Abstract
Development of brown and beige/brite adipocytes increases thermogenesis and helps to reduce obesity and metabolic syndrome. Our previous study suggests that dietary raspberry can ameliorate metabolic syndromes in diet-induced obese mice. Here, we further evaluated the effects of raspberry on energy expenditure and adaptive thermogenesis and determined whether these effects were mediated by AMP-activated protein kinase (AMPK). Mice deficient in the catalytic subunit of AMPKα1 and wild-type (WT) mice were fed a high-fat diet (HFD) or HFD supplemented with 5% raspberry (RAS) for 10 weeks. The thermogenic program and related regulatory factors in adipose tissue were assessed. RAS improved the insulin sensitivity and reduced fat mass in WT mice but not in AMPKα1−/− mice. In the absence of AMPKα1, RAS failed to increase oxygen consumption and heat production. Consistent with this, the thermogenic gene expression in brown adipose tissue and brown-like adipocyte formation in subcutaneous adipose tissue were not induced by RAS in AMPKα1−/− mice. In conclusion, AMPKα1 is indispensable for the effects of RAS on brown and beige/brite adipocyte development, and prevention of obesity and metabolic dysfunction.
Keywords: AMP-activated protein kinase (AMPK) α1, Browning, Obesity, Raspberry, Thermogenesis
Introduction
The prevalence of obesity is increasing rapidly worldwide and is recognized as a major risk factor leading to metabolic diseases such as type 2 diabetes, cancer and cardiovascular disease [1, 2]. Adipose tissues have been considered as master regulators of energy homeostasis. In mammals, white adipose tissue (WAT) is the major site of excess energy storage in the form of triglycerides [3]. Differently, two populations of uncoupling protein 1 (UCP1)-expressing thermogenic adipocytes, called classical brown adipocytes from brown adipose tissue (BAT) and brite/beige (brown in white) adipocytes from WAT, dissipate energy as heat through non-shivering thermogenesis [3, 4]. The amounts of thermogenic brown/beige adipocytes are inversely correlated with obesity in adult humans [5]. Thus, pharmacologic or nutritional enhancement of BAT development and thermogenic activity and browning of WAT may be one of the most promising strategies to counteract obesity and metabolic disease [6].
Raspberry (RAS), a fruit enriched with polyphenols of high digestibility, has been shown to ameliorate high-fat diet-induced obesity and the accompanying metabolic dysfunction in mammals [7, 8] and exert health-beneficial effects [9–11]. In addition, fruit polyphenolic compounds are known to prevent obesity by promoting thermogenic program in BAT and WAT and inducing beige adipocyte development within WAT [12, 13]. Although RAS has preventive or therapeutic effects on diet-induced obesity, little is known about the role of RAS on the formation and function of brown and beige adipocytes.
AMP-activated protein kinase (AMPK) is a key regulator of cell metabolism, composed of α, β and γ subunits [14]. The catalytic subunit of AMPK has two isoforms, α1 and α2, which display different tissue expression patterns. The α1 isoform is prominently expressed in progenitor cells and adipose tissue [15], whereas the α2 subunit is the predominant isoform expressed in muscle [16]. Moreover, we previously found that AMPKα1 participates in resveratrol stimulated brown and beige adipogenesis [13, 17]. Here, we hypothesized that RAS regulates adaptive thermogenesis through promoting brown adipocyte function and white adipocyte browning, a process mediated by AMPKα1. Thus, the objective of the present study is to investigate the effects of RAS supplementation of high-fat diet-fed mice on BAT metabolic function and browning of WAT and to explore the role of AMPKα1 in mediating the beneficial effects of dietary RAS.
Materials and methods
Animal, diet and experimental design
All animal experimental and care procedures were conducted in accordance with the guidelines of the National Institutes of Health and approved by the Animal Use and Care Committee of Washington State University (Permit No. 04719). To achieve AMPKα1 conditional knockout, AMPKα1flox/flox C57BL/6 mice (Stock No: 014141, Jackson Lab, Bar Harbor, Maine) were cross-bred with tamoxifen-inducible Rosa-Cre C57BL/6 mice (Stock No: 004847, Jackson Lab) to generate RosaCre/AMPKα1flox/flox. Breeding was performed in house. RosaCre/AMPKα1flox/flox mice were injected intraperitoneally with tamoxifen (75 μg/g body weight) for 4 continuous days to induce AMPKα1 knockout (AMPKα1−/−, α1KO) [18, 19]. To avoid possible tamoxifen effect on brown/beige adipogenesis [20], AMPKα1flox/flox mice treated with tamoxifen were used as controls (Wild-type, WT); as previously characterized, the Rosa-Cre gene does not affect brown/beige adipogenesis [21]. Mice were allowed to rest for at least 3 days after the last tamoxifen injection before dietary treatments in order to minimize possible confounding changes.
