Abstract
PURPOSE
The purpose of this study was to provide estimates of post-operative opioid usage after hip arthroscopy for femoroacetabular impingement syndrome and to identify risk factors for increased post-operative opioid usage.
METHODS
All patients who were at least eighteen years old undergoing hip arthroscopy for femoroacetabular impingement syndrome with one of two hip preservation surgeons between November 2015 and August 2016 were eligible for inclusion in this study. Target minimum enrollment was set at 30 patients per surgeon based on an a priori sample size calculation. Enrolled patients completed the International Hip Outcome Tool, visual analog pain scale, Pain Catastrophizing Scale, abbreviated Patient Health Questionnaire, and questions regarding demographics and opioid and anti-inflammatory usage. Opioid consumption was assessed through pill counting at 2-week and 6-week post-operative appointments. Of 80 patients enrolled, 67 patients had complete 2-week and 6-week opioid usage data. Patient and operative factors were correlated with outcomes in multivariable models.
RESULTS
Opioid usage in the 2 weeks prior to surgery was significantly associated with higher post-operative opioid usage at 2-weeks (253.8 additional OME’s, 95% CI 171.2–336.5, p<0.0001, n=73) and 6-weeks (385.3 additional OME’s, 95% CI 241.6–529.0, p<0.0001, n=67) post-operative. By 6-weeks post-operative, 41/52 (79%) of patients without opioid usage in the 2 weeks prior to surgery used 30 or fewer oxycodone 5-mg pills compared to only 2/15 (13%) of patients with pre-operative use (24.9 odds ratio, 95% CI 4.2–148.5, p<0.0001).
CONCLUSION
Among patients undergoing hip arthroscopy for femoroacetabular impingement syndrome, any opioid usage in the two weeks preceding surgery was the strongest predictor of opioid usage after hip arthroscopy. The impact of pre-operative opioid usage far exceeded the impact of other baseline patient and operative factors. Assessment of pre-operative opioid usage could be an important factor in guiding post-operative opioid prescribing.
LEVEL OF EVIDENCE
II, prospective observational study
INTRODUCTION
The United States is in an epidemic of opioid misuse and abuse1–4, and orthopaedic surgeons are the third highest prescribers of opioids5. Previous studies have reported that patients undergoing routine surgical procedures are over-prescribed pain medication after surgery and are left with a substantial amount of opioid pain medication6–8. Over-prescribing of opioids is likely multifactorial in nature but may stem from inadequate research into tailoring pain medication prescriptions to individual patient needs after specific surgeries. To publicly address the opioid misuse and abuse epidemic, the American Academy of Orthopaedic Surgeons (AAOS) and Institute of Medicine (IOM) have advocated for instituting evidence-based opioid prescription guidelines for specific clinical situations that take into account patient factors that may affect potential abuse3, 9–11.
Patients ages 20–39 report the highest rate of illicit drug use and are the same age group that most commonly undergoes hip arthroscopy12, 13. Post-operative pain after hip arthroscopy has been shown to be modulated by several operative factors such as infusion pressures and extent of bony and soft tissue debridement14, 15. In other areas of orthopaedics, biopsychosocial factors such as chronic pain, pain catastrophizing, psychiatric disease, and gender are related to the development of persistent post-operative pain and opioid usage16–18. Furthermore, chronic pain medication usage prior to orthopaedic surgeries, including hip, knee, or ankle arthroplasty, is associated with increased pain sensitivity (hyperalgesia), persistent post-operative pain, and increased opioid demand19–21. Despite increased research into opioid requirements within the operating room22 and recent advances in local and regional anesthesia aimed at reducing perioperative pain15, 23, 24, patients undergoing hip arthroscopy often still require powerful analgesia in the post-operative period while recovering at home7, 25, 26.
In light of the opioid misuse and abuse crisis, orthopaedic surgeons are in need of evidence-based post-operative opioid prescription protocols and risk factor identification mechanisms to predict increased usage so that opioid prescriptions can be titrated to individual patient needs5, 10. Hip arthroscopy currently has no evidence to guide post-operative opioid prescriptions. The purpose of this study was to provide estimates of post-operative opioid usage after hip arthroscopy for femoroacetabular impingement syndrome (FAI) and to identify risk factors for increased post-operative usage. This study hypothesized that post-operative opioid usage may be driven by biopsychosocial factors such as patient characteristics, psychiatric scores, and prior opioid usage.
MATERIALS AND METHODS
Study design
This prospective, observational study underwent IRB approval and evaluated opioid usage following arthroscopic treatment for FAI syndrome. The study was conducted between November 2015 and July 2016; patients were enrolled from the clinics of two established hip preservation surgeons with standardized operative and post-operative treatment protocols at a high-volume academic, tertiary care center. Target enrollment was set at 30 patients per surgeon based on an a priori sample size calculation described further in the study size sub-section. The study was designed and reported in accordance with the STROBE statement for cohort studies, which provides guidance for strengthening observational studies27.
Usual practice
Patients with FAI syndrome were considered surgical candidates if they had minimal evidence of pre-existing osteoarthritis and had failed conservative management consisting of at least 6 months of treatment, including physical therapy, corticosteroid injection, rest, and anti-inflammatory medication. Patients were not routinely prescribed opioid analgesia by their surgeon as part of conservative treatment although some patients received opioid prescriptions from outside providers. Surgical treatment was dictated by intra-operative findings and included labral repair, acetabular rim trimming, femoral osteochondroplasty, and/or microfracture (Table 1).
Table 1.
Baseline, operative, and post-operative factors with averages and standard deviations or proportions and percentages.
| Baseline characteristics | Entire sample (n=73) |
|---|---|
| Age (years) | 36.5 (11.3) |
| Female gender | 55/73 (75.3%) |
| Caucasian race | 63/73 (86.3%) |
| BMI | 27.1 (5.6) |
| ASA | 1.8 (0.5) |
| Opioid usage in the 2 weeks prior to surgery? | 16/73 (21.9%) |
| Anti-inflammatory usage in the 2 weeks prior to surgery? | 37/73 (50.6%) |
| Pre-operative pain (out of 10) | 5.4 (2.3) |
| iHOT-12 (out of 100) | 30.7 (18.5) |
| PHQ (out of 24) | 5.8 (5.5) |
| PCS (out of 52) | 16.3 (14.7) |
| Prior ipsilateral hip surgery | 5/73 (6.8%) |
| Nerve block | 22/73 (30.1%) |
| Procedure duration (hours) | 2.2 (0.5) |
| Additional procedure (see below) | 5/73 (6.8%) |
| Acetabular rim trimming | 61/73 (100%) |
| Labral repair | 72/73 (99%) |
| Femoral osteochondroplasty | 68/73 (93%) |
| Acetabular microfracture | 3/73 (4%) |
| Hamstring repair | 1/73 (1%) |
| Trochanteric bursectomy | 1/73 (1%) |
| CPM, compressive icing, and hip brace (vs. active ROM and ice packs)* | 38/73 (52%) |
denotes surgeon-dependent factors.
Consistent with usual practice regarding peri-operative and post-operative anesthesia, anesthesiologists dictated acute pain medication administration surrounding surgery, including use of peri-operative nerve blocks. All patients received general anesthesia with analgesia provided by IV fentanyl. Patients stayed in hospital for a 23-hour observational period, during which they could receive oral and/or IV analgesia. All patients received prescriptions for oral 5-milligram (mg) oxycodone unless they had pre-existing opioid preferences. Prescription amount was decided on a case-by-case basis. All patients received prescriptions for 500-mg naproxen for heterotopic ossification prophylaxis. Other standardized discharge medications are listed in the appendix (see “Post-operative medications”). One surgeon prescribed a continuous passive motion (CPM) device (Kinetec Spectra, Jackson, WI), hip brace, and compressive ice device while the other surgeon prescribed gentle active range of motion (ROM) and ice packs. All patients were encouraged to start formal physical therapy in the first week after hip arthroscopy. Operative and post-operative protocols along with medication instructions are displayed in the appendix (see “Surgical and post-operative technique”).
