hematologic
The impact of body size on RFS and OS (median follow-up of 2.4 yrs) was evaluated. Examining BSA quartiles, RFS differed by BSA percentile in univariate analysis with the most favorable outcomes in 25th-50th percentile patients (p=0.042); this only showed a trend in multivariate analysis (p=0.06) (Figure 1). Similarly, OS was superior in the 25th–50th percentile group, in both univariate and multivariate analyses (p=0.007 and p=0.009, respectively) (Figure 1). Both RFS and OS were shortest in the lowest BSA quartile. When assessing RFS and OS by BMI categories, no differences in RFS and OS (Figures 2,3) were found in either univariate or multivariate analyses.
Figure 1.
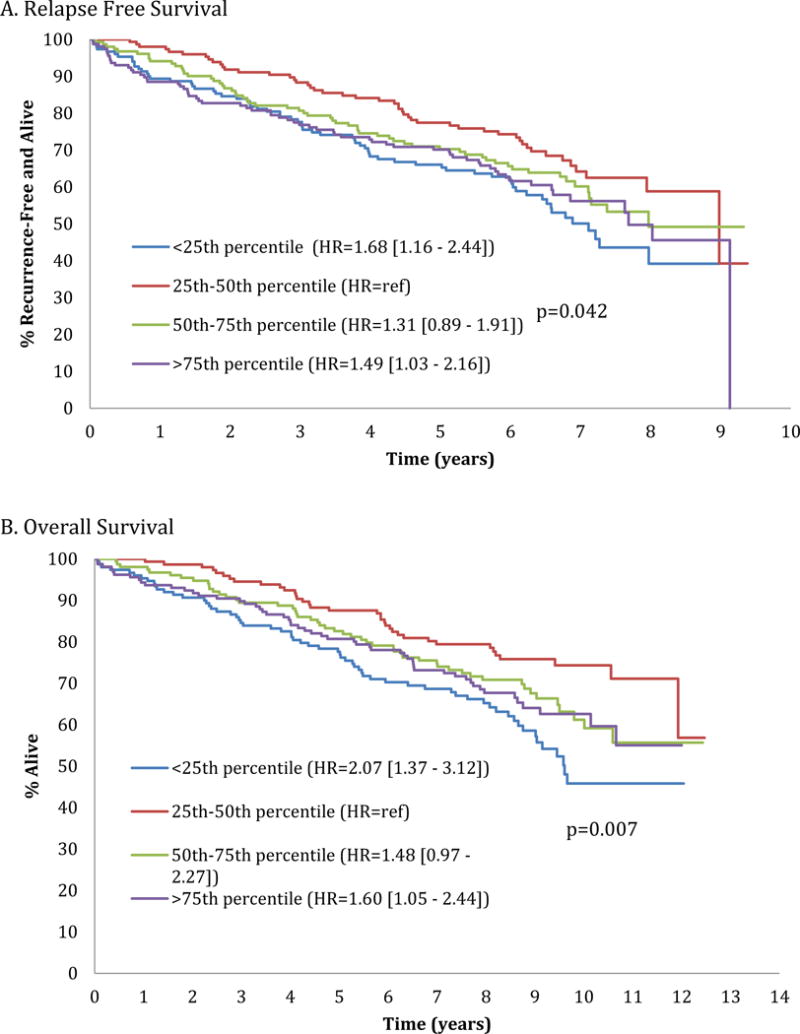
Relapse free (Figure A) and overall (Figure B) survival by body surface area [BSA] quartile category (<25th percentile, 25th-50th percentile, 50th-75th percentile, >75th percentile).
Figure 2.