Wild-type and AMPKα1−/− C57BL/6 male mice (2 months old) were fed a high-fat diet (HFD; 60% energy from fat, D12492; Research Diets, New Brunswick, NJ, USA) or a HFD diet supplemented with freeze dried raspberry (5% of dry feed weight, red raspberry powder) for 10 weeks (n = 6 per group). To prepare raspberry diet, organic frozen red raspberries were purchased from a local Safeway supermarket (Pullman, WA), originally distributed by Lucerne Foods, Inc. (Boise, ID, USA). RAS were freeze-dried using VirTis freeze drier (Vertis, Gardiner, NY, USA) and ground into powder. The RAS powder contains 1.09 ± 0.05% gallic acid equivalent polyphenolics, 4.24 ± 0.12% protein, 1.91 ± 0.03% fat, 0.81 ± 0.02% ash, and 16.14 ± 0.45% moisture. According to a recent publication, freeze-dried RAS contains 35.1% dietary fiber, in which soluble fiber is 1.6 ± 0.2% and insoluble fiber is 33.5 ± 0.1% [9]. The RAS powder was shipped to the Research Diets, Inc. for making customized research diets containing 5% of RAS on dry weight basis [7]. Diets were vaccum-packaged and frozen in −80 °C until used and fresh diets were provided weekly. We chose 5% RAS supplementation, because dietary supplementation of 5% RAS effectively ameliorated HFD-induced adiposity [7]. Mice were maintained in a temperature-controlled conditions (23 ± 2 °C, 12:12-h light-dark cycle) with free access to food and water. Feed intake and body weight were monitored weekly during dietary treatments. After 10 weeks of treatments, mice were euthanized by carbon dioxide anesthesia followed by cervical dislocation. Blood samples were collected by cardiac puncture and centrifuged at 4°C to collect serum. The interscapular BAT, inguinal WAT (IngWAT) and epididymal WAT (EpiWAT) were rapidly isolated and weighed. Tissues from one side were fixed in 4% paraformaldehyde for sectioning and staining, and from the other side, they were rapidly frozen in liquid nitrogen and stored at −80°C until further analysis. A portion of IngWAT was used for tissue oxygen consumption measurement.
Glucose tolerance test (GTT)
At 1 week before the mice were killed, following an overnight fasting, mice were i.p. injected with D-glucose (2 g/kg). Blood samples were collected from the tail vein at 0, 15, 30, 60 and 90 min after injection and glucose concentrations were measured using a glucometer (Bayer Contour, Tarrytown, NY, USA), and the area under the curve was quantified [19, 22].
Serum analysis
The content of serum free fatty acids was determined using an EnzyChrom™ Free Acid Assay Kit (BioAssay System, CA, USA). Serum triglyceride and total cholesterol levels were measured using colourimetric assay kits (Thermo-Scientific, Ann Arbor, MI, USA). Serum insulin concentration was measured using a Mouse Ultrasensitive Insulin ELISA Kit (ALPCO Diagnostics, Salem, NH, USA).
Whole-body metabolic analysis
The whole-body metabolic rate [oxygen consumption (VO2), carbon dioxide production (VCO2), respiratory exchange ratio (RER) and heat production] of mice during the day (quiescent stage) and night (active stage) was measured using an Oxymax indirect open-circuit calorimetry system (Columbus Instruments, Columbus, OH, USA) installed in a constant environmental temperature and light regimen (12 h light and 12 h dark). Mice in each chamber had free access to food and water.
Oxygen consumption assay
IngWAT was isolated, weighed, and 50 mg of tissue was minced in a respiration buffer (2% BSA, 1.1 mM sodium pyruvate, and 25 mM glucose in PBS). Oxygen consumption was measured using an Orion 3-Star Dissolved Oxygen Meter (Thermo Scientific, Waltham, MA, USA) for approximately 25 min. Oxygen consumption was normalized to minced tissue weight [23].
Histological analysis
Paraffin-embedded WAT and BAT sections (5-μm thick) were either stained with Hematoxylin and Eosin (H&E) or used for UCP1 immunohistochemical (IHC) staining as previously described [24]. Imaging was performed with an EVOS microscope (Advanced Microscopy Group, Bothell, WA, USA). At least four images per section and four sections from each individual mouse were analyzed. Adipocyte diameters were measured by Image-Pro Plus 6.0 (Media Cybernetics, Inc., Rockville, MD, USA).
Quantitative real-time PCR (qRT-PCR) analysis
Total RNA was extracted from adipose tissues by TRIzol reagent (Sigma, Saint Louis, MO, USA), and cDNA was synthesized with the iScriptTM cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). qRT-PCR was carried out on the CFX RT-PCR detection system (Bio-Rad) with 18S rRNA used as a reference gene as described previously [13]. Relative expression of mRNA was determined using the method of 2−ΔΔCt [25]. The primer sequences are shown in Table 1.
Table 1.
Primer sequences used for real-time quantitative PCR
| Gene | Forward (5′-3′) | Reverse (5′-3′) | Size (bp) | Access No. |
|---|---|---|---|---|
| 18s | GTAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGCG | 151 | NR_046233.2 |
| UCP1 | ACTGCCACACCTCCAGTCATT | CTTTGCCTCACTCAGGATTGG | 123 | NM_009463.3 |
| PRDM16 | CAGCACGGTGAAGCCATTC | GCGTGCATCCGCTTGTG | 87 | NM_001291029.1 |
| PGC-1α | CCCTGCCATTGTTAAGACC | TGCTGCTGTTCCTGTTTTC | 161 | XM_006503779.1 |
| Cidea | ATCACAACTGGCCTGGTTACG | TACTACCCGGTGTCCATTTCT | 136 | NM_007702.2 |
| Elovl3 | GATGGTTCTGGGCACCATCTT | CGTTGTTGTGTGGCATCCTT | 73 | XM_006526624.1 |
| Cox7a1 | CAGCGTCATGGTCAGTCTGT | AGAAAACCGTGTGGCAGAGA | 112 | NM_009944.3 |
| CD137 | GTCGACCCTGGACGAACTGCTCT | CCTCTGGAGTCACAGAAATGGTGGTA | 132 | NM_001077590.1 |
| Tbx1 | TGAAGAAGAACCCGAAGGTGG | ACTTGGAACGTGGGGAACATT | 133 | XM_006536887.1 |
| TMEM26 | GAAACCAGTATTGCAGCACCCAAT | AATATTAGCAGGAGTGTTTGGTGGA | 205 | NM_177794.3 |
Immunoblotting analysis
Immunoblotting analyses were performed as previously described [24]. Briefly, protein samples were extracted from adipose tissues and separated by 10% SDS-PAGE followed with nitrocellulose membrane transferring. After blocking in a blocking solution consisting of 5% nonfat dry milk in TBS with 0.1% Tween 20 (TBST), membranes were overnight incubated with the selected primary antibodies at 4 °C, followed by incubation with either IRDye 800CW goat anti-rabbit or IRDye 680 goat anti-mouse secondary antibodies at room temperature for 60 min. Finally, membranes were visualized using the Odyssey Infrared Image System (LI-COR Biosciences, Lincoln, NE, USA). Band density of target protein was quantified and then normalized to β-tubulin content. UCP1 and PR domain-containing 16 (PRDM16) polyclonal antibodies were purchased from Thermo Scientific (Waltham, MA, USA) and were diluted 1:1000. Antibodies against cytochrome c (Cyto C), AMPKα, phosphorylated-AMPKα (p-AMPKα) at Thr172 and β-tubulin were purchased from Cell Signaling (Danver, MA, USA) and were diluted 1:1000. IRDye 800CW goat anti-rabbit and IRDye 680 goat anti-mouse secondary antibodies were purchased from LI-COR (Lincoln, NE, USA) and were diluted 1:15,000.
