Abstract
Implementation science is a rapidly developing field dedicated to the scientific investigation of strategies to facilitate improvements in healthcare delivery. These strategies have been shown in several settings to lead to more complete and sustained change. In this essay, we discuss how refined surveillance recommendations for non-muscle-invasive bladder cancer, which involve a complex interplay between providers, healthcare facilities, and patients, could benefit from use of implementation strategies derived from the growing literature of implementation science. These surveillance recommendations are based on international consensus and indicate that the frequency of surveillance cystoscopy should be aligned with each patient's risk for recurrence and progression of disease. Risk-aligned surveillance entails cystoscopy at 3 and 12 months followed by annual surveillance for low-risk cancers, with surveillance every three months reserved for high-risk cancers. However, risk-aligned care is not the norm. Implementing risk-aligned surveillance could curtail overuse among low-risk patients, while curbing underuse among high-risk patients. Despite clear direction from respected and readily available clinical guidelines, there are multiple challenges to implementing risk-aligned surveillance in a busy clinical setting. Here, we describe how implementation science methods can be systematically used to understand determinants of care and to develop strategies to improve care. We discuss how the Tailored Implementation for Chronic Diseases framework can facilitate systematic assessment and how Intervention Mapping can be used to develop implementation strategies to improve care. Taken together, these implementation science methods can help facilitate practice transformation to improve risk-aligned surveillance for bladder cancer.
Keywords: bladder cancer, surveillance, cystoscopy, implementation science
Bladder Cancer is a Common and Expensive Disease
Bladder cancer is the third most prevalent non-cutaneous cancer in the United States (US), with a prevalence of ~600,000 and only surpassed by prostate cancer (in men), breast cancer (in women), and colorectal cancer (in both sexes) [1]. It is expected to become even more common over the next decade [2], because it primarily affects older patients (median age at diagnosis 73 [1]) and the US population is aging. The majority of bladder cancer patients – approximately 75% - are diagnosed with early stage non-muscle invasive bladder cancer (NMIBC) [3] and live many years with their disease (median survival after diagnosis of NMIBC >9 years [4]).
Patients with NMIBC are at risk for recurrence and progression of disease. Thus, they undergo an intensive surveillance regimen of cystoscopy, cytology, office visits and, in many cases, upper tract imaging. Furthermore, a high recurrence rate often necessitates repeat surgical resections and intravesical therapies. Within the Department of Veterans Affairs (VA) healthcare system, cystoscopy is the most common surgical procedure performed with approximately 80,000 procedures annually [5]. Thus, it is not surprising, that bladder cancer is an expensive cancer to diagnose and treat when considering per patient spending from diagnosis to death [6]. Given its high prevalence and cost, providing the most appropriate and efficient care for each patient is a high priority for patients, urologists, and healthcare systems.
Risk-Aligned Bladder Cancer Surveillance Care
All patients with NMIBC are at risk for recurrence and progression of disease. However, this risk varies widely. For example, 5-year risk for recurrence ranges from less than 30% for a patient with a solitary newly diagnosed low-grade non-invasive urothelial carcinoma to more than 50% for a patient with recurrent, multifocal high-grade non-invasive carcinoma [7,8]. Similarly, risk for progression ranges from a few percent at 5 years for patients with newly diagnosed low-grade non-invasive carcinoma to ~20% at 5 years for those with recurrent multifocal high-grade disease [7].
Over the last decade, guidelines for NMIBC bladder cancer have refined their recommendations to better align the frequency of cystoscopic surveillance with each patient's risk for recurrence and progression of disease. The National Comprehensive Cancer Network (NCCN) guidelines have recommended to perform surveillance cystoscopy for low-risk patients “at 3 months initially and then at increasing intervals” and for high-risk patients “at 3-month intervals for the first 2 years” dating back to at least 2000 (Table 1) [9]. The first specific recommendation for a risk-aligned surveillance approach was from the First International Consultation on Bladder Tumors in 2005. This consensus conference was convened by the World Health Organization (WHO) and the Société Internationale d’Urologie (SIU), and included experts from 3 continents and 7 countries [10]. They recommended cystoscopy at 3 and 12 months for low-risk patients and then annually thereafter, and cystoscopy every 3 months during the first 2 years for high-risk patients (Table 1) [10–12]. One year later, the European Association of Urology adopted these recommendations in their bladder cancer guidelines [13]. Since then, similar recommendations have been issued in the United Kingdom and in the United States (Table 1). Based on these recommendations, risk-aligned surveillance consists of cystoscopy at 3 and 12 months followed by annual surveillance for low-risk cancers, with surveillance every three months reserved for high-risk cancers.