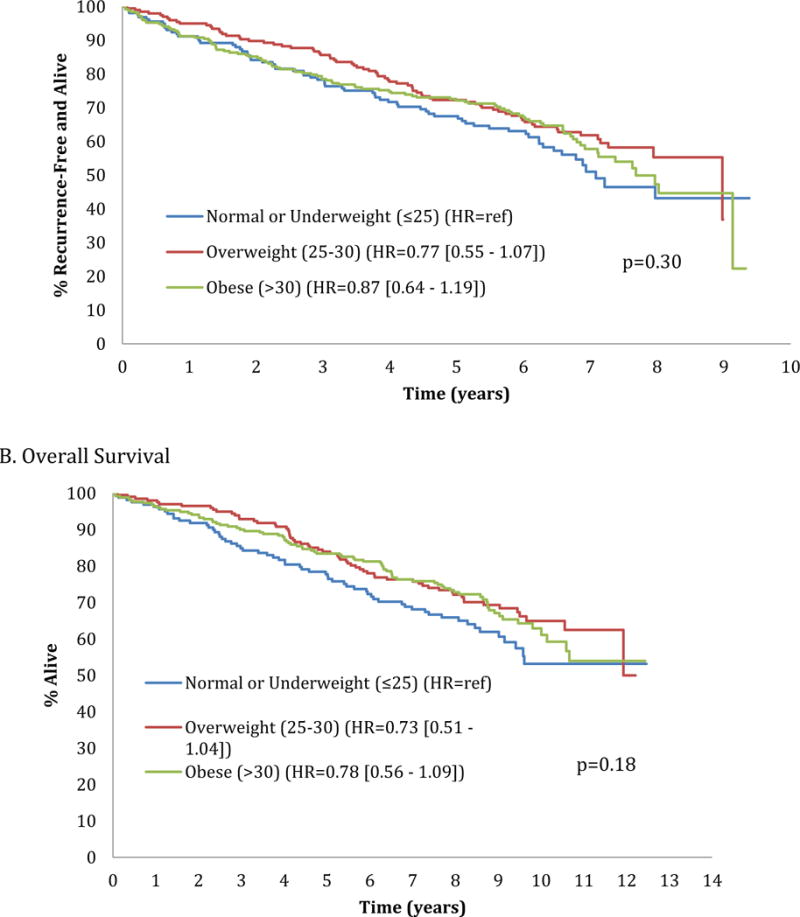
Relapse free (Figure A) and overall (Figure B) survival by standard body mass index [BMI] category (normal or underweight [BMI ≤25], overweight [BMI 25–30], obese [BMI >30]).
Figure 3.
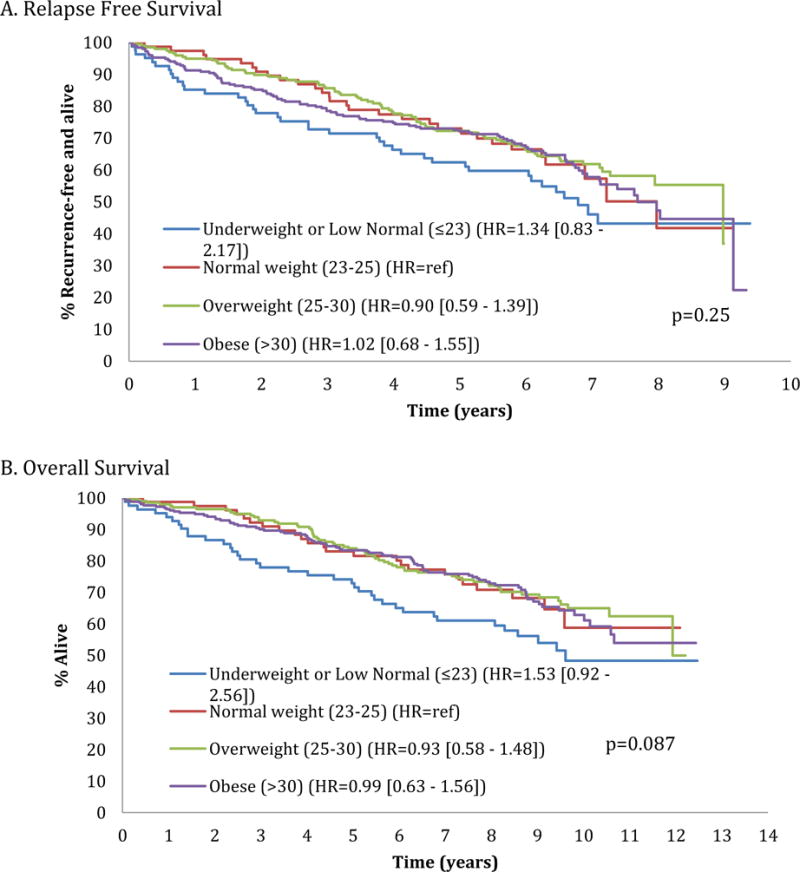
Relapse free (Figure A) and overall (Figure B) survival by redefined body mass index [BMI] category (underweight or low normal [BMI ≤23], normal [BMI 23–25], overweight [BMI 25–30], obese [BMI >30]).