Statistical analysis
All data sets were normally distributed, thus, the general linear model and Duncan’s multiple range test were used to analyze the data and to determine the significance of differences among means of different treatments, using SAS 9.0 (SAS Institute Inc., Cary, NC, USA). Results are expressed as mean ± SEM. A significant difference was considered as P < 0.05.
Results
Raspberry does not reduce body weight gain and adiposity in AMPKα1−/− mice
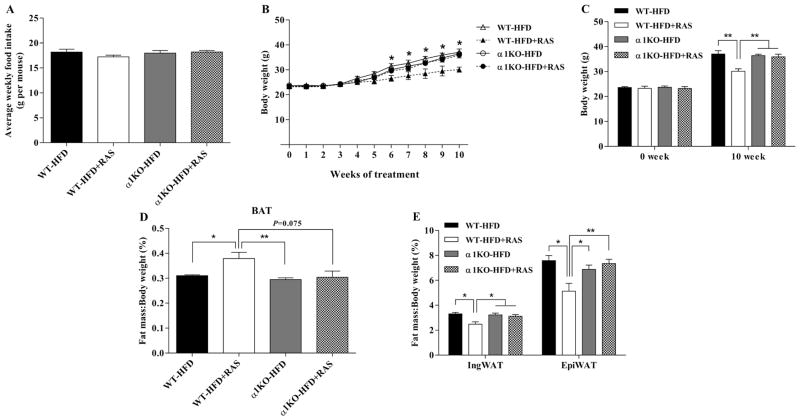
The average weekly food intake was similar for WT and AMPKα1−/− mice and was not affected significantly by RAS (Fig. 1A). Dietary RAS supplementation reduced the body weight of WT mice but not that of AMPKα1−/− mice (Fig 1. B and C). Consistently, RAS increased the BAT mass (Fig. 1D, P = 0.037) and decreased the IngWAT (P = 0.011) and EpiWAT (P = 0.016) mass (Fig. 1E) in WT mice. These data showed that AMPKα1 might be required for the anti-obesity effect of RAS.
Figure 1. Raspberry does not reduce body weight gain and adiposity in AMPKα1−/− mice challenged with HFD.
To achieve AMPKα1 conditional knockout, AMPKα1flox/flox mice (WT) and RosaCre/AMPKα1flox/flox (AMPKα1−/−, α1KO) mice were injected intraperitoneally with tamoxifen (75 μg/g body weight) for 4 continuous days. Then, mice were fed with HFD with/without 5% raspberry for 10 weeks. A, weekly food intake. B and C, body weight changes. D, ratio of brown adipose tissue to body weight. E, ratio of white adipose tissue to body weight. *P < 0.05 and **P < 0.01. Data are expressed as means ± SEM (n = 6).
Raspberry does not improve insulin sensitivity in AMPKα1−/− mice challenged with HFD
To investigate the role of AMPKα1 deficiency on obesity-associated insulin resistance, we determined the metabolic parameters of WT and AMPKα1−/− mice challenged with HFD. Glucose tolerance was similar between WT and AMPKα1−/− mice on HFD alone. However, glucose tolerance was improved by RAS in WT mice but not in AMPKα1−/− mice (Fig. 2A). RAS reduced the contents of serum free fatty acids (Fig. 2B, P = 0.008), triglycerides (Fig. 2C, P = 0.008), cholesterol (Fig. 2D, P = 0.014) and insulin (Fig. 2E, P = 0.001) in WT mice but not in AMPKα1−/− mice. These results suggested that RAS-induced improvements in metabolic homeostasis requires AMPKα1.
Figure 2. Raspberry does not improve insulin sensitivity in AMPKα1−/− mice challenged with HFD.
Mice of both WT and AMPKα1−/− with/without raspberry treatments were subjected to glucose tolerance test at 9 weeks of dietary treatment. A, glucose tolerance test and area under the curve. B, serum free fatty acid concentrations. C, serum triglyceride concentrations. D, serum cholesterol concentrations. E, serum insulin concentrations. *P < 0.05 and **P < 0.01. Data are expressed as means ± SEM (n = 6).