Table 1.
Summary and timeline of guideline recommendations for risk-aligned bladder cancer care. Note: National Institute for Health and Care Excellence (NICE) review was rated as highest quality guideline in a recent Belgian assessment, using the Appraisal of Guidelines for REsearch & Evaluation (AGREE) Instrument [42].
| Year | Panel [citation] | Risk-aligned recommendation | Level of evidence or comment |
|---|---|---|---|
| 2000 | NCCN [9] | Low-risk: cystoscopy at 3 months, then at increasing intervals High-risk: cystoscopy every 3–6 months during first 2 years |
Category 2A: “uniform NCCN consensus, based on lower-level evidence” |
| 2005 | First International Consultation on Bladder Tumors [10–12] | Low-risk: cystoscopy at 3 & 12 months, then yearly x 5y High-risk: cystoscopy every 3 months during first 2 years |
Level 3 (good-quality retrospective case-control studies or case series) |
| 2006 | European Association of Urology [13] | Low-risk: cystoscopy at 3 & 12 months, then yearly x 5y High-risk: cystoscopy every 3 months during first 2 years |
Level 3 (good-quality retrospective case-control studies or case series) |
| 2011 | International Bladder Cancer Group (IBCG) [14] | Low-risk: cystoscopy at 3 & 12 months, then yearly x 5y High-risk: cystoscopy every 3 months during first 2 years |
International consensus based on review of current evidence. Recommendations follow EAU guidelines. |
| 2015 | Canadian Association of Urology [43] | Low-risk: may undergo cystoscopy at 3 & 12 months, then stop High-risk: cystoscopy every 3 months during first 2 years |
Level 3 evidence based on Oxford Centre for Evidence-based Medicine classification |
| 2015 | NICE [44] | Low-risk: cystoscopy at 3 & 12 months, then stop High-risk: cystoscopy every 3 months during first 2 years |
Grading of Recommendations, Assessment, Development and Evaluation (GRADE): low to moderate “The Guideline Development Group considered that there was insufficient evidence to be able to support recommendations for radical changes to follow-up for patients with high-risk bladder cancer. For low and intermediate risk groups, the clinical experience of the group and the limited evidence available were felt to be sufficient to make recommendations for a change in practice.” (page 249) |
| 2016 | International Bladder Cancer Network [15] | Low-risk: cystoscopy at 3 & 12 months, then yearly x 5y High-risk: cystoscopy every 3 months during first 2 years |
International consensus based on review of current evidence. |
| 2016 | American Urological Association / Society of Urologic Oncology Guideline on Diagnosis and Treatment of non- muscle invasive bladder cancer [45] | Low-risk: cystoscopy at 3 & 12 months, then yearly x 5y High-risk: cystoscopy every 3 months during first 2 years |
Moderate Recommendation; Evidence Strength: Grade C. Thus “applies to most patients in most circumstances but better evidence is likely to change confidence”. |
| 2017 | NCCN [46] | Low-risk: cystoscopy at 3 & 12 months, then yearly x 5y High-risk: cystoscopy every 3 months during first 2 years |
Category 2A: “Uniform NCCN consensus, based on lower-level evidence” |
There is now broad international consensus that risk-aligned surveillance care should be provided. Three international panels have met over the last decade and all of them have recommended risk-aligned surveillance. This includes the First International Consultation on Bladder Tumors in 2005 [10–12], the International Bladder Cancer Group in 2011 [14], and the International Bladder Cancer Network in 2016 [15]. Over time, the language used by these experts has become stronger, with the International Bladder Cancer Network stating that “a risk-based approach [to surveillance] is paramount” [15].
Despite these recommendations, the care that patients with NMIBC receive is frequently not risk-aligned. This is demonstrated by the fact that tumor characteristics such as stage and grade are only minimally associated with intensity of surveillance [16,17]. Instead, there is substantial variation in surveillance among providers [16,17], suggesting both overuse and underuse of optimal care and therefore an opportunity to utilize implementation science to better align practice patterns with risk-aligned surveillance recommendations.
Both overuse among low-risk and underuse among high-risk patients have several undesirable consequences for patients. Among low-risk patients, unnecessary surveillance cystoscopy procedures lead to more anxiety, discomfort, travel and opportunity costs [18]. Additionally, unnecessary cystoscopy procedures lead to more bladder biopsies and, subsequently, more complications [19–21]. They are also associated with excessive resource use and cost. Among high-risk patients, underuse of surveillance can be dangerous, as delays in timely diagnosis and treatment have been associated with increased mortality [22,23].