Discussion
The results of our retrospective study of a large cohort of older women receiving breast cancer adjuvant chemotherapy provide verification of the ASCO clinical guideline that actual weight-based cytotoxic chemotherapy doses should be used to treat older obese patients with cancer, especially in the curative setting. A unique feature of our study was that it focused solely on patients ≥65 years of age. We found no increase in grade ≥3 toxicities (hematologic, non-hematologic) in overweight or obese patients as assessed by both BSA and BMI. Myelosuppression was more common in normal/underweight BMI patients, specifically grade ≥3 anemia and neutropenia. Such anemia was exceedingly uncommon, making this finding less impactful. When analyzed by treatment received, grade ≥3 neutropenia differences were confined to those patients receiving capecitabine.
We found that the lowest BSA quartile category had the shortest RFS and OS. Though not significant, the same pattern was seen for the underweight-normal weight BMI category. Likewise, when re-examining lower BMI individuals, the same pattern was seen in OS for those in the underweight/low normal BMI category by univariate and multivariate analyses (p=0.087 and p=0.096, respectively). These differences may not have reached statistical significance due to the small number of underweight/low normal weight BMI patients in our study. In addition, our arbitrary BSA quartiles may have been skewed toward “heavier” patients, with BMI categories more accurately delineating patient subgroups by body “size”. Others have reported poorer outcomes in older underweight patients [6–8]. All-cause mortality was significantly increased for individuals with either BMI <23 or BMI >33 in a meta-analysis of 197,940 community-based patients ≥65 years old [6]. In a longitudinal series of 70-year olds, 15-year mortality risk was greatest in those with BMI 14.1-22.5 [7]. In another series of >65 year old patients, the probability of death was greater with BMI <24, and increased two-fold in men of BMI <22 and women of BMI <20 [8]. Low BMI was associated with increased all-cause mortality in a large patient series ≥40 years old [9]. In contrast, in an adjuvant breast cancer study (CALGB 9741), each five-unit increase in BMI was associated with approximately 8% increased risk of cancer recurrence and death [10].
Unfortunately, our underweight BMI category of patients (n=9) was quite small, resulting in less meaningful analyses. We thus re-examined BMI in underweight/low normal (BMI<23) and normal weight (BMI 23-25) categories, and found that any grade ≥3 adverse events, anemia, and neutropenia were more common in underweight/low normal weight individuals, indicating this subgroup’s potential increased vulnerability to therapy-related toxicities. There was no impact by treatment arm on toxicity or outcome in the revised BMI categories except for anemia and neutropenia, with small patient numbers in both categories. No differences in grade ≥3 toxicities by age were found in our sub-analyses focusing on age strata, supporting that chemotherapy dose reductions should not be based on age alone.
Despite ASCO guidelines, actual weight-based dosing is not yet widely accepted among oncologists. Arbitrary dose adjustments due to concern for increased drug-related toxicities result in reduced dose intensity in obese patients with cancer [2,3]. Obesity is a significant issue, as >67% of US adults are overweight/obese (BMI >25), and 6% are morbidly obese (BMI >40) [11]. Drug metabolism and clearance are altered in obese individuals [12,13].
Both BSA and BMI have been utilized in dose calculations [14]. BSA dosing is associated with high pharmacokinetic (PK) variability [15–21]. BMI (body mass divided by height squared) reflects body shape and fat content, with different implications than BSA, and is used in nutritional and epidemiological studies [22–24]. Differences between ABW (“full-weight”) and IBW become greater as BMI increases [25]. As pharmacokinetics of anticancer agents in obese and non-obese patients may vary by agent and gender, empiric dose reductions in obese patients are discouraged [26].
Variability in breast cancer adjuvant chemotherapy dosing by body size has been reported in several series [10,26–41]. In 9672 women receiving adjuvant AC chemotherapy, doses were reduced in 11% overweight, 20% obese, and 37% severely obese patients [27]. With the importance of maintaining dose intensity, data support the use of actual weight-based dosing in heavier patients to optimize disease outcome, with no greater hematologic/non-hematologic toxicities seen [26–34]. The relationship between toxicities, outcome, and obesity has been examined in several adjuvant breast cancer trials. In CALGB 8541, obese patients did not sustain excess cycle one toxicities, and failure-free survival (FFS) was not negatively impacted with weight-based dosing [29]. However in CALGB 9741, baseline BMI was predictive for RFS and OS, with each five-unit increase in BMI associated with an 8% increased risk of cancer recurrence and death [10]. Another review of three cooperative group adjuvant chemotherapy trials found that increasing BMI within obese (BMI ≥30) and overweight (BMI 25-29.9) patients was associated with inferior DFS and OS among hormone-receptor positive and HER-2-negative patients [35]. Being overweight/obese (BMI >25) has been found to negatively impact breast cancer-specific and all-cause mortality in other series [36–41].