Raspberry does not increase whole-body energy expenditure in AMPKα1−/− mice challenged with HFD
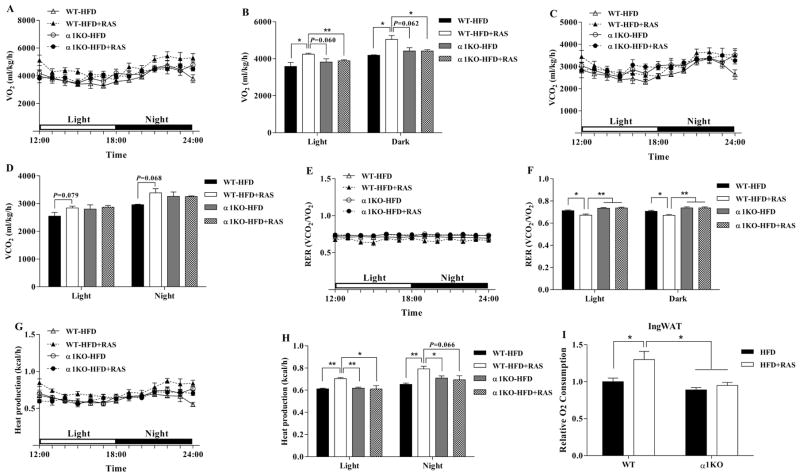
To explore why AMPKα1−/− mice on RAS did not lose weight, we measured their whole-body energy expenditure. The O2 consumption (Fig. 3A and B) and CO2 production (Fig. 3C and D) during light and dark phases were increased by RAS in WT mice but not in AMPKα1−/− mice. As a result, the increased RER was decreased by RAS in WT mice but not in AMPKα1−/− mice (Fig. 3E and F), suggesting RAS-induced lipid oxidation required AMPKα1. Furthermore, consistent with O2 consumption, RAS increased the heat production in WT mice but not in AMPKα1−/− mice (Fig. 3G and H).
Figure 3. Raspberry does not increase energy expenditure in AMPKα1−/− mice challenged with HFD.
A–H, Before necropsy, mice were subjected to the analysis of whole-body metabolic rate [oxygen consumption (VO2), carbon dioxide production (VCO2), respiratory exchange ratio (RER) and heat production] during the day (quiescent stage) and night (active stage) using an Oxymax indirect open-circuit calorimetry system (Columbus Instruments, Columbus, OH, USA). VO2 during a 6 h light-6 h dark cycle measured in a metabolic cage (A) and the average values (B). VCO2 during a 6 h light-6 h dark cycle (C) and the average values (D). The values of RER (ratio of VCO2 to VO2) were calculated from metabolic chamber data during a 6 h light-6 h dark cycle. Heat production during a 6 h light-6 h dark cycle (G) and the average values (H). I, IngWAT was isolated right after necropsy and subjected to basal oxygen consumption in vitro. *P < 0.05 and **P < 0.01. Data are expressed as means ± SEM (n = 6).
To analyze the impact of RAS on adipose tissue, IngWAT was separated for direct O2 consumption analysis. Consistently, RAS increased the basal O2 consumption in IngWAT of WT mice (Fig. 3I, P = 0.048). However, RAS did not increase the basal O2 consumption of IngWAT in AMPKα1−/− mice in vitro. Taken together, these results showed that RAS-induced improvements in metabolic activity required AMPKα1.
Raspberry does not increase BAT metabolic activity in AMPKα1−/− mice challenged with HFD
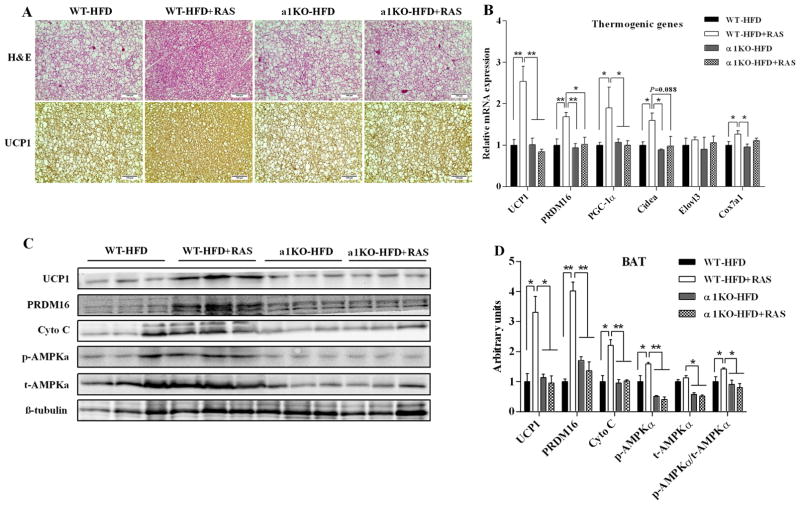
To determine the source of the increased energy expenditure in WT mice treated with RAS, we measured BAT metabolic function in these mice. Histological analysis showed that RAS reduced lipid deposits and increased UCP1 expression in BAT of WT mice. However, the morphology and UCP1 protein staining were not affected by RAS in BAT of AMPKα1−/− mice (Fig. 4A). Consistent with these findings, RAS increased the UCP1 mRNA (Fig. 4B, P = 0.008) and protein expression (Fig. 4C and D, P = 0.017) in BAT of WT mice but not in AMPKα1−/− mice. Correspondingly, the mRNA expression of PRDM16 (P = 0.008), peroxisomal proliferator-activated receptor γ coactivator-1 α (PGC-1α, P = 0.012), cell death-inducing DFFA-like effector A (Cidea, P = 0.026) and cytochrome c oxidase subunit Vlla polypeptide 1 (Cox7al, P = 0.011) were also increased by RAS in WT mice but not in AMPKα1−/− mice (Fig. 4B). In addition, RAS increased p-AMPKα protein content in BAT of WT mice but not that of AMPKα1−/− mice (Fig. 4C and D, P = 0.046). A similar pattern of changes was observed for PRDM16 and cytochrome c (Cyto C) in WT mice but absence in AMPKα1−/− mice (Fig. 4C and D). These data provide evidence that RAS-induced improvements in BAT thermogenic activity require AMPKα1.