Variables
The primary study outcome was opioid pain medication usage measured by pill counting at the 2-week and 6-week post-operative time points. To reduce the impact of opioid usage outliers, post-operative opioid usage was also analyzed in a binary fashion for intake exceeding 225 oral morphine equivalents (OME’s) or the equivalent of 30 5-mg oxycodone pills. Secondary outcomes included intra-operative opioid usage, post-operative in-hospital opioid usage, opioids remaining at 6-weeks post-operative, prescribed opioids up to the 6-week and 90-day post-operative time points, and the binary outcome of additionally prescribed opioids between 6-weeks and 90-days post-operative.
Since opioid usage may be dependent on a number of patient and operative factors, multiple input variables were measured: age, gender, race, body mass index (BMI), American Society of Anesthesiologists (ASA) score, and prior ipsilateral hip surgery. Patients were asked to report daily dosages, routes, and types of opioid and anti-inflammatory medications they consumed in the 2 weeks prior to surgery (pre-operative opioid and pre-operative anti-inflammatory usage). Patients were considered to have “pre-operative opioid usage” or “pre-operative anti-inflammatory usage” if they reported using opioids or anti-inflammatory medications in the 2 weeks prior to surgery. Participants also completed a series of patient reported outcome (PRO) measures including the International Hip Outcome Tool (iHOT-12, hip functional measure)28, visual analog scale (VAS) for pain29, Pain Catastrophizing Scale (PCS)30, and Patient Health Questionnaire 8 (PHQ-8)31. The PCS rated respondents’ psychological state through a 13-question assessment with a total PCS score of 30 representing a clinically relevant level of catastrophizing. For the PHQ-8, scores of 5, 10, 15, and 20 represented mild, moderate, moderately severe, and severe depression31. Operative and post-operative characteristics included procedure time, placement of nerve block, operative interventions, and prescribed opioid and rehabilitation.
Measurements
Intra-procedure and post-procedure in-hospital opioid usage were tabulated from the electronic medical record. Patients recorded their daily opioid usage and pain on a booklet that was provided to them to take home. Patients were reminded multiple times throughout the first two weeks by study staff to be filling out their booklet on a daily basis. At the 2-week and 6-week post-operative visits, opioid usage was measured through pill counting, performed primarily by clinicians in the office setting. However, if patients were unable to come to clinic, they were allowed to count pills and report their count to the research staff (25 of 140 pill counts, 18%). Total OME’s prescribed and remaining at various time points were also recorded. Because patients were not all prescribed the same dosage and type of medication, all opiate dosages were converted to OME’s for comparison using standard conversion factors32 (Appendix Table 1).
Post-operative pain and functional measurements included VAS pain, which was re-measured at the 2-week and 6-week visits, and the iHOT-12, which was re-measured at the 6-week visit. To align with recommendations on evaluating pain and functional outcomes in orthopaedics, a minimal clinically important difference (MCID) of 10% reduction of pre-operative to post-operative pain was selected for evaluation based on previous reports in mild to moderate hip osteoarthritis33, 34. Because the iHOT-12 had no reported MCID, the iHOT-33 MCID of 6.135 was scaled to the iHOT-12 yielding an MCID of 2.2 on the 12-point scale.
Study size
Since the surgeons involved in the study used different post-operative rehabilitation protocols and rehabilitation could potentially affect outcomes, sample size was determined to detect a difference in proportions of patients meeting the MCID pain reduction at 2-weeks post-operative. Prior to study initiation, each surgeon reviewed a consecutive sample of their patients to determine the rate of patients achieving the MCID pain reduction threshold. In this analysis, 9/10 (90%) of one surgeon’s patients met this threshold compared to 6/11 (55%) of the other surgeon’s patients. Using a standard, publicly-available sample size calculator for differences in proportions comparing 2 independent samples (www.stat.ubc.ca/~rollin/stats/ssize/b2.html), greater than or equal to 30 patients per group would be needed to detect a significant difference in the 2-week pain outcome at a power of 0.80 and an alpha of 0.05.
Statistical analysis
Averages and standard deviations or proportions and percentages were calculated for baseline characteristics and outcomes. Univariate tests of significance were carried out between all pre-operative variables and all study outcomes using JMP Pro version 13.0.0 (Statistical Analysis Software, Cary, NC). Univariate statistical tests included Student’s t-tests and Pearson correlation for continuous study outcomes (OME usage outcomes) and chi-square analysis for binary study outcomes, including intake of the OME equivalent of more than 30 5-mg oral oxycodone pills by the 2-week and 6-week post-operative visit. Pre-operative covariates with univariate p-values less than 0.1 were incorporated into multivariable main effects linear (continuous outcomes) or logistic (binary outcomes) regression models and reported as adjusted estimates, odds ratios, or odds ratios per unit change in predictor. Predictors in multivariable models with p-values less than 0.05 were reported as significant. 95% confidence intervals were displayed for all factors in multivariable models.
A Kaplan-Meier curve was constructed to display the time-dependent proportion of patients achieving their first day without opioid usage and meeting the MCID pain threshold after surgery up to 2-weeks post-operative. Cox proportional hazard models were calculated in the same manner as for the multivariate regression model.
RESULTS
A total of 80 patients were enrolled for this study. To enroll 80 patients, we approached 103 patients who were screened and potentially eligible for participation in the study; however, 23 declined to participate (Figure 1). Seven of 80 patients (9%) who consented to the study were withdrawn for the following reasons: three of those patients (4%, two from the CPM surgeon and one from the non-CPM surgeon) did not return for in-office appointments, two (3%, two from the CPM surgeon) did not bring their opioid prescription to any follow-up visits, and two (3%, two from the CPM surgeon) used opioid medication from outside providers. 73 of 80 patients (91%, 38 from the CPM surgeon and 35 from the non-CPM surgeon) had at least one study outcome available for analysis. 67 of 80 patients (84%, 35 from the CPM surgeon and 32 from the non-CPM surgeon) had complete data for the primary outcomes of 2-week and 6-week opioid usage. 64 of 80 patients (80%, 32 from the CPM surgeon and 32 from the non-CPM surgeon) completed the home booklet. Six of 80 patients (8%, three from the CPM surgeon and three from the non-CPM surgeon) did not provide any study data at the 6-week visit but were included in the 2-week analyses. For all outcomes, enrollment exceeded the per-surgeon targeted threshold (minimum 30 patients per surgeon).
Figure 1.
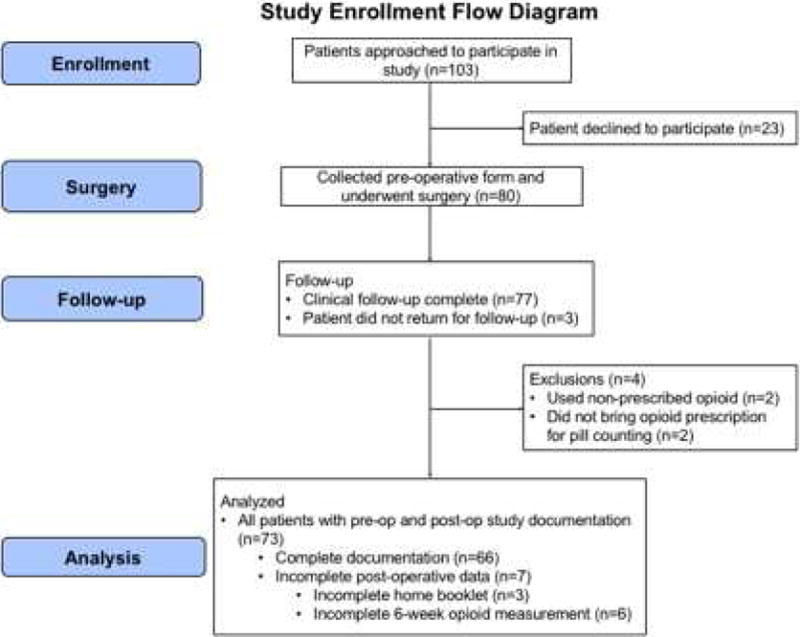
Study enrollment flow diagram.