The impact of body size on adverse events and outcome has been reported in other solid tumors [12,25,42–51]. In one series using ABW-based dosing, grade three/four toxicities were significantly less common in obese versus normal weight patients, with comparable survival outcomes [42]. However, in another series of obese patients with cancer, grade four hematologic toxicities were more common in patients dosed by ABW than by IBW [25]. Increased BMI has been associated with shorter survival and increased recurrence risk in patients with breast and colon cancer, and with improved survival in patients with kidney and lung cancer [43–46]. In a small cell lung cancer series treated with ABW-based doses, no significant differences in survival or toxicities were found by BMI [47]. Several gynecologic malignancy studies found no increased grade three/four toxicities with ABW-based dosing in obese patients [12,48]. Fully dosed obese patients with colorectal cancer had no more toxicities than those given reduced doses, with shorter survival in dose-reduced, versus fully-dosed, patients [49]. In two colorectal cooperative group studies, therapy-related toxicities were no greater among overweight/obese patients, although obesity was associated with shorter survival and disease recurrence [50,51].
The impact of weight-based dosing on toxicity and survival has been examined more limitedly in hematologic malignancy patients. Some series found no increased therapy-related toxicities in obese non-Hodgkin lymphoma (NHL) or myeloma patients [25,52,53]. However, more grade three/fourtoxicities and infections occurred in overweight/obese patients in other NHL series [54]. Weight-based dosing of yttrium-90 ibritumomab tiuxetan has no correlation with grade three/four hematologic/non-hematologic toxicities [55]. OS was not compromised in overweight/obese NHL or Hodgkin lymphoma patients receiving ABW dosing, compared with normal weight patients [53,54,56,57]. Two recent series suggest that increased BMI was associated with prolonged survival in large cell NHL [58,59]. Likewise in a myeloma series, increased BMI was not associated with shorter survival [60]. In acute myeloid leukemia (AML) series, OS was briefer in normal, compared to overweight/obese, patients [61], and no increase in hematologic/non-hematologic toxicities was found in overweight/obese patients receiving ABW-based dosing [61–65].
Our study has several potential limitations. Analyses were retrospective (post hoc), as the trial was not designed to assess impact of dose on toxicities and outcome. Enrolled patients were highly selected with limited comorbidities and most being Caucasian with PS 0-1, which may not reflect real world experience. Some data subsets, including underweight individuals and those ≥80 years of age, were too small to analyze separately. As previously noted, our BSA quartiles were an arbitrary division, and as the study had fewer “smaller” patients (at least by BMI), it is likely that the BSA quartiles were “skewed” toward heavier patients, overall. Last, no pharmacokinetic analyses were included in this study.
In conclusion, findings of our large study in a uniquely older population support the safety, tolerability and efficacy of actual weight-based therapy in this curative treatment setting. BSA and BMI had no linear correlation with an adverse relapse/survival outcome, and grade ≥3 therapy-related toxicities were not greater with full dose therapy in obese patients. Our results add to evidence supporting the use of actual weight-based dosing as per the 2012 ASCO clinical practice guideline for appropriate chemotherapy dosing, including older patients with cancer for whom little prior evidence exists. As new agents are added to the oncologic therapeutic armamentarium, it will be of importance to include older adults in clinical trials examining such agents.
Acknowledgments
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under the Award Number UG1CA189823 (Alliance for Clinical Trials in Oncology NCORP Grant), U10CA016450, U10CA031946, U10CA032291, U10CA047559, U10CA047577, U10CA180790, U10CA180838, U10CA180857, and U10CA180867. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ClinicalTrials.gov Identifier: NCT00024102 (49907)
Conflict of Interest and Disclosure Statement
There are no conflict of interest to report.