Figure 4. Raspberry does not increase BAT metabolic activity in AMPKα1−/− mice challenged with HFD.
AMPKα1flox/flox mice (WT) and RosaCre/AMPKα1flox/flox (AMPKα1−/−, α1KO) mice were fed with HFD with/without 5% raspberry for 10 weeks, and BAT were sampled for histochemical and biochemical analyses. A, representative H&E staining and UCP1 staining for BAT sections. B, mRNA expression of thermogenic genes in BAT. C, representative images of immunoblotting. D, contents of thermogenic proteins and AMPKα. *P < 0.05 and **P < 0.01. Data are expressed as means ± SEM (n = 6).
Raspberry does not induce brown fat-like changes in WAT of AMPKα1−/− mice challenged with HFD
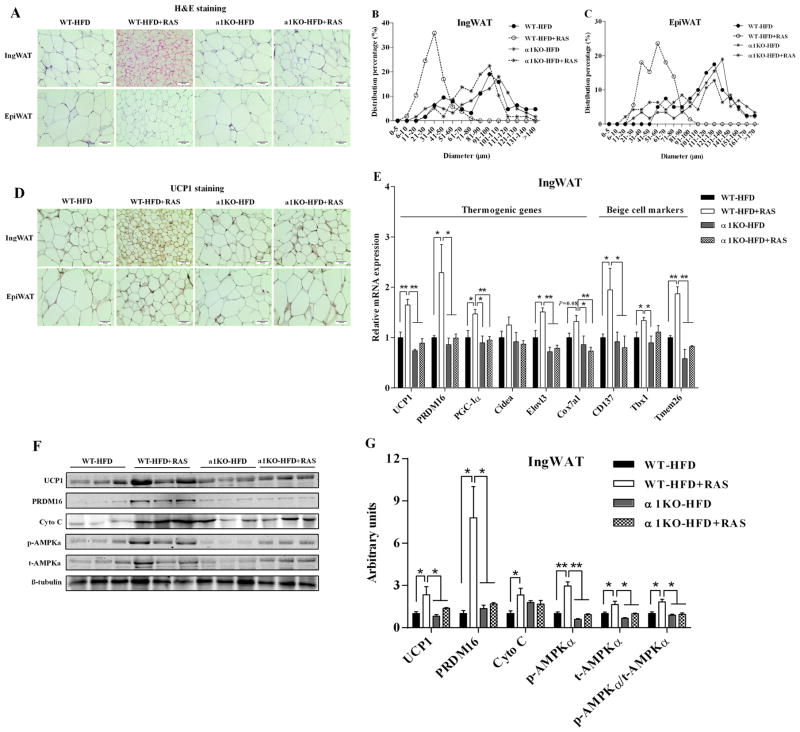
Besides BAT activity, browning of WAT is another important process in adaptive thermogenesis. H&E staining results revealed that the average adipocyte diameters were decreased by RAS in WAT of WT mice but not in AMPKα1−/− mice (Fig. 5A). Small adipocytes were most abundant in WT mice treated with RAS (Fig. 5B and C). Moreover, beige adipocyte numbers (Fig. 5A) and UCP1-positive areas (Fig. 5D) were increased by RAS in IngWAT of WT mice but not in AMPKα1−/− mice. However, RAS did not affect the beige adipocyte formation (Fig. 5A) and UCP1 protein staining (Fig. 5D) in EpiWAT of both WT mice and AMPKα1−/− mice, which implies that EpiWAT might be not sensitive enough to browning-associated stimuli. Correspondingly, UCP1 mRNA (Fig. 5E, P = 0.005) and protein expression (Fig. 5F and G, P = 0.014) were also increased by RAS in IngWAT of WT mice but not in AMPKα1−/− mice. RAS increased the expression of a series of thermogenic genes in IngWAT of WT mice but not in AMPKα1−/− mice, including PRDM16 (P = 0.022), PGC-1α (P = 0.032) and elongation of very long-chain fatty acids protein 3 (Elovl3, P = 0.019) (Fig. 5E). Moreover, RAS also induced the mRNA expression of beige adipocyte-selective markers in IngWAT of WT mice, including cluster of differentiation 137 (CD137, P = 0.036), T-box1 (Tbx1, P = 0.049) and transmembrane protein 26 (Tmem26, P = 0.004) (Fig. 5E). However, RAS did not affect the mRNA expression of these genes in IngWAT of AMPKα1−/− mice (Fig. 5E). In addition, PRDM16 (P = 0.011) and Cyto C (P = 0.037) protein contents were increased by RAS in IngWAT of WT mice but not in AMPKα1−/− mice (Fig. 5F and G). A similar pattern of changes was observed for p-AMPKα (Fig. 5F and G). These results suggested that RAS-induced IngWAT browning requires AMPKα1.
Figure 5. Raspberry does not induce brown fat-like changes in white adipose tissue of AMPKα1−/− mice challenged with HFD.