Baseline patient characteristics are displayed in Table 1. On average, patients reported a VAS pain score of 5.4 out of 10, iHOT-12 score of 30.7, depression score (PHQ) of 5.8, and a catastrophization score (PCS) of 16.3. 5 patients had prior ipsilateral hip surgery, and 5 required an operative intervention during their index procedure in addition to labral repair, acetabular rim trimming, and femoral osteochondroplasty. Surgical and post-operative interventions were recorded. All patients underwent acetabular rim trimming, 72 of 73 (99%) underwent labral repair, and 68 of 73 (93%) underwent femoral osteochondroplasty. 3 of 73 (4%) underwent acetabular microfracture, 1 of 73 (1%) underwent hamstring repair, and 1 of 73 (1%) underwent trochanteric bursectomy.
Due to the considerable impact of opioid usage prior to surgery on post-operative opioid usage (see Table 3), Table 2 displays the 2-week and 6-week post-operative opioid usage outcomes in OME’s for the entire study sample as well as for patients with and without opioid usage in the 2 weeks preceding surgery. For reference, 7.5 OME’s is equivalent to 1 oral 5-mg oxycodone pill (Appendix Table 1, conversion factors list). Compared with patients not reporting pre-operative opioid usage, patients with pre-operative opioid usage took 3.3 times as much in the first 2 weeks after surgery and took 3.9 times as much in the first 6 weeks after surgery. In terms of oral oxycodone 5-mg pills, patients with pre-operative opioid usage consumed an average of nearly 79 pills (Q1 - 55 pills, Q3 111 pills) compared to approximately 20 pills consumed (Q1 - 1 pill, Q3 28 pills) for patients without pre-operative opioid usage. Patients without pre-operative opioid usage consumed less than 225 OME’s (30 oxycodone 5-mg pills) about 80% of the time compared to less than 20% of the time for patients with pre-operative opioid usage. Results of further opioid measurements such as in-hospital opioid usage, prescribed amounts, and remaining opioid is described in Appendix Table 3.
Table 3.
Multivariable outcome modeling incorporating all pre-operative and operative factors from Table 1 that met a univariate significance threshold of 0.1 for 2-week and 6-week post-operative opioid usage outcomes.
| Outcome | Patient or operative characteristic | Adjusted estimate or odds ratio | P-value |
|---|---|---|---|
| 2-week OME’s (n=73) | Opioid usage in 2 weeks prior to surgery | 253.84 (171.22, 336.46) | <0.001 |
| PCS (out of 52) | 2.45/point (−0.44, 5.33) | 0.096 | |
| PHQ (out of 24) | −1.19/point (−8.75, 6.37) | 0.75 | |
| iHOT-12 (out of 100) | 0.23/point (−1.88, 2.33) | 0.83 | |
| ASA | −1.62/ASA (−68.82, 65.59) | 0.96 | |
| 6-week OME’s (n=67) | Opioid usage in 2 weeks prior to surgery | 385.29 (241.64, 528.95) | <0.001 |
| Prior ipsilateral hip surgery | 161.31 (−52.23, 374.86) | 0.136 | |
| Active ROM surgeon | 66.3 (−40.12, 172.72) | 0.22 | |
| PCS (out of 52) | 2.58/point (−1.96, 7.11) | 0.26 | |
| PHQ (out of 24) | 4.78/point (−8.3, 17.86) | 0.47 | |
| ASA | 33.75/point (−73.07, 140.57) | 0.53 | |
| BMI | −2.11/point (−12.06, 7.84) | 0.67 | |
| Procedure duration (hours) | −7.05/hour (−131.23, 117.13) | 0.91 | |
| iHOT-12 (out of 100) | 0.11/point (−3.22, 3.43) | 0.95 | |
| 2-week OME’s >225? (n=73) | Opioid usage in 2 weeks prior to surgery | 17.14 odds ratio (3.74, 78.56) | <0.001 |
| Prior ipsilateral hip surgery | 9.61 odds ratio (0.78, 118.22) | 0.077 | |
| Active ROM surgeon | 2.52 odds ratio (0.7, 9.07) | 0.156 | |
| PCS (out of 52) | 0.99 unit odds ratio/point (0.93, 1.04) | 0.61 | |
| iHOT-12 (out of 100) | 1.00 unit odds ratio/point (0.96, 1.05) | 0.88 | |
| PHQ (out of 24) | 0.99 unit odds ratio/point (0.86, 1.15) | 0.91 | |
| 6-week OME’s >225? (n=67) | Opioid usage in 2 weeks prior to surgery | 24.87 odds ratio (4.16, 148.51) | <0.001 |
| Prior ipsilateral hip surgery | 9.28 odds ratio (0.71, 120.72) | 0.074 | |
| Active ROM surgeon | 1.96 odds ratio (0.51, 7.49) | 0.32 | |
| PCS (out of 52) | 0.98 unit odds ratio/point (0.92, 1.03) | 0.43 | |
| PHQ (out of 24) | 0.99 unit odds ratio/point (0.84, 1.15) | 0.87 | |
| Procedure duration (hours) | 1.12 unit odds ratio/hour (0.22, 5.78) | 0.89 | |
| iHOT-12 (out of 100) | 1 unit odds ratio/point (0.96, 1.04) | 0.92 |
Table 2.
2-week and 6-week Opioid usage for entire study sample and divided by patients with and without opioid usage in the two weeks prior to surgery (unadjusted results).
| Outcome | Entire sample | Without pre-op opioid | With pre-op opioid |
|---|---|---|---|
| 2-week OME’s (n=73) | 172.3 (173) | 113.8 (120.7) | 380.8 (172.9) |
| 6-week OME’s (n=67) | 250.8 (278.6) | 152.5 (181.4) | 591.7 (292.9) |
| 2-week OME’s <225? (n=73) | 49/73 (32.8%) | 46/57 (80.7%) | 3/16 (18.8%) |
| 6-week OME’s <225? (n=67) | 43/67 (35.8%) | 41/52 (78.8%) | 2/15 (13.3%) |
Multivariable outcome models were constructed that incorporated all pre-operative and operative factors that first met a univariate threshold of p<0.1 (Table 3). All univariate p-values are displayed in Appendix Table 2. In multivariable models, patients reporting opioid usage in the 2 weeks preceding surgery demonstrated significantly increased 2-week opioid usage (p<0.001), 6-week opioid usage (p<0.001), rates of 2-week opioid usage exceeding 225 OME’s (equivalent to 30 oxycodone 5-mg pills) (p<0.001), and rates of 6-week opioid usage exceeding 225 OME’s (p<0.001). Further results regarding the other opioid measurement outcomes (in-hospital usage, prescribed amounts, and remaining amounts) are described in Appendix Table 4.
Patients’ time to their first day with no opioid usage after surgery was analyzed against patient characteristics (Figure 2). 64 of 73 patients (88%) completed the home booklet and were included in the analysis. For patients without pre-operative opioid usage, greater than 50% of patients achieved their first day without opioid usage between days 4 and 5 compared to days 12 and 13 for patients with pre-operative opioid usage. Univariate Cox proportional hazard ratios were calculated for each characteristic listed in Table 1, and all factors achieving a p-value less than 0.1 in univariate analysis were included in a multivariable model. Pre-operative opioid usage and active ROM rehabilitation patients were the only factors that achieved the univariate significance threshold for increasing the time it took for patients to cease opioid usage, and both of these factors were significantly associated in a multivariable Cox proportional hazards model.