Authorship Contributions
| Study concepts | Morrison, Muss, Jatoi, Cohen, Hurria |
| Study design | Morrison, Muss, Jatoi, Cohen, Hurria |
| Data acquisition | Morrison, McCall, Muss, Cirrincione |
| Quality control of data and algorithms | Morrison, McCall, Cirrincione, Lafky, Hurria |
| Data analysis and interpretation | Morrison, McCall, Muss, Jatoi, Cohen, Cirrincione, Ligibel, Lafky, Hurria |
| Statistical analysis | McCall, Cirrincione |
| Manuscript preparation | Morrison, Hurria |
| Manuscript editing | Morrison, McCall, Muss, Jatoi, Cohen, Cirrincione, Ligibel, Lafky, Hurria |
| Manuscript review | Morrison, McCall, Muss, Jatoi, Cohen, Cirrincione, Ligibel, Lafky, Hurria |
References
- 1.Griggs JJ, Mangu PB, Anderson H, et al. Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2012;30:1553–61. doi: 10.1200/JCO.2011.39.9436. [DOI] [PubMed] [Google Scholar]
- 2.Lyman GH, Dale DC, Crawford J. Incidence and predictors of low dose-intensity in adjuvant breast cancer chemotherapy: A nationwide study of community practices. J Clin Oncol. 2003;21:4524–31. doi: 10.1200/JCO.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Shayne M, Crawford J, Dale DC, et al. Predictors of reduced dose intensity in patients with early-stage breast cancer receiving adjuvant chemotherapy. Breast Cancer Res Treat. 2006;100:255–62. doi: 10.1007/s10549-006-9254-4. [DOI] [PubMed] [Google Scholar]
- 4.Muss HB, Berry DA, Cirrincione CT, et al. Adjuvant chemotherapy in older women with early-stage breast cancer. N Engl J Med. 2009;360:2055–65. doi: 10.1056/NEJMoa0810266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Physical status: the use of interpretation of anthropometry: report of a WHO expert committee. World Health Organ Tech Rep Ser. 1995;854:1–452. [PubMed] [Google Scholar]
- 6.Winter JE, MacInnis RJ, Wattanapenpaiboon N, et al. BMI and all-cause mortality in older adults: a meta-analysis. Am J Clin Nutr. 2014;99:875–90. doi: 10.3945/ajcn.113.068122. [DOI] [PubMed] [Google Scholar]
- 7.Heiat A, Vaccarino V, Krumholz HM. An evidence-based assessment of federal guidelines for overweight and obesity as they apply to elderly persons. Arch Int Med. 2001;161:1194–1203. doi: 10.1001/archinte.161.9.1194. [DOI] [PubMed] [Google Scholar]
- 8.Dey DK, Rothenberg E, Sundh V, et al. Body mass index, weight change and mortality in the elderly. A 15 year longitudinal population study of 70 yr olds. J Europ J Clin Nutrition. 2001;55:482–92. doi: 10.1038/sj.ejcn.1601208. [DOI] [PubMed] [Google Scholar]
- 9.Padwal R, Leslie WD, Lix LM, et al. Relationship among body fat percentage, body mass index, and all-cause mortality: A cohort study. Ann Intern Med. 2016;164:532–41. doi: 10.7326/M15-1181. [DOI] [PubMed] [Google Scholar]
- 10.Ligibel JA, Cirrincione CT, Liu M, et al. Body mass index, PAM50 subtype, and outcomes in node-positive breast cancer: CALGB 9741 (Alliance) J Natl Cancer Inst. 2015;107(9):djv179. doi: 10.1093/jnci/djv179. First published online June 25, 2015. JNCI 2016;107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogden CL, Carroll MD, Kit BK, et al. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen J, Stephan JM, Freesmeier M, et al. The effect of weight-based chemotherapy dosing in a cohort of gynecologic oncology patients. Gynecologic Oncology. 2015;138:154–8. doi: 10.1016/j.ygyno.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 13.Han PY, Duffull SB, Kirkpatrick CM, et al. Dosing in obesity: A simple solution to a big problem. Clin Pharmacol Ther. 2007;82:505–8. doi: 10.1038/sj.clpt.6100381. [DOI] [PubMed] [Google Scholar]
- 14.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. Nutrition. 1916;5:303–311. discussion 1989;312–13. [PubMed] [Google Scholar]
- 15.Beumer JH, Chu E, Salamone SJ. Body-surface area–based chemotherapy dosing: Appropriate in the 21st century? J Clin Oncol. 2012;30:3896–7. doi: 10.1200/JCO.2012.44.2863. [DOI] [PubMed] [Google Scholar]