AMPKα1flox/flox mice (WT) and RosaCre/AMPKα1flox/flox (AMPKα1−/−, α1KO) mice were fed with HFD with/without 5% raspberry for 10 weeks, and IngWAT and EpiWAT were sampled for histochemical and biochemical analyses. A, representative H&E staining in IngWAT and EpiWAT sections. B and C, percentage distribution of adipocyte diameters of IngWAT (B) and EpiWAT (C). D, representative UCP1 staining in IngWAT and EpiWAT sections. E, mRNA expression of thermogenic genes and beige adipocyte-selective markers in IngWAT. F, representative images of immunoblotting. G, contents of thermogenic proteins and AMPKα. *P < 0.05 and **P < 0.01. Data are expressed as means ± SEM (n = 6).
Discussion
The imbalance between energy intake and energy expenditure induces animal overweight and obesity leading to metabolic diseases [26]. The development and thermogenic function of BAT plays an important role in an adequate neonatal response to prevent hypothermia. BAT burns fatty acids and glucose for heat production and is able to produce 300 times more heat than other tissues per unit mass [27]. Thus, brown and beige adipose tissues have been identified as promising therapeutic targets against diet-induced obesity and diabetes [28]. RAS, which contains high contents of both polyphenols and dietary fibers, has shown to be beneficial for the prevention and treatment of obesity and related chronic diseases including diabetes, oxidative stress and heart diseases [8, 9, 29, 30]. In our previous studies, we observed that RAS ameliorates adiposity and metabolic syndromes in diet-induced obese mice [7]. However, to our knowledge, no study assessed RAS’s effects on brown and beige adipogenesis and thermogenesis. In the present study, we used the HFD-induced obesity mice model to investigate the effects of RAS on BAT/beige adipocyte function and thermogenesis and explored the underlying mechanism. Our results showed that RAS increased energy expenditure and adaptive thermogenesis by promoting BAT metabolic activity and beige adipocyte formation through the activation of AMPKα1.
It has been reported that increased energy expenditure contributes to body weight loss and protects against diet-induced obesity [31]. As determined by an indirect calorimetry, in our study, the increased metabolic rate and decreased fat mass induced by RAS are related, at least partly, to the increased whole-body energy expenditure. More importantly, after AMPKα1 knockout, the promotional effects of RAS on the oxygen consumption and heat production were abolished. In accordance, our in vitro data further suggested RAS fails to increase the basal oxygen consumption of IngWAT in AMPKα1−/− mice. The RER is commonly used to analyze the sources of fuel. A high RER suggests that carbohydrates are being predominantly utilized, whereas a low RER indicates fatty acid oxidation [32]. In this study, the decreased RER in RAS-treated WT mice suggests that a higher ratio of lipids was being oxidized. Together, these findings suggest that AMPKα1 mediates RAS-induced energy expenditure.
Enhanced BAT metabolic function and browning of IngWAT could lead to increased oxygen consumption and heat production. The differentiation and function of brown/beige adipocytes are regulated by a complex network of transcription factors and signaling pathway components. PRDM16 is a crucial transcription factor in brown and beige adipogenesis [33]. The expression of PRDM16 results in up-regulation of genes involved in BAT/beige adipocyte function and thermogenesis, including UCP1, PGC-1α and Cyto C [34, 35]. AMPK, an important energy sensor, mediates dietary polyphenols-induced anti-obesity effects [13, 24]. In our previous studies, we found that AMPK promotes PRDM16 expression [21], and resveratrol, a representative polyphenol present in grape and some berries, activates AMPK [13]. To evaluate the possibility that RAS, which contains a high level of polyphenols, activates AMPKα1 for brown/beige fat activation, we studied the effects of RAS in mice deficient in AMPKα1. As expected, only trace amount of p-AMPKα was detected in BAT and IngWAT of AMPKα1−/− mice, suggesting that α1 isoform accounts for most of the total AMPKα activity. More importantly, RAS increased the phosphorylated AMPKα level in WT mice and RAS-induced increase of the expression of PRDM16 and other thermogenic genes in BAT and IngWAT are abolished after knockout of AMPKα1, showing that the biological effects of RAS on BAT metabolic function and browning of IngWAT are largely mediated through AMPKα1.
Based on lineage tracing, brown adipocytes are derived from MYF5 (myogenic factor 5)-expressing cells [36], whereas most of the beige adipocytes in WAT are developed from platelet-derived growth factor receptor α positive (PDGFRα+) cells [37, 38]. The density of progenitor cells varies among different fat depots. Compared with EpiWAT, IngWAT possesses higher density of PDGFRα+ progenitor cells and higher expansion capacity, and may be more sensitive to browning-associated stimuli [22, 39]. The low density of PDGFRα+ progenitor cells in EpiWAT limits de novo formation of beige adipocytes, which might explain the lack of changes in EpiWAT beige adipogenesis of both WT mice and AMPKα1−/− mice, due to new beige adipocytes are mainly derived from PDGFRα+ progenitor cells [40].
In conclusion, we have demonstrated that RAS promotes a thermogenic program in BAT and WAT in HFD-fed mice by stimulating the expression of thermogenic genes and beige adipocyte formation, which are mediated by AMPKα1. This study provides new insights into the regulation of brown/beige adipocyte formation and thermogenesis by dietary RAS and suggests the potential application of RAS as a nutritional intervention strategy for the prevention and therapeutic of obesity and related metabolic diseases.
Acknowledgments
This study was supported by grants from the National Institutes of Health (R01-HD067449 and R21-AG049976) and a grant from the National Processed Raspberry Council to M.D.