Figure 2.
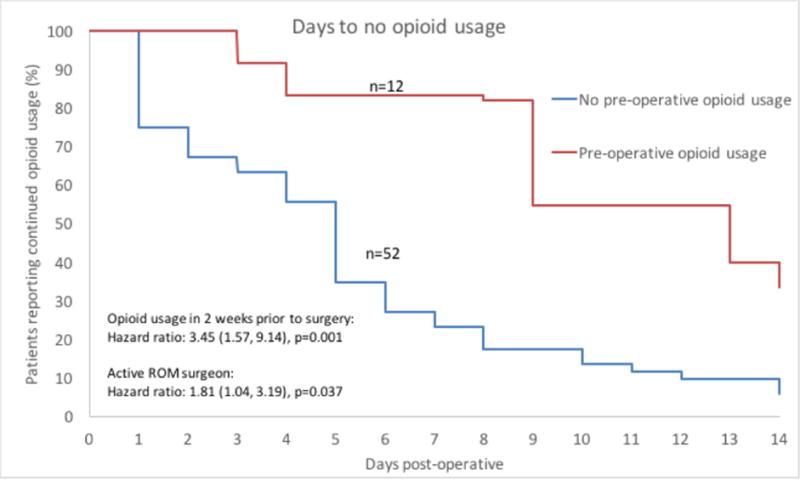
Kaplan-Meier curve showing differential rates of patients achieving their first day with no reported opioid usage between patients with and without pre-operative opioid usage among all patients completing the home booklet (n=64). Results of multivariable significance testing is displayed in the bottom left corner of the figure indicating that prior opioid usage and treatment by the active ROM surgeon were significantly associated with increased time to the first day without opioid usage. The figure does not display results broken down by treating surgeon.
Daily opioid usage for all patients that completed the home booklet (n=64 of 73, 88%) was displayed from the pre-operative to 14-days post-operative (Figure 3) period and broken down based on patient-reported opioid usage in the two weeks prior to surgery. Univariate Student t-tests were carried out between the two groups with p-values displayed above each day. Patients with opioid usage in the two weeks prior to surgery consumed significantly more opioid at all time points except for post-operative days 13 and 14.
Figure 3.
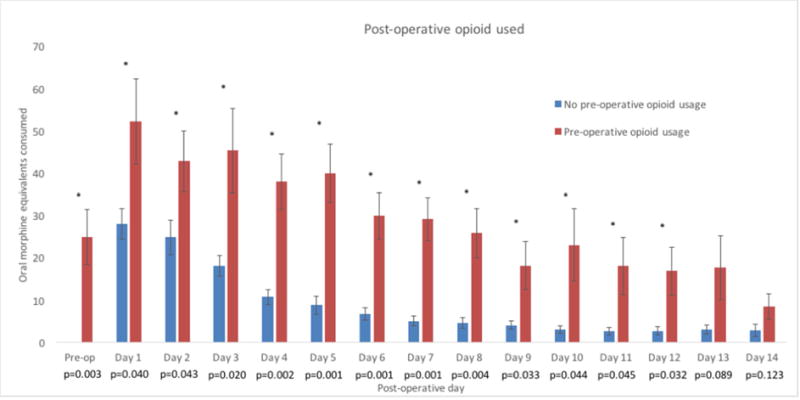
14-day opioid usage in patients with and without pre-operative opioid usage (n=64 completed booklets, 12 with pre-operative opioid usage and 52 without pre-operative opioid usage). “*” indicates p-value less than 0.05. P-values are displayed below each day label.
Appendix Table 5 describes the relationships between baseline pre-operative patient characteristics and the risk factor of pre-operative opioid usage. All factors with univariate p-values less than 0.1 were included in a multivariable logistic regression model describing each factor’s association with pre-operative opioid usage. Higher BMI and ASA scores were significantly associated with pre-operative opioid usage in the multivariable model. To provide further clinical context of the impact of opioid usage prior to surgery on post-operative functional outcomes, Appendix Table 6 displays functional outcomes divided by patients with and without pre-operative opioid usage among the 70 of 73 patients (96%) with complete functional outcomes at 2-weeks post-operative and the 67 of 73 patients (92%) with complete functional outcomes at 6-weeks post-operative. Univariate Student’s t-tests or chi-square analysis were performed to assess the differences between these two samples. Pain and functional outcomes were not significantly different between these groups.
DISCUSSION
Opioid usage in the two weeks prior to surgery was the major risk factor for increased post-operative opioid usage and was associated with patients consuming significantly more (3.9 times) as much opioid (591.7 vs. 152.5 OME’s) as patients without pre-operative opioid usage by 6-weeks post-operative. Opioid prescribing patterns should align with individual patient needs. This study assessed typical post-operative opioid usage and correlated pre-operative and operative factors to post-operative opioid usage and prescribing after hip arthroscopy for FAI syndrome. Consistent with previous reports in total joint arthroplasty36, pre-operative opioid usage proved to be the main determinant of post-operative opioid usage. This risk factor was reported in 22% of patients. 13 of 15 patients (86.6%) with opioid usage in the 2 weeks prior to surgery used more than 30 oxycodone 5-mg pills (225 OME’s) in the first 6 weeks after surgery compared with only 11 of 52 patients (21.1%) without opioid usage in the 2 weeks prior to surgery. Further, patients without pre-operative opioid usage consumed very little pain medication between the 2-week and 6-week visit (38.7 OME’s or 5.2 oxycodone 5-mg pills) compared to patients with pre-operative opioid usage (210.9 OME’s or 28.1 oxycodone 5-mg pills). The average patient had 376.1 OME’s (50.1 oxycodone 5-mg pills) remaining at the 6-week post-operative visit. Despite increased opioid usage, 2-week and 6-week pain and functional improvements were similar between these groups.
Besides the clear relationship between prior opioid usage and post-operative opioid usage, several other factors were also associated with outcomes. Prior ipsilateral hip surgery was associated with higher odds of requesting additional opioid between the 6-week visit and 90-days post-operative. However, only 5 of 73 patients (6.8%) had this risk factor, and 2 of these 5 reported prior opioid usage. Intra-procedure opioid use was significantly increased with elevated patient BMI, and post-operative in-hospital opioid usage was significantly higher with younger patients and among patients treated by the surgeon that prescribed active ROM as part of their rehabilitation protocol. The surgeon that prescribed active ROM for rehabilitation also prescribed significantly more opioid to his patients by 6-weeks post-operative. However, differences in usage between the surgeons were not statistically significant in multivariable analysis. Further, home opioid usage was not significantly affected by surgeon’s preference of rehabilitation strategy.
Several psychological and physiological factors could partially explain the difference in opioid consumption between patients with and without pre-operative opioid usage. First, patients with pre-operative opioid usage reported lower function along with higher pain, depression, and pain catastrophization than patients without pre-operative opioid usage. The combination of these factors could contribute to heightened pain leading to greater opioid usage after surgery16–19 and may have been the reason that some of these factors were associated with opioid usage in univariate analysis. However, we did not find that these factors remained correlated in multivariable models of opioid usage, suggesting that their effect was either minimal or encompassed by the effect of pre-operative opioid usage. Second, patients with pre-operative opioid usage may have developed tolerance to the analgesic effect of opioids37. Lastly, extended usage of opioids has been associated with heightened pain sensation20 which could lead to greater post-operative opioid consumption, although we acknowledge that we did not record the duration of time that patients were on narcotics prior to surgery.