- 16.Baker SD, Verweij J, Rowinsky EK, et al. Role of body surface area in dosing of investigational anticancer agents in adults, 1991–2001. J Natl Cancer Inst. 2002;94:1883–8. doi: 10.1093/jnci/94.24.1883. [DOI] [PubMed] [Google Scholar]
- 17.deJongh FE, Verweij J, Loos WJ, et al. Body-surface area-based dosing does not increase accuracy of predicting cisplatin exposure. J Clin Oncol. 2001;19:3733–9. doi: 10.1200/JCO.2001.19.17.3733. [DOI] [PubMed] [Google Scholar]
- 18.Mathijssen RHJ, Verweij J, de Jonge MJA, et al. Impact of body-size measures on irinotecan clearance: alternative dosing recommendations. J Clin Oncol. 2002;20:81–7. doi: 10.1200/JCO.2002.20.1.81. [DOI] [PubMed] [Google Scholar]
- 19.Loos WJ, Gelderblom H, Sparreboom A, et al. Inter- and intrapatient variability in oral topotecan pharmacokinetics: implications for body-surface area dosage regimens. Clin Cancer Res. 2000;6:2685–9. [PubMed] [Google Scholar]
- 20.Etienne MC, Chatelut E, Pivot X, et al. Co-variables influencing 5-fluorouracil clearance during continuous venous infusion. A NONMEM analysis. Eur J Cancer. 1998;34:92–7. doi: 10.1016/s0959-8049(97)00345-6. [DOI] [PubMed] [Google Scholar]
- 21.Pai MP. Drug dosing based on weight and body surface area: mathematical assumptions and limitations in obese adults. Pharmacotherapy. 2012;32:856–68. doi: 10.1002/j.1875-9114.2012.01108.x. [DOI] [PubMed] [Google Scholar]
- 22.National Institutes of Health. Health implications of obesity: National Institutes of Health Concensus Development Conference Statement. Ann Intern Med. 1985;103:1073–7. [PubMed] [Google Scholar]
- 23.Kuczmarski RJ, Flegal KM, Campbell SM, et al. Increasing prevalence of overweight among US adults: The national health and nutrition examination surveys, 1960 to 1991. JAMA. 1994;272:205–11. doi: 10.1001/jama.272.3.205. [DOI] [PubMed] [Google Scholar]
- 24.Williamson DF. Descriptive epidemiology of body weight and weight change in US adults. Ann Intern Med. 1993;119:646–9. doi: 10.7326/0003-4819-119-7_part_2-199310011-00004. [DOI] [PubMed] [Google Scholar]
- 25.Miyahara T, Mochinaga S, Kimura S, et al. Effects of tumor type, degree of obesity, and chemotherapy regimen on chemotherapy dose intensity in obese cancer patients. Cancer Chemother Pharmacol. 2013;71:175–82. doi: 10.1007/s00280-012-1994-8. [DOI] [PubMed] [Google Scholar]
- 26.Sparreboom A, Wolff AC, Mathijssen RHJ, et al. Evaluation of alternate size descriptors for dose calculation of anticancer drugs in the obese. J Clin Oncol. 2007;25:4707–13. doi: 10.1200/JCO.2007.11.2938. [DOI] [PubMed] [Google Scholar]
- 27.Griggs JJ, Sorbero ME, Lyman GH. Undertreatment of obese women receiving breast cancer chemotherapy. Arch Intern Med. 2005;165:1267–73. doi: 10.1001/archinte.165.11.1267. [DOI] [PubMed] [Google Scholar]
- 28.Greenman CG, Jagielski CH, Griggs JJ. Breast cancer adjuvant chemotherapy dosing in obese patients. Dissemination of information from clinical trials to clinical practice. Cancer. 2008;112:2159–65. doi: 10.1002/cncr.23416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosner GL, Hargis JB, Hollis DR, et al. Relationship between toxicity and obesity in women receiving adjuvant chemotherapy for breast cancer: Results from Cancer and Leukemia Group B study 8541. J Clin Oncol. 1996;14:3000–8. doi: 10.1200/JCO.1996.14.11.3000. [DOI] [PubMed] [Google Scholar]
- 30.Colleoni M, Li S, Gelber RD, et al. Relation between chemotherapy dose, oestrogen receptor expression, and body-mass index. Lancet. 2005;366:1108–10. doi: 10.1016/S0140-6736(05)67110-3. [DOI] [PubMed] [Google Scholar]
- 31.Poikonen P, Blomqvist C, Joensuu H. Effect of obesity on the leukocyte nadir in women treated with adjuvant cyclophosphamide, methotrexate, and fluorouracil dosed according to body surface area. Acta Oncol. 2001;40:67–71. doi: 10.1080/028418601750071082. [DOI] [PubMed] [Google Scholar]