Given these undesirable consequences, there is a need to systematically develop strategies that make it easier to get the right care to the right patient every single time. These strategies would lead to reduction of overuse among patients with low-risk cancer and of underuse among those with high-risk cancer, contributing to higher value and more patient-centered care.
What are the Determinants of Risk-Aligned Bladder Cancer Surveillance?
To develop strategies to get the right care to the right patient, we need a clear understanding of the determinants of risk-aligned surveillance. Historically, implementation strategies for healthcare settings were based on a variety of approaches, such as continuing medical education, educational conferences, physician professionalism, employer mandates, and reimbursement structures. While these are still important drivers of staying “up to date”, concerted and organized efforts to change practice patterns often relied on best guesses, a method humorously referred to as the “ISLAGIATT principle”, that is “It seemed like a good idea at the time” [24]. This often led to interventions that may not have addressed the most important problem in a given setting. For example, a systematic review of 15 cluster randomized trials of clinical reminders found a median improvement in process measures of 14% but with a wide range from 1% deterioration to 34% improvement [25]. Thus, the lack of a full understanding of which strategy works in which setting has hampered the broader role-out of implementation strategies to improve care.
A more efficient and rigorous approach is to use frameworks built on theory and a growing body of evidence to systematically understand problems and develop implementation strategies to change care. Using a framework can provide guidance when understanding determinants of evidence-based care and can then be used to develop and test implementation strategies that mitigate specific barriers to care. Importantly, when frameworks and theory are used in this manner, selection of implementation strategies will be based on previous strong cumulative evidence demonstrating that the intervention is likely to be successful in addressing a specific barrier to evidence-based care [26].
One such comprehensive framework is the Tailored Implementation for Chronic Diseases (TICD) framework, which was specifically designed to address challenges in managing chronic diseases [27,28]. This makes it well suited for addressing challenges in managing NMIBC, which is similar to managing a chronic disease given that a majority of patients live with it for more than 9 years rather than quickly dying from it [4]. The TICD framework is based on a systematic review of the literature, incorporating 12 prior frameworks [28]. Since its publication in 2013, it has been widely used (>200 citations).
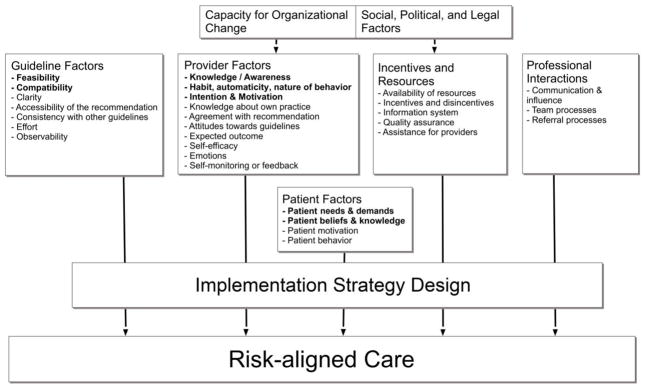
The Figure shows an example of how this framework can be applied to the provision of risk-aligned bladder cancer care [28]. It is the underpinning for the development of a systematic understanding of determinants of risk-aligned surveillance and can guide the selection, operationalization, and specification of implementation strategies that address these determinants. Determinants of risk-aligned surveillance are the factors that might prevent or enable improvements [27]. As shown in the Figure, the provision of risk-aligned surveillance is affected by guideline and provider factors, incentives and resources, professional interactions, and patient factors. For example, providers may lack detailed knowledge of the guidelines or may not have a habit of following the guidelines. A relevant incentive in a fee-for-service environment may be that providers are paid more for doing more cystoscopic surveillance regardless of risk – and so far incentives have been minimally affected by the introduction of Accountable Care Organizations [29]. Using the TICD framework, one can also explore how much such incentives matter in providers’ decision making and whether changes in reimbursement – for example the decrease in reimbursement for cystoscopy earlier this year [30,31] – modify these effects. Similarly, patient preferences and beliefs can be assessed. For example, patients may not follow through with risk-aligned care – even if recommended by providers – because their needs and demands are not met (e.g. rationale for the recommendations is not conveyed).
Figure.
Tailored Implementation for Chronic Diseases (TICD) framework [28] showing factors informing implementation strategy design and affecting risk-aligned bladder cancer care. We hypothesize the bolded determinants as salient.
How can we Develop Strategies to Improve Care?
A systematic framework-based approach as described above allows for organized development of implementation strategies to improve care. One such approach is Intervention Mapping, which uses a stepwise process to develop theory- and evidence-based implementation programs [32]. The steps entail (1) a needs assessment which is based on an understanding of the salient determinants of care, (2) defining proximal program objectives for the implementation strategies, (3) identifying theoretical and practical strategies to deliver risk-aligned bladder cancer care, (4) prioritizing strategies and producing materials, and (5) specifying implementation strategies and planning for implementation. We will briefly describe how these steps could be applied towards the development of implementation strategies for risk-aligned bladder cancer surveillance.