In addition to assessing opioid usage in the 2 weeks preceding surgery, the following 2 strategies could potentially reduce the amount of left-over opioid medication: 1) provide patients with several opioid prescriptions for smaller individual amounts6 and 2) provide patients with explicit instructions on appropriate opioid disposal guidelines7. For reference, the Food and Drug Administration currently recommends patients either flush their unused opioids down the sink or toilet or return their opioids to a medicine take-back program or Drug Enforcement Agency authorized collector38. Lastly, several studies have evaluated peri-operative analgesic strategies that may reduce short-term post-operative opioid usage39, 40. Further evaluation could determine analgesic strategies that reduce home opioid usage in addition to reducing immediate post-operative opioid need.
LIMITATIONS
Opioid prescription amounts were not standardized. Though standardizing opioid prescriptions was considered during study design, there was no data on which to gauge an appropriate prescription amount. Therefore, as was part of usual care, prescription amounts were decided upon in a case-by-case fashion based on surgeon anticipation of potential patient need. Patients who were initially prescribed more pain medication may have used more pain medication simply due to the greater availability. However, despite the lack of standardization, many patients ended the 6-week post-operative period with a considerable amount of left-over pain medication. Further, although patients received oxycodone by default, some patients preferred alternate oral opioid medications other than oxycodone (i.e. hydrocodone or hydromorphone). However, this study accounted for these differences in prescribing patterns through converting opioid usage into standard oral morphine equivalents. Second, patients at this institution routinely stayed in hospital overnight for monitoring. Patients at other institutions may routinely discharge patients the same day after surgery. To address this limitation, intra-procedure and post-hospital opioids were tabulated. This in-hospital post-procedure use was predominantly comprised of oral oxycodone and IV nurse-administered hydromorphone. To provide data for those surgeons whose patients are discharged without using IV or in-hospital analgesia, the average patient in this study consumed an OME amount equivalent to 10 oral oxycodone 5-mg pills while still in recovery after the procedure.
A further limitation of this study is that 23 patients from the 103 patients (22%) eligible for study inclusion declined to participate. Because these patients did not consent to research, it is not possible to analyze them to determine whether or not these patients represented a distinct sub-population with potentially different baseline characteristics and responses to opioid medication. This could expose the study to selection bias. Further, despite considerable effort on the part of clinical and research staff to ensure study follow-up, three patients of 80 (4%) did not return for follow-up, two patients of 80 (3%) never brought their opioid prescription for pill counting, and two patients of 80 (3%) reported using opioids prescribed from other providers which invalidated their counts of the surgeon-prescribed opioid. Therefore, before consideration of incomplete study data, 73 of 80 patients (91%) enrolled into the study had useable study data. However, 6 patients had incomplete primary outcome data during at least one post-operative data collection time point. This meant that there was a minimal effective follow-up rate 67 of 80 patients (84%). However, each patient with follow-up data from the 73 of 80 patients that completed the study was included in every outcome for which they had complete data (i.e. even if a patient did not complete their home booklet, they were not excluded from contributing to 2-week and 6-week opioid usage outcomes). Patients declining participation, losses to follow-up, exclusions, and incomplete documentation contribute to potential selection and transfer bias.
As noted above, despite repeated reinforcement regarding the importance of accurate measurement of post-operative opioid usage, two patients reported that they used opioids from outside providers. This invalidated their results since an accurate count of post-operative opioid usage could not be made. It is conceivable that other patients could also have used outside opioids. However, to the authors’ knowledge, there is not an efficient and affordable way to be absolutely certain that patients are taking opioids prescribed by a specific provider. Additionally, although opioid usage was closely tracked, post-operative NSAID usage was not measured in this study due to the potential high rate of patients using previously-purchased supplies of over-the-counter NSAID rather than the naproxen that was prescribed. This could have prevented obtaining reliable estimates of post-operative NSAID usage. Pre-operative NSAID usage was not associated with post-operative opioid usage in multivariable models. However, post-operative NSAID usage could have an effect on post-operative opioid usage. Lastly, the results of a high-volume, academic, tertiary-care center may not be applicable to all hip arthroscopy providers. Surgeons should validate study findings in their own patient population since there may be differences from our institution in terms of patient characteristics and operative and post-operative treatment.
CONCLUSION
Among patients undergoing hip arthroscopy for femoroacetabular impingement syndrome, any opioid usage in the two weeks preceding surgery was the strongest predictor of opioid usage after hip arthroscopy. The impact of pre-operative opioid usage far exceeded the impact of other baseline patient and operative factors including psychiatric scores. Assessment of pre-operative opioid usage could be an important factor in guiding post-operative opioid prescribing.
Supplementary Material
Surgical and post-operative technique.
Patient positioning
- Traction boots applied
-
○One investigator routinely applied boots pre-operatively while the other applied the boots in the operating room
-
○
Patient positioned supine on HANA traction bed
Perineal post applied
Non-operative leg abducted
Coviden sequential compression devices placed bilaterally
Operative area prepped with chlorhexidine
Operative area draped
Ioban tape applied to operative area
Traction applied to operative leg to open joint space
Internal rotation to align landmarks for first portal
Anterolateral portal placement
First portal (anterolateral) established through intermuscular plane between TFL and Sartorius under x-ray guidance
Blunt dissection used to widen portal track
Anterior portal placed under arthroscopic guidance
Capsulotomy
- Smooth, continuous release of anterior capsule from both anterior ports
-
○One investigator routinely shaved capsular edges while the other investigator did not
-
○
Capsule suspended with sutures
Chondro-labral junction evaluation and labral repair
Osteochondral separation stabilized as clinically indicated
Acetabular rim prepared using shaver, radiofrequency ablation, and burr
- Labrum repaired with suture anchors
-
○One investigator used loop sutures while the other investigator used mattress sutures
-
○
Femoral neck
- Femoral osteochondroplasty achieved using radiofrequency ablation and burr
-
○One investigator used a T-capsulotomy while the other did not
-
○
- Traction released
-
○One investigator also routinely released boot straps following femoral osteochondroplasty
-
○
Capsular closure
Complete capsular repair with non-absorbable suture. Intra-operative factors such as joint laxity encouraged further water-tight closure.
Port site closure
- Dexamethasone and novocaine injected
-
○One investigator injected at port sites while the other injected intra-articularly
-
○
Disposition
- All patients stayed <24 hours in observation
-
○Inpatient medications were ordered at the discretion of the orthopaedic house staff and anesthesia staff on duty
-
○
All patients discharged to home
Physical therapy
- Patients instructed to begin early ROM exercises as indicated based on rehabilitation protocol
-
○CPM
-
■CPM 4–6 hours per day with progressively increasing flexion arc for 3–4 weeks
-
■Belly laying for 2 hours per day
-
■Hip brace to be worn at night (0 – 20 degrees flexion)
-
■
-
○Active ROM
-
■See plan entitled “Active ROM Exercise Plan” below
-
■
-
○
All patients instructed to begin formal physical therapy within 1 week following surgery
Post-operative medications
- Oxycodone 5 – 10 mg every 4 hours as needed for pain
-
○Patients were allowed to request other opioid pain medication based on prior experience (e.g. tramadol, hydrocodone-acetaminophen, dilaudid)
-
○
Tylenol ER as needed for pain
Phenergan as needed for nausea
Naproxen 500 mg once daily for heterotopic ossification prophylaxis
Aspirin 325 mg once daily for DVT prophylaxis
Gabapentin and pregabalin only ordered if clinically indicated
Meloxicam and indomethacin only ordered based on patient request
Active ROM Exercise Plan.
WEEK 1
Perform exercises 1, 2 and 3 every hour, 15 repetitions each.
Perform exercises 4 and 5, 10–20 repetitions, 2–3 times per day.
Ankle pumps – Move both feet up and down and around in circles.
Quadriceps setting - Tighten the muscle in the front of your thigh by pushing the back of your knee down, and hold for 5 seconds without holding your breath.
Gluteal setting – Tighten your buttocks muscles and hold for 5 seconds without holding your breath.