- 32.Poikonen P, Saarto T, Lundin J, et al. Leucocyte nadir as a marker for chemotherapy efficacy in node-positive breast cancer treated with adjuvant CMF. Br J Cancer. 1999;80:1763–6. doi: 10.1038/sj.bjc.6690594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jenkins P, Elyan S, Freeman S. Obesity is not associated with increased myelosuppression in patients receiving chemotherapy for breast cancer. Eur J Cancer. 2006;43:544–8. doi: 10.1016/j.ejca.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 34.Baker SD, Grochow LB, Donehower RC. Should anticancer drug doses be adjusted in the obese patient? J Natl Cancer Inst. 1995;87:333–4. doi: 10.1093/jnci/87.5.333. [DOI] [PubMed] [Google Scholar]
- 35.Sparano JA, Wang M, Zhao F, et al. Obesity at diagnosis is associated with inferior outcomes in hormone receptor-positive operable breast cancer. Cancer. 2012;118:5937–46. doi: 10.1002/cncr.27527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamineni A, Anderson ML, White E, et al. Body mass index, tumor characteristics, and prognosis following diagnosis of early-stage breast cancer in a mammographically screened population. Cancer Causes Control. 2013;24:305–12. doi: 10.1007/s10552-012-0115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niraula S, Ocana A, Ennis M, et al. Body size and breast cancer prognosis in relation to hormone receptor and menopausal status: a meta-analysis. Breast Cancer Res Treat. 2012;134:769–81. doi: 10.1007/s10549-012-2073-x. [DOI] [PubMed] [Google Scholar]
- 38.Cheraghi Z, Poorolajal J, Hashem T, et al. Effect of body mass index on breast cancer during premenopausal and postmenopausal periods: a meta-analysis. PLoS One. 2012;7:e51446. doi: 10.1371/journal.pone.0051446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kwan ML, Chen WY, Kroenke CH, et al. Pre-diagnosis body mass index and survival after breast cancer in the After Breast Cancer Pooling Project. Breast Cancer Res Treat. 2012;132:729–39. doi: 10.1007/s10549-011-1914-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2010;123:627–35. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- 41.Azrad M, Demark-Wahnefried W. The association between adiposity and breast cancer recurrence and survival: A review of the recent literature. Curr Nutr Rep. 2014;3:9–15. doi: 10.1007/s13668-013-0068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hourdequin KC, Schpero WL, McKenna DR, et al. Toxic effect of chemotherapy dosing using actual body weight in obese versus normal-weight patients: a systematic review and meta-analysis. Ann Oncol. 2013;24:2952–62. doi: 10.1093/annonc/mdt294. [DOI] [PubMed] [Google Scholar]
- 43.Ewertz M, Jensen MB, Gunnarsdottir KA, et al. Effect of obesity on prognosis after early-stage breast cancer. J Clin Oncol. 2011;29:25–31. doi: 10.1200/JCO.2010.29.7614. [DOI] [PubMed] [Google Scholar]
- 44.Dignam JJ, Polite BN, Yothers G, et al. Body mass index and outcomes in patients who receive adjuvant chemotherapy for colon cancer. J Natl Cancer Inst. 2006;98:1647–54. doi: 10.1093/jnci/djj442. [DOI] [PubMed] [Google Scholar]
- 45.Waalkes S, Merseburger AS, Kramer MW, et al. Obesity is associated with improved survival in patients with organ-confined clear-cell kidney cancer. Cancer Causes Control. 2010;21:1905–10. doi: 10.1007/s10552-010-9618-2. [DOI] [PubMed] [Google Scholar]
- 46.Yang R, Cheung MC, Pedroso FE, et al. Obesity and weight loss at presentation of lung cancer are associated with opposite effects on survival. J Surg Res. 2011;170:e75–83. doi: 10.1016/j.jss.2011.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Georgiasis MS, Steinberg SM, Hankins LA, et al. Obesity and therapy-related toxicity in patients treated for small-cell lung cancer. J Nat Ca Inst. 1995;87:361–6. doi: 10.1093/jnci/87.5.361. [DOI] [PubMed] [Google Scholar]
- 48.Schwartz J, Toste B, Dizon DS. Chemotherapy toxicity in gynecologic cancer patients with a body surface area (BSA) > 2 m2. Gynecol Oncol. 2009;114:53–6. doi: 10.1016/j.ygyno.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 49.Chambers P, Daniels SH, Thompson LC, et al. Chemotherapy dose reductions in obese patients with colorectal cancer. Ann Oncol. 2012;23:748–53. doi: 10.1093/annonc/mdr277. [DOI] [PubMed] [Google Scholar]