Step 1: Understanding the salient determinants of care
Once a framework such as the TICD is chosen, this framework can guide exploration of determinants of care. For example, semi-structured interviews with providers and patients help understand determinants of the care received. The interview guide can be based on the domains outlined in the framework, such that each domain can be explored during the interviews. To understand which domain may be most salient, it is recommended to start with a broad open-ended question. This will then provide insight into what first comes to providers' and patients' minds when discussing risk-aligned bladder cancer surveillance care. Following this, specific domains can be explored with questions that target each of the areas specified in the framework (Figure). The results from this qualitative work will be information on salient determinants of care that can be used to develop strategies to improve care in the steps that follow.
Step 2: Defining proximal program objectives for the implementation strategies
The next step is focused on defining proximal program objectives for the implementation strategies. These are developed within a matrix of specific performance objectives (rows) by TICD domains and determinants (see Table 2 for example) [33]. Performance objectives can be based on findings from interviews with providers and patients and include (1) facilitators and (2) barriers reframed into desirable objectives [32]. For each performance objective and TICD determinant, one can then identify a proximal program objective in each cell, specified as a change objective (what needs to be changed related to a determinant to accomplish the performance objective, see example matrix in Table 2) [32].
Table 2.
Example Matrix for defining program objectives during Intervention Mapping. One hypothetical performance objective is shown with a small selection of TICD domains and determinants. For each determinant, a proximal program objective is defined in each cell. TICD domains and determinants are listed as Domain – Determinant.
| Performance Objective | Proximal Program Objectives | |||
|---|---|---|---|---|
| Guideline – Feasibility | Provider – Nature of behavior | Patient – Knowledge | Patient – Demands | |
| Provider communicates cancer risk and follow- up recommendation clearly to patient. | Easy access to risk factors and cancer risk in electronic health record. | Automate risk assessment within provider workflow. | Provider effectively educates patient about their cancer risk. | Patient understands what a given risk means for them. |
Step 3: Identifying theoretical and practical strategies to deliver risk-aligned bladder cancer care
In the next step, one can draw upon the TICD and on other theories such as the Theoretical Domains Framework [34] or the Behavior Change Wheel [35] to identify theory- and evidence-based change techniques that match the proximal program objectives defined in the prior step. For each change technique, one can map practical implementation strategies and include suggestions obtained during qualitative interviews. Thus, one can build a matrix of proximal program objectives (rows), theory-based change techniques, practical strategies, and specific features or components suggested by providers and patients during the semi-structured interviews (brief example in Table 3). To enhance comparability across different implementation studies, it is recommended that practical implementation strategies be labeled using standardized nomenclature, for example using terms from the Expert Recommendations for Implementing Change (ERIC) [36].
Table 3.
Example Matrix describing the identification of implementation strategies to improve care. Two hypothetical proximal program objectives are shown together with potential strategies and hypothetical suggestions from interviews.
| Proximal Program Objective | Theory-based change technique | Practical strategy – ERIC label [36] | Suggestions from interviews |
|---|---|---|---|
| Automate risk assessment within provider workflow. | Prompt that triggers attention [26] | Implement prompt in provider workflow – Reminder for providers | Template prompts provider to assess and document risk |
| Provider effectively educates patient about their cancer risk. | Specify goal and increase skills [26] | Supply risk-communication materials to support patient education – Intervene with patients to enhance adherence | Pictogram conveys risk to patient in easy to understand format |
Step 4: Prioritizing strategies and producing materials
In Step 4, one can preferentially select strategies that have (1) the potential to affect multiple determinants, (2) have proven to be effective based on the published literature [25,37], and (3) have the highest potential to change practice. For example, assuming the hypothetical results shown in tables 2 and 3, a template prompting providers to assess risk could serve as a reminder for providers – an evidence-based strategy [37] – and would address two proximal program objectives, including easy access to risk factors and automation of work flow. It would thus be prioritized and created.
Step 5: Specifying implementation strategies and planning for implementation
Step 5 is focused on systematically specifying implementation strategies for risk-aligned bladder cancer care. Clear specification of strategies helps with initial implementation and enhances reproducibility in other contexts. One systematic way to specify strategies was described by Proctor and colleagues (see Table 4) [38]. Once specified, the strategies can be codified in a detailed implementation manual, describing exactly how each of them should be enacted [38].
Table 4.