Abbreviations
- AMPK
AMP-activated protein kinase
- BAT
brown adipose tissue
- CD137
cluster of differentiation 137
- Cidea
cell death-inducing DFFA-like effector A
- Cox7al
cytochrome c oxidase subunit Vlla polypeptide 1
- Cyto C
cytochrome c
- Elovl3
elongation of very long-chain fatty acids protein 3
- EpiWAT
epididymal white adipose tissue
- GTT
glucose tolerance test
- HFD
high-fat diet
- IngWAT
inguinal white adipose tissue
- PGC-1α
peroxisomal proliferator-activated receptor γ coactivator-1
- PRDM16
PR domain-containing 16
- RAS
raspberry
- RER
respiratory exchange ratio
- Tbx1
T-box 1
- Tmem26
transmembrane protein 26
- UCP1
uncoupling protein 1
Footnotes
Author contributions
T.Z. and M.D. conceived the project, designed the experiments, analyzed data and wrote the manuscript. T.Z., B.W. and Q.Y. performed the experiments. J.M.A. assisted with the mouse experiments and metabolic rate analyses. M.J.Z., J.Y. and D.C. contributed to the discussion and reviewed and edited the manuscript.
Conflicts of interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ding J, Reynolds LM, Zeller T, Müller C, Lohman K, Nicklas BJ, et al. Alterations of a Cellular Cholesterol Metabolism Network Are a Molecular Feature of Obesity-Related Type 2 Diabetes and Cardiovascular Disease. Diabetes. 2015;64:3464–74. doi: 10.2337/db14-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garg SK, Maurer H, Reed K, Selagamsetty R. Diabetes and cancer: two diseases with obesity as a common risk factor. Diabetes Obes Metab. 2014;16:97–110. doi: 10.1111/dom.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giralt M, Villarroya F. White, brown, beige/brite: different adipose cells for different functions? Endocrinology. 2013;154:2992–3000. doi: 10.1210/en.2013-1403. [DOI] [PubMed] [Google Scholar]
- 4.Wu J, Boström P, Sparks L, Ye L, Choi JH, Giang AH, et al. Beige Adipocytes Are a Distinct Type of Thermogenic Fat Cell in Mouse and Human. Cell. 2012;150:366–76. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saito M, Okamatsuogura Y, Matsushita M, Watanabe K, Yoneshiro T, Niokobayashi J, et al. High Incidence of Metabolically Active Brown Adipose Tissue in Healthy Adult Humans. Diabetes. 2009;58:1526–31. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonet ML, Oliver P, Palou A. Pharmacological and nutritional agents promoting browning of white adipose tissue. BBA-Mol Cell Biol L. 2013;1831:969–85. doi: 10.1016/j.bbalip.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Zhu MJ, Kang Y, Xue Y, Liang X, García MPG, Dan R, et al. Red raspberries suppress NLRP3 inflammasome and attenuate metabolic abnormalities in diet-induced obese mice. J Nutr Biochem. 2018;53:96–103. doi: 10.1016/j.jnutbio.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Luo T, Miranda-Garcia O, Adamson A, Sasaki G, Shay NF. Development of obesity is reduced in high-fat fed mice fed whole raspberries, raspberry juice concentrate, and a combination of the raspberry phytochemicals ellagic acid and raspberry ketone. J Berry Research. 2016;6:213–23. [Google Scholar]
- 9.Noratto GD, Chew BP, Atienza LM. Red raspberry (Rubus idaeus L.) intake decreases oxidative stress in obese diabetic (db/db) mice. Food Chem. 2017;227:305–14. doi: 10.1016/j.foodchem.2017.01.097. [DOI] [PubMed] [Google Scholar]
- 10.Noratto G, Chew BP, Ivanov I. Red raspberry decreases heart biomarkers of cardiac remodeling associated with oxidative and inflammatory stress in obese diabetic db/db mice. Food Funct. 2016;7:4944–55. doi: 10.1039/c6fo01330a. [DOI] [PubMed] [Google Scholar]
- 11.Ghalayini IF, Al-Ghazo MA, Harfeil MN. Prophylaxis and therapeutic effects of raspberry (Rubus idaeus) on renal stone formation in Balb/c mice. Int Braz J Urol. 2011;37:259–67. doi: 10.1590/s1677-55382011000200013. [DOI] [PubMed] [Google Scholar]
- 12.Andrade JMO, Frade ACM, Guimaraes JB, Freitas KM, Lopes MTP, Guimarães ALS, et al. Resveratrol increases brown adipose tissue thermogenesis markers by increasing SIRT1 and energy expenditure and decreasing fat accumulation in adipose tissue of mice fed a standard diet. Euro J Nutr. 2014;53:1503–10. doi: 10.1007/s00394-014-0655-6. [DOI] [PubMed] [Google Scholar]
- 13.Wang S, Liang X, Yang Q, Fu X, Rogers C, Zhu M, et al. Resveratrol induces brown-like adipocyte formation in white fat through activation of AMP-activated protein kinase (AMPK) α1. Int J Obesity. 2015;39:967–76. doi: 10.1038/ijo.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Bio. 2012;13:251–62. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quentin T, Kitz J, Steinmetz M, Poppe A, Bär K, Krätzner R. Different expression of the catalytic alpha subunits of the AMP activated protein kinase--an immunohistochemical study in human tissue. Histol Histopathol. 2011;26:589–96. doi: 10.14670/HH-26.589. [DOI] [PubMed] [Google Scholar]
- 16.Stapleton D, Mitchelhill KI, Gao G, Widmer J, Michell BJ, Teh T, et al. Mammalian AMP-activated Protein Kinase Subfamily. J Biol Chem. 1996;271:611–4. doi: 10.1074/jbc.271.2.611. [DOI] [PubMed] [Google Scholar]