Short-arc quad - Place a small roll or pillow under your knee. Lift foot off of bed and straighten knee. Hold knee straight for 5 seconds, then slowly lower foot down to bed.
Lie face down on your stomach in prone position or "on your belly" so your thigh is straight in line with your upper body. Do this on a comfortable surface. Work up to lying in prone position for 2 hours a day for the first two weeks after your surgery. This helps to stretch the tissue about the hip joint.
Stationary bike without resistance for 15–20 minutes (if you have access to one)
WEEK 2
With both hands, hold onto a stable support such as a countertop or door frame.
Perform exercises 1, 3 through 7 10–20 repetitions, 2–3 times per day on the operated leg.
Perform these exercises only on the operated leg, as exercises on the non-operative hip will cause you to weight bear on the operated side
Hip abduction gravity eliminated with mild resistance – While lying on your back, slide your leg out to the side and then return to starting position. Only do this exercise while lying down. Move the operative leg away from mid-line without lifting the leg off of the surface. Keep knee straight and pointed to the ceiling.
Hip and knee bending – While lying on your back, slide your heel along the bed so that the hip and knee bend, then slide foot back down.
Standing hip flexion – Move your leg forward, keeping knee straight, and return to starting position. Do not lean backwards.
Standing knee flexion – Bend your knee so your foot moves towards buttocks. Keep thigh straight and do not let it extend backwards.
WEEK 3
Begin to add stretching
Hamstring – Stand with heel propped on low table, knee straight, as shown. Gently and slowly lead forward at waist. Hold stretch for 30 seconds.
Standing hip/knee flexion – Bend hip and knee of involved leg up as if marching in place.
WEEK 4
Standing hip abduction – Move your leg straight out to the side and then return to starting position. Do not move your body or let your leg turn inward or outward. Do not add extra weight to your leg.
Hip abduction gravity eliminated with mild resistance – While lying on your back, slide your leg out to the side and then return to starting position – this time add a thera-band or resistance rubber band from about your ankles. Only do this exercise while lying down. Make a loop out of thera-band or a lightly resistant elastic material. Place the loop around both legs at the ankle level. Keep the non-operative leg still – as a “post” for the thera-band. Move the operative leg away from mid-line. Keep knee straight and pointed to the ceiling.
Acknowledgments
Funding provided in part by the National Institutes of Health Clinical and Translational Sciences Awards (TL1TR001116). The funding source played no role in the investigation.
Appendix Table 1.
Oral morphine equivalent dosage conversion chart
| Medication | Oral morphine mg equivalent/1 unit medication |
|---|---|
| IV fentanyl (mcg) | 0.25 |
| IV hydromorphone (mg) | 20 |
| oral codeine (mg) | 0.15 |
| oral hydrocodone (mg) | 1 |
| oral hydromorphone (mg) | 4 |
| oral meperidine (mg) | 0.1 |
| oral oxycodone (mg) | 1.5 |
| oral oxycontin (mg) | 1.5 |
| oral tramadol (mg) | 0.1 |
Appendix Table 2.
Univariate significance testing of baseline and operative characteristics with study outcomes. Factors with univariate significance less than 0.1 were included in multivariable outcome models.
| Character istic |
Intra- proced ure OME’s (n=73) |
Post-op in- hospita l OME’s (n=73) |
2- wee k OM E’s (n=7 3) |
Prescrib ed OME’s to 6- week visit (n=73) |
Prescrib ed OME’s to 90d post-op (n=73) |
Prescribed additional narcotics at/after 6-week visit up to 90d post-op? (n=73) |
6- wee k OM E’s (n=6 7) |
Remaini ng OME’s at 6- week visit (n=67) |
2-week OME’s > 1.5*5*30 (30 oxycodone 5mg pills) |
6-week OME’s > 1.5*5*30 (30 oxycodone 5mg pills) |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 0.2901 | 0.0806 | 0.3279 | 0.7134 | 0.9632 | 0.9844 | 0.8601 | 0.2447 | 0.5228 | 0.3489 |
| Female gender | 0.3456 | 0.7498 | 0.9192 | 0.6144 | 0.8042 | 0.8287 | 0.9041 | 0.5709 | 0.592 | 0.8727 |
| Caucasian race | 0.2466 | 0.6909 | 0.495 | 0.6804 | 0.7558 | 0.6499 | 0.6746 | 0.2855 | 0.8338 | 0.6738 |
| BMI | 0.0193 | 0.6533 | 0.2964 | 0.4254 | 0.3314 | 0.5293 | 0.0828 | 0.2265 | 0.2151 | 0.2783 |
| ASA | 0.6178 | 0.1349 | 0.0464 | 0.3206 | 0.1882 | 0.223 | 0.0056 | 0.1313 | 0.1209 | 0.2792 |
| Any opioid usage in 2 weeks prior to surgery | 0.5942 | 0.5694 | <0.0001 | <0.0001 | <0.0001 | 0.056 | <0.0001 | 0.2449 | <0.0001 | <0.0001 |
| Anti-inflammatory usage | 0.6159 | 0.0594 | 0.658 | 0.2532 | 0.5281 | 0.3002 | 0.8064 | 0.2154 | 0.9347 | 0.9273 |
| Pain (out of 10) | 0.2248 | 0.8734 | 0.6408 | 0.7059 | 0.6822 | 0.6566 | 0.2585 | 0.4557 | 0.4499 | 0.6458 |
| iHOT-12 (out of 100) | 0.3459 | 0.5792 | 0.083 | 0.2056 | 0.1688 | 0.3549 | 0.01 | 0.4381 | 0.0954 | 0.0726 |
| PHQ (out of 24) | 0.5785 | 0.4664 | 0.0602 | 0.0433 | 0.036 | 0.6348 | 0.0052 | 0.8303 | 0.0517 | 0.0728 |
| PCS (out of 52, n=71) | 0.7552 | 0.3474 | 0.0071 | 0.083 | 0.0894 | 0.2669 | 0.004 | 0.5655 | 0.053 | 0.0456 |
| Prior ipsilateral hip surgery | 0.6507 | 0.056 | 0.181 | 0.1786 | 0.0246 | 0.0152 | 0.0251 | 0.4211 | 0.0243 | 0.035 |
| Nerve block | 0.389 | 0.6682 | 0.3742 | 0.7376 | 0.6302 | 0.6302 | 0.2502 | 0.2915 | 0.3418 | 0.1773 |
| Procedure duration | 0.6687 | 0.2796 | 0.4258 | 0.0037 | 0.001 | 0.2107 | 0.0062 | 0.388 | 0.2283 | 0.0369 |
| Additional procedure | 0.873 | 0.8447 | 0.5745 | 0.5754 | 0.2887 | 0.1568 | 0.2478 | 0.5296 | 0.5084 | 0.8406 |
| (see next table for details) | ||||||||||
| Surgeon using CPM | 0.1292 | 0.0021 | 0.1392 | 0.0019 | 0.0037 | 0.6345 | 0.0295 | 0.254 | 0.0241 | 0.0702 |
Appendix Table 3.
Opioid usage for entire study sample and divided by patients with and without opioid usage in the two weeks prior to surgery (unadjusted results) for outcomes except for 2-week and 6-week post-operative opioid usage outcomes.
| Outcome | Entire sample | Without pre-op opioid | With pre-op opioid |
|---|---|---|---|
| OME’s per day pre-op | 5 (13.4) | 0 (0) | 23 (20.6) |
| Intra-operative OME’s (n=73) | 63.9 (28.2) | 64.8 (30.1) | 60.5 (20.9) |
| Post-op in-hospital OME’s (n=73) | 44.8 (47.8) | 43.1 (49.1) | 50.9 (43.9) |
| Prescribed OME’s to 6-week visit (n=73) | 617.5 (315.6) | 542.8 (286.2) | 883.9 (274.6) |
| Prescribed OME’s to 90d post-op (n=73) | 657.4 (362.5) | 566.7 (302.7) | 980.8 (382) |
| Remaining OME’s at 6-week visit (n=67) | 376.1 (262.9) | 396.2 (269.7) | 306.1 (232.5) |
| Prescribed additional narcotics at/after 6-week visit up to 90d post-op? (n=73) | 11/73 (15%) | 6/57 (10.5%) | 5/16 (31.2%) |
Appendix Table 4.