Example showing how an implementation strategy will be specified according to seven dimensions [38]. Here, we show implementation of a prompt in providers' workflow as a hypothetical example.
| Proctor et al. dimensions [38] | Strategy: Implement prompt in provider workflow |
|---|---|
| Actors | Clinician champion at each site |
| Actions | Incorporate prompt into Electronic Health Record, educates local clinicians about purpose of prompt, collaborates with local clinicians to adapt prompt to local needs |
| Target of the action | Clinicians who provide cystoscopic bladder cancer surveillance care |
| Temporality | Prompt will be implemented in work-flow just prior to requesting the next follow-up cystoscopy |
| Dose | Prompt will be used in every cystoscopy encounter |
| Implementation outcomes affected | Adoption of risk-aligned care, feasibility, and fidelity of providing risk-aligned care |
| Justification | Behavior change theory[26] |
Product of this systematic process
The product of this systematic process is an implementation manual that includes highly specified implementation strategies for risk-aligned care. These can then be piloted and further refined in an iterative fashion. Generally, it is advisable to involve providers and patients in the step-wise process outlined above, for example in a steering and peer review group [39]. This involvement will increase the likelihood for success [40]. Each of the practical implementation strategies should have a theoretical justification and should be labeled according to the Expert Recommendations for Implementing Change (ERIC) [36]. For illustration purposes, we show a list of potential practical implementation strategies in Table 5 along with the domains and determinants that they address, their theoretical justification, and their ERIC label.
Table 5.
Potential implementation strategies, domains and determinants they address, theoretical justification, and ERIC label. ERIC = Expert Recommendations for Implementing Change [36].
| Practical implementation strategy | Domain – Determinant addressed | Theoretical justification | ERIC label [36] |
|---|---|---|---|
| Prompt in provider workflow | Provider – Nature of behavior Provider – Knowledge |
Behavior change theory: prompt triggers attention [26] Psychological capability (comprehension, reasoning) [35] |
Reminder for providers |
| Distribute diagnostic algorithm to assign risk | Guideline – Feasibility | Guideline implementation tool framework: point of care tools that integrate recommendations [47] | Distribute educational materials / toolkits |
| Audit and Feedback | Provider–Knowledge about own practice Provider–Self-monitoring or feedback |
Feedback intervention theory [48] | Audit and provide feedback |
| Academic Detailing | Guideline – accessibility Provider – knowledge Provider – motivation |
Social marketing [49] Behavior change theory: persuasive communication [26] |
Conduct educational outreach visits |
| Supply risk-communication materials to support patient education | Patient – demands Patient – knowledge Patient – motivation |
Evidence-based recommendation on how to communicate cancer risk to patients [50] | Intervene with patients to enhance adherence |
Summary
At first glance, following guideline recommendations and providing risk-aligned surveillance care may appear simple. However, doing it consistently in a busy clinical setting, as is true for many other changes in healthcare delivery, is challenging. The field of implementation science has developed frameworks and systematic methods that can help us think through the evaluation of a specific problem and the development of strategies to improve care. Here, we have described this systematic process, using implementation of risk-aligned bladder cancer surveillance care as an example.
As outlined above, specific strategies for the improvement of care can be developed both for providers and patients. These strategies have been shown to facilitate getting the right care to the right patient. For patients, they can help with addressing specific barriers that may lead to patients not following providers' recommendations. We believe that the use of a systematic scientifically rigorous approach to develop such implementation strategies will lead to strategies that have the highest likelihood for success. In addition, if a systematic process was used for development, adaptation of strategies to specific settings will be easier, because one can follow a similar process during adaptation [41]. Taken together, we believe that use of rigorous implementation science methods such as those described in this essay will have a meaningful impact on the hundreds of thousands of patients living with bladder cancer.
Highlights.
Implementing risk-aligned surveillance could curb overuse among low-risk patients.
It also will curtail underuse among high-risk patients.
Multiple challenges exist to implementing risk-aligned surveillance in practice.
Implementation science methods can be used to understand determinants of care.
Based on this understanding, strategies to improve care can be developed.
Acknowledgments
Funding: FRS is supported by the Department of Veterans Affairs, Veterans Health Administration, VISN1 Career Development Award, by a Conquer Cancer Foundation Career Development Award, and by the Dow-Crichlow Award of the Department of Surgery at the Dartmouth-Hitchcock Medical Center. FRS AND JBS are supported by the Mentored Training in Implementation Research in Cancer (MT-DIRC), National Cancer Institute grant number 5R25CA171994 (PI Brownson).
Footnotes
Disclaimer: Opinions expressed in this manuscript are those of the authors and do not constitute official positions of the U.S. Federal Government or the Department of Veterans Affairs.