- 50.Meyerhardt JA, Tepper JE, Niedzwiecki D, et al. Impact of body mass index on outcomes and treatment-related toxicity in patients with stage II and III rectal cancer: findings from Intergroup Trial 0114. J Clin Oncol. 2004;22:648–57. doi: 10.1200/JCO.2004.07.121. [DOI] [PubMed] [Google Scholar]
- 51.Meyerhardt JA, Catalano PJ, Haller DG, et al. Influence of body mass index on outcomes and treatment-related toxicity in patients with colon carcinoma. Cancer. 2003;98:484–95. doi: 10.1002/cncr.11544. [DOI] [PubMed] [Google Scholar]
- 52.Kuribyashi Kuribayashi T, Kondo M, et al. Feasibility of CHOP chemotherapy-With special reference to age, diabetes mellitus, liver cirrhosis, and obesity. Nippon Gan Chiryo Gakkai Shi. 1989;24:109–16. [PubMed] [Google Scholar]
- 53.Ganti A, Liu W, Luo S, et al. Impact of body mass index on incidence of febrile neutropenia and treatment-related mortality in United States veterans with diffuse large B-cell lymphoma receiving rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone. Br J Haematol. 2014;167:699–702. doi: 10.1111/bjh.13026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones JA, Fayad LE, Elting LS, et al. Body mass index and outcomes in patients receiving chemotherapy for intermediate-grade B-cell non-Hodgkin lymphoma. Leuk Lymphoma. 2010;51:1649–57. doi: 10.3109/10428194.2010.494315. [DOI] [PubMed] [Google Scholar]
- 55.Wiseman GA, Conti PS, Vo K, et al. Weight-based dosing of yttrium 90 ibritumomab tiuxetan in patients with relapsed or refractory B-cell non-Hodgkin lymphoma. Clin Lymphoma Myeloma. 2007;7:514–7. doi: 10.3816/clm.2007.n.035. [DOI] [PubMed] [Google Scholar]
- 56.Hong F, Habermann TM, Gordon LI, et al. The role of body mass index in survival outcome for lymphoma patients: US intergroup experience. Ann Oncol. 2014;25:669–74. doi: 10.1093/annonc/mdt594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weiss L, Melchardt T, Habringer S, et al. Increased body mass index is associated with improved overall survival in diffuse large B-cell lymphoma. Ann Oncol. 2014;25:171–6. doi: 10.1093/annonc/mdt481. [DOI] [PubMed] [Google Scholar]
- 58.Jones JA, Fayad LE, Elting LS, et al. Body mass index and outcomes in patients receiving chemotherapy for intermediate-grade B-cell non-Hodgkin lymphoma. Leuk Lymphoma. 2010;51:1649–57. doi: 10.3109/10428194.2010.494315. [DOI] [PubMed] [Google Scholar]
- 59.Carson KR, Bartlett NL, McDonald JR, et al. Increased body mass index is associated with improved survival in United States veterans with diffuse large B-cell lymphoma. J Clin Oncol. 2012;30:3217–22. doi: 10.1200/JCO.2011.39.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumar M, Nooka A, Langston A, et al. Impact of body mass index (BMI) on overall survival in myeloma. Proc Am Soc Hematol. 2012 abstr 4289. [Google Scholar]
- 61.Wenzell CM, Gallagher EM, Earl M, et al. Outcomes in obese and overweight acute myeloid leukemia patients receiving chemotherapy dosed according to actual body weight. Am J Hematol. 2013;88:906–9. doi: 10.1002/ajh.23530. [DOI] [PubMed] [Google Scholar]
- 62.Lee HJ, Licht AS, Hyland AJ, et al. Is obesity a prognostic factor for acute myeloid leukemia outcome? Ann Hematol. 2012;91:359–65. doi: 10.1007/s00277-011-1319-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Medeiros BC, Othus M, Estey EH, et al. Impact of body- mass index on the outcome of adult patients with acute myeloid leukemia. Haematologica. 2012;97:1401–4. doi: 10.3324/haematol.2011.056390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin A, Othus M, McQuary A, et al. Influence of obesity on efficacy and toxicity of induction chemotherapy in patients with newly diagnosed acute myeloid leukemia. Leuk Lymphoma. 2013;54:541–6. doi: 10.3109/10428194.2012.717278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Breccia M, Mazzarella L, Bagnardi V, et al. Increased BMI correlates with higher risk of disease relapse and differentiation syndrome in patients with acute promyelocytic leukemia treated with the AIDA protocols. Blood. 2012;119:49–54. doi: 10.1182/blood-2011-07-369595. [DOI] [PubMed] [Google Scholar]