Multivariable outcome modeling incorporating all pre-operative and operative factors from Table 1 that met a univariate significance threshold of 0.1 for outcomes other than 2-week and 6-week opioid usage outcomes.
| Outcome | Patient or operative characteristic | Adjusted estimate or odds ratio | P-value |
|---|---|---|---|
| Intra-operative OME’s (n=73) | BMI | 1.36/point (0.23, 2.49) | 0.019 |
| Post-op inhospital OME’s (n=73) | Active ROM surgeon | 27.49 (5.45, 49.53) | 0.015 |
| Age | −1.05/year (−1.96, −0.14) | 0.025 | |
| Prior ipsilateral hip surgery | 40.97 (−0.33, 82.28) | 0.052 | |
| Anti-inflammatory usage in 2 weeks prior to surgery | 11.6 (−10.14, 33.33) | 0.29 | |
| iHOT-12 (out of 100) | 0.11/point (−3.22, 3.43) | 0.95 | |
| Prescribed OME’s to 6-week visit (n=73) | Opioid usage in 2 weeks prior to surgery | 269.4 (105.21, 433.6) | 0.002 |
| Active ROM surgeon | 154.53 (18.61, 290.45) | 0.026 | |
| Procedure duration (hours) | 76.26/hour (−53.3, 205.82) | 0.24 | |
| PHQ (out of 24) | 2.67/point (−12.8, 18.14) | 0.73 | |
| PCS (out of 52) | 0.55/point (−5.13, 6.23) | 0.85 | |
| Prescribed OME’s to 90d post-op (n=73) | Opioid usage in 2 weeks prior to surgery | 322.8 (137.99, 507.62) | <0.001 |
| Active ROM surgeon | 141.07 (−12.41, 294.54) | 0.071 | |
| Procedure duration (hours) | 98.19/hour (−51.82, 248.2) | 0.196 | |
| Prior ipsilateral hip surgery | 186.08 (−113.68, 485.84) | 0.22 | |
| PHQ (out of 24) | 2.38/point (−15.07, 19.84) | 0.79 | |
| PCS (out of 52) | 0.65/point (−5.75, 7.05) | 0.84 | |
| Prescribed additional narcotics at/after 6-week visit up to 90d post-op? (n=73) | Prior ipsilateral hip surgery | 10.29 odds ratio (1.37, 77.18) | 0.023 |
| Opioid usage in 2 weeks prior to surgery | 3.57 odds ratio (0.84, 15.14) | 0.084 | |
| Remaining OME’s at 6-week visit (n=67) | No univariate significance | N/A | N/A |
Appendix Table 5.
Pain and functional outcomes for patients with and without pre-operative opioid usage.
| Outcomes | Without pre-op opioid | With pre-op opioid | P-value |
|---|---|---|---|
| 2-week pain MCID | 44/54 (81.4%) | 12/16 (75%) | 0.58 |
| 2-week pain change | −2.6 (−4.7, −0.5) | −2.4 (−6, 1.2) | 0.77 |
| 6-week pain MCID | 46/52 (88.4%) | 12/15 (80%) | 0.42 |
| 6-week pain change | −3.4 (−6.2, −0.5) | −3.6 (−10.3, 3.1) | 0.75 |
| 6-week iHOT-12 MCID | 31/52 (59.6%) | 8/15 (53.3%) | 0.66 |
| 6-week iHOT-12 change | 25.2 (19.8, 30.6) | 29.5 (12.4, 46.6) | 0.51 |
Appendix Table 6.
Baseline pre-operative characteristics for the samples with and without pre-operative opioid usage. “n/s” indicates that the factor did not meet the univariate significance threshold of 0.1.
| Baseline characteristics | Without pre-op opioid | With pre-op opioid | Univariate p-value | Multivariable p-value | Estimate |
|---|---|---|---|---|---|
| Age (years) | 35.8 (11.5) | 39.2 (10.1) | 0.29 | n/s | n/s |
| Female gender | 44/57 (77.1%) | 11/16 (68.7%) | 0.50 | n/s | n/s |
| Caucasian race | 50/57 (87.7%) | 13/16 (81.2%) | 0.52 | n/s | n/s |
| BMI | 25.9 (5.1) | 31.1 (5.8) | 0.002 | 0.016 | 1.16 unit odds ratio/point (0.76, 0.97) |
| ASA | 1.7 (0.4) | 2.1 (0.5) | <0.001 | 0.043 | 5.81 unit odds ratio/point (0.03, 0.95) |
| Anti-inflammatory usage in the 2 weeks prior to surgery? | 28/57 (49.1%) | 9/16 (56.2%) | 0.61 | n/s | n/s |
| Pre-operative pain (out of 10) | 5.2 (2.4) | 6.2 (2.1) | 0.125 | n/s | n/s |
| iHOT-12 (out of 100) | 33.1 (18.3) | 22.5 (17.2) | 0.036 | 0.91 | 1.00 unit odds ratio/point (0.96, 1.04) |
| PHQ (out of 24) | 5.1 (5.4) | 8.2 (5.5) | 0.057 | 0.74 | 1.03 unit odds ratio/point (0.84, 1.13) |
| PCS (out of 52) | 14.5 (14.7) | 22.7 (13.2) | 0.054 | 0.32 | 1.03 unit odds ratio/point (0.92, 1.03) |
| Prior ipsilateral hip surgery | 3/57 (5.2%) | 2/16 (12.5%) | 0.34 | n/s | n/s |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Manchikanti L, Helm S, 2nd, Fellows B, et al. Opioid epidemic in the United States. Pain Physician. 2012;15:ES9–38. [PubMed] [Google Scholar]
- 2.Okie S. A flood of opioids, a rising tide of deaths. The New England journal of medicine. 2010;363:1981–1985. doi: 10.1056/NEJMp1011512. [DOI] [PubMed] [Google Scholar]
- 3.Pizzo PA, Clark NM. Alleviating suffering 101–pain relief in the United States. The New England journal of medicine. 2012;366:197–199. doi: 10.1056/NEJMp1109084. [DOI] [PubMed] [Google Scholar]
- 4.Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. Jama. 2008;300:2613–2620. doi: 10.1001/jama.2008.802. [DOI] [PubMed] [Google Scholar]
- 5.Morris BJ, Mir HR. The opioid epidemic: impact on orthopaedic surgery. J Am Acad Orthop Surg. 2015;23:267–271. doi: 10.5435/JAAOS-D-14-00163. [DOI] [PubMed] [Google Scholar]
- 6.Bates C, Laciak R, Southwick A, Bishoff J. Overprescription of postoperative narcotics: a look at postoperative pain medication delivery, consumption and disposal in urological practice. J Urol. 2011;185:551–555. doi: 10.1016/j.juro.2010.09.088. [DOI] [PubMed] [Google Scholar]
- 7.Macintyre PE, Huxtable CA, Flint SL, Dobbin MD. Costs and consequences: a review of discharge opioid prescribing for ongoing management of acute pain. Anaesth Intensive Care. 2014;42:558–574. doi: 10.1177/0310057X1404200504. [DOI] [PubMed] [Google Scholar]
- 8.Bailey JE, Campagna E, Dart RC, Investigators RSPC The underrecognized toll of prescription opioid abuse on young children. Ann Emerg Med. 2009;53:419–424. doi: 10.1016/j.annemergmed.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 9.States EOotPotU. Epidemic: Responding to America’s Prescription Drug Abuse Crisis. 2011 [Google Scholar]
- 10.AAOS. Opioid use, misuse, and abuse in orthopaedic practice. American Academy of Orthopaedic Surgeons. 2015 [Google Scholar]
- 11.Frieden TR, Houry D. Reducing the Risks of Relief–The CDC Opioid-Prescribing Guideline. The New England journal of medicine. 2016;374:1501–1504. doi: 10.1056/NEJMp1515917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montgomery SR, Ngo SS, Hobson T, et al. Trends and demographics in hip arthroscopy in the United States. Arthroscopy: the journal of arthroscopic & related surgery: official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2013;29:661–665. doi: 10.1016/j.arthro.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Administration SAaMHS. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. (Vol HHS Publication No. (SMA) 11-4658: NSDUH Series H-41).Substance Abuse and Mental Health Services Administration. 2011 [Google Scholar]