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, et al. SEER Cancer Statistics Review, 1975–2013. Bethesda MD: Natl Cancer Inst; 2016. [accessed 01/16/2017]. Available at http://seer.cancer.gov/csr/1975_2013/ [Google Scholar]
- 2.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103:117–28. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scosyrev E, Noyes K, Feng C, Messing E. Sex and racial differences in bladder cancer presentation and mortality in the US. Cancer. 2009;115:68–74. doi: 10.1002/cncr.23986. [DOI] [PubMed] [Google Scholar]
- 4.Ries LAG, Young JL, Keel GE, Eisner M, Lin YD, Horner M-J. SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988–2001, Patient and Tumor Characteristics. Bethesda, MD: NIH; 2007. Pub. No. 07-6215. [Google Scholar]
- 5.Department of Veteran Affairs. VHA Support Service Center (VSSC) 2015. [Google Scholar]
- 6.Riley GF, Potosky AL, Lubitz JD, Kessler LG. Medicare payments from diagnosis to death for elderly cancer patients by stage at diagnosis. Med Care. 1995;33:828–41. doi: 10.1097/00005650-199508000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Cambier S, Sylvester RJ, Collette L, Gontero P, Brausi MA, van Andel G, et al. EORTC Nomograms and Risk Groups for Predicting Recurrence, Progression, and Disease-specific and Overall Survival in Non–Muscle-invasive Stage Ta–T1 Urothelial Bladder Cancer Patients Treated with 1–3 Years of Maintenance Bacillus Calmette-Guérin. Eur Urol. 2016;69:60–9. doi: 10.1016/j.eururo.2015.06.045. [DOI] [PubMed] [Google Scholar]
- 8.Rieken M, Shariat SF, Kluth L, Crivelli JJ, Abufaraj M, Foerster B, et al. Comparison of the EORTC tables and the EAU categories for risk stratification of patients with nonmuscle-invasive bladder cancer. Urol Oncol Semin Orig Investig. 2017 doi: 10.1016/j.urolonc.2017.08.027. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: bladder cancer Version 2000. Available via NCCN archives upon request. [Google Scholar]
- 10.Oosterlinck W, Solsona E, Akaza H, Busch C, Goebell PJ, Malmström P-U, et al. Low-grade Ta (noninvasive) urothelial carcinoma of the bladder. Urology. 2005;66:75–89. doi: 10.1016/j.urology.2005.07.063. [DOI] [PubMed] [Google Scholar]
- 11.Sylvester RJ, van der Meijden A, Witjes JA, Jakse G, Nonomura N, Cheng C, et al. High-grade Ta urothelial carcinoma and carcinoma in situ of the bladder. Urology. 2005;66:90–107. doi: 10.1016/j.urology.2005.06.135. [DOI] [PubMed] [Google Scholar]
- 12.Nieder AM, Brausi M, Lamm D, O’Donnell M, Tomita K, Woo H, et al. Management of stage T1 tumors of the bladder: International Consensus Panel. Urology. 2005;66:108–25. doi: 10.1016/j.urology.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 13.Oosterlinck W, van der Meijden A, Sylvester R, Böhle A, Rintala E, Narvón ES, et al. Guidelines on TaT1 (Non-muscle invasive) Bladder Cancer. [accessed 12/04/2017];European Association of Urology. 2006 Available at http://uroweb.org/wp-content/uploads/EAU-Guidelines-TaT1-Bladder-Cancer-2006.pdf.