- 17.Wang S, Liang X, Yang Q, Fu X, Zhu M, Rodgers B, et al. Resveratrol enhances brown adipocyte formation and function by activating AMP- activated protein kinase (AMPK) α1 in mice fed high- fat diet. Mol Nutr Food Res. 2017;61:1600746. doi: 10.1002/mnfr.201600746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayashi S, Mcmahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244:305–28. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- 19.Fu X, Zhu M, Zhang S, Foretz M, Viollet B, Du M. Obesity impairs skeletal muscle regeneration via inhibition of AMP-activated protein kinase. Diabetes. 2015;65:188–200. doi: 10.2337/db15-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hesselbarth N, Pettinelli C, Gericke M, Berger C, Kunath A, Stumvoll M, et al. Tamoxifen affects glucose and lipid metabolism parameters, causes browning of subcutaneous adipose tissue and transient body composition changes in C57BL/6NTac mice. Biochem Biophys Res Commun. 2015;464:724–9. doi: 10.1016/j.bbrc.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Yang Q, Liang X, Sun X, Zhang L, Fu X, Rogers CJ, et al. AMPK/α-ketoglutarate axis dynamically mediates DNA demethylation in the Prdm16 promoter and brown adipogenesis. Cell Metab. 2016;24:542–54. doi: 10.1016/j.cmet.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang X, Yang Q, Fu X, Rogers CJ, Wang B, Pan H, et al. Maternal obesity epigenetically alters visceral fat progenitor cell properties in male offspring mice. J Physiol. 2016;594:4453–66. doi: 10.1113/JP272123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harms MJ, Ishibashi J, Wang W, Lim H-W, Goyama S, Sato T, et al. Prdm16 Is Required for the Maintenance of Brown Adipocyte Identity and Function in Adult Mice. Cell Metab. 2014;19:593–604. doi: 10.1016/j.cmet.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zou T, Chen D, Yang Q, Wang B, Zhu MJ, Nathanielsz PW, et al. Resveratrol supplementation to high fat diet-fed pregnant mice promotes brown and beige adipocyte development and prevents obesity in male offspring. J Physiol. 2017;595:1547–62. doi: 10.1113/JP273478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–43. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 27.Symonds ME. Brown Adipose Tissue Growth and Development. Scientifica. 2013;2013:305763. doi: 10.1155/2013/305763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19:1252–63. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 29.Noratto G, Chew B, Mencia A. Effects of Raspberry Dietary Supplementation on Risk Biomarkers of Diabetes Related Complications and Heart Disease in Diabetic Mice. Faseb J. 2016;30(1 Supplement):692.23. [Google Scholar]
- 30.Carvalho E, Franceschi P, Feller A, Palmieri L, Wehrens R, Martens S. A targeted metabolomics approach to understand differences in flavonoid biosynthesis in red and yellow raspberries. Plant Physiol Biochem. 2013;72:79–86. doi: 10.1016/j.plaphy.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 31.Schneider K, Valdez J, Nguyen J, Vawter M, Galke B, Kurtz TW, et al. Increased Energy Expenditure, Ucp1 Expression, and Resistance to Diet-induced Obesity in Mice Lacking Nuclear Factor-Erythroid-2-related Transcription Factor-2 (Nrf2) J Biol Chem. 2016;291:7754–66. doi: 10.1074/jbc.M115.673756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramosjiménez A, Hernándeztorres RP, Torresdurán PV, Romerogonzalez J, Mascher D, Posadasromero C, et al. The Respiratory Exchange Ratio is Associated with Fitness Indicators Both in Trained and Untrained Men: A Possible Application for People with Reduced Exercise Tolerance. Clin Med Circ Respirat Pulm Med. 2008;2:1–9. doi: 10.4137/ccrpm.s449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hondares E, Rosell M, Díaz-Delfín J, Olmos Y, Monsalve M, Iglesias R, et al. Peroxisome proliferator-activated receptor α (PPARα) induces PPARγ coactivator 1α (PGC-1α) gene expression and contributes to thermogenic activation of brown fat involvement of PRDM16. J Biol Chem. 2011;286:43112–22. doi: 10.1074/jbc.M111.252775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest. 2011;121:96–105. doi: 10.1172/JCI44271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–7. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seale P, Kajimura S, Spiegelman BM. Transcriptional control of brown adipocyte development and physiological function—of mice and men. Genes Dev. 2009;23:788–97. doi: 10.1101/gad.1779209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McDonald ME, Li C, Bian H, Smith BD, Layne MD, Farmer SR. Myocardin-related transcription factor A regulates conversion of progenitors to beige adipocytes. Cell. 2015;160:105–18. doi: 10.1016/j.cell.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tharp KM, Jha AK, Kraiczy J, Yesian A, Karateev G, Sinisi R, et al. Matrix-assisted transplantation of functional beige adipose tissue. Diabetes. 2015;64:3713–24. doi: 10.2337/db15-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang X, Zhang Q, Wang X, Zhang L, Qu W, Bao B, et al. Dietary luteolin activates browning and thermogenesis in mice through an AMPK/PGC1α pathway-mediated mechanism. Int J Obesity. 2016;40:1841–9. doi: 10.1038/ijo.2016.108. [DOI] [PubMed] [Google Scholar]
- 40.Lee YH, Petkova AP, Konkar AA, Granneman JG. Cellular origins of cold-induced brown adipocytes in adult mice. Faseb J. 2015;29:286–99. doi: 10.1096/fj.14-263038. [DOI] [PMC free article] [PubMed] [Google Scholar]