- 14.Tan CO, Chong YM, Tran P, Weinberg L, Howard W. Surgical predictors of acute postoperative pain after hip arthroscopy. BMC Anesthesiol. 2015;15:96. doi: 10.1186/s12871-015-0077-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ward JP, Albert DB, Altman R, Goldstein RY, Cuff G, Youm T. Are femoral nerve blocks effective for early postoperative pain management after hip arthroscopy? Arthroscopy: the journal of arthroscopic & related surgery: official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2012;28:1064–1069. doi: 10.1016/j.arthro.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Chapman CR, Davis J, Donaldson GW, Naylor J, Winchester D. Postoperative pain trajectories in chronic pain patients undergoing surgery: the effects of chronic opioid pharmacotherapy on acute pain. J Pain. 2011;12:1240–1246. doi: 10.1016/j.jpain.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–1625. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 18.Helmerhorst GT, Vranceanu AM, Vrahas M, Smith M, Ring D. Risk factors for continued opioid use one to two months after surgery for musculoskeletal trauma. J Bone Joint Surg Am. 2014;96:495–499. doi: 10.2106/JBJS.L.01406. [DOI] [PubMed] [Google Scholar]
- 19.Lee D, Armaghani S, Archer KR, et al. Preoperative Opioid Use as a Predictor of Adverse Postoperative Self-Reported Outcomes in Patients Undergoing Spine Surgery. J Bone Joint Surg Am. 2014;96:e89. doi: 10.2106/JBJS.M.00865. [DOI] [PubMed] [Google Scholar]
- 20.Hina N, Fletcher D, Poindessous-Jazat F, Martinez V. Hyperalgesia induced by low-dose opioid treatment before orthopaedic surgery: An observational case-control study. Eur J Anaesthesiol. 2015;32:255–261. doi: 10.1097/EJA.0000000000000197. [DOI] [PubMed] [Google Scholar]
- 21.Rozet I, Nishio I, Robbertze R, Rotter D, Chansky H, Hernandez AV. Prolonged opioid use after knee arthroscopy in military veterans. Anesth Analg. 2014;119:454–459. doi: 10.1213/ANE.0000000000000292. [DOI] [PubMed] [Google Scholar]
- 22.Baker JF, Byrne DP, Hunter K, Mulhall KJ. Post-operative opiate requirements after hip arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2011;19:1399–1402. doi: 10.1007/s00167-010-1248-4. [DOI] [PubMed] [Google Scholar]
- 23.Bech NH. Perioperative pain management in hip arthroscopy, what options are there? Journal of Hip Preservation Surgery. 2016 doi: 10.1093/jhps/hnw015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cogan CJ, Knesek M, Tjong VK, et al. Assessment of Intraoperative Intra-articular Morphine and Clonidine Injection in the Acute Postoperative Period After Hip Arthroscopy. Orthopaedic journal of sports medicine. 2016;4 doi: 10.1177/2325967116631335. 2325967116631335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alam A, Gomes T, Zheng H, Mamdani MM, Juurlink DN, Bell CM. Long-term analgesic use after low-risk surgery: a retrospective cohort study. Arch Intern Med. 2012;172:425–430. doi: 10.1001/archinternmed.2011.1827. [DOI] [PubMed] [Google Scholar]
- 26.Wunsch H, Wijeysundera DN, Passarella MA, Neuman MD. Opioids Prescribed After Low-Risk Surgical Procedures in the United States, 2004–2012. Jama. 2016;315:1654–1657. doi: 10.1001/jama.2016.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 28.Griffin DR, Parsons N, Mohtadi NG, Safran MR, Multicenter Arthroscopy of the Hip Outcomes Research N A short version of the International Hip Outcome Tool (iHOT-12) for use in routine clinical practice. Arthroscopy: the journal of arthroscopic & related surgery: official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2012;28:611–616. doi: 10.1016/j.arthro.2012.02.027. quiz 616–618. [DOI] [PubMed] [Google Scholar]
- 29.Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP) Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S240–252. doi: 10.1002/acr.20543. [DOI] [PubMed] [Google Scholar]
- 30.Osman A, Barrios FX, Gutierrez PM, Kopper BA, Merrifield T, Grittmann L. The Pain Catastrophizing Scale: further psychometric evaluation with adult samples. J Behav Med. 2000;23:351–365. doi: 10.1023/a:1005548801037. [DOI] [PubMed] [Google Scholar]
- 31.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Devin CJ, Lee DS, Armaghani SJ, et al. Approach to pain management in chronic opioid users undergoing orthopaedic surgery. J Am Acad Orthop Surg. 2014;22:614–622. doi: 10.5435/JAAOS-22-10-614. [DOI] [PubMed] [Google Scholar]
- 33.Katz NP, Paillard FC, Ekman E. Determining the clinical importance of treatment benefits for interventions for painful orthopedic conditions. J Orthop Surg Res. 2015;10:24. doi: 10.1186/s13018-014-0144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stauffer ME, Taylor SD, Watson DJ, Peloso PM, Morrison A. Definition of nonresponse to analgesic treatment of arthritic pain: an analytical literature review of the smallest detectable difference, the minimal detectable change, and the minimal clinically important difference on the pain visual analog scale. Int J Inflam. 2011;2011:231926. doi: 10.4061/2011/231926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohtadi NG, Griffin DR, Pedersen ME, et al. The Development and validation of a self-administered quality-of-life outcome measure for young, active patients with symptomatic hip disease: the International Hip Outcome Tool (iHOT-33) Arthroscopy: the journal of arthroscopic & related surgery: official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2012;28:595–605. doi: 10.1016/j.arthro.2012.03.013. quiz 606–510 e591. [DOI] [PubMed] [Google Scholar]
- 36.Zarling BJ, Yokhana SS, Herzog DT, Markel DC. Preoperative and Postoperative Opiate Use by the Arthroplasty Patient. J Arthroplasty. 2016;31:2081–2084. doi: 10.1016/j.arth.2016.03.061. [DOI] [PubMed] [Google Scholar]
- 37.Collett BJ. Opioid tolerance: the clinical perspective. Br J Anaesth. 1998;81:58–68. doi: 10.1093/bja/81.1.58. [DOI] [PubMed] [Google Scholar]
- 38.Administration FaD. Disposal of unused medicines: What you should know. 2016 [Google Scholar]
- 39.Baker JF, McGuire CM, Byrne DP, Hunter K, Eustace N, Mulhall KJ. Analgesic control after hip arthroscopy: a randomised, double-blinded trial comparing portal with intra-articular infiltration of bupivacaine. Hip Int. 2011;21:373–377. doi: 10.5301/HIP.2011.8390. [DOI] [PubMed] [Google Scholar]
- 40.Lee EM, Murphy KP, Ben-David B. Postoperative analgesia for hip arthroscopy: combined L1 and L2 paravertebral blocks. J Clin Anesth. 2008;20:462–465. doi: 10.1016/j.jclinane.2008.04.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