- 14.Brausi M, Witjes JA, Lamm D, Persad R, Palou J, Colombel M, et al. A Review of Current Guidelines and Best Practice Recommendations for the Management of Nonmuscle Invasive Bladder Cancer by the International Bladder Cancer Group. J Urol. 2011;186:2158–2167. doi: 10.1016/j.juro.2011.07.076. [DOI] [PubMed] [Google Scholar]
- 15.Kassouf W, Traboulsi SL, Schmitz-Dräger B, Palou J, Witjes JA, van Rhijn BWG, et al. Follow-up in non–muscle-invasive bladder cancer—International Bladder Cancer Network recommendations. Urol Oncol Semin Orig Investig. 2016;34:460–8. doi: 10.1016/j.urolonc.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 16.Hollingsworth JM, Zhang Y, Krein SL, Ye Z, Hollenbeck BK. Understanding the variation in treatment intensity among patients with early stage bladder cancer. Cancer. 2010;116:3587–94. doi: 10.1002/cncr.25221. [DOI] [PubMed] [Google Scholar]
- 17.Chamie K, Saigal CS, Lai J, Hanley JM, Setodji CM, Konety BR, et al. Compliance with guidelines for patients with bladder cancer. Cancer. 2011;117:5392–401. doi: 10.1002/cncr.26198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koo K, Zubkoff L, Sirovich BE, Goodney PP, Robertson DJ, Seigne JD, et al. The Burden of Cystoscopic Bladder Cancer Surveillance: Anxiety, Discomfort, and Patient Preferences for Decision Making. Urology. 2017;108:122–8. doi: 10.1016/j.urology.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher ES, Welch HG. Avoiding the unintended consequences of growth in medical care: how might more be worse? JAMA J Am Med Assoc. 1999;281:446–53. doi: 10.1001/jama.281.5.446. [DOI] [PubMed] [Google Scholar]
- 20.Kulkarni GS, Finelli A, Fleshner NE, Jewett MAS, Lopushinsky SR, Alibhai SMH. Optimal management of high-risk T1G3 bladder cancer: a decision analysis. PLoS Med. 2007;4:e284. doi: 10.1371/journal.pmed.0040284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pruthi RS, Baldwin N, Bhalani V, Wallen EM. Conservative management of low risk superficial bladder tumors. J Urol. 2008;179:87–90. doi: 10.1016/j.juro.2007.08.171. [DOI] [PubMed] [Google Scholar]
- 22.van den Bosch S, Alfred Witjes J. Long-term cancer-specific survival in patients with high-risk, non-muscle-invasive bladder cancer and tumour progression: a systematic review. Eur Urol. 2011;60:493–500. doi: 10.1016/j.eururo.2011.05.045. [DOI] [PubMed] [Google Scholar]
- 23.Kulkarni GS, Urbach DR, Austin PC, Fleshner NE, Laupacis A. Longer wait times increase overall mortality in patients with bladder cancer. J Urol. 2009;182:1318–24. doi: 10.1016/j.juro.2009.06.041. [DOI] [PubMed] [Google Scholar]
- 24.Atkins L, Michie S. Designing interventions to change eating behaviours. Proc Nutr Soc. 2015;74:164–70. doi: 10.1017/S0029665115000075. [DOI] [PubMed] [Google Scholar]
- 25.Grimshaw J, Thomas R, MacLennan G, Fraser C, Ramsay CR, Vale L, et al. Effectiveness and Efficiency of Guideline Dissemination and Implementation Strategies. Health Technol Assess. 2004;8:1–72. doi: 10.3310/hta8060. [DOI] [PubMed] [Google Scholar]
- 26.Michie S, Johnston M, Francis J, Hardeman W, Eccles M. From Theory to Intervention: Mapping Theoretically Derived Behavioural Determinants to Behaviour Change Techniques. Appl Psychol. 2008;57:660–80. doi: 10.1111/j.1464-0597.2008.00341.x. [DOI] [Google Scholar]
- 27.Wensing M, Oxman A, Baker R, Godycki-Cwirko M, Flottorp S, Szecsenyi J, et al. Tailored Implementation For Chronic Diseases (TICD): a project protocol. Implement Sci. 2011;6:103. doi: 10.1186/1748-5908-6-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flottorp SA, Oxman AD, Krause J, Musila NR, Wensing M, Godycki-Cwirko M, et al. A checklist for identifying determinants of practice: A systematic review and synthesis of frameworks and taxonomies of factors that prevent or enable improvements in healthcare professional practice. Implement Sci. 2013;8:35. doi: 10.1186/1748-5908-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Neil B, Graves AJ, Barocas DA, Chang SS, Penson DF, Resnick MJ. Doing More for More: Unintended Consequences of Financial Incentives for Oncology Specialty Care. JNCI J Natl Cancer Inst. 2016:108. doi: 10.1093/jnci/djv331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Urological Association. [accessed 11/27/17];CMS Releases Proposed Rule for the 2017 Physician Fee Schedule. 2016 https://www.auanet.org/advocacy/advocacy-by-topic/physician-payment-and-coverage-issues/cms-releases-proposed-rule-for-the-2017-physician-fee-schedule.
- 31.Centers for Medicare & Medicaid Services. [accessed 11/27/17];Physician Fee Schedule Search. 2017 https://www.cms.gov/apps/physician-fee-schedule.
- 32.Bartholomew LK, Parcel GS, Kok G. Intervention mapping: a process for developing theory and evidence-based health education programs. Health Educ Behav. 1998;25:545–563. doi: 10.1177/109019819802500502. [DOI] [PubMed] [Google Scholar]
- 33.Schmid AA, Andersen J, Kent T, Williams LS, Damush TM. Using intervention mapping to develop and adapt a secondary stroke prevention program in Veterans Health Administration medical centers. Implement Sci. 2010;5:97. doi: 10.1186/1748-5908-5-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cane J, O’Connor D, Michie S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement Sci. 2012;7:37. doi: 10.1186/1748-5908-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michie S, van Stralen MM, West R. The behaviour change wheel: A new method for characterising and designing behaviour change interventions. Implement Sci. 2011;6:42. doi: 10.1186/1748-5908-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Powell BJ, Waltz TJ, Chinman MJ, Damschroder LJ, Smith JL, Matthieu MM, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci. 2015;10:21. doi: 10.1186/s13012-015-0209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prior M, Guerin M, Grimmer-Somers K. The effectiveness of clinical guideline implementation strategies – a synthesis of systematic review findings. J Eval Clin Pract. 2008;14:888–97. doi: 10.1111/j.1365-2753.2008.01014.x. [DOI] [PubMed] [Google Scholar]
- 38.Proctor EK, Powell BJ, McMillen JC. Implementation strategies: recommendations for specifying and reporting. Implement Sci. 2013;8:1. doi: 10.1186/1748-5908-8-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waldron N, Johnson CE, Saul P, Waldron H, Chong JC, Hill A-M, et al. Development of a video-based education and process change intervention to improve advance cardiopulmonary resuscitation decision-making. BMC Health Serv Res. 2016;16:555. doi: 10.1186/s12913-016-1803-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marshall M, de Silva D, Cruickshank L, Shand J, Wei L, Anderson J. What we know about designing an effective improvement intervention (but too often fail to put into practice) BMJ Qual Saf. 2017;26:578–82. doi: 10.1136/bmjqs-2016-006143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Highfield L, Hartman MA, Mullen PD, Leerlooijer JN. Using Intervention Mapping to adapt evidence-based interventions. In: Bartholomew LK, Markham C, Ruiter RAC, Fernandez ME, Kok G, Parcel GS, editors. Plan Health Promot Programs Interv Mapp Approach. 4. San Francisco, CA, USA: John Wiley & Sons, Ltd; 2016. pp. 597–649. [Google Scholar]
- 42.Belgian Health Care Knowledge Centre. [accessed 12/04/2017];Bladder Cancer: an Assessment of International Practice Guidelines. 2015 Available at https://kce.fgov.be/sites/default/files/page_documents/KCE_247_Bladder_cancer_Report.pdf.
- 43.Kassouf W, Traboulsi SL, Kulkarni GS, Breau RH, Zlotta A, Fairey A, et al. CUA guidelines on the management of non-muscle invasive bladder cancer. Can Urol Assoc J. 2015;9:690. doi: 10.5489/cuaj.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.National Institute for Health and Care Excellence (NICE) [accessed 12/04/2017];Bladder Cancer: Diagnosis and Management. 2015 Available at https://www.nice.org.uk/guidance/ng2/evidence. [PubMed]
- 45.Chang SS, Boorjian SA, Chou R, Clark PE, Siamak D, Konety BR, et al. [accessed 05/03/2016];Non-Muscle Invasive Bladder Cancer: American Urological Association / SUO Guideline. 2016 https://www.auanet.org/education/guidelines/non-muscle-invasive-bladder-cancer.cfm.
- 46.The National Comprehensive Cancer Network. [accessed 01/29/17];NCCN Clinical Practice Guidelines in Oncology: bladder cancer Version 1. 2017 https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf.
- 47.Gagliardi AR, Brouwers MC, Bhattacharyya OK Guideline Implementation Research and Application Network. A framework of the desirable features of guideline implementation tools (GItools): Delphi survey and assessment of GItools. Implement Sci IS. 2014;9:98. doi: 10.1186/s13012-014-0098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hysong SJ, Kell HJ, Petersen LA, Campbell BA, Trautner BW. Theory-based and evidence-based design of audit and feedback programmes: examples from two clinical intervention studies. BMJ Qual Saf. 2017;26:323–34. doi: 10.1136/bmjqs-2015-004796. [DOI] [PubMed] [Google Scholar]
- 49.Soumerai SB, Avorn J. Principles of Educational Outreach ('Academic Detailing’) to Improve Clinical Decision Making. JAMA. 1990;263:549–56. doi: 10.1001/jama.1990.03440040088034. [DOI] [PubMed] [Google Scholar]
- 50.Fagerlin A, Zikmund-Fisher BJ, Ubel PA. Helping Patients Decide: Ten Steps to Better Risk Communication. J Natl Cancer Inst. 2011;103:1436–43. doi: 10.1093/jnci/djr318. [DOI] [PMC free article] [PubMed] [Google Scholar]